Abstract
The presented work demonstrates the capability of obtaining composite powder, silver-fullerene soot, by the electrolytic deposition of silver from an aqueous solution of silver nitrate. The morphology of particles was studied as a function of fullerene soot concentration and current density. The microstructure of compact materials obtained by hot pressing was investigated. The hardness of the compact material increased up to 30% and the same corrosion properties relative to pure silver were obtained using a similar technology.
1. Introduction
Silver has the highest electrical and thermal conductivity of any metal and is widely used to create electrical contacts, jewelry and cutlery. Since silver has a low strength, and alloying leads to a decrease in conductivity and corrosion properties, dispersion hardening can be used to increase the mechanical properties with little loss of conductivity and corrosion properties The addition of reinforcement to the silver matrix compensates for the performance deficiencies of the matrix and saves the comprehensive electrical, mechanical, anticorrosion, and thermal properties of the composite materials [1,2,3].
Apart from the addition of oxide and ceramic particles into the silver matrix, a number of works suggest the addition of carbon nanostructures instead of them. The addition of nanodiamonds [4] and carbon nanotubes (CNTs) [5,6] into the silver matrix made it possible to increase the strength characteristics. In most works on the addition of carbon nanostructures into a metal matrix, a high-energy treatment [4,5,6,7,8] is mentioned, the disadvantage of which is the energy consumption and contamination of the matrix with the material of the grinding media.
In work [9], Ag/CNT composite films were obtained electrochemically using an iodide electroplating bath. A slight increase in hardness (from 60.4 to 63.2 HV) and electrical resistance (from 1.8 to 1.9 μΩ cm) and a decrease in the coefficient of friction were noted.
To date, there is no work devoted to Ag-based materials reinforced with fullerene soot (mainly amorphous carbon nanoparticles). In previous works, we showed, using the example of copper–fullerene soot materials, that it is possible to obtain composites with a combination of hardness–thermal conductivity properties comparable to copper-CNT materials [10]. In the presented work, a method is proposed for obtaining a composite powder material, silver–fullerene soot (FS), by electrolytic deposition from an aqueous solution of silver nitrate.
2. Materials and Methods
Silver powder was obtained galvanostatically. Titanium plates were used as the cathode, and silver (fused powder of technical-grade reduced silver oxalate) was used as the anode. Electrolyte composition was AgNO3—25 g/L, HNO3—15 g/L. The current density varied from 2 to 6 A/dm2. The process temperature was 20–30 °C. Fullerene soot (Suzhou Dade Carbon Nanotechnology Co., Suzhou, Jiangsu, China) in amounts of 0.05 and 0.2 g/L was additionally introduced into the electrolyte directly during the electrochemical process of obtaining silver powder. Fullerene soot consists of particles with an average size of 40 nm [10]. In this case, the electrolyte was mixed with an electromagnetic stirrer with a speed of 500 rpm for a uniform supply of carbon nanostructures to the cathode. Electrochemical deposition continued for 2 h, and then the electrolyte was filtered. The FS content in mass% was calculated by dividing the mass of FS by that of the Ag/FS powder. The samples were compacted by cold pressing at a pressure of 700 MPa, followed by hot pressing at 720 °C and a pressure of 350 MPa. To select the compacting modes, the data of paper [11] were taken into account.
To reveal the microstructure, the polished samples were subjected to electrochemical etching in a commercial electrolyte PLS-12 (NTC Technokom AS, Moscow, Russia) at a current density of 10 A/dm2, and the cathode was a titanium plate. Scanning electron microscopy (SEM) images were obtained on Phenom ProX. The hardness was determined using ZWICK ZHU via the Vickers method at a load of 10 kg, and the load time was 15 s. XRD was carried out on a Bruker D8 Advance diffractometer using Cu Kα monochromatic radiation (λ = 1.5406 Å). Polarization measurements of the films were conducted in a 0.01 M Na2S aqueous solution [6] at 25 °C using an electrochemical measurement system (IRC-Pro). The Ag/FS composite or Ag composite, a graphite rod, and a Ag/AgCl electrode (+0.199 V vs. standard hydrogen electrode (SHE)) were used as the working electrode, counter electrode, and the reference electrode, respectively.
