Metal Recovery from Wastewater Using Electrodialysis Separation
Abstract
:1. Introduction
2. Methodology and Review Structure
3. Fundamentals of Electrodialysis Processes
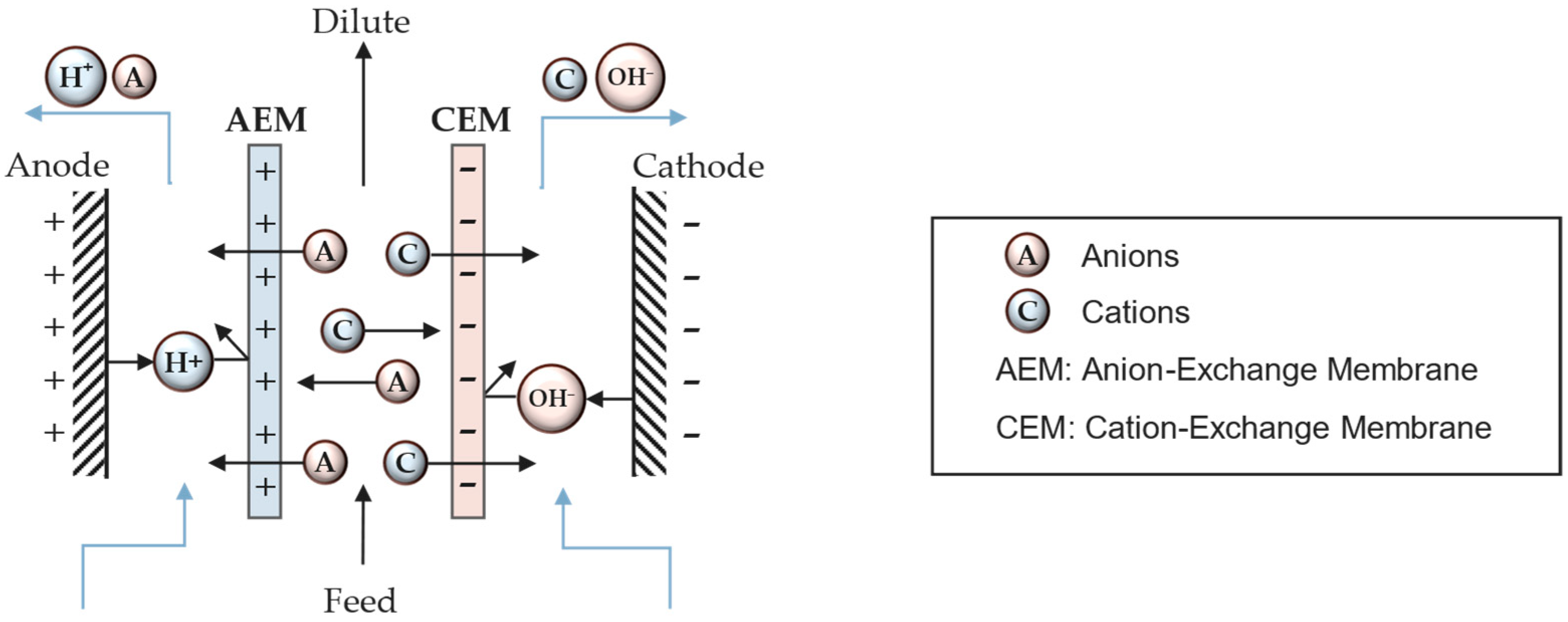
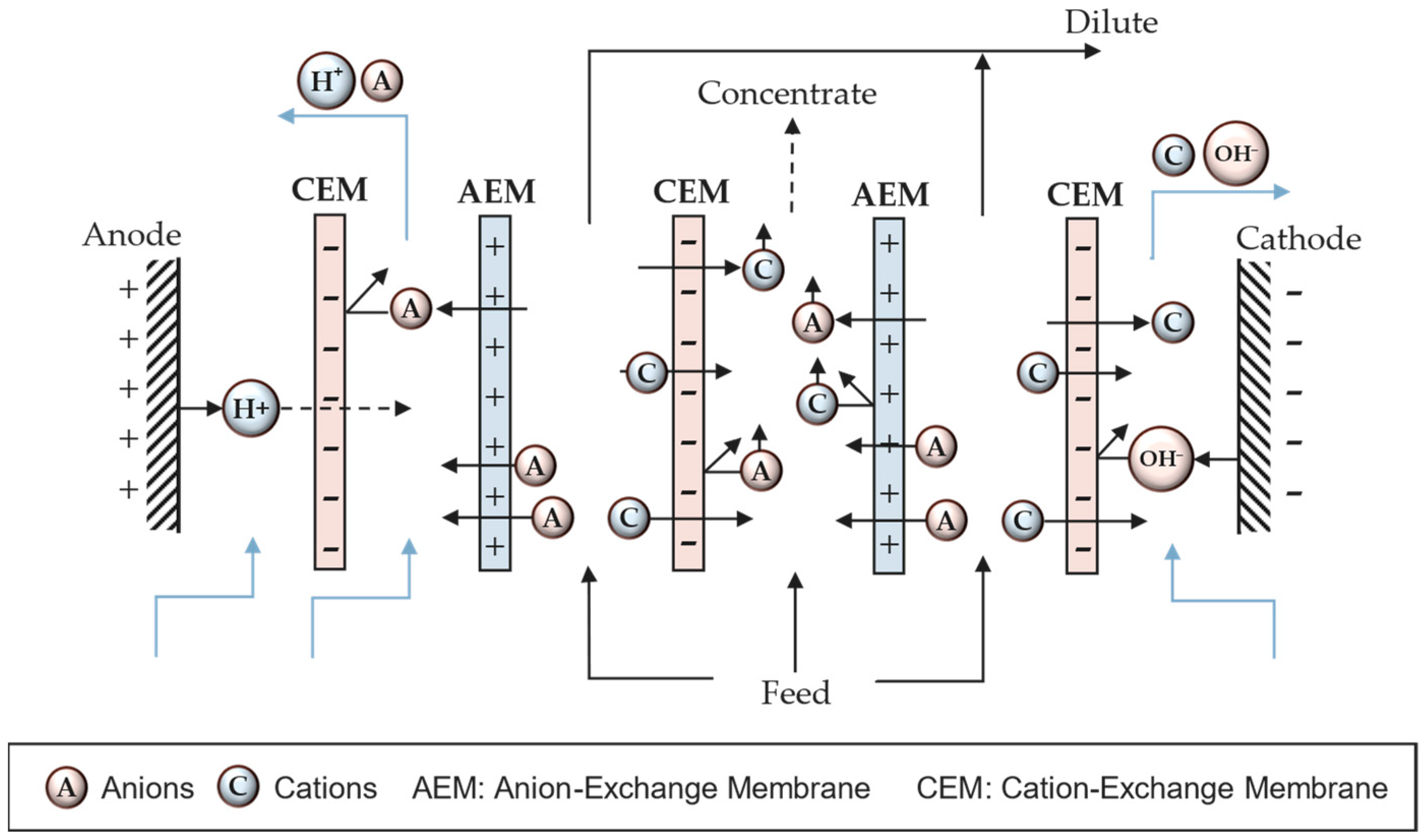
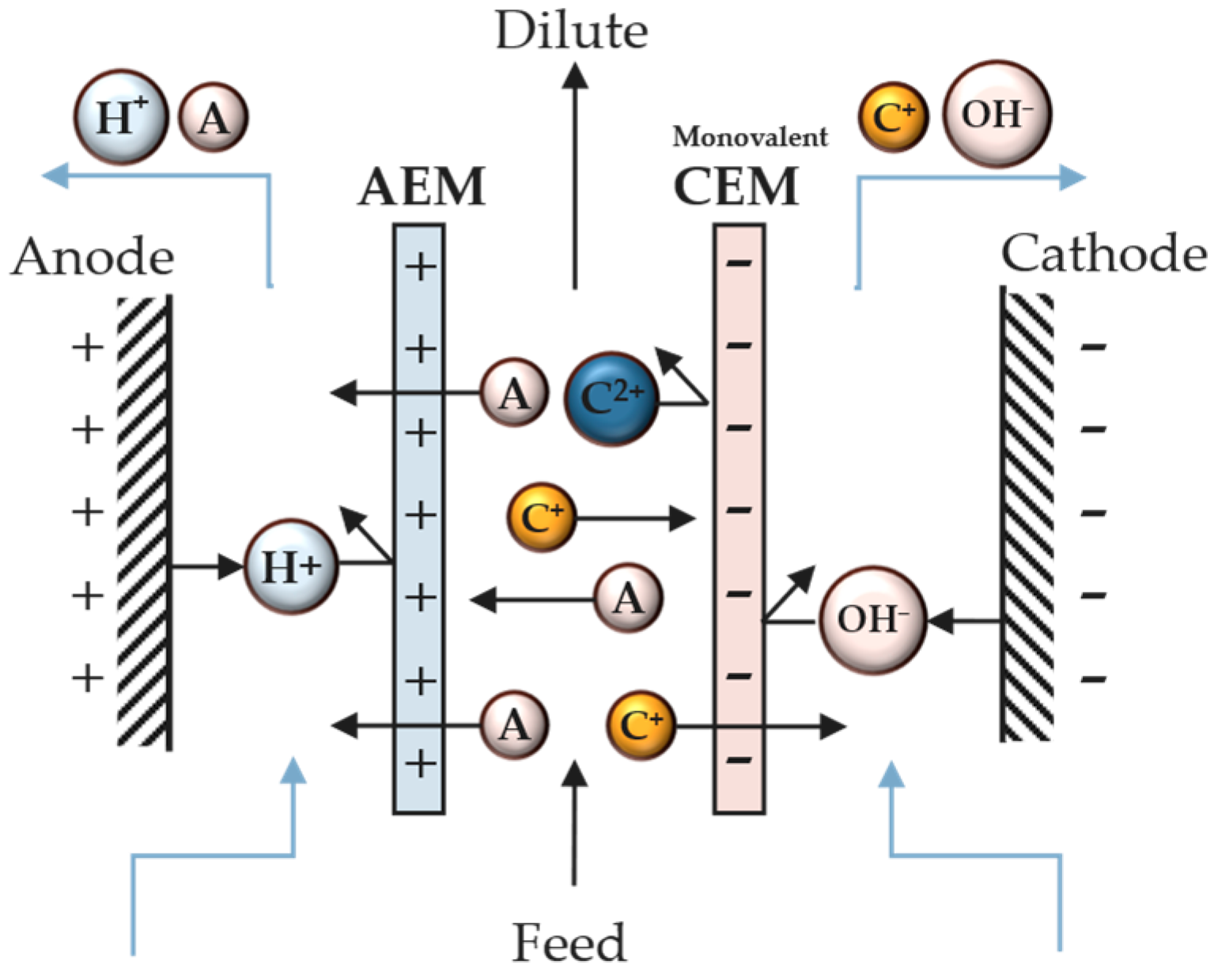
3.1. Operational Principle
3.2. Mass Transport Mechanism
4. Application of the Technique
4.1. General Application
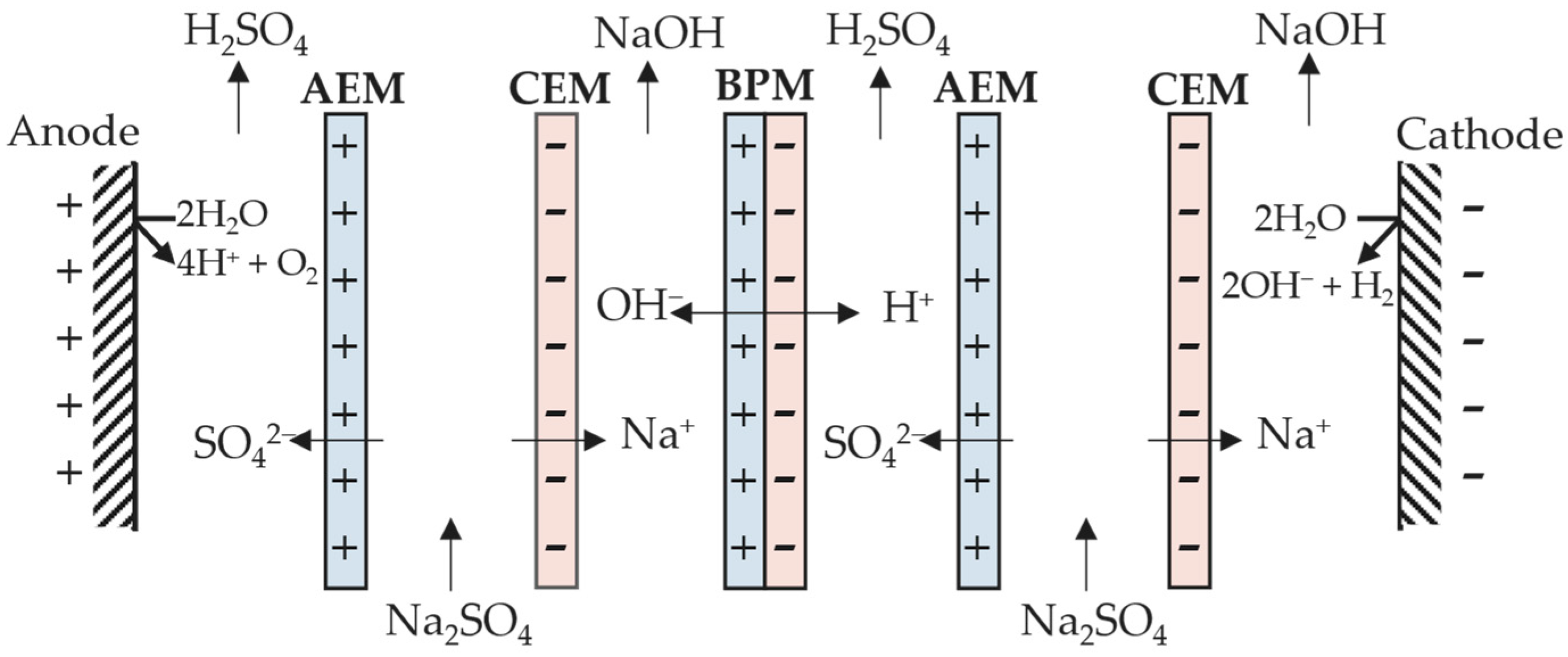
4.2. Applications to Wastewater Containing Metals
4.2.1. Single Metal Recovery from Aqueous Effluents
4.2.2. Multiple Metal Recovery from Aqueous Effluents
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, J.; Thakur, A.; Goyal, A. Chapter 1: Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; The Royal Society of Chemistry: London, UK, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Cerrillo-Gonzalez, M.M.; Paz-Garcia, J.M.; Rodriguez-Maroto, J.M.; Arhoun, B. Valorization of Lemon Peel Waste as Biosorbent for the Simultaneous Removal of Nickel and Cadmium from Industrial Effluents. Environ. Technol. Innov. 2021, 21, 101380. [Google Scholar] [CrossRef]
