Electrochemical Determination of Ascorbic Acid by Mechanically Alloyed Super Duplex Stainless Steel Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of SDSS Powders by Mechanical Alloying
2.2. Preparation of Carbon Paste Electrodes
3. Results
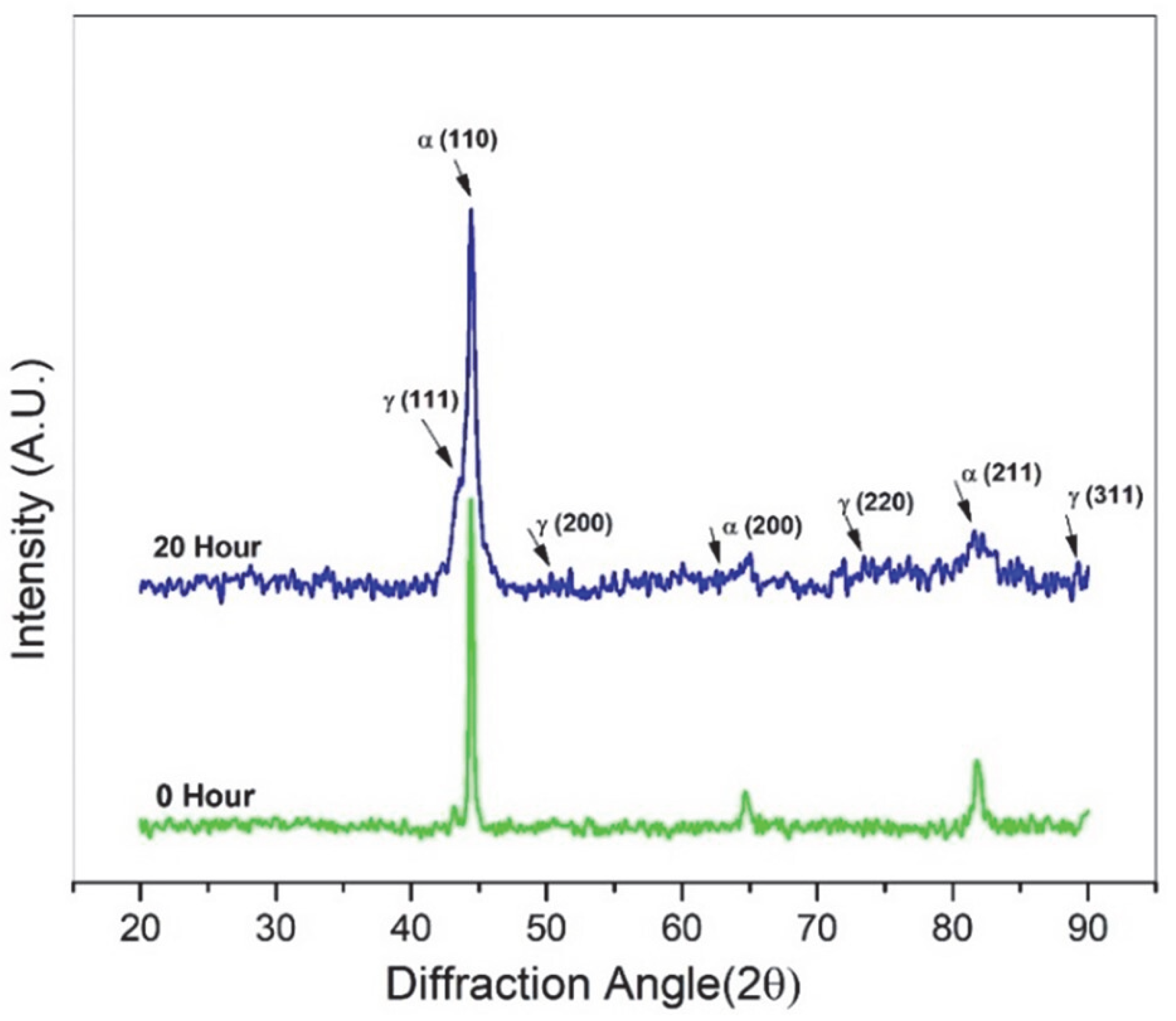
3.1. XRD Phase Analysis
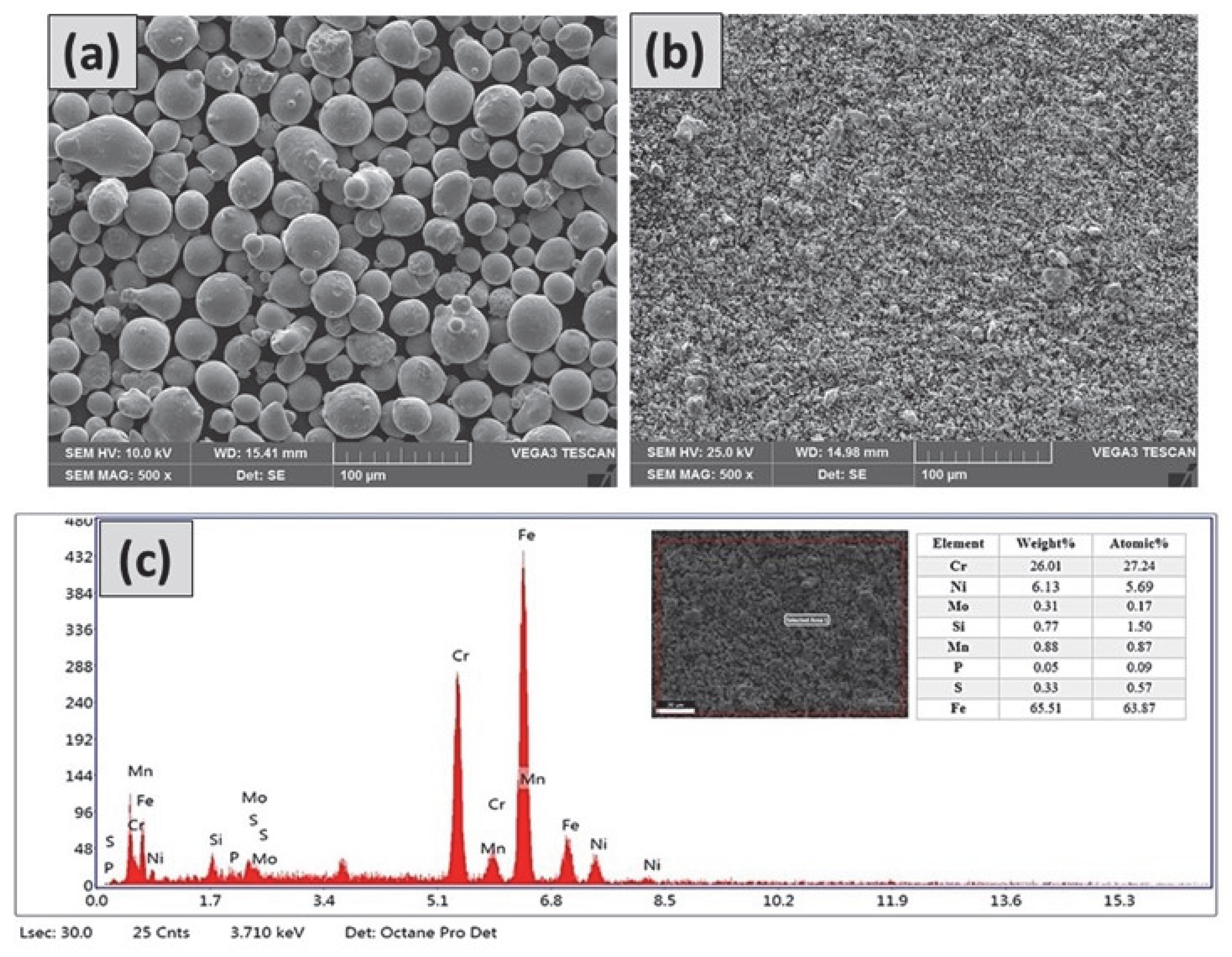
3.2. Powder Morphology Studies
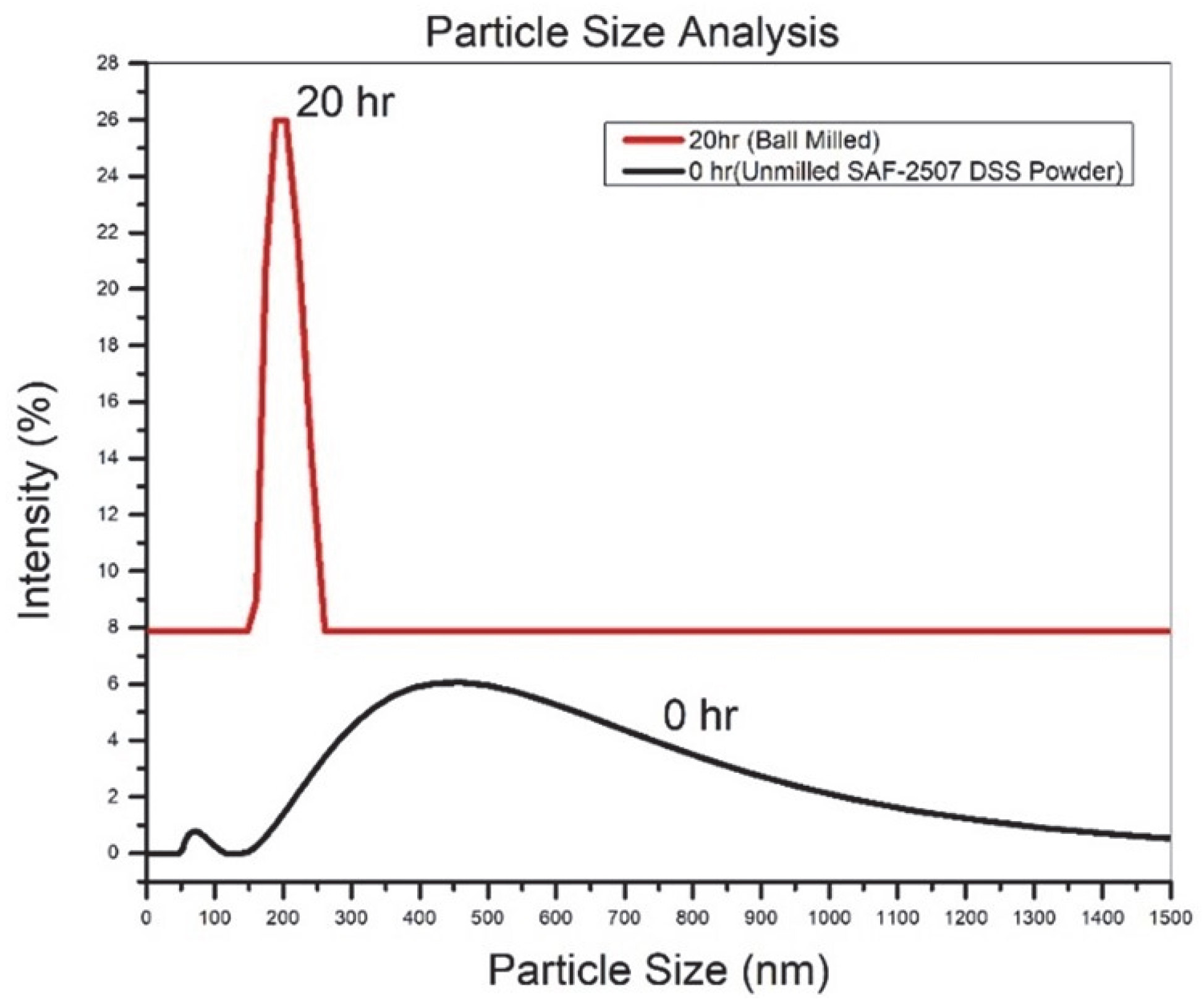
3.3. Particle Size Analysis
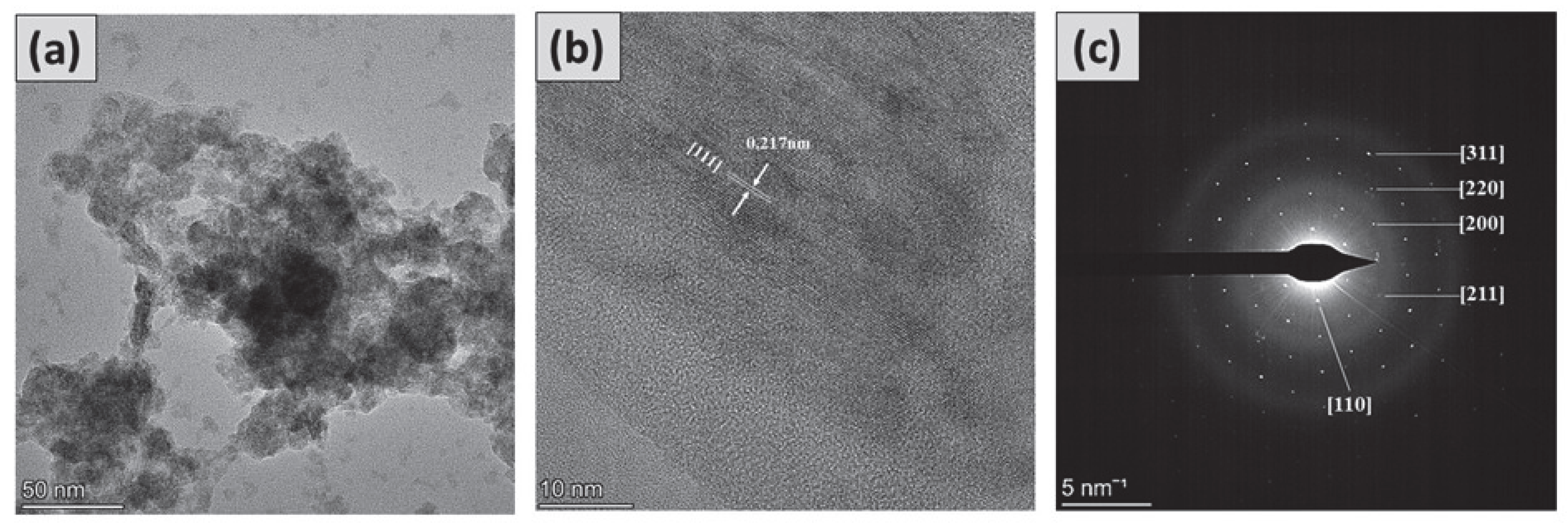
3.4. High-Resolution Transmission Electron Microscopy
3.5. Investigation of Electrochemical Sensor Applications
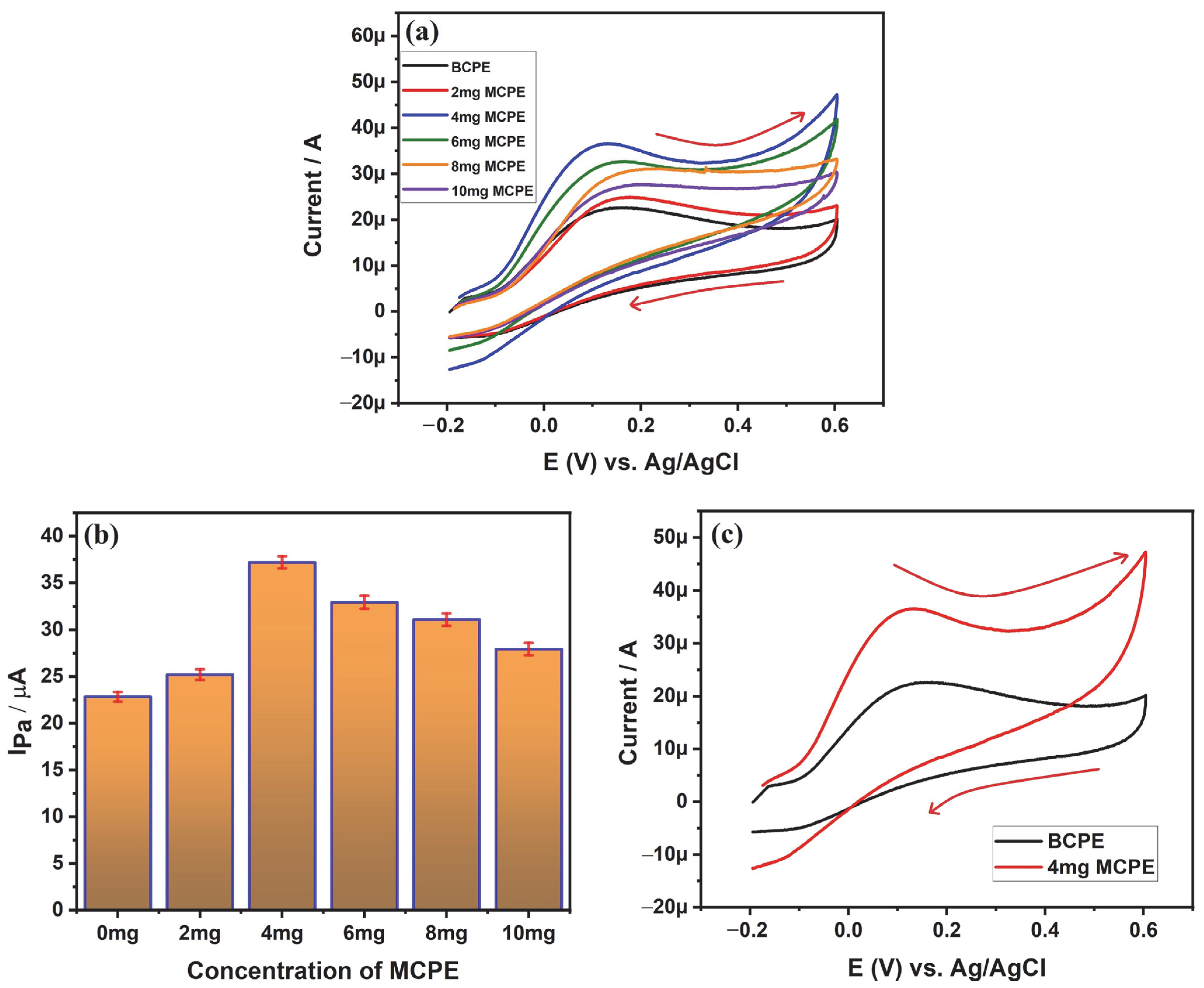
3.5.1. Electro-Oxidation of Ascorbic Acid at Different Concentrations of SDSS-MCPE
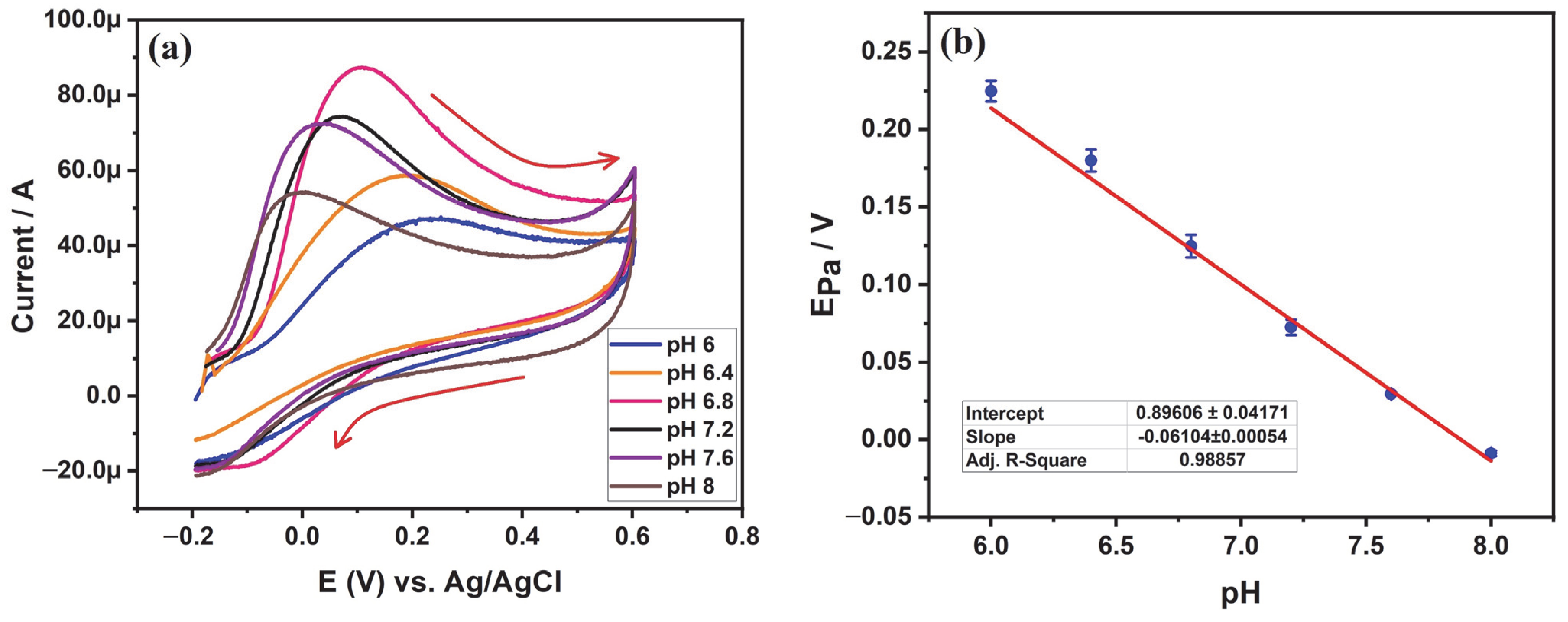
3.5.2. Investigating the Influence of pH
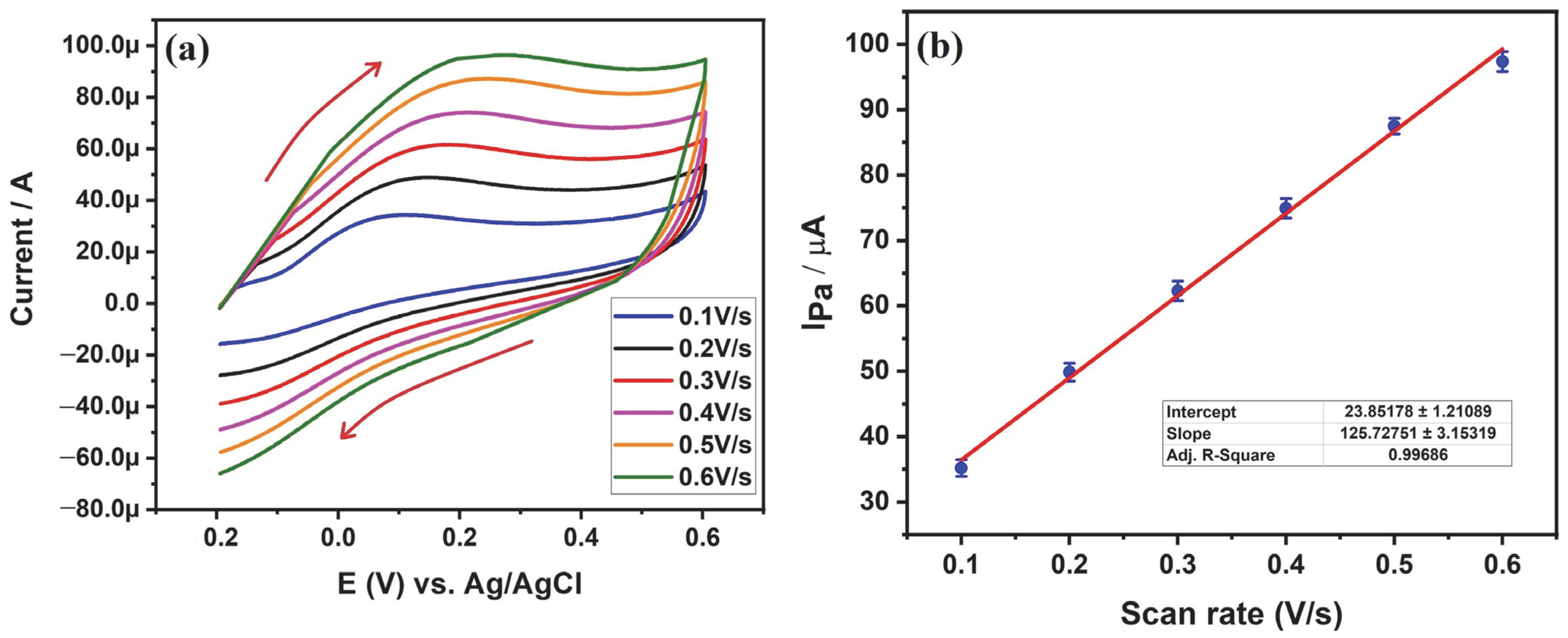
3.5.3. Influence of Scan Rate
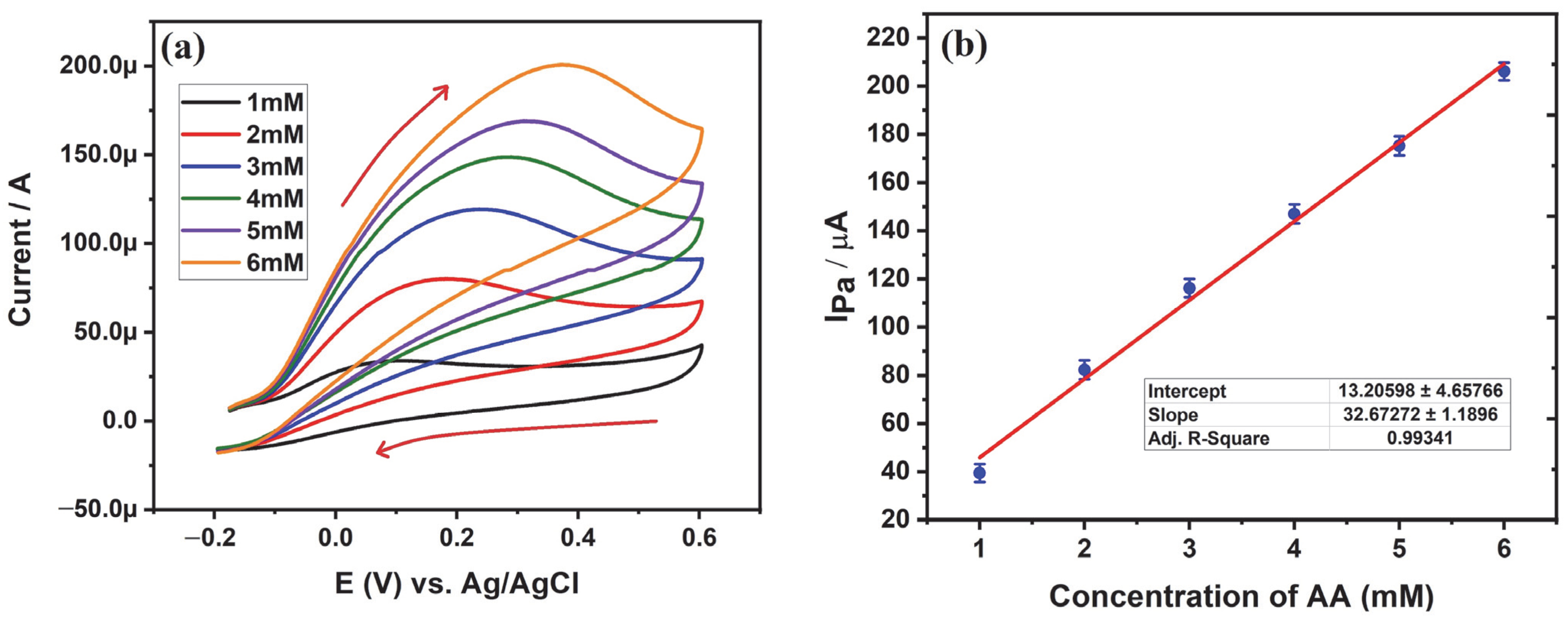
3.5.4. Influence of Concentration of AA
3.5.5. Interference Ions’ Influence on AA during Its Electro-Oxidation at 4 mg SDSS-MCPE
3.5.6. Repeatability, Reproducibility, and Stability of 4 mg SDSS-MCPE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philip Selvaraj, D.; Chandramohan, P.; Mohanraj, M. Optimization of surface roughness, cutting force and tool wear of nitrogen alloyed duplex stainless steel in a dry turning process using Taguchi method. Measurement 2014, 49, 205–215. [Google Scholar] [CrossRef]
- Jan, O.; Malin, S. Duplex—A new generation of stainless steels for desalination plants. Desalination 2007, 25, 104–113. [Google Scholar]
- Wang, Y.Q.; Yang, B.; Han, J.; Dong, F.; Wang, Y.L. Localized corrosion of thermally aged cast duplex stainless steel for primary coolant pipes of nuclear power plant. Procedia Eng. 2012, 36, 88–95. [Google Scholar] [CrossRef]
- Schofield, M.J.; Bradsha, R.; Cottis, R.A. Stress Corrosion cracking of duplex stainless-steel weldments in sour conditions. Mater. Perform. 1996, 36, 65–70. [Google Scholar]
- Shashanka, R.; Chaira, D. Effects of Nano-Y2O3 and Sintering Parameters on the Fabrication of PM Duplex and Ferritic Stainless Steels. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 58–71. [Google Scholar] [CrossRef]
- Tuba, K.; Hayriye, E.E.; Mustafa, T.; Ramazan, K. Strengthening of AISI 2205 duplex stainless steel by strain ageing. Mater. Des. 2014, 55, 250–256. [Google Scholar] [CrossRef]
