Abstract
Metal surfaces can become damaged by corrosion when they interact with their surroundings, leading to huge financial losses. The use of corrosion inhibitors is one of the most crucial ways to combat the risky and hazardous effects of corrosion. In the present research, electrochemical techniques and surface analysis have been used to characterize the inhibition properties of (3-hydroxy-4-((4-nitrophenyl)diazenyl)-5-(phenylamino)thiophen-2-yl)(phenyl)methanone for the corrosion of carbon steel in an aerated 1.0 M HCl solution. Steel’s corrosion resistance was discovered to be improved by the presence of the examined inhibitor in 1.0 M HCl medium through the adsorption of the inhibitor species to create a barrier layer. The findings showed that when inhibitor concentrations increased and solution temperatures decreased, the inhibition performance (%IE) of the compound under study enhanced. In the light of inhibitor probe’s chemical makeup and theoretical analysis, the mechanism of the inhibition process was addressed. In a 1.0 M HCl solution containing 5 × 10−5 M of the inhibitor, the inhibition performance, at room temperature, was found to be almost 97%. The electrochemical results revealed that the examined compound successfully prevented carbon steel corrosion as a mixed-type inhibitor. The Langmuir and Freundlich isotherms are pursued by the adsorption of the examined inhibitor. Additionally, using Arrhenius and transition state equations, the activation thermodynamic parameters ΔEa, ΔH*, and ΔS* were determined and explained. The adsorption process was illustrated using DFT computation and MC simulations. The experimental findings and theoretical simulations concurred surprisingly well. Finally, the paper presents a discussion of the inhibitory mechanism.
1. Introduction
In both daily life and industrial operations, metal corrosion is a common and well-established occurrence. It can decrease a metal or alloy’s functionality, reduce its lifespan, and increase maintenance costs. Additionally, engineering quality, public safety, and considerable financial loss are all at serious risk from corrosion [1,2]. Because of its beneficial characteristics, such as superior mechanical pliability, superior cost-effective performance, and strong thermal and electrical conductivity, the petrochemical sector, pipeline transportation industry, power plants, the navy, and the military commonly use carbon steel. Numerous circumstances, including an acidic solution, a microbiological environment, an oxygen-rich medium, and a medium with high conductivity, can cause steel to corrode [3,4,5,6,7,8]. One example is pickling with HCl, which is frequently used in industrial processes including chemical cleaning, alloy pickling, the descaling of boilers, and the acidification of oil wells [9]. In comparison with other acids, HCl acid is favored considerably since the salts produced through the method are soluble in water [10,11,12,13,14]. Although corrosion cannot be completely eliminated, its rate can be reduced by implementing efficient protective measures. It is predicted that if proper preventative measures are taken, 20–30% of corrosion losses could be prevented [15,16]. One of the most useful and economical approaches now taken in business is the usage of corrosion inhibitors. The adsorption of corrosion inhibitors onto metal surfaces creates a barrier that protects them from corrosive media. Therefore, there is always a significant demand for the discovery and development of new inhibitors with high inhibition capacities [17,18,19,20,21,22]. Corrosion inhibitors are often organic substances with heteroatoms (O, N, S, and/or P) and aromatic rings or long alkyl chains [23,24,25,26,27,28]. In acidic settings, several N-heterocyclic compounds act as powerful corrosion inhibitors because they contain polar groups and/or π electrons. An inhibitor can stop the corrosion of metals by physically or chemically adhering to the surface of the alloy [29]. In terms of application, heteroatoms and other compounds with functional groups that may donate a single pair of electrons are advantageous as inhibitors of metal corrosion [30].
Numerous studies have examined the impact of synthetic organic compounds, polymers, drugs, and plant extracts on the inhibition of metals in hydrochloric acid corrosion. This trait has prompted researchers to explore the potential use of drugs as corrosion inhibitors. D-penicillamine, a penicillin breakdown product, and L-cysteine, an amino acid frequently found in living organisms, were tested for their ability to suppress mild steel corrosion in a 1.0 M HCl solution using electrochemical techniques. These tests were conducted using a range of concentrations, immersion periods, and temperatures. Through a polarization analysis, the maximal %IE value of 5 mM L-cysteine in 1.0 M HCl solutions at 4 h immersion durations were calculated to be 91% [31]. Espinoza-Vázquez et al. [32] investigated the effect of fluconazole and its fragments as corrosion inhibitors of API 5L X52 steel in 1.0 M HCl solution, where it appeared to be an active chemical at its low concentrations. According to Samide et al. [33], the corrosion of carbon steel in 1.0 M HCl solution was inhibited by sulfacetamide and attained an efficiency of 84.7% at a concentration of 10 mM. There are four main chemical components in a magnolia grandiflora leaf: 3,7-dimethyl-2,6-octadien-1-ol (DTO), santamarine (STA), lanuginosine (LGS), and anonaine (ANI). In a study by Chen et al. [34], magnolia grandiflora leaf extract was made using the water extraction method, and the extract’s effectiveness at preventing Q235 steel corrosion in 1.0 M HCl was assessed using several techniques. The outcomes demonstrated that the extract acted as a mixed-type corrosion inhibitor, and that at 298 K and 500 mg/L, where LGS contributed the most to corrosion protection, its corrosion inhibition efficiency exceeded 85%. Wang et al. [35] also demonstrated the potential of Ficus tikoua leaf extract as a corrosion inhibitor for carbon steel in 1.0 M HCl solution. By using quantum chemical calculations, the authors determined that the 5-methoxypsoralen (5-MOP) components of the extract performed the most significant impact in the suppression of steel corrosion. This extract demonstrated outstanding protective effectiveness at 200 mg/L in the temperature range of 298 K to 318 K, with respective values of 95.8% (298 K), 95.7% (308 K), and 95.0% (318 K). A new organic epoxy resin, N, N, 1-tri(oxiran-2-ylmethoxy)-5-((oxiran-2-ylmethoxy)thio)-1H-1,2,4-triazol-3-amine, was synthesized by Damej et al. in recent studies [36,37] and was employed as a corrosion inhibitor for carbon steel C38 in 1.0 M HCl solution. Unpredictably, through stationary and transiency measurements, the inhibition efficiency of this new epoxy resin reached a value of 92% at a concentration of only 1 mM. Additionally, it was noted that the surface morphology of C38 steel in the presence of the inhibitor remained clean after immersion in 1.0 M HCl solution, demonstrating that this epoxy resin effectively protected the C38 steel against corrosion. Also, according to earlier investigations, thiophene derivatives effectively suppress corrosion [38,39,40,41,42]. Thiophene and its replaced derivatives are widely utilized in medicinal chemistry as anti-microbial, antioxidant, anti-corrosion, and anti-cancer compounds due to their safety [43,44].
This study examines the safe chemical (3-hydroxy-4-((4-nitrophenyl)diazenyl)-5-(phenylamino)thiophen-2-yl)(phenyl)methanone as an inhibitor to control the corrosion of carbon steel in aerated 1.0 M HCl solution. The choice of this molecule depends on its chemical composition, as it contains many effective functional groups and heteroatoms such as OH, CO, -N=N-, NO2, S and N, which help enhance its adsorption strength on the alloy surface and ensure its safety. Researchers have examined the effects of temperature and concentration on the efficiency of inhibition and the pace of corrosion. Additionally, kinetic and thermodynamic characteristics have been calculated. To comprehend the inhibitory mechanism, DFT computations and MC simulations are used for theoretical foretelling.
2. Experimental Details
2.1. Preparation of the Testing Media and the Investigated Surface
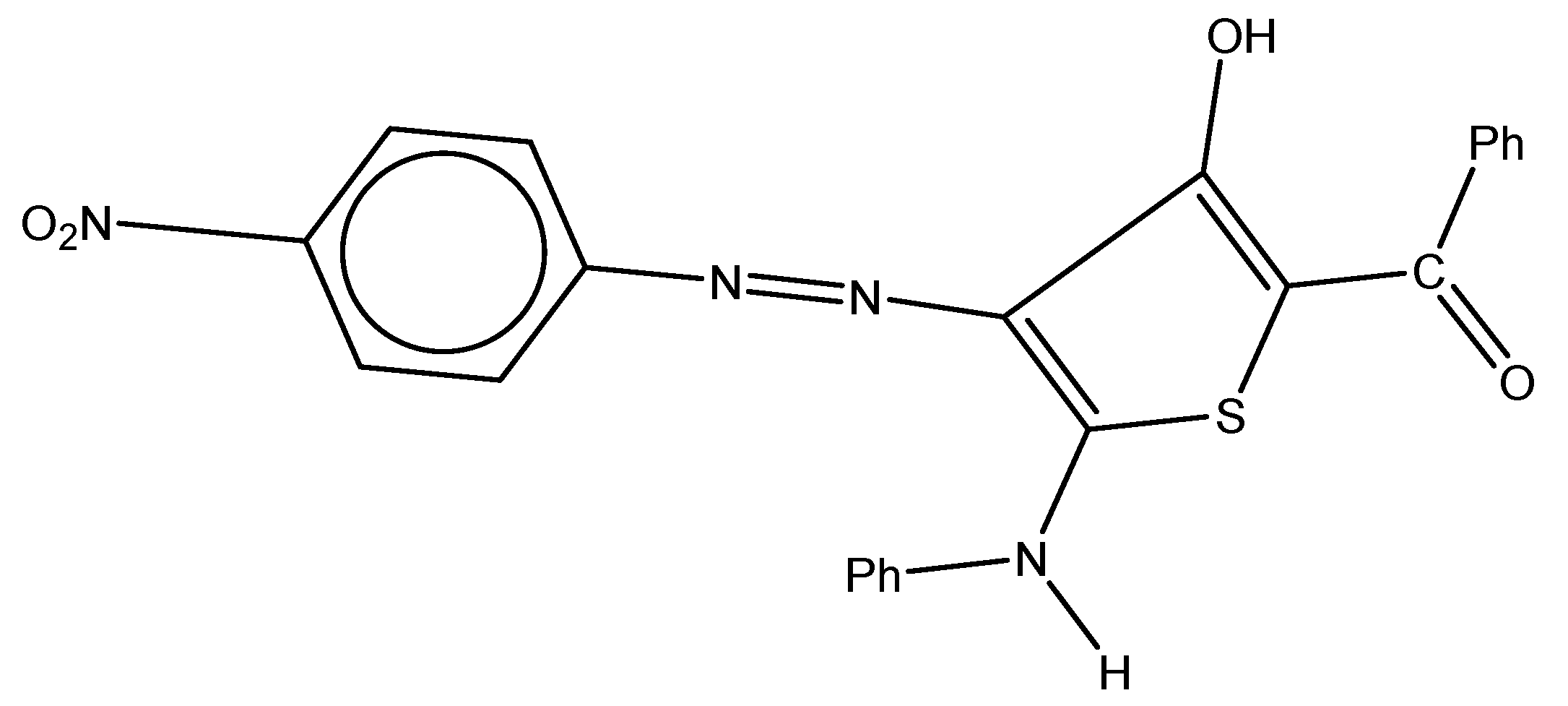
The molecule used as a corrosion inhibitor in this work (Figure 1) was synthesized as described elsewhere [45]. The investigated molecule was immersed in 1.0 M HCl solution at its concentration range of 1 × 10−6 to 5 × 10−5 M. The inhibitor was then dissolved in 3 mL of ethanol before the acid was added. The tested corrosive medium was a 1.0 M HCl solution, which is made by diluting 37% hydrochloric acid in bi-distilled water. The solution was standardized using a 1 M Na2CO3 standard solution. This solution can be made without an inhibitor or with varied amounts of an inhibitor. The alloy used in this study was carbon steel, which has a conformation (wt%) of Si (0.002%), P (0.007%), Mn (0.910%), C (0.200%), and leftover iron. Following the 120-, 240-, 400-, 600-, 800-, and 1200-grit emery paper abrasion procedure, each coupon was washed with distilled water and acetone before being allowed to air dry in accordance with ASTM guidelines before the experiments began [46].
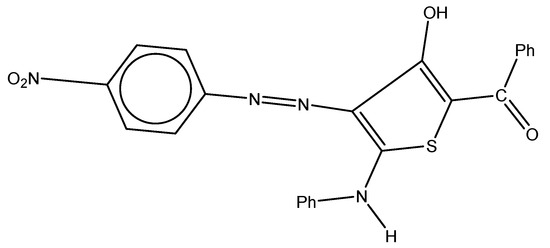
Figure 1.
Chemical structure of the inhibitor: (3-hydroxy-4-((4-nitrophenyl)diazenyl)-5-(phenylamino)thiophen-2-yl)(phenyl)methanone.
2.2. Electrochemical Analysis
The electrochemical studies were carried out using a Gamry device of the Potentiostat/Galvanostat/ZRA (REF 600) type (Gamry, Warminster, PA, USA). A circular carbon steel specimen with an exposed area of 1 cm2 served as the working electrode for the measurements, while a saturated calomel electrode and a platinum rod (also with an approximate 1 cm2 surface area) served as the reference and counter electrodes (SCE). In order to achieve a steady-state open-circuit potential (OCP) and ensure that the working electrode’s surface had acquired a steady-state potential, the carbon steel samples were submerged in a corrosive environment for 30 min prior to the measurement. The potentiodynamic measurements assess the corrosion current potential and inhibition effectiveness of carbon steel that has been exposed to acidic environments both in the presence and absence of an inhibitor within a range of −850 to −100 mV vs. open-circuit potential (OCP) and at scan rate of 0.1 mV s−1. The polarization cell received a predetermined amount (100 mL) of the test fluid. The %η (percent inhibition efficiency calculated by corrosion current density values) can be calculated using Equation (1).
In the absence and presence of an inhibitor, the corrosion current densities, individually, are and .
2.3. Methodologies for Surface Characterization
2.3.1. Atomic Force Microscopy (AFM) Analysis
Atomic force microscopy (AFM) was used to determine the morphological properties of the carbon steel surface. In the absence of the inhibitor and in the case of the maximum concentration of this inhibitor (5 × 10−5 M), one molar of hydrochloric acid was used for the analysis. A silicon nitride probe (MLCT type; Bruker) was used, and the AFM analysis was carried out in contact mode. The Proscan software, version 1.8, was used to monitor the scanning settings, and picture processing was carried out using IP version 2.1. The carbon steel sheet was subjected to three different tests: one where the object was polished, another that involved immersing it in 1 molar hydrochloric acid, and a third in which it was submerged in a solution containing 1.0 M acid and 5 × 10−5 M of the compound. Next, the results of the three tests were contrasted.
2.3.2. Scanning Electron Microscopy (SEM)
In order to compare the surfaces of the carbon steel coupons with and without 5 × 10−5 M of the compound, the testers were allowed to interact with the tested solutions for one day. With the aid of a JEOL scanning electron microscope model T-200 (×500), an expansion of the morphology was investigated.
2.4. Molecular Dynamic and DFT Computations
Utilizing the Dmol3 and adsorption locator components of the Materials Studio V.7.0 program from Accelrys Inc., San Diego, CA, USA, DFT computations and molecular dynamic simulations were performed.
2.4.1. DFT Calculations
The inhibitor compound was improved using the GGA/BLYP foundation that was established in the aqueous phase [47]. The following equations were used to compute the ionization potential (I), electron affinity (A), electronegativity (χ), global hardness (η), and global softness (σ) [48].
I = −EHOMO
A = −ELUMO
σ = 1/η
The fraction of electrons that were adsorption-transferred from the inhibitor molecules to the metallic surface (ΔN) can be calculated using the equation below.
ΔN = (χFe − χinh)/2(ηFe + ηinh)
χFe, χinh, ηFe, and ηinh are the values for the electronegativity and hardness of the inhibitor molecules and Fe, respectively. χFe is worth 7.00 eV, while ηFe is worthless [49].
2.4.2. Simulation of Molecular Dynamics (MD)
The adsorption finder determines the potential adsorption configurations for the molecules in the (3-hydroxy-4-((4-nitrophenyl)diazenyl)-5-(phenylamino)thiophen-2-yl)(phenyl)methanone compound for the purpose of determining the effectiveness of the inhibition using molecular dynamic simulation on the Fe (1 1 0) surface [50]. In a simulated box (32.27 32.27 50.18) with periodic boundary conditions, the interactions between the (3-hydroxy-4-((4-nitrophenyl)diazenyl)-5-(phenylamino)thiophen-2-yl)(phenyl)methanone compound and the iron surface (110) were investigated. The optimization of the examined compound molecular energy was carried out using the traditional simulation engine Forcite [51]. Utilizing the layer creator, the corrosion system in the aqueous environment was developed, which is made up of molecules of the perfect inhibitor substance, water, and Fe (110) surface. The inhibitor’s ability to bind to the iron surface (110) was modeled using the COMPASS emulation research through force field [52].
