Abstract
Cord steel is used for making tire frames and wire saws for cutting silicon wafers. The diameter of mainstream cutting wire has been developed to be lower than 100 μm. The size and deformation ability of inclusions are very important to the wire breaking rate of cord steel during the drawing process. In order to improve the deformation ability of the inclusions in cord steel, alkali metal oxide was added into the molten steel to improve the inclusions in the steel so as to obtain good, plastic, low-melting-point inclusions. Mass fractions of 0.3%, 0.5% and 1.0% K2CO3, Na2CO3 and B2O3 were added into cord steel, which were melted in 10 furnaces (including 0% alkali metal oxides, mass fractions of 0.3%/0.5%/1.0% K2CO3, Na2CO3 and B2O3). The morphology and composition of inclusions were observed by SEM-EDS. Factsage phase diagram calculations and experimental results show that, with the increase in Na2CO3 content in cord steel, the aluminum content in the inclusions gradually decreased. When the mass fraction of Na2CO3 was 0.5% per ton, most of the inclusions in the steel fell in the low melting point region (less than 1300 °C). With the increase in K2CO3 content in cord steel, the silicon content in the inclusions decreased gradually. When the mass fraction of K2CO3 was 0.5% per ton, most of the inclusions in the steel fell in the low melting point region. The deformation ability of the inclusions added with 0.5% Na2CO3 in the steel during forging was better than that of the inclusions added with 0.5% K2CO3. After adding B2O3, the inclusions in the steel were SiO2-MnO-Al2O3 inclusions or inclusions with SiO2-MnO-Al2O3 as the core and BN wrapped around. Boron could not be dissolved into the inclusions for plastic modification.
1. Introduction
Cord steel wire is widely used in industry because of its excellent performance, a d is used in the bridge industry to make the main load-bearing component cables, in the tire industry to make tire skeletons [1,2] and in the photovoltaic industry for cutting silicon wafers [3,4]. The diameter of steel cord is 80~200 μm, which is for the main skeleton of tires, and the diameter of cutting steel wire is 50~80 μm, which is mainly used for cutting precious materials such as solar silicon wafers, gems and aviation semiconductors [5]. In the process of cutting silicon wafers, the diameter of the cutting steel wire determines the amount of debris loss of material. Therefore, in order to reduce the amount of debris loss as much as possible, the smaller the diameter of the cutting wire, the better. At present, the diameter of mainstream cutting steel wire in the market has reached lower than 100 μm. The most common reason for the fracture of steel cord and cutting steel wire is the presence of large hard inclusions in steel. Si-Mn combined deoxidization or Si-Ca combined deoxidization is usually used in the refining process of cord steel, and the deoxidization products are a MnO-Al2O3-SiO2 system and a CaO-Al2O3-SiO2 system [6,7,8,9,10]. Among them, Al2O3 inclusion is the most important component, which occupies a high content; it most affects the deformation of cord steel in the processing process and the wire breaking rate in the process of drawing and hot rolling. So, on the one hand, we want to reduce the content of Al2O3 in the inclusion because the high content of Al2O3 makes the inclusion harder. On the other hand, we also hope that the size of these kinds of hard, high-melting-point inclusions can be reduced, and the wire breaking rate of cord steel can be reduced to a certain extent. But neither of these two aspects can solve the final problem. We think the most important thing is to reduce the melting point of the inclusion so that it can be softened or even liquid at rolling temperature, with good deformability—that is, the wire of the cord steel can be drawn thinner and continuously [11].
The control of inclusions is not only limited to the refining [12,13,14,15,16,17] and solidification processes [18,19,20], but also needs to pay attention to the evolution behavior of inclusions in the hot working process [21]. And in the study of steel samples, it was also found that there are hard, high-melting-point Al2O3 inclusions. The melting point of the inclusion decreases, and it softens at the rolling temperature, which makes it easier to deform. The increase in inclusion deformability will reduce the wire breaking rate of cord steel and make its diameter smaller. Although the inclusion elongates and becomes larger in the rolling direction after the melting point of the inclusion is reduced, the size of the inclusion on the cross-section becomes smaller, so the wire breaking rate of the cord steel can be reduced. In recent years, the method of adding alkali metals to molten steel to modify inclusions in steel has attracted the attention of metallurgical workers for the purpose of improving the deformability of inclusions in cord steel [22,23]. Chen et al. [24] studied the inclusions in modified low-carbon steel by boron treatment and on this basis, they applied it to the smelting of cord steel [25]. By adding boron to molten steel, the low melting point regions of the inclusions in SiO2-MnO-Al2O3 and SiO2-CaO-Al2O3 systems were significantly expanded, thus improving the deformability of inclusions. However, the hot workability was easy to deteriorate when the mass fraction of boron in the steel was too high, so it must be strictly controlled at 0.005%. Chen et al. [26] studied the modification mechanism of inclusions in cord steel treated with Na2CO3 and K2CO3, and pointed out that the addition of Na2CO3 to molten steel could significantly improve the deformability of inclusions [27]. The main mechanism was that Na2O could sharply reduce the melting point of SiO2-MnO-Al2O3 inclusions and significantly expanded the low melting point region of SiO2-MnO-Al2O3 inclusions. Chen et al. [28] studied the effect of NaF treatment on inclusions in cord steel. The experimental results showed that after NaF treatment, the number of inclusions in the steel decreased, the average diameter decreased, a large number of inclusions containing Na appeared in the steel, and the melting point of the inclusions containing Na was lower, which was beneficial for improving the deformability of the inclusions. When smelting superfine steel wire [26,27,28,29], the effect of non-metallic inclusions in cord steel modified by alkali metals such as Na, K, B and their oxides was remarkable. In this paper, with the help of the Shagang Group, the effect of alkali metal carbonate on cord steel inclusions was studied.
To sum up, the influence of the types and contents of alkali metals on the inclusions in a SiO2-MnO-Al2O3 system still needs to be further studied. We aim to study which type of alkali metal is more beneficial for improving the deformability of cord steel inclusions and what is the most suitable content. Alkali metal was added to cord steel to reduce the melting point of its inclusions so as to improve the deformability of the inclusions and reduce the wire breaking rate of cord steel. In this paper, the influence of Na2CO3, K2CO3 and B2O3 addition on the composition of SiO2-MnO-Al2O3 inclusions in steel is studied, and the evolution law of the inclusions after adding alkali metals is pointed out, which provides research basis and theoretical guidance for the production of cord steel.
2. Experimental
The raw material of cord steel comes from the Shagang Iron and Steel Group, and its production process was KR (Kambara reactor) → BOF (basic oxygen furnace) → LF (ladle furnace) → CC (continuous casting). Among them, the KR process was desulfurized for molten iron and then transported to a converter for desilication, decarbonization and dephosphorization, and low titanium and low aluminum ferrosilicon were added in the converter tapping → silicomanganese → high-carbon ferrochrome → deoxidation and alloying of synthetic slag and slagging. Then, it was transported to the LF for refining, fine-tuning the composition of molten steel and slag, electrifying to adjust the temperature, and then it was transported to continuous casting and pouring after reaching the standard. The compositions of cord steel and refining slag are shown in Table 1 and Table 2, respectively.

Table 1.
Chemical composition of cord steel/mass%.

Table 2.
Chemical composition of slag/mass%.
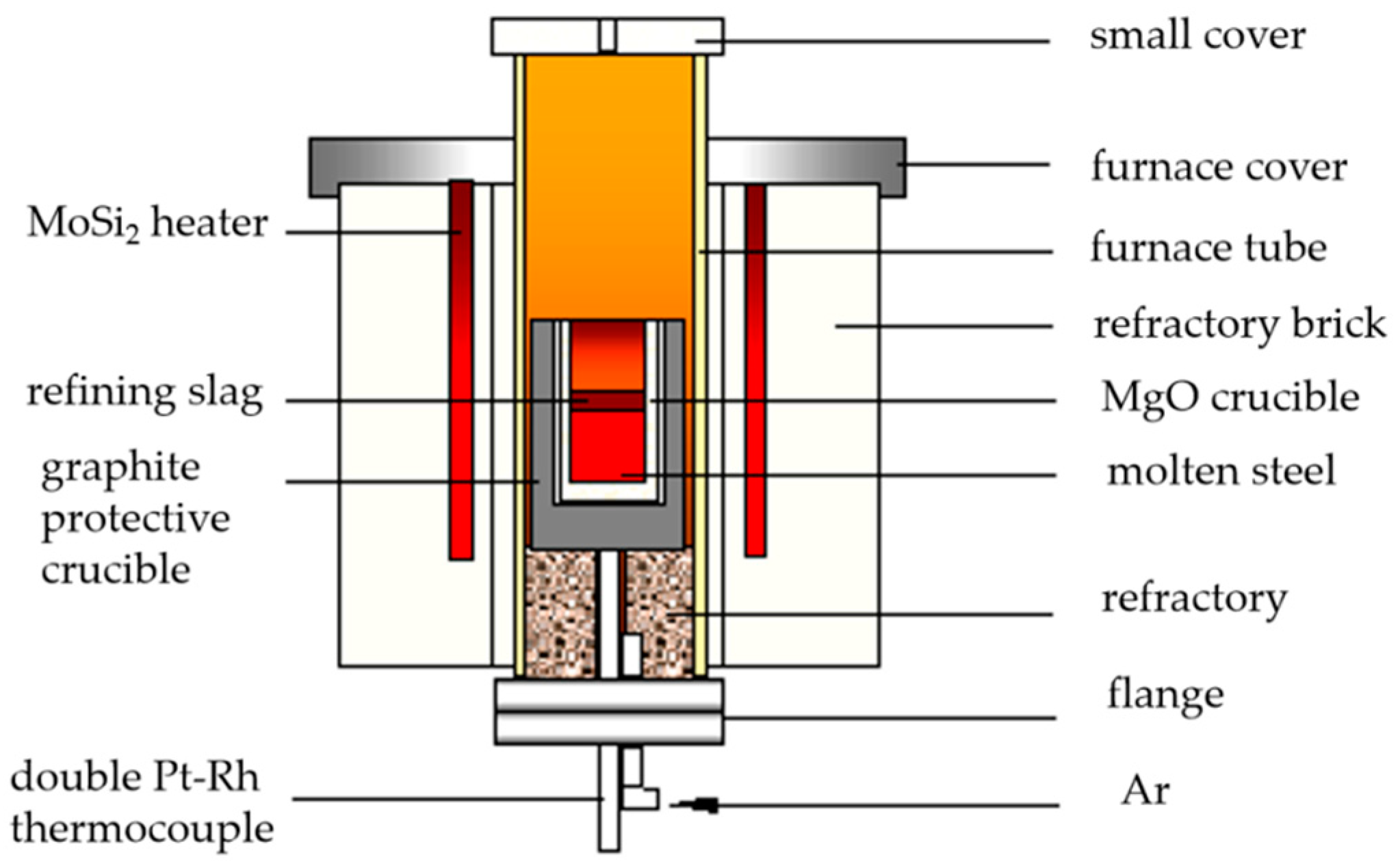
The experiment of adding alkali metal carbonate to cord steel was carried out in a laboratory resistance furnace, and the device is shown in Figure 1. The test steps were as follows:
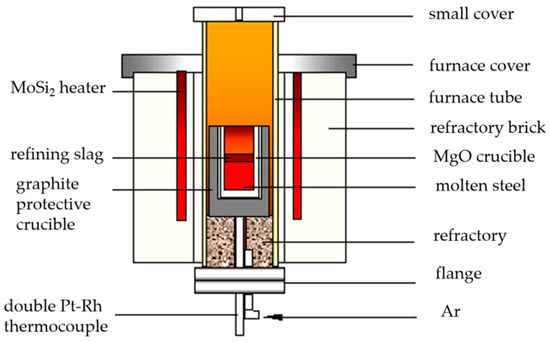
Figure 1.