- Herenu, S.; Alvarez, A.; Armas, A.F. The influence of dynamic strain aging on the low cycle fatigue of duplex stainless steel. Scr. Mater. 2001, 45, 739–745. [Google Scholar] [CrossRef]
- Hiroyuki, M.; Takuro, M.; Satoshi, H. Superplastic deformation of micro-specimens of duplex stainless steel. Mater. Sci. Eng. A 2001, 319–321, 779–783. [Google Scholar] [CrossRef]
- Solomon, H.D.; Devine, T.M. Duplex Stainless Steels; American Society of Metals: Metals Park, OH, USA, 1983; pp. 693–756. [Google Scholar]
- Marina, K.; Michael, P. Duplex Steels: Part I: Genesis, Formation, Structure. Metallogr. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Development of nano-structured duplex and ferritic stainless steels by pulverisette planetary milling followed by pressureless sintering. Mater. Charact. 2015, 99, 220–229. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D.; Dibyendu, C. Fabrication of Nano-Yttria Dispersed Duplex and Ferritic Stainless Steels by Planetary Milling Followed by Spark Plasma Sintering and Non-Lubricated Sliding Wear Behaviour Study. J. Mater. Sci. Eng. B 2016, 6, 111–125. [Google Scholar] [CrossRef]
- Vannevik, H.; Nilsson, J.O.; Frodigh, J.; Kangas, P. Effect of Elemental Partitioning on Pitting Resistance of High Nitrogen Duplex Stainless Steels. ISIJ Int. 1996, 36, 807–812. [Google Scholar] [CrossRef]
- Wang, J.; Uggowitzer, P.J.; Magdowski, R.; Speidel, M.O. Nickel-Free Duplex Stainless Steels. Scr. Mater. 1998, 40, 123–129. [Google Scholar] [CrossRef]
- Kurzydlowski, K.J. Microstructural refinement and properties of metals processed by severe plastic deformation. Bull. Pol. Acad. Sci. 2004, 52, 301–311. [Google Scholar]
- Shashanka, R. Non-lubricated dry sliding wear behavior of spark plasma sintered nano-structured stainless steel. J. Mater. Environ. Sci. 2019, 10, 767–777. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Oleszak, D.; Grabias, A.; Pekala, M.; Swiderska-Sroda, A.; Kulik, T. Evolution of structure in austenitic steel powders during ball milling and subsequent sintering. J. Alloys Compd. 2007, 434–435, 340–343. [Google Scholar] [CrossRef]
- Rayappa, S.M.; Shashanka, R.; Shamanth, V.; Hemanth, K.; Nithin, S.K.; Sharath, P.C.; Adarsh, P. Technology and Challenges in Additive Manufacturing of Duplex Stainless Steels. Biointerface Res. Appl. Chem. 2022, 12, 1110–1119. [Google Scholar] [CrossRef]