3. Results and Analysis
3.1. Potentiodynamic Polarization (PDP) Investigation
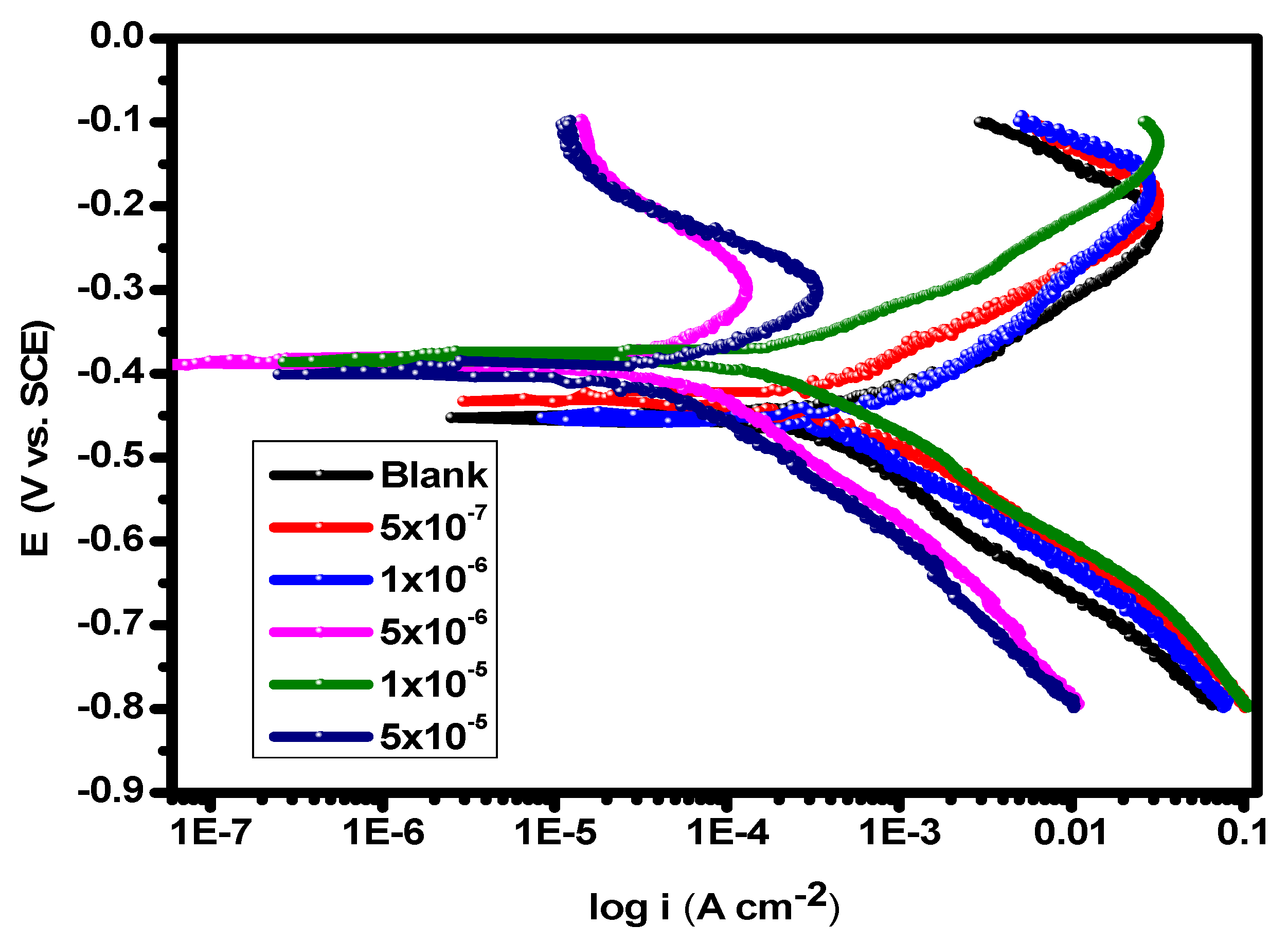
Potentiodynamic polarization experiments were carried out in 1.0 M HCl solution at 25 °C in the absence and presence of various concentrations of the substance (5 × 10–7–5 × 10–5 M) shown in Figure 2. The Icorr (corrosion current density) and the corrosion potential (Ecorr) of carbon steel in 1.0 M HCl solution without and with the inhibitor, as well as the inhibition efficiencies of the inhibitor (%η), are among the data derived from the PDP experiments that are included in Table 1. The difference between the Ecorr values of the utilized inhibitor and the blank solution was found to be less than 85 mV, demonstrating the mixed type of the inhibitors and lowering of the values of both the anodic and cathodic Tafel slope [53]. Figure 2 shows that the anodic metal dissolution and cathodic H2 reduction processes were controlled by adding the inhibitor to 1.0 M hydrochloric acid solution. Additionally, the significance of this inhibition increased with higher inhibitor dosages. According to the calculations, when the cathodic curves produce almost parallel lines, the hydrogen discharge process becomes slower. The presence of the inhibitor in an aggressive medium controls the activation of this reaction [54]. The results for βa and βc presented in Table 1 demonstrate that the dissolving mechanism remained constant despite a modest variation in the reactions of cathodic hydrogen reduction and anodic alloy dissolution [55,56]. By increasing the compound’s concentration, as depicted in Figure 3, its adsorption increased, improving the polarization resistance on the surface of the carbon steel [57]. The repeatability of an experiment is established by conducting it three times.
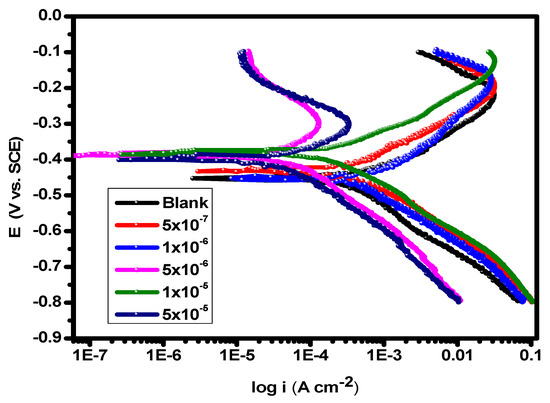
Figure 2.
PDP plots of carbon steel in 1.0 M HCl solution and with varying concentrations of the inhibitor at 25 °C.

Table 1.
PDP characteristics of carbon steel at different temperatures, in 1.0 M HCl solution, and with various inhibitor doses.
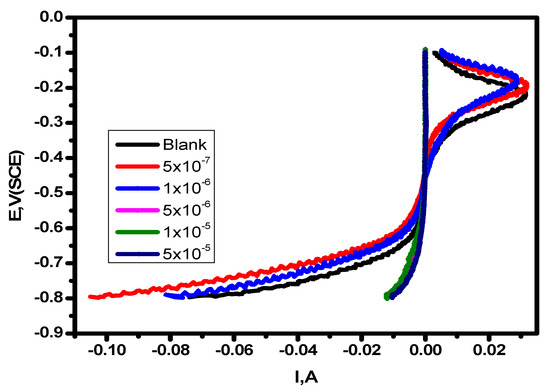
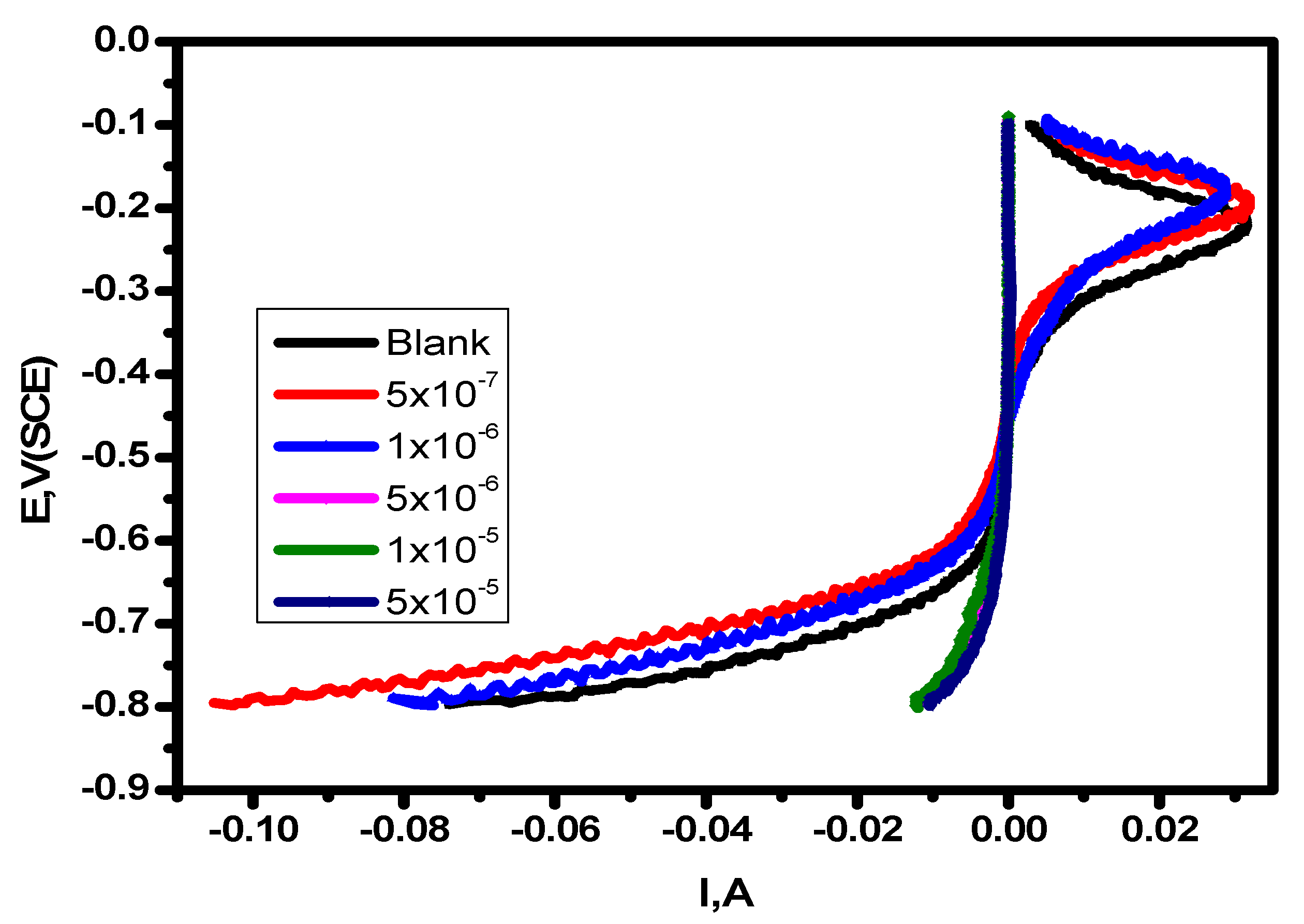
Figure 3.
Linear sweep voltammetry of alloy in 1.0 M HCl solution and with varying concentrations of the inhibitor at 25 °C.
3.2. Adsorption Studies
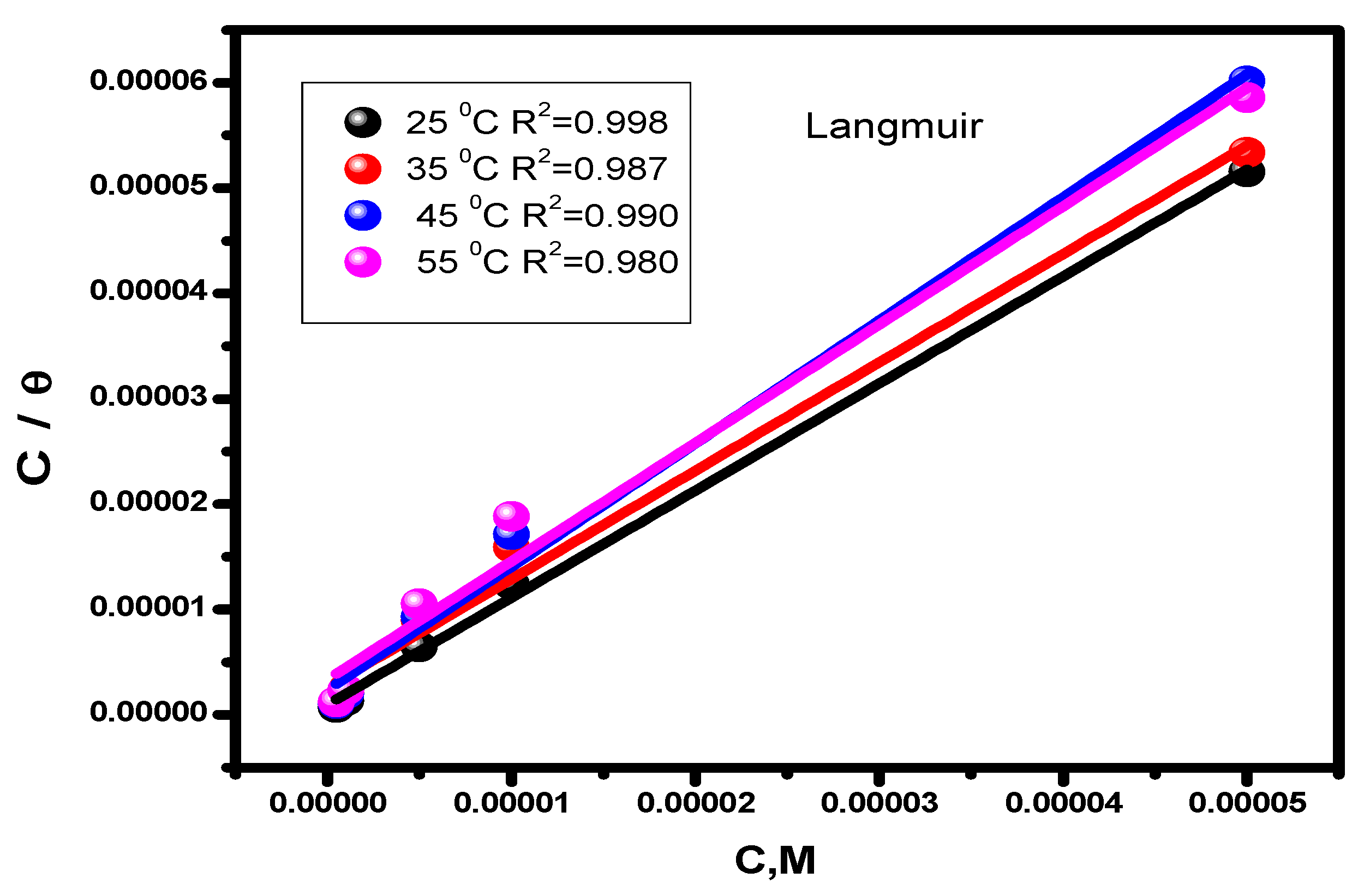
In order to protect the alloy surface from acidic attack, corrosion inhibitors adsorb protective layers onto it. This process can provide precise descriptions of the nature of the interaction between the molecules of the examined inhibitor and the steel surface [58]. For our new inhibitor, the experimental findings from the Tafel extrapolation method were fitted to various isotherms [59]. The data that the thermal model most closely resembles are given by Langmuir (Figure 4) and Henry’s (Figure 5) equations:
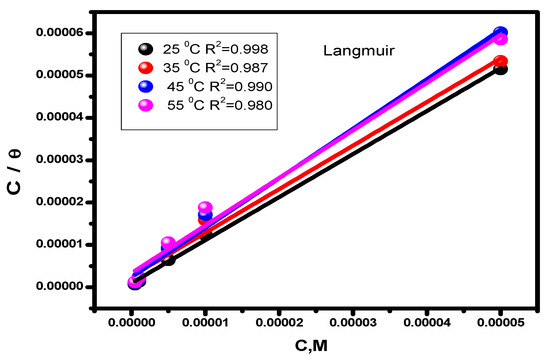
Figure 4.
Inhibitor Langmuir adsorption isotherm (C/θ vs. C) for alloy corrosion at different temperatures.
