Crystallization of Zr-Based Amorphous Alloys in Laser Welding
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Original Specimens
2.2. Detection Method
3. Simulation
3.1. CHT Curve Fitting
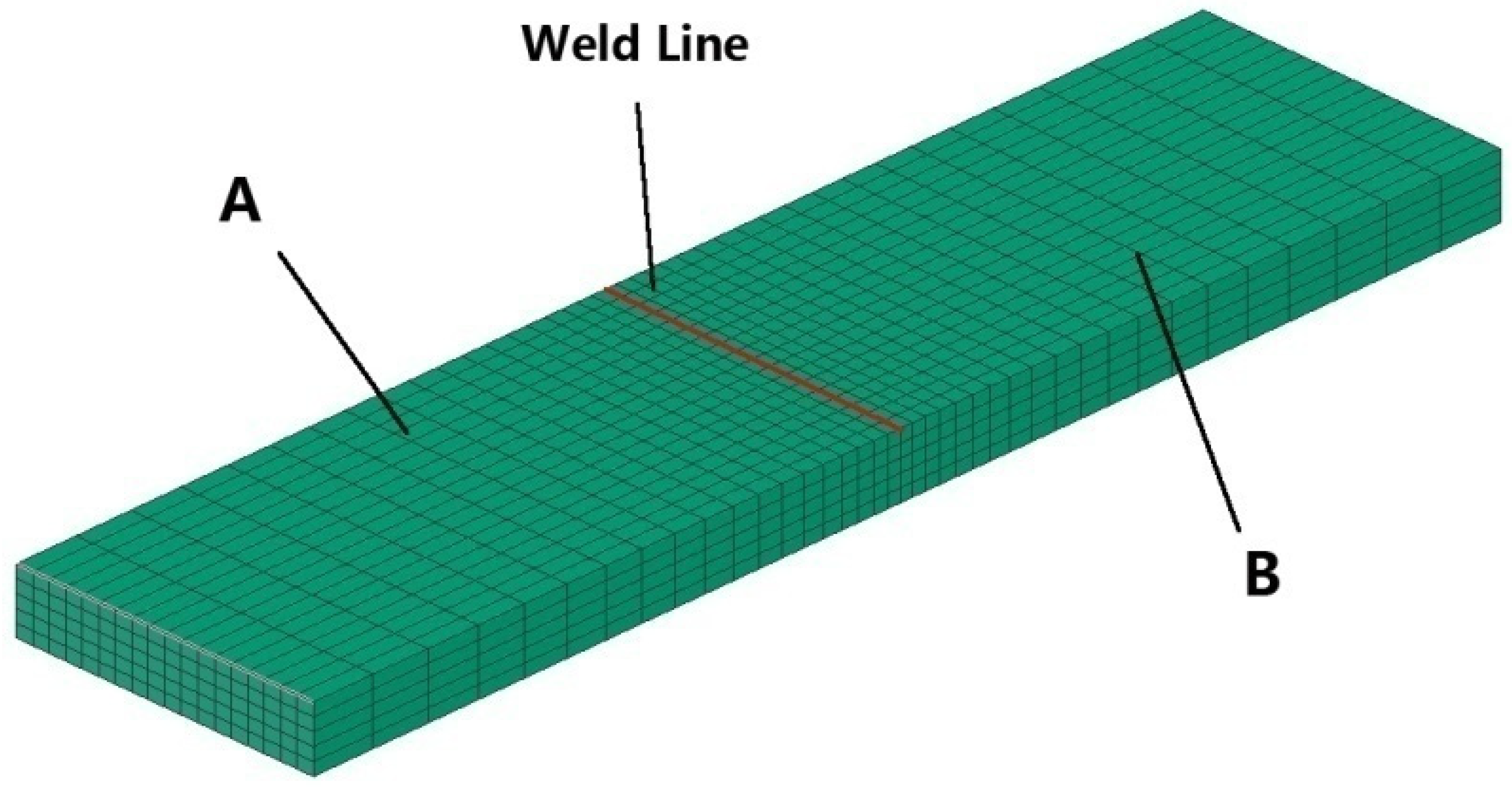
3.2. Simulation of Temperature Field
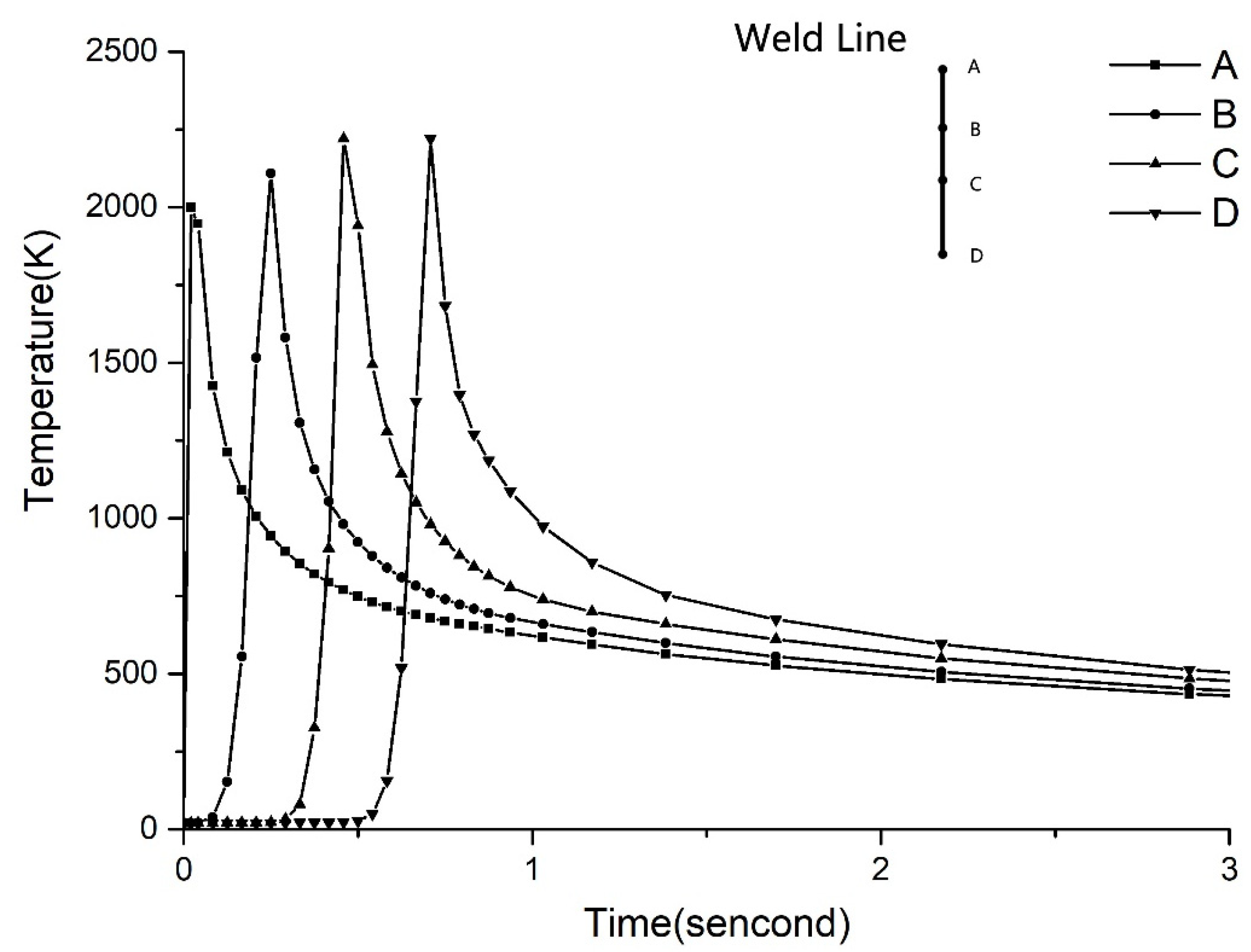
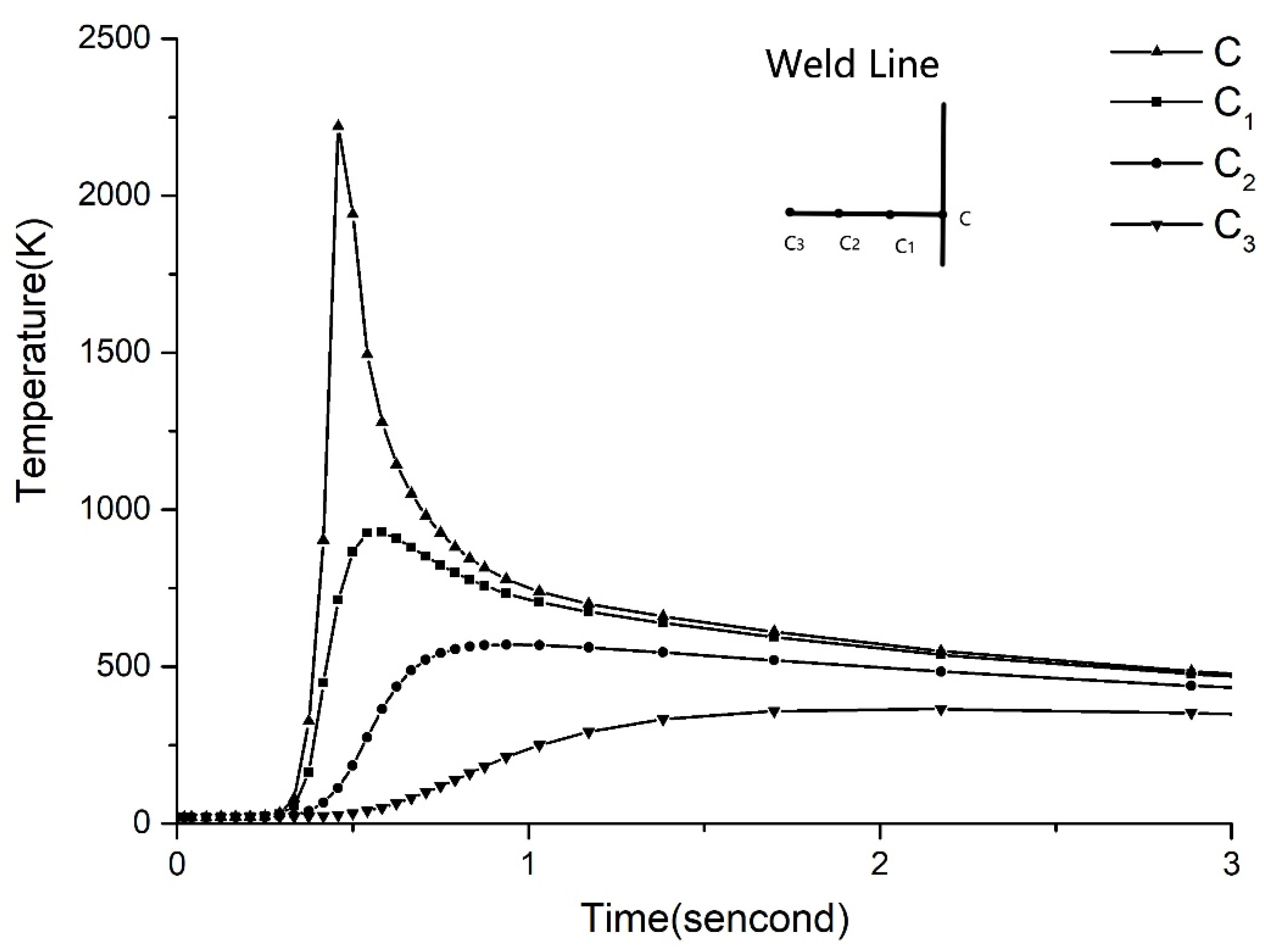
3.3. Results and Discussion
4. Laser Welding Experiments
4.1. Procedure
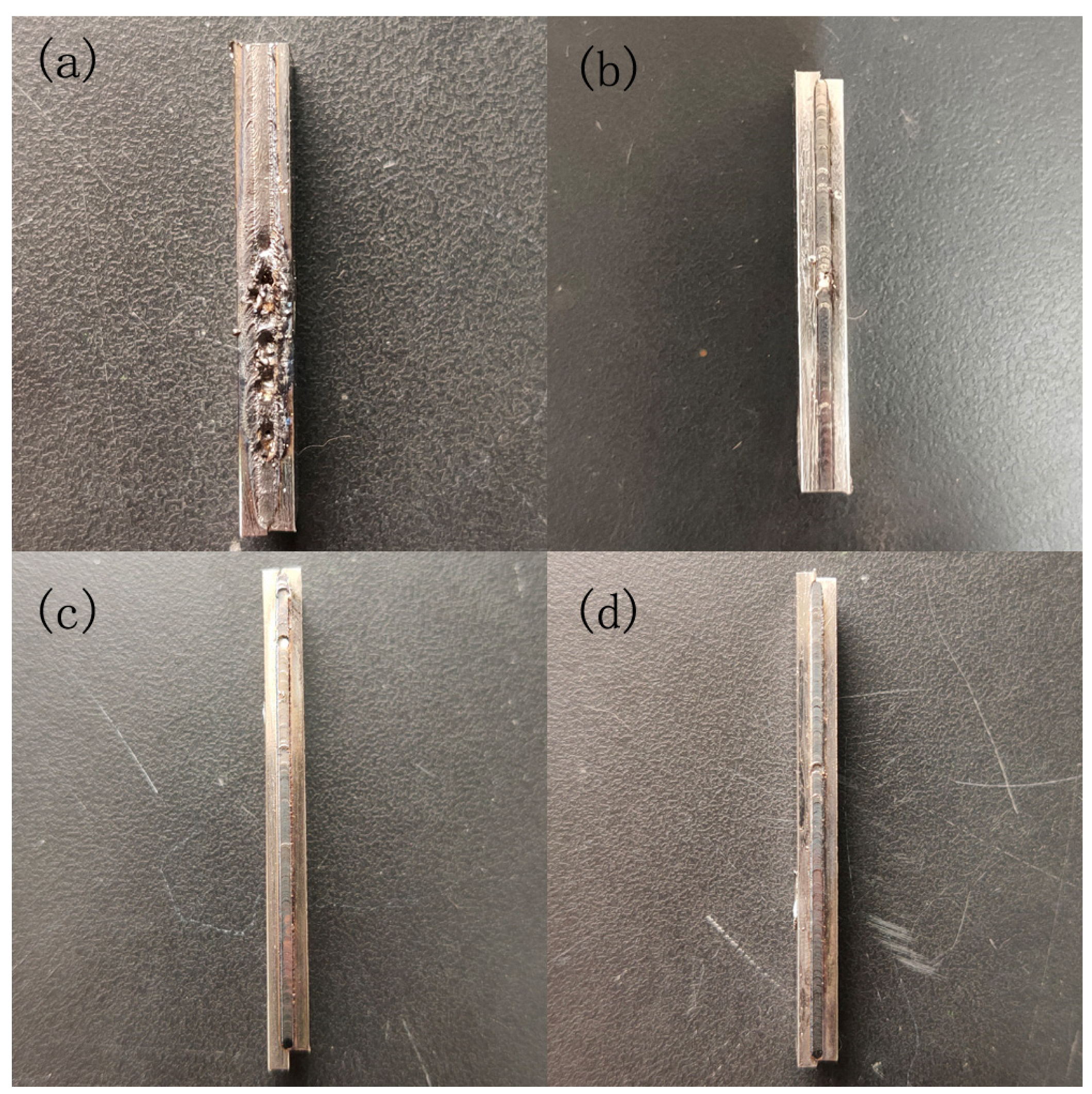
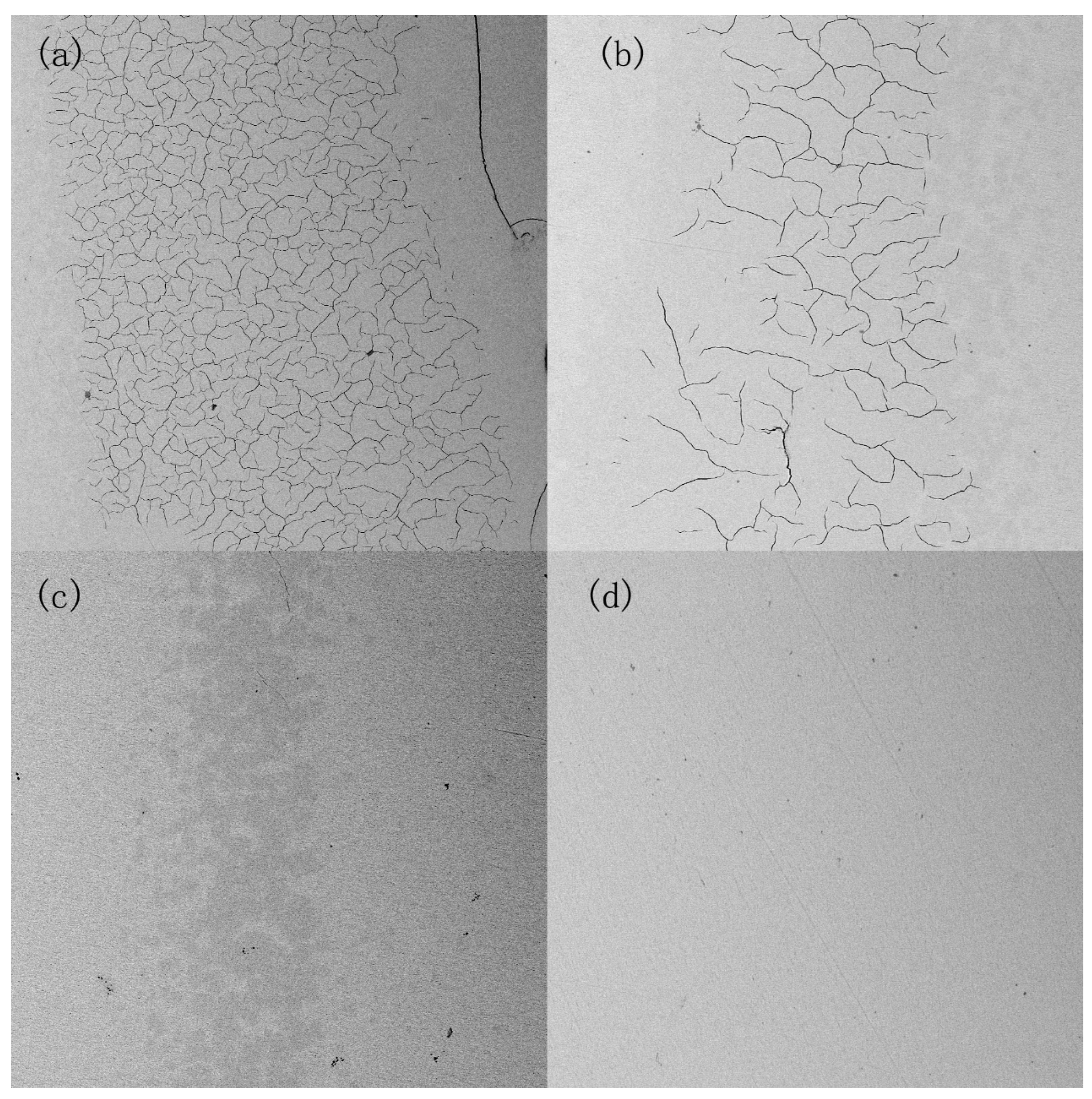
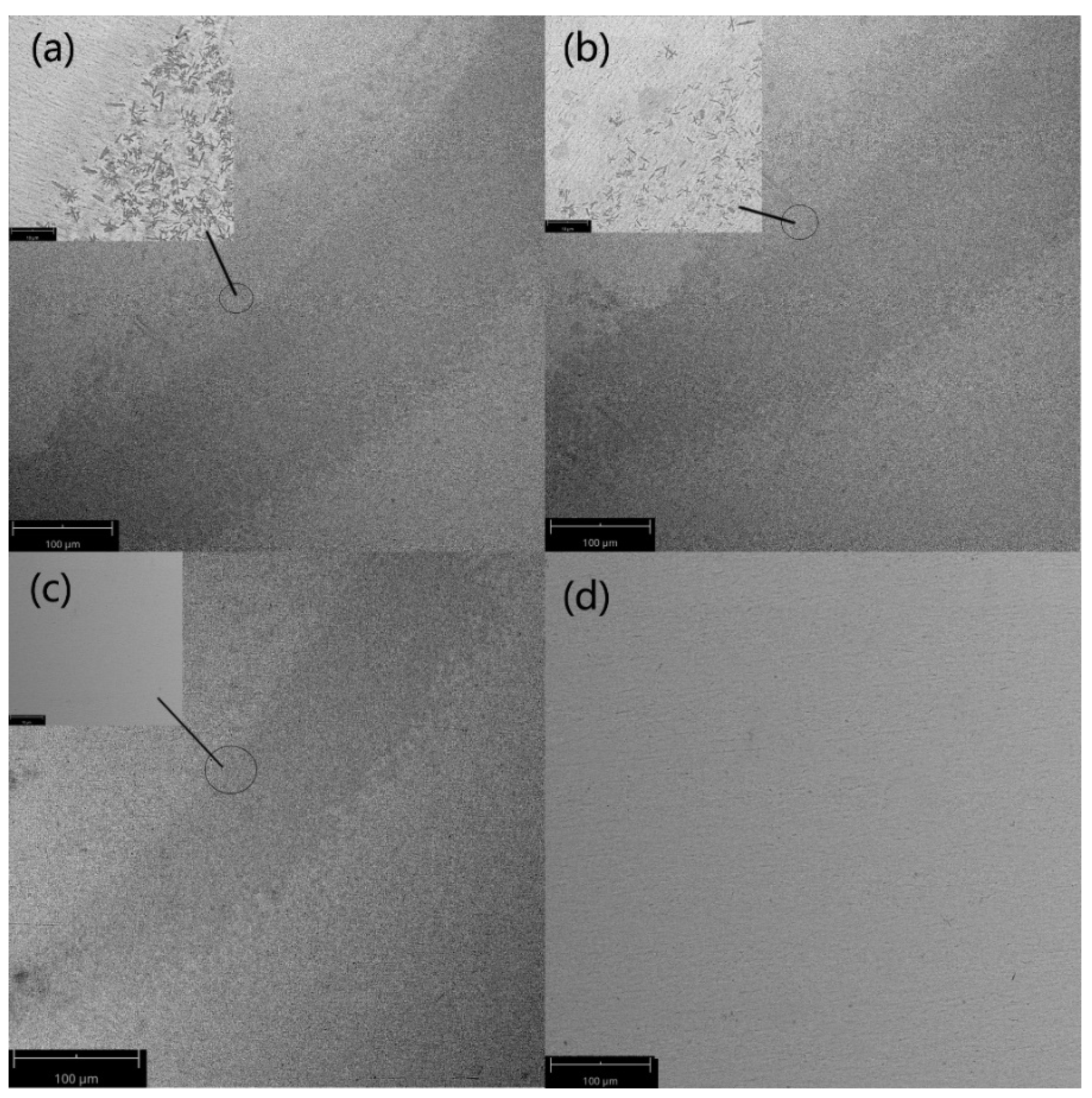
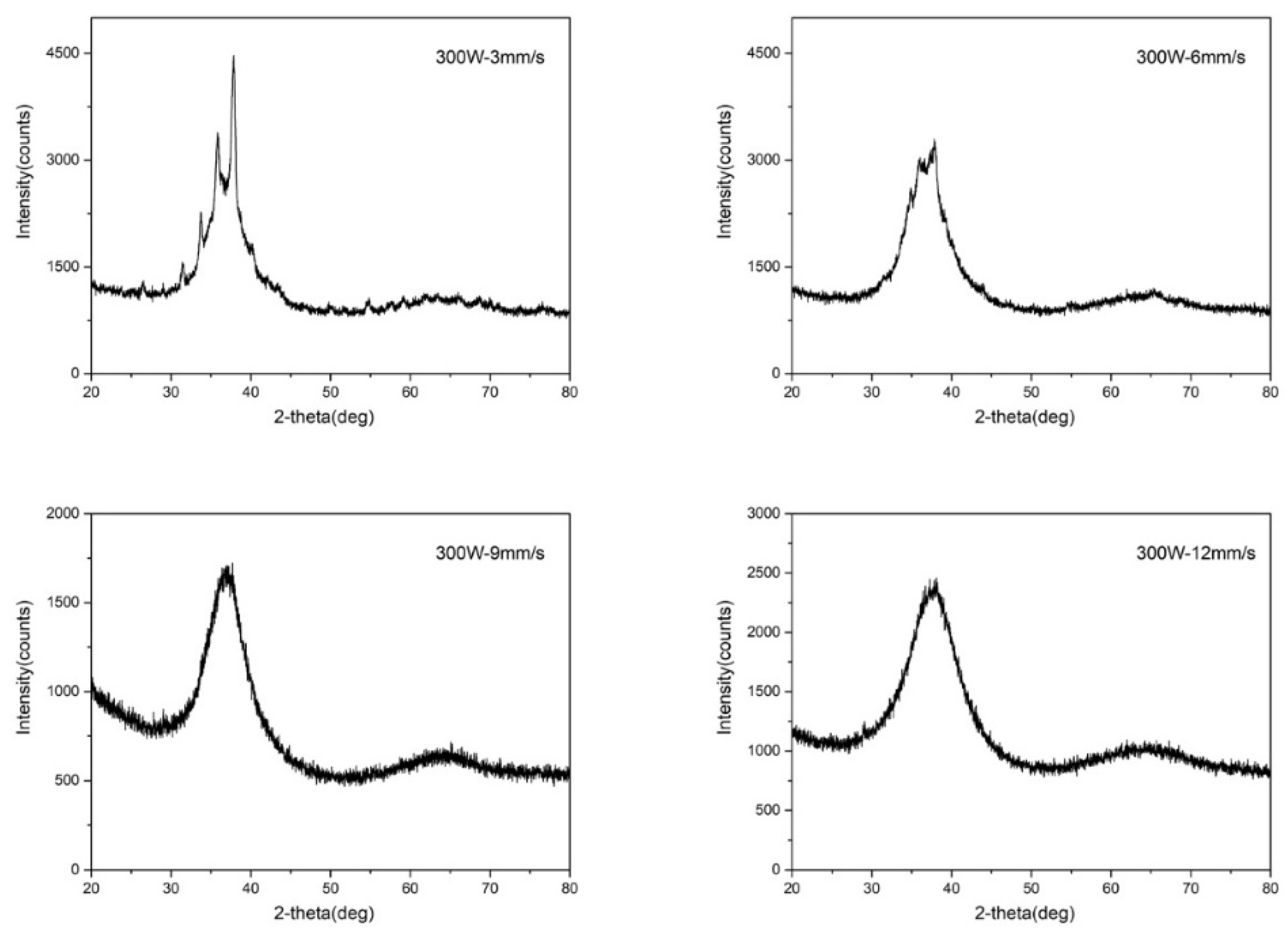
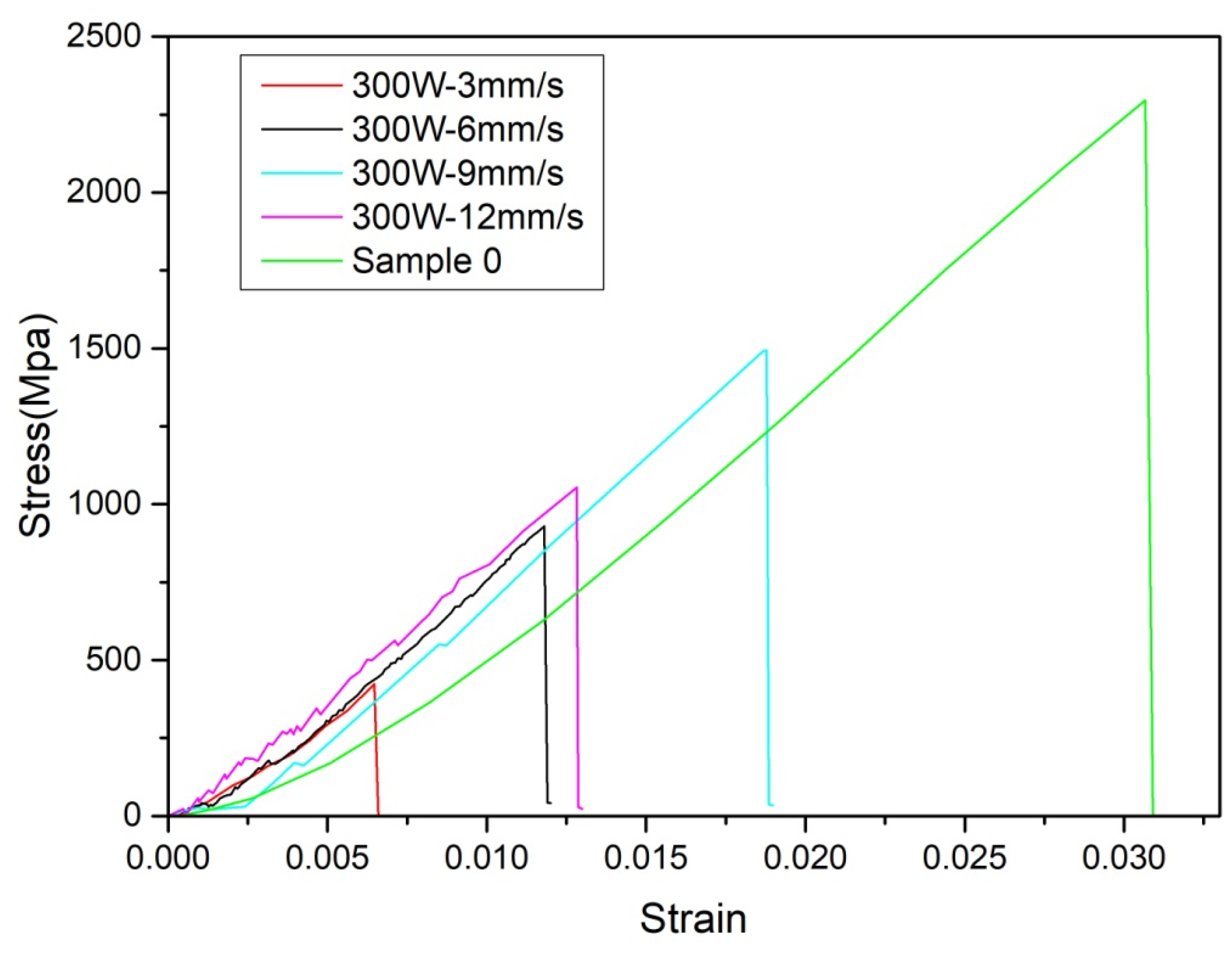
4.2. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Han, P.; Yin, S.; Niu, W.-J.; Zhai, L.; Li, X.; Mao, X.; Han, Y. Current Research Status on Cold Sprayed Amorphous Alloy Coatings: A Review. Coatings 2021, 11, 206. [Google Scholar] [CrossRef]
- Shen, J.; Lopes, J.G.; Zeng, Z.; Choi, Y.T.; Maawad, E.; Schell, N.; Kim, H.S.; Mishra, R.S.; Oliveira, J.P. Deformation behavior and strengthening effects of an eutectic AlCoCrFeNi2.1 high entropy alloy probed by in-situ synchrotron X-ray diffraction and post-mortem EBSD. Mater. Sci. Eng. A 2023, 872, 144946. [Google Scholar] [CrossRef]