- Rayappa, S.M.; Shamanth, V.; Sharath, P.C.; Shashanka, R.; Hemanth, K. A Review on Spark Plasma Sintering of Duplex Stainless Steels. Mater. Today Proc. 2021, 45, 138–144. [Google Scholar] [CrossRef]
- Rayappa, S.M.; Shamanth, V.; Hemanth, K.; Sharath, P.C.; Shashanka, R. Mechanical Testing of Spark Plasma Sintered Materials: A Review. AIP Conf. Proc. 2022, 2469, 020026. [Google Scholar] [CrossRef]
- Ravnsbaek, D.B.; Sorensen, L.H.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Screening of metal borohydrides by mechanochemistry and diffraction. Angew. Chem. 2012, 51, 3582–3586. [Google Scholar] [CrossRef]
- Morten, B.L.; Dorthe, B.R.; Yaroslav, F.; Lee, Y.-S.; Raphael, J.; Young, W.C.; Jorgen, S.; Torben, R.J. LiCe(BH4)3Cl a new lithium-ion conductor and hydrogen storage material with isolated tetranuclear anionic clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbaek, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Ravnsbaek, D.B.; Nickels, E.A.; Cerny, R.; Olesen, C.H.; David, W.I.F.; Edwards, P.P.; Filinchuk, Y.; Jensen, T.R. Novel Alkali Earth Borohydride Sr(BH4)2 and Borohydride-Chloride Sr(BH4)Cl. Inorg. Chem. 2013, 52, 10877–10885. [Google Scholar] [CrossRef]
- Radovan, C.; Pascal, S.; Yolanda, S.; Katarina, S.; Lubomir, S.; Ritcher, B.; Torben, R.J. Trimetallic borohydride Li3MZn5(BH4)15 (M=Mg, Mn) containing two weakly interconnected frameworks. Inorg. Chem. 2013, 52, 9941–9947. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Phase transformation and microstructure study of nano-structured austenitic and ferritic stainless-steel powders prepared by planetary milling. Powder Technol. 2014, 259, 125–136. [Google Scholar] [CrossRef]
- Haghir, T.; Abbasi, M.H.; Golozar, M.A.; Panjepour, M. Investigation of α to γ transformation in the production of a nanostructured high-nitrogen austenitic stainless-steel powder via mechanical alloying. Mater. Sci. Eng. A 2009, 507, 144–148. [Google Scholar] [CrossRef]
- Amini, R.; Salahinejad, E.; Hadianfard, M.J.; Marasi, M.; Sritharan, T. Characterization of Fe–Cr–Mn–N amorphous powders with a wide supercooled liquid region developed by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 1135–1142. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Wang, L.; Xu, Z.; Ji, V.; Jiang, C. Residual stress and microstructure evolutions of SAF 2507 duplex stainless steel after shot peening. Appl. Surf. Sci. 2018, 459, 155–163. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Xiao, X.; Zhao, J.; Jiang, L. On the behavior of nitrogen in a low-Ni high-Mn super duplex stainless steel. Mater. Des. 2011, 32, 2199–2205. [Google Scholar] [CrossRef]
