Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodized Process
2.3. Microstructural Characterization
2.4. Electrochemical Measurements
3. Results
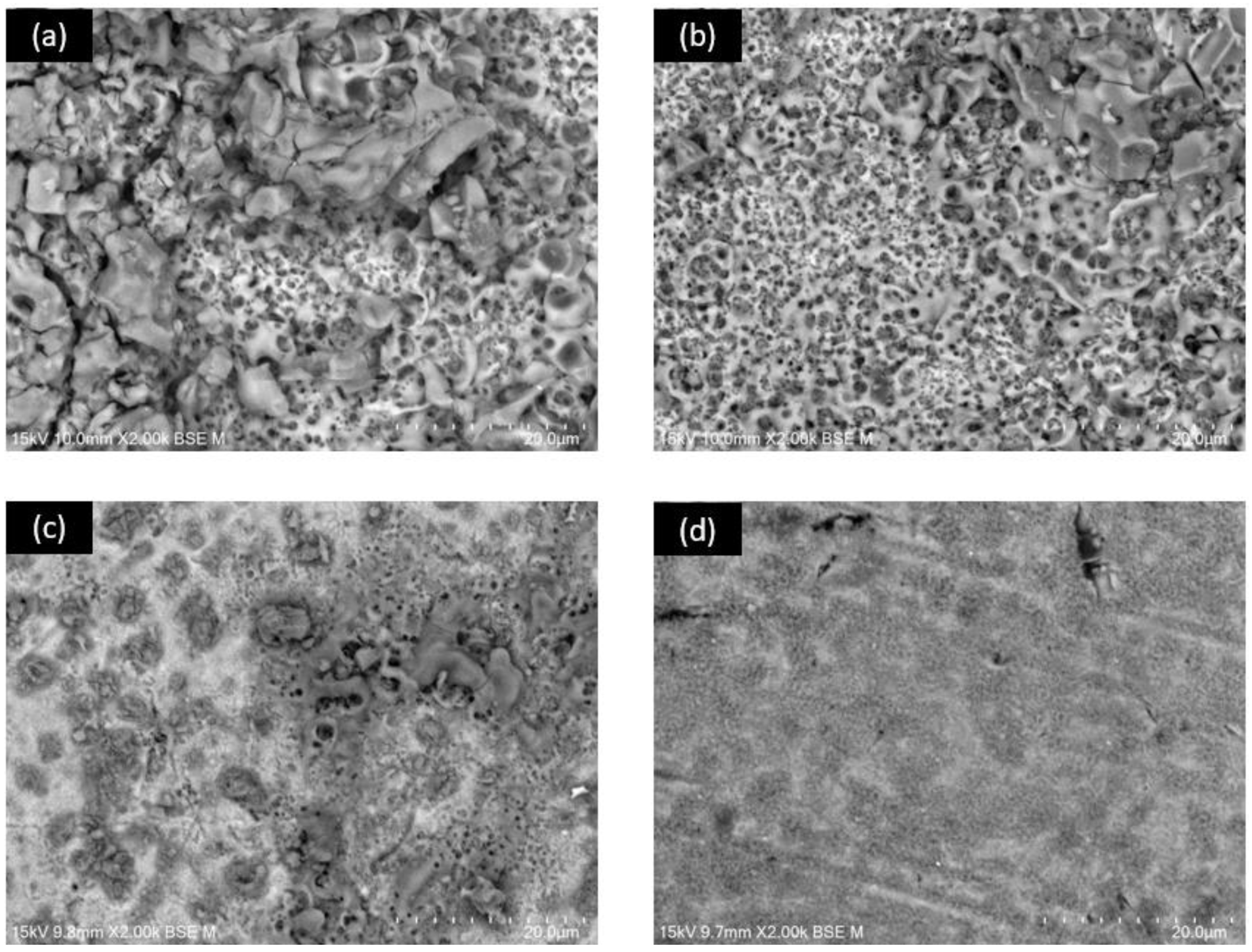
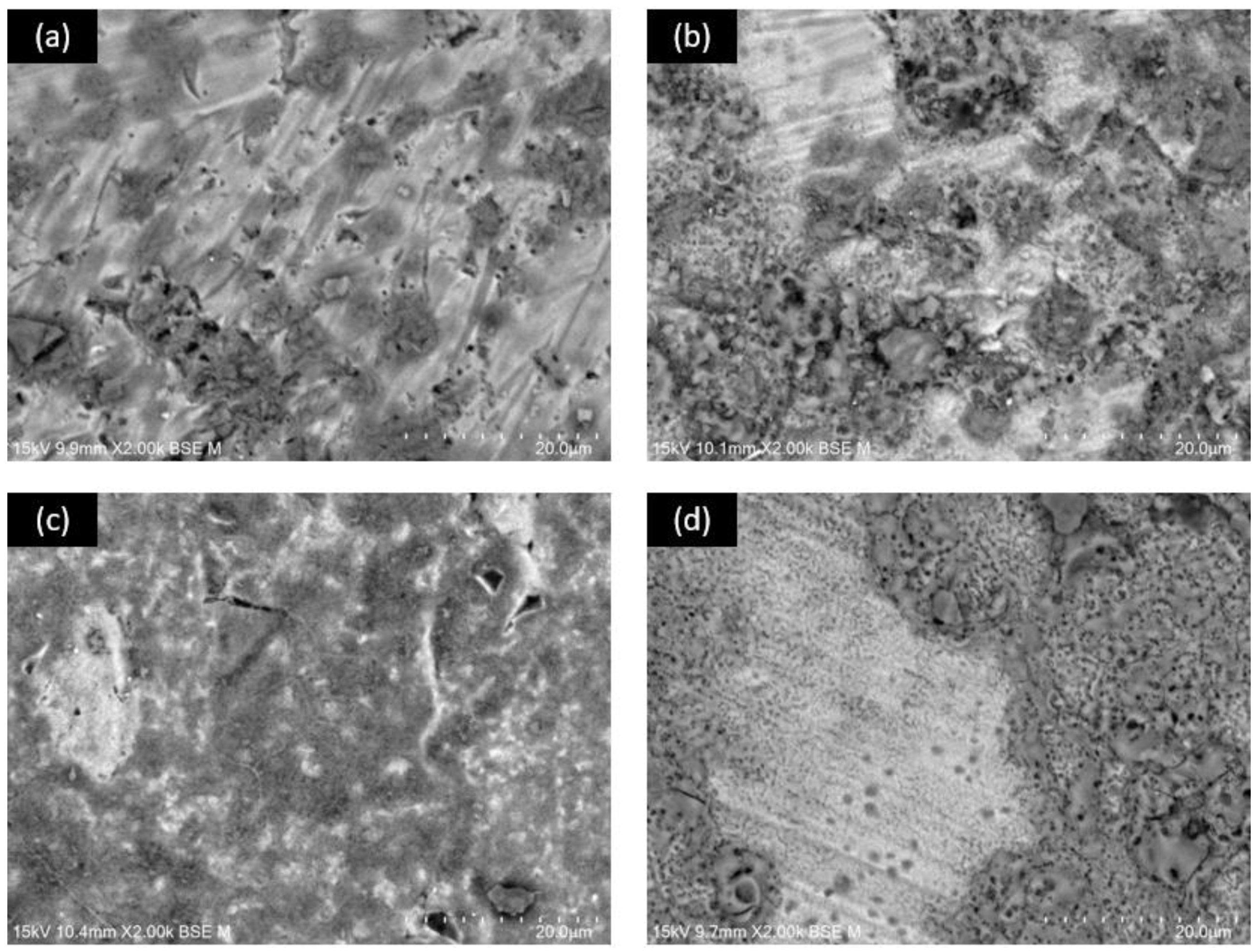
3.1. SEM Superficial Analysis
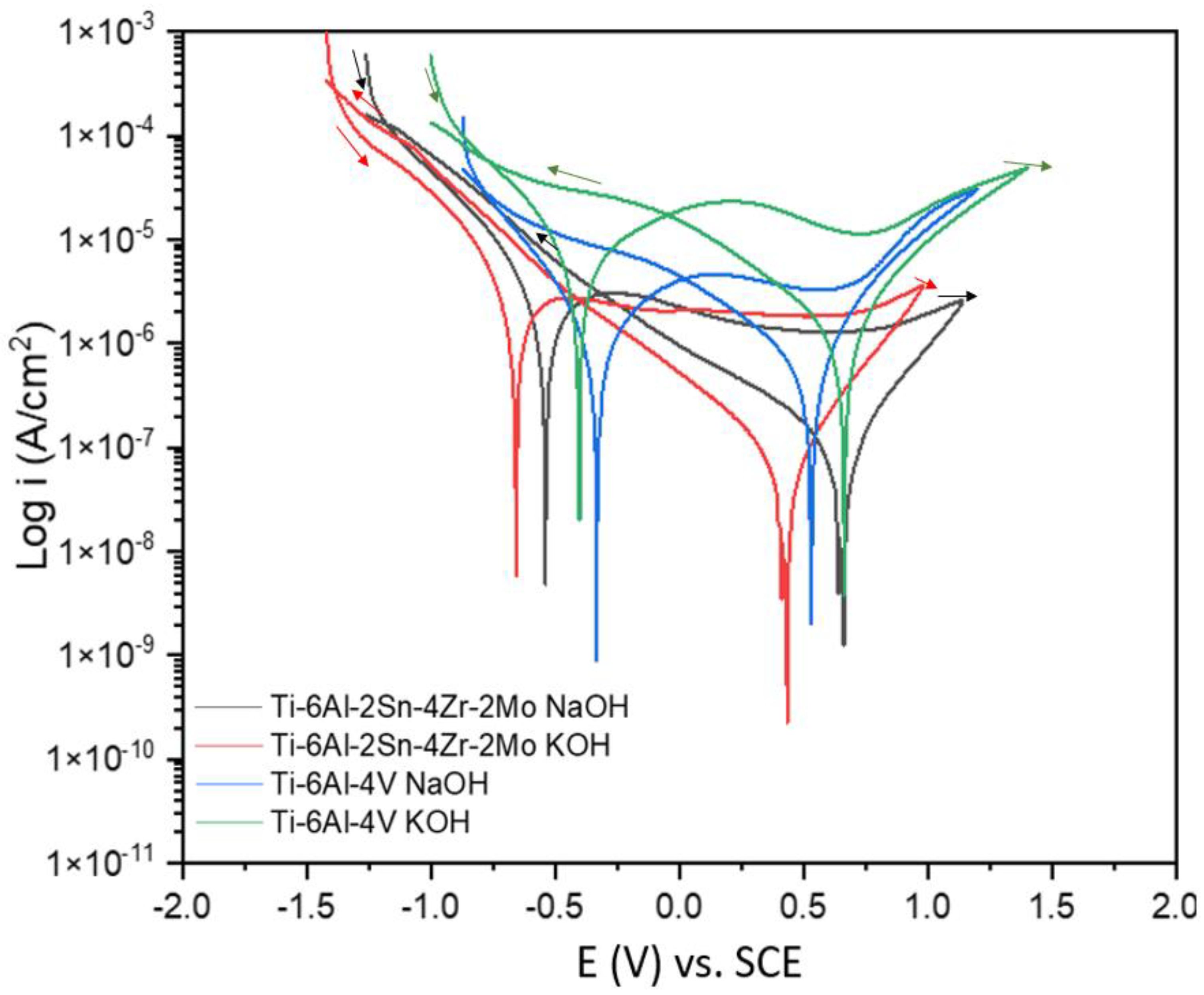
3.2. Cyclic Potentiodynamic Polarization
3.3. Electrochemical Noise
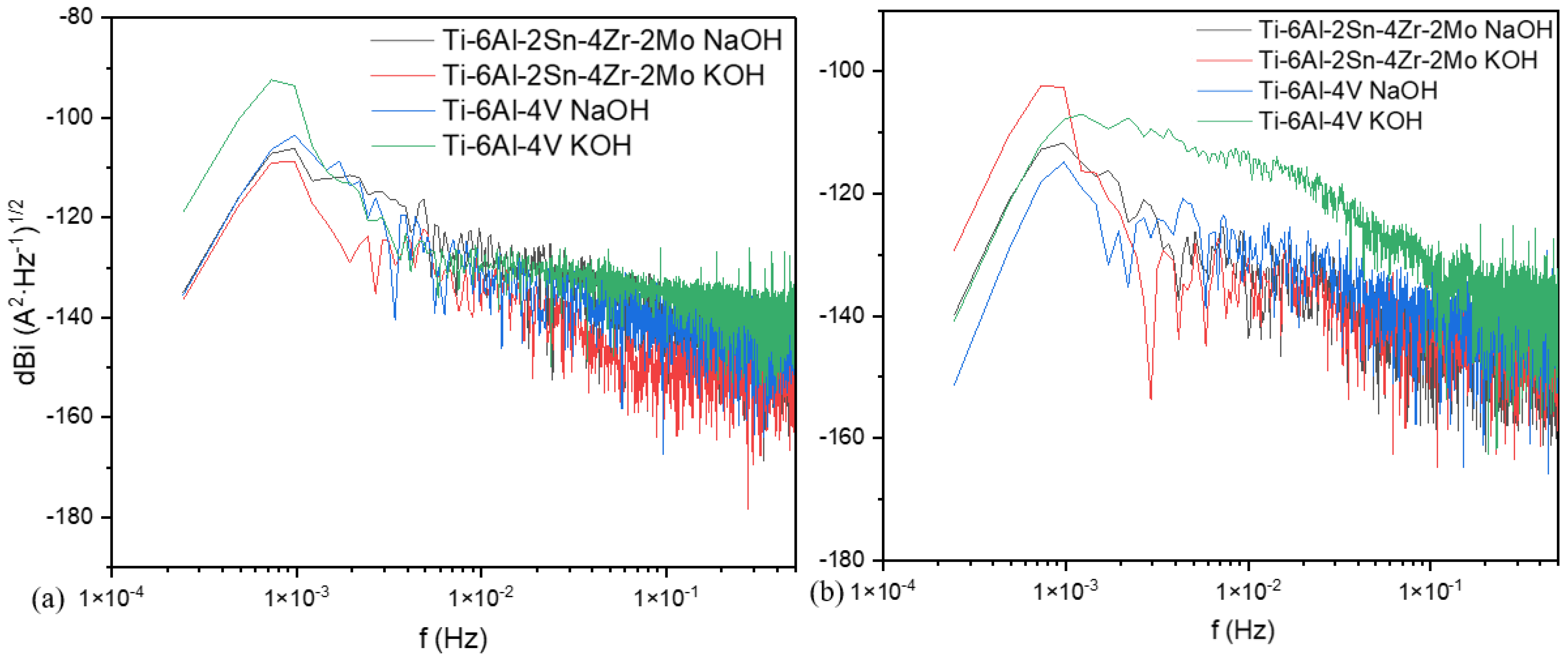
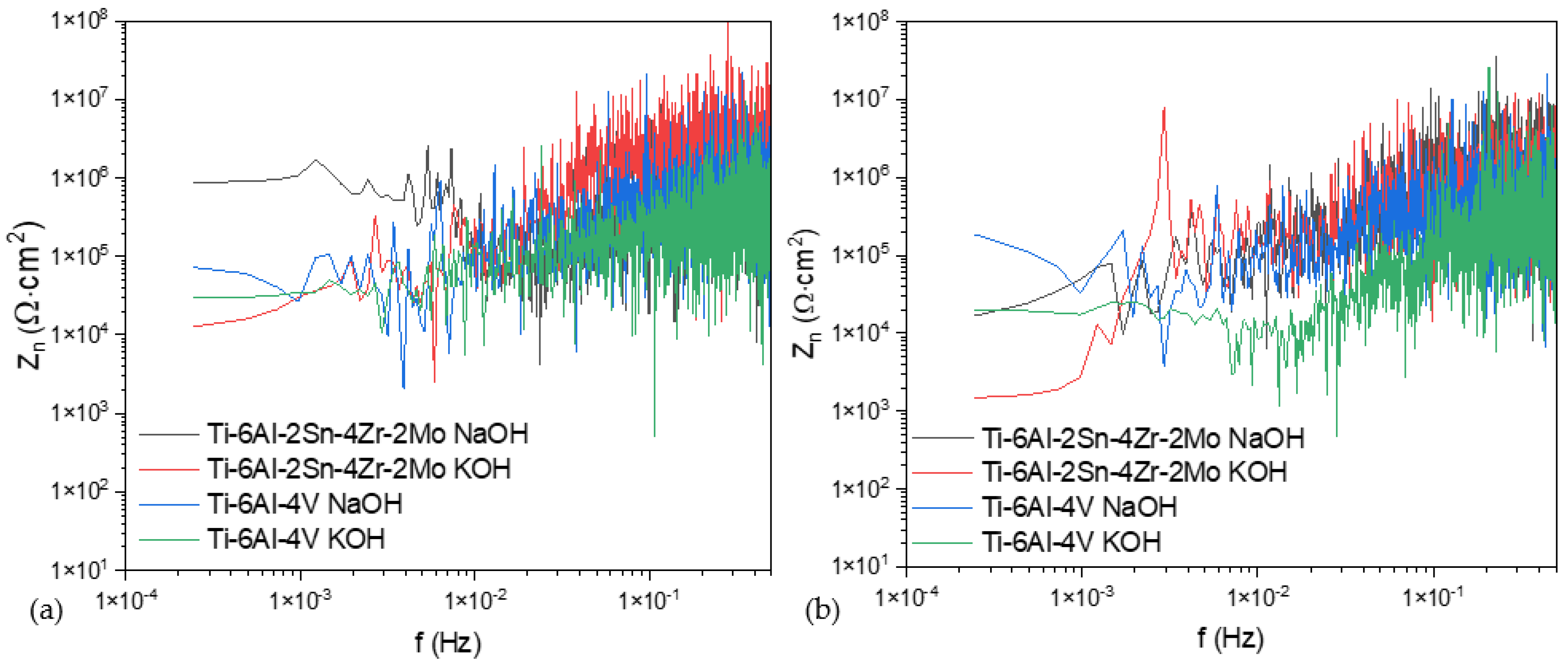
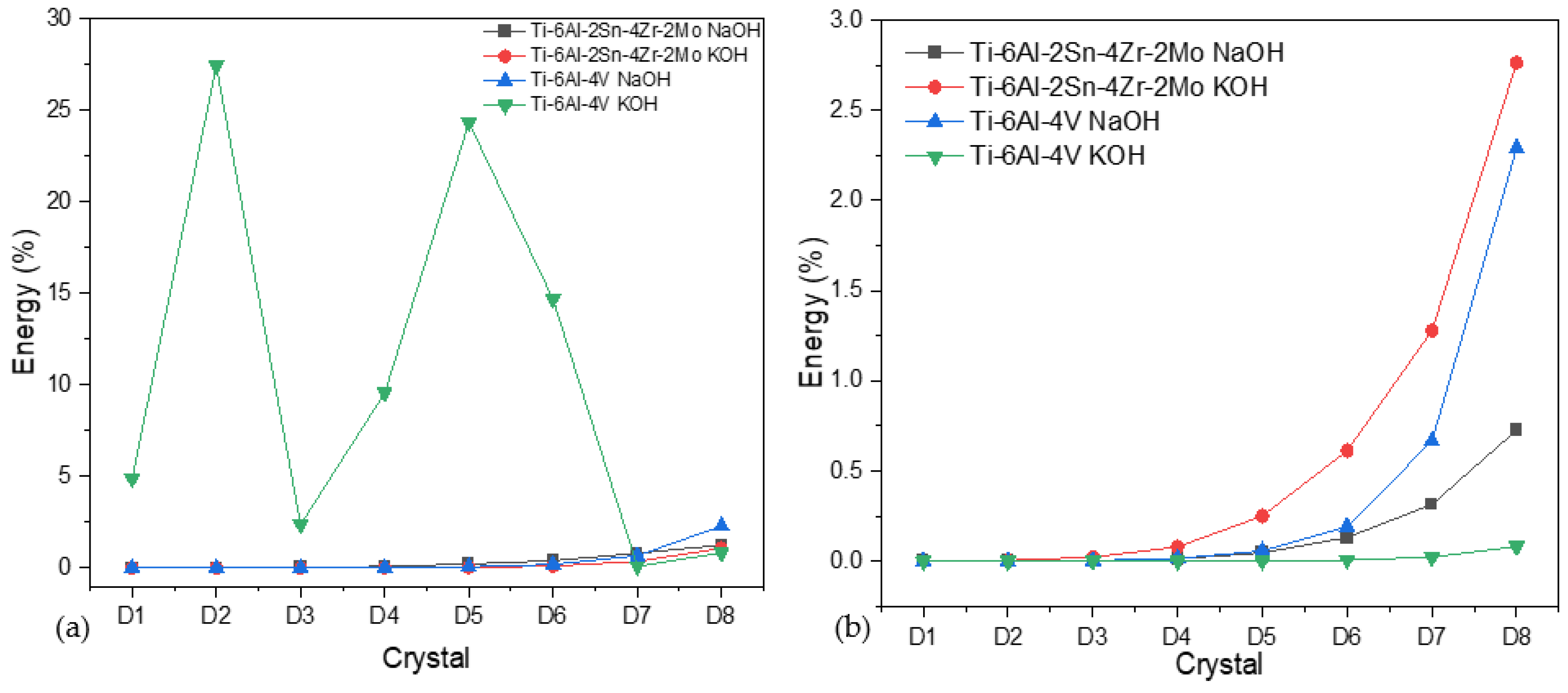
3.3.1. Power Spectral Density (PSD) and Noise Impedance (Zn)
3.3.2. Wavelets Analysis
3.4. SEM after Corrosion
4. Discussion
5. Conclusions
- Results indicated that the titanium alloys anodized on NaOH presented better electrochemical behavior against corrosion. In both techniques, the anodized alloys showed the best behavior.
- The anodized NaOH presented a high passivity range and a minor passivation current, which means a more stable anodized in this medium. The Ti-6Al-2Sn-4Zr-2Mo presented 1.27 V of passivation range.
- The Ti-6Al-2Sn-4Zr-2Mo anodized in NaOH presented the lowest values of ipass even in NaCl and H2SO4, with values of 3.03 × 10−6 and 1.67 × 10−7 A/cm2.
- The decrease in ipass for Ti-6Al-2Sn-4Zr-2Mo anodized in KOH exposed to H2SO4 is related to the formation of an oxide layer at the surface that protects the anodized.
- The alloy Ti-6Al-2Sn-4Zr-2Mo presented better properties when anodized; this behavior is related to the predominance of the α phase.
- The analysis by power spectral density showed that in a chloride system, the anodized material presented more susceptibility to being attacked by localized processes due to the interstitial role of Cl−.
- The alloy Ti-6Al-4V presented a more heterogeneous surface according to both techniques in CPP, with a lower passivity range and a high current passivation demand (2.28 × 10−5 A/cm2). In PSD and Zn, an abrupt slope change was presented.
- Both techniques contribute to characterizing anodized surfaces and, therefore, to both methods’ ability to corroborate information. In electrochemical noise, PSD and wavelets are required to match results due to the complexity of signals (chaotic systems).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys. In Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9783030244392. [Google Scholar]
- Mouritz, A.P. Introduction to Aerospace Materials; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9781855739468. [Google Scholar]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and Applications of Titanium Alloys: A Brief Review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium—A Technical Guide; ASM International: The Netherlands, 2000; Volume 55, ISBN 978-0-87170-686-7. [Google Scholar]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Giriga, S.; Mudali, U.K.; Raju, V.R.; Raj, B. Electrochemical Noise Technique for Corrosion Assessment—A Review. Corros. Rev. 2005, 23, 107–170. [Google Scholar] [CrossRef]
