Effect of Mg-Ce Treatment on Inclusion Characteristics and Pitting Corrosion Behavior in EH420 Marine Steel
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Procedure
2.2. Analysis Methods
2.3. Computational Details
3. Results and Discussion
3.1. Evolution of Inclusion in Marine Steel after Mg-Ce Addition
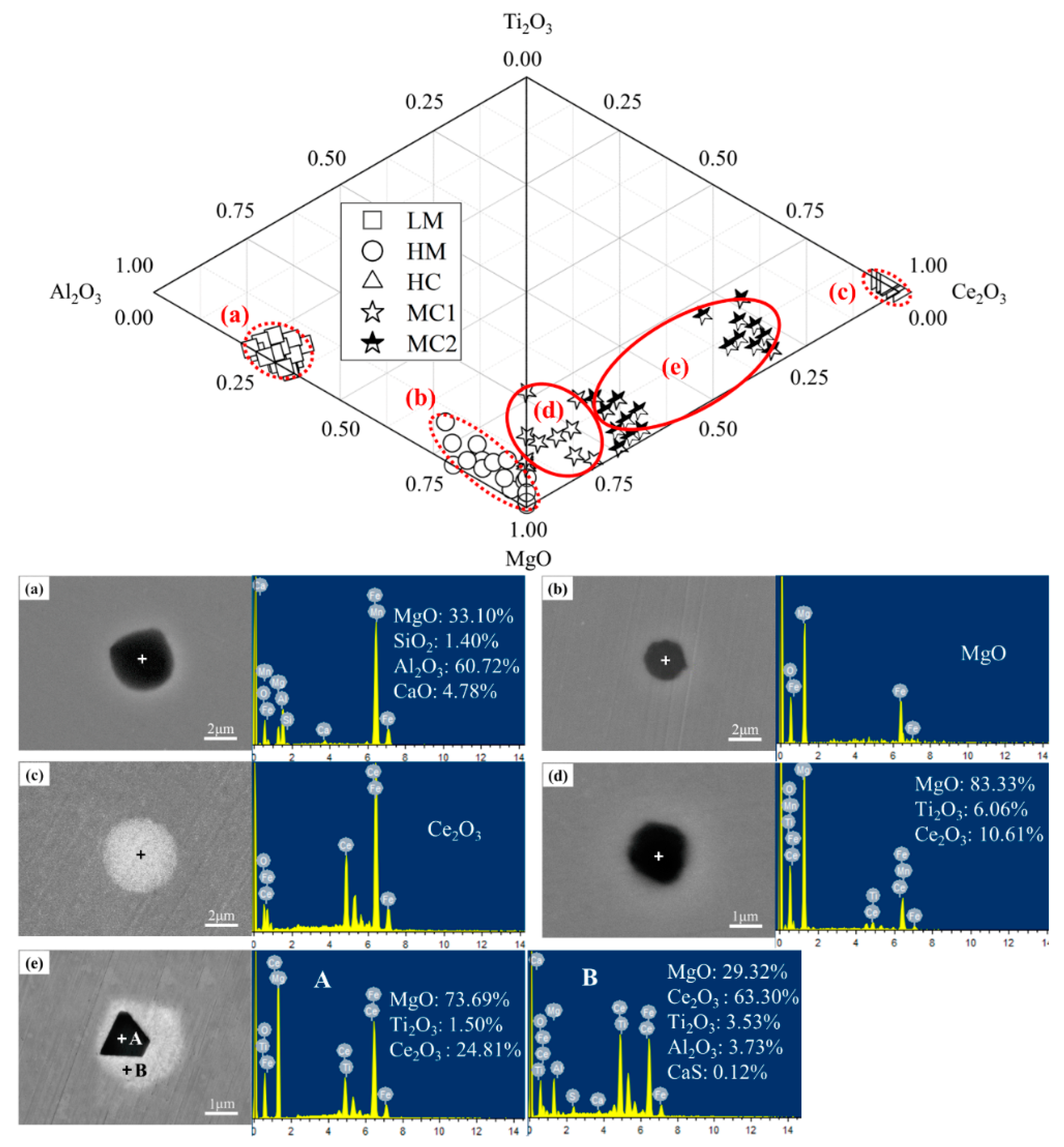
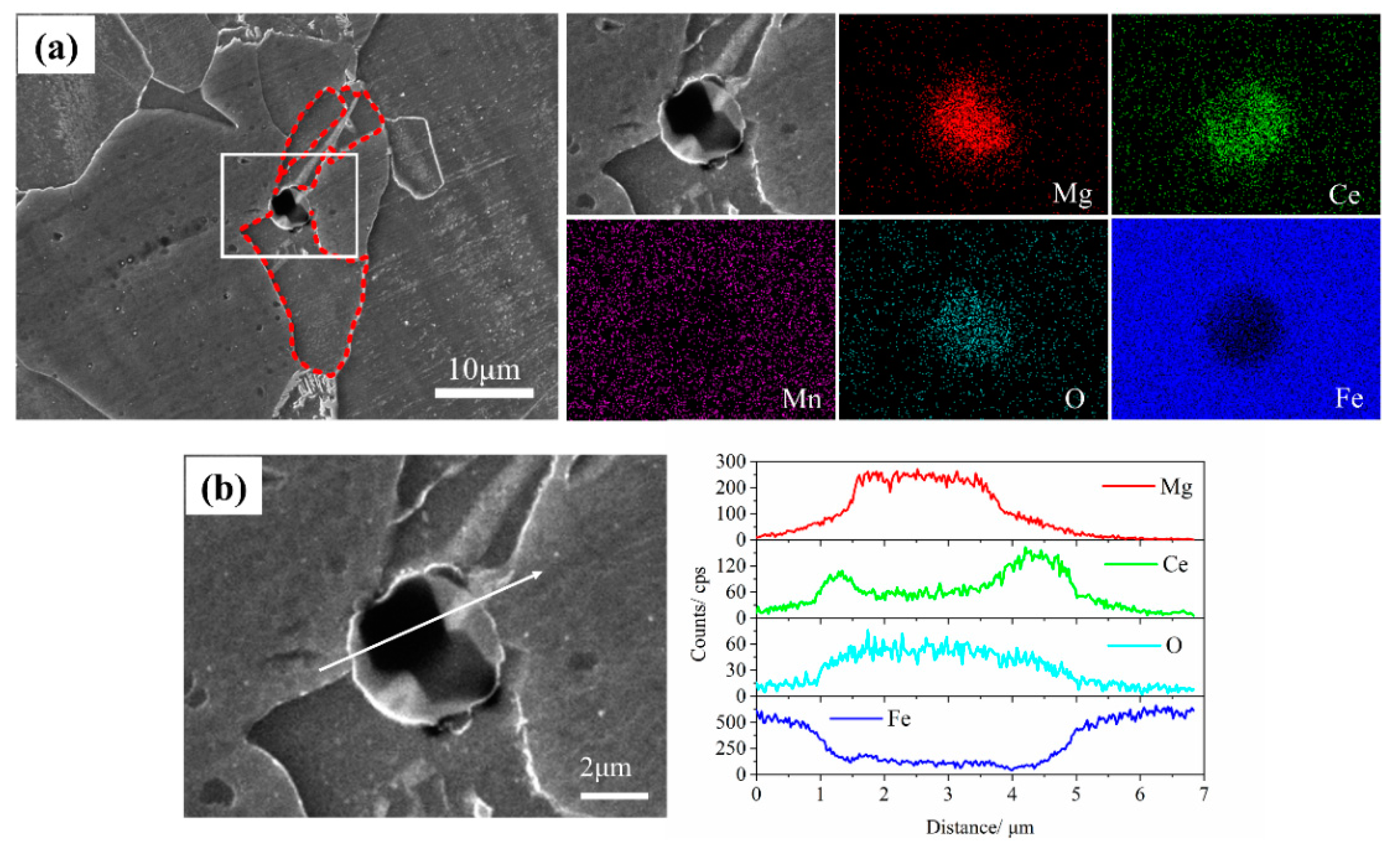
3.1.1. Effect of the Mg-Ce Addition on the Characteristics of Inclusions
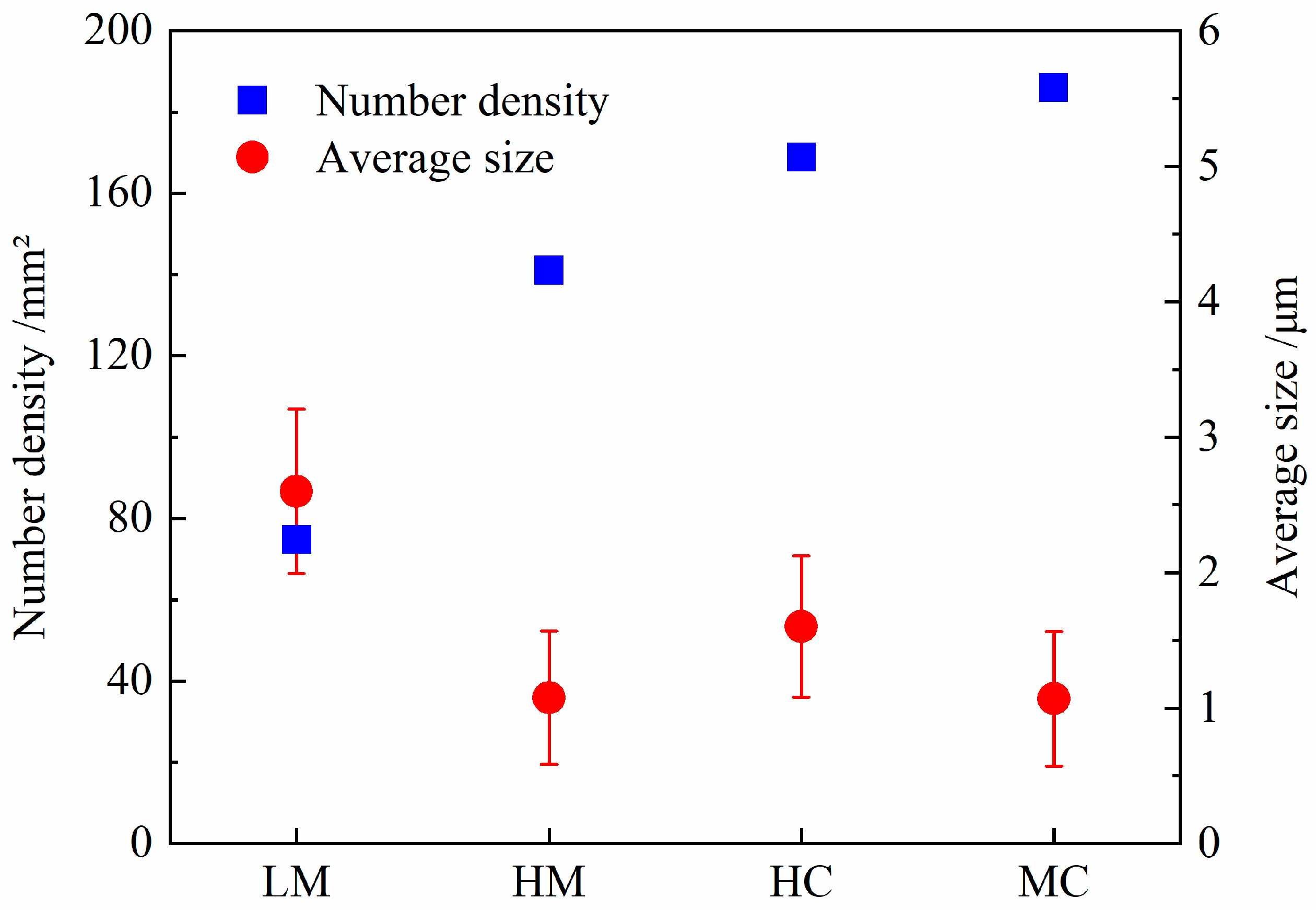
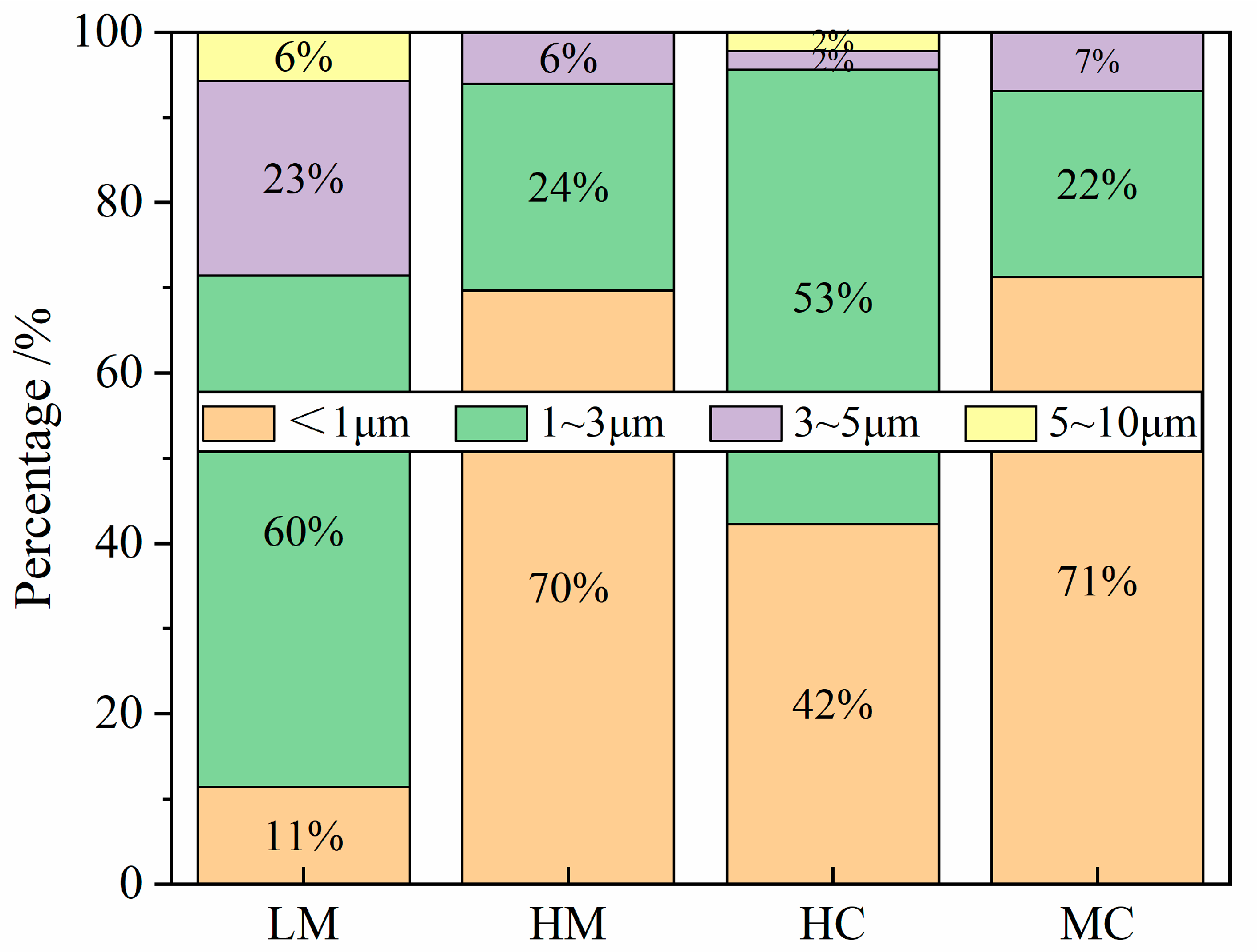
3.1.2. Effect of Mg-Ce Addition on the Number and Size Distribution of the Inclusions
3.2. Analysis of the Thermodynamic Mechanism of Inclusion Evolution
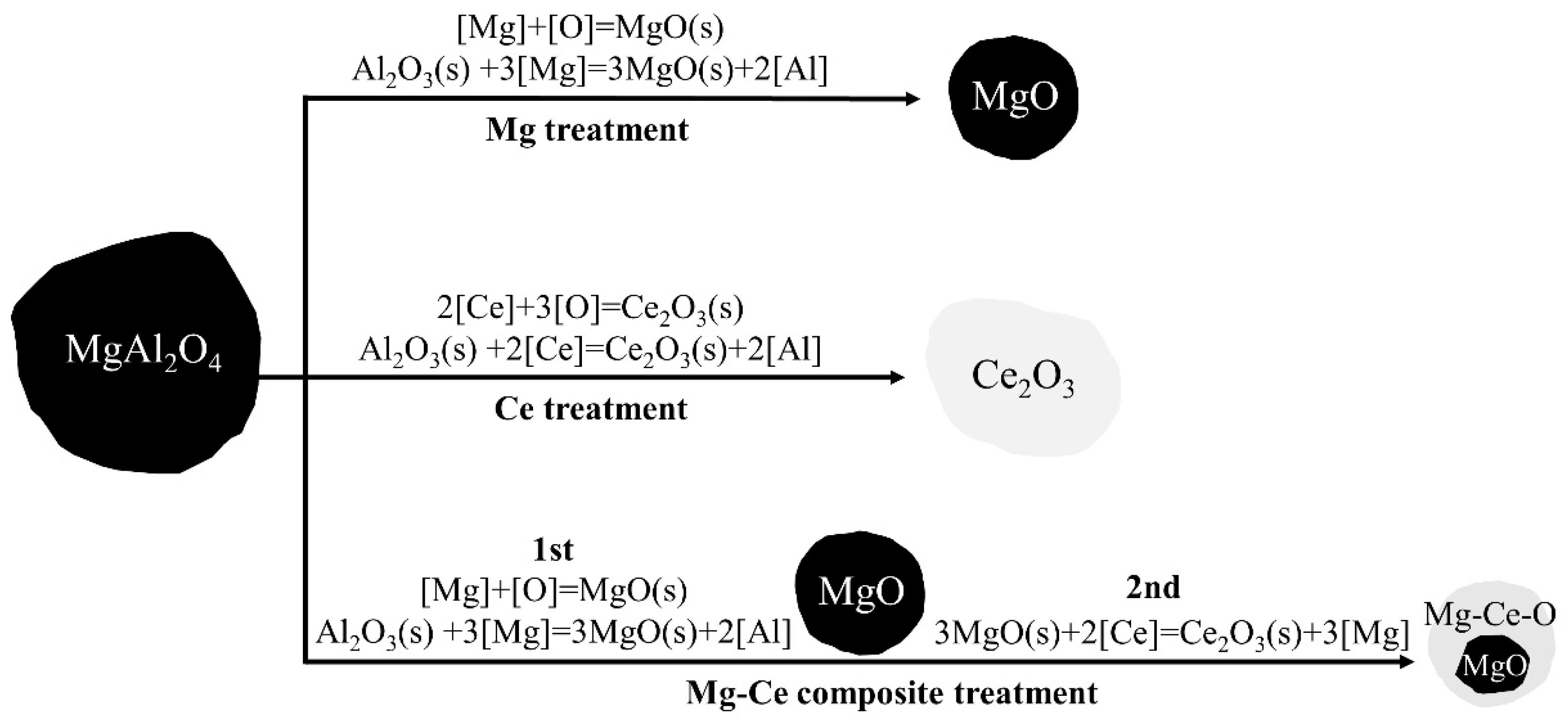
3.2.1. Analysis of Equilibrium Inclusion Evolution Based on Phase Diagram
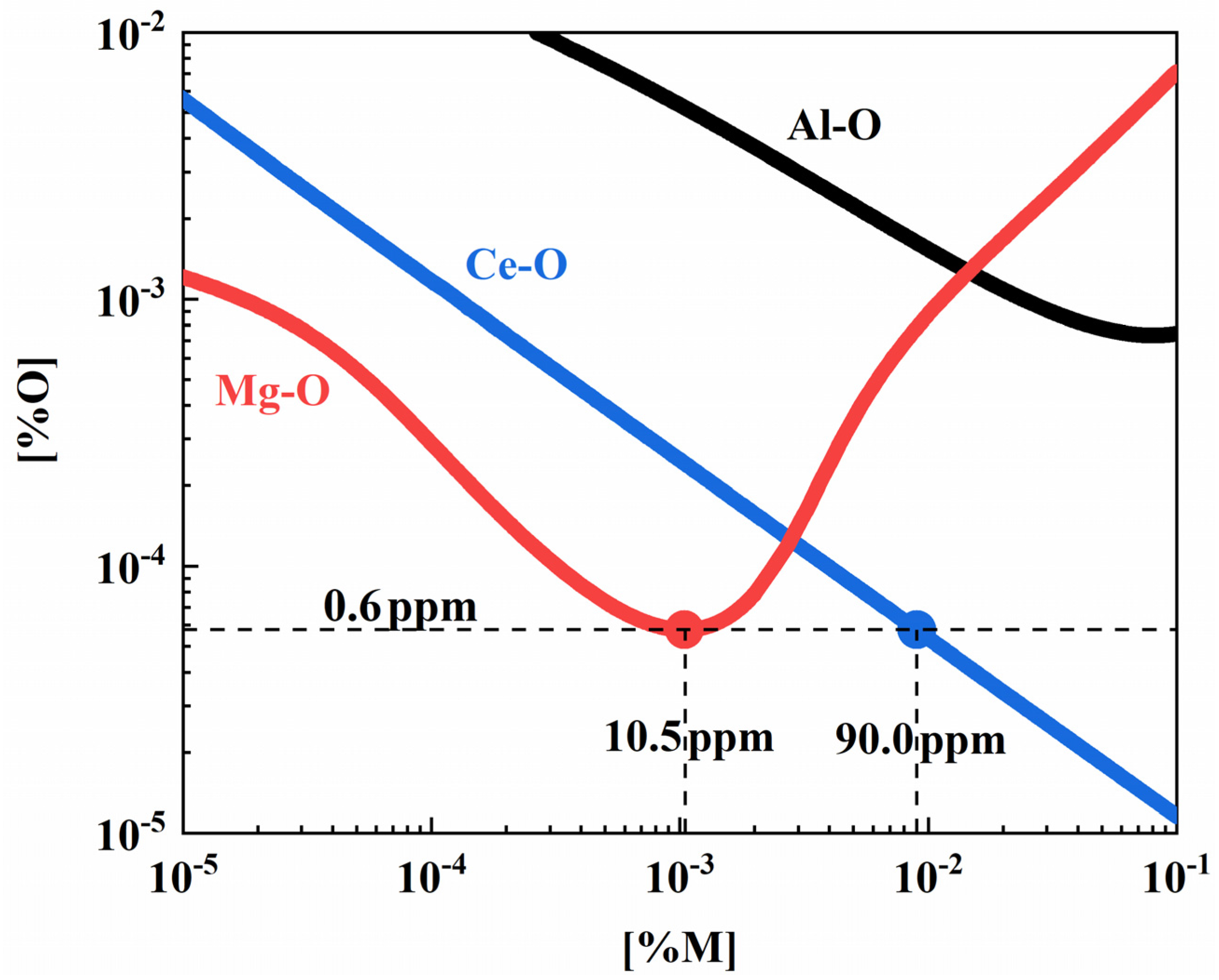
3.2.2. Analysis of Inclusion Modification Based on Reducing Ability
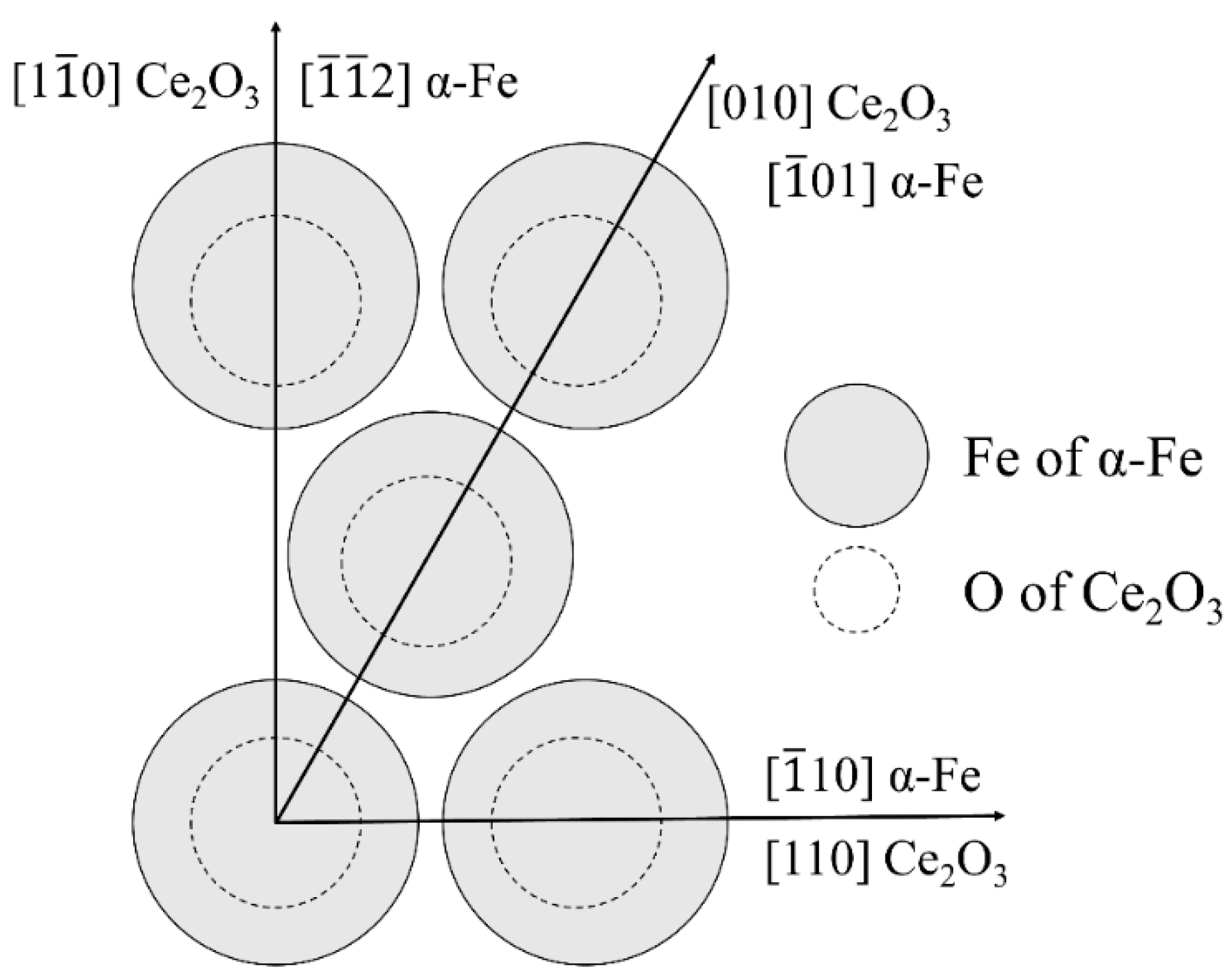
3.3. Analysis of Mg-Ce-O Composite Inclusion-Induced Acicular Ferrite Nucleation Ability
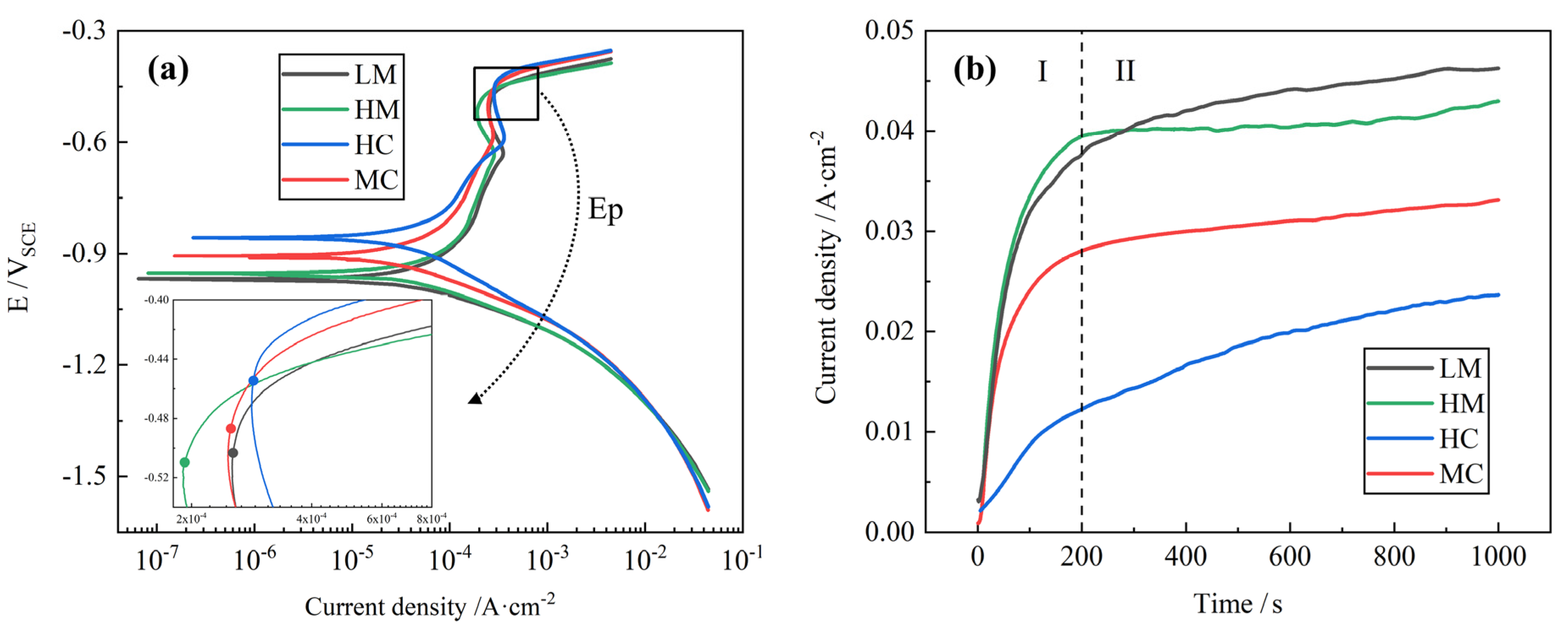
3.4. Effect of Inclusion Type on the Resistance to Pitting Corrosion
3.5. Analysis of Inclusions for Pitting Corrosion Resistance Based on Electronic Work Function
4. Conclusions
- The typical inclusions in EH420 marine steel are MgAl2O4. After Mg treatment, the inclusions can be transformed into MgO-dominated inclusions. After Ce treatment, the inclusions can be transformed into Ce2O3 inclusions. After Mg-Ce composite treatment, the inclusions can be transformed into Mg-Ce-O composite inclusions and MgO-dominated inclusions with trace Ti2O3. The experimental results are consistent with the thermodynamic calculation results;
- Mg treatment, Ce treatment, and Mg-Ce composite treatment can increase the number of inclusions and refine the size of the inclusions. Among them, the effect of Mg-Ce composite treatment is the most significant. After Mg-Ce composite treatment, the inclusion number density in MC steel is increased by 2.5 times, and the average size is reduced to 2/5 of standard steel;