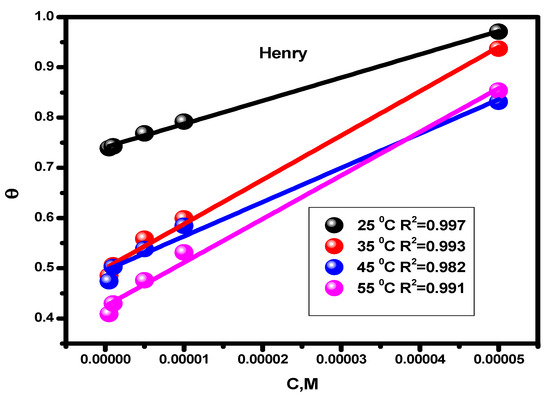
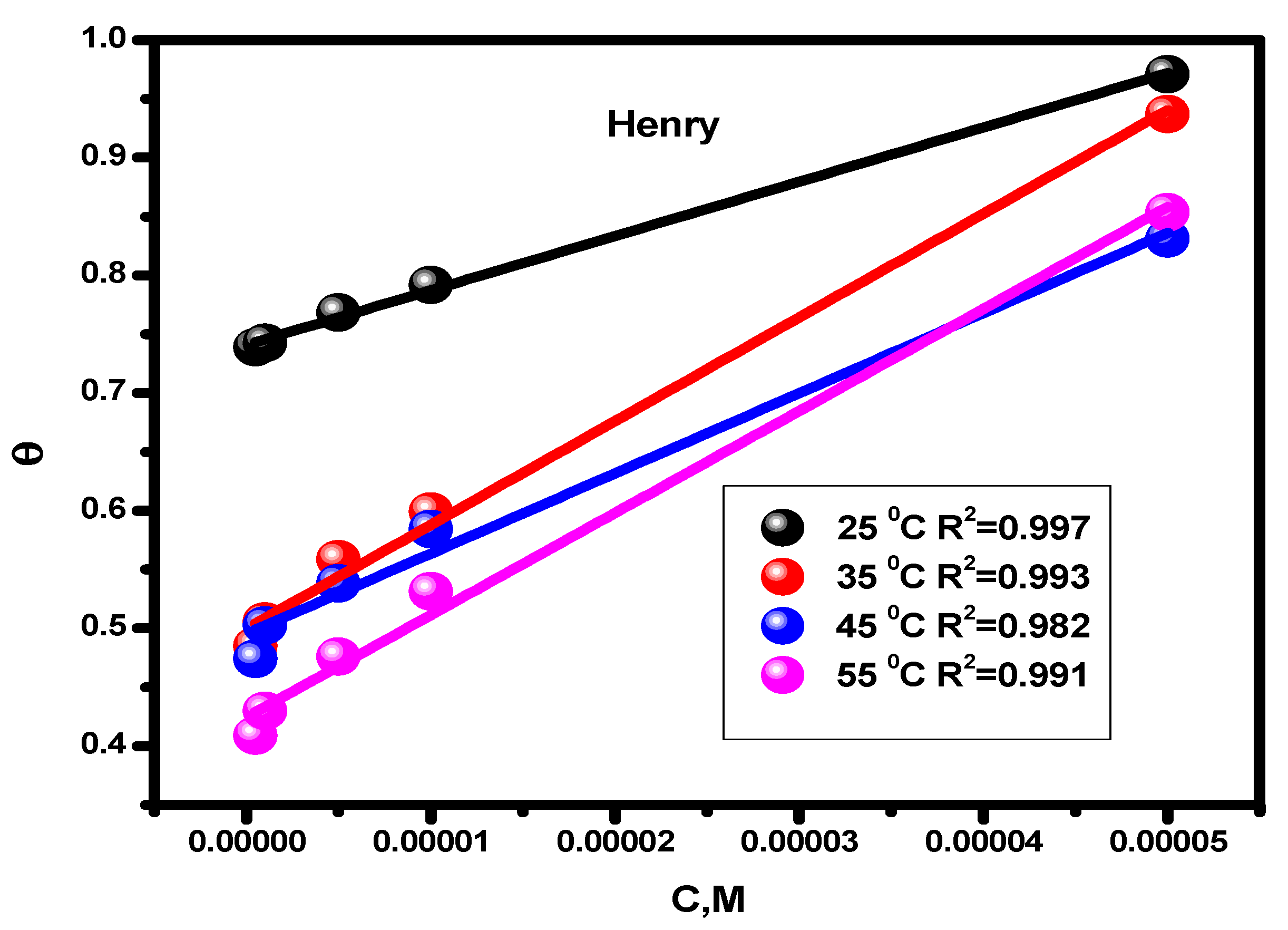
Figure 5.
Temperature-dependent inhibitor Henry adsorption isotherm for alloy corrosion in a 1 M HCl solution.
The adsorption constant, surface coverage, and inhibitor concentration are all individually represented by the letters Kads, θ, and C. The inhibitor’s adsorption constant (Kads) was calculated using the slope and intercept of the straight lines (Henry and Langmuir isotherms, separately) that intersect the x-axis. The values are shown in Table 2. The standard free energy values (ΔGads) were derived using Equation (10), which illustrates how the values of Kads affects ΔGads. In this formula, T (K) denotes temperature, R (J mol/K) denotes the universal gas constant, and water has a 55.5 molar content [60].
ΔGads = −RT ln (55.5 Kads)
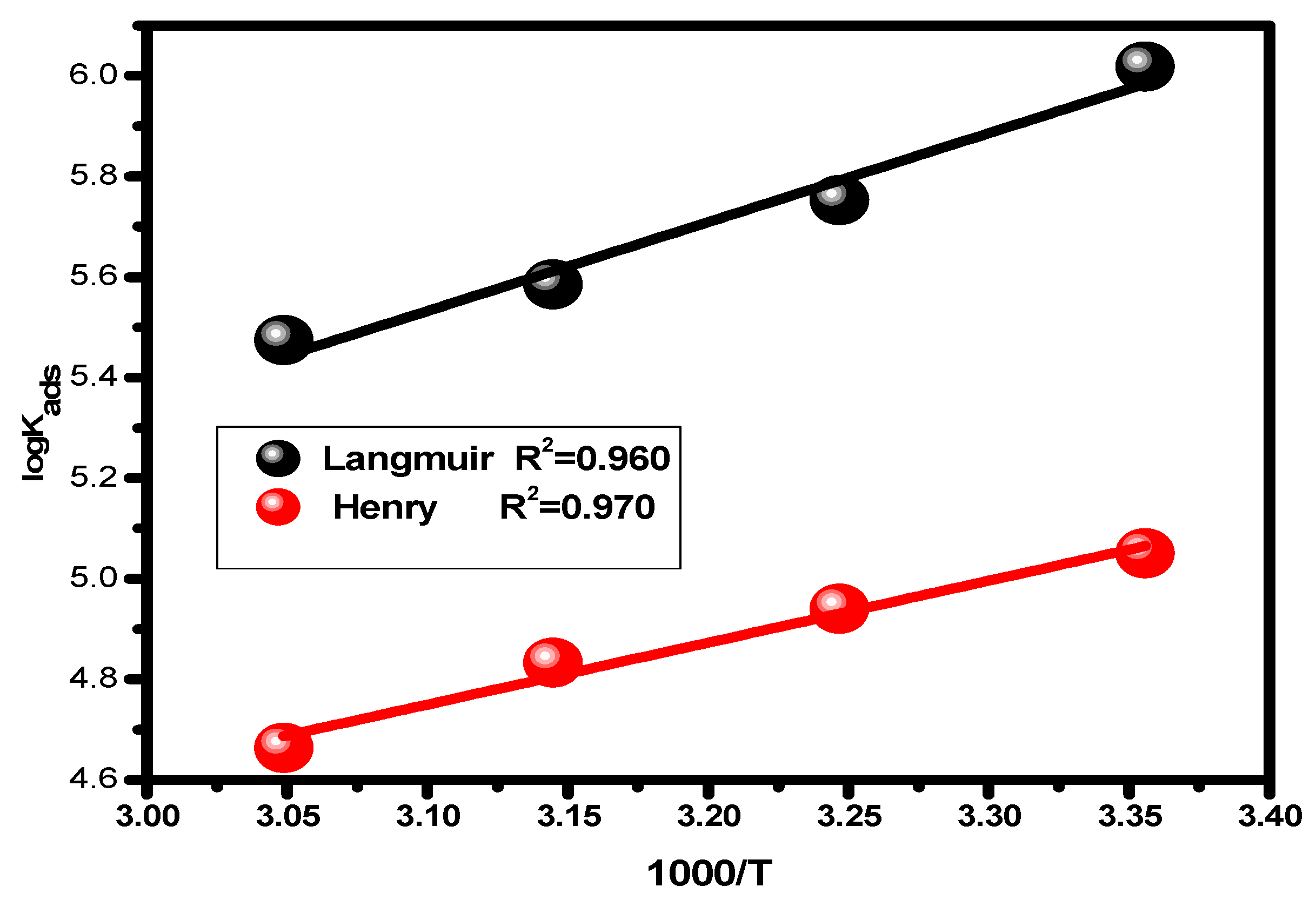
The enthalpy (ΔHads) value for the adsorption process was obtained by plotting log Kads vs. 1/T, Equation (10), Figure 6. Following the application of Equation (11), employing the enthalpy and standard free energy values, the entropy values (ΔSads) were intended [61,62].
log Kads = (−ΔHads/2.303 RT) + Constant
ΔGads = ΔHads − TΔSads
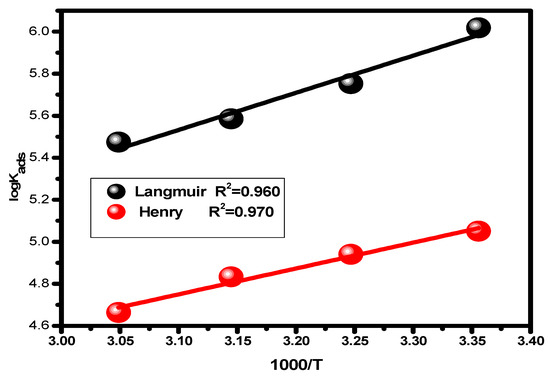
Figure 6.
Van’t Hoff plots showing the relationship between the adsorption equilibrium constant and the absolute temperature.
Table 2 gives the predicted thermodynamic characteristics of the adsorption process. This includes the enthalpy (ΔHads), adsorption equilibrium constant (Kads), entropy (ΔSads), and Gibbs free energy (ΔGads). Based on the highest (Kads) value discovered at 25 °C, it is thought that the best inhibitor adsorption on the surface of carbon steel takes place at this temperature, creating the best barrier that protects the metal surface from the aggressive acidic medium [57]. The nature and spontaneity of the adsorption process can be identified. Both Kads and ΔGads values affect these parameters, with negative ΔGads data showing the spontaneous nature of the compound adsorption process on the substrate’s surface. The physisorption (electrostatic interaction between the charged substrate surface and charged inhibitor molecules) is regarded to be present when the ΔGads data is less than −20 kJ mol−1. In addition, a result higher than −40 kJ mol−1 is considered to be a sign of chemisorption (charge allocation and/or electron transfer between the orbitals of the chemical molecules and those of the alloy to establish coordinate connection) [63,64]. Given this, the study’s findings regarding the values of ΔGads show that the compound molecules are adsorbed onto the substrate surface by both chemisorption and physisorption methods, with chemisorption dominating (mixed-type adsorption). The negative ΔHads value, which demonstrates that the chemical molecules are adsorbed on the alloy surface through an exothermic adsorption mechanism, provides an explanation for why the adsorption decreases at higher temperatures [65]. According to the declining values of ΔSads [66], the molecules of the inhibitor become more ordered as the adsorption process progresses.
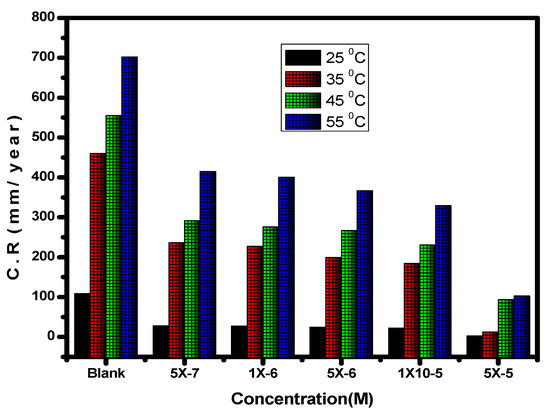
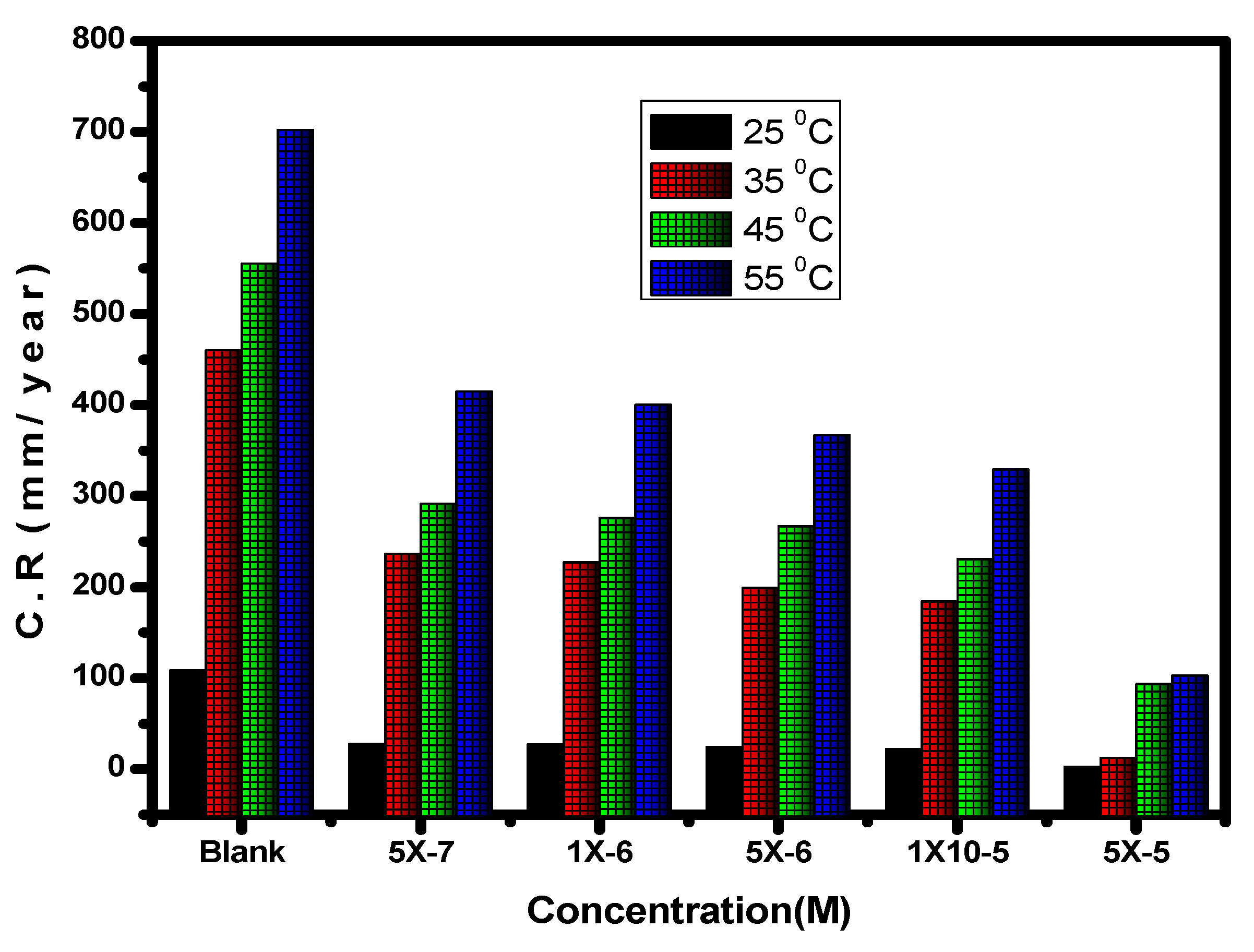
Figure 7.
Impact of the inhibitor concentration on the corrosion rate (C.R) of carbon steel in 1.0 M HCl solution at various temperatures.