- Pathania, A.; Kumar, S.A.; Nagesha, B.K.; Barad, S.; Suresh, T.N. Reclamation of Titanium Alloy Based Aerospace Parts Using Laser Based Metal Deposition Methodology. Mater. Today Proc. 2021, 45, 4886–4892. [Google Scholar] [CrossRef]
- Du, X.Q.; Yang, Q.S.; Chen, Y.; Yang, Y.; Zhang, Z. Galvanic Corrosion Behavior of Copper/Titanium Galvanic Couple in Artificial Seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 570–581. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; De Los Rios, J.P.F.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A Review of Growth Mechanism, Structure and Crystallinity of Anodized TiO2 Nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.R.; Stevens, R.; Allsopp, D. Macro, Micro and Nanostructure of TiO2 Anodised Films Prepared in a Fluorine-Containing Electrolyte. J. Mater. Sci. 2007, 42, 6729–6734. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; dos Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and Characterization of TiO2 Thin Films by Sol-Gel Method. J. Sol-Gel Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Bahri, Y.A.; Sherif, E.-S.M. Influence of Zirconium on the Corrosion Passivation of Titanium in Simulated Body Fluid. Crystals 2021, 11, 1391. [Google Scholar] [CrossRef]
- Qin, P.; Chen, L.Y.; Liu, Y.J.; Jia, Z.; Liang, S.X.; Zhao, C.H.; Sun, H.; Zhang, L.C. Corrosion and Passivation Behavior of Laser Powder Bed Fusion Produced Ti-6Al-4V in Static/Dynamic NaCl Solutions with Different Concentrations. Corros. Sci. 2021, 191, 109728. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, M.K.; Jung, G.C.; Vang, M.S.; Park, Y.J. The Effects of Spark Anodizing Treatment of Pure Titanium Metals and Titanium Alloys on Corrosion Characteristics. Surf. Coat. Technol. 2007, 201, 8738–8745. [Google Scholar] [CrossRef]
- Xia, D.H.; Qin, Z.; Song, S.; Macdonald, D.; Luo, J.L. Combating Marine Corrosion on Engineered Oxide Surface by Repelling, Blocking and Capturing Cl−: A Mini Review. Corros. Commun. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.Á.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; los Rios, J.P.F.-D.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Estupinán-López, F.; Orquiz-Muela, C.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Bautista-Margulis, R.G.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Almeraya-Calderón, F.; Lopes, A.J. Oxidation Kinetics of Ti-6Al-4V Alloys by Conventional and Electron Beam Additive Manufacturing. Materials 2023, 16, 1187. [Google Scholar] [CrossRef]
