Fabrication of High-Entropy Alloys Using a Combination of Detonation Spraying and Spark Plasma Sintering: A Case Study Using the Al-Fe-Co-Ni-Cu System
Abstract
:1. Introduction
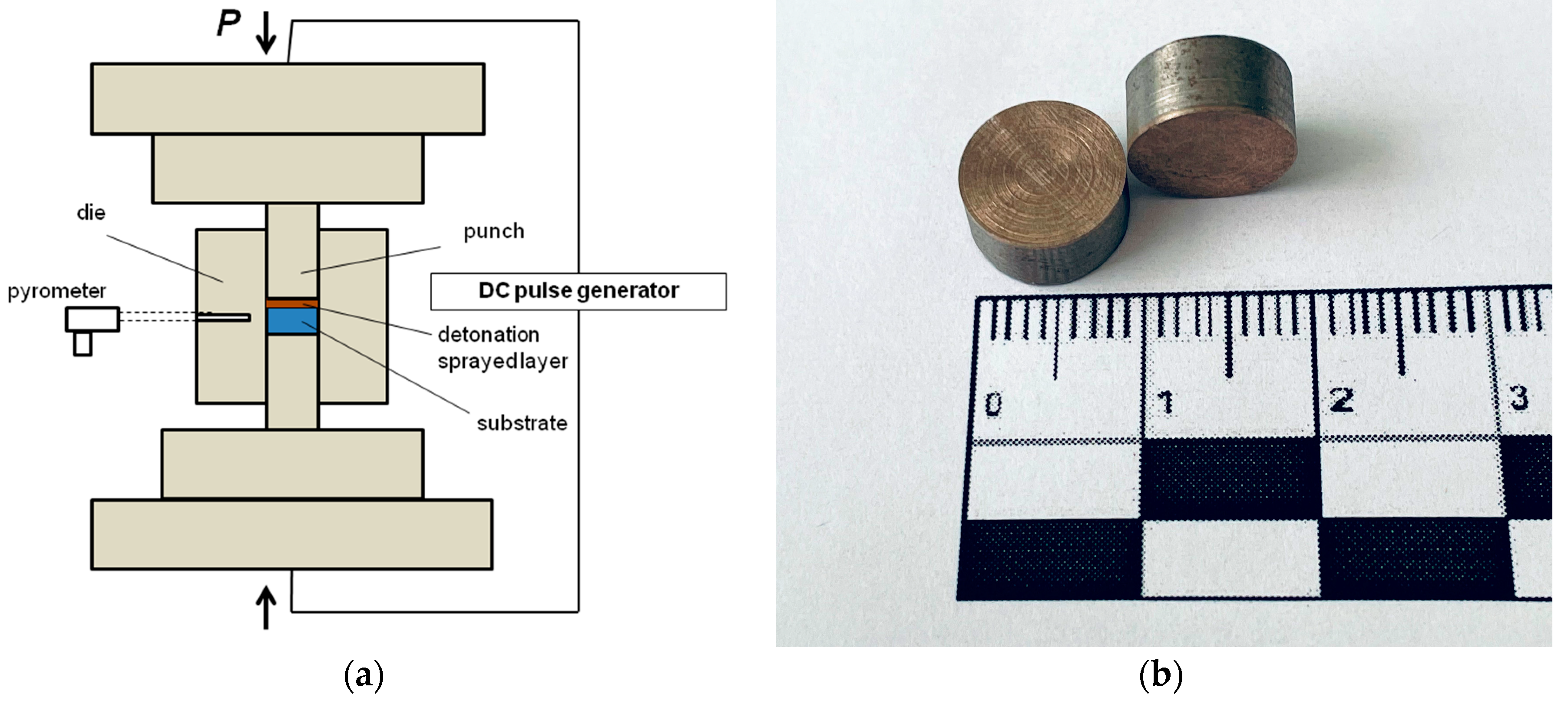
2. Materials and Methods
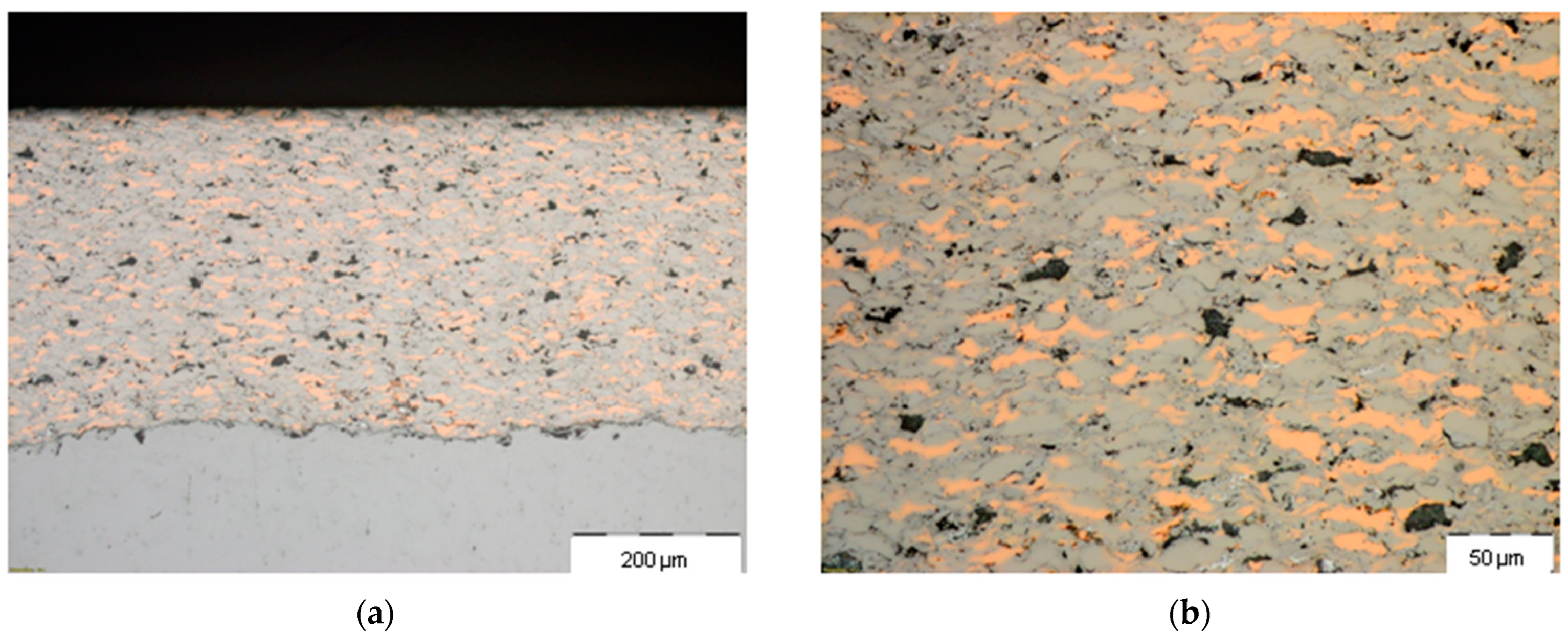
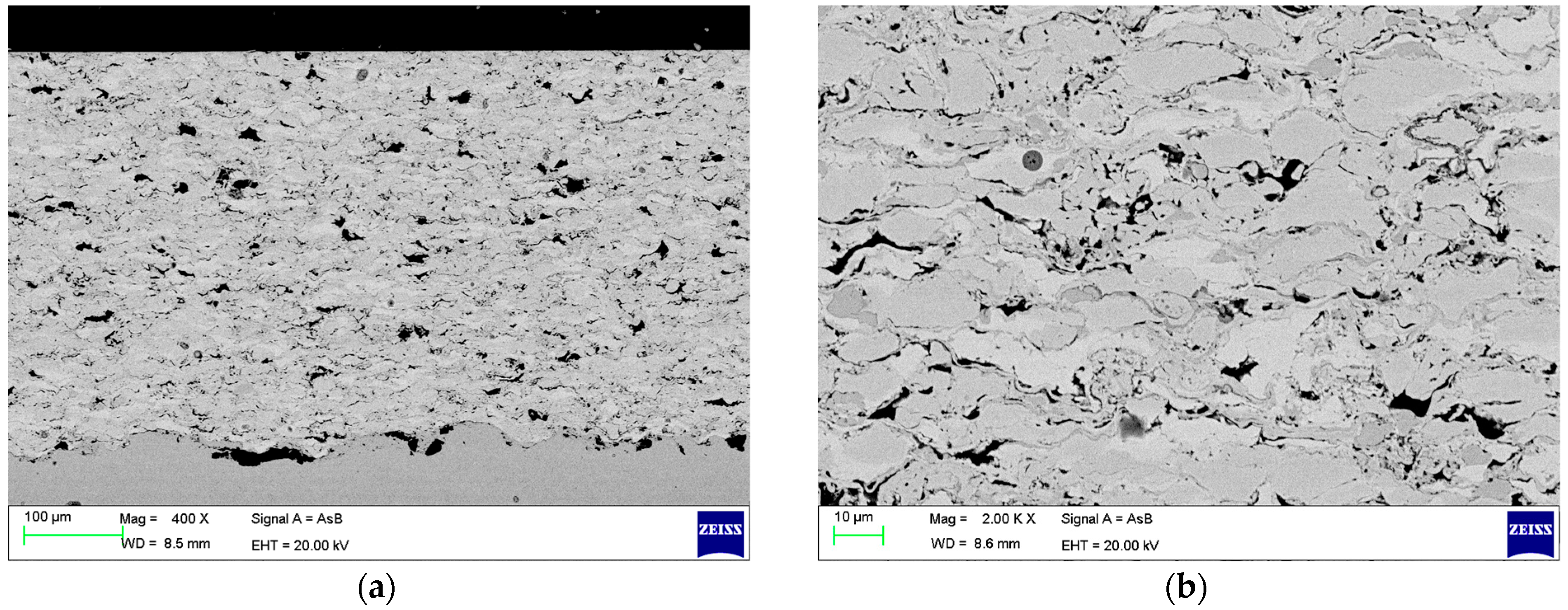
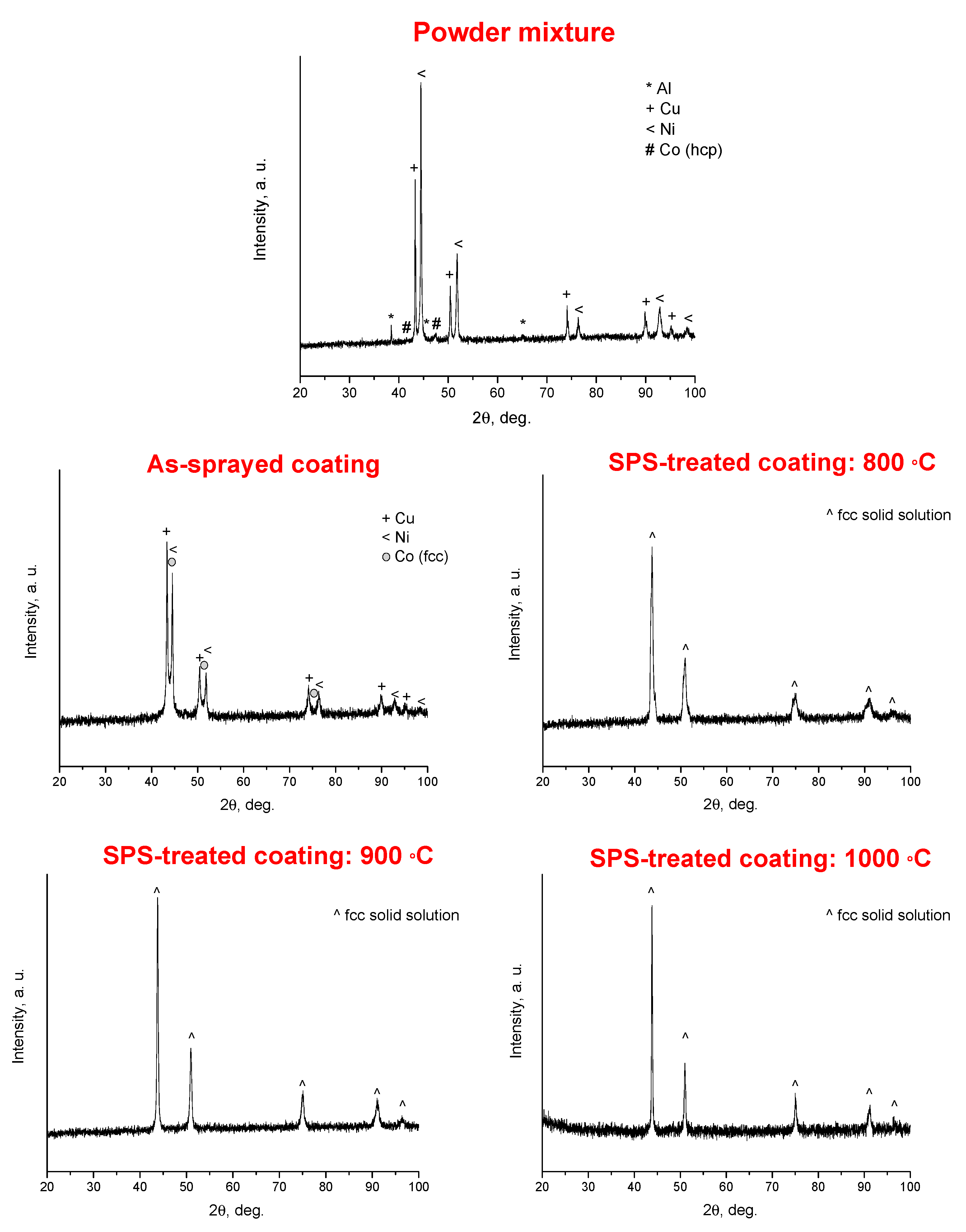
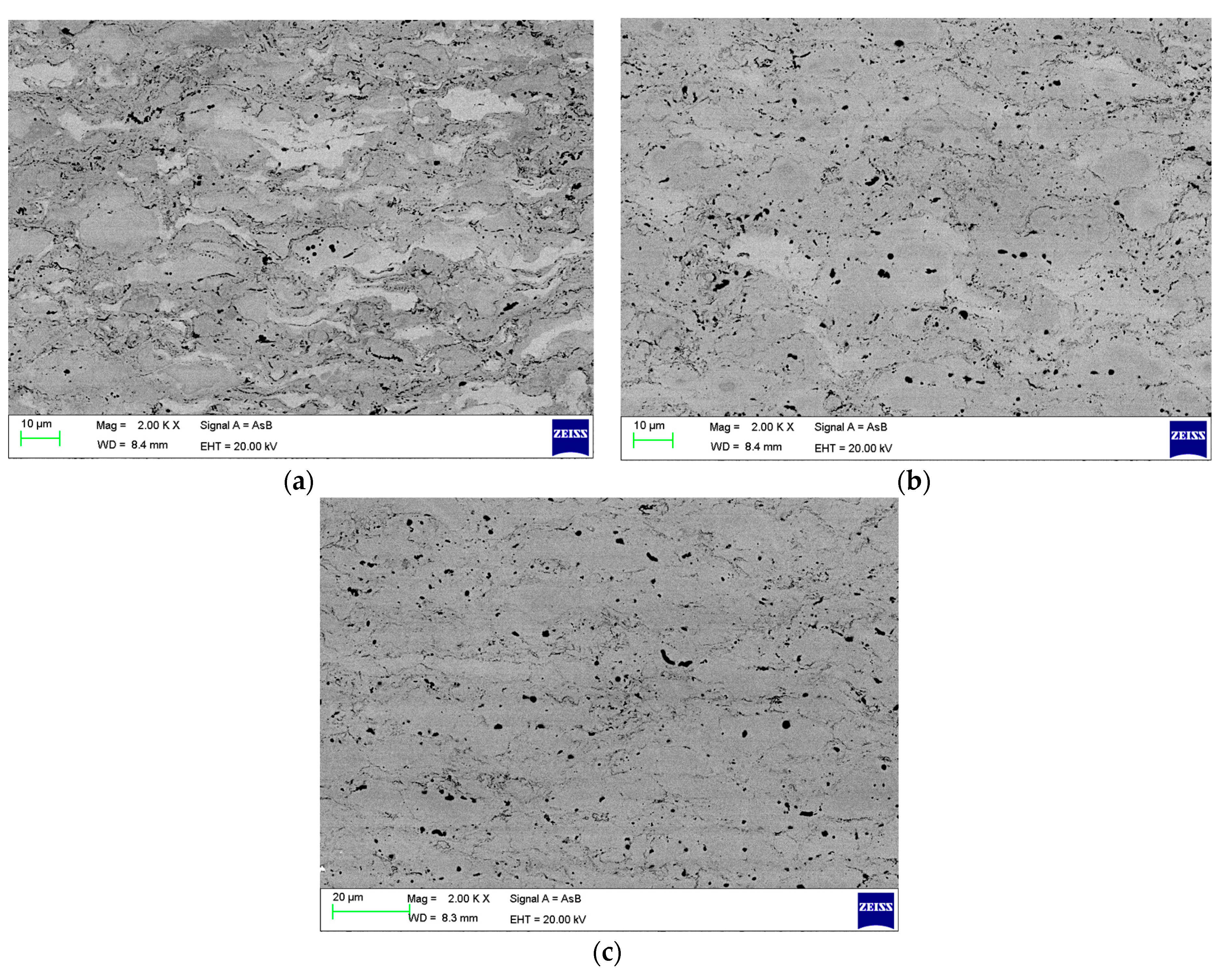
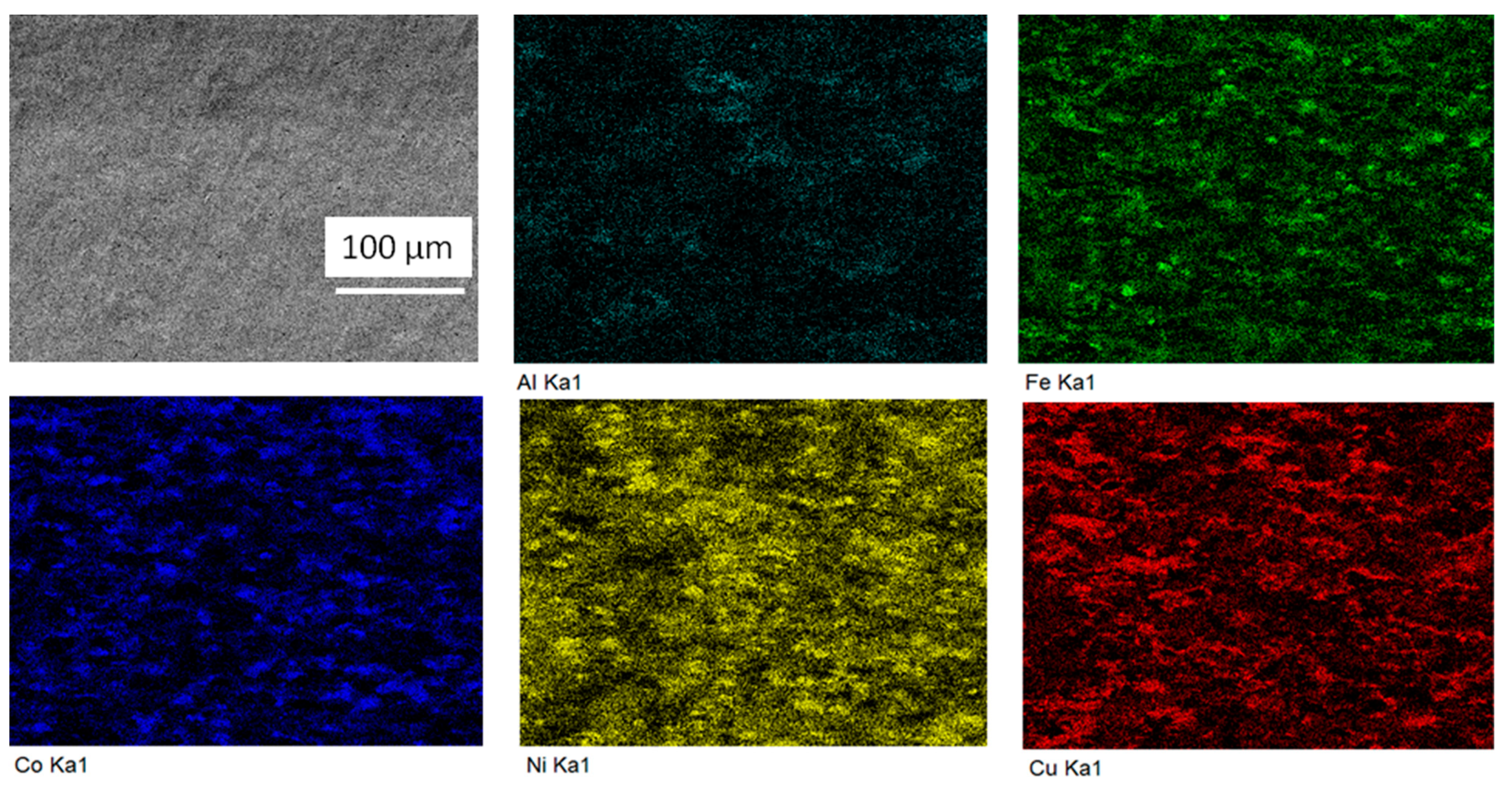
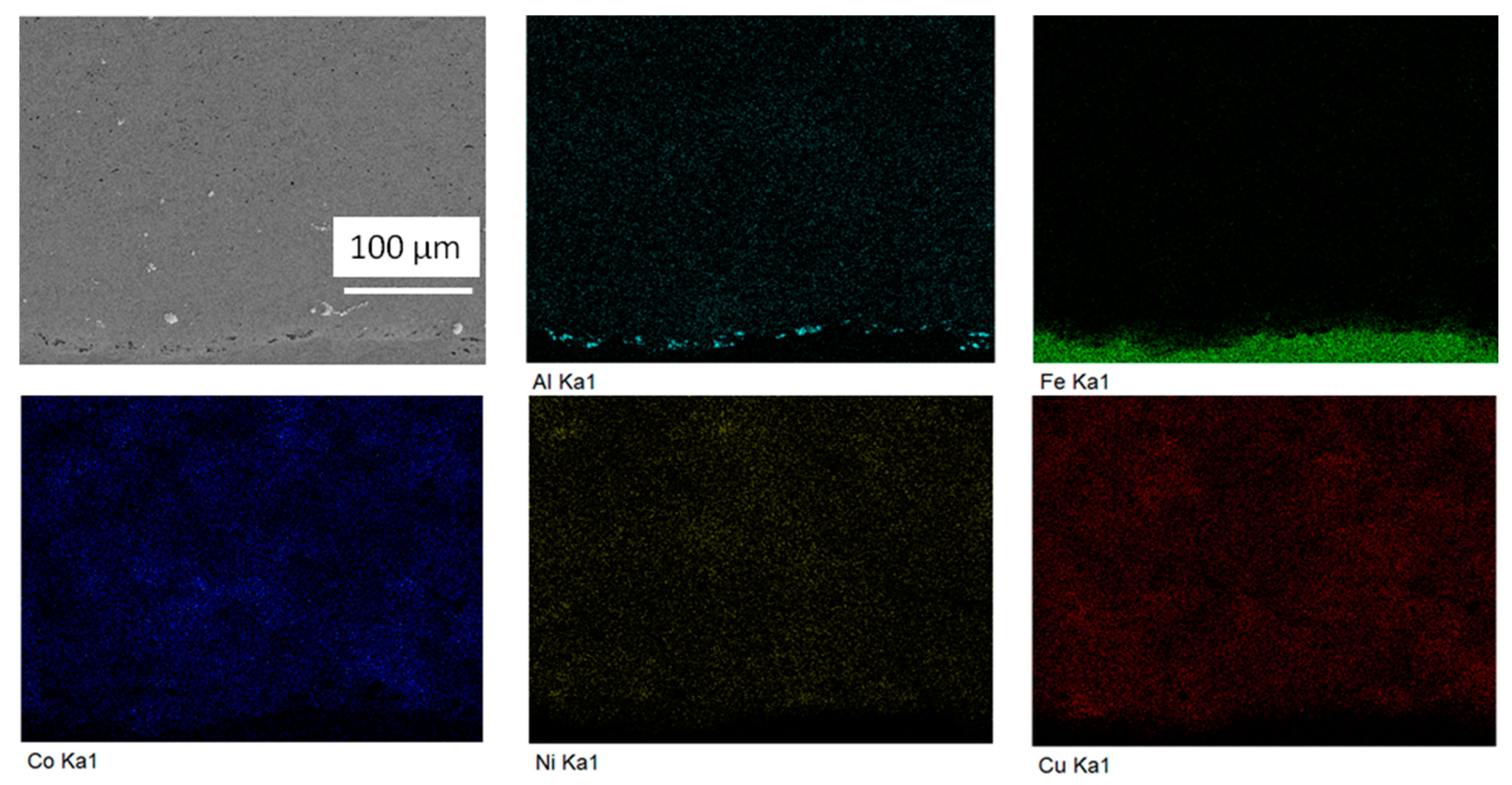
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Tokita, M. Progress of spark plasma sintering (SPS) method, systems, ceramics applications and industrialization. Ceramics 2021, 4, 160–198. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Rogachev, A.S.; Moskovskikh, D.O.; Yermekova, Z.S. Reactive spark plasma sintering of exothermic systems: A critical review. Ceram. Int. 2022, 48, 2988–2998. [Google Scholar] [CrossRef]
- Mulukutla, M.; Singh, A.; Harimkar, S. Spark Plasma Sintering for multi-scale surface engineering of materials. JOM 2010, 62, 65–71. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V. Joining processes for dissimilar and advanced materials. In Electric Current-Assisted Joining of Similar/Dissimilar Materials; Chapter 7 (Woodhead Publishing Reviews: Mechanical Engineering Series); Elsevier: Amsterdam, The Netherlands, 2022; pp. 151–176. [Google Scholar]
