An Electrochemical Approach to the Recovery of Metals Typical of Battery Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrolytes and Materials
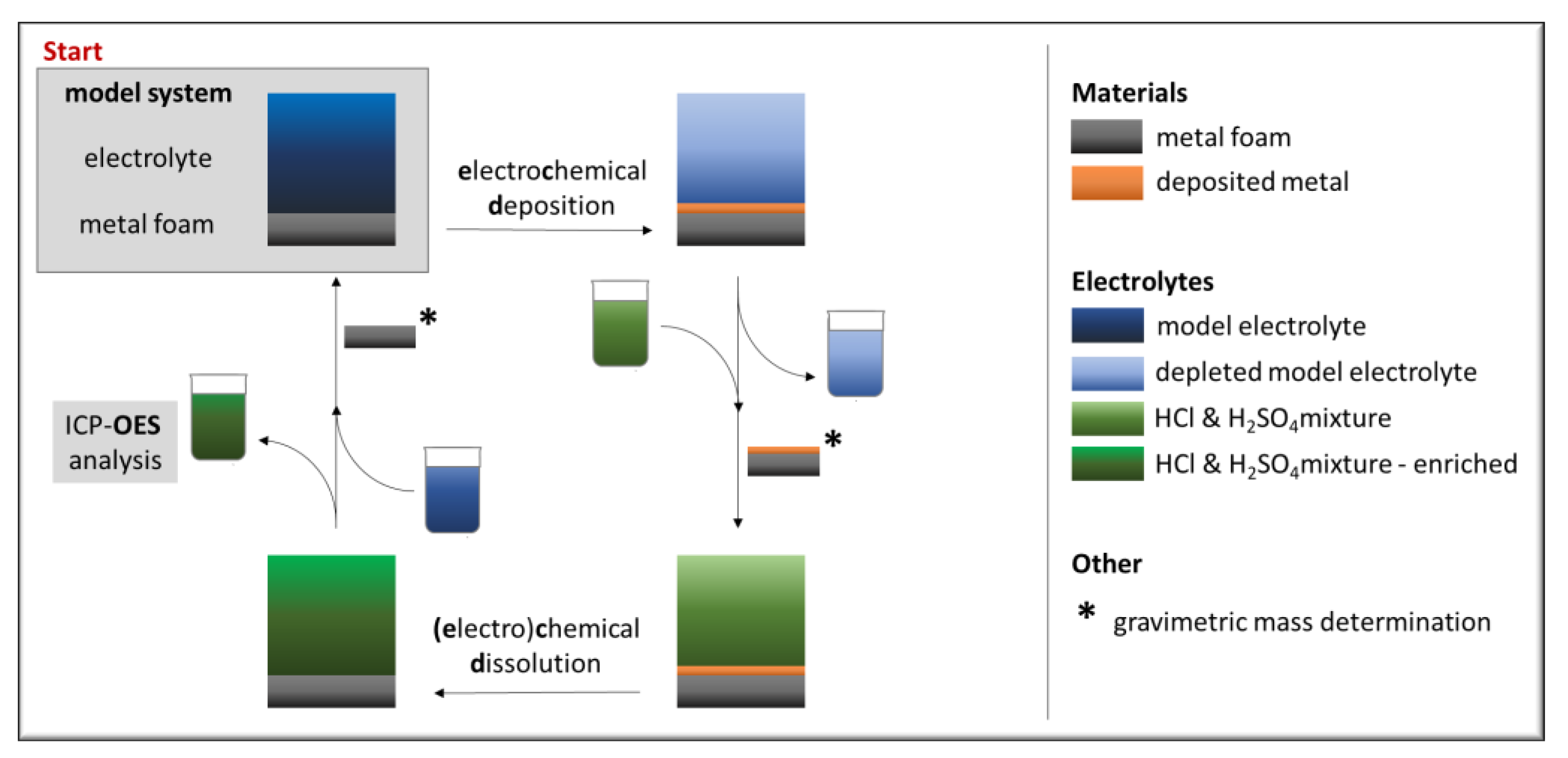
2.2. Methods
