Abstract
The RecoDust process is a pyrometallurgical process for treating steel mill dusts that cannot be recycled internally due to their zinc content, providing numerous benefits compared to conventional processes. State-of-the-art processes often face the problem of recycling only zinc, but not iron, which is frequently landfilled and withdrawn from a closed loop. Furthermore, these processes are also often limited to a specific zinc content in the feedstock. Within the described RecoDust smelting campaigns, basic oxygen converter dust with about 15 wt.% zinc was taken as feedstock. After high-temperature treatment of the input material, the RecoDust slag (RDS) has a zinc content of 0.4 wt.% and the crude zinc oxide (CZO) has a ZnO content of up to 80 wt.%. The RDS is suitable for use in the sinter plant as a secondary raw material. To investigate the influence of adding RDS to a common sintering mixture, sintering pot tests followed by RDI (reduction disintegration indices) tests were carried out. The influence of the admixture of RDS on the RDI values is not detectable. The CZO was fed into a soda extraction system for halogen removal. The halogen removal of this two-stage leaching was highly efficient with over 90% for chlorine.
1. Introduction
Besides the reduction of carbon dioxide emissions, from an environmental point of view, an increase in the resource efficiency is an important goal for metallurgical processes. European steel producers have increased recycling rates of by-products over the last decades to improve the circular economy, which is one of the important pillars to enhance sustainability in steelmaking processes [1,2].
Zinc is a widely used metal with a production of about 14 million tons per year; its main application is the galvanization of steel [3]. After the end of life, galvanized steel scrap is brought back into the basic oxygen furnace (BOF) or the electric arc furnace (EAF). The proportion of scrap in the BOF is between 20 and 30 wt.%. Steel mill dusts are still by-products, which are problematic regarding recycling, due to their high zinc content. Most of the literature discusses the possibilities of EAF dust recycling [4].
For BOF dusts, three main mechanisms of dust formation are described in literature [5]. With an average of 67 wt.%, the ejection of metal and slag is the main mechanism. The second one is the vaporization of metals and elements such as zinc, lead, and chlorine, which accounts for a share between 14 and 36 wt.%, and the entrainment of charged material is the third, with an amount of 0–18 wt.%, [5].
The most common process for the treatment of steel mill dust is the Waelz process; a rotary kiln is fed with pelletized materials such as coal or lime/sand. Natural gas and air generate the reducing/oxidizing atmospheres. The Waelz kiln has a typical annual capacity of 60–220 kt and recovers only one metal—zinc. Iron is collected in the Waelz slag, which can in general be used as construction material, and zinc is collected in the Waelz oxide and can be used as a secondary zinc resource [6,7]. The main disadvantage of this process is that only one valuable metal, zinc, is recycled. Iron is lost, and high maintenance costs, low productivity, and a requirement of 16 wt.% zinc make the process unusable for low-zinc-content dusts.
For low zinc-containing dusts, mostly originating from the BOF process, other recycling techniques must be used. Another different technology operated in various specific systems is the rotary hearth furnace (RHF). The RHF produces a zinc-rich dust and direct reduced iron. The most established RHF process is FASTMET, where the feed is a mixture of pelletized iron oxides and a carbon source. The temperature in the RHF has a maximum of 1300 °C for 6 to 12 min [8]. The main disadvantages of this technology are the high investment and operating costs and the high temperature requirements. This technology was suited for recycling EAF dusts with a zinc content below 5 wt.%; Due to problems such as ZnO blockages at higher zinc content, it is being reengineered to overcome these problems [9,10].
Other recycling techniques are using a shaft furnace and producing hot metal and a zinc-rich dust. These treatment solutions are the DK process and the OxyCup process; in both processes steel mill dusts are mixed with other residues, such as mill scale, BOF sludge, sinter dust, secondary dusts, ore fines, carbon fines, and cement [11,12]. In the case of the DK process, a blast furnace is used with a typical amount of about 50 wt.% BOF dust. This blast furnace allows the use of high zinc loads to be processed; the maximum zinc load of almost 40 kg/t (which corresponds to 4 wt.%) hot metal is up to 400 times higher than the average of European blast furnaces [13]. Other drawbacks of this process are the necessary agglomeration step of the feed material, the costs for the smelting step and the high energy consumption [4,14].