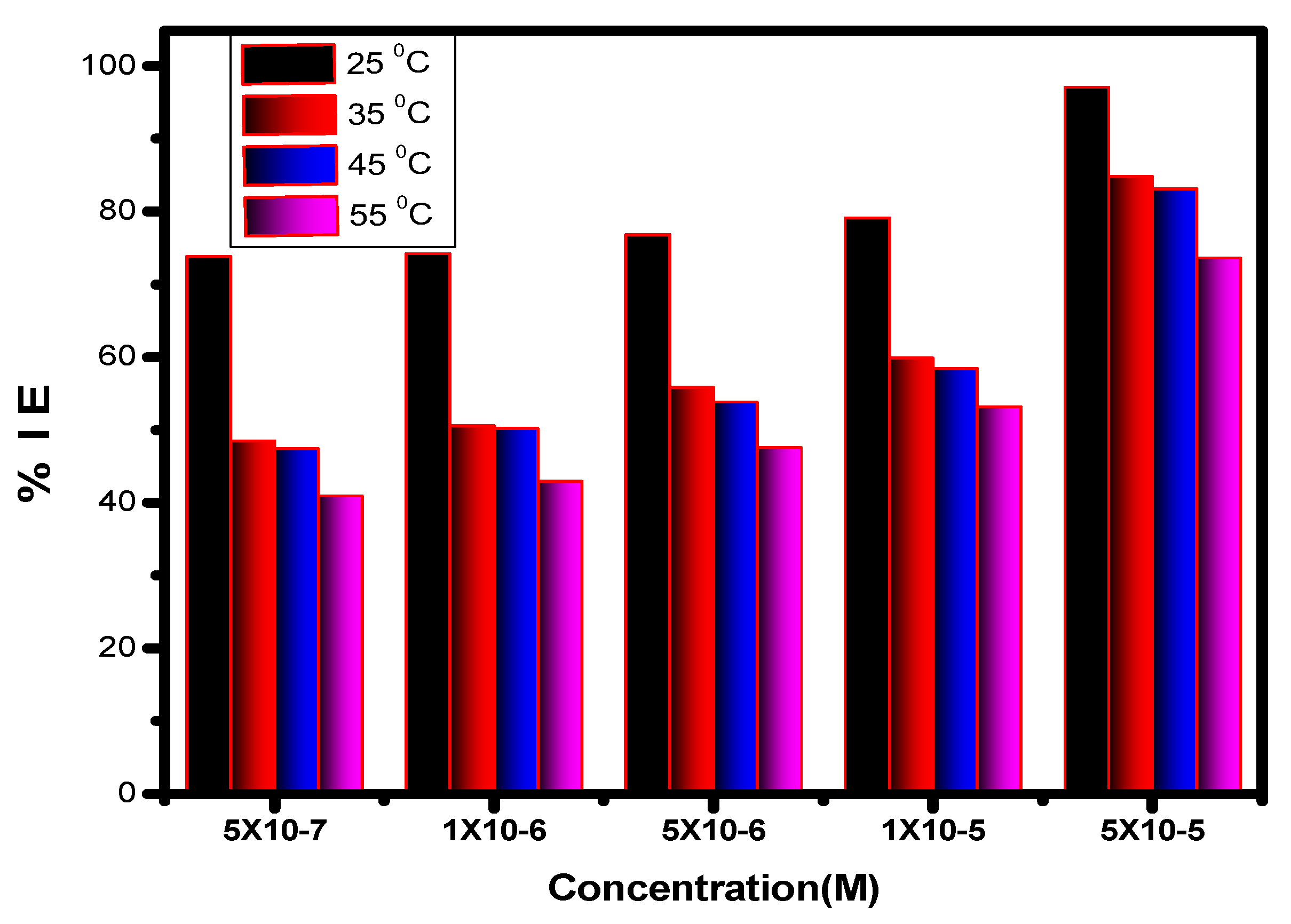
Figure 8.
Impact of the inhibitor concentration on its inhibition efficiency (%IE) for the corrosion of alloy in 1.0 M HCl solution at several temperatures.
| Isotherm | Temp. (K) | Log Kads | R2 | −ΔG°ads (KJ/mol) | −ΔH0ads (KJ/mol) | ΔS°ads (J/mol) |
|---|---|---|---|---|---|---|
| Langmuir | 298 | 4.77 | 0.998 | 37.16 | 33.85 | 11.12 |
| 308 | 4.624 | 0.987 | 37.55 | 12.01 | ||
| 318 | 4.585 | 0.990 | 38.54 | 14.74 | ||
| 328 | 4.474 | 0.980 | 39.05 | 15.8 | ||
| Henry | 298 | 3.95 | 0.997 | 32.49 | 23.58 | 29.899 |
| 308 | 3.94 | 0.993 | 33.52 | 32.27 | ||
| 318 | 3.833 | 0.982 | 33.96 | 32.64 | ||
| 328 | 3.764 | 0.991 | 34.59 | 33.56 |
3.3. Effect of Temperature
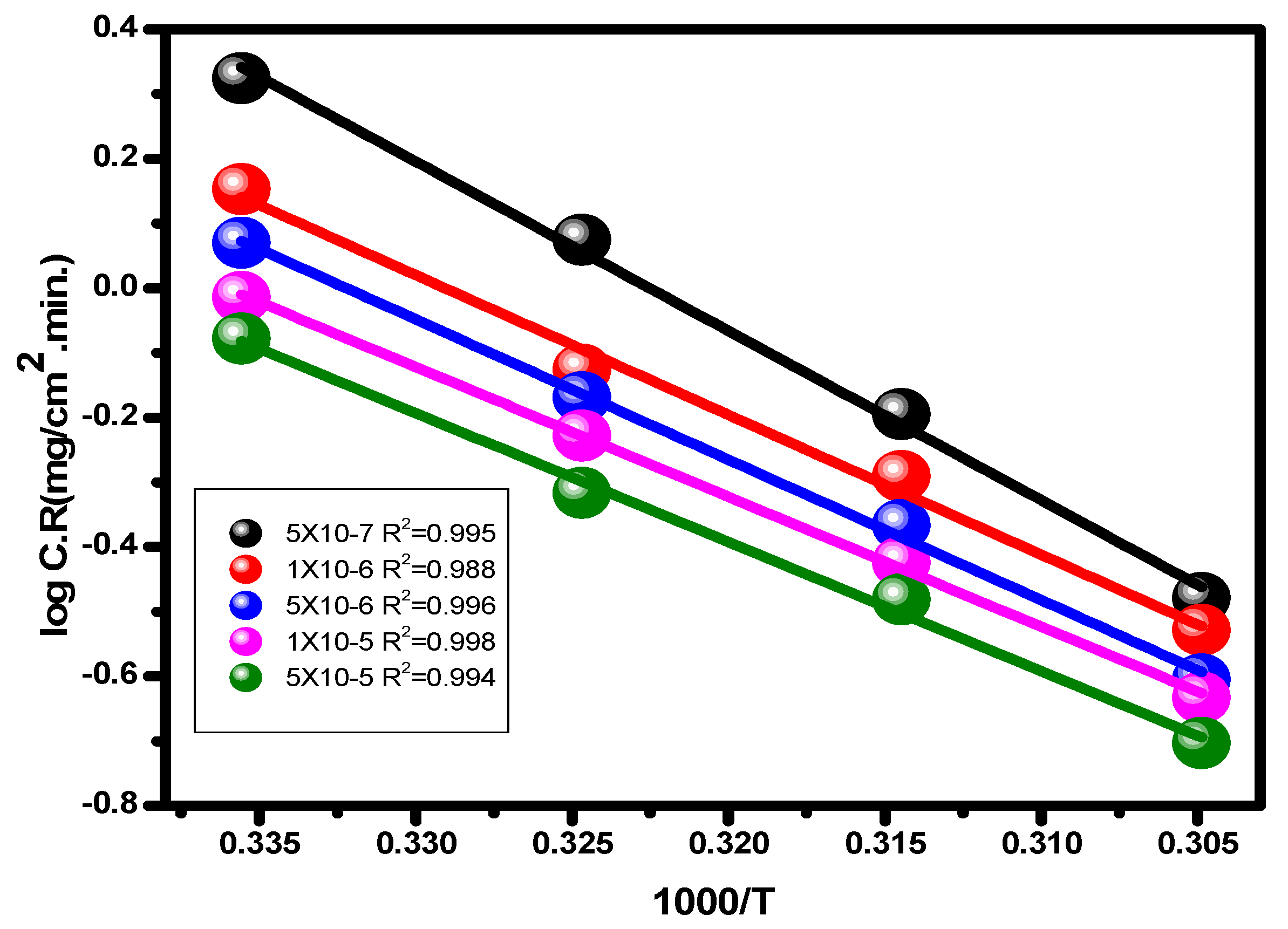
With the use of the potentiodynamic polarization method, comparing the degrees of alloy corrosion in 1.0 M hydrochloric acid and with the compound at various temperatures was possible. The temperature range was between 25 and 55 °C. Table 1 and Figure 7 and Figure 8 demonstrate how temperature affects the values for inhibitory efficiency and corrosion rate. Figure 9 shows the corrosion degree (log C.R) vs. time (1/T) for the alloy at 1.0 M HCl solution according to the presence or absence of the different quantities of the inhibitor. Given that all linear degradation coefficients are quite close to 1, the kinetic model may be able to adequately explain the carbon steel metal corrosion in 1.0 M HCl solution. This picture shows how the Arrhenius equation is applied to reach intersection A and how the slopes of the straight lines are sloped: [37].
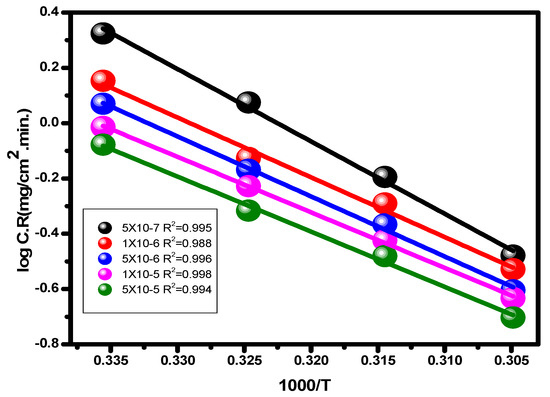
Figure 9.
Arrhenius plots (log C.R versus 1/T) for alloy in 1.0 M HCl solution and through various concentrations of the examined inhibitor.
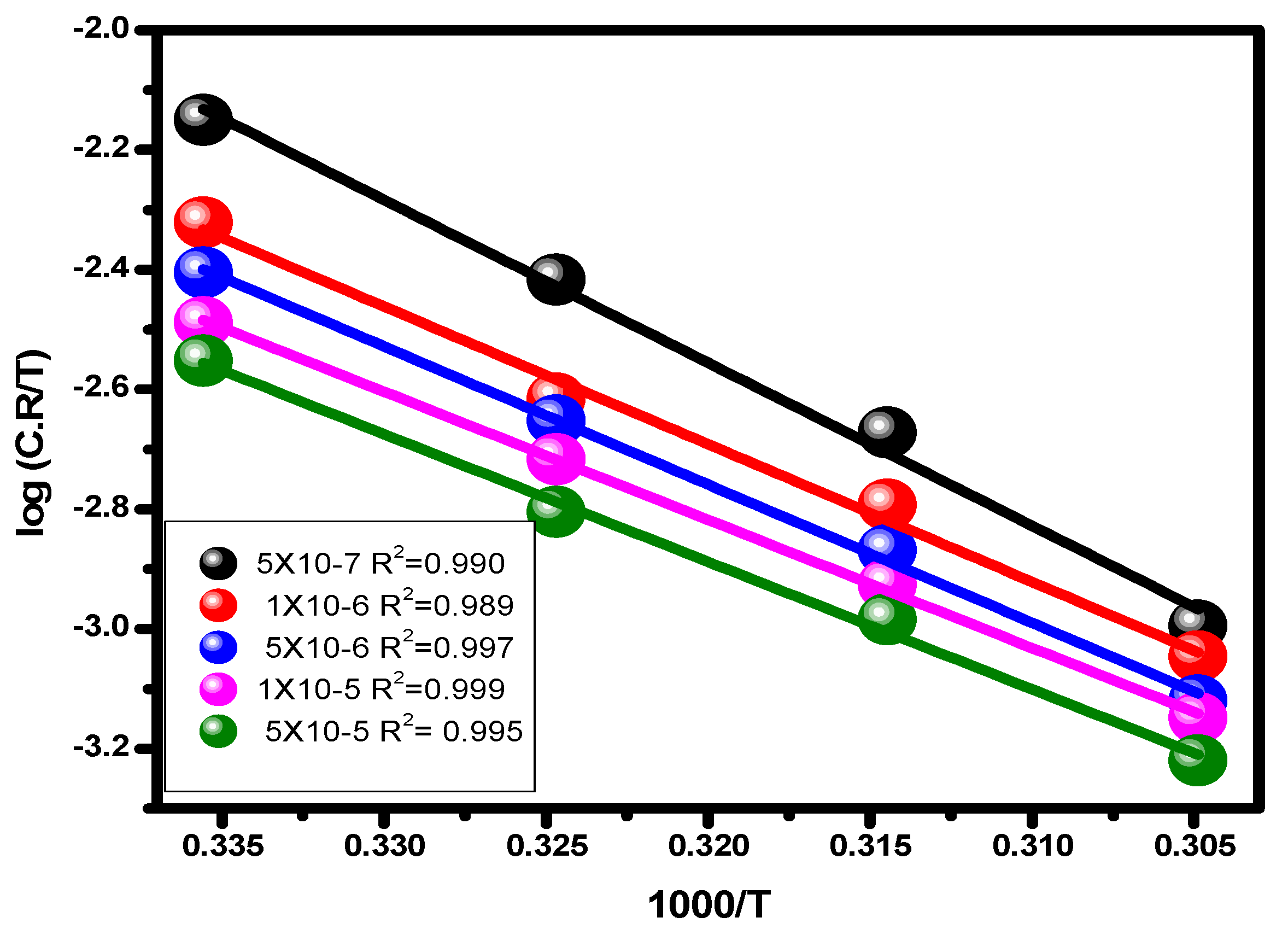
The letters A, E*a, and C.R stand for a constant that depends on the kind of metal, electrolyte, and apparent activation energy. The association between log (C.R/T) vs. 1/T for carbon steel in 1.0 M hydrochloric acid solution and at different dosages of the substance is shown in Figure 10. The transition condition is shown to be associated with the slope (−ΔH/2.303R) and the intercept (log (R/Nh) + ΔS/2.303R) [67].
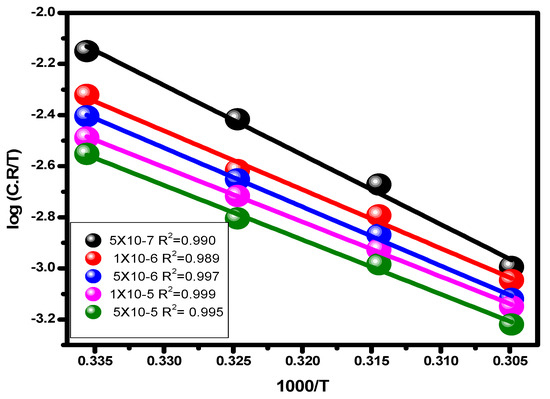
Figure 10.
Transition state plots (C.R/T vs. 1/T) for alloy in 1.0 M HCl solution and with various concentrations of the examined inhibitor.
The Planck constant is h, the entropy of activation is ΔS*, the enthalpy of activation is ΔH*, the Avogadro number is N, and the entropy of activation is ΔS*. In Table 3, the computed activation energy (E*a), activation enthalpy (ΔH*), and activation entropy (ΔS*) values are given. According to the data and our research, these additions raise the activation energy, activation entropy, and activation enthalpy of reactions, all of which inhibit corrosion development. The amount of inhibitor added to the acid solution boosts the active enthalpy (ΔH*), indicating that the energy barrier in the corrosion reaction is somewhat controlled by the inhibitor’s presence and is increased by doing so. The blank sample and the inhibitory solution’s negative and considerable entropy activation demonstrate that the activated molecule differentiates itself through the binding procedure as opposed to the dissociation stage. Compared to their initial state, the activated molecules had a strong organizational structure [68].

Table 3.
Effect of inhibitor concentration on the thermodynamic activation parameters of carbon steel corrosion in 1.0 M HCl solution.