3. Results
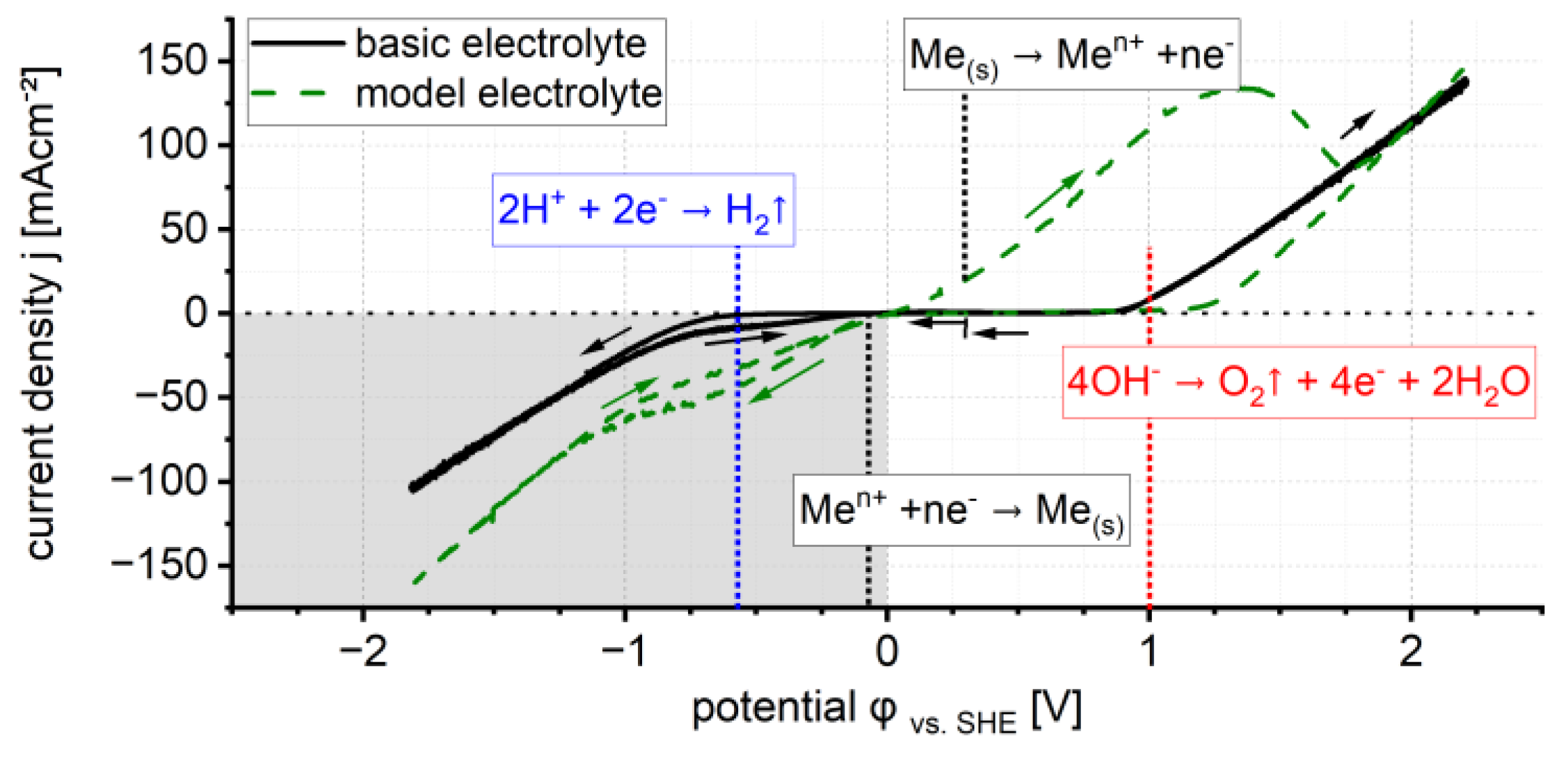
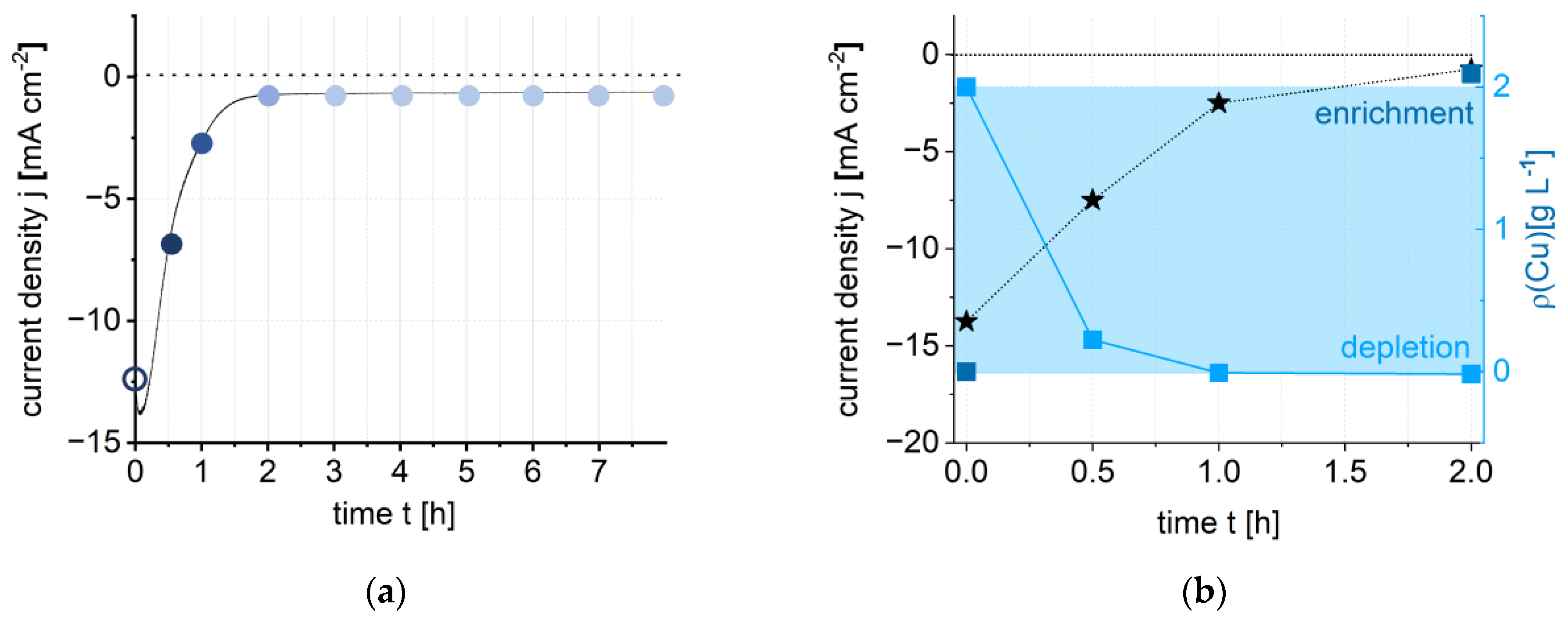