- Terekhov, S.V. Single- and Multistage Crystallization of Amorphous Alloys. Phys. Met. Metallogr. 2020, 121, 664–669. [Google Scholar] [CrossRef]
- Kuvandikov, O.K.; Subkhankulov, I.; Amonov, B.U.; Imamnazarov, D.H. Physical Properties of High-Cobalt Amorphous Alloys. Metallofiz. I Noveishie Tekhnol. 2021, 43, 1601–1609. [Google Scholar] [CrossRef]
- Meagher, P.; O’Cearbhaill, E.D.; Byrne, J.H.; Browne, D.J. Bulk Metallic Glasses for Implantable Medical Devices and Surgical Tools. Adv. Mater. 2016, 28, 5755–5762. [Google Scholar] [CrossRef]
- Loye, A.M.; Kwon, H.K.; Dellal, D.; Ojeda, R.; Lee, S.; Davis, R.; Nagle, N.; Doukas, P.G.; Schroers, J.; Lee, F.Y.; et al. Biocompatibility of platinum-based bulk metallic glass in orthopedic applications. Biomed. Mater. 2021, 16, 045018. [Google Scholar] [CrossRef]
- Rajan, S.T.; Arockiarajan, A. Thin film metallic glasses for bioimplants and surgical tools: A review. J. Alloys Compd. 2021, 876, 159939. [Google Scholar] [CrossRef]
- Rai, N.; Das, P.; Gollapudi, S. Can an amorphous alloy crystallize into a high entropy alloy? Model. Simul. Mater. Sci. Eng. 2022, 30, 025007. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Wang, X.; Salloom, R.; Xia, Z.; Schroers, J.; Mukherjee, S. Nanomanufacturing of Non-Noble Amorphous Alloys for Electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 12099–12107. [Google Scholar] [CrossRef]