- High MgO inclusions formed after Mg treatment are prone to pitting corrosion. The inclusions containing rare earth elements after Ce treatment can significantly improve the pitting resistance of steel and reduce the corrosion rate. Compared with Mg treatment, Mg-Ce composite treatment can be used to improve the corrosion resistance of steel;
- Based on first-principles calculations, it was determined that the average order of the electron work function is ΦMgO < ΦCe2O3 < Φα-Fe < ΦAl2O3. As a result of its low electron work function value, MgO can dissolve and cause pitting corrosion. In contrast, Ce2O3 has a similar electron work function value to that of the steel matrix, making it hard for it to induce pitting corrosion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Zhang, P.; Peng, X.; Yan, L.; Li, G. Comparison of microstructure and mechanical properties of high strength and toughness ship plate steel. Materials 2021, 14, 5886. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Z.; Wang, Q.; Li, X. Improving the resistance of high-strength steel to SCC in a SO2-polluted marine atmosphere through Nb and Sb microalloying. Corros. Sci. 2020, 170, 108693. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Park, J.H.; Jönsson, P.G. Non-metallic inclusions in different ferroalloys and their effect on the steel quality: A review. Metall. Mater. Trans. B 2021, 52, 2892–2925. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Jönsson, P.G. Comparison of nonmetallic inclusion characteristics in metal samples using 2D and 3D methods. Steel Res. Int. 2020, 91, 1900669. [Google Scholar] [CrossRef]
- Lou, H.; Wang, C.; Wang, B.; Wang, Z.; Misra, R.D.K. Evolution of inclusions and associated microstructure in Ti-Mg oxide metallurgy steel. ISIJ Int. 2019, 59, 312–318. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Zhu, L. Effect of inclusion size and type on the nucleation of acicular ferrite in high strength ship plate steel. ISIJ Int. 2018, 58, 965–969. [Google Scholar] [CrossRef]