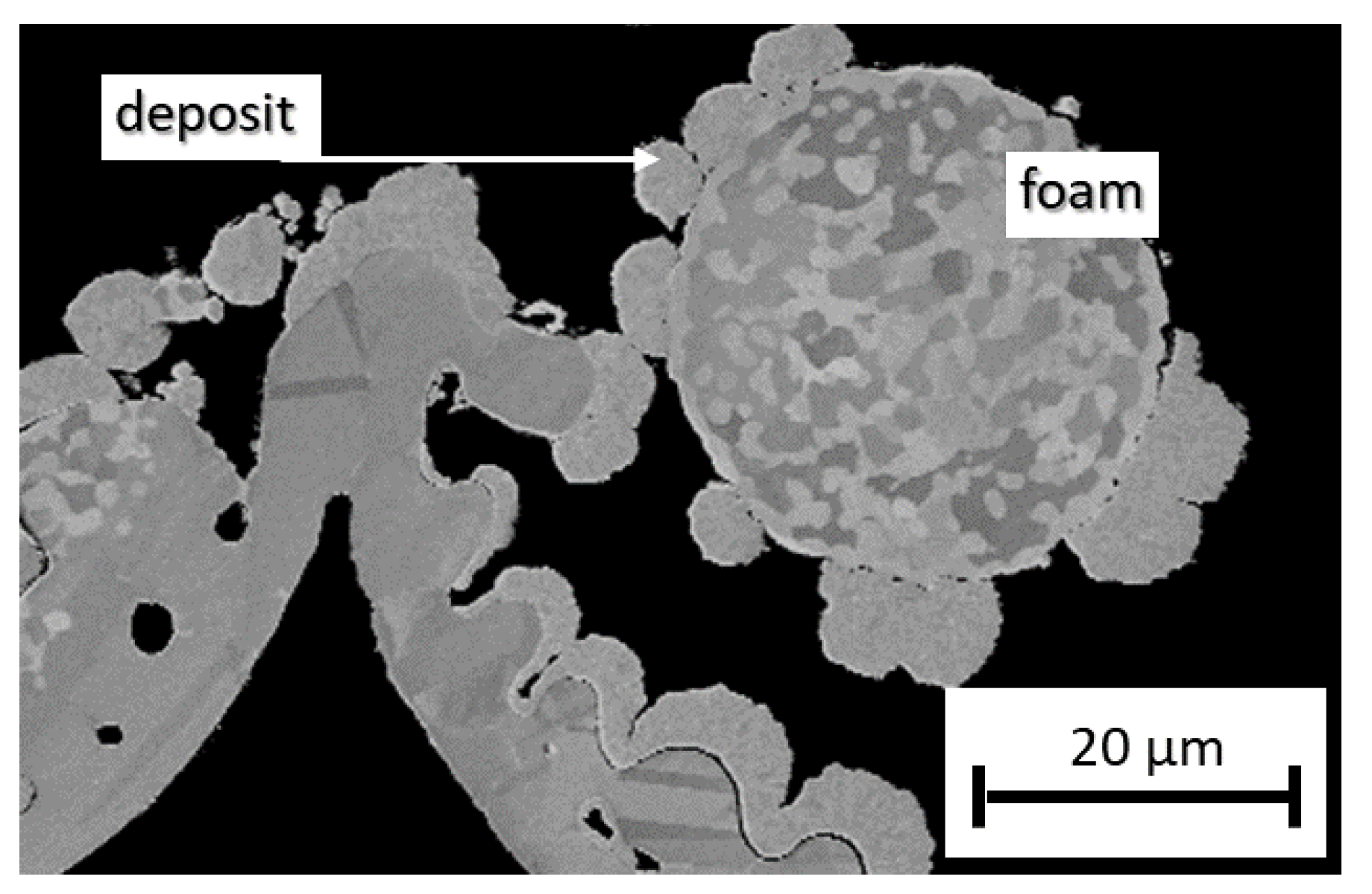
3.1. Stationary Electrochemical Deposition