3. Results and Discussion
SEM images of silver particles obtained by electrolysis without additives and with FS additives at a current density of 2 A/dm2 are shown in Figure 1. Silver particles have granular forms (Figure 1a–c). The mechanisms of silver deposition involve three-dimensional nucleation and growth as well as the layer-by-layer deposition of {111} close-packed planes, manifested by the presence of 111 surface steps [12]. The processes of silver electrodeposition were studied in detail by the authors of the works [12,13,14].
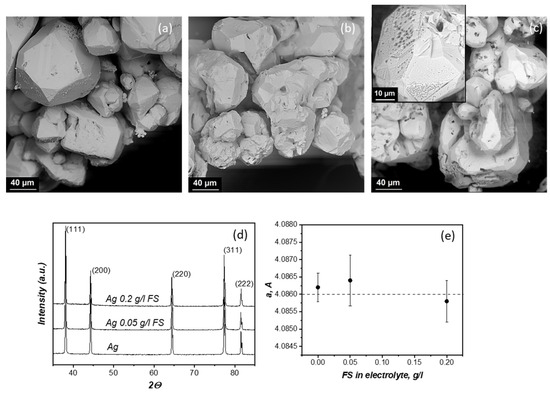
Figure 1.
SEM images of silver particles obtained by electrolysis without additives (a) and with 0.05 g/L (b) and 0.2 g/L (c) of FS at current density of 2 A/dm2. XRD of powders (d) and lattice parameter (e).
Fullerene soot from the electrolyte is likely deposited on the surface of the growing faces. At an FS concentration of 0.2 g/L, pronounced pyramidal pores are observed (inset of Figure 1c). A large amount of fullerene soot makes it difficult to reduce silver ions directly on the surface of silver particles, which may explain the presence of pores on the surface of the particles.
It is also worth paying attention to the fact that with an increase in the content of carbon structures in the electrolyte, the particle size does not change significantly.
The powders were examined by XRD, and the results are shown in Figure 1d. It can be seen that the diffraction pattern contains peaks corresponding to the crystallographic planes of silver; no other phases are found. Carbon in silver practically does not dissolve. A solid solution with a content of 0.036 atomic % is formed by peritectic transformation at 962 °C. According to the XRD data, the lattice parameter is determined, which varies within the error (Figure 1e).
In the second stage, for a concentration of 0.05 g/L, electrolysis was carried out with varying current density. This concentration was chosen because, with a higher content of fullerene soot in the electrolyte, a large amount of carbon is located on the surface of silver particles. Increasing the current density contributes to the production of smaller particles (Figure 2a,b). The average particle size decreases from 45 µm at 2 A/dm2 to 42 µm at 3 A/dm2, 35 µm at 4 A/dm2, and 15 µm at 6 A/dm2. At 6 A/dm2, a large amount of fine dendritic deposits is observed (Figure 2c).
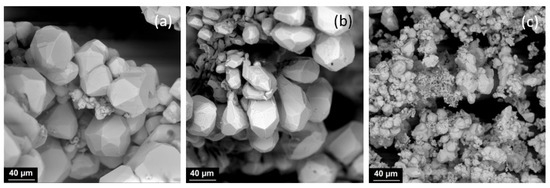
Figure 2.
SEM images of silver particles obtained by electrolysis with 0.05 g/L of FS at current densities 3 A/dm2 (a), 4 A/dm2 (b), 6 A/dm2 (c).
The resulting powder materials were compacted by hot pressing. The microstructures of the etched samples are shown in Figure 3a–e.
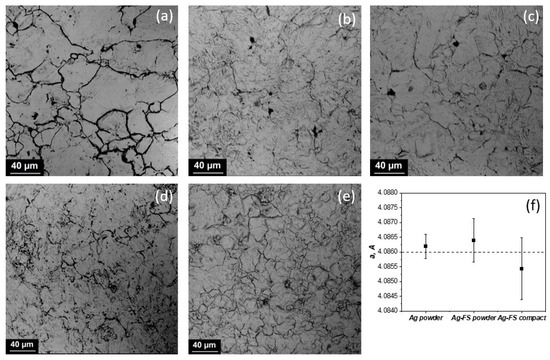
Figure 3.