3.4. Surface Investigations
3.4.1. SEM Analysis
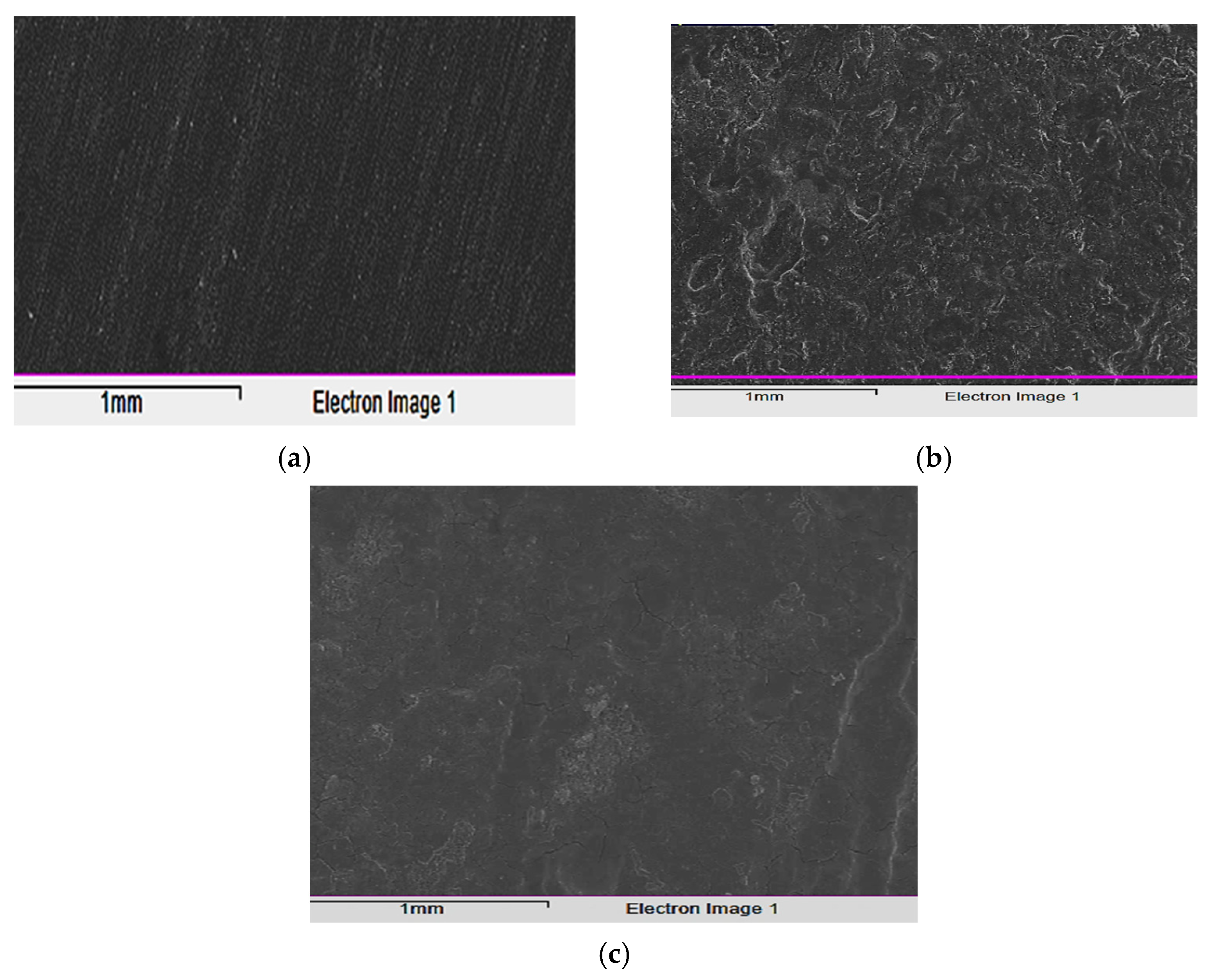
To evaluate the corrosive effects on the alloy surface both with and without the tested inhibitor (5 × 10−5 M), before and after being immersed in the acidic solution for 24 h at 298 K, the test specimens were captured in images using a scanning electron microscope (SEM). Figure 11a illustrates how the carbon steel sample appears smooth before immersion and how some surface scratches indicate the metal’s smooth surface. This demonstrates that no corrosion-related byproducts or inhibitor complexes developed on the carbon steel surface. After being exposed to the corrosive fluid, the sample was photographed to reveal the extent to which corrosion had damaged the unconstrained specimen’s surface. Additionally, the morphology of the corrosion products is quite irregular and cube-shaped. The fact that the damage was greatly lessened after the inhibitor was introduced to the mixture demonstrates how effectively this chemical inhibits corrosion and shields the alloy surface from corrosive agents (Figure 11). When exposed to the aggressive solution containing the inhibitor, the carbon steel surface behaved differently than in the absence of inhibitor, appearing flat and tightly packed rather than uneven and potholed. This suggests that an adsorption layer formed on the surface, shielding the metal from aggressive corrosion.
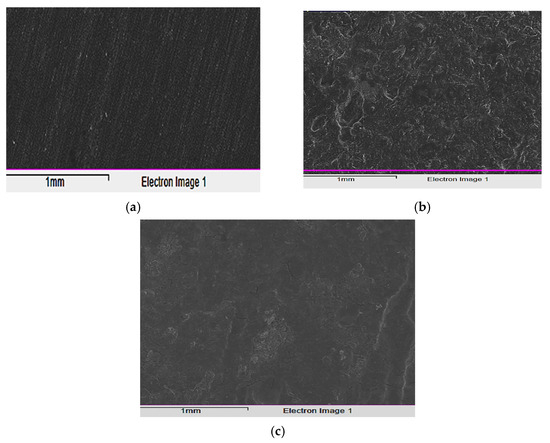
Figure 11.
SEM images of: (a) alloy surface, (b) corroded surface in 1.0 M HCl solution (lacking inhibitor), and (c) with the inhibitor.
3.4.2. Analysis with Atomic Force Microscopy (AFM)
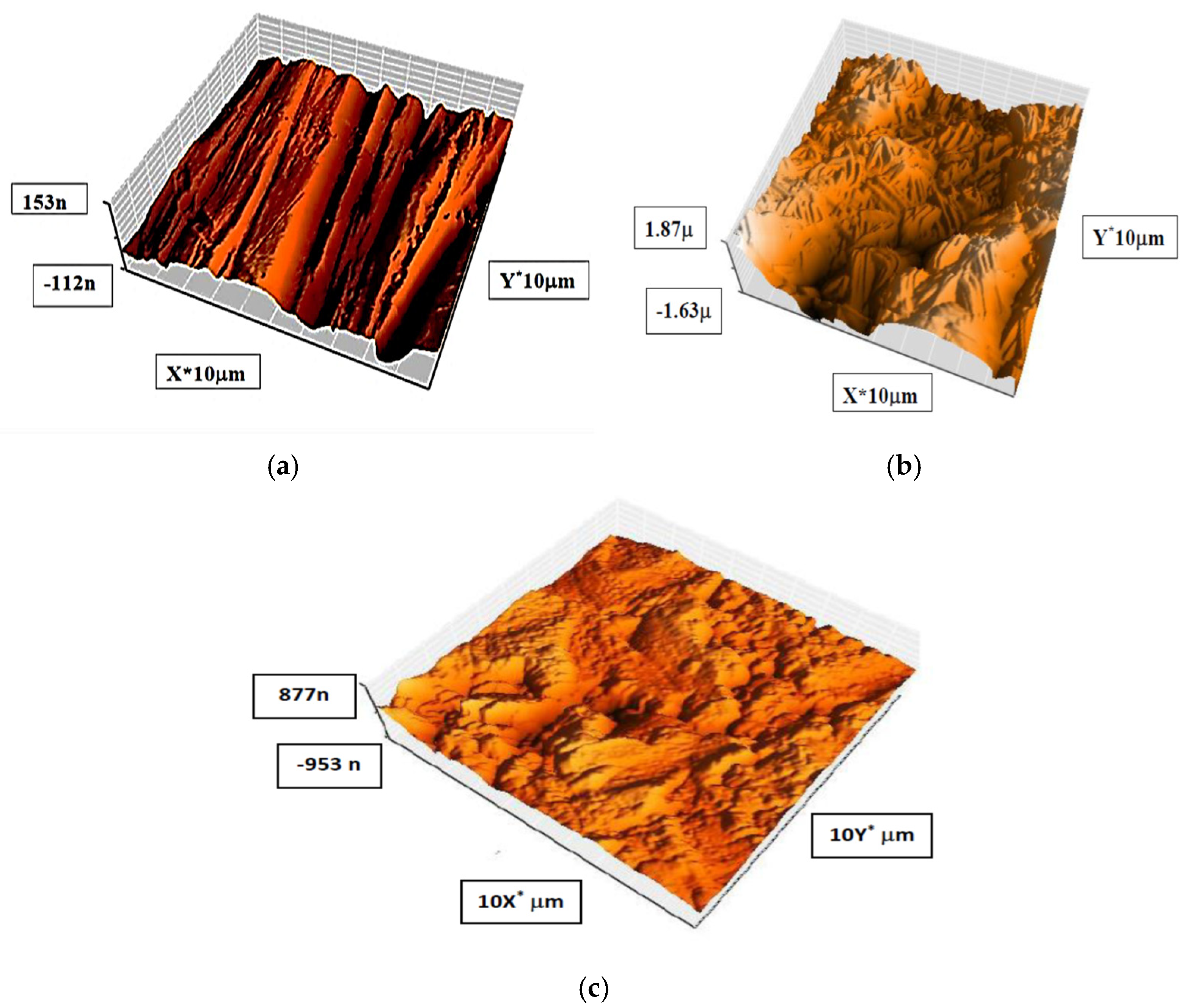
Atomic force microscopy (AFM) has been found to be one of the most precise techniques in this inquiry for verifying previously obtained results. This scan is supplemented through a continued resolution that is 1000 times the optical diffraction limit for organizing nanoscale fractions [69]. Table 4 displays the findings of this survey. Sq stands for root-mean-square roughness, and it represents the height deviations’ average measured both inside and outside of the assessment length and measured from the mean line. The average roughness and the maximum single peak-to-valley height in the neighboring sampling heights, respectively, are signified by the symbols P-V and Sa. Sa also shows the average deviation of the roughness profiles of all of the spots across the evaluation time from a mean line. Figure 12 displays three examples of an alloy. The first model is a pure alloy piece, the second one is an alloy that has spent 24 h in a 1.0 M HCl solution, and the third one is a piece of carbon steel that has spent 24 h in a 1.0 M HCl solution in which the inhibitor is present at the highest concentration possible (5 × 10−5 M). The small imperfection on the polished alloy surface is the result of atmospheric corrosion. The material that was immersed in 1 molar of hydrochloric acid is missing from Figure 12, exposing the corroded alloy surface. The Sq, Sa, and P-V alloy surfaces have heights of 592.05 nm, 486.76 nm, and 3842.0 nm, respectively. These findings demonstrate that the refined alloy’s surface is smoother than the surface of the alloy that was dipped in 1.0 M HCl. The average surface roughness of the carbon steel decreased while immersed in 1 M HCl, going from 486.76 to 187.40 nm, when 5 × 10−5 M of the inhibitor was present. Because the inhibitor molecules adsorbed onto the substrate surface and reduced the interaction between the alloy and hydrochloric acid, the alloy surface was prevented from uniformly corroding because the highest concentration smoothed the surface (Figure 12 and Table 4). This outcome was consistent with the value of ΔS*, which demonstrated that species ordering increased in the presence of the compound. Similar findings were seen in other research as well [67,68].

Table 4.
Data on roughness for carbon steel after one day of immersion in a 1 M HCl solution and with a 1 × 10−5 M compound.

Figure 12.
Pictures of AFM for a carbon steel surface showing it in three different states: uninhibited (a), corroded (b), and inhibited (c) (5 × 10−5 M).
3.5. Theoretical Investigations
3.5.1. Calculations of DFT
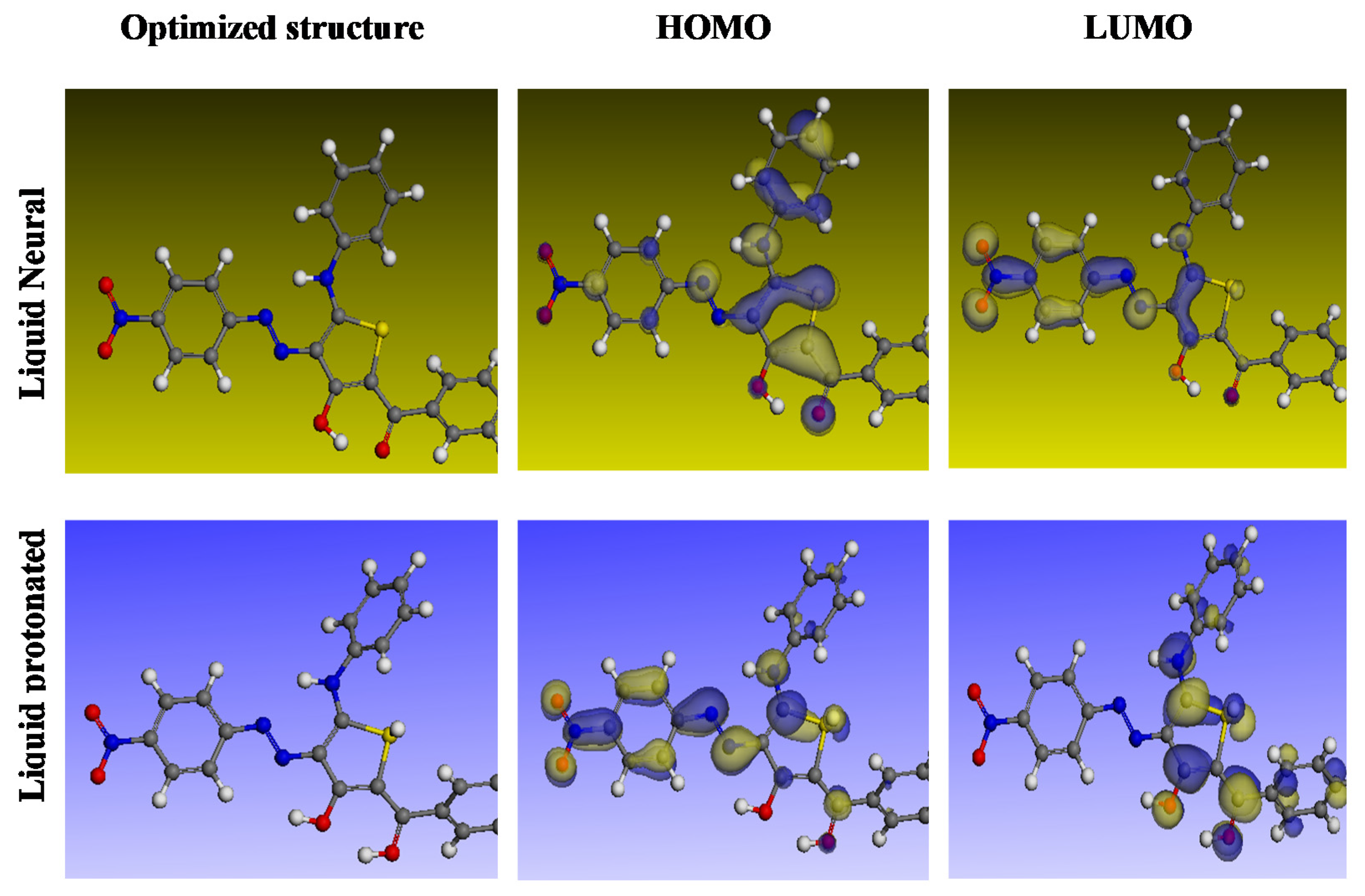
In order to understand the nature of the interaction between the inhibitor molecules’ adsorption centers and the alloy surface, DFT analyses were conducted. The ideal inhibitor molecules’ neutral and protonated structures are shown in Figure 13 along with their HOMO and LUMO distributions, and their quantum chemical characteristics are listed in Table 5. The frontier orbital theory states that the HOMO and LUMO energies govern the interaction between the inhibitor molecule and the metal [65]. Chemistry uses the terms EHOMO and ELUMO to refer to a molecule’s ability to give and obtain electrons separately [70]. Smaller ELUMO values indicate a compound’s capacity to receive electrons, whereas larger EHOMO values indicate a compound’s capacity to donate electrons to the open d-orbital on the surface of carbon steel [71]. It was also suggested that an inhibitor molecule with a higher efficacy in preventing corrosion would have a smaller energy hole among the LUMO and HOMO energies (ΔE). Due to the fact that removing an electron from its outer shell orbital only necessitates a small amount of ionization energy, an electron from the EHOMO state is provided to the ELUMO state. The EHOMO and ELUMO values in Table 5 show that the inhibitor can provide or take electrons, which is significant. The proton form of the compound has a smaller energy gap (ΔE) than the neutral form, indicating that the inhibitor reacts with the steel surface more forcefully [72]. Small energy gap molecules are more flexible than large energy gap ones. Because the electrons travel to the acceptor so quickly, the molecule is more reactive than harder materials. A substance’s composition, stability, and reactivity can all be determined using the absolute hardness (η) and softness (σ) values. The only part of the inhibitor molecule that can adsorb is the one with the greatest value for simple electron transfer [73]. The inhibitor molecules are converted to Lewis bases in the corrosion system, while alloy molecules are converted to Lewis acids. For avoiding the soft acid corrosion of bulk metals, soft-base inhibitors are the most effective. Information about structural organization is disseminated and rationalized using the dipole moment, the third important property [74,75]. Another indicator that is frequently used to forecast the direction of corrosion inhibition is the dipole moment (μ). It measures the polarity of the connection and reveals the number of electrons scattered within it. Strong dipole–dipole interactions between the inhibitor and iron surface result in better inhibition and long-lasting adsorption on the surface of carbon steel [76]. An organic dipole’s electrostatic attraction to a charged alloy surface speeds up the actual adsorption of the organic retarder onto the metal surface. Therefore, large dipole moments are favorable for adsorption molecules. The dipole torque in protons altered dramatically, as seen by analyzing the dipole moments, proving that the inhibitor is actually physically adsorbed on the steel surface in the form of a proton can molecule [77]. However, the research does not provide any indication of a connection between inhibitory efficacy [78]. It is asserted that the electronic inhibitor on the metal surface will be more effective at suppressing the reaction if ΔN is lower than 3.6 [79]. An electron’s ability to bind inhibitors (χ) is referred to as its electronegativity. As the attraction of an electron to a carbon steel surface grows stronger, the value of inh increases (the protonated version has a high value for electronegativity). Since the inhibitory particles are in stronger contact with the more electroactive carbon steel surface, there is higher inhibition, according to previous studies [79,80,81,82]. According to Table 5, the examined compound is a suitable inhibitor. By increasing the region where chemical molecules are in contact with the surface of carbon steel, the inhibition is more effective.