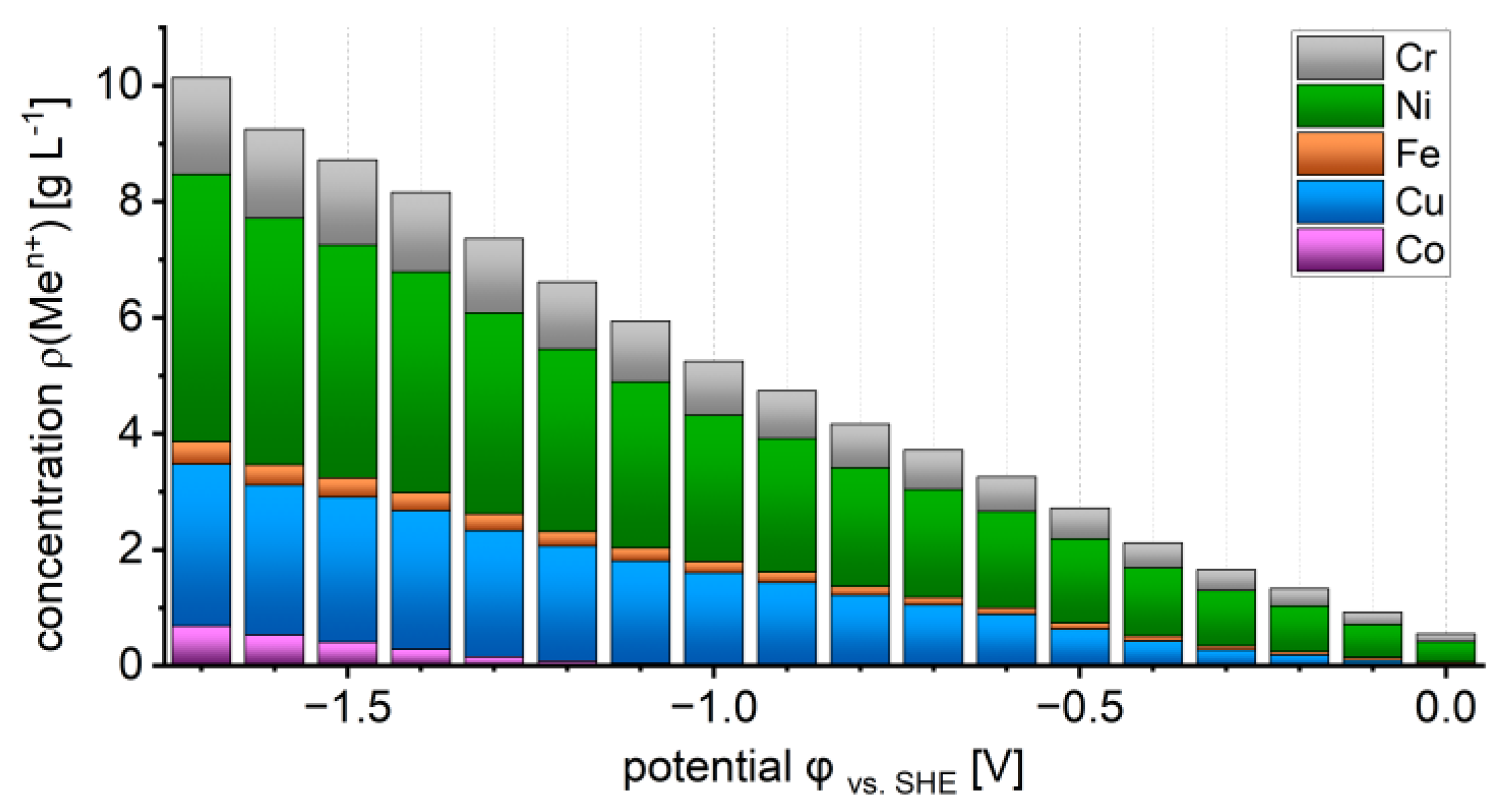
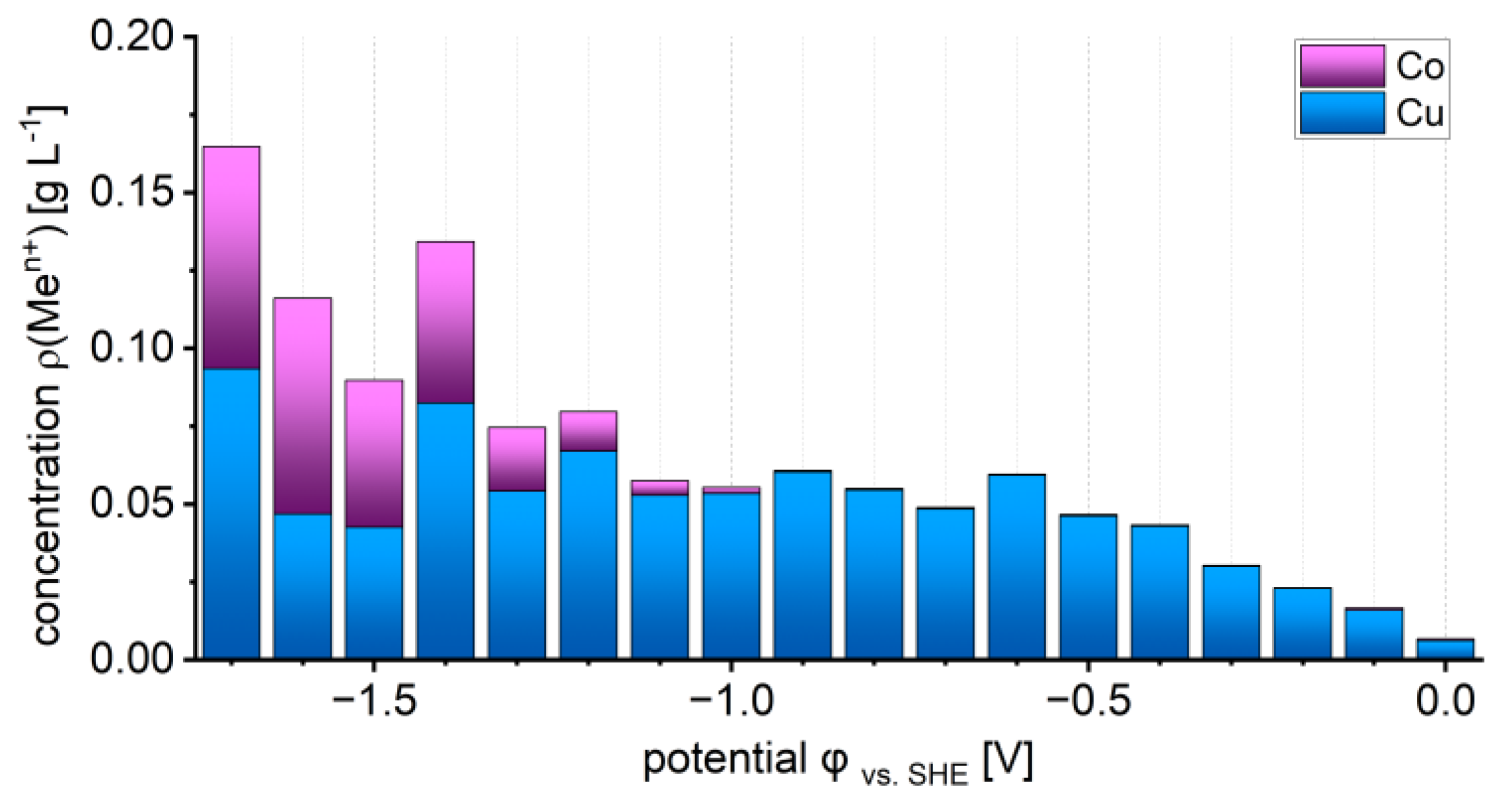
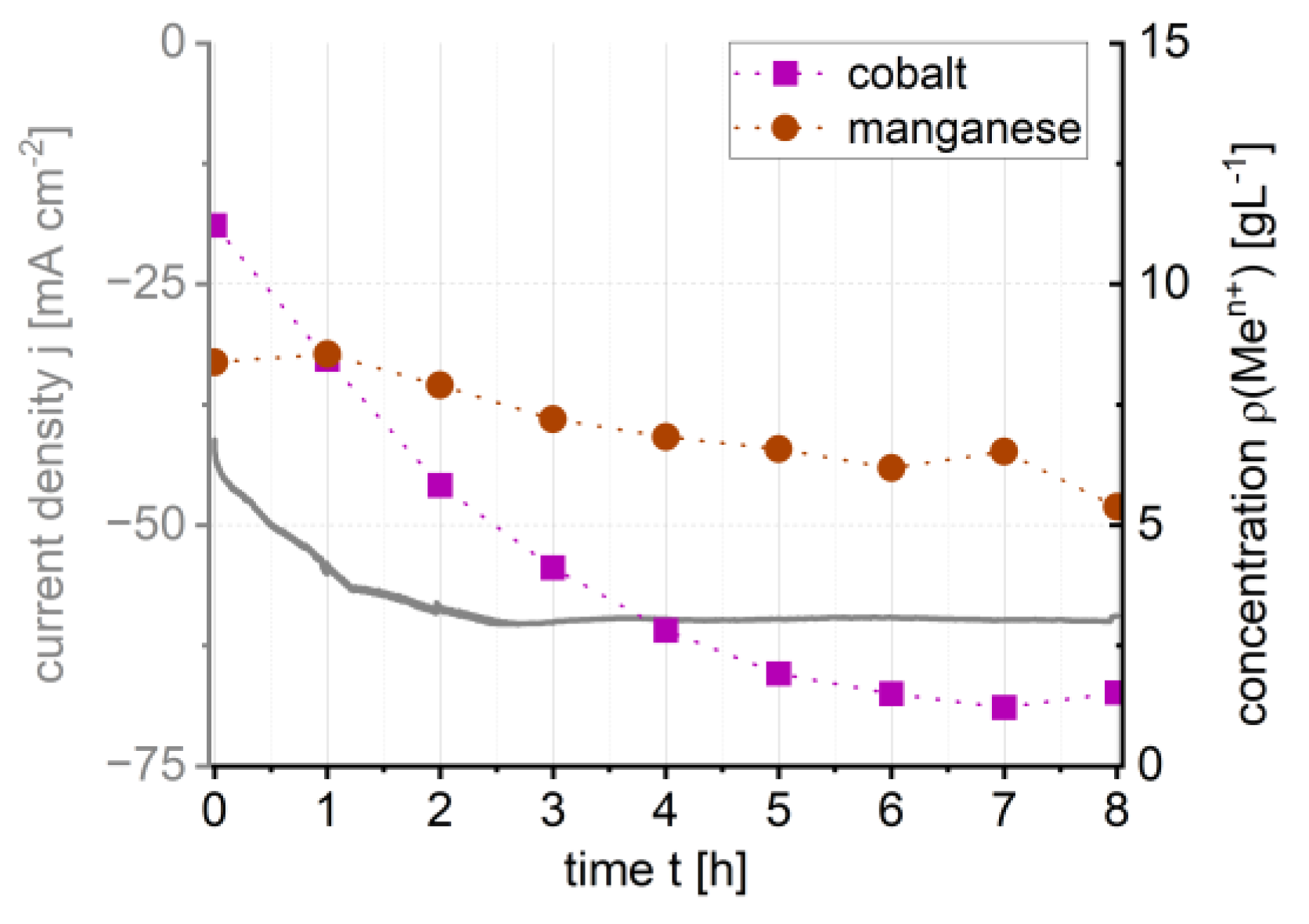
3.2. Metal Recovery and Electrolyte Depletion
4. Conclusions
- Copper starts at 0 V (vs. SHE).