- Zhang, G.; He, X.; Zhang, Q.; Wang, W.; Wang, M. Comparison of microstructure and heat treatment distortion of gear steels with and without Nb addition. J. Iron Steel Res. Int. 2021, 28, 488–495. [Google Scholar] [CrossRef]
- Sun, F.; Jordan, L.; Albin, V.; Lair, V.; Ringuedé, A.; Prima, F. On the high sensitivity of corrosion resistance of NiTi stents with respect to inclusions: An experimental evidence. ACS Omega 2020, 5, 3073–3079. [Google Scholar] [CrossRef]
- Zhou, X.; Shao, Z.; Tian, F.; Hopper, C.; Jiang, J. Microstructural effects on central crack formation in hot cross-wedge-rolled high-strength steel parts. J. Mater. Sci. 2020, 55, 9608–9622. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; Lu, B.; Zhi, J.; Zhang, S.; Liu, Y. Analysis on clogging of submerged entry nozzle in continuous casting of high strength steel with rare earth. J. Iron Steel Res. Int. 2022, 29, 34–43. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Ahmed, F.; Channa, I.A.; Inam, A.; Riaz, F.; Siyal, S.H.; Shar, M.A.; Alhazaa, A. Effect of Multiple Thermal Cycles on the Microstructure and Mechanical Properties of AISI 1045 Weldments. ACS Omega 2022, 7, 42313–42319. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, D.; Lei, H.; Li, Y.; Wang, R.; Wang, J.; Jiang, Z.; Zhang, H. Effect of addition ZrO2 nanoparticles on inclusion characteristics and microstructure in low carbon microalloyed steel. ISIJ Int. 2020, 60, 1948–1956. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, L.; Xiong, X.; Chen, L. Effect of yttrium treatment on alumina inclusions in high carbon steel. J. Iron Steel Res. Int. 2022, 29, 655–664. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.; Zhi, J.; Duan, C.; Gao, S.; Wang, M. Effect of rare earth Ce on the morphology and distribution of Al2O3 inclusions in high strength IF steel containing phosphorus during continuous casting and rolling process. ISIJ Int. 2021, 61, 657–666. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.; Cui, S. Effect of rare earth-magnesium alloy on inclusion evolution in industrial production of die steel. Steel Res. Int. 2019, 90, 1900103. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Chen, C.; Li, J.; Li, X. Effect of Mg Treatment on the Nucleation and Ostwald Growth of Inclusions in Fe-O-Al-Mg Melt. Materials 2020, 13, 3355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, C.; Jiang, M. Effect of Mg on behavior and particle size of inclusions in Al-Ti deoxidized molten steels. Metall. Mater. Trans. B 2016, 47, 2253–2262. [Google Scholar] [CrossRef]
- Kimura, S.; Nakajima, K.; Mizoguchi, S. Behavior of alumina-magnesia complex inclusions and magnesia inclusions on the surface of molten low-carbon steels. Metall. Mater. Trans. B 2001, 32, 79–85. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, L.; Xin, J.; Tian, H.; Wang, X.; Cui, Z. Corrosion evolution and stress corrosion cracking of E690 steel for marine construction in artificial seawater under potentiostatic anodic polarization. Constr. Build. Mater. 2020, 238, 117763. [Google Scholar] [CrossRef]
- Yang, C.; Luan, Y.; Li, D.; Li, Y. Effects of rare earth elements on inclusions and impact toughness of high-carbon chromium bearing steel. J. Mater. Sci. Technol. 2019, 35, 1298–1308. [Google Scholar] [CrossRef]
- Farzi, G.; Davoodi, A.; Ahmadi, A.; Neisiany, R.E.; Anwer, M.K.; Aboudzadeh, M.A. Encapsulation of Cerium Nitrate within Poly (urea-formaldehyde) Microcapsules for the Development of Self-Healing Epoxy-Based Coating. ACS Omega 2021, 6, 31147–31153. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Song, B.; Song, M.; Song, G. Effect of cerium on characteristic of inclusions and grain boundary segregation of arsenic in iron melts. Steel Res. Int. 2015, 86, 1430–1438. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W. Effect of cerium on the behavior of inclusions in H13 steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Sun, M.; Chen, C.; Jiang, Z. Experimental study on rare earth and magnesium composite treatment of 49MnVS3 non-quenched and Tempered Steel. Steel Res. Int. 2021, 92, 2100190. [Google Scholar] [CrossRef]
- Wang, C.; Hao, J.; Kang, J.; Yuan, G.; Misra, R.D.K.; Wang, G. Tailoring the microstructure of coarse-grained HAZ in steel for large heat input welding: Effect of Ti-Mg-Ce-V inclusion/precipitation particles. Metall. Mater. Trans. A 2021, 52, 3191–3197. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Choi, M.S.; Kim, J.S.; Park, Y.S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of pitting corrosion in hyperduplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]
- Liu, Z.; Song, B.; Yang, Z.; Cui, X.; Li, L.; Wang, L.; Song, Z. Effect of cerium content on the evolution of inclusions and formation of acicular ferrite in Ti-Mg-killed EH36 steel. Metals 2020, 10, 863. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, L.; Zhang, L.; Jiang, Z.; Wang, E. Influence of Ce Addition on Microstructure and Corrosion Resistance of 2101 Duplex Stainless Steel. Steel Res. Int. 2021, 92, 2100003. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Basinski, Z.S.; Hume-Rothery, W.; Sutton, A.L. The lattice expansion of iron. Proc. R. Soc. Lond. Ser. A 1955, 229, 459–467. [Google Scholar]
- Finger, L.W.; Hazen, R.M. Crystal structure and compression of ruby to 46 kbar. J. Appl. Phys. 1978, 49, 5823–5826. [Google Scholar] [CrossRef]
- Bragg, W.L. Crystal structure. Nature 1920, 105, 646–648. [Google Scholar] [CrossRef]
- Hirosaki, N.; Ogata, S.; Kocer, C. Ab initio calculation of the crystal structure of the lanthanide Ln2O3 sesquioxides. J. Alloys Compd. 2003, 351, 31–34. [Google Scholar] [CrossRef]