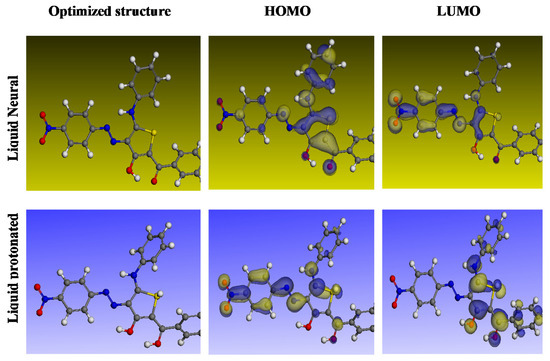
Figure 13.
Best molecular forms of the inhibitor and its HOMO and LUMO identified using DFT calculations in the aqueous phase.

Table 5.
Inhibitor structure in the aqueous phase and its quantum chemical parameters.
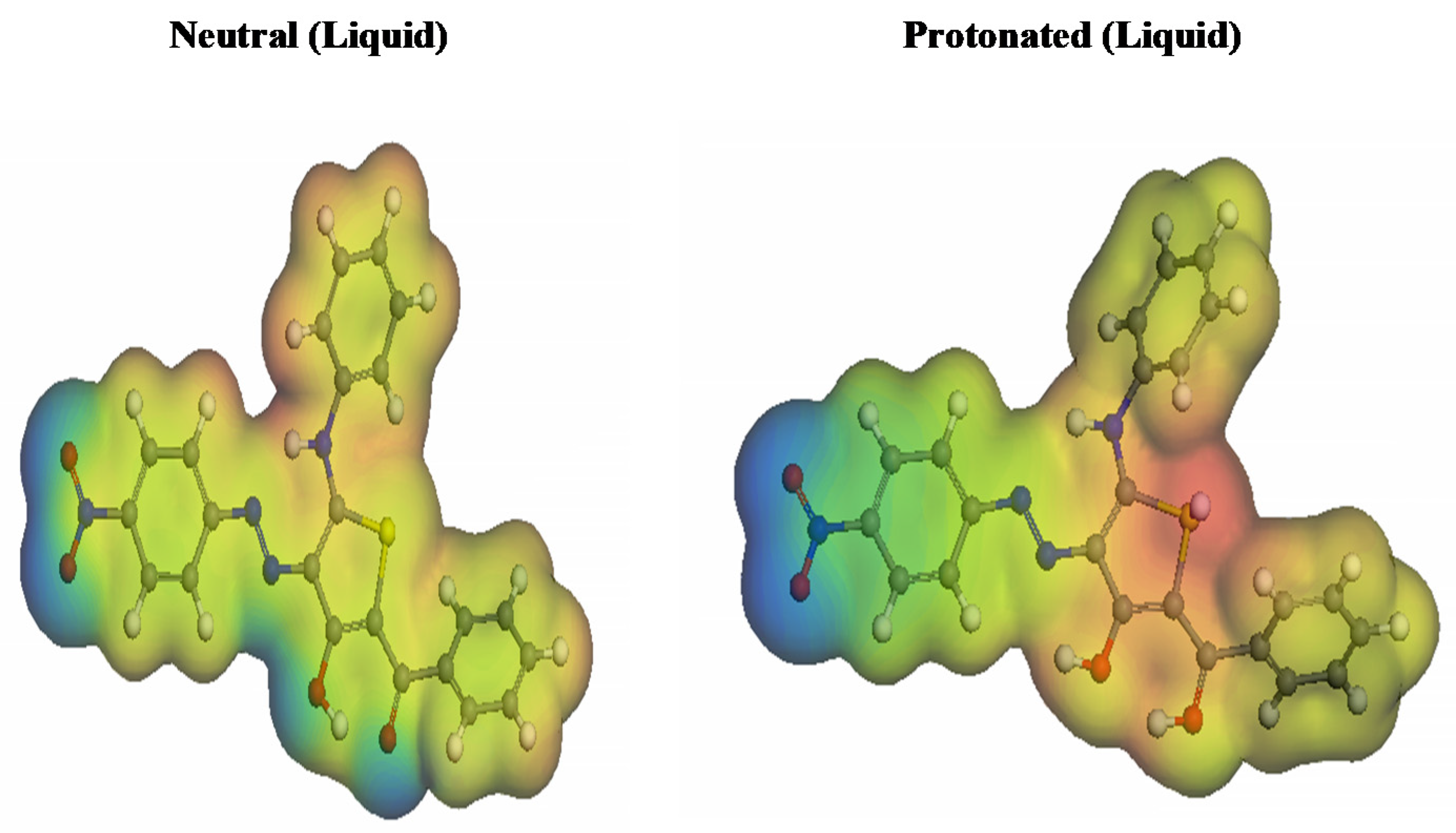
3.5.2. Molecule Electrostatic Potential (MESP)
The effective 3D visual method known as MESP mappings can be used to differentiate between the total charge dispersion and the net electrostatic impact created over a molecule [83]. It is possible to discern the maximum electron density in Figure 14’s red hues, with MEP standing for the most significant negative (nucleophilic reaction). On the other side, the blue colours represent the electrophilic reaction [84]. Heteroatoms and double conjugate bonds are particularly prevalent in regions with high electron densities. Unfavorable positions are indicated by sulfur, nitrogen, and oxygen groups, which facilitate electrophilic attacks. The positive indications are the blue-colored hydrogen atoms, which are more vulnerable to nucleophilic assaults.
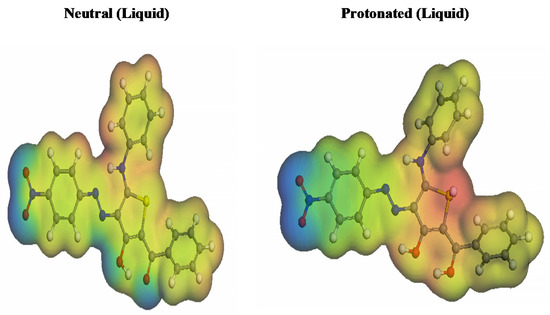
Figure 14.
Inhibitor molecule’s protonated and unprotonated electrostatic potentials.
3.5.3. Fukui Indices and Mulliken Charges
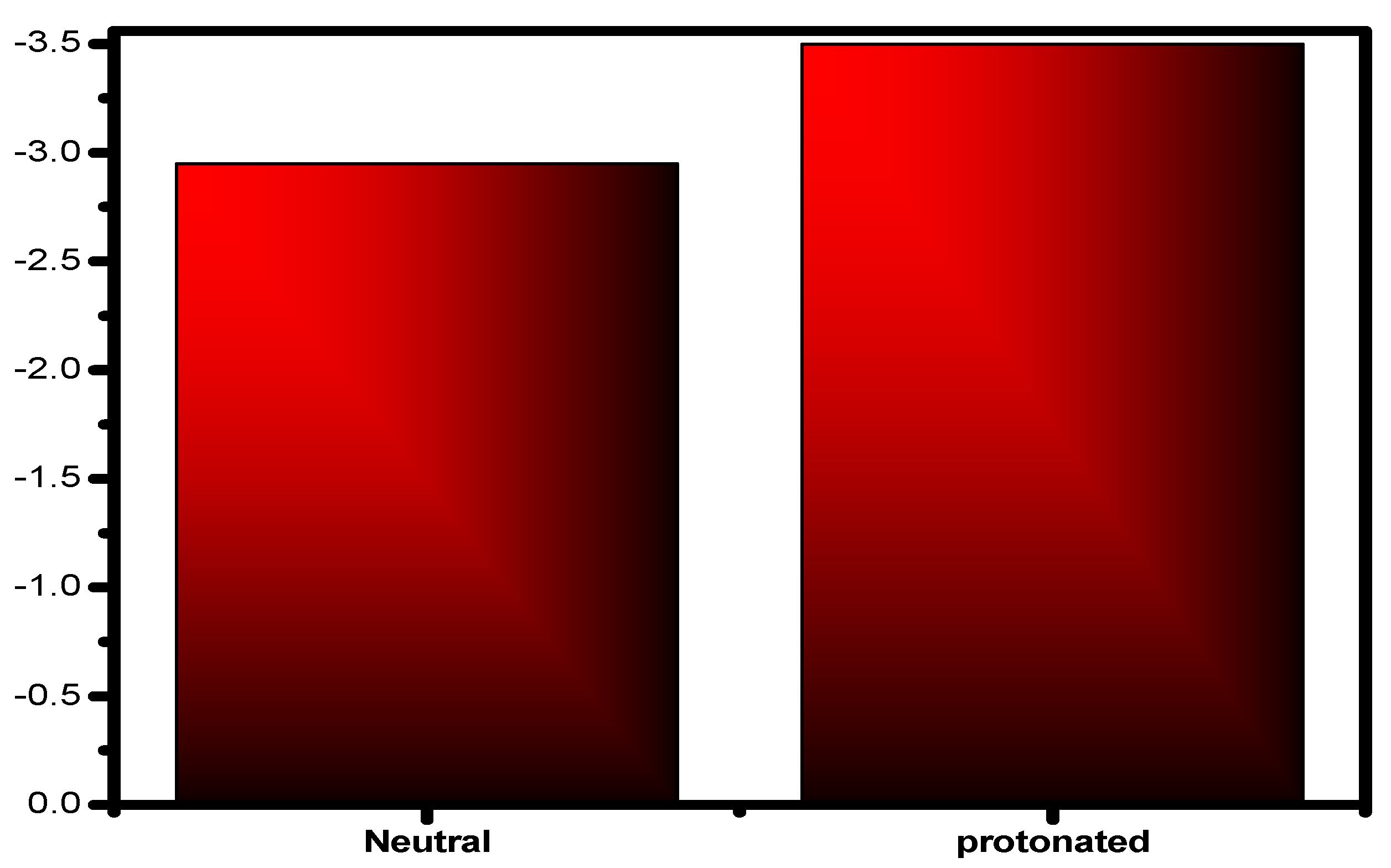
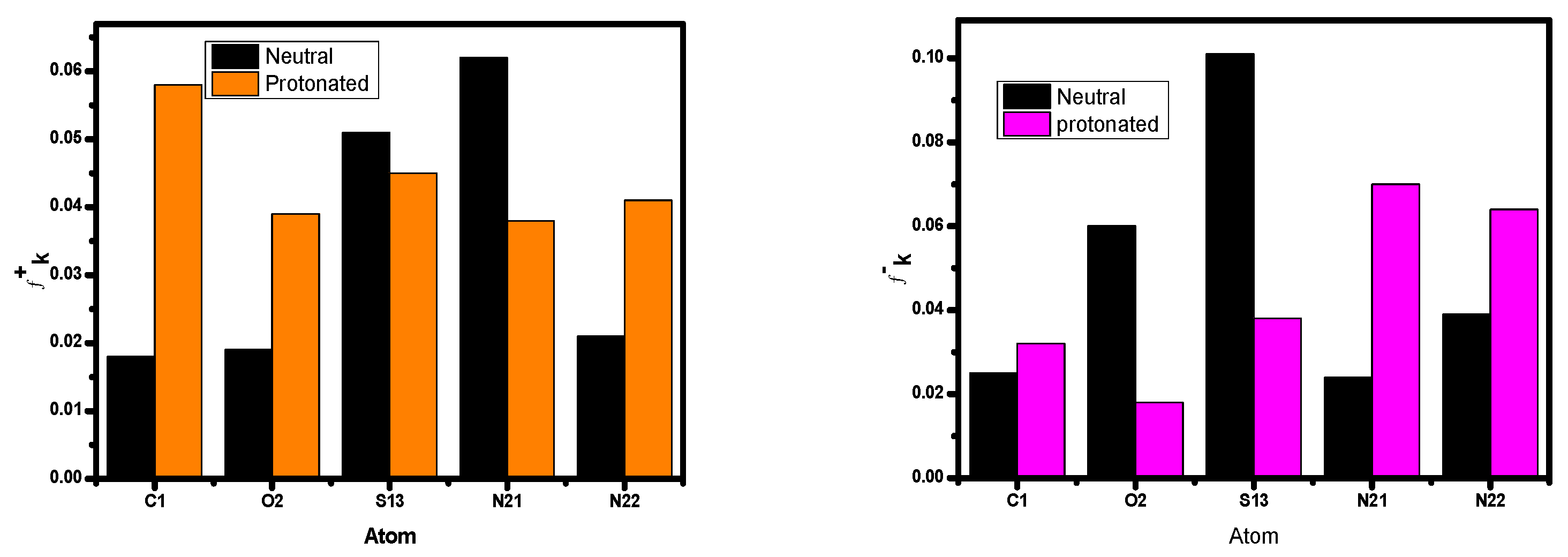
The locations of the donor and acceptor of the molecule’s active centers can be determined using Mulliken atomic charges and Fukui indices. The results showed that the inhibitor neutral and protonated particles were distributed in various electrophilic and nucleophilic sites. The outcomes show that these species are more reactive to the carbon steel atoms due to the active sites (donor–acceptor) in the inhibitor. There are both negative and positive numbers in the Mulliken distribution. Due to this finding, all of the proton molecule locations significantly shifted in the discovery’s favor, showing that neutral molecules are flexible and that there was an increase in the amount of chemical interaction through the proton inhibitor. The inhibitor’s total negative charge (TNC) increased as it protonated (Figure 15), demonstrating similar behavior [85]. This demonstrates the increased frequency of structural interactions between protonated particles. The inhibitor has the ability to protonate at these sites in conditions with high acid (HCl) concentrations, creating molecules that are positively charged. This chemical contains a minimum of one heteroatom. As a result, they engage in interactions with the anions (Cl−), which are widely dispersed on the alloy’s surface. A concept will be used to investigate how protonation affects the nearby inhibitory substance centers. The atoms of the examined inhibitor are shown in Figure 16 with added markers (Fukui’s index (FI)) that have higher values of fk+ and fk− in both protonated and non-protonated forms. This image’s comprehensive analysis demonstrates that for assault systems that employ both electrophilic and nucleophilic ions, every molecule has a unique atom composition. The highest Fukui function (fk+) LUMO-connected atom in the compound’s molecules, which improves reactivity to the donor reagent, is the most sought-after target for nucleophilic assault. The atom in the compound with the maximum value of the Fukui function (fk−) is chosen for electrophilic attack because it is related to the HOMO and provides the reactivity towards an acceptor reagent. After protonation, the fk+ values of some atoms increased because they could now take electrons; however, the data for their fk− values fell because their donor characteristics were lost as their centers were blocked by H+ protons (Figure 16). The changed atoms within a same compound with changing f values further supports the hypothesis that the same compound molecules can take and give electrons in two different forms (the neutral state and protonated form).