- Doble, M.; Kumar, A. Treatment of Waste from Metal Processing and Electrochemical Industries. In Biotreatment of Industrial Effluents; Butterworth-Heinemann: Oxford, UK, 2005; pp. 145–155. [Google Scholar] [CrossRef]
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Membrane Technology: A Versatile Tool for Saline Wastewater Treatment and Resource Recovery. Desalination 2022, 521, 115377. [Google Scholar] [CrossRef]
- Arana Juve, J.M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for Metal Removal and Recovery: A Review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Keele, UK, 2004; Volume 33. [Google Scholar]
- Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis for Wastewater Treatment—Part I: Fundamentals and Municipal Effluents. In Current Trends and Future Developments on (Bio-) Membranes: Membrane Technology for Water and Wastewater Treatment—Advances and Emerging Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–192. ISBN 9780128168240. [Google Scholar]
- Van der Bruggen, B. Ion-Exchange Membrane Systems—Electrodialysis and Other Electromembrane Processes. In Fundamental Modeling of Membrane Systems: Membrane and Process Performance; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–300. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar Membranes: A Review on Principles, Latest Developments, and Applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of Ion Exchange Membranes Used in the Electrodialysis Reversal Advanced Water Treatment: A Review. Chemosphere 2021, 263, 127951. [Google Scholar] [CrossRef]
- Tanaka, Y. Chapter 2: Electrodialysis Reversal. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12, pp. 383–404. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-Determining Membrane Properties in Reverse Electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S.; Burheim, O.S.; Deng, L. Cation Exchange Membranes and Process Optimizations in Electrodialysis for Selective Metal Separation: A Review. Membranes 2023, 13, 566. [Google Scholar] [CrossRef]
- Moura Bernardes, A.; Zoppas Ferreira, J.; Siqueira Rodrigues, M.A. Electrodialysis and Water Reuse: Novel Approaches; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642402494. [Google Scholar]
- Ebbers, B.; Ottosen, L.M.; Jensen, P.E. Electrodialytic Treatment of Municipal Wastewater and Sludge for the Removal of Heavy Metals and Recovery of Phosphorus. Electrochim. Acta 2015, 181, 90–99. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Peng, C.; Almeria, O.J.; Xu, H. Effect of PH on Separation of Pb (II) and NO3- from Aqueous Solutions Using Electrodialysis. Desalination 2012, 285, 46–53. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill Professional Publishing: New York, NY, USA, 1999; ISBN 0-07-016384-7. [Google Scholar]
- Strathmann, H. Electrodialysis, a Mature Technology with a Multitude of New Applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Dow Water & Process Solutions Aumenta La Eficiencia de La Planta de Agua Municipal MasPalomas I|IAgua. Available online: https://www.iagua.es/noticias/espana/dow-water-and-process-solutions/17/02/22/dow-water-process-solutions-aumenta (accessed on 26 May 2022).