Schematic diagram of MoSi2 resistance furnace.
- (1)
- Mix and briquette the alkali metal carbonate and iron powder at a mass ratio of 1:1.
- (2)
- Put the cord steel sample with the mass of 600 g (the original sample comes from the hot-rolled bar produced by Shagang) into an MgO crucible, and put the outer graphite crucible into the MoSi2 resistance furnace to heat up with electricity. The argon flow rate is 2.5 L/min.
- (3)
- Raise the temperature to 1540 °C, and keep the temperature constant for 10 min.
- (4)
- Add the mixed compact of alkali metal and iron powder into the molten steel for 20 min, and stop the power supply and cool after the test.
- (5)
- Use a scanning electron microscope SEM-EDS (Carl Zeiss Microscopy, Oberkochen, Germany) to analyze the composition and types of inclusions in the steel samples cut from a 600 g cord steel ingot.
- (6)
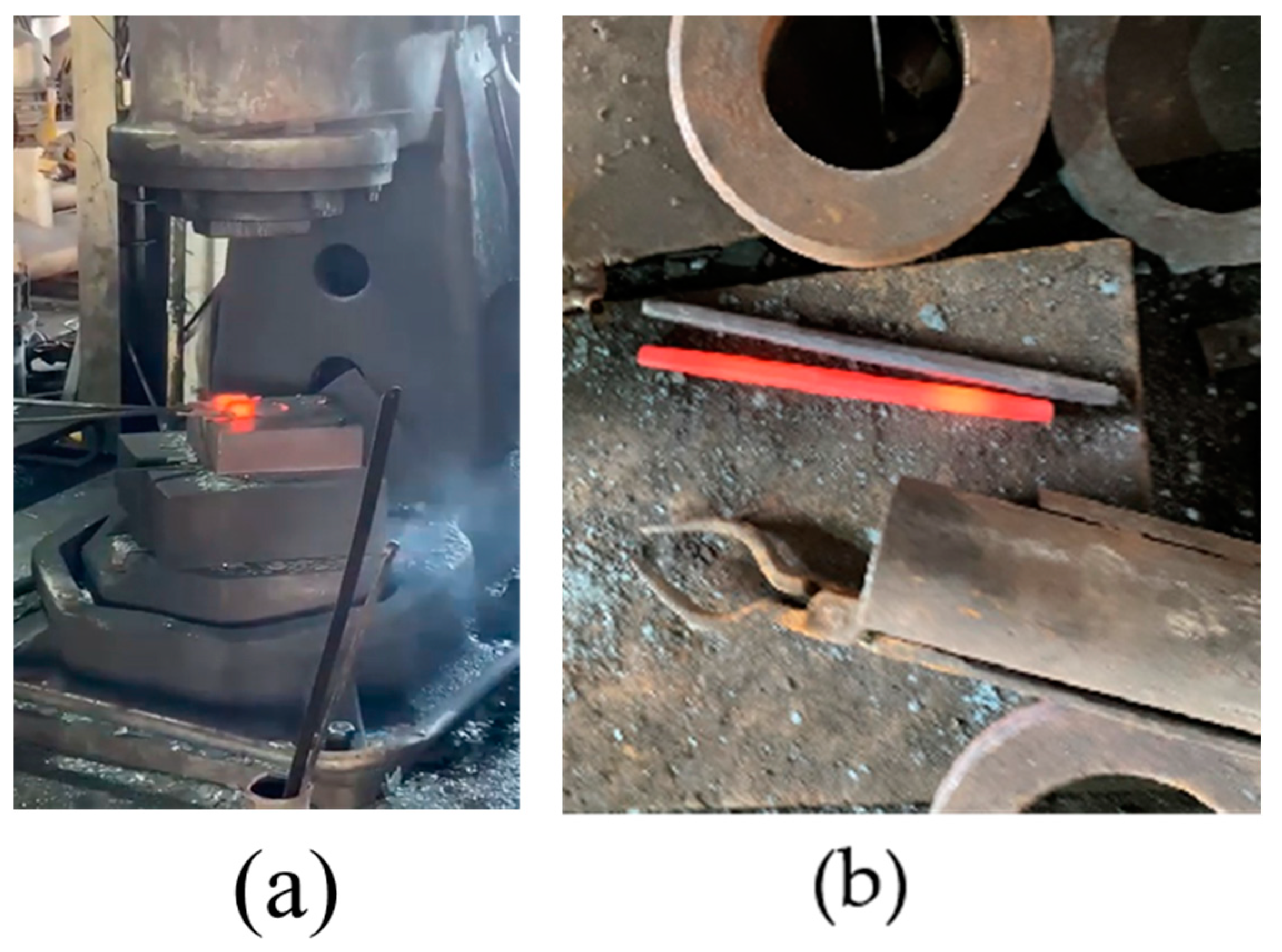
- Forge the solidified 600 g cord steel after holding at 1200 °C for 1 h, as shown in Figure 2b.
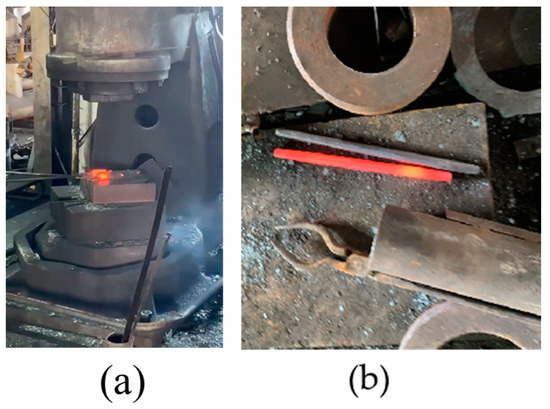 Figure 2. (a) Forging process of 600 g steel sample, and (b) pictures after forging.
Figure 2. (a) Forging process of 600 g steel sample, and (b) pictures after forging. - (7)
- Analyze the deformability of inclusions during thermal deformation with a SEM-EDS.
2.1. Test Process of Adding Na2CO3 to Cord Steel
The mass ratio of Na2CO3 and iron powder was 1:1 for mixed briquetting, and the additional amounts of Na2CO3 were 0.3%, 0.5% and 1.0% of the steel sample. Totals of 1.8 g, 3.0 g and 6.0 g of Na2CO3 were added to the 600 g steel sample, that is, 3.6 g, 6.0 g and 12.0 g of Na2CO3 and iron powder-mixed compacts were added to the 600 g steel sample, and three groups of experiments were conducted to add Na2CO3 to the cord steel.
2.2. Test Process of Adding K2CO3 to Cord Steel
The mass ratio of K2CO3 and iron powder was 1:1 for mixed briquetting, and the additional amounts of K2CO3 were 0.3%, 0.5% and 1.0% of the steel sample. Totals of 1.8 g, 3.0 g and 6.0 g of K2CO3 were added to the 600 g steel sample, that is, 3.6 g, 6.0 g and 12.0 g of K2CO3 and iron powder-mixed compacts were added to the 600 g steel sample, and three groups of experiments were conducted to add K2CO3 to the cord steel.
2.3. Test Process of Adding B2O3 to Cord Steel
The mass ratio of B2O3 and iron powder was 1:1 for mixed briquetting, and the additional amounts of B2O3 were 0.3%, 0.5% and 1.0% of the steel sample. Totals of 1.8 g, 3.0 g and 6.0 g of B2O3 were added to the 600 g steel sample, that is, 3.6 g, 6.0 g and 12.0 g of B2O3 and iron powder-mixed compacts were added to the 600 g steel sample, and three groups of B2O3 added to the cord steel were tested.
3. Results and Discussion
3.1. Inclusion Types of Na2CO3, K2CO3 and B2O3 Added to Cord Steel
- (1)
- The influence of Na2CO3 addition on inclusion types of cord steel.