- Dziewoński, P.M.; Grzeszczuk, M. Deposition of Thin TiO2 Layers on Platinum by Means of Cyclic Voltammetry of Selected Complex Ti(IV) Media Leading to Anatase. Electrochim. Acta 2009, 54, 4045–4055. [Google Scholar] [CrossRef]
- Löbl, P.; Huppertz, M.; Mergel, D. Nucleation and Growth in TiO2 Films Prepared by Sputtering and Evaporation. Thin Solid Film. 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Gong, T.; Su, J.; Zhang, W.; Song, Y.; Zhu, X. Comparative Study on the Anodizing Process of Ti and Zr and Oxide Morphology. Ceram. Int. 2021, 47, 23332–23337. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Reactivity of Porous Titanium Oxide Film and Chitosan Layer Electrochemically Formed on Ti-6Al-4V Alloy in Biological Solution. Surf. Coat. Technol. 2018, 354, 145–152. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of Titanium Alloys for Orthopedic Applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Constant-Phase Elements. In Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 395–419. [Google Scholar]
- Schultze, J.W.; Lohrengel, M.M.; Ross, D. Nucleation and Growth of Anodic Oxide Films. Electrochim. Acta 1983, 28, 973–984. [Google Scholar] [CrossRef]
- Seçkin, E.; Ürgen, M. A Kinetic Model for Determining Morphology Transitions and Growth Kinetics of Titania Nanotubes during Anodization of Titanium in Ethylene Glycol Based Electrolytes. Surf. Coat. Technol. 2021, 409, 126840. [Google Scholar] [CrossRef]
- Nakajima, M.; Miura, Y.; Fushimi, K.; Habazaki, H. Spark Anodizing Behaviour of Titanium and Its Alloys in Alkaline Aluminate Electrolyte. Corros. Sci. 2009, 51, 1534–1539. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Vázquez-Arenas, J.G.; Cabrera-Sierra, R.; Lartundo-Rojas, L.; González, I. Hydration and Structural Transformations during Titanium Anodization under Alkaline Conditions. ECS Trans. 2013, 50, 21–32. [Google Scholar] [CrossRef]
- Uzal, H.; Döner, A. Corrosion Behavior of Titanium Dioxide Nanotubes in Alkaline Solution. Prot. Met. Phys. Chem. Surf. 2020, 56, 311–319. [Google Scholar] [CrossRef]
- Attabi, S.; Mokhtari, M.; Taibi, Y.; Abdel-Rahman, I.; Hafez, B.; Elmsellem, H. Electrochemical and Tribological Behavior of Surface-Treated Titanium Alloy Ti–6Al–4V. J. Bio- Tribo-Corros. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Ormellese, M.; Prando, D.; Nicolis, D.; Bolzoni, F.; Pedeferri, M.P. Chemical Oxidation as Repairing Technique to Restore Corrosion Resistance on Damaged Anodized Titanium. Surf. Coat. Technol. 2019, 364, 225–230. [Google Scholar] [CrossRef]