- Generous, M.M.; Qasem, N.A.A.; Akbar, U.A.; Zubair, S.M. Techno-Economic Assessment of Electrodialysis and Reverse Osmosis Desalination Plants. Sep. Purif. Technol. 2021, 272, 118875. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy Consumption and Environmental Impact Assessment of Desalination Plants and Brine Disposal Strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Zhang, Z.; Xu, T. Electrodialysis of Concentrated Brine from RO Plant to Produce Coarse Salt and Freshwater. J. Membr. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Ma, G.; Wang, H.; Jiang, W.; He, Q.; Nirmalakhandan, N.; Xu, P. Study of Polyethyleneimine Coating on Membrane Permselectivity and Desalination Performance during Pilot-Scale Electrodialysis of Reverse Osmosis Concentrate. Sep. Purif. Technol. 2018, 207, 396–405. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Wu, L.; Shehzad, M.A.; Jiang, C.; Fu, R.; Liu, Z.; Xu, T. Multistage-Batch Electrodialysis to Concentrate High-Salinity Solutions: Process Optimisation, Water Transport, and Energy Consumption. J. Membr. Sci. 2019, 570–571, 245–257. [Google Scholar] [CrossRef]
- Korngold, E.; Aronov, L.; Daltrophe, N. Electrodialysis of Brine Solutions Discharged from an RO Plant. Desalination 2009, 242, 215–227. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard, V.J.H. The Benefits of Hybridising Electrodialysis with Reverse Osmosis. J. Membr. Sci. 2014, 469, 326–335. [Google Scholar] [CrossRef]
- Soliman, M.; Eljack, F.; Kazi, M.K.; Almomani, F.; Ahmed, E.; El Jack, Z. Treatment Technologies for Cooling Water Blowdown: A Critical Review. Sustainability 2021, 14, 376. [Google Scholar] [CrossRef]
- Gally, C.R.; Benvenuti, T.; Da Trindade, C.D.M.; Rodrigues, M.A.S.; Zoppas-Ferreira, J.; Pérez-Herranz, V.; Bernardes, A.M. Electrodialysis for the Tertiary Treatment of Municipal Wastewater: Efficiency of Ion Removal and Ageing of Ion Exchange Membranes. J. Environ. Chem. Eng. 2018, 6, 5855–5869. [Google Scholar] [CrossRef]
- Goodman, N.B.; Taylor, R.J.; Xie, Z.; Gozukara, Y.; Clements, A. A Feasibility Study of Municipal Wastewater Desalination Using Electrodialysis Reversal to Provide Recycled Water for Horticultural Irrigation. Desalination 2013, 317, 77–83. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Llanos, J.; Cotillas, S.; Cañizares, P.; Rodrigo, M.A. Novel Electrodialysis–Electrochlorination Integrated Process for the Reclamation of Treated Wastewaters. Sep. Purif. Technol. 2014, 132, 362–369. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpää, M. A Systematic Review and Statistical Analysis of Nutrient Recovery from Municipal Wastewater by Electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Mohammadi, R.; Ramasamy, D.L.; Sillanpää, M. Enhancement of Nitrate Removal and Recovery from Municipal Wastewater through Single- and Multi-Batch Electrodialysis: Process Optimisation and Energy Consumption. Desalination 2021, 498, 114726. [Google Scholar] [CrossRef]
- Cai, Y.; Han, Z.; Lin, X.; Duan, Y.; Du, J.; Ye, Z.; Zhu, J. Study on Removal of Phosphorus as Struvite from Synthetic Wastewater Using a Pilot-Scale Electrodialysis System with Magnesium Anode. Sci. Total Environ. 2020, 726, 138221. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus Recovery from Low Phosphate-Containing Solution by Electrodialysis. J. Membr. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, L.; Ke, X.; Ding, J.; Wu, X.; Chen, R.; Ding, R.; Van der Bruggen, B. Arsenic and Cation Metal Removal from Copper Slag Using a Bipolar Membrane Electrodialysis System. J. Clean. Prod. 2022, 338, 130662. [Google Scholar] [CrossRef]