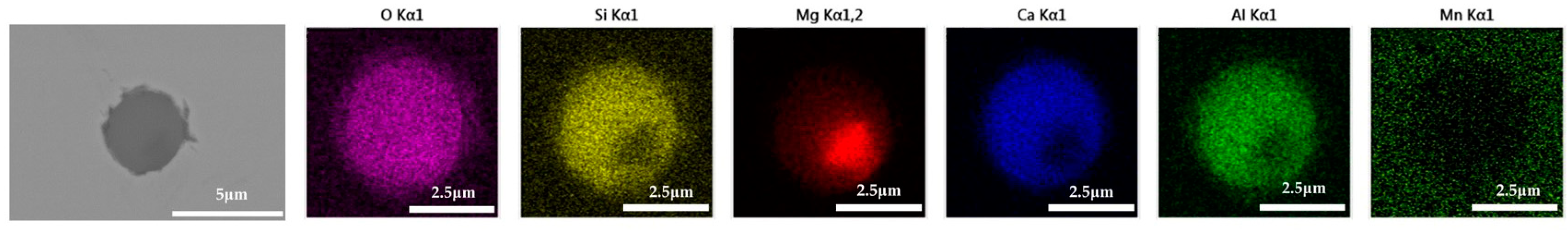
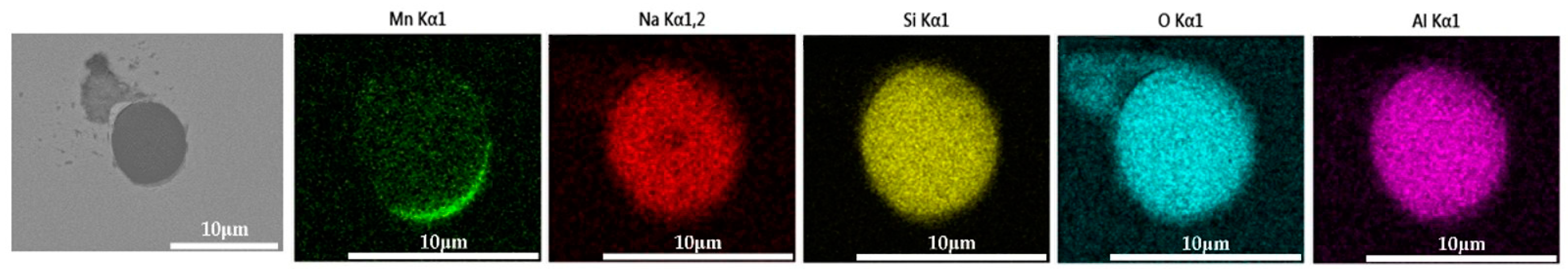
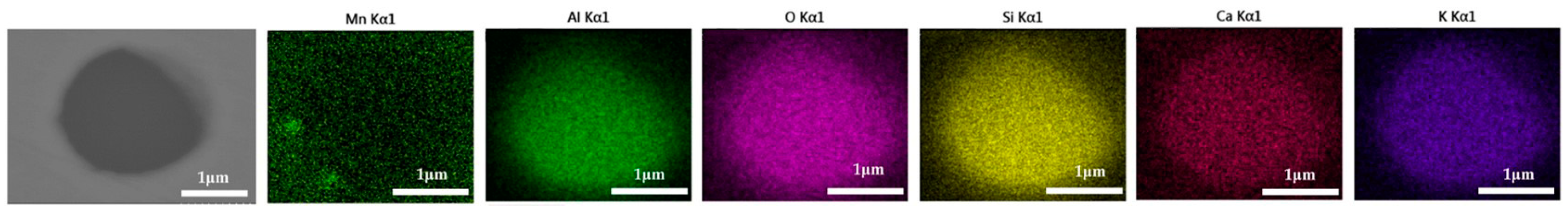
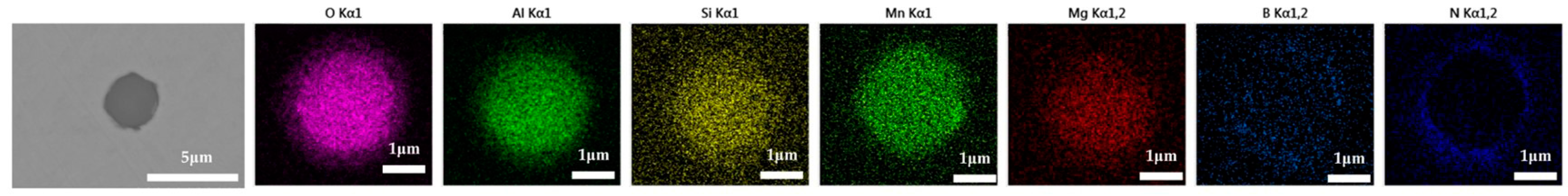
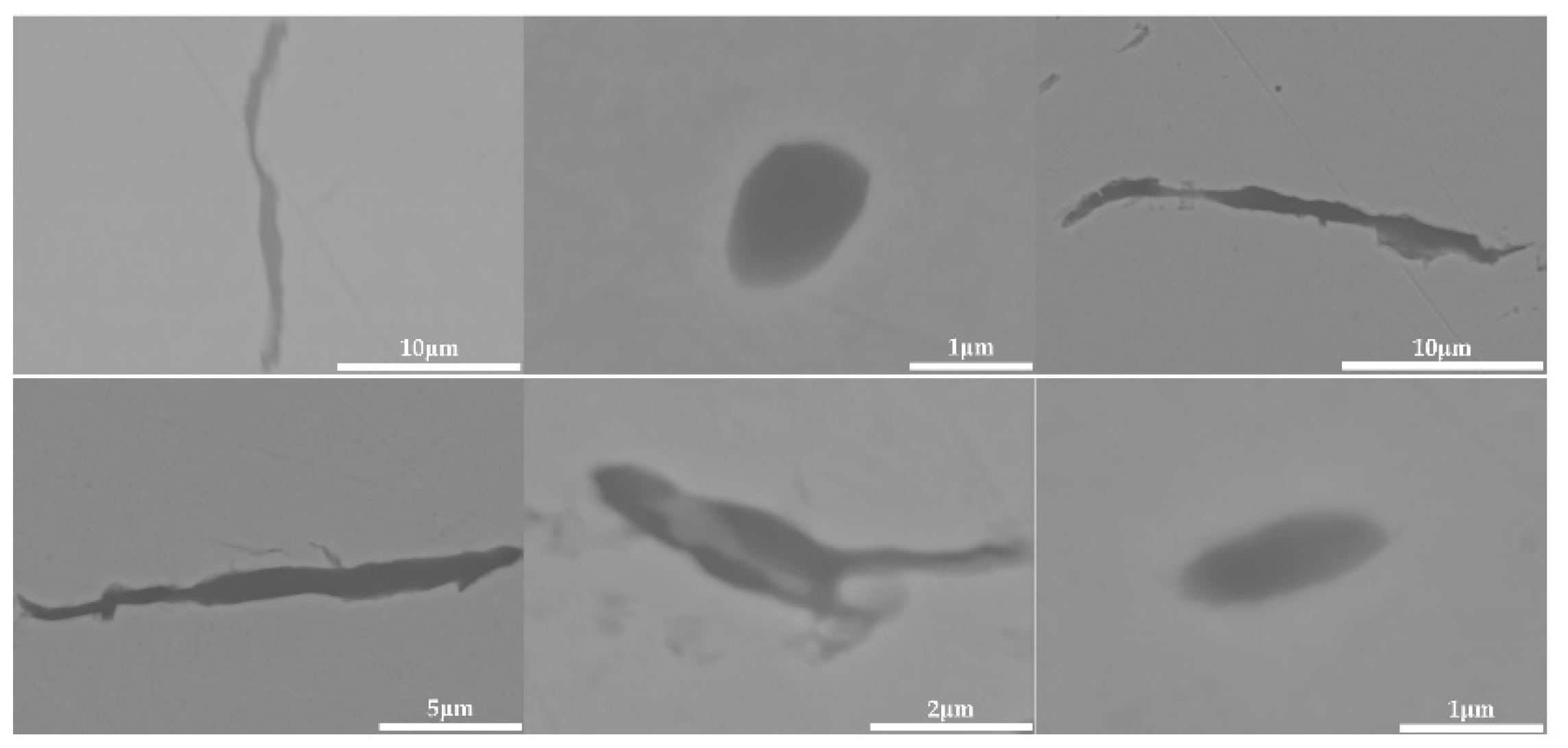
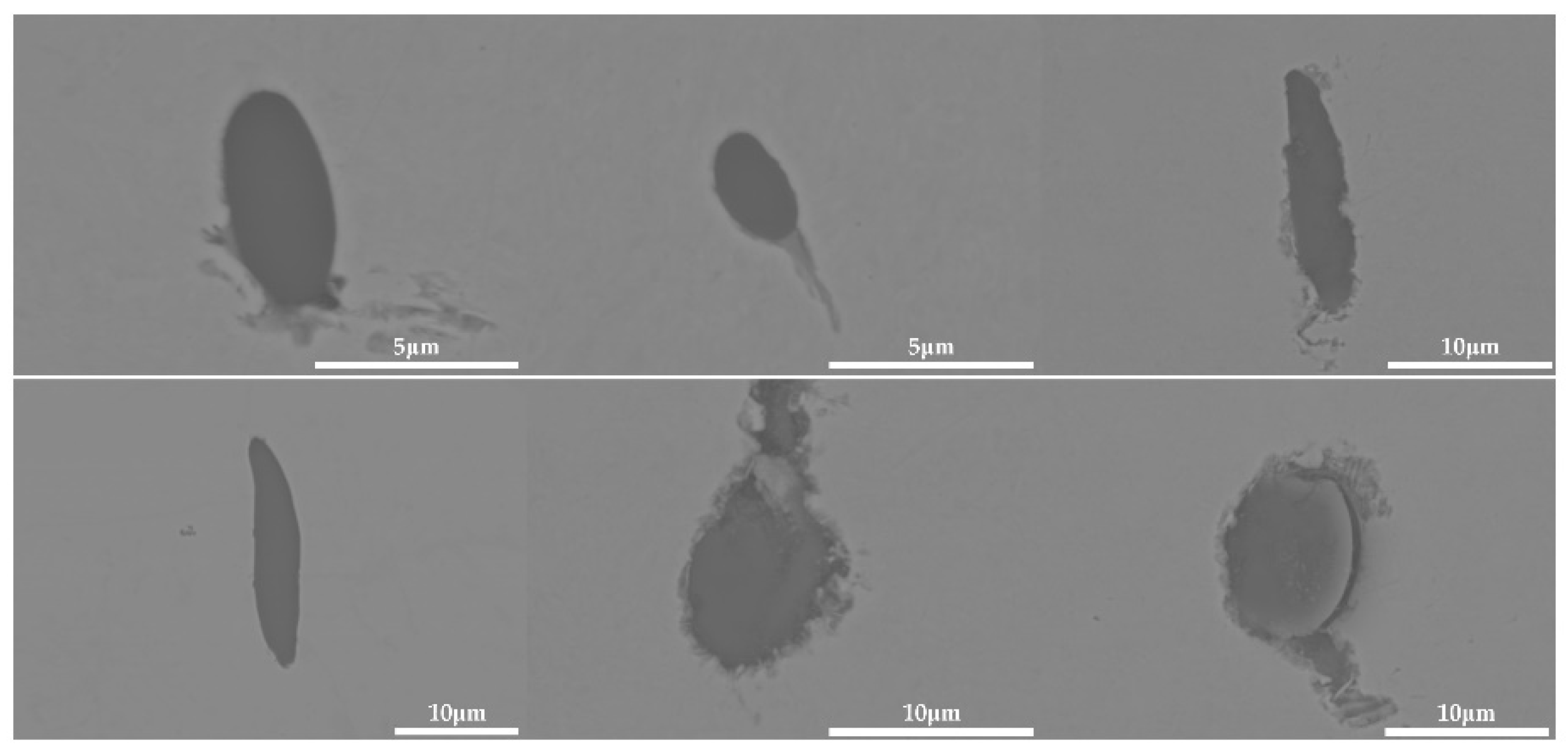
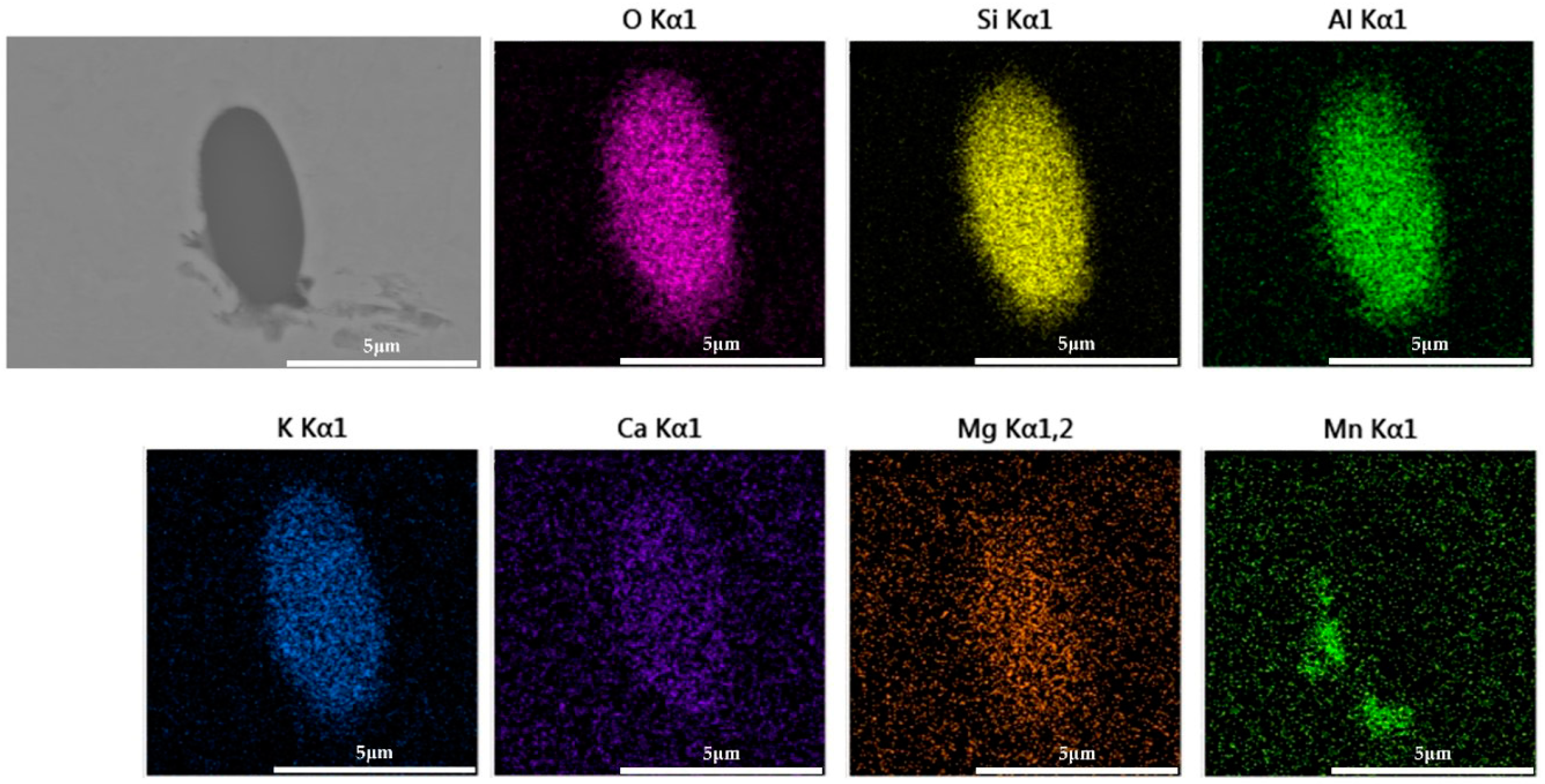
The 600 g steel sample without Na2CO3 added during the resistance furnace experiment process was analyzed by SEM-EDS, and the typical inclusion is shown in Figure 3. The scanning results of the inclusions in the steel sample with 0.3%, 0.5% and 1.0% Na2CO3 added are shown in Figure 4, Figure 5 and Figure 6, respectively. After adding alkali carbonate, the content of calcium in the inclusions in the sample decreased significantly (CaO from 15% to 8%). And with the increase in Na2CO3 content, the content of Na in the inclusions also increased significantly (Na2O from 0% to 6%). After comparing the inclusions obtained by SEM analysis between the blank samples and the samples with a different alkali metal to sodium carbonate, it was found that the size of the inclusion did not change significantly with the increase in the amount of sodium carbonate, but only increased slightly.