The microstructures of hot-pressed materials: Ag (a); Ag obtained at current density 2 A/dm2 with 0.05 g/L (b) and 0.2 g/L (c) of FS in electrolyte; Ag with 0.05 g/L of FS in electrolyte obtained at current densities 4 A/dm2 (d) and 6 A/dm2 (e); lattice parameter (f).
The average grain size of pure silver was 33 µm; for samples with fullerene soot at 0.05 g/L, it was 27 µm; and for 0.2 g/L, it was 22 µm. Increasing the current density led to a significant decrease in particle size, but not such a significant reduction in grain size. For 4 and 6 A/dm2, the average grain size was 21 and 19 µm, respectively. The FS content in mass% was calculated by dividing the mass of FS by that of the Ag/FS powder. During electrolysis with the addition of 0.05 g/L FS and 0.2 g/L, the amount of carbon was 0.5 and 0.2 wt %. The lattice parameter of pure silver powder, silver with fullerene soot, and silver-FS compact material was within the error (Figure 3f).
The hardness values of the resulting compacts are shown in Figure 4a. During deposition at a current density of 2 A/dm2, the maximum increase in hardness was 19% at an FS concentration in the electrolyte of 0.05 g/L (relatively pure silver obtained by a similar technology). An increase in the FS concentration in the electrolyte led to a decrease in hardness, despite a decrease in the grain size. This may be caused by the agglomeration of nanocarbon in the pores of silver particles. An increase in the current density during the deposition of composite particles in an electrolyte containing 0.05 g/L FS led to an increase in hardness by 30% at 4 A/dm2. The relative density of samples determined by hydrostatic weighing, using Ag and FS densities of 10.49 and 1.56 g cm−3 [15], respectively, was more than 97.5%.
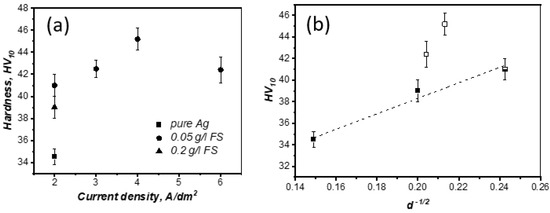
Figure 4.
Hardness values of hot-pressed materials (a). Hall–Petch dependence (b) (closed dots—current density of 2 A/dm2, open dots—current density of 3–6 A/dm2).
The literature describes a number of mechanisms for strengthening a metal matrix with carbon nanostructures [16]. The Hall–Petch and Orowan mechanisms are the most common and applicable for the strengthening phase in the form of nanoparticles.
The grain size directly depends on the length of the grain boundaries, and the influence of dislocations interacting with the grain boundaries affects the strength of the composite. The strength of the material will increase as the grain size decreases. This important and general mechanism for strengthening materials is noted in [17], where the carbon phase prevents grain growth during sintering or high-temperature annealing, which may be the primary cause of strengthening. Therefore, the relationship between the yield stress of the composite and the average grain diameter (d) can be expressed by the Hall–Petch relation according to Equation (1):
where σ0 is the friction stress required for dislocation glide in a single crystal, and K is an individual constant for each material, also called the “Hall-Petch coefficient”. The hardness of the material correlates with the yield strength of the material [18].
Dispersion strengthening according to the Orowan mechanism is such that dispersed particles are obstacles in the path of dislocations moving in their slip planes.
Silver is not a carbide-forming element and does not form solid solutions with carbon, so the Orowan dispersion strengthening mechanism can be considered. In [19], hardening by the Orowan–Ashby mechanism is presented in the form of Equation (2):
where Gm is the shear modulus of the matrix, b is the Burgers vector, Lp is the average distance between particles of the strengthening phase, and r is the radius of the particle.