- Dudina, D.V.; Georgarakis, K.; Olevsky, E.A. Progress in aluminium and magnesium matrix composites obtained by spark plasma, microwave and induction sintering. Int. Mater. Rev. 2023, 68, 225–246. [Google Scholar] [CrossRef]
- Monchoux, J.P.; Couret, A.; Durand, L.; Voisin, T.; Trzaska, Z.; Thomas, M. Elaboration of metallic materials by SPS: Processing, microstructures, properties, and shaping. Metals 2021, 11, 322. [Google Scholar] [CrossRef]
- Abedi, M.; Sovizi, S.; Azarniya, A.; Giuntini, D.; Seraji, M.E.; Hosseini, H.R.M.; Amutha, C.; Ramakrishna, S.; Mukasyan, A. An analytical review on Spark Plasma Sintering of metals and alloys: From processing window, phase transformation, and property perspective. Crit. Rev. Solid State Mater. Sci. 2023, 48, 169–214. [Google Scholar] [CrossRef]
- Abedi, M.; Asadi, A.; Sovizi, S.; Moskovskikh, D.; Vorotilo, S.; Mukasyan, A. Influence of pulsed direct current on the growth rate of intermetallic phases in the Ni–Al system during reactive spark plasma sintering. Scr. Mater. 2022, 216, 114759. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Yadav, S.; Biswas, K.; Kumar, A. Spark plasma sintering of high entropy alloys. In Spark Plasma Sintering of Materials; Cavaliere, P., Ed.; Springer: Cham, Switzerland, 2019; pp. 539–571. [Google Scholar]