Figure 3.
SEM-EDS images of samples without Na2CO3.

Figure 4.
SEM-EDS images of samples with 0.3% Na2CO3.

Figure 5.
SEM-EDS images of samples with 0.5% Na2CO3.
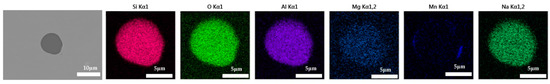
Figure 6.
SEM-EDS images of samples with 1.0% Na2CO3.
- (2)
- The influence of K2CO3 addition on inclusion types of cord steel.
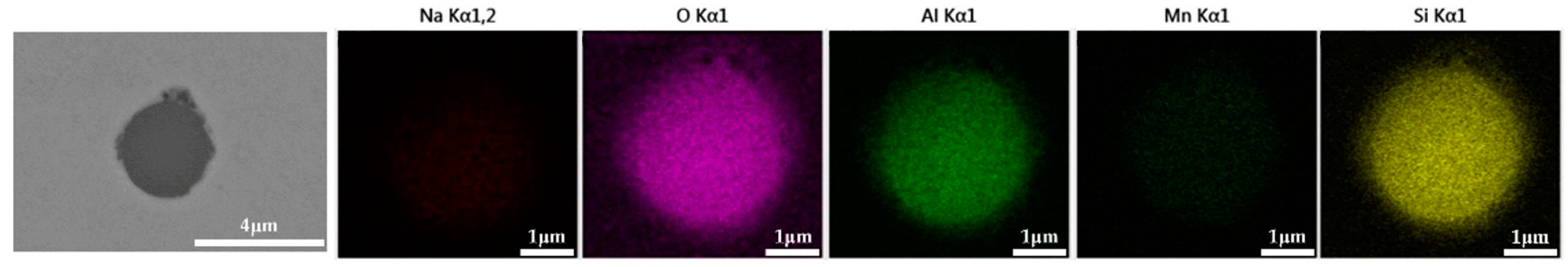
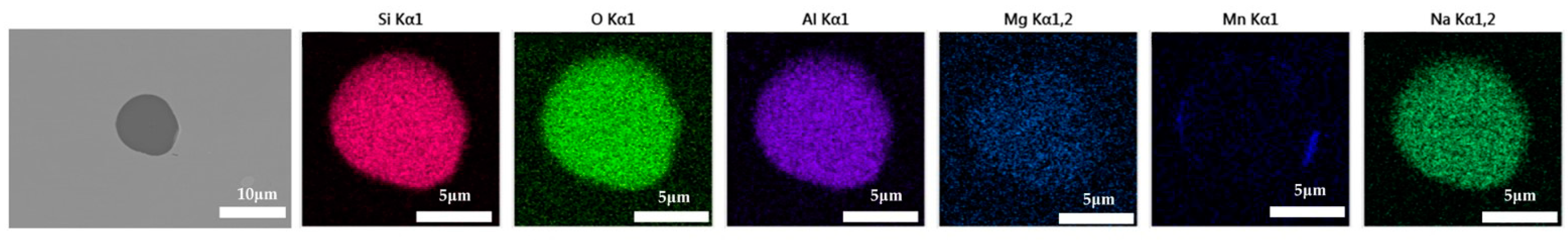
The 600 g steel sample without K2CO3 added during the resistance furnace experiment process was analyzed by SEM-EDS, and the typical inclusion is shown in Figure 7. The morphologies of the samples with 0.3%, 0.5% and 1.0% K2CO3 in the steel are shown in Figure 8, Figure 9 and Figure 10, respectively. After adding alkali carbonate, the calcium content in the inclusions in the sample decreased significantly (CaO from 15% to 8%). And with the increase in K2CO3 content, the content of K in the inclusions also increased significantly (from 0% to 6%). After comparing the inclusions obtained by SEM analysis between the blank samples and the samples with a different alkali metal to K2CO3, it was found that, similar to the addition of Na2CO3, the size of the inclusion in the sample with the addition of K2CO3 did not change significantly with the increase in potassium carbonate, but only increased slightly.

Figure 7.
SEM-EDS images of samples without K2CO3.

Figure 8.
SEM-EDS images of samples with 0.3% K2CO3.
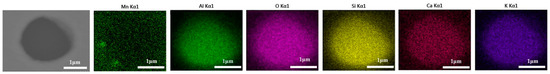
Figure 9.
SEM-EDS images of samples with 0.5% K2CO3.
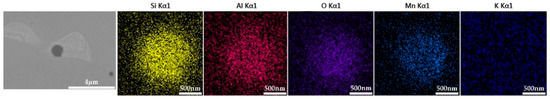
Figure 10.
SEM-EDS images of samples with 1.0% K2CO3.
- (3)
- The influence of B2O3 addition on inclusion types of cord steel.
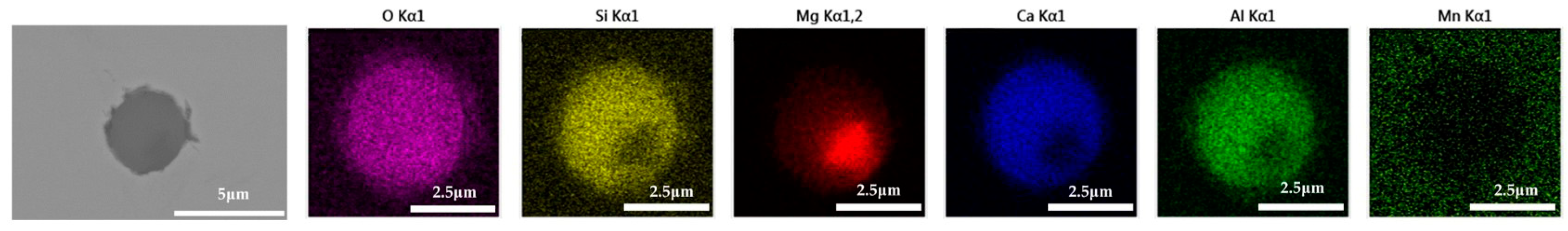
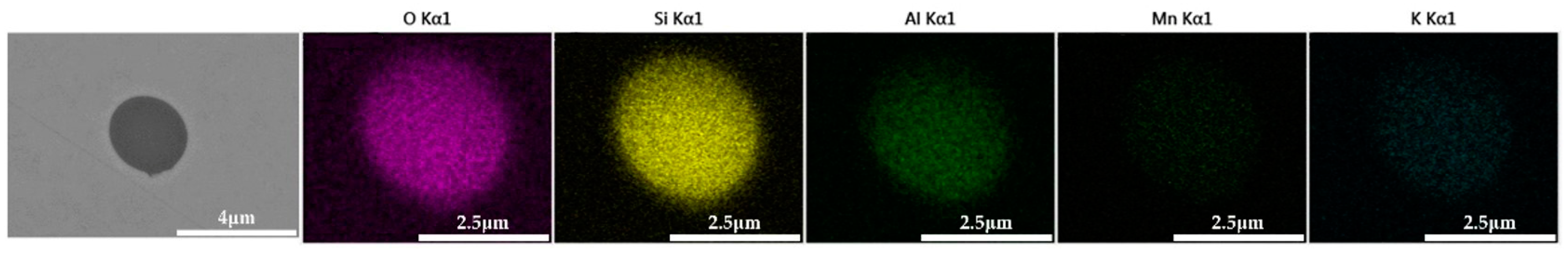
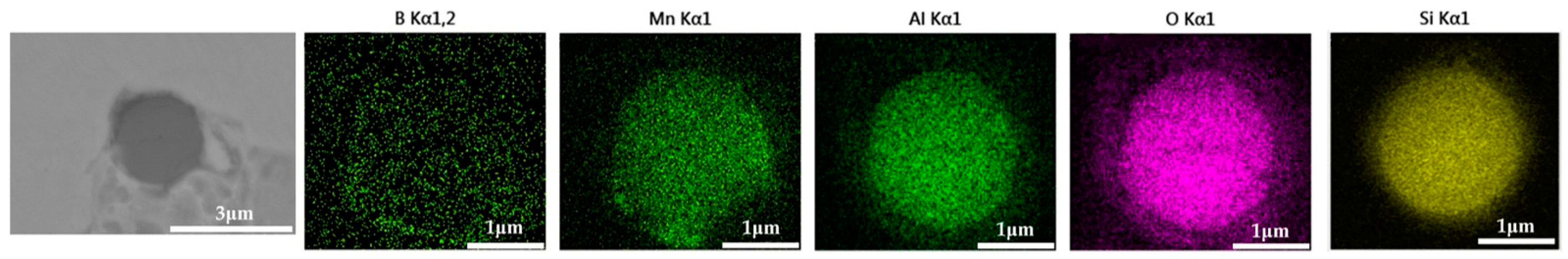
The 600 g steel sample with 0.3% and 1.0% B2O3 added during the resistance furnace experiment process was analyzed by SEM-EDS, and the typical inclusions are shown in Figure 11 and Figure 12, respectively. When the amount of B2O3 was small, the inclusions were boron-free inclusions in the SiO2-MnO-Al2O3 system. When excessive B2O3 was added, the inclusions were composite inclusions with SiO2-MnO-Al2O3 as the core and BN wrapped around. The boron element could not be incorporated into the original inclusion of SiO2-MnO-Al2O3 when adding a small amount or an excessive amount B2O3, so adding B2O3 could not reduce the melting point of the inclusion and could not effectively modify it.