Based on the observations and the Hall–Petch dependence (Figure 4b), it can be assumed that carbon nanoparticles are located mainly along grain boundaries at a current density of 2 A/dm2, preventing grain growth. It is worth noting that the size of the composite particles averages 40–80 µm (Figure 1), and the grain size is significantly lower (up to 33 µm for pure silver, Figure 3). In this case, during hot pressing, recrystallization of silver occurs and the growth of new grains is inhibited by the presence of fullerene soot; this can explain the decrease in grain size in the composite with an increasing content of fullerene soot and constant current density. For samples obtained at 3–6 A/dm2 (the particle size is smaller and decreases from 45 to 15 μm with increasing current density), no direct dependence of hardness on grain size is observed, and the hardness values are higher and do not fall into the Hall–Petch relationship, which may indicate a contribution to the strengthening of the Orowan mechanism.
Figure 5 shows the polarization curves for the composites. The corrosion potential of both the Ag and the Ag/FS composite was approximately −152 mV, and their cathode and anode polarization curves were also similar. Thus, the sulfidation resistance of the Ag composite and that of the Ag/FS composite differed only slightly.
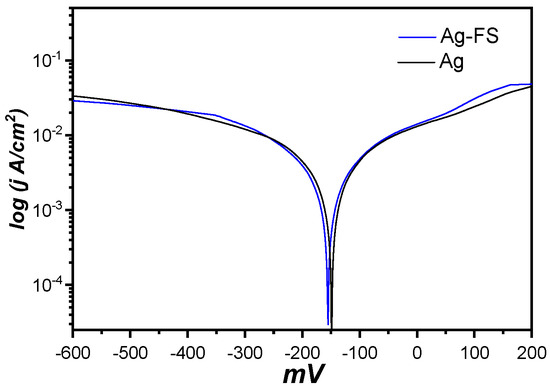
Figure 5.
Polarization curves for the Ag and Ag/FS composite.
4. Conclusions
A method is proposed for obtaining a composite powder material silver–fullerene soot (FS) by electrolytic deposition from an aqueous solution of silver nitrate with suspended carbon nanoparticles in an electrolyte. With an increase in the content of carbon structures in the electrolyte, the particle size does not change significantly. At an FS concentration of 0.2 g/L, pronounced pyramidal pores are observed. Increasing the current density leads to a decrease in the average particle size from 45 µm at 2 A/dm2 to 15 µm at 6 A/dm2.
Compact silver-FS materials obtained by hot pressing have a finer grain structure compared to pure silver. The maximum increase in hardness is 30% relative to silver. The density of composites is more than 97.5%. X-ray diffraction analysis showed that no additional phases and solid solutions are formed. Full strengthening of the composites is mainly attributed to the Orowan strengthening and the grain-size refinement. The corrosion potential of both the Ag and the Ag/FS composite is approximately −152 mV, and their cathode and anode polarization curves are also similar.
Author Contributions
Conceptualization, T.S.K.; methodology, V.A.P.; investigation, V.A.T.; data curation, E.V.B.; writing—original draft preparation, O.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research is partially funded by the Ministry of Science and Higher Education of the Russian Federation as a part of the World-class Research Center program: Advanced Digital Technologies (contract No. 075-15-2022-311 dated 20 April 2022).
Data Availability Statement
Data is contained within the article.
