1. Introduction
In contrast to traditional manufacturing techniques, Additive Manufacturing (AM) relies on the sequential addition and consolidation of powdered feedstock in small increments. This layer-by-layer approach is employed to fabricate intricate shapes and achieve high density in the final parts [
1,
2]. Laser Powder Bed Fusion (LPBF), also known as Selective Laser Melting (SLM), is an Additive Manufacturing (AM) technique that involves melting a thin layer of powder using a high-power laser beam. This process takes place under an inert gas atmosphere, while the laser beam scans across the powder bed according to a predetermined scanning pattern [
3].
Moreover, the LPBF process is distinguished by the swift melting and subsequent solidification of small material volumes, leading to significant thermal gradients. High localized heating and cooling rates control the resulting microstructure, grain size and orientation and may cause compositional segregation or the formation of non-equilibrium trapped phases. Cooling rates as high as 10
6 K/s, owing to small melt pool size and high laser scanning speeds, result in fine and anisotropic microstructures, typically dominated by cellular or dendritic solidification structures [
4,
5,
6,
7,
8].
Under these high cooling rates, DSS and SDSS fabricated using LPBF predominantly exhibit a ferritic structure (>95%) [
9,
10]; it is well known that ferritic stainless steels exhibit a significantly lower elongation at fracture. This limits the utilization of their full property potential, which is derived from the two-phase microstructure consisting of a balanced fraction of ferrite and austenite. Nitrogen (N) supersaturation in the ferrite matrix causes precipitation of chromium nitride [
11], which contributes to increased strength but decreased ductility in LPBF DSS compared to conventionally manufactured DSS. Ferrite is the dominant phase in SDSS in the as-built condition and to restore the balance between austenite and ferrite, and therefore regain desired ductility, high-temperature annealing at approximately 1100 °C followed by water quenching are common post-processing steps. The austenitic phase contributes to toughness and resistance against corrosion whilst ferrite improves strength but decreases crack susceptibility and toughness. Hence, the above unique properties offered by the balanced phase fraction, together with good weldability and higher strength than any comparable austenitic or ferritic stainless steels, make DSS and SDSS suitable for applications in the chemical and petrochemical industry, pulp and paper industry, power generation, marine constructions and offshore structural industries [
12]. Due to their lower thermal expansion coefficient and relatively higher thermal conductivity in comparison to austenitic stainless steels, DSSs are well suited for additive manufacturing in applications requiring complex and thin-walled geometries, such as heat exchangers [
13]. The volume fraction, morphology and chemistry of the two phases can be adjusted primarily by controlling the chemical composition and the path of the ferrite-to-austenite phase transformation [
14,
15]. Interestingly, although heat-treated duplex steels show a high elongation at fracture, the thermal effect decreases the dislocation density and thus lowers strength [
16].
Conventional wrought or cast alloys are not always suitable for metal AM processes due to the trade-offs between fundamental material properties and manufacturability under rapid cooling rates. As a result, the pursuit of new alloys for AM presents an opportunity to tap into the growing AM market, enabling part manufacturers to tailor the composition of the feedstock material for developing an optimal alloy for their specific applications. A key advantage of studying alloy development through powder blending and screening lies in the mitigation of potential uncertainties and costs associated with their production. These uncertainties, primarily linked to the atomization process, encompass factors such as the feasibility of atomizing a novel alloy, process yield, compatibility of powder morphology with LPBF process specifications and compositional consistency of the powder batch. These uncertainties often impose significant lead times and expenses on innovative projects, impeding their market viability. By adopting such an accelerated alloy screening methodology, it becomes possible to gain deeper understanding of the intricate relationship between process-driven phenomena and material structure. This, in turn, sheds light on how these interactions influence part performance while enabling the construction of a comprehensive testing matrix for novel materials, allowing for the identification of robust additive manufacturing (AM) parameters tailored to yield desirable material properties.
This efficient approach enables the development of new alloys suitable for LPBF by dry mixing of elemental powders and/or pre-alloyed powders, allowing us to create custom blends with tailored chemical compositions. This approach facilitates high-throughput alloy design and screening of new material compositions, featuring mechanical and functional property combinations not found in conventional, commercial alloys. These properties can be intentionally varied by modifying the concentration of one or more of the elements towards engineered microstructures of interest resulting from the LPBF process [
17].