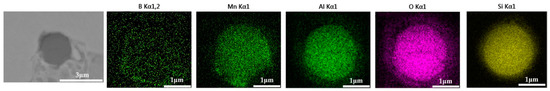
Figure 11.
SEM-EDS images of samples with 0.3% B2O3.
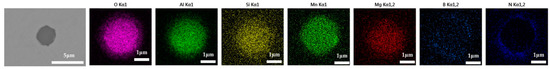
Figure 12.
SEM-EDS images of samples with 1.0% B2O3.
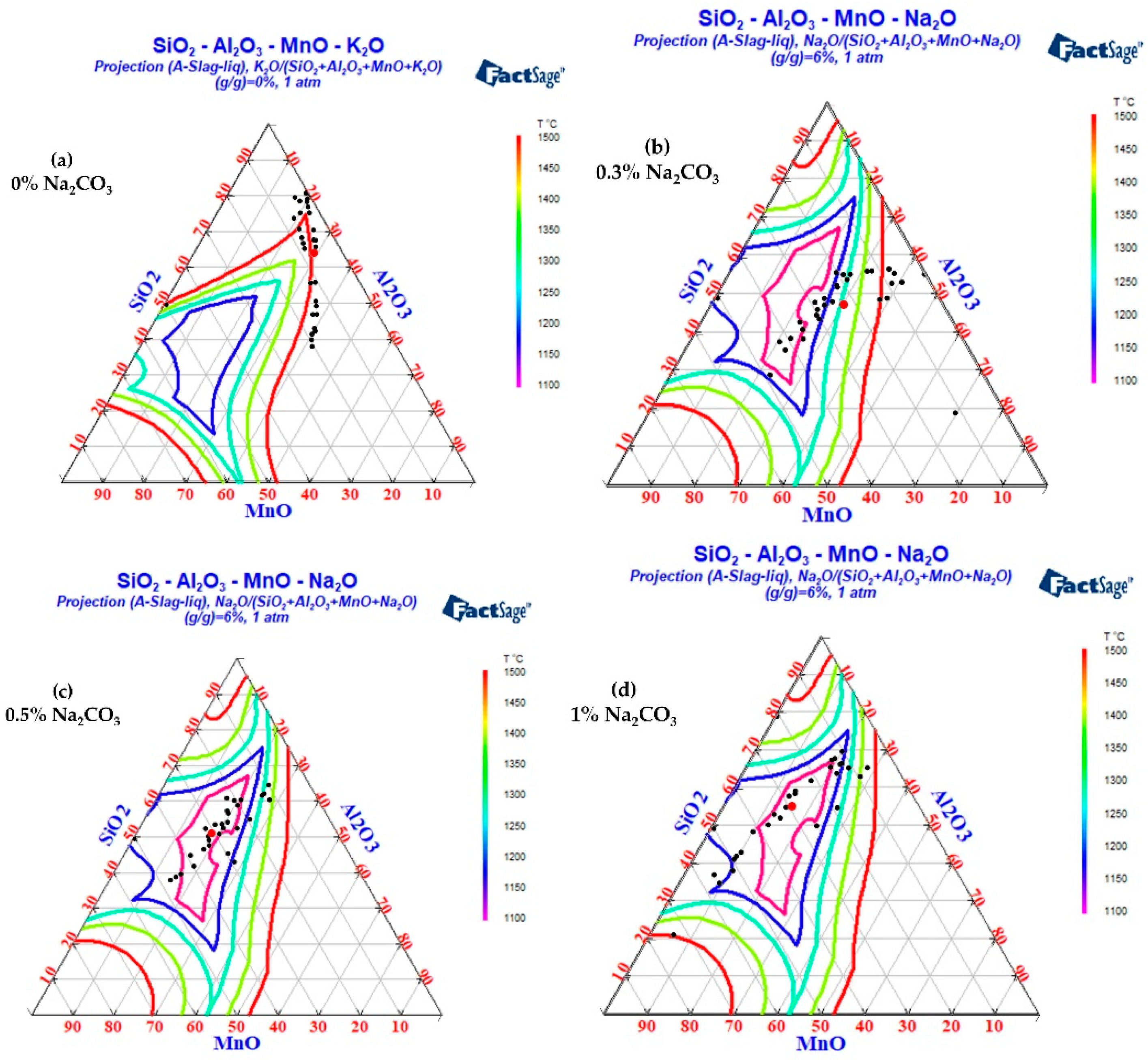
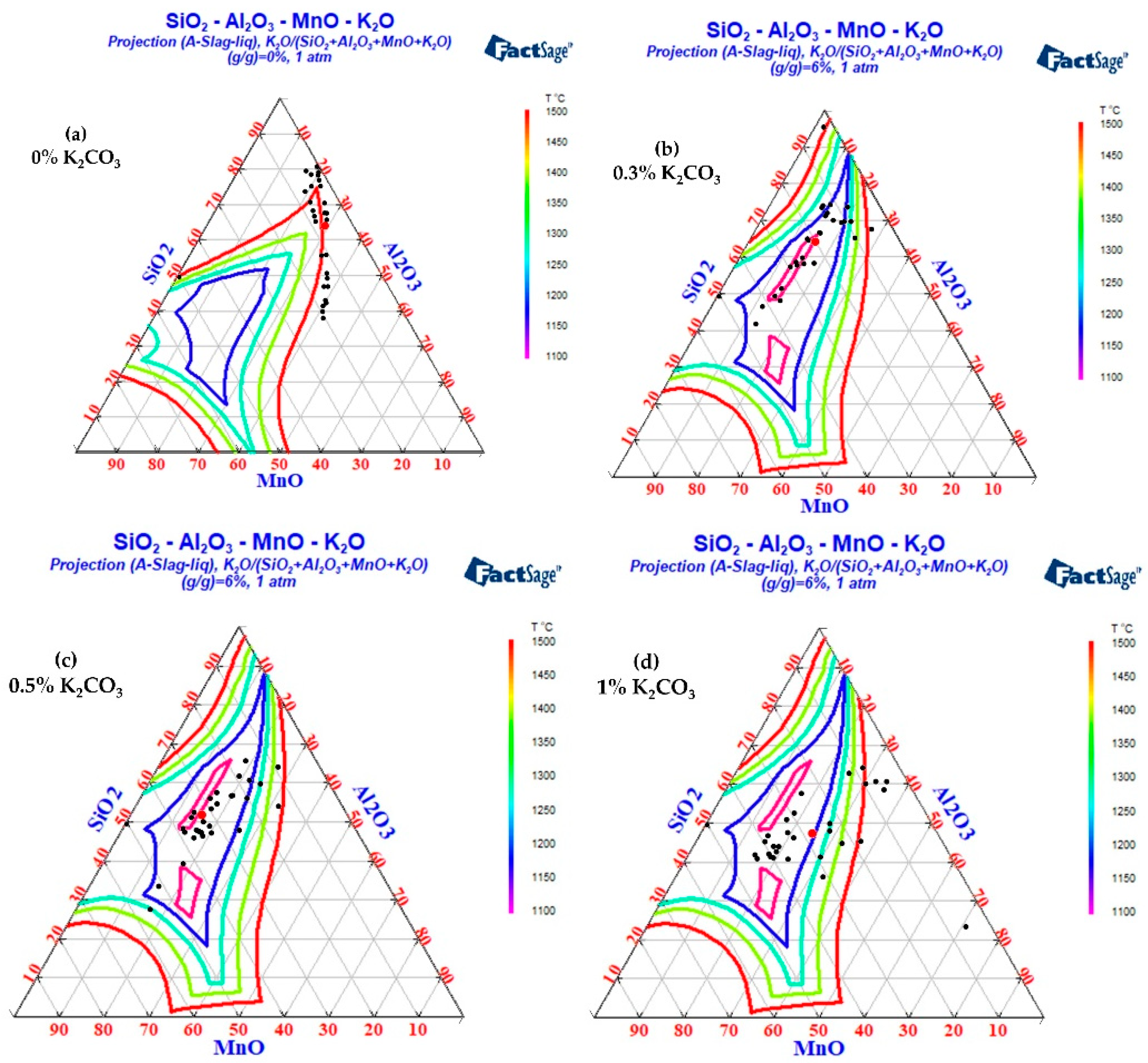
3.2. The Change in Inclusion Composition and Factsage Thermodynamic Calculation and Analysis after Na2CO3 and K2CO3 Are Added to Cord Steel
The FactPS and FToxid databases in Factsage thermodynamic software were used to calculate the phase diagrams of the SiO2-Al2O3-MnO-Na2O and SiO2-Al2O3-MnO-K2O systems. The inclusion components in the test results were counted into the phase diagram, as shown in Figure 13 and Figure 14. Because the addition of B2CO3 cannot be incorporated into the inclusions in the steel, the effect of B2CO3 on the low melting point phase diagram of the SiO2-Al2O3-MnO inclusions is not carried out here. When Na2CO3/K2CO3 was added to the steel, the inclusion composition also tended to the low melting point area in the phase diagram, and the experimental results were in good agreement with the calculation of the Factsage phase diagram. In addition, due to the low content of sodium and potassium in the steel and the limitations of the detection methods, it was difficult to accurately detect the content of sodium and potassium in the steel. In the experiment of adding 1.0% Na2CO3 and K2CO3, the mass fractions of sodium and potassium in the steel were 0.0012% and 0.0014%, respectively.