- Rajendrachari, S. An overview of high-entropy alloys prepared by mechanical alloying followed by the characterization of their microstructure and various properties. Alloys 2022, 1, 116–132. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Kochetov, N.A.; Panteleeva, A.V.; Kuskov, K.V.; Kovalev, D.Y.; Shchukin, A.S.; Vadchenko, S.G.; Scheck, Y.B. High-energy ball milling and spark plasma sintering of the CoCrFeNiAl high-entropy alloy. Metals 2020, 10, 1489. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Meng, J.; Su, B.; Li, P. Rapid preparation of AlCoCrFeNi high entropy alloy by spark plasma sintering from elemental powder mixture. Mater. Lett. 2016, 181, 82–85. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Powder metallurgy processing of a WxTaTiVCr high-entropy alloy and its derivative alloys for fusion material applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef] [PubMed]
- Anupam, A.; Kottada, R.S.; Kashyap, S.; Meghwal, A.; Murty, B.S.; Berndt, C.C.; Ang, A.S.M. Understanding the microstructural evolution of high entropy alloy coatings manufactured by atmospheric plasma spray processing. Appl. Surf. Sci. 2020, 505, 144117. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal spray high-entropy alloy coatings: A review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Supekar, R.; Nair, R.B.; McDonald, A.; Stoyanov, P. Sliding wear behavior of high entropy alloy coatings deposited through cold spraying and flame spraying: A comparative assessment. Wear 2023, 516–517, 204596. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, J.; Wang, H.; Luo, C. Sliding wear of spark plasma sintered CrFeCoNiCu high entropy alloy coatings with MoS2 and WC additions. Int. J. Adv. Manuf. Technol. 2018, 96, 1685–1691. [Google Scholar] [CrossRef]
- Prawara, B.; Yara, H.; Miyagi, Y.; Fukushima, T. Spark plasma sintering as a post-spray treatment for thermally-sprayed coatings. Surf. Coat. Technol. 2003, 162, 234–241. [Google Scholar] [CrossRef]
- Ito, K.; Ogawa, K. Effects of spark-plasma sintering treatment on cold-sprayed copper coatings. J. Therm. Spray Technol. 2014, 23, 104–113. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Bokhonov, B.B.; Ukhina, A.V.; Esikov, M.A.; Shikalov, V.S.; Kosarev, V.F. Spark plasma sintering treatment of cold sprayed materials for synthesis and structural modification: A case study using TiC-Cu composites. Mater. Lett. X 2022, 14, 100140. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Dudina, D.V.; Shtertser, A.A.; Smurov, I. Computer-controlled detonation spraying: Flexible control of the coating chemistry and microstructure. Metals 2019, 9, 1244. [Google Scholar] [CrossRef]
- Liao, W.B.; Wu, Z.X.; Lu, W.; He, M.; Wang, T.; Guo, Z.; Huang, J. Microstructures and mechanical properties of CoCrFeNiMn high-entropy alloy coatings by detonation spraying. Intermetallics 2021, 132, 107138. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Rybin, D.K.; Sova, A.; Moghaddam, A.O.; Samodurova, M.; Doubenskaia, M.; Trofimov, E. Formation of metal composites by detonation spray of powder mixtures. Int. J. Adv. Manuf. Technol. 2021, 117, 81–95. [Google Scholar] [CrossRef]
- Batraev, I.S.; Ulianitsky, V.Y.; Sova, A.A.; Samodurova, M.N.; Trofimov, E.A.; Pashkeev, K.Y.; Malikov, A.G.; Dudina, D.V.; Ukhina, A.V. A Feasibility study of high-entropy alloy coating deposition by detonation spraying combined with laser melting. Materials 2022, 15, 4532. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nishad, S.K.; Robi, P.S. A new high-entropy alloy of Al–Fe–Co–Ni–Cu possessing single face-centered cubic crystal structure and excellent mechanical properties at room temperature. Phys. Status Solidi A 2021, 218, 2000825. [Google Scholar] [CrossRef]
- Edalati, P.; Mohammadi, A.; Ketabchi, M.; Edalati, K. Microstructure and microhardness of dual-phase high-entropy alloy by high-pressure torsion: Twins and stacking faults in FCC and dislocations in BCC. J. Alloys Compd. 2022, 894, 162413. [Google Scholar] [CrossRef]