- Nishi, T.; Shinme, K. Formation of spinel inclusions in molten stainless steel under Al deoxidation with slags. Tetsu Hagané 1998, 84, 837–843. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Q.; Chen, D. Dissolution equilibrium of magnesium V por in liquid iron. Metall. Mater. Trans. B 1991, 22, 918–921. [Google Scholar] [CrossRef]
- Han, Q.; Feng, X.; Liu, S.; Niu, H.; Tang, Z. Equilibria between cerium or neodymium and oxygen in molten iron. Metall. Trans. B 1990, 21, 295–302. [Google Scholar] [CrossRef]
- Hino, M.; Ito, K. Thermodynamic Date for Steelmaking; Tohoku University Press: Sendai, Japan, 2010. [Google Scholar]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Lee, I.S.; Park, Y.S. Effects of sulfur addition on pitting corrosion and machinability behavior of super duplex stainless steel containing rare earth metals: Part 2. Corros. Sci. 2010, 52, 3537–3547. [Google Scholar] [CrossRef]
- Ren, P.; Tang, X.; Qin, Z.; Wang, Y.; Cai, J. Coupling Effect of Hydrostatic Pressure and Erosion on Corrosion Behavior of X70 Steel in Simulated Seawater. ACS Omega 2022, 7, 44033–44046. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, D.Y. Variations of work function and corrosion behaviors of deformed copper surfaces. Appl. Surf. Sci. 2005, 240, 388–395. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhang, J.; Zhang, L.; Ge, Y. First-principle study of the effect of cerium on the modification and corrosion of nonmetal inclusions in steel. Chin. J. Eng. 2022, 44, 1516–1528. [Google Scholar]
- Yuan, X.; Xiao, Y.; Wang, G.; Zhang, L. TiN inducing ferrite nucleation based on the bcc-Fe/TiN interfaces formation at atomic scale by first-principles calculation. Comput. Mater. Sci. 2021, 197, 110570. [Google Scholar]
- Zaid, H.; Tanaka, K.; Ciobanu, C.V.; Yang, J.M.; Kindlund, H. Growth of elastically-stiff, nanostructured, high-entropy alloy nitride, (VNbTaMoW)N/Al2O3 thin film. Scr. Mater. 2021, 197, 113813. [Google Scholar] [CrossRef]