- Ayoub, H.; Lair, V.; Griveau, S.; Brunswick, P.; Bedioui, F.; Cassir, M. Electrochemical Characterization of Stainless Steel as a New Electrode Material in a Medical Device for the Diagnosis of Sudomotor Dysfunction. Electroanalysis 2012, 24, 1324–1333. [Google Scholar] [CrossRef]
- Kitte, S.A.; Gao, W.; Zholudov, Y.T.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Stainless steel electrode for sensitive luminol electrochemiluminescence detection of H2O2, glucose, and glucose oxidase activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef] [PubMed]
- Bimakr, F.; Ginige, M.P.; Kaksonen, A.H.; Sutton, D.C.; Puzon, G.J.; Cheng, K.Y. Assessing graphite and stainless-steel for electrochemical sensing of biofilm growth in chlorinated drinking water systems. Sens. Actuators B Chem. 2018, 277, 526–534. [Google Scholar] [CrossRef]
- Faria, R.A.D.; Luiz, G.; Dias, H.; Luiz, G.D.H.; Vanessa de, F.C.L. AISI 304 Stainless Steel as a Transducer Substrate in Electrochemical Biosensors for Medical Applications. Biomed. J. Sci. Tech. Res. 2019, 18, 13382–13388. [Google Scholar] [CrossRef]
- Martínez-Ibernón, A.; Lliso-Ferrando, J.; Gandía-Romero, J.M.; Soto, J. Stainless Steel Voltammetric Sensor to Monitor Variations in Oxygen and Humidity Availability in Reinforcement Concrete Structures. Sensors 2021, 21, 2851. [Google Scholar] [CrossRef]
- González, E.N.A.; Puente, D.S.L.; Muñiz, F.M.C.; Flores, S.E.; Martínez-Luévanos, A. Electrochemical Fabrication of a GO-NiO/AISI 316L Electrode and Its Evaluation for Glucose Detection. Int. J. Electrochem. Sci. 2021, 16, 21066. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D.; Kumara Swamy, B.E. Electrochemical investigation of duplex stainless steel at carbon paste electrode and its application to the detection of dopamine, ascorbic and uric acid. Int. J. Sci. Eng. Res. 2015, 6, 1863–1871. [Google Scholar]
- Shashanka, R.; Chaira, D.; Kumara Swamy, B.E. Electrocatalytic Response of Duplex and Yittria Dispersed Duplex Stainless Steel Modified Carbon Paste Electrode in Detecting Folic Acid Using Cyclic Voltammetry. Int. J. Electrochem. Sci. 2015, 10, 5586–5598. [Google Scholar] [CrossRef]