Several previous studies have demonstrated the successful utilization of powder blends as input materials for LPBF for all alloy classes [
18]; to name a few, in [
19] the primary focus was on the processability of elemental powder blends of a Al-C-Co-Fe-Mn-Ni system and the influence of process parameters on the mechanical properties of lattice structures; results were found comparable to high-Manganese steel samples, achieving an increase in energy absorption equal to 75% in comparison with the commonly used 316L. They also highlighted the influence of higher energy density on the chemical homogeneity and elemental distribution of the studied high entropy alloys. According to another study [
20], an alternative alloying concept for Aluminum-based alloys was demonstrated to combat the challenges present in its AM processing such as its low laser absorption, high thermal conductivity and reduced powder flowability, responsible for its poor processability [
21]. Through the addition of nanodiamonds and graphene powder, the processing of AlSiMg alloy powder was successful at lower energy densities, since the energy absorption of the material was effectively increased. Nevertheless, it was concluded that the decrease in relative density was due to the addition of graphene, which might cause fusion defects. Most importantly, the formation of a graphene network in the material structure and the in situ formation of Al
4C
3 phases was identified and linked to the Orowan strengthening mechanism, leading to >40% increase in material hardness compared to the virgin AlSi10Mg alloy. However, the authors concluded that additional efforts are needed for the optimization of the blended fractions to secure a homogeneous distribution of the strengthening phases [
20]. In similar studies, Montero Sistiaga et al. and Aversa et al. [
22,
23] modified the composition of the 7075 alloy, increasing the Si content to change the solidification range and the fluidity of the molten phase, Martin et al. [
24] introduced hydrogen stabilized Zr nanoparticles to the powder batches in aluminum series 7075 and 6061, Zhang et al. [
25] and Nie et al. [
26] introduced Zr into Al-Cu-Mg powder samples for grain refinement through the Al
3Zr precipitates which act as seeds for the heterogeneous nucleation, while Wang et al. [
27] investigated the addition of Si and in Al-Cu-Mg alloy and its effect on its processability, consolidation and microstructure. Hence, numerous concepts can be found in the literature for the modification of aluminum alloys towards developing new compositions specifically designed for laser-based powder bed AM processes. However, to the best of our knowledge, limited publications exist for duplex and super duplex stainless-steel powder blends.
In the work of Köhler et al. [
28], various mixture ratios of austenitic stainless-steel powder (AISI 316L) with a duplex stainless-steel powder (AISI 318LN) were studied in order to attain a duplex microstructure in the as-built state with the use of different energy densities during LPBF. The authors reported that the addition of 30 wt% AISI 318LN to AISI 316L led to the formation of a duplex (roughly 50/50) microstructure in the as-built condition. The strength and corrosion resistance of this new alloy was superior to that of pure AISI 316L. In addition, it was shown that the formation of the duplex microstructure was extremely sensitive to different energy inputs. It was concluded that different cooling rates along with different cooling modes caused by the varying energy inputs, had the main impact on the microstructural phase formation. Cui et al. [
29] demonstrated the successful AM processing of different ratios of X2CrNiMo17-12-2 powder and the super duplex stainless-steel X2CrNiMoN25-7-4 powder for tuning the solidification and percentages of ferrite/austenite microstructural phases. Chemical homogeneity of the powder mixture is another factor determining phase formation during fabrication of the parts. For this reason, it is of interest to gain a deeper understanding of how different chemical compositions and different laser energy inputs influence the phase composition of LPBF processed DSS and SDSS alloys. In this context, it is important to understand how energy density-dependent chemical heterogeneities at the powder bed affect the phase formation when using powder mixtures [
29].
Although the mechanical properties of DSSs have been studied at the macroscale, the number of works involving low-scale mechanical characterization is quite low for such a material [
30,
31,
32]. In this regard, the reported results of mechanical properties of constitutive phases (ferrite or austenite) at the nanoscale are diverse. The austenitic phase may exhibit a higher, lower or equal hardness/elastic modulus when compared with the ferritic phase, depending on different variables such as selected manufacturing process, formulation/chemical compositions (mostly N content) and microstructural/crystallographic texture of the individual phases (e.g., orientation). For this reason, the aim in [
10,
11] was to achieve a two-phase microstructure of ferrite and austenite (in the PBF-LB state) to combine the high strength in the LPBF state resulting from the high cooling rates (high dislocation density and small grains) with the aforementioned advantages of a two-phase microstructure [
10,
11].