- Cobalt starts at −1.1 V (vs. SHE).
- Nickel starts at −1.4 V (vs. SHE).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthes, S.; Grewe, V.; Sausen, R.; Roelofs, G.-J. Global impact of road traffic emissions on tropospheric ozone. Atmos. Chem. Phys. 2007, 7, 1707–1718. [Google Scholar] [CrossRef]
- Matthes, S. Globale Auswirkung des Straßenverkehrs auf die Chemische Zusammensetzung der Atmosphäre. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2023. [Google Scholar]
- McDuffie, E.E.; Smith, S.J.; O’Rourke, P.; Tibrewal, K.; Venkataraman, C.; Marais, E.A.; Zheng, B.; Crippa, M.; Brauer, M.; Martin, R.V. A global anthropogenic emission inventory of atmospheric pollutants from sector- and fuel-specific sources (1970–2017): An application of the Community Emissions Data System (CEDS). Earth Syst. Sci. Data 2020, 12, 3413–3442. [Google Scholar] [CrossRef]
- Situ, L. Electric Vehicle Development: The Past, Present & Future. In Proceedings of the 3rd International Conference on Power Electronics Systems and Applications (PESA), Hong Kong, China, 20 May 2009; Available online: https://www.researchgate.net/publication/224585932_Electric_Vehicle_development_The_past_present_future (accessed on 3 September 2023).
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Iyer-Raniga, U. Lithium-Ion Battery Recycling in the Circular Economy: A Review. Recycling 2022, 7, 33. [Google Scholar] [CrossRef]
- Doose, S.; Mayer, J.K.; Michalowski, P.; Kwade, A. Challenges in Ecofriendly Battery Recycling and Closed Material Cycles: A Perspective on Future Lithium Battery Generations. Metals 2021, 11, 291. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]
- Peschel, C.; van Wickeren, S.; Preibisch, Y.; Naber, V.; Werner, D.; Frankenstein, L.; Horsthemke, F.; Peuker, U.; Winter, M.; Nowak, S. Comprehensive Characterization of Shredded Lithium-Ion Battery Recycling Material. Chem. Eur. J. 2022, 28, e202200485. [Google Scholar] [CrossRef] [PubMed]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Mohr, M.; Weil, M.; Peters, J.; Wang, Z. Recycling of Lithium-Ion Batteries. In Encyclopedia of Electrochemistry; Bard, A.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for re-cycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environ-mental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-ion Batteries. Miner. Process. Extr. Met. Rev. 2020, 42, 451–472. [Google Scholar] [CrossRef]
- Arshad, F.; Li, L.; Amin, K.; Fan, E.; Manurkar, N.; Ahmad, A.; Yang, J.; Wu, F.; Chen, R. A Comprehensive Review of the Advancement in Recycling the Anode and Electrolyte from Spent Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13527–13554. [Google Scholar] [CrossRef]
- Fan, X.; Song, C.; Lu, X.; Shi, Y.; Yang, S.; Zheng, F.; Huang, Y.; Liu, K.; Wang, H.; Li, Q. Separation and recovery of valuable metals from spent lithium-ion batteries via concen-trated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2. J. Alloys Compd. 2021, 863, 158775. [Google Scholar] [CrossRef]
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag. 2021, 125, 192–203. [Google Scholar] [CrossRef]
- Walther, G.; Klöden, B.; Büttner, T.; Weißgärber, T.; Kieback, B.; Böhm, A.; Naumann, D.; Saberi, S.; Timberg, L. A New Class of High Temperature and Corrosion Resistant Nickel-Based Open-Cell Foams. Adv. Eng. Mater. 2008, 10, 803–811. [Google Scholar] [CrossRef]
- Harald Pihl, Inconel 625. Available online: https://www.haraldpihl.com/de/products/Nickellegierungen/inconel-625 (accessed on 5 September 2023).