- Caballero, R.; Quintanar, C.; Köster, A.M.; Khanna, S.N.; Reveles, J.U. Structural and Electronic Properties of Au and Au2 on an MgO(100) Surface: A DFT Cluster Embedding Approach. J. Phys. Chem. C 2008, 112, 14919–14928. [Google Scholar] [CrossRef]
- Xiao, W.; Guo, Q.; Wang, E.G. Transformation of CeO2(1 1 1) to Ce2O3(0 0 0 1) films. Chem. Phys. Lett. 2003, 368, 527–531. [Google Scholar] [CrossRef]


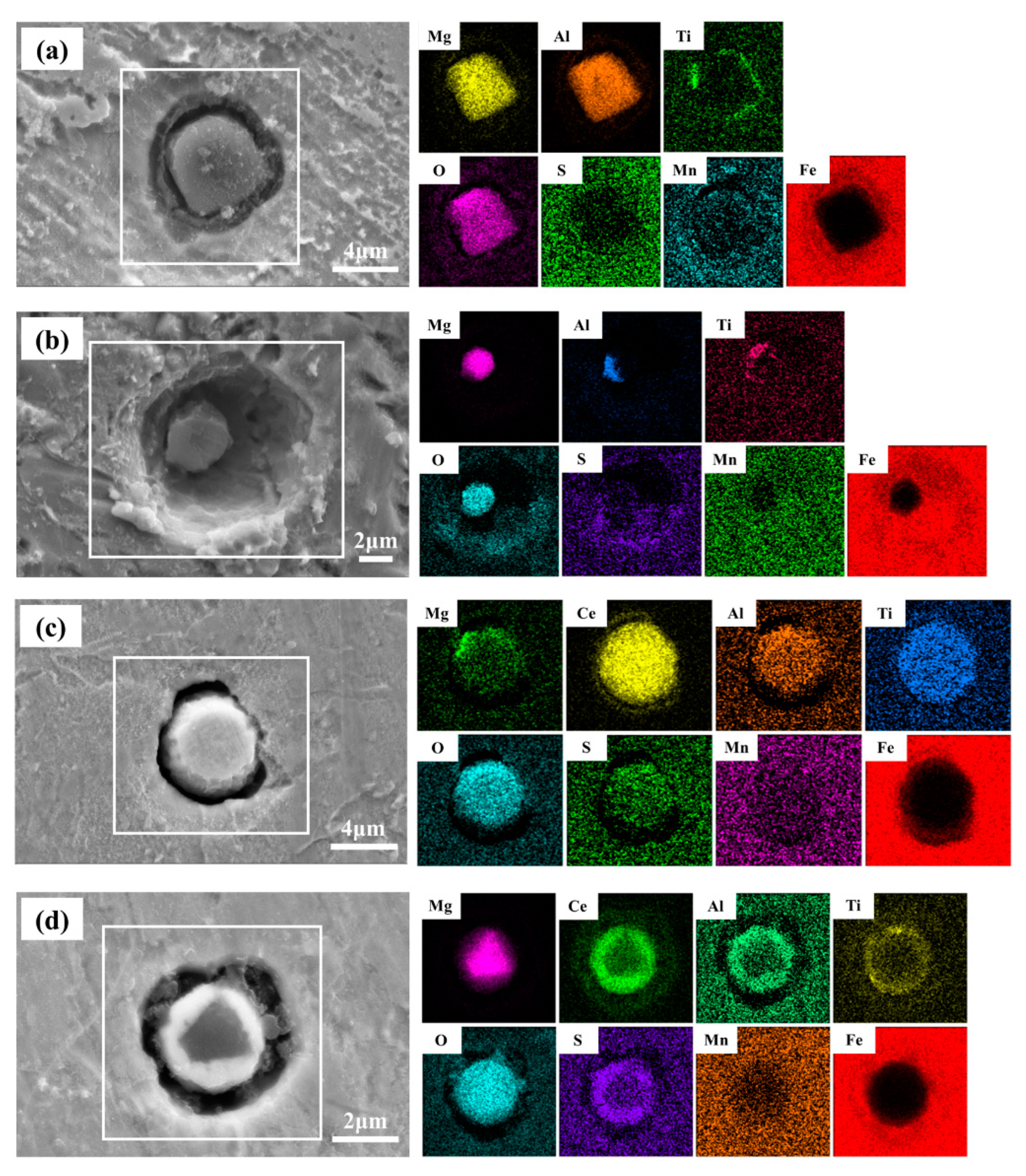
| Steel | C | Si | Mn | Ni | Cu | P | S | Al | Ti | T.O. | T.N. | Mg | Ce |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LM | 0.08 | 0.34 | 1.68 | 0.31 | 0.23 | 0.0081 | 0.0040 | 0.010 | 0.015 | 0.004 | 0.0033 | 0.0006 | — |
| HM | 0.07 | 0.38 | 1.72 | 0.28 | 0.20 | 0.0072 | 0.0039 | 0.011 | 0.010 | 0.003 | 0.0032 | 0.0022 | — |
| HC | 0.06 | 0.42 | 1.72 | 0.29 | 0.20 | 0.0077 | 0.0035 | 0.011 | 0.011 | 0.003 | 0.0030 | 0.0008 | 0.034 |
| MC | 0.07 | 0.38 | 1.72 | 0.29 | 0.20 | 0.0070 | 0.0030 | 0.012 | 0.010 | 0.003 | 0.0028 | 0.0032 | 0.014 |
| Oxides | Pearson Symbol | Space Group (NO) | Atom Positions | Lattice Parameters (Å) |
|---|---|---|---|---|
| α-Fe [33] | cl2 | Im-3m (229) | Fe 0, 0, 0 | a = 2.866, b = 2.866, c = 2.866, α = 90°, β = 90°, γ = 120° |
| Al2O3 [34] | hR30 | R-3c (167) | Al 0, 0, 0.3520 O 0.3057, 0, 0.2500 | a = 4.747, b = 4.747, c = 12.954, α = 90°, β = 90°, γ = 120° |
| MgO [35] | cF8 | Fm-3m (225) | Mg 0, 0, 0 O 0.5, 0.5, 0.5 | a = 4.220, b = 4.220, c = 4.220, α = 90°, β = 90°, γ = 90° |
| Ce2O3 [36] | P-3m | P-3m1 (164) | Ce 0.3333, 0.6667, 0.2481 O 0.3333, 0.6667, 0.6447 O 0, 0, 0 | a = 3.941, b = 3.941, c = 6.182, α = 90°, β = 90°, γ = 120° |
| Reaction | △Gθ/(J·mol−1) |
|---|---|
| Al2O3(s) = 2[Al] + 3[O] | 1202000-386.3T |
| MgO(s) = [Mg] + [O] | 728600-238.4T |
| Ce2O3 = 2[Ce] + 3[O] | 1827424-643.8T |
| C | Si | Mn | Ni | P | S | Al | Ti | O | N | Mg | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 0.091 | 0.056 | −0.004 | −0.0173 | 0.0046 | 0.030 | 0.045 | −6.6 | −0.004 | −0.13 | |
| Mg | −0.24 | −0.088 | −0.012 | −0.12 | −0.64 | −404 | |||||