- Beyramali Kivy, M.; Asle Zaeem, M.; Lekakh, S. Investigating phase formations in cast AlFeCoNiCu high entropy alloys by combination of computational modeling and experiments. Mater. Des. 2017, 127, 224–232. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Microstructure and corrosion performance of AlFeCoNiCu high entropy alloy coatings by addition of graphene oxide. Materialia 2019, 8, 100459. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, H.; Zhou, R.; Zhang, N.; Yin, Y.; Liang, L.; Liu, Y.; Li, J.; Shan, Q.; Li, Q.; et al. Microstructures and tribological properties of TiC reinforced FeCoNiCuAl high-entropy alloy at normal and elevated temperature. Metals 2020, 10, 387. [Google Scholar] [CrossRef]
- Batraev, I.S.; Prokhorov, E.S.; Ul’yanitskii, V.Y. Acceleration and heating of powder particle by gas detonation products in channels with a conical passage. Combust. Explos. Shock Waves 2014, 50, 315–322. [Google Scholar] [CrossRef]
- Ye, X.; Ma, M.; Liu, W.; Li, L.; Zhong, M.; Liu, Y.; Wu, Q. Synthesis and characterization of high-entropy alloy AlXFeCoNiCuCr by laser cladding. Adv. Mater. Sci. Eng. 2011, 2011, 485942. [Google Scholar] [CrossRef]
- Uporov, S.; Bykov, V.; Pryanichnikov, S.; Shubin, A.; Uporova, N. Effect of synthesis route on structure and properties of AlCoCrFeNi high-entropy alloy. Intermetallics 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Chen, Z.; Wen, H.; Lavernia, E.J. Influence of Ti addition and sintering method on microstructure and mechanical behavior of a medium-entropy Al0.6CoNiFe alloy. Mater. Sci. Eng. A 2014, 619, 137–145. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Z.; Wang, W.; Chen, S.; Zhou, J. Understanding the spark plasma sintering from the view of materials joining. Scr. Mater. 2016, 123, 118–121. [Google Scholar] [CrossRef]
- Fujii, T.; Tohgo, K.; Goto, K.; Shimamura, Y. Interfacial properties of bonded dissimilar materials fabricated via Spark Plasma Sintering. Mater. Trans. 2021, 62, 1102–1108. [Google Scholar] [CrossRef]
- Li, R.; Yuan, T.; Liu, X.; Zhou, K. Enhanced atomic diffusion of Fe–Al diffusion couple during spark plasma sintering. Scr. Mater. 2016, 110, 105–108. [Google Scholar] [CrossRef]
- Rudinsky, S.; Gauvin, R.; Brochu, M. The effects of applied current on one-dimensional interdiffusion between copper and nickel in spark plasma sintering. J. Appl. Phys. 2014, 116, 154901. [Google Scholar] [CrossRef]


| Coating | Porosity, % | Vickers Hardness, HV0.3 * | Vickers Hardness, HV0.3 ** |
|---|---|---|---|
| As-sprayed | 3 | 320 ± 15 | 315 ± 15 |
| SPS 800 °C | <1 | 305 ± 25 | 320 ± 20 |
| SPS 900 °C | <1 | 330 ± 30 | 330 ± 20 |
| SPS 1000 °C | <1 | 350 ± 20 | 340 ± 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batraev, I.S.; Ulianitsky, V.Y.; Shtertser, A.A.; Dudina, D.V.; Ivanyuk, K.V.; Kvashnin, V.I.; Lukyanov, Y.L.; Samodurova, M.N.; Trofimov, E.A. Fabrication