- Pham, M.T.; Nishihama, S.; Yoshizuka, K. Effect of Operational Conditions on Arsenic Removal from Aqueous Solution Using Electrodialysis. Solvent Extr. Ion. Exch. 2021, 39, 655–667. [Google Scholar] [CrossRef]
- Benredjem, Z.; Delimi, R.; Khelalfa, A.; Saaidia, S.; Mehellou, A. Coupling of Electrodialysis and Leaching Processes for Removing of Cadmium from Phosphate Ore. Sep. Sci. Technol. 2016, 51, 718–726. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Zhang, M.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y. Cr(VI) Recovery from Chromite Ore Processing Residual Using an Enhanced Electrokinetic Process by Bipolar Membranes. J. Membr. Sci. 2018, 566, 190–196. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Liu, Y.; Chen, R.; Qian, Q.; Van der Bruggen, B. Cr(III) Recovery in Form of Na2CrO4 from Aqueous Solution Using Improved Bipolar Membrane Electrodialysis. J. Membr. Sci. 2020, 604, 118097. [Google Scholar] [CrossRef]
- Dai, L.; Ding, J.; Liu, Y.; Wu, X.; Chen, L.; Chen, R.; Van der Bruggen, B. Recovery of Cr(VI) and Removal of Cationic Metals from Chromium Slag Using a Modified Bipolar Membrane System. J. Membr. Sci. 2021, 639, 119772. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, M.; Wu, X.; Ding, J.; Dai, L.; Xue, H.; Ye, X.; Chen, R.; Ding, R.; Liu, J.; et al. Recovery of Copper from Electroplating Sludge Using Integrated Bipolar Membrane Electrodialysis and Electrodeposition. J. Colloid Interface Sci. 2023, 642, 29–40. [Google Scholar] [CrossRef]
- Hernández, J.; Tapia, J. Direct Copper Recovery from Pregnant Leaching Solutions (PLS), Using a Custom Electrolytic Cell, Based on Reactive Electrodialysis (RED). Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2023, 132, 110–116. [Google Scholar] [CrossRef]
- Yan, K.; Huang, P.; Xia, M.; Xie, X.; Sun, L.; Lei, W.; Wang, F. An Efficient Two-Chamber Electrodeposition-Electrodialysis Combination Craft for Nickel Recovery and Phosphorus Removal from Spent Electroless Nickel Plating Bath. Sep. Purif. Technol. 2022, 295, 121283. [Google Scholar] [CrossRef]
- Liu, Y.; Lian, R.; Wu, X.; Dai, L.; Ding, J.; Wu, X.; Ye, X.; Chen, R.; Ding, R.; Liu, J.; et al. Nickel Recovery from Electroplating Sludge via Bipolar Membrane Electrodialysis. J. Colloid Interface Sci. 2023, 637, 431–440. [Google Scholar] [CrossRef]
- Voutetaki, A.; Plakas, K.V.; Papadopoulos, A.I.; Bollas, D.; Parcharidis, S.; Seferlis, P. Pilot-Scale Separation of Lead and Sulfate Ions from Aqueous Solutions Using Electrodialysis: Application and Parameter Optimization for the Battery Industry. J. Clean. Prod. 2023, 410, 137200. [Google Scholar] [CrossRef]
- Babilas, D.; Dydo, P. Selective Zinc Recovery from Electroplating Wastewaters by Electrodialysis Enhanced with Complex Formation. Sep. Purif. Technol. 2018, 192, 419–428. [Google Scholar] [CrossRef]
- Xing, Z.; Srinivasan, M. Lithium Recovery from Spent Lithium-Ion Batteries Leachate by Chelating Agents Facilitated Electrodialysis. Chem. Eng. J. 2023, 474, 145306. [Google Scholar] [CrossRef]
- Hansen, H.K.; Ottosen, L.M.; Kliem, B.K.; Villumsen, A. Electrodialytic Remediation of Soils Polluted with Cu, Cr, Hg, Pb and Zn. J. Chem. Technol. Biotechnol. 1997, 70, 67–73. [Google Scholar] [CrossRef]