The focus of this study is to minimize or even eliminate the post-process annealing and achieve a dual phase microstructure of LPBF processed DSS and SDSS parts by blending pre-alloyed DSS/SDSS powders with austenitic stainless-steel 316L (SS316L) powder. The primary idea was to increase the nickel content, to induce in situ austenite formation at the end of solidification since Ni is an austenite stabilizer. SS316L powder was selected, due to its higher nickel content in comparison to DSS/SDSS [
33]. Moreover, its low concentration of Mo, Cr, C, N chemical elements, will not affect the final microstructure of the blends with deleterious intermetallic phases and carbide/nitride precipitates [
34,
35,
36]. Nanoindentation studies on custom powder blends reveal the mechanical behavior of constituent phases at the micro-scale as a key parameter for optimizing the microstructural design of these materials, while a comprehensive literature review on the subject is provided. In order to attain reliable values of the hardness and elastic modulus of each constitutive phase, a coupling of techniques is here employed for assessing small-scale mechanical properties, providing information about the content and distribution of constitutive phases by linking different discerned responses exhibited by distinct mechanical phases. The methodology can be replicated for a variety of pre-alloyed/elemental powders as a rapid alloy screening workflow via LPBF, enabled through powder blending. This approach facilitates the creation of a targeted Design of Experiments (DOE) matrix for the purpose of developing a comprehensive ‘powder toolkit’. This toolkit establishes a link between composition, properties and microstructure, thereby aiding in the selection of optimal alloys for parts processed through Additive Manufacturing (AM) prior to selected atomization experiments for producing pre-alloyed powders.
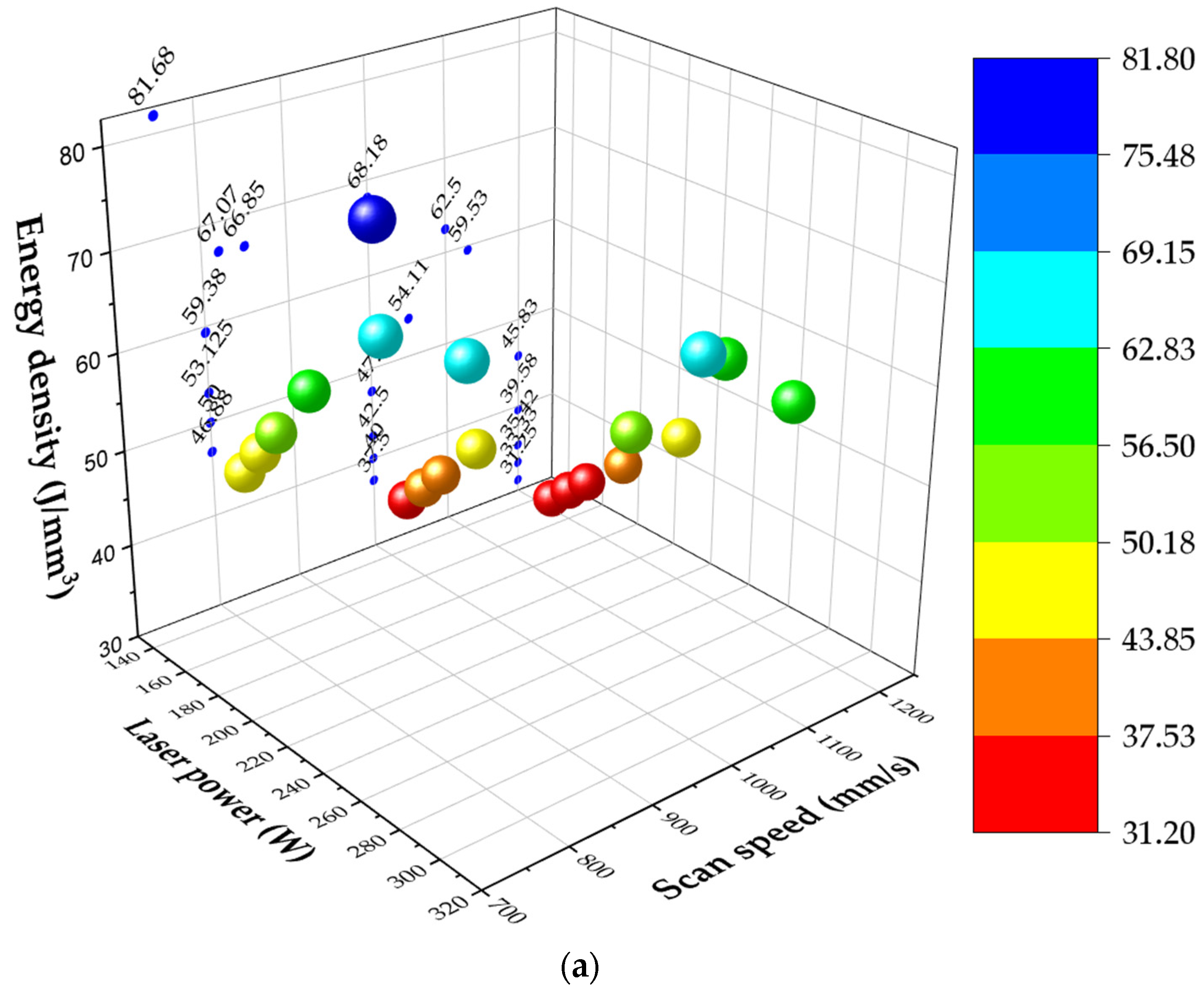
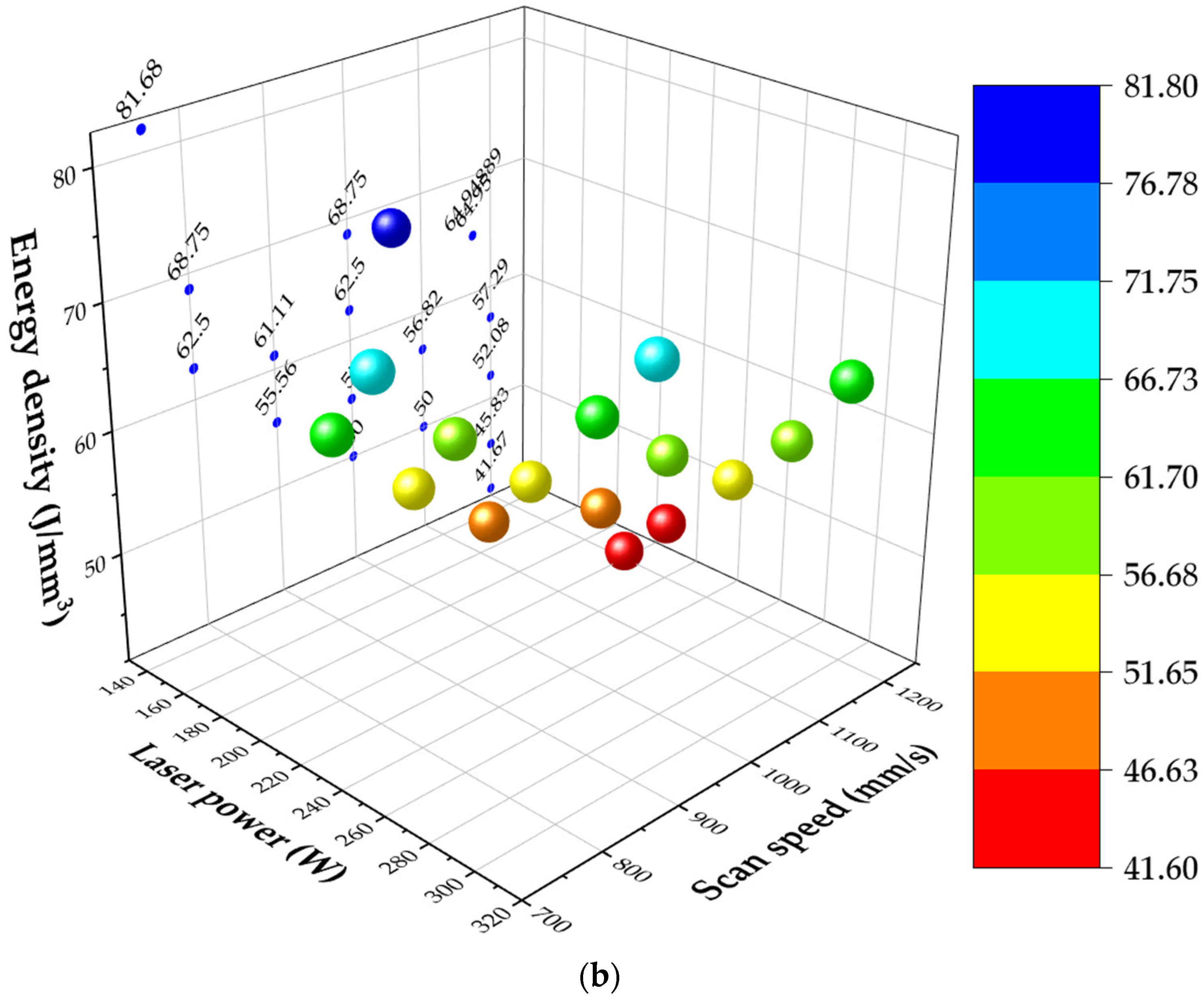
4. Discussion
The microstructure of both alloys revealed increased austenite content due to the addition of austenitic stainless-steel powder compared to LPBF as-built DSS and SDSS microstructures which are mentioned in the literature [
48,
49,
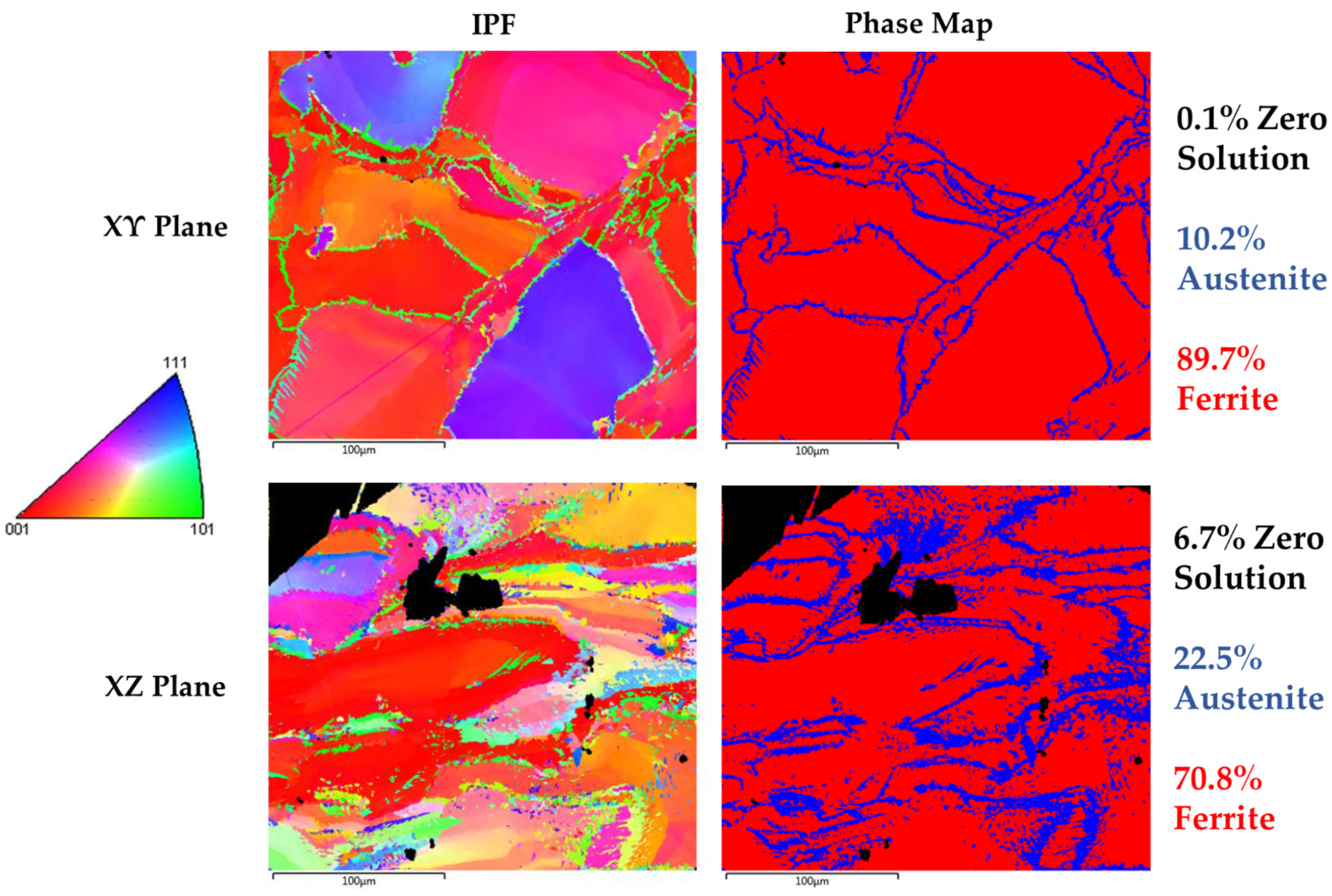
50]. Blend 1 presented higher austenite content in comparison to Blend 2. EBSD confirmed the austenite and ferrite phases that were observed with LOM and SEM. More specifically, EBSD phase maps (
Figure 7) showed an austenite content equal to 10.2% at the XY plane and 22.5% at the XZ plane for Blend 1 while, for Blend 2, the EBSD phase map (
Figure 9) showed an austenite content of 5% at the XY plane and 3.6% at the XZ plane. The phase characterization was validated by EDS, where the ferrite exhibited higher Mo content (ferrite stabilizer) in comparison to the austenite for both blends. The higher nickel content in Blend 1 compared to Blend 2 can justify the increased austenite volume fraction in the as-built microstructure, as shown in
Table 3 for the calculated theoretical chemical composition of blended powder mixtures. The higher content of SS316L in the blend resulted in the generation of higher nickel content and in turn in higher austenite content in the as-built microstructure of Blend 1 compared to Blend 2. The composition equivalents, nickel (Ni
eq) and chromium (Cr
eq), were calculated, where all alloying elements in steels are grouped into Ni-like and Cr-like elements, so-called austenite and ferrite stabilizers, and their contributions to Ni
eq and Cr
eq are weighted by certain empirical coefficients obtained from mass of experiments [
51]. Based on the nominal material compositions shown in
Table 3, the ferrite/austenite contents of the LPBF samples were estimated by the Schaeffler diagram, as shown in
Figure 13.