- Martínez-Aparicio, B.; Martínez-Bastidas, D.; Gaona-Tiburcio, C.; Martin, U.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Localized corrosion of 15–5 PH and 17–4 PH stainless steel in NaCl solution. J. Solid State Electrochem. 2023. [Google Scholar] [CrossRef]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- SAE. Anodic Treatment of Titanium and Titanium Alloys Solution PH 12.4 Maximum; SAE: Warrendale, PA, USA, 2018; pp. 1–7. [Google Scholar]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron, Nickel or Cobalt Based Aalloys. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM G5-14; Standard Reference Test Method for Making Potentiodynamic Anodic Polarization. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2020.
- Apaza-Bedoya, K.; Tarce, M.; Benfatti, C.A.M.; Henriques, B.; Mathew, M.T.; Teughels, W.; Souza, J.C.M. Synergistic Interactions between Corrosion and Wear at Titanium-Based Dental Implant Connections: A Scoping Review. J. Periodontal Res. 2017, 52, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Loch, J.; Łukaszczyk, A.; Vignal, V.; Krawiec, H. Corrosion Behaviour of Ti6Al4V and TiMo10Zr4 Alloys in the Ringer’s Solution: Effect of PH and Plastic Strain. Solid State Phenom. 2015, 227, 435–438. [Google Scholar] [CrossRef]
- Xu, Y.F.; Xiao, Y.F.; Yi, D.Q.; Liu, H.Q.; Wu, L.; Wen, J. Corrosion Behavior of Ti–Nb–Ta–Zr–Fe Alloy for Biomedical Applications in Ringer’s Solution. Trans. Nonferrous Met. Soc. China 2015, 25, 2556–2563. [Google Scholar] [CrossRef]
- López, M.F.; Jiménez, J.A.; Gutiérrez, A. Corrosion Study of Surface-Modified Vanadium-Free Titanium Alloys. Electrochim. Acta 2003, 48, 1395–1401. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Ragab, S.A.; Abdo, H.S. Role of Vanadium Additions on the Corrosion Mitigation of Ti-6Al-XV Alloy in Simulated Body Fluid. Metals 2020, 10, 903. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Liao, F.; Li, G.; Zhao, C.; Cui, C.; Mei, J.; Zhao, Y. Bridging Effects of Sulfur Anions at Titanium Oxide and Perovskite Interfaces on Interfacial Defect Passivation and Performance Enhancement of Perovskite Solar Cells. ACS Omega 2021, 6, 34485–34493. [Google Scholar] [CrossRef]
- Yan, S.; Song, G.L.; Li, Z.; Wang, H.; Zheng, D.; Cao, F.; Horynova, M.; Dargusch, M.S.; Zhou, L. A State-of-the-Art Review on Passivation and Biofouling of Ti and Its Alloys in Marine Environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Cui, W.F.; Dong, Y.Y.; Bao, Y.C.; Qin, G.W. Improved Corrosion Resistance of Dental Ti50Zr Alloy with (TiZr)N Coating in Fluoridated Acidic Artificial Saliva. Rare Met. 2021, 40, 2927–2936. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Shao, M.; Zhang, Z.; Wang, C.; Yan, J.; Lu, J.; Zhang, L.; Xie, B.; He, Y.; et al. Characterization and Electrochemical Behavior of a Multilayer-Structured Ti–N Layer Produced by Plasma Nitriding of Electron Beam Melting TC4 Alloy in Hank’s Solution. Vacuum 2023, 208, 111737. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.M.; Ungureanu, G.; Gloriant, T. Comparative Corrosion Study of Ti–Ta Alloys for Dental Applications. Acta Biomater. 2009, 5, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, C.; Chen, Y. Surface Determination and Electrochemical Behavior of IrO2-RuO2-SiO2 Ternary Oxide Coatings in Oxygen Evolution Reaction Application. Electrochim. Acta 2018, 264, 350–357. [Google Scholar] [CrossRef]
- Delgado, D.; Minakshi, M.; Kim, D.-J. Electrochemical Impedance Spectroscopy Studies on Hydrogen Evolution from Porous Raney Cobalt in Alkaline Solution. Int. J. Electrochem. Sci. 2015, 10, 9379–9394. [Google Scholar] [CrossRef]
- Liu, B.; Ma, B.; Chen, Y.; Wang, C. Corrosion Mechanism of Ti/IrO2-RuO2-SiO2 Anode for Oxygen Evolution in Sulfuric Acid Solution. Corros. Sci. 2020, 170, 108662. [Google Scholar] [CrossRef]
- Fekry, A.M. The Influence of Chloride and Sulphate Ions on the Corrosion Behavior of Ti and Ti-6Al-4V Alloy in Oxalic Acid. Electrochim. Acta 2009, 54, 3480–3489. [Google Scholar] [CrossRef]
- Yan, J.; Shao, M.; Zhou, Z.; Zhang, Z.; Yi, X.; Wang, M.; Wang, C.; Fang, D.; Wang, M.; Xie, B.; et al. Electrochemical Corrosion Behavior of Ti-N-O Modified Layer on the TC4 Titanium Alloy Prepared by Hollow Cathodic Plasma Source Oxynitriding. Metals 2023, 13, 1083. [Google Scholar] [CrossRef]
- Cottis, R.; Turgoose, S. Electrochemical Impedance and Noise; NACE International: Houston, TX, USA, 1999; p. 153. [Google Scholar]
- Eden, D.A.; Rothwell, A.N. Electrochemical Noise Data: Analysis, Interpretation and Presentation; NACE International: Houston, TX, USA, 1992; pp. 1–12. [Google Scholar]
- Coakley, J.; Vorontsov, V.A.; Littrell, K.C.; Heenan, R.K.; Ohnuma, M.; Jones, N.G.; Dye, D. Nanoprecipitation in a Beta-Titanium Alloy. J. Alloys Compd. 2015, 623, 146–156. [Google Scholar] [CrossRef]
- Wei, D.B.; Chen, X.H.; Zhang, P.Z.; Ding, F.; Li, F.K.; Yao, Z.J. Plasma Surface Tantalum Alloying on Titanium and Its Corrosion Behavior in Sulfuric Acid and Hydrochloric Acid. Appl. Surf. Sci. 2018, 441, 448–457. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Z.; Dong, J.; Ariyanti, D.; Niu, Z.; Huang, S.; Zhang, W.; Gao, W. Self-Organized ZnO Nanorods Prepared by Anodization of Zinc in NaOH Electrolyte. RSC Adv. 2016, 6, 72968–72974. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, Y.; Zhang, J.; Wang, L.; Zhang, H.; Wan, Q.; Li, W.; Wang, L.; Lv, Z. Growing Carbon Nanotubes In-Situ via Chemical Vapor Deposition and Resin Pre-Coating Treatment on Anodized Ti-6Al-4V Titanium Substrates for Stronger Adhesive Bonding with Carbon Fiber Composites. Surf. Coat. Technol. 2023, 457, 129296. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Zhao, X.; Sheng, Y.; Li, W.; Chang, C.L.; Zhang, Q.; Yu, Z.; Wang, X. Microstructure, Mechanical Properties and Corrosion Behaviors of Biomedical Ti-Zr-Mo-XMn Alloys for Dental Application. Corros. Sci. 2019, 161, 108195. [Google Scholar] [CrossRef]