of High-Entropy Alloys Using a Combination of Detonation Spraying and Spark Plasma Sintering: A Case Study Using the Al-Fe-Co-Ni-Cu System. Metals 2023, 13, 1519. https://doi.org/10.3390/met13091519
Batraev IS, Ulianitsky VY, Shtertser AA, Dudina DV, Ivanyuk KV, Kvashnin VI, Lukyanov YL, Samodurova MN, Trofimov EA. Fabrication of High-Entropy Alloys Using a Combination of Detonation Spraying and Spark Plasma Sintering: A Case Study Using the Al-Fe-Co-Ni-Cu System. Metals. 2023; 13(9):1519. https://doi.org/10.3390/met13091519
Chicago/Turabian StyleBatraev, Igor S., Vladimir Yu. Ulianitsky, Alexandr A. Shtertser, Dina V. Dudina, Konstantin V. Ivanyuk, Vyacheslav I. Kvashnin, Yaroslav L. Lukyanov, Marina N. Samodurova, and Evgeny A. Trofimov. 2023. "Fabrication of High-Entropy Alloys Using a Combination of Detonation Spraying and Spark Plasma Sintering: A Case Study Using the Al-Fe-Co-Ni-Cu System" Metals 13, no. 9: 1519. https://doi.org/10.3390/met13091519
APA StyleBatraev, I. S., Ulianitsky, V. Y., Shtertser, A. A., Dudina, D. V., Ivanyuk, K. V., Kvashnin, V. I., Lukyanov, Y. L., Samodurova, M. N., & Trofimov, E. A. (2023). Fabrication of High-Entropy Alloys Using a Combination of Detonation Spraying and Spark Plasma Sintering: A Case Study Using the Al-Fe-Co-Ni-Cu System. Metals, 13(9), 1519. https://doi.org/10.3390/met13091519