- Gerstl, S.S.A.; Schaublin, R.; Loffler, J. Nanoscale Clusters and Heterogeneities in Engineering and Amorphous Alloys. Microsc. Microanal. 2022, 28, 712–713. [Google Scholar] [CrossRef]
- Karna, S.; Cheepu, M.; Venkateswarulu, D.; Srikanth, V. Recent Developments and Research Progress on Friction Stir Welding of Titanium Alloys: An Overview. IOP Conf. Ser. Mater. Sci. Eng. 2018, 330, 012068. [Google Scholar] [CrossRef]
- Shanjeevi, C.; Arputhabalan, J.J.; Dutta, R.; Pradeep. Investigation on the Effect of Friction Welding Parameters on Impact Strength in Dissimilar Joints. IOP Conf. Ser. Mater. Sci. Eng. 2017, 197, 012069. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Huang, B.M.; Jiang, Z.J.; Lu, C.; Dai, L.H. Joining of bulk metallic glass to brass by thick-walled cylinder explosion. Scr. Mater. 2015, 97, 17–20. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, P.; An, E.; Feng, J. Experimental Study on the Explosive Welding of Thin Al/Cu Composite Plates. Mater. Sci. Forum 2018, 910, 52–57. [Google Scholar] [CrossRef]
- Tariq, N.H.; Shakil, M.; Hasan, B.A.; Akhter, J.I.; Haq, M.A.; Awan, N.A. Electron beam brazing of Zr62Al13Ni7Cu18 bulk metallic glass with Ti metal. Vacuum 2014, 101, 98–101. [Google Scholar] [CrossRef]
- Qiao, J.; Yu, P.; Wu, Y.; Chen, T.; Du, Y.; Yang, J. A Compact Review of Laser Welding Technologies for Amorphous Alloys. Metals 2020, 10, 1690. [Google Scholar] [CrossRef]
- Yao, Y.; Tang, J.; Zhang, Y.; Hu, Y.; Wu, D. Development of Laser Fabrication Technology for Amorphous Alloys. Chin. J. Lasers-Zhongguo Jiguang 2021, 48, 174–189. [Google Scholar]
- Caiazzo, F.; Caggiano, A. Investigation of Laser Welding of Ti Alloys for Cognitive Process Parameters Selection. Materials 2018, 11, 632. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, Y.X.; Liu, H.B.; Oliveira, J.P.; Tan, C.W.; Xia, H.B.; Yang, J. Dissimilar laser spot welding of aluminum alloy to steel in keyhole mode. J. Non-Cryst. Solids 2022, 34, 012009. [Google Scholar] [CrossRef]
- Sun, J. Mechanical manufacturing technology and precision machining technology. In Proceedings of the Second International Conference on Cloud Computing and Mechatronic Engineering (I3CME 2022), Chendu, China, 17–19 June 2022; Volume 12339. [Google Scholar] [CrossRef]