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: New York, NY, USA, 1966; ISBN -13. [Google Scholar]
- Bockris, J.O.M. Electrolytic polarisation—I. The overpotential of hydrogen on some less common metals at high current densities. Influence of current density and time. Trans. Faraday Soc. 1947, 43, 417–429. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, A.J.J. Electrode Materials in Modern Organic Electrochemistry. Angew. Chem. Int. Ed. 2020, 59, 18866–18884. [Google Scholar] [CrossRef]
- Haynes, W.M. Crc Handbook of Chemistry and Physics, 97th ed.; CRC Press LLC Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN -13: 978-1-4987-5429-3. [Google Scholar]
- Han, P.; Bartels, D.M. Temperature Dependence of Oxygen Diffusion in H2O and D2O. J. Phys. Chem. 1996, 100, 5597–5602. [Google Scholar] [CrossRef]
- The Engineering ToolBox. Oxygen–Solubility in Fresh and Sea Water vs. Temperature. Available online: https://www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html (accessed on 6 September 2023).
- Schneider, M.; Langklotz, U.; Labus, M.; Arnold, B.; Michaelis, A. Investigation of corrosion products formed on C35–AlMgSi0·5 friction welds during natural weathering in marine climate. Corros. Eng. Sci. Technol. 2012, 47, 312–319. [Google Scholar] [CrossRef]
- Rode, S.; Henninot, C.; Vallières, C.; Matlosz, M. Complexation Chemistry in Copper Plating from Citrate Baths. J. Electrochem. Soc. 2004, 151, C405–C411. [Google Scholar] [CrossRef]
- Zelenin, O.Y. Interaction of the Ni2+ ion with citric acid in an aqueous solution. Russ. J. Coord. Chem. 2007, 33, 346–350. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. Order of Stability of Metal Complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Daniele, P.G.; Ostacoli, G.; Zerbinati, O.; Sammartano, S.; De Robertis, A. Mixed metal complexes in solution. Thermodynamic and spectrophotometric study of copper(II)-citrate heterobinuclear complexes with nickel(II), zinc(II) or cadmium(II) in aqueous solution. Transit. Met. Chem. 1988, 13, 87–91. [Google Scholar] [CrossRef]


| Type of Element | ρ (Element) g L−1 | (Metallic) Salt Used | ρ (Salt) g L−1 | c mol L−1 |
|---|---|---|---|---|
| Co | 10 | CoSO4 7H2O | 47.7 | 0.17 |
| Ni | 9 | NiSO4 6H2O | 40.3 | 0.15 |
| Mn | 8 | MnSO4 H2O | 24.61 | 0.15 |
| Cu | 2 | CuSO4 5H2O | 7.86 | 0.03 |
| P | 2 | H3PO4 (85 wt.%) | 4.41 cm3 L−1 | 0.07 |
| Al | 0.3 | Al2(SO4)3 16H2O | 3.5 | 0.01 |
| Li | 0.1 | LiCl | 0.61 | 0.01 |
| H2SO4 (96 wt.%) | 5.6 cm3 L−1 | 0.1 | ||
| citric acid | 50 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutzer-Schulze, C.; Schmidt, H.; Weiser, M.; Büttner, T.; Schneider, M.; Michaelis, A. An Electrochemical Approach to the Recovery of Metals Typical of Battery Waste. Metals 2024, 14, 109. https://doi.org/10.3390/met14010109
Kutzer-Schulze C, Schmidt H, Weiser M, Büttner T, Schneider M, Michaelis A. An Electrochemical Approach to the Recovery of Metals Typical of Battery Waste. Metals. 2024; 14(1):109. https://doi.org/10.3390/met14010109
Chicago/Turabian StyleKutzer-Schulze, Claudia, Hannes Schmidt, Mathias Weiser, Tilo Büttner, Michael Schneider, and Alexander Michaelis. 2024. "An Electrochemical Approach to the Recovery of Metals Typical of Battery Waste" Metals 14, no. 1: 109. https://doi.org/10.3390/met14010109
APA StyleKutzer-Schulze, C., Schmidt, H., Weiser, M., Büttner, T., Schneider, M., & Michaelis, A. (2024). An Electrochemical Approach to the Recovery of Metals Typical of Battery Waste. Metals, 14(1), 109. https://doi.org/10.3390/met14010109