Acknowledgments
Experimental studies were carried out on the equipment of the Core shared research facilities “Composition, structure and properties of structural and functional materials” of the NRC “Kurchatov Institute”—CRISM “Prometey”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, W.; Yu, H.; Wang, L.; Wu, X.; Ouyang, C.; Zhang, Y.; He, J. State of the art and prospects in sliver- and copper-matrix composite electrical contact materials. Mater. Today Commun. 2023, 37, 107256. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W. A facile fabrication of Ag/SiC composite coating with high mechanical properties and corrosion resistance by electroless plating. Mater. Today Commun. 2023, 36, 106737. [Google Scholar] [CrossRef]
- Cusma, A.; Sebastiani, M.; De Felicis, D.; Basso, A.; Bemporad, E. Study on the Correlation between Microstructure Corrosion and Wear Resistance of Ag-Cu-Ge Alloys. Coatings 2015, 5, 78–94. [Google Scholar] [CrossRef]
- Katzensteiner, A.; Rosalie, J.M.; Pippan, R.; Bachmaier, A. Synthesis of nanodiamond reinforced silver matrix nanocomposites: Microstructure and mechanical properties. Mater. Sci. Eng. A 2020, 782, 139254. [Google Scholar] [CrossRef]
- Zhao, Q.; Tan, S.; Xie, M.; Liu, Y.; Yiet, J. A study on the CNTs-Ag composites prepared based on spark plasma sintering and improved electroless plating assisted by ultrasonic spray atomization. J. Alloys Compd. 2018, 737, 31–38. [Google Scholar] [CrossRef]
- Li, A.; Xie, M.; Yang, Y.; Zhang, J.; Wang, S.; Chen, Y.; Zhou, W. Effect of CNTs content on the mechanical and arc-erosion performance of Ag-CNTs composites. Diam. Relat. Mater. 2022, 128, 109211. [Google Scholar] [CrossRef]
- Larionova, N.S.; Nikonova, R.M.; Lad’yanov, V.I.; Ul’yanov, A.L.; Mikheev, K.G. Structural changes of fullerite C60/70 during mechanosynthesis of copper and iron based composites. Adv. Powder Technol. 2019, 30, 1724–1728. [Google Scholar] [CrossRef]
- Akbarpour, M.R.; Alipour, S.; Farvizi, M.; Kim, H.S. Mechanical, tribological and electrical properties of Cu-CNT composites fabricated by flake powder metallurgy method. Arch. Civ. Mech. Eng. 2019, 19, 694–706. [Google Scholar] [CrossRef]
- Arai, S.; Kikuhara, T.; Shimizu, M.; Horita, M. Electrodeposition of Ag/CNT Composite Films from Iodide Plating Baths. J. Electrochem. Soc. 2020, 167, 122515. [Google Scholar] [CrossRef]
- Koltsova, T.; Bobrynina, E.; Vozniakovskii, A.; Larionova, T.; Klimova-Korsmik, O. Thermal Conductivity of Composite Materials Copper-Fullerene Soot. Materials 2022, 15, 1415. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, V.A.; Zabelin, S.F.; Ankudinov, A.B.; Tregubova, I.V. The Study of Mechanical and Technological Properties of Dispersion-Strengthened Electric-Based Materials on the Base of Composite Powder Argent. Sci. Notes ZabGU 2015, 3, 59–69. [Google Scholar]
- Radmilovic, V.; Popov, K.I.; Pavlovic, M.G.; Dimitrov, A.A.; Hadzi Jordanov, S. The mechanism of silver granular electrodeposits formation. J. Solid State Electrochem. 1998, 2, 162–169. [Google Scholar] [CrossRef]
- Avramovic, L.; Pavlovic, M.M.; Maksimovic, M.M.; Vukovic, M.; Stevanovic, J.S.; Bugarin, M.; Nikolic, N. Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles. Metals 2017, 7, 160. [Google Scholar] [CrossRef]
- Dimitrov, A.T.; Jordanov, S.H.; Popov, K.I.; Pavlovic, M.G.; Radmilovic, V. Electrodeposition of Ag from nitrate solutions: Part I. Effect of phosphate ions on morphology. J. Appl. Electrochem. 1998, 28, 791–796. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Thornton, T.; Smith, D. Density of fullerene containing soot as determined by helium pycnometry. Chem. Phys. Lett. 1991, 186, 456–458. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Lahiri, D.; Agarwal, A. Carbon Nanotube Reinforced Metal Matrix Composites—A Review. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Yoo, S.J.; Han, S.H.; Kim, W.J. A combination of ball milling and high-ratio differential speed rolling for synthesizing carbon nanotube/copper composites. Carbon 2013, 61, 487–500. [Google Scholar] [CrossRef]
- Vandeperre, L.J.; Clegg, W.J. The Correlation between Hardness and Yield Strength of Hard Materials. Mater. Sci. Forum 2005, 492–493, 555–560. [Google Scholar] [CrossRef]
- Dieter, G. Mechanical Metallurgy, 3rd ed.; McGraw-Hill: New York, NY, USA, 1986; p. 751. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).