- Wu, W.F.; Li, Y. Bulk metallic glass formation near intermetallic composition through liquid quenching. Appl. Phys. Lett. 2009, 95, 011906. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Glass forming properties of Zr-based bulk metallic alloys. J. Non-Cryst. Solids 2003, 315, 206–210. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhang, S.; Shi, Z.L.; Wang, F.L.; Wei, C.; Ma, M.Z.; Liu, R.P. Corrosion behavior and mechanical properties of Vit1 metallic glasses prepared at different cooling rates. J. Alloys Compd. 2023, 934, 167848. [Google Scholar] [CrossRef]
- Ghosh, P.S.; Sen, A.; Chattopadhyaya, S.; Sharma, S.; Singh, J.; Dwivedi, S.P.; Saxena, A.; Khan, A.M.; Pimenov, D.Y.; Giasin, K. Prediction of Transient Temperature Distributions for Laser Welding of Dissimilar Metals. Appl. Sci. 2021, 11, 5829. [Google Scholar] [CrossRef]
- Liu, W.M.; Guo, C.; Wu, S.S.; Li, Y.; Ying, M.; Kang, T.Y. Influence of Laser Pulse Welding Power on Welding Joints of Zr-Based Amorphous Alloys. Cryst. Res. Technol. 2022, 57, 2200063. [Google Scholar] [CrossRef]
- Chen, B.; Shi, T.; Li, M.; Wen, C.; Liao, G. Crystallization of Zr55Cu30Al10Ni5 Bulk Metallic Glass in Laser Welding: Simulation and Experiment. Adv. Eng. Mater. 2015, 17, 483–490. [Google Scholar] [CrossRef]