- Liu, H.; Syama, L.; Zhang, L.; Lee, C.; Liu, C.; Dai, Z.; Yan, Q. High-entropy alloys and compounds for electrocatalytic energy conversion applications. SusMat 2021, 1, 482–505. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D.; Kumara Swamy, B.E. Fabrication of yttria dispersed duplex stainless steel electrode to determine dopamine, ascorbic and uric acid electrochemically by using cyclic voltammetry. Int. J. Sci. Eng. Res. 2016, 7, 1275–1285. [Google Scholar]
- Rajendrachari, S.; Adimule, V.M.; Jayaprakash, G.K.; Pandith, A. Electrochemical oxidation of methylene blue dye in wastewater using mechanically alloyed high entropy alloy modified carbon paste electrode using cyclic voltammetry. Mater. Res. Express 2023, 10, 054003. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Kudur Jayaprakash, G.; Pandith, A.; Karaoglanli, A.C.; Uzun, O. Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles. Catalysts 2022, 12, 994. [Google Scholar] [CrossRef]
- Shashanka, R.; Jayaprakash, G.K.; Prakashaiah, B.G.; Kumar, M.; Swamy, B.K. Electrocatalytic determination of Ascorbic Acid using a Green Synthesised Magnetite Nano-Flake Modified Carbon Paste Electrode by Cyclic Voltammetric method. Mater. Res. Innov. 2021, 26, 229–239. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.K.; Rajendrachari, S.; Sharma, S.C.; Roberto, F.-M. Dual Descriptor Analysis of Cetylpyridinium modified Carbon Paste Electrodes for Ascorbic Acid sensing Applications. J. Mol. Liq. 2021, 334, 116348. [Google Scholar] [CrossRef]
- Mendonça, C.D.S.P.; Oliveira, A.F.; Oliveira, L.A.; da Silva, M.R.; Melo, M.D.L.N.M.; Silva, G. Structural and Magnetic Properties of Duplex Stainless steel (UNS S31803) Powders Obtained by high Energy Milling of Chips with Additions of NbC. Mater. Res. 2018, 21, e20170717. [Google Scholar] [CrossRef]
- Torres, C.; Johnsen, R.; Iannuzzi, M. Crevice corrosion of solution annealed 25Cr duplex stainless steels: Effect of Won critical temperatures. Corros. Sci. 2020, 178, 109053. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Chen, H.; Xiao, X.; Zhao, J.; Jiang, L. New Economical 19Cr Duplex Stainless Steels. Met. Mater. Trans. A 2011, 43, 428–436. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef]