| Ce | −0.077 | 0.13 | 1.746 | −39.8 | −2.25 | −5.03 | −6.599 | ||||
| O | −0.45 | −0.131 | −0.021 | 0.006 | −0.3 | −0.133 | −3.9 | −0.34 | −0.20 | −266 |
| Steel | LM | HM | HC | MC |
|---|---|---|---|---|
| Ep/mV | −503 | −509 | −456 | −487 |
| KI/mA·cm−2·s−1 | 0.173 | 0.193 | 0.052 | 0.136 |
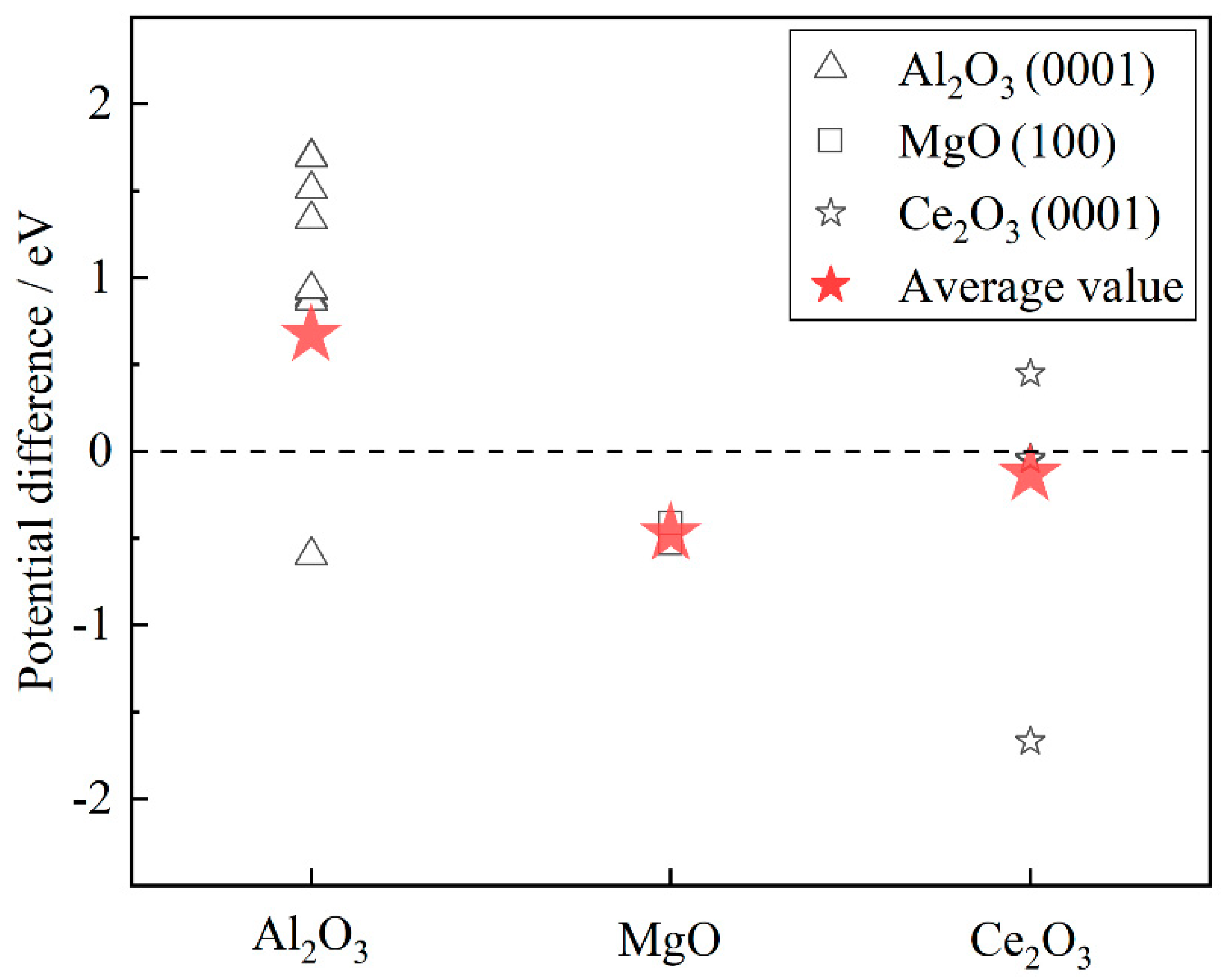
| Surfaces | Terminated Plane | Electronic Work Function/eV |
|---|---|---|
| α-Fe(110) | 1 | 4.706 |
| Al2O3(0001) | 1 | 5.636 |
| 2 | 6.404 | |
| 3 | 4.108 | |
| 4 | 5.632 | |
| 5 | 6.215 | |
| 6 | 4.108 | |
| 7 | 5.594 | |
| 8 | 6.399 | |
| 9 | 4.108 | |
| 10 | 6.043 | |
| 11 | 6.216 | |
| 12 | 4.108 | |
| 13 | 5.573 | |
| 14 | 6.401 | |
| 15 | 4.108 | |
| 16 | 5.638 | |
| 17 | 6.398 | |
| 18 | 4.108 | |
| MgO(100) | 1 | 4.174 |
| 2 | 4.291 | |
| Ce2O3(0001) | 1 | 3.036 |
| 2 | 4.655 | |
| 3 | 5.360 | |
| 4 | 5.152 | |
| 5 | 4.664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Liu, E.; Wang, Q.; Lou, X.; Liu, H.; Zheng, Y.; Wang, B.; Zhu, L. Effect of Mg-Ce Treatment on Inclusion Characteristics and Pitting Corrosion Behavior in EH420 Marine Steel. Metals 2023, 13, 1244. https://doi.org/10.3390/met13071244
Guo Z, Liu E, Wang Q, Lou X, Liu H, Zheng Y, Wang B, Zhu L. Effect of Mg-Ce Treatment on Inclusion Characteristics and Pitting Corrosion Behavior in EH420 Marine Steel. Metals. 2023; 13(7):1244. https://doi.org/10.3390/met13071244
Chicago/Turabian StyleGuo, Zhihong, Erkang Liu, Qi Wang, Xiangjie Lou, Hongbo Liu, Yaxu Zheng, Bo Wang, and Liguang Zhu. 2023. "Effect of Mg-Ce Treatment on Inclusion Characteristics and Pitting Corrosion Behavior in EH420 Marine Steel" Metals 13, no. 7: 1244. https://doi.org/10.3390/met13071244
APA StyleGuo, Z., Liu, E., Wang, Q., Lou, X., Liu, H., Zheng, Y., Wang, B., & Zhu, L. (2023). Effect of Mg-Ce Treatment on Inclusion Characteristics and Pitting Corrosion Behavior in EH420 Marine Steel. Metals, 13(7), 1244. https://doi.org/10.3390/met13071244