- Xia, L.; Ding, D.; Shan, S.T.; Dong, Y.D. Evaluation of the thermal stability of Nd60Al20Co20 bulk metallic glass. Appl. Phys. Lett. 2007, 90, 111903. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Han, F.; Xie, H.; Wen, C.e. Thermal stability of the Al70Ni10Ti10Zr5Ta5 amorphous alloy powder fabricated by mechanical alloying. J. Alloys Compd. 2010, 496, 313–316. [Google Scholar] [CrossRef]
- He, M.; Zhang, Y.; Xia, L.; Yu, P. Kinetics and thermal stability of the Ni62Nb38–xTax(x=5, 10, 15, 20 and 25) bulk metallic glasses. Sci. China Phys. Mech. Astron. 2017, 60, 076111. [Google Scholar] [CrossRef]
- Shi, J.; Cui, C.; Zhao, L.; Ding, J.; Cui, S.; Liu, S.; Sun, Y. Thermodynamic calculation and thermal stability of Al-Y-Ce-Ni metallic glass. Mater. Res. Express 2018, 5, 025205. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, W. Effect of room temperature ageing on structure and thermal stability of as-cast and deformed Pd40Ni40P20 metallic glass. Int. J. Mod. Phys. B 2018, 32, 1850225. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Wen, P.; Pan, M.X.; Zhao, D.; Wang, W.; Wang, W.L. A highly glass-forming alloy with low glass transition temperature. Appl. Phys. Lett. 2003, 82, 4699–4701. [Google Scholar] [CrossRef]