- He, L.; Wu, X.; Zhang, Z.; Li, J. Microstructure Evolution and Corrosion Behavior of Duplex Stainless Steel During Isothermal Aged at 650 °C. Int. J. Electrochem. Sci. 2016, 11, 8046–8056. [Google Scholar] [CrossRef]
- Li, Z.; Wei, F.; La, P.; Wang, H.; Wei, Y. Enhancing Ductility of 1045 Nanoeutectic Steel Prepared by Aluminothermic Reaction through Annealing at 873K. Adv. Mater. Sci. Eng. 2017, 2017, 5392073. [Google Scholar] [CrossRef]
- Mao, Y.; Zheng, Y.; Shi, Y.; Zhu, M.; Saitejin; Liu, S.; Lin, X.; La, P. Effect of rolling deformation on microstructure and mechanical properties of 2205 duplex stainless steel with micro-nano structure. Mod. Phys. Lett. B 2020, 34, 2050269. [Google Scholar] [CrossRef]
- Nayak, A.K.; Shashanka, R.; Chaira, D. Effect of Nanosize Yittria and Tungsten Addition to Duplex Stainless Steel During High Energy Planetary Milling. IOP Conf. Ser. Mater. Sci. Eng. 2016, 115, 012008. [Google Scholar] [CrossRef]
- Shashanka, R. Synthesis of nano-structured stainless-steel powder by mechanical alloying-an overview. Int. J. Sci. Eng. Res. 2017, 8, 588–594. [Google Scholar]
- Dharmalingam, G.; Prasad, M.A.; Salunkhe, S. Optimization of milling speed and time in mechanical alloying of ferritic ODS steel through taguchi technique. Int. J. Simul. Multidisci. Des. Optim. 2021, 12, 25. [Google Scholar] [CrossRef]
- Gupta, S.; Shashanka, R.; Chaira, D. Synthesis of nano-structured duplex and ferritic stainless-steel powders by planetary milling: An experimental and simulation study. IOP Conf. Ser. Mater. Sci. Eng. 2014, 75, 012033. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Basavegowda, N.; Vinaykumar, R.; Narsimhachary, D.; Somu, P.; Lee, M.-J. Electrocatalytic determination of methyl orange dye using mechanically alloyed novel metallic glass modified carbon paste electrode by cyclic voltammetry. Inorg. Chem. Commun. 2023, 155, 111010. [Google Scholar] [CrossRef]
- Reddy, S.; Kumara Swamy, B.E.; Aruna, S.; Mohan, K.; Shashanka, R.; Jayadevappa, H. Preparation of NiO/ZnO hybrid nanoparticles for electrochemical sensing of dopamine and uric acid. Chem. Sens. 2012, 2, 7. [Google Scholar]
- Rajendrachari, S.; Adimule, V.; Gulen, M.; Khosravi, F.; Somashekharappa, K.K. Synthesis and Characterization of High Entropy Alloy 23Fe-21Cr-18Ni-20Ti-18Mn for Electrochemical Sensor Applications. Materials 2022, 15, 7591. [Google Scholar] [CrossRef]