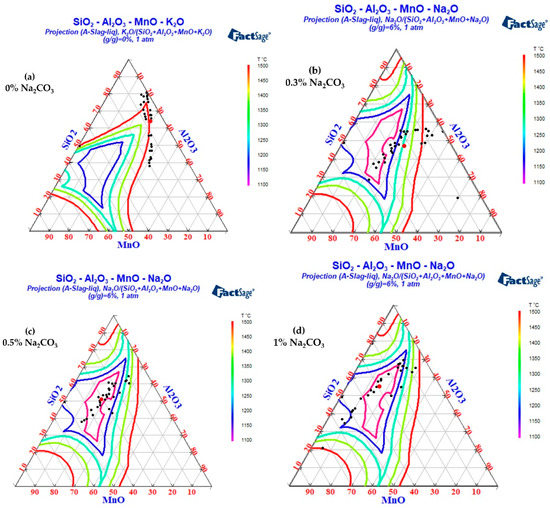
Figure 13.
The changes in inclusions with different Na2CO3 quantities added into steel.
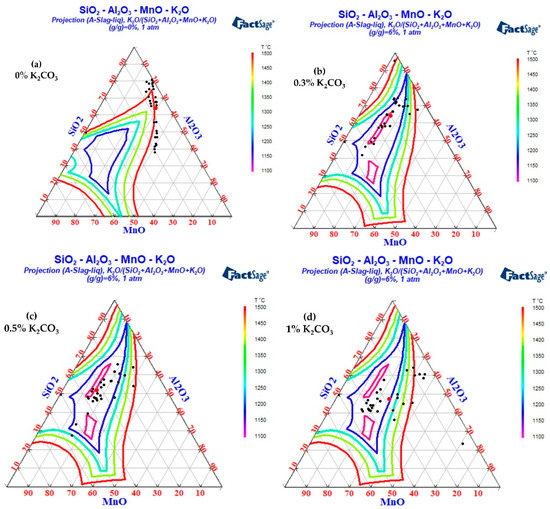
Figure 14.
The change in inclusion with different K2CO3 added into steel.
- (1)
- Influence of Na2CO3 addition on inclusion composition of cord steel.
As can be seen from Figure 13, when Na2CO3 was added to steel, the mass fraction of Na2O in the inclusions was about 6%, with little change. The differences were as follows: (1) When the mass fraction of Na2CO3 was 0%, the inclusions in the steel were brittle with a high melting point near SiO2 and SiO2-MnO-Al2O3 inclusions with high melting points of about 1500 °C. (2) With the increase in Na2CO3 content, the aluminum content in the inclusions decreased significantly. (3) When the mass fraction of Na2CO3 was 0.3%, the inclusions were completely modified, and there were still some high-alumina, high-melting-point, SiO2-Al2O3-MnO inclusions. (4) When the mass fraction of Na2CO3 was 0.5%, the inclusions were completely modified, and almost all the inclusions fell in the low melting point region (less than 1300 °C). (5) When excessive Na2CO3 (the mass fraction was 1%) was added, the aluminum content in the inclusions further decreased, and the inclusions began to move away from the low melting point region.
To sum up, the aluminum content in the inclusions gradually decreased with the increase in Na2CO3. When the mass fraction of Na2CO3 was 0.5%, most inclusions in the steel fell in the low melting point region, which was beneficial to the deformation of inclusions during the hot rolling process.
- (2)
- Influence of K2CO3 addition on inclusion composition of cord steel.
As can be seen from Figure 14, when K2CO3 was added to steel, the mass percentage of K2O in the inclusions was about 3%. With the increase in K2CO3 content, the content of potassium in the inclusions did not increase significantly. The differences in the influence of K2CO3 addition on the inclusions were as follows: (1) When K2CO3 addition was 0%, the inclusions in the steel were divided into brittle inclusions with a high melting point near SiO2 and SiO2-MnO-Al2O3 inclusions with a high melting point of about 1500 °C. (2) With the increase in K2CO3 content, the silicon content in the inclusions decreased significantly. (3) When the mass fraction of K2CO3 was 0.3%, the inclusion modification was incomplete, and some high-silicon SiO2-Al2O3-MnO inclusions still existed. (4) When the mass fraction of K2CO3 was 0.5%, the inclusions were completely modified, and almost all the inclusions fell in the low melting point region. (5) When excessive (1% by mass) K2CO3 was added, the silicon content in the inclusions decreased slightly, but the aluminum content in some inclusions increased, and some inclusions were far away from the low melting point area.
To sum up, with the increase in K2CO3, the silicon content in the inclusions gradually decreased. When the mass fraction of K2CO3 was 0.5%, most of the inclusions in the steel fell in the low melting point region, which was beneficial to the deformation of inclusions during the hot rolling process.
3.3. Thermodynamic Calculation and Reaction Mechanism of Inclusion Transformation in Molten Steel
Based on the steel composition in Table 1 and the activity interaction coefficients [30] of various elements in molten steel at 1600 °C in Table 3, the activity coefficient of C in steel was calculated to be 0.445 according to Equation (1):

Table 3.
Activity interaction coefficient of each element for carbon in molten steel.
In order to reveal the modification mechanism of inclusions in steel by adding alkali metal carbonate into molten steel, thermodynamic analysis was carried out [27].
Na2CO3 is added to the molten steel and decomposed into Na2O at a high temperature, which reacts with C in the molten steel to generate Na in the molten steel, as shown in Equations (2)–(5). The thermodynamic calculation for the generation of gaseous Na(g) is shown in Equations (6)–(9).
K2CO3 is added to the molten steel and decomposed into K2O at a high temperature, which reacts with C in the molten steel to generate K in the molten steel, as shown in Equations (10)–(13). The thermodynamic calculation for the generation of gaseous K(g) is shown in Equations (14)–(17).
In Equations (1)–(17), : activity; : partial pressure; : standard atmosphere; : mass fraction; : activity coefficient.
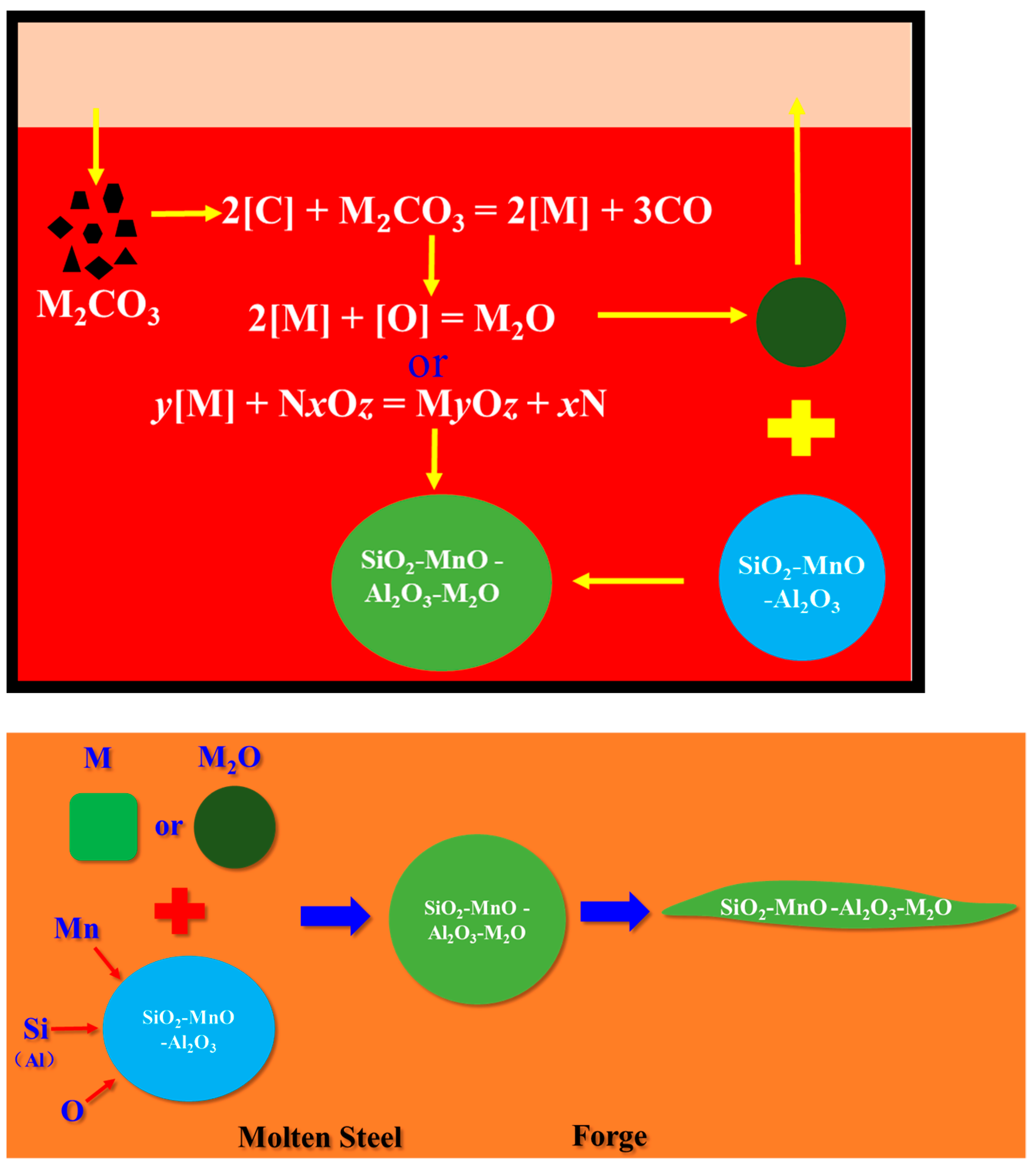
The above calculation results show that Na2CO3 and K2CO3 added to the molten steel decomposed into Na2O and K2O, and may theoretically be reduced by C in the steel and enter the molten steel in the form of Na and K. Since Na and K elements are very reactive, they will react quickly with a small amount of free oxygen in the surrounding molten steel or react with oxide inclusion: 2[M] + [O] = M2O or y[M] + NxOz = MyOz + xN, M: Na, K; N: Mn, Si, Al.
High-carbon steel wire such as cord steel and spring steel and low-titanium and low-aluminum ferrosilicon and manganese are used for deoxidizing and alloying, and the aluminum content in the alloy is very low so the deoxidized inclusions produced in the molten steel are mainly SiO2-MnO-Al2O3 clips. When Na and K elements first react with free oxygen, part of the oxide is formed to float into the slag, and the other part may collide and fuse with the SiO2-MnO-Al2O3 inclusion to form a SiO2-MnO-Al2O3-M2O complex inclusion. When Na and K elements react directly with oxide inclusion to form a SiO2-MnO-Al2O3-M2O complex inclusion, the contents of Na2O and K2O in the complex inclusion increase, and the contents of one or more components of SiO2, MnO and Al2O3 decrease. The modification and deformation mechanisms of Na2CO3 and K2CO3 on SiO2-MnO-Al2O3 inclusions are shown in Figure 15.