- Sun, J.; Su, X.; Liu, Z.; Liu, J.; Ma, Z.; Sun, Y.; Gao, X.; Gao, J. Removal of Mercury (Hg(II)) from Seaweed Extracts by Electrodialysis and Process Optimization Using Response Surface Methodology. J. Ocean Univ. China 2020, 19, 135–142. [Google Scholar] [CrossRef]
- Kırmızı, S.; Karabacakoğlu, B. Performance of Electrodialysis for Ni(II) and Cr(VI) Removal from Effluents: Effect of Process Parameters on Removal Efficiency, Energy Consumption and Current Efficiency. J. Appl. Electrochem. 2023, 53, 2039–2055. [Google Scholar] [CrossRef]
- Shestakov, K.V.; Lazarev, S.I.; Polyanskii, K.K.; Ignatov, N.N. Recovery of Iron, Nickel, and Copper in Waste Water from Printed Circuit Board Manufacture by Electrodialysis Method. Russ. J. Appl. Chem. 2021, 94, 555–559. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Membrane Extraction of Ag(I), Co(II), Cu(II), Pb(II), and Zn(II) Ions with Di(2-Ethylhexyl)Phosphoric Acid under Conditions of Electrodialysis with Metal Electrodeposition. Theor. Found. Chem. Eng. 2021, 55, 1204–1220. [Google Scholar] [CrossRef]
- Aliaskari, M.; Ramos, R.L.; Schäfer, A.I. Removal of Arsenic and Selenium from Brackish Water Using Electrodialysis for Drinking Water Production. Desalination 2023, 548, 116298. [Google Scholar] [CrossRef]
- Min, K.J.; Choi, S.Y.; Jang, D.; Lee, J.; Park, K.Y. Separation of Metals from Electroplating Wastewater Using Electrodialysis. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2471–2480. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of Lithium, Nickel, Manganese, and Cobalt from Waste Lithium-Ion Batteries Using Electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Recovery of Li and Co from LiCoO2 via Hydrometallurgical–Electrodialytic Treatment. Appl. Sci. 2020, 10, 2367. [Google Scholar] [CrossRef]
- Siekierka, A.; Yalcinkaya, F.; Bryjak, M. Recovery of Transition Metal Ions with Simultaneous Power Generation by Reverse Electrodialysis. J. Environ. Chem. Eng. 2023, 11, 110145. [Google Scholar] [CrossRef]
| Industries | Al | As | Cd | Cr | Cu | Hg | Pb | Ni | Zn | Co | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper mills | x | x | x | x | x | x | x | |||||
| Organic chemistry | x | x | x | x | x | x | x | |||||
| Fertilizer | x | x | x | x | x | x | x | x | x | |||
| Petroleum refinery | x | x | x | x | x | x | x | x | ||||
| Steel works | x | x | x | x | x | x | x | x | x | |||
| Aircrafts | x | x | x | x | x | x | x | |||||
| Textile mills | x | |||||||||||
| Power plants | x | |||||||||||
| Pharmaceutical | x | x | x | x | x | x | x | |||||
| Engineering | x | x | x | x | x | x | x | |||||
| Metal smelters | x | |||||||||||
| Electroplating | x | x | x | |||||||||
| Mining | x | |||||||||||
| Ferromanganese production | x | x |
| Research Questions | |
|---|---|
| Q1 | What are the fundamentals of electrodialysis? |
| Q2 | Can electrodialysis be used to treat wastewater? |
| Q3 | Is electrodialysis used to separate metals from industrial wastewater? |
| Database (2008–2023) | Electrodialysis and Metals | Electrodialysis and Metals and Wastewater | Electrodialysis and Metals and Wastewater and Recovery |
|---|---|---|---|
| Scopus | 676 | 239 | 111 |
| Web of Science | 648 | 185 | 111 |
| Sciencedirect | 227 | 80 | 44 |