- Manjunatha, J.G.; Deraman, M.; Basri, N.H.; Talib, I.A. Fabrication of poly (Solid Red A) modified carbon nano tube paste electrode and its application for simultaneous determination of epinephrine, uric acid and ascorbic acid. Arab. J. Chem. 2018, 11, 149–158. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Raril, C.; Tighezza, A.M.; Albaqami, M.D.; Mika, S. Electrochemically polymerized glutamine-activated graphite paste surface as a green biosensor for sensitive catechol detection in water samples. J. Mater. Sci. Mater. Electron. 2023, 34, 533. [Google Scholar] [CrossRef]
- Alemu, T.; Zelalem, B.; Amare, N. Voltammetric Determination of Ascorbic Acid Content in Cabbage Using Anthraquinone Modified Carbon Paste Electrode. J. Chem. 2022, 2022, 7154170. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Nagaraj, B.; Adimule, V.M.; Baris, A.; Prathap, S.; Saravana Kumar, R.M.; Kwang-Hyun, B. Assessing the Food Quality Using Carbon Nanomaterial Based Electrodes by Voltammetric Techniques. Biosensors 2022, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsabour, M.; Keriman, M.; Abd-Elsabur, F.H.; Hasan, A.; Ibrahem, M.A. An Electrochemical Sensor Based on Poly(methyl orange) Modified Glassy Carbon Electrode for Simultaneous Determination of Vitamins B2 and C in Aqueous Solution. Anal. Bioanal. Chem. Res. 2022, 9, 259–268. [Google Scholar]


| Elemental Composition | Electrochemical Technique | Chemical Constituent Used | Observations | Reference No. |
|---|---|---|---|---|
| Fe-18Cr-13Ni | Cyclic voltammetry | Dopamine, ascorbic acid, and uric acid | Since the 4 mg duplex modified carbon paste electrode exhibits a maximal anodic peak current of 25.61 µA, it is utilized as a modifier to examine the electrochemical characteristics of dopamine, ascorbic acid, and uric acid. | [39] |
| Fe-18Cr-13Ni | Cyclic voltammetry | Folic acid | The electrode process is controlled by electrode diffusion, and the anodic peak current rises linearly with correlation coefficient. The oxidation peak current is found to be 8.67 µA at 50 mV s−1 and 17.32 µA at 300 mV s−1, respectively. | [40] |
| 23Fe-21Cr-18Ni-20Ti-18Mn High Entropy Alloy | Cyclic voltammetry | Ascorbic Acid | For the concentration of 8 mg modifier, a maximum peak current of 104.07 µA was measured. For the high entropy alloy modified carbon paste electrode and the bare carbon paste electrode, the active surface areas for the electron transfer process of ascorbic acid are calculated to be 0.0014 cm2 and 0.0027 cm2, respectively. | [41] |