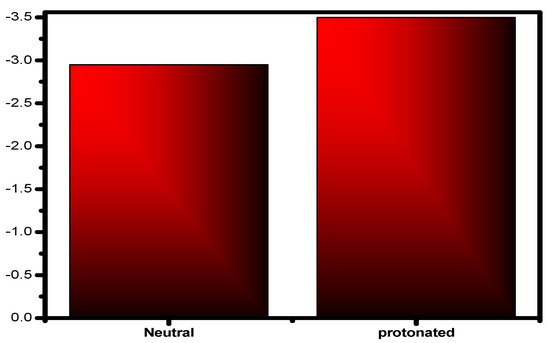
Figure 15.
Inhibitor molecules, both protonated and unprotonated, have a total negative charge (TNC).
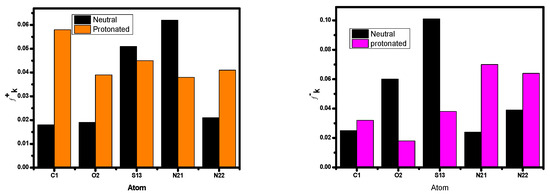
Figure 16.
Graphical representations of the Fukui indices for the most reactive atoms in the protonated and unprotonated forms of the inhibitor molecule.
3.5.4. Calculations Based on Molecular Dynamics
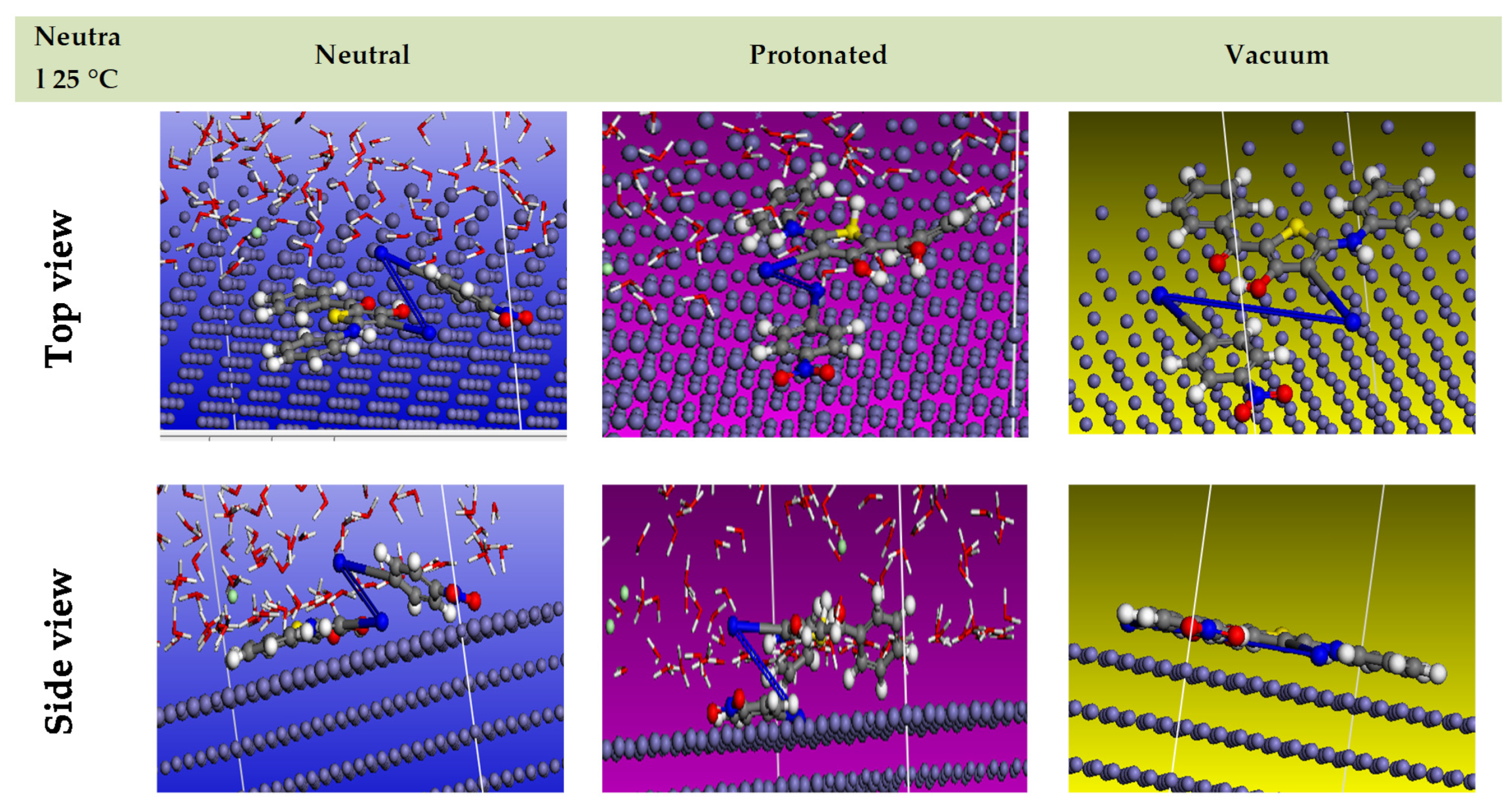
Through applying MD simulations and maintaining the adsorption locator module in situ, one may theoretically explain how the examined inhibitor chemicals interact with the alloy surface. Because of this, Figure 17 depicts the inhibitor molecules’ most effective adsorption configurations on the alloy surface, which are positioned in a flat or nearly parallel arrangement, exhibiting an expansion of the spectrum of adsorption and the extreme surface coverage [86,87,88,89]. In addition, Table 6 includes the data of the molecular dynamic simulation; this contains the adsorption energy for restful adsorbate molecules when the process is neutral, protonated, or vacuum, the rigid adsorption energy for unrelaxed adsorbate molecules, and the deformation energy for relaxed adsorbate molecules [80]. Contrary to what was shown in earlier studies, Table 6 shows that the inhibitor has a higher adsorption energy for both neutral and protonated forms [38]. According to this study, the compound strongly adheres to the surface of the alloy, generating immobile adsorbed coats that protect the alloy from corrosion. Table 6’s results show that this inhibitor has a higher inhibitory efficiency than other substances that have been studied in the past [38], accounting for the higher adsorption energies of its relaxed and unrelaxed forms. This is true both before and after the geometry optimization process. dEads/dNi (kcal mol−1) measures the amount of energy that is created when one of the adsorbate molecules splits from the formation of the adsorbent substrate [90,91,92,93]. The fact that water has low dEads/dNi values—around 11.67 kcal mol−1 in a neutral system and 8.63 kcal mol−1 in a protonated arrangement—indicates that the inhibitor’s molecules can take the place of water molecules. Research in theory and practice demonstrates that the inhibitor molecules are subsequently forcedly adsorbed on the carbon steel surface and produce a strong adsorbed protective coat, creating a corrosion barrier for the alloy surface in challenging circumstances. As the adsorption energy in an aqueous solution rises over the vacuum value, the particle’s efficacy is increased on the alloy surface (Table 6).

Figure 17.
Simulations using molecular dynamics for the compound molecule on the carbon steel substrate (1 1 0) (adsorption locator module).

Table 6.
Information and specifications obtained from a molecular dynamic simulation of the compound (neutral–protonated–vacuum) adsorption on alloy surface.
4. Conclusions
- ∗
- The investigated substance was found to prevent carbon steel from corroding in 1.0 M HCl medium and adheres to the Langmuir and Henry isotherms for adsorption, where both isotherms denote both physical and chemical adsorption, but chemical adsorption is the predominant type.
- ∗
- Electrochemical experiments revealed that while the inhibitory efficacy diminishes as the temperature rises, it increases when the concentration of the examined substance increases. This feature facilitates the molecules’ binding on the steel surface.
- ∗
- Potentiodynamic polarization measurements show that the employed inhibitor operates as a mixed-type inhibitor.
- ∗
- SEM and AFM studies substantially validate the electrochemical and theoretical findings, and they also verify the inhibitor’s adsorption of the inhibitor molecules on alloy surfaces.
- ∗
- The investigation’s results and theoretical analysis provided evidence in favor of the claim that the compound is a potent corrosion inhibitor.
- ∗
- According to a Fukui function analysis, the main reactive sites for corrosion inhibitors are phenyl carbon atoms, oxygen, and nitrogen.
Author Contributions
A.T.: conceptualization, data curation, investigation, methodology, writing—original draft, writing—review and editing, and formal analysis; H.S.G.: conceptualization, data curation, investigation, methodology, writing—original draft, writing—review and editing, and formal analysis. A.F.: investigation, methodology, writing—review and editing, and formal analysis. H.A.: funding acquisition, validation, software, writing—review and editing. H.S.: investigation, software, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023054. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia, for funding this research through the project number IFP-IMSIU-2023054. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Fawzy, A.; Toghan, A. Inhibition evaluation of chromotrope dyes for the corrosion of mild steel in acidic environment: Thermodynamic and kinetic aspects. ACS Omega 2021, 6, 4051–4061. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Fawzy, A. Unraveling the Adsorption Mechanism and Anti-Corrosion Functionality of Dextrin and Inulin as Eco-Friendly Biopolymers for the Corrosion of Reinforced Steel in 1.0 M HCl: A Thermodynamic and Kinetic Approach. Polymers 2023, 15, 3144. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Fawzy, A.; Alakhras, A.I.; Farag, A.A. Electrochemical and theoretical examination of some imine compounds as corrosion inhibitors for carbon steel in oil wells formation water. Int. J. Electrochem. Sci. 2022, 17, 2212108. [Google Scholar] [CrossRef]
- Manh, T.D.; Huynh, T.L.; Thi, B.V.; Lee, S.; Yi, J.; Dang, N.N. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Environments Containing Sonneratia caseolaris Leaf Extract. ACS Omega 2022, 7, 8874–8886. [Google Scholar] [CrossRef]
- Alamry, K.A.; Khan, A.; Aslam, J.; Hussein, M.A.; Aslam, R. Corrosion inhibition of mild steel in hydrochloric acid solution by the expired Ampicillin drug. Sci. Rep. 2023, 13, 6724. [Google Scholar] [CrossRef]
- Rahimi, A.; Farhadian, A.; Berisha, A.; Shaabani, A.; Mehmeti, V.; Zhong, X.; Yousefzadeh, S.; Djimasbe, R. Novel sucrose derivative as a thermally stable inhibitor for mild steel corrosion in 15% HCl medium: An experimental and computational study. Chem. Eng. J. 2022, 446, 136938. [Google Scholar] [CrossRef]
- Dahmani, K.; Galai, M.; Cherkaoui, M.; El Hasnaoui, A.; El Hessni, A. Cinnamon essential oil as a novel eco-friendly corrosion inhibitor of copper in 0.5 M sulfuric acid medium. J. Mater. Environ. Sci. 2017, 8, 1676–1689. [Google Scholar]
- El Hajjaji, F.; Salim, R.; Messali, M.; Hammouti, B.; Chauhan, D.S.; Almutairi, S.M.; Quraishi, M.A. Electrochemical studies on new pyridazinium derivatives as corrosion inhibitors of carbon steel in acidic medium. J. Bio-Tribo-Corros. 2018, 5, 4. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene)rhodamine. Corros. Sci. 2014, 79, 169–176. [Google Scholar] [CrossRef]
- Eldesoky, A.M.; Nozha, S.G. The adsorption and corrosion inhibition of 8-hydroxy-7-quinolinecarboxaldehyde derivatives on Csteel surface in hydrochloric acid. Chin. J. Chem. Eng. 2017, 25, 1256–1265. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Fawzy, A.; Alakhras, A.I.; Sanad, M.M.S.; Khairy, M.; Farag, A.A. Correlating Experimental with Theoretical Studies for a New Ionic Liquid for Inhibiting Corrosion of Carbon Steel during Oil Well Acidification. Metals 2023, 13, 862. [Google Scholar] [CrossRef]
- Akpan, E.D.; Dagdag, O.; Ebenso, E.E. Recent progress on the anticorrosion activities of acridine and acridone derivatives: A review. J. Mol. Liq. 2022, 361, 119686. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent advances of metal-organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Cen, H.; Wu, C.; Chen, Z. Co-doped carbon coated MnS/MnO/Mn nanoparticles as a novel corrosion inhibitor for carbon steel in CO2-saturated NaCl solution. Coll. Surf. A 2021, 630, 127528. [Google Scholar] [CrossRef]
- Verma, C.; Alrefaee, S.H.; Quraishi, M.A.; Ebenso, E.E.; Hussain, C.M. Recent developments in sustainable corrosion inhibition using ionic liquids: A review. J. Mol. Liq. 2021, 321, 114484. [Google Scholar] [CrossRef]
- Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Adesina, A.Y.; Saleh, T.A. The synergistic influence of polyethyleneimine-grafted graphene oxide and iodide for the protection of steel in acidizing conditions. RSC Adv. 2020, 10, 17739–17751. [Google Scholar] [CrossRef]
- Cen, H.; Cao, J.; Chen, Z. Functionalized carbon nanotubes as a novel inhibitor to enhance the anticorrosion performance of carbon steel in CO2-saturated NaCl solution. Corros. Sci. 2020, 177, 109011. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.A.; Ebenso, E.E. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Wang, C.L.; Qian, B. A novel l-histidine based ionic liquid (LHIL) as an efficient corrosion inhibitor for mild steel. RSC Adv. 2022, 12, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Wu, Y. Novel pHresponsive self-healing anti-corrosion coating with high barrier and corrosion inhibitor loading based on reduced graphene oxide loaded zeolite imidazole framework. Colloids Surf. A 2022, 642, 128641. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Cherkaoui, M. Imidazole derivatives as efficient and potential class of corrosion inhibitors for metals and alloys in aqueous electrolytes: A review. J. Mol. Liq. 2022, 345, 117815. [Google Scholar] [CrossRef]
- Verma, C.; Abdellattif, M.H.; Alfantazi, A.; Quraishi, M.A. N-heterocycle compounds as aqueous phase corrosioninhibitors: A robust, effective and economic substitute. J. Mol. Liq. 2021, 340, 117211. [Google Scholar] [CrossRef]
- Aslam, R.; Serdaroglu, G.; Zehra, S.; Verma, D.K.; Aslam, J.; Guo, L.; Verma, C.; Ebenso, E.E.; Quraishi, M.A. Corrosion inhibition of steel using different families of organic compounds: Past and present progress. J. Mol. Liq. 2022, 348, 118373. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: Design and applications. Coord. Chem. Rev. 2021, 446, 214105. [Google Scholar] [CrossRef]
- Alqarni, N.; El-Gammal, B.; Fawzy, A.; Al Bahir, A.; Toghan, A. Investigation of expired ticarcillin and carbenicillin drugs for inhibition of aluminum corrosion in hydrochloric acid solution. Int. J. Electrochem. Sci. 2022, 17, 2212113. [Google Scholar] [CrossRef]
- Toghan, A.; Fawzy, A.; Al Bahir, A.; Alqarni, N.; Sanad, M.M.S.; Khairy, M.; Alakhras, A.I.; Farag, A.A. Computational foretelling and experimental implementation of the performance of polyacrylic acid and polyacrylamide polymers as eco-friendly corrosion inhibitors for Copper in nitric acid. Polymers 2022, 14, 4802. [Google Scholar] [CrossRef]
- Farahati, R.; Mousavi-Khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Prog. Org. Coat. 2020, 142, 105567. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F.; Negrón-Silva, G.; González-Olvera, R.; Ángeles-Beltrán, D.; Palomar-Pardavé, M.; Miralrio, A.; Castro, M. Fluconazole and fragments as corrosion inhibitors of API 5L X52 steel immersed in 1M HCl. Corros. Sci. 2020, 174, 108853. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Negrila, C.; Trandafir, I.; Maxut, A. Effect of sulfacetamide on the composi-tion of corrosion products formed onto carbon steel surface in hydrochloric acid. Dig. J. Nanomater. Bios. 2011, 6, 663–673. [Google Scholar]
- Chen, S.; Chen, S.; Zhu, B.; Huang, C.; Li, W. Magnolia grandiflora leaves extract as a novel environmentally friendly inhibitor for Q235 steel corrosion in 1 M HCl: Combining experimental and theoretical researches. J. Mol. Liq. 2020, 311, 113312. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, B.; Bao, H.; Xie, Y.; Mou, Y.; Li, P.; Chen, D.; Shi, Y.; Li, X.; Yang, W. Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 2019, 128, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Luft, J.H. Improvements in Epoxy Resin Embedding Methods. J. Cell Biol. 1961, 9, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Damej, M.; Hsissou, R.; Berisha, A.; Azgaou, K.; Sadiku, M.; Benmessaoud, M.; Labjar, N.; El Hajjaji, S. New epoxy resin as a corrosion inhibitor for the protection of carbon steel C38 in 1M HCl experimental and theoretical studies (DFT, MC, and MD). J. Mol. Struct. 2022, 1254, 132425. [Google Scholar] [CrossRef]
- Ouici, H.B.; Benali, O.; Harek, Y.; Larabi, L.; Hammouti, B.; Guendouzi, A. Inhibition of mild steel corrosion in 5% HCl solution by 5-(2-hydroxyphenyl)-1,2,4-triazole-3-thione. Res. Chem. Intermed. 2013, 39, 2777–2793. [Google Scholar] [CrossRef]
- Paul, P.K.; Yadav, M. Investigation on corrosion inhibition and adsorption mechanism of triazine-thiourea derivatives at mild steel/HCl solution interface: Electrochemical, XPS, DFT and Monte Carlo simulation approach. J. Electroanal. Chem. 2020, 877, 114599. [Google Scholar] [CrossRef]
- Toghan, A.; Dardeer, H.M.; Gadow, H.S.; Elabbasy, H.M. New promising halogenated cyclic imides derivatives as potential corrosion inhibitors for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2021, 325, 115136. [Google Scholar] [CrossRef]
- Arrousse, N.; Fernine, Y.; Al-Zaqri, N.; Boshaala, A.; Ech-chihbi, E.; Salim, R.; El Hajjaji, F.; Alami, A.; Touhami, M.E.; Taleb, M. Correction: Thiophene derivatives as corrosion inhibitors for 2024-T3 aluminum alloy in hydrochloric acid medium. RSC Adv. 2022, 12, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Abo-baker, A.M.; Rageh, H.M.; Abd-Elsabour, M. Green electrochemical strategy for one-step synthesis of new catechol derivatives. RSC Adv. 2019, 9, 13145. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Verma, P.K. Synthesis of thiophene derivatives and their anti-microbial, antioxidant, anticorrosion and anticancer activity. BMC Chem. 2019, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, E.; Amer, F.A. Synthesis of some 4-arylazo-3-hydroxythiophene disperse dyes for dyeing polyester fabrics. Mon. Chem. 2008, 139, 561–567. [Google Scholar] [CrossRef]
- ASTM, AST G 31-72; Standard Recommended Practice for the Laboratory Immersion Corrosion Testing of Metals. American Society for Testing and Materials: Philadelphia, PA, USA, 2004.