As previously reported [
52], the use of a Schaeffler diagram to estimate the ferrite/austenite fraction has revealed two drawbacks. First, the strongly austenite-stabilizing element N is not considered in the Ni equivalent. Second, the austenite-stabilizing effect of Mn on the high-temperature reaction δ-Fe → γ-Fe is overestimated. The Ni equivalent based on the Schaeffler diagram equation was slightly higher for Blend 2 than Blend 1. The lower Cr equivalent and the slightly higher Ni equivalent of Blend 1 in comparison to Blend 2 can be considered as an indication of the theoretical higher austenite content in Blend 1. On the contrary, the Schaeffler diagram does not consider the solidification rate (cooling rate) of the LPBF part. The high solidification rates alter the as-built microstructure by suppressing the solidification mode. In our case (Blend 1 and Blend 2), the solidification mode was a suppressed ferrite to austenite (FA) solid transformation. In DSS and SDSS as-built microstructures the solidification mode FA suppressed and changed to only ferrite (F) [
53]. The change in solidification mode modifies the volume fractions of austenite and ferrite. Therefore, as-built microstructures exhibited a 80–20 and 95–5 ferrite/austenite ratio while Schaeffler predictions exhibited a 25–75 and 60–40 ferrite/austenite ratio for Blend 1 and Blend 2, respectively. There are deviations between the Schaeffler predictions and EBSD phase quantification results for as-built microstructures, but both led to the result that the austenite content of Blend 1 was higher than the austenite content of Blend 2. Hence, the Schaeffler diagrams can be considered an approximate phase prediction study for powder blending of stainless-steel alloys.
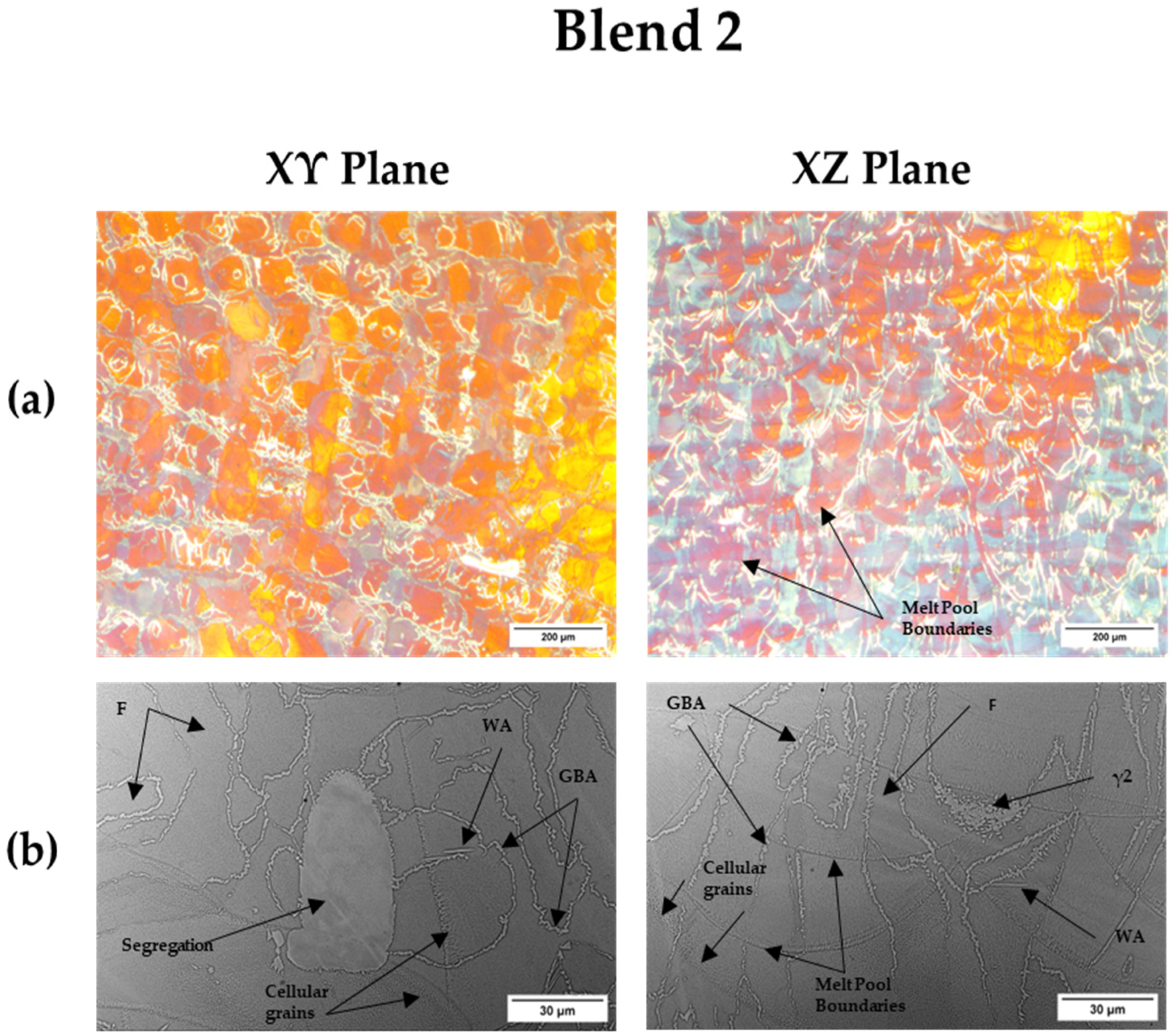
Blend 2 revealed SS316L micro-segregations in the microstructure. However, there were no traces of SS316L or DSS segregations in Blend 1. The segregations in the Blend 2 microstructure may be attributed to the fact that the amount of SDSS is higher than SS316L in the mixture and it is difficult to maintain an equal distribution between the two powders with the re-coater of the LPBF machine spreading the powder layer. In order to have an equal arrangement of particles between the two powders, it is favored to keep an equal amount, such as in the case of Blend 1 [
29]. Local inhomogeneities or micro-segregations influence the microhardness uniformity of the as-built blended material [