- Ding, D.; Xia, L.; Shan, S.-T.; Dong, Y.-D. Long-term thermal stability of binary Cu50.3Zr49.7 bulk metallic glass. Chin. Phys. Lett. 2008, 25, 306–309. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, C.; Ma, M.; Shan, D.; Guo, B. Non-isothermal crystallization kinetics of Zr41.2Ti13.8Cu12.5Ni10Be22.5 amorphous alloy. Thermochim. A Acta 2014, 587, 11–17. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kagao, S.; Kawamura, Y.; Yoshimura, K. Thermal diffusivity and conductivity of supercooled liquid in Zr41Ti14Cu12Ni10Be23 metallic glass. Appl. Phys. Lett. 2004, 84, 4653–4655. [Google Scholar] [CrossRef]
- Chen, B.; Shi, T.L.; Li, M.; Yang, F.; Yan, F.; Liao, G.L. Laser welding of annealed Zr55Cu30Ni5Al10 bulk metallic glass. Intermetallics 2014, 46, 111–117. [Google Scholar] [CrossRef]

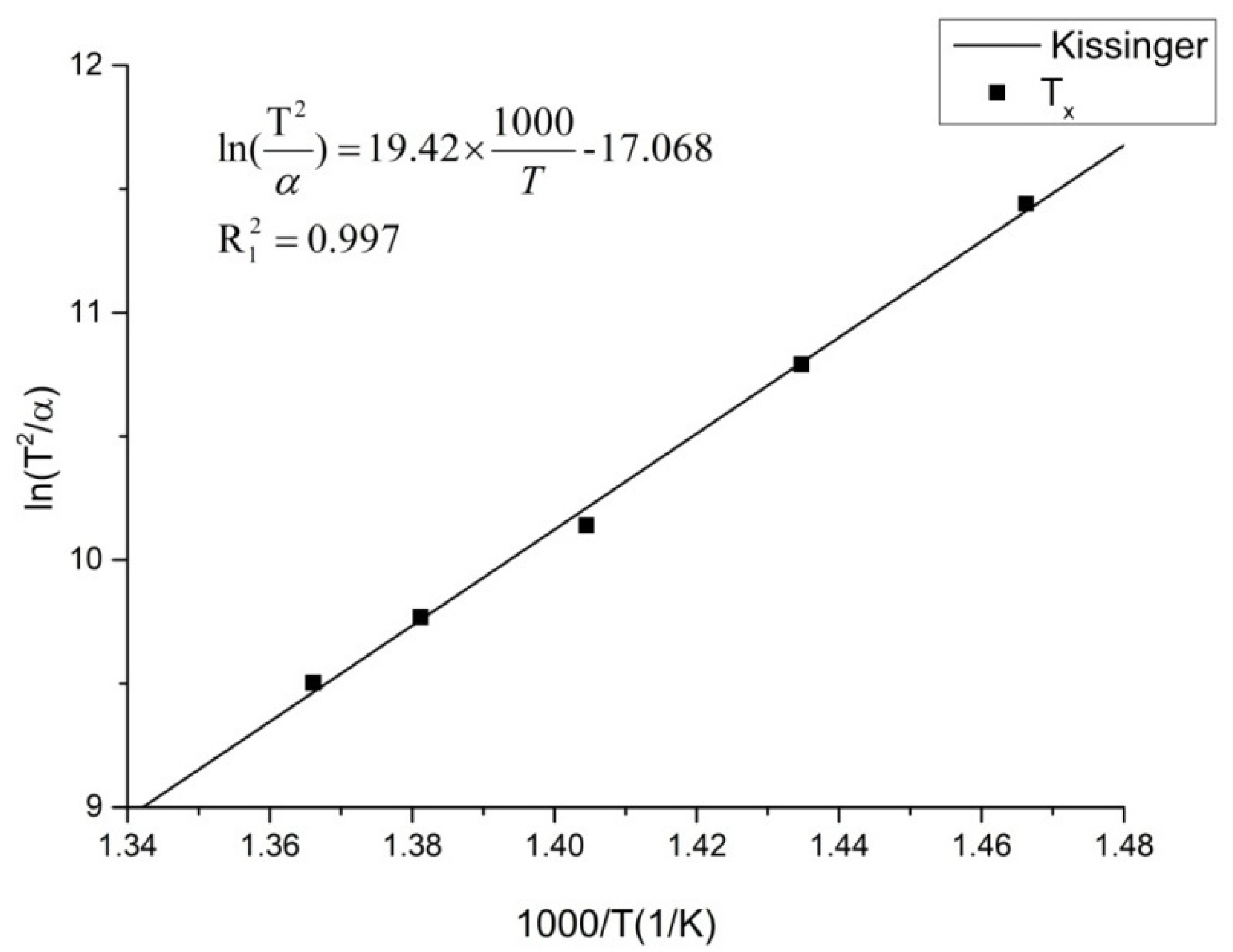
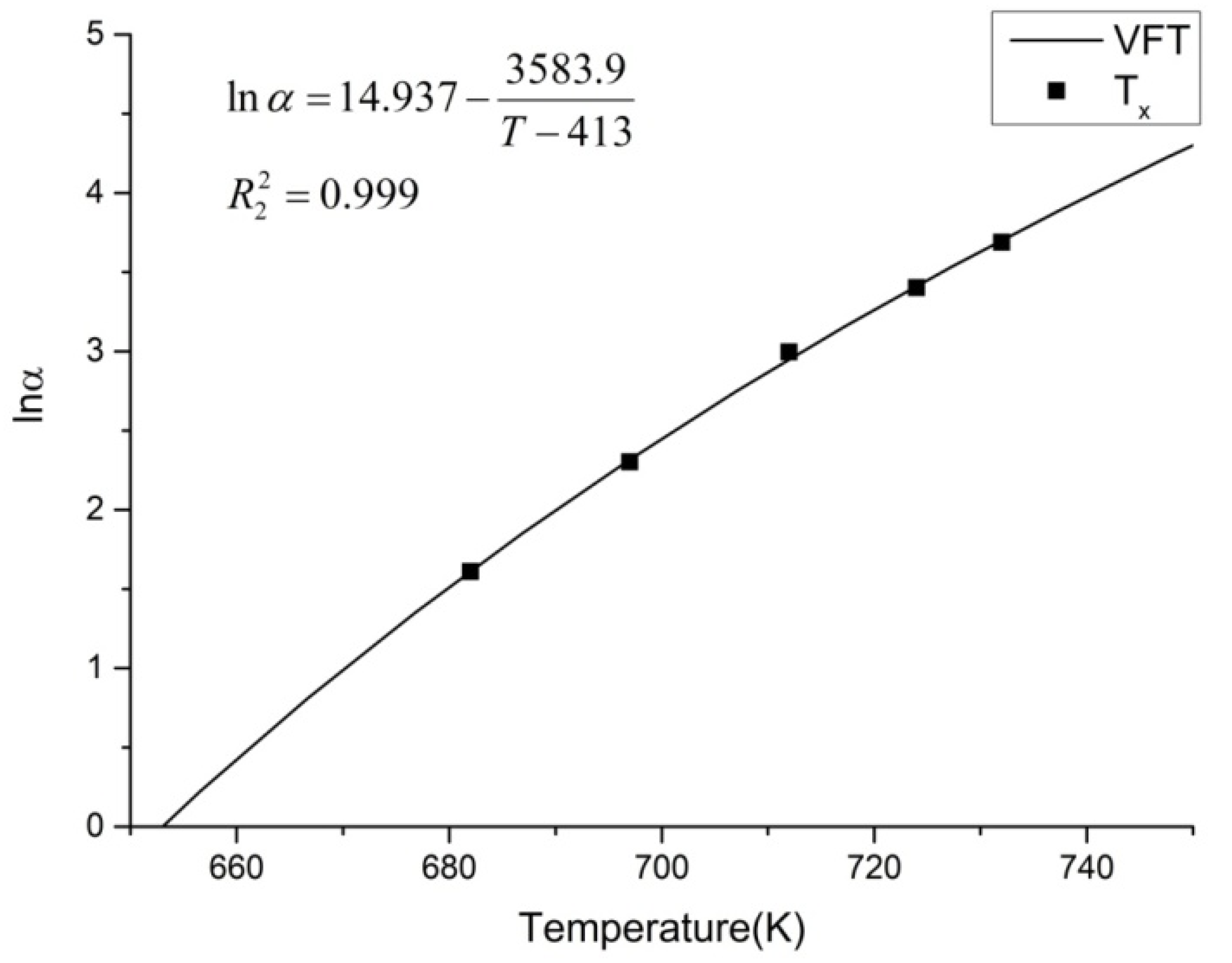

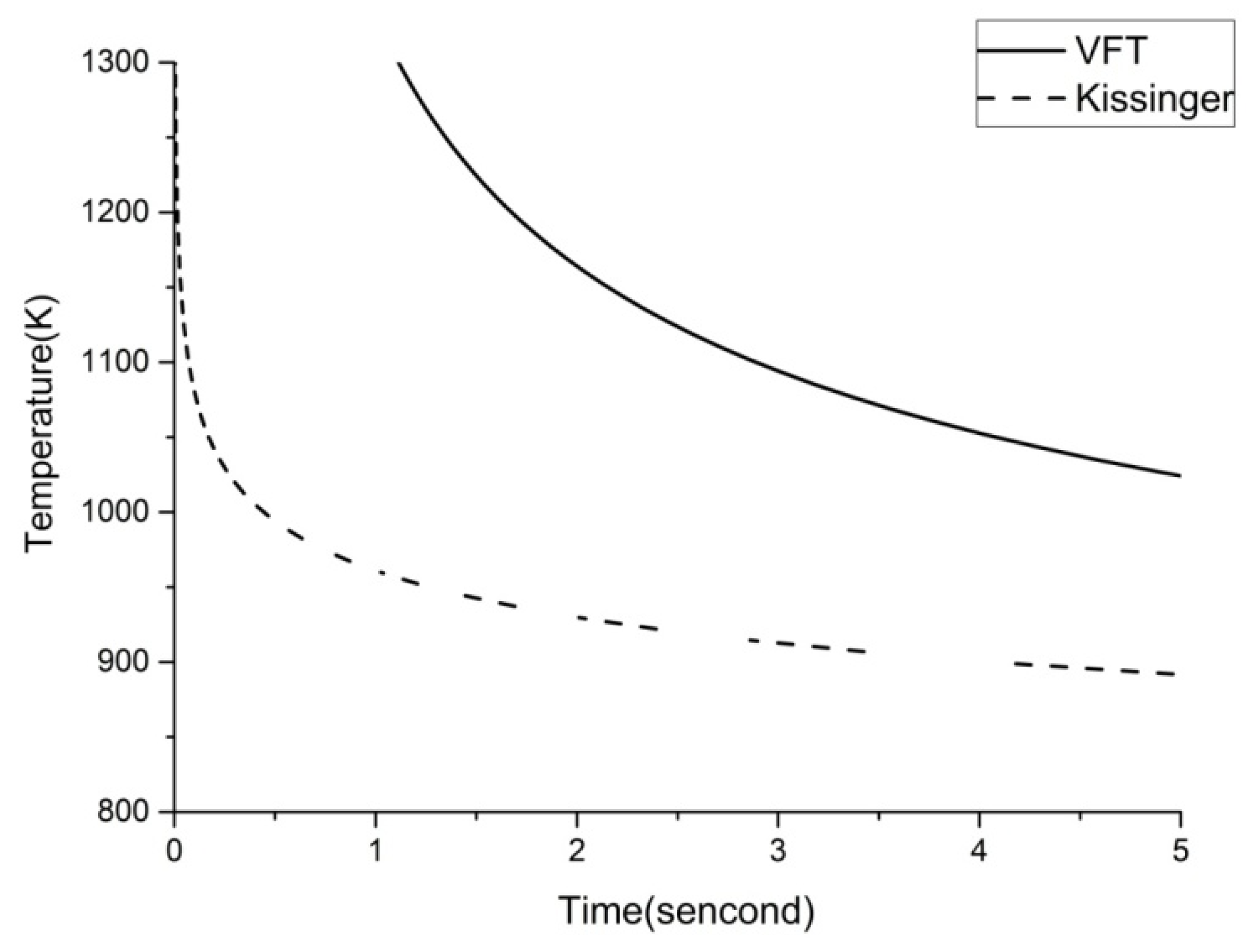


| Heating Rate (K/min) | Tg (K) | Tx (K) | Tp1 (K) | Tp2 (K) | Tp3 (K) |
|---|---|---|---|---|---|
| 5 | 623 | 682 | - | 726 | - |
| 10 | 627 | 697 | - | 735 | - |
| 20 | 630 | 712 | 726 | 746 | 802 |
| 30 | 633 | 724 | 736 | 754 | 808 |