- Marušić, K.; Otmačić Ćurković, H. Self-Assembling Monolayers of Stearic Acid in Protection of Steel. Croat. Chem. Acta 2018, 91, 427–433. [Google Scholar] [CrossRef]
- Khaled, M.; El-Khalek, A.; Shalabi, K.; Ismail, M.A.; El-Aziz, A.; Fouda, S. 5-Arylidene-1,3-dialkylbarbituric acid derivatives as efficient corrosion inhibitors for carbon steel in molar hydrochloric acid solution. RSC Adv. 2022, 12, 10443–10459. [Google Scholar]
- Eid, A.M.; Shaaban, S.; Shalabi, K. Tetrazole-based organoselenium bi-functionalized corrosion inhibitors during oil well acidizing: Experimental, computational studies, and SRB bioassay. J. Mol. Liq. 2020, 298, 111980. [Google Scholar] [CrossRef]
- Tang, J.; Hu, Y.; Han, Z.; Wang, H.; Zhu, Y.; Wang, Y.; Nie, Z.; Wanmg, Y. Experimental and Theoretical Study on the Synergistic Inhibition Effect of Pyridine Derivatives and Sulfur-Containing Compounds on the Corrosion of Carbon Steel in CO2-Saturated 3.5 wt.% NaCl Solution. Molecules 2018, 23, 3270. [Google Scholar] [CrossRef]
- Singh, A.K.; Chugh, B.; Thakur, S.; Pani, B.; Lgaz, H.; Chung, I.-M.; Pal, S.; Prakash, R. Green approach of synthesis of thiazolyl imines and their impeding behavior against corrosion of mild steel in acid medium, Colloids Surfaces a Physicochem. Eng. Asp. 2020, 599, 124824. [Google Scholar] [CrossRef]
- Fawzy, A.; Al Bahir, A.; Alqarni, N.; Toghan, A.; Khider, M.; Ibrahim, I.M.; Abulreesh, H.H.; Elbanna, K. Evaluation of synthesized biosurfactants as promising corrosion inhibitors and alternative antibacterial and antidermatophytes agents. Sci. Rep. 2023, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Dehghani, A.; Guo, L.; Ramezanzadeh, B. Pepper extract effectiveness as a natural inhibitor against corrosion of steel samples (SS) in 1 M hydrochloric acid; Theoretical (DFT calculation—MD simulation), thermodynamic, and electrochemical-surface studies. Ind. Crops Prod. 2022, 189, 115839. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shalabi, K.; Tantawy, A.H. Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: Experimental and computational investigations. New J. Chem. 2020, 44, 17791–17814. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Fouda, A.S.; Ahmed, R.E.; El-Hossiany, A. Chemical, Electrochemical and Quantum Chemical Studies for Famotidine Drug as a Safe Corrosion Inhibitor for α-Brass in HCl Solution. Prot. Met. Phys. Chem. Surf. 2021, 57, 398–411. [Google Scholar] [CrossRef]
- Migahed, M.A.; Azzam, E.M.S.; Morsy, S.M.I. Electrochemical behaviour of carbon steel in acid chloride solution in the presence of dodecyl cysteine hydrochloride self-assembled on gold nanoparticles. Corros. Sci. 2009, 51, 1636–1644. [Google Scholar] [CrossRef]
- Fergachi, O.; Benhiba, F.; Rbaa, M.; Ouakki, M.; Galai, M.; Touir, R.; Lakhrissi, B.; Oudda, H.; Touhami, M.E. Corrosion Inhibition of Ordinary Steel in 5.0 M HCl Medium by Benzimidazole Derivatives: Electrochemical, UV–Visible Spectrometry, and DFT Calculations. J. Bio Tribo-Corros. 2019, 5, 21–33. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Goulart, C.M.; Esteves-Souza, A.; Martinez-Huitle, C.A.; Rodrigues, C.J.F.; Maciel, M.A.M.; Echevarria, A. Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors. Corros. Sci. 2013, 67, 281–291. [Google Scholar] [CrossRef]
- Halambek, J.; Cindri, I.; Nin, A.; Date, R.; Date, R.; Date, A.; An, G. Characterization and study the inhibition activity of Luxol fast blue dye on corrosion of carbon steel in 1M H2SO4. J. Pre-Proof 2020, 15, 234. [Google Scholar]
- Boughoues, Y.; Benamira, M.; Messaadia, L.; Ribouh, N. Adsorption and corrosion inhibition performance of some environmental friendly organic inhibitors for mild steel in HCl solution via experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124610. [Google Scholar] [CrossRef]
- Sedik, A.; Lerari, D.; Salci, A.; Athmani, S.; Bachari, K.; Dardagan, İ.H. Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Electrochemical and surface morphological studies. J. Taiwan Inst. Chem. Eng. 2020, 107, 189–200. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Prakash, P.; Shankar, K.; Kathiresan, A. Azo Schiff Base as Antiscaling Agent for Mild Steel in Hydrochloric Acid: Electrochemical, Non-electrochemical, and DFT Studies. J. Bio-Tribo-Corros. 2019, 5, 12. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion inhibitors: Physisorbed or chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Benmahammed, I.; Douadi, T.; Issaadi, S.; Al-Noaimi, M.; Chafaa, S. Heterocyclic Schiff bases as corrosion inhibitors for carbon steel in 1 M HCl solution: Hydrodynamic and synergetic effect. J. Dispers. Sci. Technol. 2019, 41, 1002–1021. [Google Scholar] [CrossRef]
- Al Bahir, A. Estimation of the performances of creatine and creatinine as eco-friendly corrosion inhibitors for copper in sodium hydroxide solution. Int. J. Electrochem. Sci. 2023, 18, 100040. [Google Scholar] [CrossRef]
- Mazumder; Synthesis, M.A.J. Characterization and electrochemical analysis of cysteine modified polymers for corrosion inhibition of mild steel in aqueous 1 M HCl. RSC Adv. 2019, 9, 4277–4294. [Google Scholar] [CrossRef] [PubMed]
- Sliem, M.H.; Afifi, M.; Radwan, A.B.; Fayyad, E.M.; Shibl, M.F.; Heakal, F.E.T.; Abdullah, A.M. AEO7 Surfactant as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl solution. Sci. Rep. 2019, 9, 2319. [Google Scholar] [CrossRef]
- Abdallah, Y.M.; Shalabi, K.; Bayoumy, N.M. Eco-friendly synthesis, biological activity and evaluation of some new pyridopyrimidinone derivatives as corrosion inhibitors for API 5L X52 carbon steel in 5% sulfamic acid medium. J. Mol. Struct. 2018, 1171, 658–671. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 2009, 51, 1876–1878. [Google Scholar] [CrossRef]
- Bedair, M.A.; El-Sabbah, M.M.B.; Fouda, A.S.; Elaryian, H.M. Synthesis, electrochemical and quantum chemical studies of some prepared surfactants based on azodye and Schiff base as corrosion inhibitors for steel in acid medium. Corros. Sci. 2017, 128, 54–72. [Google Scholar] [CrossRef]
- Nazari, M.H.; Shihab, M.S.; Havens, E.A.; Shi, X. Mechanism of corrosion protection in chloride solution by an apple-based green inhibitor: Experimental and theoretical studies. J. Infrastruct. Preserv. Resil. 2020, 1, 7–25. [Google Scholar] [CrossRef]
- El Kacimi, Y.; Touir, R.; Alaoui, K.; Kaya, S.; Abousalem, A.S.; Ouakki, M.; Touhami, M.E. Anti-corrosion Properties of 2-Phenyl-4(3H)-quinazolinone-Substituted Compounds: Electrochemical, Quantum Chemical, Monte Carlo, and Molecular Dynamic Simulation Investigation. J. Bio-Tribo-Corros. 2020, 6, 47–71. [Google Scholar] [CrossRef]
- Koumya, Y.; Idouhli, R.; Oukhrib, A.; Khadiri, M.; Abouelfida, A.; Benyaich, A. Synthesis, Electrochemical, Thermodynamic, and Quantum Chemical Investigations of Amino Cadalene as a Corrosion Inhibitor for Stainless Steel Type 321 in Sulfuric Acid 1M. Int. J. Electrochem. 2020, 2020, 5620530–5620540. [Google Scholar] [CrossRef]
- Kumar, S.R.; RashvandAvei, M.; Vijayan, M. Novel metal complexes as antimicrobial and anticorrosion in acid media. Mater. Res. 2020, 23, e20180610. [Google Scholar]
- Mert, B.D.; Mert, M.E.; Kardas, G.; Yazici, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]
- Londonkar, R.; Kamble, A.; Reddy, V.C. Anti-Inflammatory Activity of Pandanus odoratissimus Extract. Int. J. Pharm. 2010, 6, 311–314. [Google Scholar] [CrossRef]
- Ansari, K.R.; Ramkumar, S.; Nalini, D.; Quraishi, M.A. Studies on adsorption and corrosion inhibitive properties of quinoline derivatives on N80 steel in 15% hydrochloric acid. Cogent Chem. 2016, 2, 1145032–1145045. [Google Scholar] [CrossRef]
- Vu, H.N.; Hien, P.; Mathesh, M.; Thu, V.; Nam, N. Improved Corrosion Resistance of Steel in Ethanol Fuel Blend by Titania Nanoparticles and Aganonerion polymorphum Leaf Extract. ACS Omega 2019, 4, 146–158. [Google Scholar]
- Lukovits, I.; Lalman, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, Y.; Wang, L.-J.; Wu, Y.-C.; Li, H.J. 9-Substituted acridines as effective corrosion inhibitors for mild steel: Electrochemical, surface morphology, and computational studies. New J. Chem. 2020, 44, 6464–6474. [Google Scholar] [CrossRef]
- Mary, Y.S.; Panicker, C.Y.; Sapnakumari, M.; Narayana, B.; Sarojini, B.K.; Saadi, A.A.A.; Van Alsenoy, C.; War, J.A. FT-IR, NBO, HOMO–LUMO, MEP analysis and molecular docking study of 1-[3-(4-Fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl]ethenone. Spectrochim. Acta Part A 2015, 136, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Babu, P.D.S.; Periandy, S.; Fereyduni, E. Spectrochim. Vibrational investigation, molecular orbital studies and molecular electrostatic potential map analysis on 3-chlorobenzoic acid using hybrid computational calculations. Acta Part A 2011, 84, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Boughoues, Y.; Benamira, M.; Messaadia, L.; Bouider, N.; Abdelaziz, S. Experimental and theoretical investigations of four amine derivatives as effective corrosion inhibitors for mild steel in HCl medium. RSC Adv. 2020, 10, 24145–24158. [Google Scholar] [CrossRef] [PubMed]
- Madkour, L.H.; Kaya, S.; Obot, I.B. Computational, Monte Carlo simulation and experimental studies of some arylazotriazoles (AATR) and their copper complexes in corrosion inhibition process. J. Mol. Liq. 2018, 260, 351–374. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.M.; El-Askalany, A.H.; Shahba, M.M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, İ.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
- Toghan, A.; Fawzy, A.; Alakhras, A.I.; Alqarni, N.; Zaki, M.E.A.; Sanad, M.M.S.; Farag, A.A. Experimental exploration, RSM modeling and DFT/MD simulations of the anticorrosion performance of naturally occurring amygdalin and raffinose for aluminum in NaOH solution. Coatings 2023, 13, 704. [Google Scholar] [CrossRef]
- Farag, A.A.; Abdallah, H.E.; Badr, E.A.; Mohamed, E.A.; Ali, A.I.; El-Etre, A.Y. The inhibition performance of morpholinium derivatives on corrosion behavior of carbon steel in the acidized formation water: Theoretical, experimental and biocidal evaluations. J. Mol. Liq. 2021, 341, 117348. [Google Scholar] [CrossRef]
- Farag, A.A.; Eid, A.M.; Shaban, M.M.; Mohamed, E.A.; Raju, G. Integrated modeling, surface, electrochemical, and biocidal investigations of novel benzothiazoles as corrosion inhibitors for shale formation well stimulation. J. Mol. Liq. 2021, 336, 116315. [Google Scholar] [CrossRef]
- Toghan, A.; Khairy, M.; Huang, M.; Gadow, H.S. Electrochemical, surface analysis, and theoretical investigation of 3-hydroxy-5-(phenylamino)-4-(p-tolyldiazenyl)thiophen-2-yl)(phenyl) methanone as a corrosion inhibitor for carbon steel in a molar hydrochloric acid solution. Int. J. Electrochem. Sci. 2023, 18, 100070. [Google Scholar] [CrossRef]
- Dagdag, O.; Safi, Z.; Erramli, H.; Cherkaoui, O.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; El Harfi, A. Adsorption and anticorrosive behavior of aromatic epoxy monomers on carbon steel corrosion in acidic solution: Computational studies and sustained experimental studies. RSC Adv. 2019, 9, 14782–14796. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).