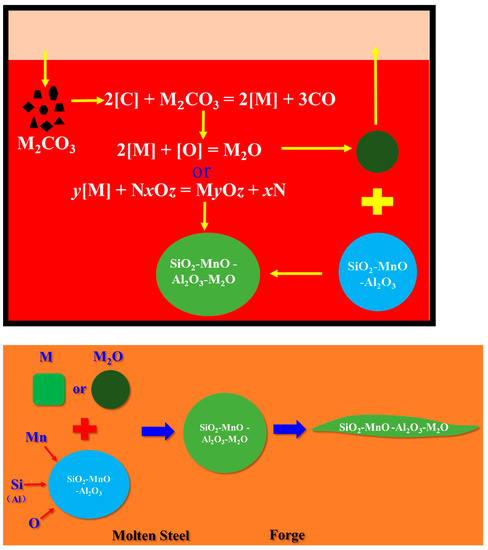
Figure 15.
Modification and deformation mechanism of SiO2-MnO-Al2O3 inclusions by Na2CO3 and K2CO3.
3.4. Effect of Na2CO3/K2CO3 Addition on Low Melting Point Region of Inclusions in Cord Steel
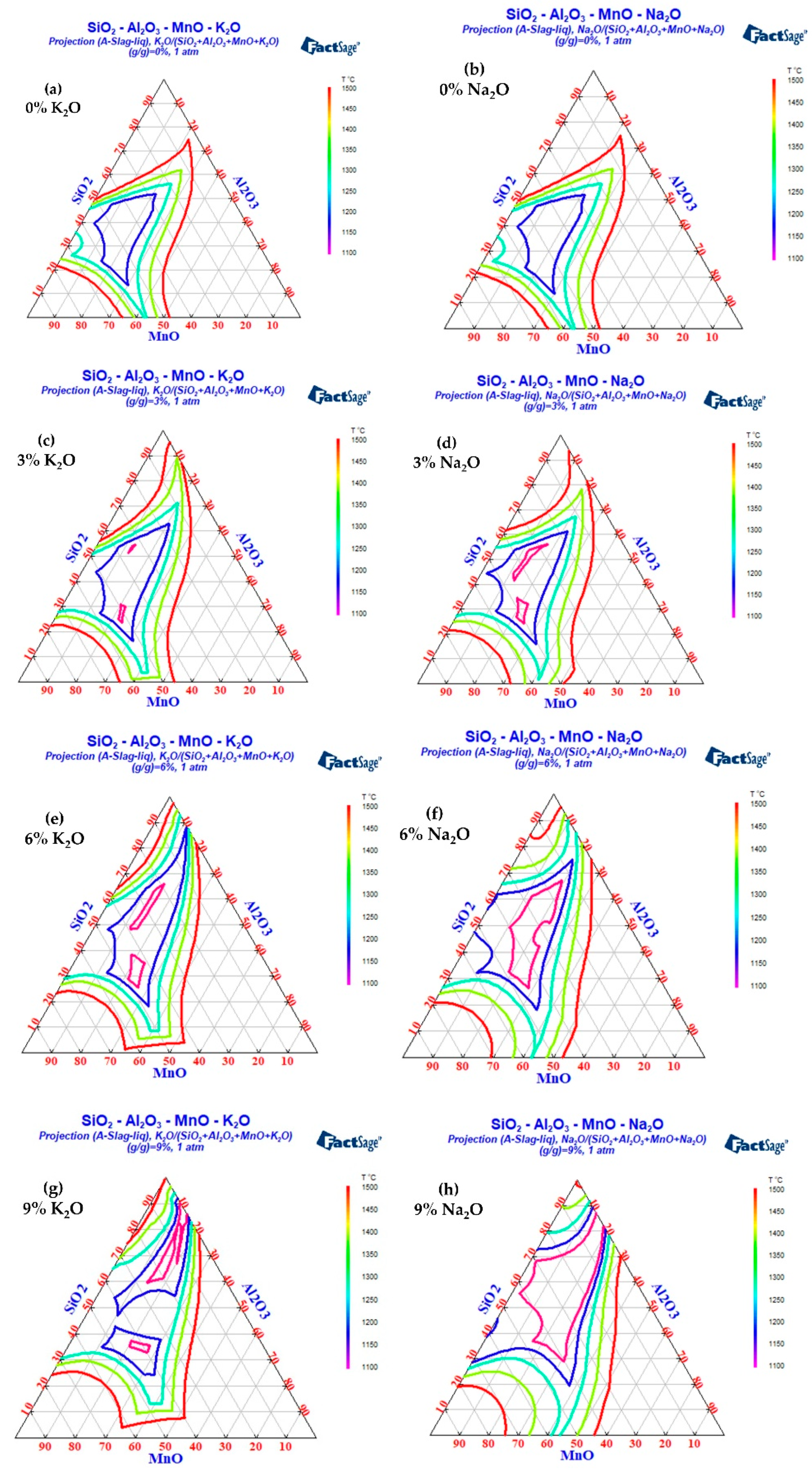
The influences of different K2O and Na2O contents on the low melting point region of SiO2-MnO-Al2O3 inclusions were calculated by Factsage 8.1, as shown in Figure 16. It can be seen that the low melting point region of SiO2-MnO-Al2O3 inclusions expands in varying degrees with the increase in alkali metal content. Among the inclusions with the same alkali metal content, Na2O had a better expansion effect on the low melting point region of SiO2-MnO-Al2O3 inclusions than K2O. At the same time, with the increase in Na2O, the melting point of SiO2-MnO-Al2O3 inclusions was lower. When the mass fraction of K2O in inclusions increased from 6% to 9%, the melting point region of inclusions below 1200 °C was divided into two parts, and the composition control region was narrowed, which was not conducive to the control of the low melting point region of the inclusions.
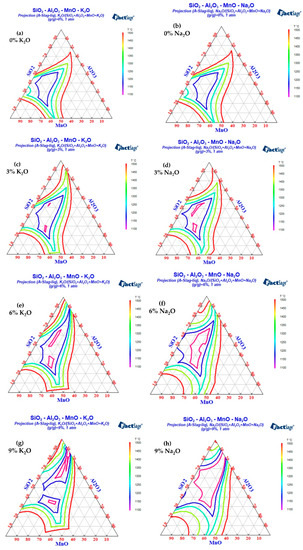
Figure 16.
The effects of different Na2O/K2O contents on low melting point area of inclusions in cord steel. (a) 0%K2O, (b) 0% Na2O, (c) 3% K2O, (d) 3% Na2O, (e) 6% K2O, (f) 6% Na2O, (g) 9% K2O, and (h) 9% Na2O.
3.5. Effect of 0.5%Na2CO3/K2CO3 Addition on Inclusion Deformability of Cord Steel after Forging
- (1)
- Analysis of inclusion deformation test results of cord steel samples with 0.5% Na2CO3.
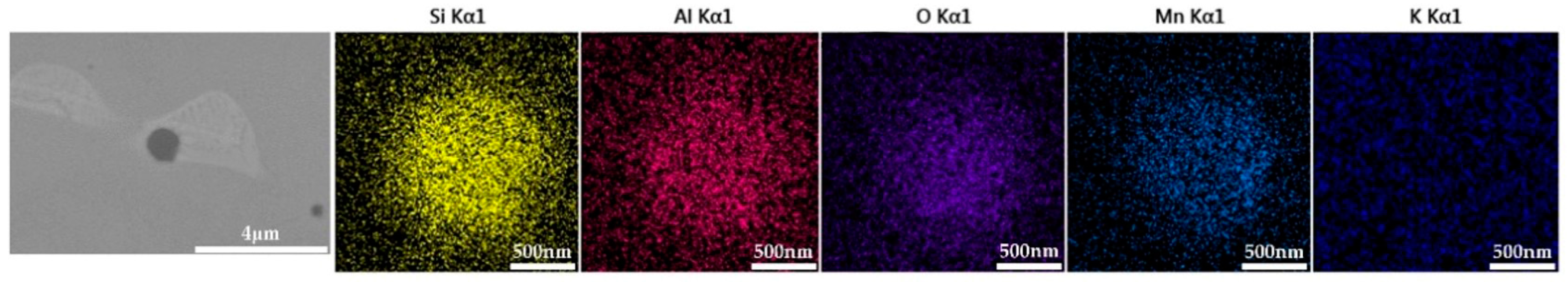
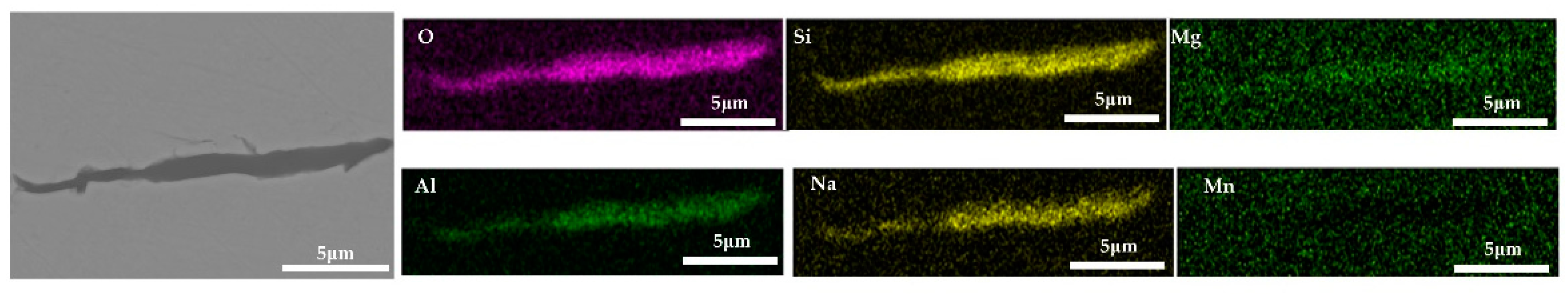
Inclusions were observed in the longitudinal section of a 600 g forged bar in Figure 2. The deformation of the inclusions after forging is shown in Figure 17, and the distribution of each element in the inclusions is shown in Figure 18, which shows obvious deformation behavior. It can be seen from Figure 17 that when Na2CO3 was added to the cord steel, after forging the inclusion changed from spherical to a long strip as cast, and has good deformability. Adding sodium carbonate to cord steel could significantly reduce the melting point of the inclusions, and almost all inclusions were deformed during hot forging. Among the thirty inclusions observed, twenty-seven inclusions had obvious deformation, and three inclusions had no obvious deformation. It could be seen from the distribution of inclusions in Figure 13c and Figure 17 that almost all inclusions in the phase diagram of MnO-Al2O3-SiO2-Na2O were located in the low melting point region. Adding sodium carbonate could significantly improve the deformation behavior of inclusions during hot forging.

Figure 17.
Deformation morphology of inclusions in longitudinal section of steel with 0.5% Na2CO3 added after forging.

Figure 18.
Composition distribution of inclusions in longitudinal section of steel with 0.5% Na2CO3 added after forging.
- (2)
- Analysis of inclusion deformation test results of cord steel samples with 0.5% K2CO3.