54]. In addition, Blend 2 cellular grains were observed near the melt pool and laser melt track boundaries. These fine cellular grain structures are typical microstructures of the LPBF process, due to constitutional supercooling together with a high solidification rate [
55,
56]. As previously reported, the fine cellular grains of LPBF as-built SS316L increase the yield strength [
57]. Other reports mentioned that the boundaries of the SS316L LPBF as-built cellular grains were enriched with high density dislocations and presented chromium and molybdenum segregations [
57,
58].
No intermetallic phases were observed in both as-built microstructures (Blend 1 and Blend 2) because the precipitates are too fine to observe under SEM; however, chromium nitride precipitation in the DSS as-built microstructure has been reported and can be observed and identified via a Transmission Electron Microscope (TEM) [
59]. Nitrogen content in the SDSS is twice as much compared to DSS and, in turn, the Blend 2 nitrogen content is twice as much compared to Blend 1. Increasing the nitrogen content in the DSS results in an increased fraction of chromium nitrides after non-equilibrium cooling rates [
60]. The precipitation of chromium nitrides increases the yield and tensile strength and reduces the ductility [
61]. Nitrogen is much more soluble in austenite than in ferrite [
62,
63]. Therefore, it is expected that chromium nitrides are present in the microstructure of both blends, and especially in Blend 2. Blend 2 contained the highest nitrogen content and showed increased ferrite content when compared to Blend 1, where a significant amount of N is assumed to be dissolved in austenite.
Both blends showed the same austenite morphologies (GBA, WA and γ2). Blend 2 exhibited a thinner GBA and reduced nucleation sites of γ2 and WA in comparison to Blend 1. The reduced nucleation sites of WA in Blend 2 are attributed to lower nickel content [
64]. Moreover, the thinner GBA of Blend 2 compared to Blend 1 could be associated with the lower amount of SS316L powder particles in the blend. The SS316L alloy has lower thermal conductivity than DSS/SDSS [
65,
66]. The overall thermal conductivity of Blend 2 will be higher compared to Blend 1. The lower thermal conductivity reduces the cooling rate of the microstructure and increases the transformation time of ferrite to austenite [
67]. Fewer powder particles of SS316L that exhibit higher thermal conductivity and lower nickel content will result in reduced volume fractions of GBA, γ2 and WA in the Blend 2 microstructure. Secondary γ2 austenite was formed at lower temperatures while WA and GBA were formed at higher temperatures [
68].