- Casanova, L.; La Padula, M.; Pedeferri, M.P.; Diamanti, M.V.; Ormellese, M. An Insight into the Evolution of Corrosion Resistant Coatings on Titanium during Bipolar Plasma Electrolytic Oxidation in Sulfuric Acid. Electrochim. Acta 2021, 379, 138190. [Google Scholar] [CrossRef]
- Homborg, A.M.; Oonincx, P.J.; Mol, J.M.C. Wavelet Transform Modulus Maxima and Holder Exponents Combined with Transient Detection for the Differentiation of Pitting Corrosion Using Electrochemical Noise. Corrosion 2018, 74, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Homborg, A.M.; van Westing, E.P.M.; Tinga, T.; Zhang, X.; Oonincx, P.J.; Ferrari, G.M.; de Wit, J.H.W.; Mol, J.M.C. Novel Time–Frequency Characterization of Electrochemical Noise Data in Corrosion Studies Using Hilbert Spectra. Corros. Sci. 2013, 66, 97–110. [Google Scholar] [CrossRef]
- Carmona-Hernández, A.; Orozco-Cruz, R.; Carpio-Santamaria, F.A.; Campechano-Lira, C.; López-Huerta, F.; Mejía-Sánchez, E.; Contreras, A.; Galván-Martínez, R. Electrochemical Noise Analysis of the X70 Pipeline Steel under Stress Conditions Using Symmetrical and Asymmetrical Electrode Systems. Metals 2022, 12, 1545. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhao, Z.; Bai, P.; Liu, B.; Tan, J.; Wu, X. In-Situ Monitoring of Pitting Corrosion of Q235 Carbon Steel by Electrochemical Noise: Wavelet and Recurrence Quantification Analysis. J. Electroanal. Chem. 2020, 879, 114776. [Google Scholar] [CrossRef]
- Mazzarolo, A.; Curioni, M.; Vicenzo, A.; Skeldon, P.; Thompson, G.E. Anodic Growth of Titanium Oxide: Electrochemical Behaviour and Morphological Evolution. Electrochim. Acta 2012, 75, 288–295. [Google Scholar] [CrossRef]
- Laurindo, C.A.H.; Torres, R.D.; Mali, S.A.; Gilbert, J.L.; Soares, P. Incorporation of Ca and P on Anodized Titanium Surface: Effect of High Current Density. Mater. Sci. Eng. C 2014, 37, 223–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, H.; Ding, X.; Yan, Q.; Wang, L.; Ma, W. Simulation of Anodizing Current-Time Curves and Morphology Evolution of TiO2 Nanotubes Anodized in Electrolytes with Different NH4F Concentrations. Electrochim. Acta 2015, 176, 1083–1091. [Google Scholar] [CrossRef]
- Burleigh, T.D.; Dotson, T.C.; Dotson, K.T.; Gabay, S.J.; Sloan, T.B.; Ferrell, S.G. Anodizing Steel in KOH and NaOH Solutions. J. Electrochem. Soc. 2007, 154, C579. [Google Scholar] [CrossRef]