Inclusions were observed in the longitudinal section of a 600 g forged bar in Figure 2, and the deformation of the inclusions after forging is shown in Figure 19 and Figure 20, which shows obvious deformation behavior. It can be seen from Figure 19 that after the addition of K2CO3 to the cord steel, the inclusion changed from spherical to a strip after forging. However, compared with the addition of Na2CO3, the deformation ability of the inclusion with K2CO3 was lower, and the deformation was not obvious. Adding potassium carbonate to cord steel could reduce the melting point of inclusions, and most inclusions were deformed during hot forging, but there were still a few inclusions that were not easy to deform. Among the 30 inclusions observed, 20 inclusions had obvious deformation and 10 inclusions had no obvious deformation. It could be seen from the distribution of inclusions in Figure 14c and Figure 19 that most of the inclusions in the phase diagram of MnO-Al2O3-SiO2-K2O were located in the low melting point region, but there were still a few inclusions in the high melting point region. Adding potassium carbonate could improve the deformation behavior of inclusions during hot forging to some extent; most inclusions were easy to deform and a few were not easy to deform.
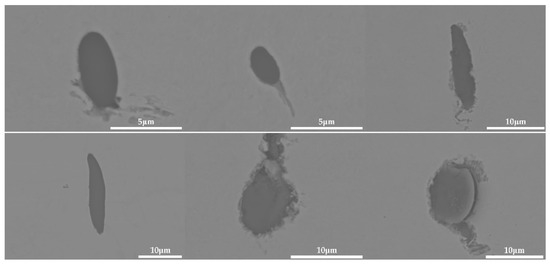
Figure 19.
Deformation morphology of inclusions in longitudinal section of steel with 0.5% K2CO3 added after forging.
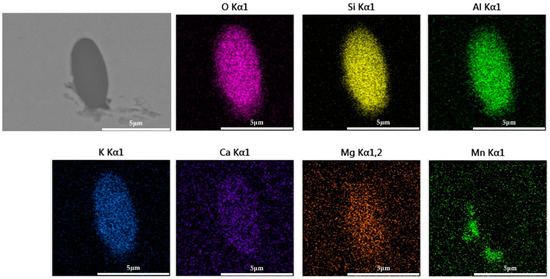
Figure 20.
Composition distribution of inclusions in longitudinal section of steel with 0.5% K2CO3 added after forging.
Therefore, after adding Na2CO3 and K2CO3 to the cord steel, the samples were forged. After SEM analysis, it was found that most of the inclusions had good deformability.
4. Conclusions
In summary, the effects of different contents of three kinds of alkali metal oxides on the inclusions of cord steel were studied in this paper. The following conclusions were drawn.
After the experiment of alkali metal carbonate treatment of inclusions in cord steel, it was found that the size of inclusions did not change significantly after Na and K treatment. However, the composition of the inclusion changed, such as the decrease in Al content. After forging, the inclusions in the longitudinal section of steel were long, and the deformability of inclusions had been greatly improved. Moreover, B had no effect on the melting point because it could not be integrated into the inclusions.
What is more, the phase diagram of FactSage (FactPS and FToxid databases) showed that Na2O had a better expansion effect on the low melting point region of SiO2-MnO-Al2O3 inclusions than K2O-treated samples. After adding K2CO3 and Na2CO3, most of the inclusions observed fell in the low melting point region (less than 1300 °C). The decrease in the melting point of the inclusions made them deform more easily. The inclusion analysis of the forged samples showed that the inclusion deformation ability of cord steel after adding Na2CO3 was better than that of K2CO3 treatment.
Author Contributions
Data curation, J.Z. and M.W.; methodology, Y.B. and D.H.; writing—original draft preparation, Y.W. and H.M.; writing—review and editing, Y.W., H.M. and H.J.; project administration, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the National Natural Science Foundation of China (Grant No. 51774031 and Grant No. 51804205) for support.
Data Availability Statement
Not applicable.
Acknowledgments
The studies were carried out on the equipment of Institute of Research of Iron & Steel and School of Iron and Steel, Soochow University. Thanks to Ma Jianchao for his guidance on the content and structure of this article as well.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.Y.; Yang, S.F.; Qu, J.L.; Li, J.S.; Dong, A.P.; Gu, Y. Effects of different melting technologies on the purity of super alloy GH4738. Materials 2018, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Pang, Z.G.; Xing, X.D.; Xu, R.S. Thermodynamic properties, viscosity, and structure of CaO-SiO2-MgO-Al2O3-TiO2-based slag. Materials 2021, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Min, D.J. A novel electrochemical process for desulfurization in the CaO-SiO2-Al2O3 system. Materials 2020, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, M.; Wang, B.; Zhou, J.; Jiang, Z. Recent advances on drawing technology of ultra-fine steel tire cord and steel Saw Wire. Metals 2021, 11, 1590. [Google Scholar] [CrossRef]
- Gao, Y.X.; Leng, M.; Chen, Y.F.; Chen, Z.C.; Li, J.L. Crystallization products and structural characterization of CaO-SiO2-based mold fluxes with varying Al2O3/SiO2 ratios. Materials 2019, 12, 206. [Google Scholar] [CrossRef]
- Leng, M.; Lai, F.F.; Li, J.L. Effect of cooling rate on phase and crystal morphology transitions of CaO-SiO2-based systems and CaO-Al2O3-based systems. Materials 2019, 12, 62. [Google Scholar] [CrossRef]
- Gu, S.P.; Wen, G.H.; Ding, Z.Q.; Tang, P.; Liu, Q. Effect of shear stress on isothermal crystallization behavior of CaO-Al2O3-SiO2 -Na2O-CaF2 Slags. Materials 2018, 11, 1085. [Google Scholar] [CrossRef]
- Shu, Q.; You, C.; Alatarvas, T.; Fabritius, T.M.J. Experimental and modeling study of deformability of glassy CaO-(MnO)-Al2O3-SiO2 inclusions. Metals 2022, 12, 522. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.-C.; Li, K.-L.; Wang, R.-G.; Wang, X.-H. Formation Mechanism of Large CaO-SiO2-Al2O3 Inclusions in Si-Deoxidized Spring Steel Refined by Low Basicity Slag. Metall. Mater. Trans. B 2021, 52, 1950–1954. [Google Scholar] [CrossRef]
- Wang, C.; Tang, W.; Zhang, J.S.; Chi, Y.G.; Chen, J.; Liu, Q. Evolution Mechanism of MgO-Al2O3-SiO2-CaO Inclusions During Continuous Casting of Si-Killed Spring Steel. Steel Res. Int. 2023, 94, 2200475. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Sun, M.; Chen, X.; Li, Y.; Jiang, Z. Recent advances in inclusions and central segregation control technology in tyre cord steel and saw wire steel. Ironmak. Steelmak. 2023, 50, 197–214. [Google Scholar] [CrossRef]
- Qiao, T.; Cheng, G.; Huang, Y.; Li, Y.; Zhang, Y.; Li, Z. Formation and removal mechanism of nonmetallic inclusions in 42CrMo4 steel during the steelmaking process. Metals 2022, 12, 1505. [Google Scholar] [CrossRef]
- Liu, J.; Tang, H.; Guo, L.; Zhang, J. Effect of deoxidizing and alloying routes on the evolution of non-metallic inclusions in 55SiCr spring steel. Metals 2022, 12, 1531. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Hu, H.; Yang, H.; Sun, M.; Jiang, Z. Effect of slag adjustment on inclusions and mechanical properties of Si-killed 55SiCr spring steel. Crystals 2022, 12, 1721. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.; Xu, Q.; Yu, Y. Effect of MgO on crystallization behavior of MnO–SiO2–Al2O3 based inclusions in tire cord steel. Heliyon 2022, 8, e11800. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.; Xu, Q.; Yu, W. Effect of MgO in low-basicity refining slag on properties of MnO-SiO2-Al2O3 inclusions in tire cord steel. Mater. Today Commun. 2022, 33, 104351. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Lai, Y.; Liao, J.; Jiang, M.; Wang, X. An innovative CaO–SiO2–MgO-refining slag containing 12 to 16 mass pct MgO for tire cord steel. Metall. Mater. Trans. B 2022, 53, 651–655. [Google Scholar] [CrossRef]
- Cui, Z.; Yan, C.; Li, Y.; Wang, X.; Zhu, L.; Zhang, Q. Control of the non-metallic inclusions near solidification front by pulsed magnetic field. Metals 2022, 12, 2008. [Google Scholar] [CrossRef]
- Chen, C.; Sun, M.; Chen, X.; Wang, B.; Zhou, J.; Jiang, Z. State of the art in control of inclusions and microalloying elements in tire cord steel and saw wire steel. Steel Res. Int. 2022, 93, 2100507. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Wang, L.; Zhang, J.; Xue, Z. Thermodynamic analysis of TiN precipitation in SWRH92A high carbon tire cord steel under the influence of solute micro-segregations during solidification. Metall. Mater. Trans. B 2021, 53, 2056–2071. [Google Scholar] [CrossRef]
- Toribio, J.; Ayaso, F.-J.; González, B. Role of non-metallic inclusions in the fracture behavior of cold drawn pearlitic steel. Metals 2021, 11, 962. [Google Scholar] [CrossRef]
- Bandyopadhyay, T.R.; Rao, P.K.; Prabhu, N. Behavior of alloying elements during electro-slag remelting of ultrahigh strength steel. Metall. Min. Ind. 2012, 4, 6–16. [Google Scholar]
- Jiang, Z.; Dong, Y.; Liang, L.; Li, Z. Hydrogen pick-up during electroslag remelting process. J. Iron Steel Res. Int. 2011, 18, 19–23. [Google Scholar] [CrossRef]
- Chui, H.Z.; Chen, W.Q. Effect of boron on morphology of inclusions in tire cord steel. J. Iron Steel Res. Int. 2012, 19, 22–27. [Google Scholar]
- Polonsky, A.T.; Echlin, M.P.; Lenthe, W.C.; Dehoff, R.R.; Kirka, M.M.; Pollock, T.M. Defects and 3D structural inhomogeneity in electron beam additively manufactured inconel 718. Mater. Charact. 2018, 143, 171–181. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Hu, Y.; Chen, Z.; Xu, Y.; Yan, W. Effect of Na2CO3 addition on inclusions in high-carbon steel for saw wire. Trans. Indian Inst. Met. 2017, 71, 383–391. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Hu, Y.; Chen, Z.; Xu, Y.; Yan, W. Effects of K2CO3 addition on inclusions in high-carbon steel for saw wire. High Temp. Mater. Process. 2018, 37, 701–709. [Google Scholar] [CrossRef]
- Chen, L.; Wan, Y.; Li, J.; Chen, W.; Yang, Y.; McLean, A. A new method for plasticization of inclusions in saw-w Recent advances ire steel by NaF addition. Metals 2020, 10, 704. [Google Scholar] [CrossRef]
- Yang, J.; Du, J.; Chen, B.; Wu, J. Effect of calcium treatment on oxide inclusions during ultra-low oxygen refining. Iron Steel 2015, 50, 19–26. [Google Scholar]
- The Japan Society for the Promotion of Science. The 19th Committee on Steelmaking: Steelmaking Data Sourcebook; Gordon and Breach Science Publishers: New York, NY, USA, 1988.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).