| Metal | Sources | Recovery (%) | Time | Max V | Current Density (mA cm−2) | Energy (kWh/g) | Feed | pH | Membrane | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| As | Copper slag | 96.50 | 45 h | 9 | 3 | - | - | - | BPMs | [40] |
| As | Geothermal water | 91 As(III) 98 As(V) | 60 min | 25 | - | - | 5 mg/L As(III) 60 mg/L As(V) | 8 | CMB and AHA | [41] |
| Cd | Phosphate ore | 84.30 | 24 h | 8 | 10 | - | 50 mL 0.5 M Acetic acid + 2 g phosphate ore | 4.5 | CEM and AEM | [42] |
| Cr | Chromite ore | 82 | 350 h | 5 | 3 | 0.395 | 50 mL water NaNO3 + 50 g solid | 13.5 | BPMs | [43] |
| Cr | Aqueous solution | 87.8 Cr(III) | 24 h | 4 | 0.5 | 0.73 | 5 g/L Na2SO4 | 12 | BPM, CEM and AEM | [44] |
| Cr | Chromium slag | 70.6 Cr(VI) | 300 h | - | 3 | - | 50 mL water + 50 g solid | - | BPMs | [45] |
| Cu | Electroplating sludge | 96.4 | 5 h | - | 50 | 5.3 | 4 g/L Cu2+ | 0.5 | BPM, CEM, AEM | [46] |
| Cu | Pregnant leaching solutions | 99.2 | 5 h | 2.5 | 8 | 2.11 | 2.5 g/L Cu2+ | - | - | [47] |
| Ni | Electroless plating bath | 82.34 | 3 h | - | 3.5 | 0.0182 | 325 mg/L Ni2+ | 3 | AEM | [48] |
| Ni | Electroplating sludge | 94 | 28 h | - | 20 | - | S/L ratio 1:15 | - | BPM and CEM | [49] |
| Pb | Lead battery manufacture | 75 | 4 h | 40 | - | 7 kWh/m3 | 5 mg/L Pb2+ 1000 mg/L SO4− | - | AEM: PC SA CEM: PC SK | [50] |
| Zn | Electroplating waste | 86.6 | 60 min | - | 2.5 | - | 0.748 M ZnSO4·7H2O + Citric acid | 4 | CMH-AMH Ralex membranes | [51] |
| Li | Spent LIB leachate | 63.91 | 3 h | 15 | - | - | 7 | - | [52] |
| Metal | Sources | Recovery (%) | Time | Max V | Current Density (mA cm−2) | Energy | Feed | Stage | Membrane | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Cr(VI) Ni | Industrial effluent | 97.9 97.1 | 90 min | 25 | - | 38.57 Wh/L | 50 mg/L 50 mg/L | 1 | Ionac MC 3470 Ionac MA 3475 | [55] |
| Fe Ni Cu | Printed circuit boards | - | 50 min | 30 | 50 | - | - | 1 | PC Acid 60 (PCCell GmbH)CMH RALEX® (MEGA | [56] |
| Co Zn | Sulfuric acid solution | 93 100 | 5 h | - | 5 | - | 0.01 M 0.01 M | 1 | MK-40 CEM MK-40 AEM | [57] |
| As(III) Se(IV) Se(VI) | Brackish water | 58 80 80 | - | 25 | - | - | 50–1000 µg/L | 1 | - | [58] |
| Cu Ni | Electroplating wastewater | 90.7 90.2 | 25 min | 12 | 22.5 | - | 22.3 mg/L 24.4 mg/L | 1 | AMX Astom CMX Astom | [59] |
| Ni Co Li Mn | Lithium-ion battery leaching solution | 99.8 87.3 99 99 | 180 min | 18 | - | 9.65 kWh/mol 15.3 kWh/mol - - | 0.01 M 0.003 M 0.003 M 0.003 M | 3 | Neosepta AMXNeosepta CMXPCA PC 400D | [60] |
| Li Co | Lithium-ion battery leaching solution | 66 33 | 144 h | - | 1 | - | 1.75 g LiCoO2 350 mL 0.1 M HCl | 1 | Neosepta CMX | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal Recovery from Wastewater Using Electrodialysis Separation. Metals 2024, 14, 38. https://doi.org/10.3390/met14010038
Cerrillo-Gonzalez MdM, Villen-Guzman M, Rodriguez-Maroto JM, Paz-Garcia JM. Metal Recovery from Wastewater Using Electrodialysis Separation. Metals. 2024; 14(1):38. https://doi.org/10.3390/met14010038
Chicago/Turabian StyleCerrillo-Gonzalez, Maria del Mar, Maria Villen-Guzman, Jose Miguel Rodriguez-Maroto, and Juan Manuel Paz-Garcia. 2024. "Metal Recovery from Wastewater Using Electrodialysis Separation" Metals 14, no. 1: 38. https://doi.org/10.3390/met14010038
APA StyleCerrillo-Gonzalez, M. d. M., Villen-Guzman, M., Rodriguez-Maroto, J. M., & Paz-Garcia, J. M. (2024). Metal Recovery from Wastewater Using Electrodialysis Separation. Metals, 14(1), 38. https://doi.org/10.3390/met14010038