- Hsu, H.C.; Hsu, S.K.; Wu, S.C.; Hung, Y.H.; Ho, W.F. Surface Modification of Nanotubular Anodized Ti–7.5Mo Alloy Using NaOH Treatment for Biomedical Application. Thin Solid Film. 2020, 710, 138273. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Takadama, H.; Matsushita, T.; Nakamura, T.; Kokubo, T. Preparation of Bioactive Ti-15Zr-4Nb-4Ta Alloy from HCl and Heat Treatments after an NaOH Treatment. J. Biomed. Mater. Res. Part A 2011, 97A, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Wu, S.-C.; Hsu, S.-K.; Chuang, S.-H.; Ho, W.-F. Surface Modification of Commercially Pure Ti Treated with Aqueous NaOH Treatment and Ethyl Alcohol Aging. J. Med. Biol. Eng. 2013, 33, 331–336. [Google Scholar] [CrossRef]
- Martinez, A.L.; Flamini, D.O.; Saidman, S.B. Corrosion Resistance Improvement of Ti-6Al-4V Alloy by Anodization in the Presence of Inhibitor Ions. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2022, 32, 1896–1909. [Google Scholar] [CrossRef]
- Prando, D.; Nicolis, D.; Pedeferri, M.P.; Ormellese, M. Pitting Corrosion on Anodized Titanium: Effect of Halides. Mater. Corros. 2018, 69, 1441–1446. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between Constant-Phase Element (CPE) Parameters and Physical Properties of Films with a Distributed Resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
- Rajan, S.T.; Anusha, T.V.V.; Terada-Nakaishi, M.; Chen, P.; Hanawa, T.; Nandakumar, A.K.; Subramanian, B. Zirconium-Based Metallic Glass and Zirconia Coatings to Inhibit Bone Formation on Titanium. Biomed. Mater. 2020, 15, 065019. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of Titanium: Part 1: Aggressive Environments and Main Forms of Degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef]
- Madina, V.; Azkarate, I. Compatibility of Materials with Hydrogen. Particular Case: Hydrogen Embrittlement of Titanium Alloys. Int. J. Hydrogen Energy 2009, 34, 5976–5980. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; Noël, J.J.; Hardie, D.; Ikeda, B.M. Hydrogen Absorption and the Lifetime Performance of Titanium Nuclear Waste Containers. Corros. Rev. 2000, 18, 331–359. [Google Scholar] [CrossRef]
- Hua, F.; Mon, K.; Pasupathi, P.; Gordon, G.; Shoesmith, D. Modeling the Hydrogen-Induced Cracking of Titanium Alloys in Nuclear Waste Repository Environments. JOM 2005, 57, 20–26. [Google Scholar] [CrossRef]
- Christ, H.J.; Senemmar, A.; Decker, M.; Prüßner, K. Effect of Hydrogen on Mechanical Properties of β -Titanium Alloys. Sadhana 2003, 28, 453–465. [Google Scholar] [CrossRef]
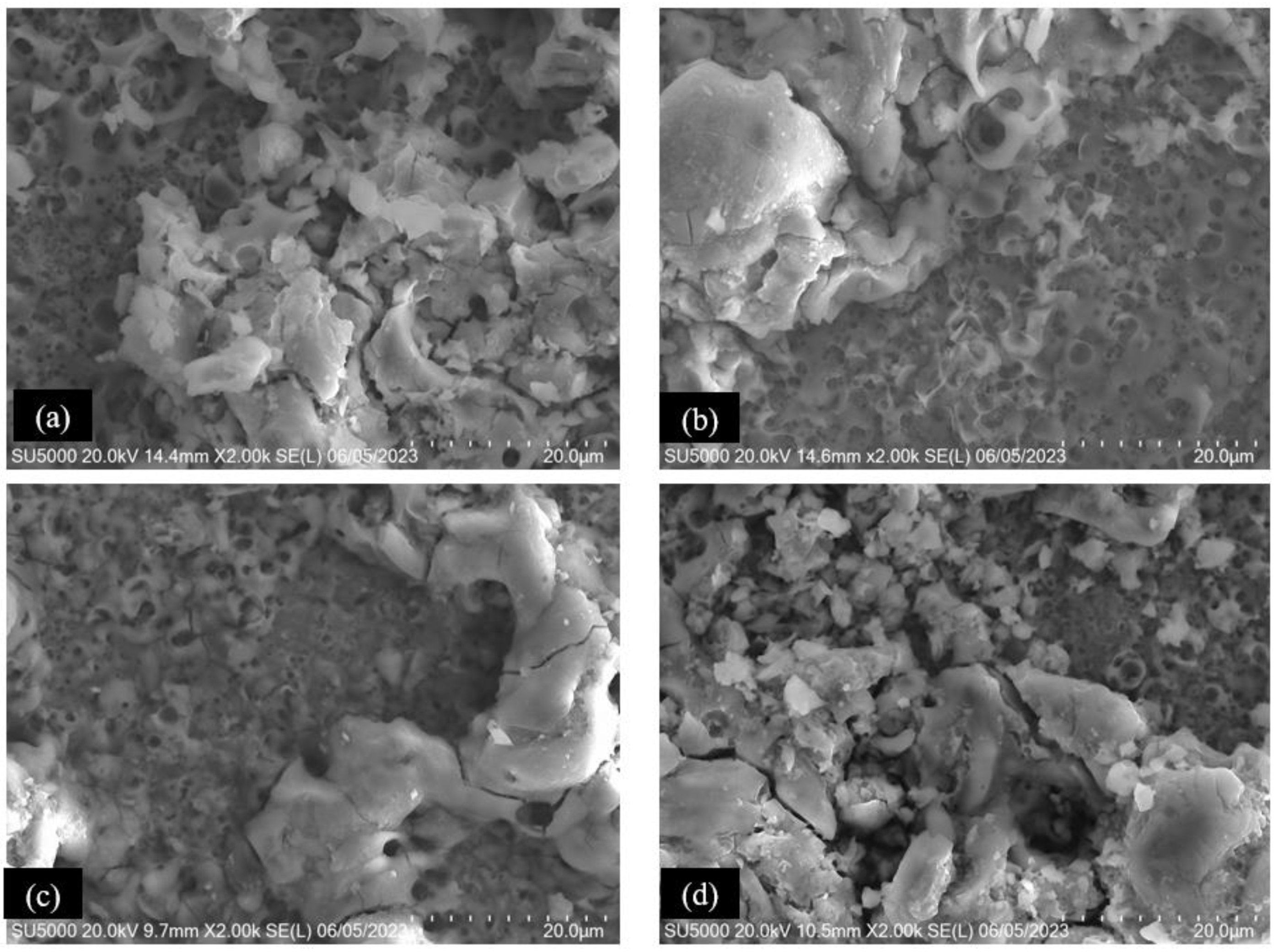

| Elements | Ti-6Al-2Sn-4Zr-2Mo AMS 4917 | Ti-6Al-4V AMS 4911 |
|---|---|---|
| Ti | 84.65 ± 0.19 | 87.71 ± 0.36 |
| Al | 6.75 ± 0.20 | 7.14 ± 0.37 |
| Sn | 2.08 ± 0.01 | – |
| V | – | 4.03 ± 0.08 |
| Zr | 4.18 ± 0.01 | – |
| Mo | 1.99 ± 0.008 | – |
| Alloys | Anodizing Electrolyte | Ecorr (V) | icorr (A/cm2) | Epit (V) | Rpass (V) | ipass (A/cm2) | Hysteresis |