| 40 | 636 | 732 | 744 | 759 | 815 |
| Temperature (K) | Specific Heat (J·g−1·K−1) | Thermal Conductivity (W·m−1·K−1) | Density (×10−6, kg·mm−3) |
|---|---|---|---|
| 300 | 0.382 | 4.5 | 6.12 |
| 440 | 0.411 | 6.5 | 6.12 |
| 580 | 0.523 | 10.8 | 6.12 |
| 650 | 0.724 | 14.6 | 6.13 |
| 700 | 0.543 | 17.0 | 6.19 |
| Sample Number | Power (W) | Speed (mm/s) |
|---|---|---|
| 1 | 300 | 3 |
| 2 | 300 | 6 |
| 3 | 300 | 9 |
| 4 | 300 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Song, C.; Huang, L.; Han, L.; Wang, C. Crystallization of Zr-Based Amorphous Alloys in Laser Welding. Metals 2023, 13, 1283. https://doi.org/10.3390/met13071283
Yan S, Song C, Huang L, Han L, Wang C. Crystallization of Zr-Based Amorphous Alloys in Laser Welding. Metals. 2023; 13(7):1283. https://doi.org/10.3390/met13071283
Chicago/Turabian StyleYan, Shiju, Chengli Song, Lingling Huang, Liang Han, and Chengyong Wang. 2023. "Crystallization of Zr-Based Amorphous Alloys in Laser Welding" Metals 13, no. 7: 1283. https://doi.org/10.3390/met13071283
APA StyleYan, S., Song, C., Huang, L., Han, L., & Wang, C. (2023). Crystallization of Zr-Based Amorphous Alloys in Laser Welding. Metals, 13(7), 1283. https://doi.org/10.3390/met13071283