| Fe-18Cr-13Ni | Cyclic voltammetry | Dopamine, ascorbic acid, and uric acid | The 8 mg yttria dispersed duplex stainless steel modified carbon paste electrode has an anodic peak current of 31.01 µA, demonstrating significant electrocatalytic activity towards the oxidation of dopamine, ascorbic acid, and uric acid. | [42] |
| 25Fe-19Cr-19Ni-18Ti-19Mn High Entropy Alloy | Cyclic voltammetry | Methylene Blue | The anodic peak current of 508.4 µA was displayed by the 4 mg high entropy alloy modified carbon paste electrode, while only 99.74 µA was displayed by the bare carbon paste electrode. This significant anodic peak current difference between the two different electrodes has demonstrated the value of the modifier in enhancing the electrode sensor’s sensitivity, robustness, and selectivity. | [43] |
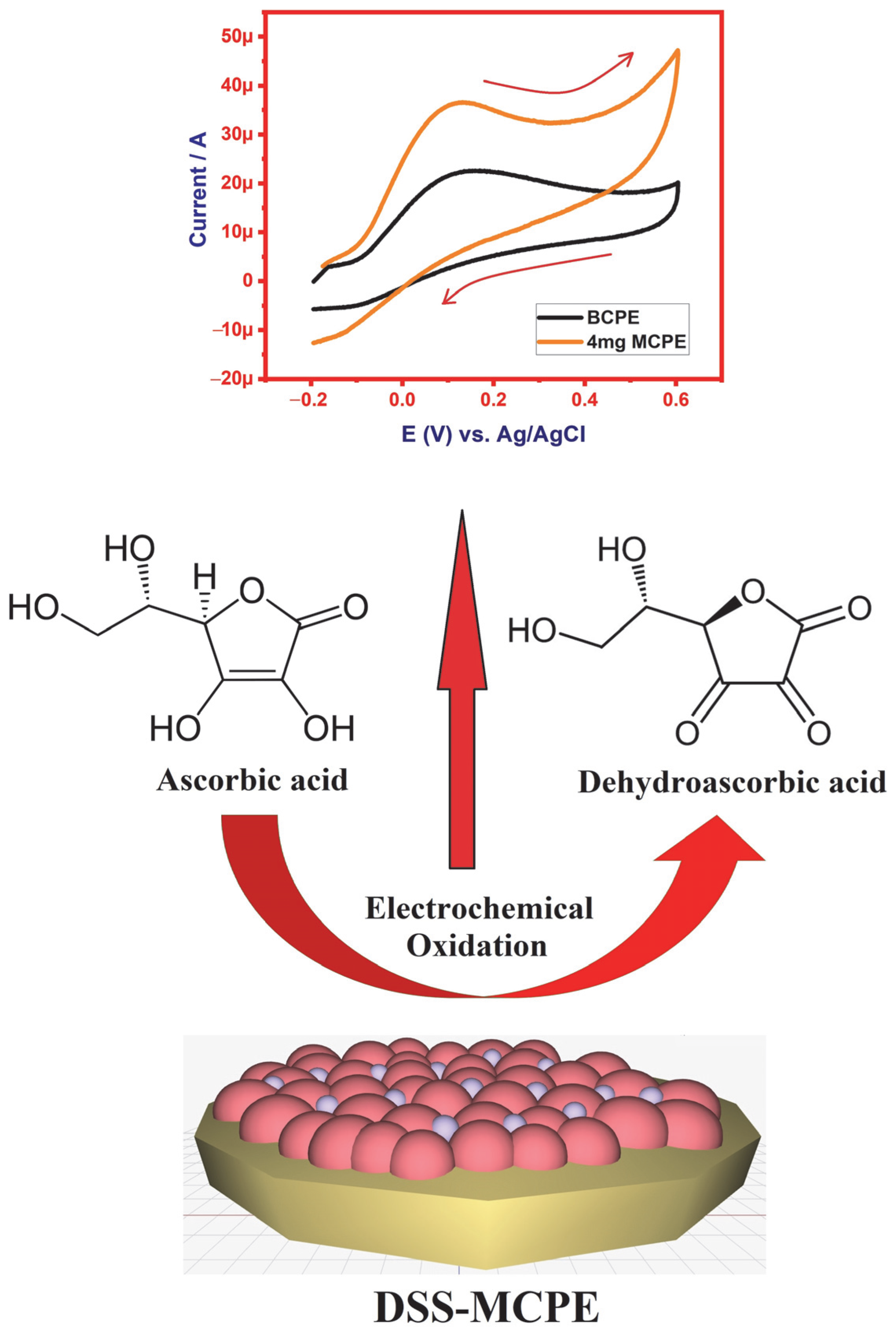
| 2507 super duplex stainless steel | Cyclic voltammetry | Ascorbic acid | BCPE has shown an anodic peak current of 22.5 µA and 4 mg SDSS-MCPE has recorded 37.2 µA of anodic peak current during the electro-oxidation of 1 mM AA | Current Paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahale, R.S.; Vasanth, S.; Chikkegouda, S.P.; Rajendrachari, S.; Narsimhachary, D.; Basavegowda, N. Electrochemical Determination of Ascorbic Acid by Mechanically Alloyed Super Duplex Stainless Steel Powders. Metals 2023, 13, 1430. https://doi.org/10.3390/met13081430
Mahale RS, Vasanth S, Chikkegouda SP, Rajendrachari S, Narsimhachary D, Basavegowda N. Electrochemical Determination of Ascorbic Acid by Mechanically Alloyed Super Duplex Stainless Steel Powders. Metals. 2023; 13(8):1430. https://doi.org/10.3390/met13081430
Chicago/Turabian StyleMahale, Rayappa Shrinivas, Shamanth Vasanth, Sharath Peramenahalli Chikkegouda, Shashanka Rajendrachari, Damanapeta Narsimhachary, and Nagaraj Basavegowda. 2023. "Electrochemical Determination of Ascorbic Acid by Mechanically Alloyed Super Duplex Stainless Steel Powders" Metals 13, no. 8: 1430. https://doi.org/10.3390/met13081430
APA StyleMahale, R. S., Vasanth, S., Chikkegouda, S. P., Rajendrachari, S., Narsimhachary, D., & Basavegowda, N. (2023). Electrochemical Determination of Ascorbic Acid by Mechanically Alloyed Super Duplex Stainless Steel Powders. Metals, 13(8), 1430. https://doi.org/10.3390/met13081430