|---|---|---|---|---|---|---|---|
| Immersed in 3.5 wt.% NaCl Solution | |||||||
| Ti-6Al-2Sn-4Zr-2Mo | NaOH | −0.52 | 6.98 × 10−7 | 0.83 | 1.27 | 3.03 × 10−6 | negative |
| Ti-6Al-2Sn-4Zr-2Mo | KOH | −0.64 | 5.55 × 10−7 | 0.73 | 1.25 | 2.58 × 10−6 | negative |
| Ti-6Al-4V | NaOH | −0.32 | 1.06 × 10−7 | 0.61 | 0.55 | 4.33 × 10−6 | negative |
| Ti-6Al-4V | KOH | −0.40 | 3.20 × 10−6 | 0.77 | 0.59 | 2.28 × 10−5 | negative |
| Immersed in 3.5 wt.% H2SO4 Solution | |||||||
| Ti-6Al-2Sn-4Zr-2Mo | NaOH | −0.19 | 5.51 × 10−7 | 1.01 | 0.82 | 1.67 × 10−7 | negative |
| Ti-6Al-2Sn-4Zr-2Mo | KOH | −0.05 | 7.53 × 10−7 | 0.72 | 0.69 | 1.75 × 10−6 | negative |
| Ti-6Al-4V | NaOH | −0.05 | 1.05 × 10−6 | 0.83 | 0.63 | 4.66 × 10−6 | negative |
| Ti-6Al-4V | KOH | −0.05 | 2.26 × 10−6 | 0.63 | 0.44 | 9.65 × 10−6 | negative |
| Alloys | Anodizing Electrolyte | Ψ0 (dBi) | Β (dB [A]) | Zn0 (Ω·cm2) |
|---|---|---|---|---|
| Immersed in 3.5 wt.% NaCl Solution | ||||
| Ti-6Al-2Sn-4Zr-2Mo | NaOH | −134 | −9.9 | 87.80 × 104 |
| Ti-6Al-2Sn-4Zr-2Mo | KOH | −136 | −7.3 | 12.46 × 103 |
| Ti-6Al-4V | NaOH | −135 | −7.2 | 72.53 × 103 |
| Ti-6Al-4V | KOH | −118 | −7.9 | 29.57 × 103 |
| Immersed in 3.5 wt.% H2SO4 Solution | ||||
| Ti-6Al-2Sn-4Zr-2Mo | NaOH | −139 | −6.3 | 16.75 × 103 |
| Ti-6Al-2Sn-4Zr-2Mo | KOH | −129 | −5.3 | 1.48 × 103 |
| Ti-6Al-4V | NaOH | −151 | −4.5 | 18.66 × 104 |
| Ti-6Al-4V | KOH | −140 | −12.7 | 19.75 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olgui-Coca, J.; Lopez-Leon, L.D.; Estupiñán-López, F.; Lira-Martínez, A.; Gaona Tiburcio, C. Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals 2023, 13, 1510. https://doi.org/10.3390/met13091510
Almeraya-Calderón F, Jáquez-Muñoz JM, Maldonado-Bandala E, Cabral-Miramontes J, Nieves-Mendoza D, Olgui-Coca J, Lopez-Leon LD, Estupiñán-López F, Lira-Martínez A, Gaona Tiburcio C. Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals. 2023; 13(9):1510. https://doi.org/10.3390/met13091510
Chicago/Turabian StyleAlmeraya-Calderón, Facundo, Jesús M. Jáquez-Muñoz, Erick Maldonado-Bandala, Jose Cabral-Miramontes, Demetrio Nieves-Mendoza, Javier Olgui-Coca, Luis Daimir Lopez-Leon, Francisco Estupiñán-López, Alejandro Lira-Martínez, and Citlalli Gaona Tiburcio. 2023. "Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions" Metals 13, no. 9: 1510. https://doi.org/10.3390/met13091510
APA StyleAlmeraya-Calderón, F., Jáquez-Muñoz, J. M., Maldonado-Bandala, E., Cabral-Miramontes, J., Nieves-Mendoza, D., Olgui-Coca, J., Lopez-Leon, L. D., Estupiñán-López, F., Lira-Martínez, A., & Gaona Tiburcio, C. (2023). Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals, 13(9), 1510. https://doi.org/10.3390/met13091510