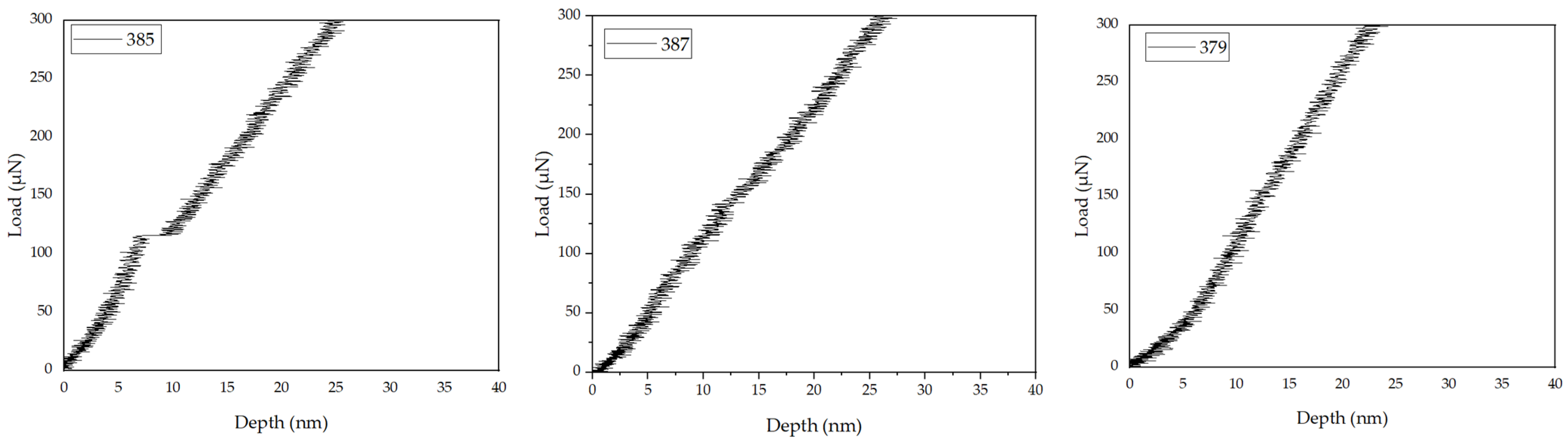
Austenite is reported to exhibit a higher H/E ratio than ferrite [
30]; one has also to consider that indentations formed also on the phase boundaries, and whose plastic zones included both phases, yielding a composite-like (containing α/γ interphase boundaries) response. Ferrite is reported to exhibit a higher E (20 GPa) due to its BCC crystal structure, which has a lower interatomic distance (smaller lattice parameter) than the FCC crystal structure of austenite; the crystallographic texture induced by the deformation itself could also be recalled as a possible reason for explaining the above literature findings. Geometrically Necessary Dislocations (GND) are developed in the sample at the indenter probe/sample interface in order to accommodate local strain conditions, resulting in significant local hardening, while at shallow indentation displacements, the proportion of work-hardened material to the total effective volume of material being probed can be considerable [
69]. The presence of grain boundaries starts to make a measurable contribution to the response as compared with its giant grain counterpart. It is important to note that, at these indentation depths (depending on the grain size), for laser-processed materials (especially SLM), the response is always a combination of grain size effects coupled with cell size effects, in addition to any interactions that may be present between the two. It is seen that, for h
c below ~150 nm, the hardness responses are statistically indistinguishable, attributed possibly to the indentation size effect whereby GNDs generated to accommodate the contact strain at or near the probe interface give rise to significant work hardening, thus dominating the overall hardness response. At indentation contact depths greater than 150 nm, a clear divergence is observed beginning at about 500 nm, with the steady-state hardness value for the nominal grain base plate exceeding that of the giant grain by approximately 30%; these size effects are due to the indenter, characteristic sub-structural length and grain size. Both the elastic recovery energy and the plastic energy dissipation of the indenter onto the austenite phase are reported to be larger than those onto ferrite phase, whereas pile-up phenomena exist in both phases. The physical explanation for this is that, when the materials were work hardened during the indentation tests, the dislocation mobility was reduced, and the dislocations were confined to the surface, causing pile up. During the quasi-static single and cyclic indentation tests, the major deformation mechanisms are the nucleation, multiplication and propagation of dislocations in both the austenite phase and ferrite phase as well as dislocation activation; especially for the case of ferrite, additional propagation of slip bands occurs. After single indentation tests, heterogeneous dislocations are concentrated under the indenter; discrete dislocations in the ferrite phase are reported to bundle and form the slip bands with a relatively higher dislocation density [
47]. In
Figure 14, plasticity and the H/E ratio distribution of the grid mapping for Blend 1 and Blend 2 in the XY and XZ planes respectively, are presented. Higher stresses are expected at high hardness to modulus ratio (also referred to as the plasticity index), in brittle or hard materials (that exhibit sink-in), and high stress concentrations develop toward the indenter probe, whereas in the case of low H/E, i.e., ductile, or soft materials (pile-up), the stresses are lower and are distributed more evenly across the cross-section of the material. When the plasticity index H/E is much less than unity, the deformation is likely to be entirely elastic, when it is significantly greater than one, the deformation is predominantly plastic; in
Figure 14b the linear trend is confirmed [
70].