The OxyCup process uses a specially designed cupola furnace for the reduction and melting, based on the production of self-reducing carbon bricks. A typical feed has a ZnO content of 1 wt.% and an iron content of about 35 wt.% [11]. Disadvantages are high costs for the smelting step and a short operating cycle of the equipment [4].
Table 1 describes the main disadvantages and advantages of the most common treatment solutions for steel mill dusts.

Table 1.
Potential pyrometallurgical treatment solutions for steel mill dusts based on [14,15,16,17,18].
The aim of the RecoDust process is to separate zinc from iron in the form of an iron-rich fraction, called RecoDust slag (RDS), and a zinc-rich fraction, called crude zinc oxide (CZO). The RecoDust process could be a technology where these dusts are transferred into two valuable products—RecoDust slag (RDS) and crude zinc oxide (CZO)—without any pre-treatment. From an operational point of view, the plant is very small, and it should be possible to install as a part of the integrated still mill. Furthermore, there are no technical limitations regarding the zinc content of the dust feed.
From the economic side, the RecoDust process has enormous potential. The revenues of the two products are considered to be EUR 420 per ton of CZO and about EUR 40 per ton RDS. The price of the CZO depends on the zinc price and on the purity. The price for the RDS is assigned to a low-grade iron ore. On the OPEX side, the costs for natural gas and oxygen are about EUR 50 and 30 per ton feed, respectively. Taking treatment charges into account being normally paid by the steel mill, the RecoDust solution should be realizable.
In this paper, the RecoDust process, results of a smelting campaign, and the evaluation of the products for recycling are described.
2. Materials and Methods
In this section, the dust feed for the Flash-Reactor and the results are described. Further treatment of the two products of the RecoDust process (RDS and CZO) are mentioned.
The flowchart of the RecoDust process is shown in Figure 1. The slagging takes place in the central part called the Flash-Reactor, which is heated by a natural gas/oxygen burner with an air excess ratio below one to provide a reducing gas atmosphere. In this step, the dust feed smelts, and the non-volatile components are collected at the bottom of the Flash-Reactor as the RDS (product #1). The volatile components are evaporated and leave the Flash-Reactor with the flue gas, which is fired in the next process step—the converter (post-combustion). After further quenching of the flue gas, the cleaning takes place, where the CZO (product #2) is collected.
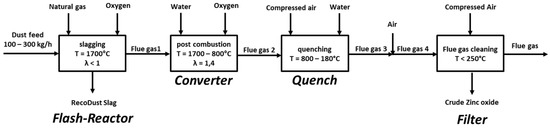
Figure 1.
Flowchart of the RecoDust process, reprinted with permission from [20], Springer, 2020.
2.1. Feed Material and Characterization
The dust feed used in the experiments is a by-product from an industrial BOF. For the use in the pneumatic conveying machine, the fraction > 400 µm was sieved out. The investigation starts with the chemical analysis of the feed material. The feed material must be dry and free-floating, and the grain size is limited to 400 µm.
2.2. RecoDust Pilot Plant and Methodology
The pyrometallurgical experiments were carried out in the RecoDust pilot plant at the Chair of Thermal Processing Technology, Montanuniversitaet Leoben (Leoben, Austria). A smelting campaign consists of up to eight trials with up to 250 kg dust feed. The dosing rate of the feed depends on the chemical composition of the dust feed and can reach a maximum of 250 kg/h. One important challenge/requirement for a high zinc separation (“dezincification”) is to reach a desired uniform dosing rate over the whole process duration. The pilot plant is shown in Figure 2. Coming from the storage tank, the BOF dust is sent pneumatically to the burner of the Flash-Reactor with natural gas. The pneumatic conveying is designed for entrained flow and the transport gas is also part of the fuel gas and reducing agent. Due to this, the input material is fed into the central part of the flame, leading to a very fast smelting and reduction/slagging of the feed material with very high chemical reaction rates. Physical properties such as grain size distribution and bulk density help to estimate the optimal pneumatic conveying conditions. The main chemical reactions in the Flash-Reactor are:
3Fe2O3 + H2 → 2Fe3O4 + H2O
Fe3O4 + H2 → 3FeO + H2O
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + CO → 3FeO + CO2
ZnO + H2 → Zn + H2O
ZnO + CO → Zn + CO2
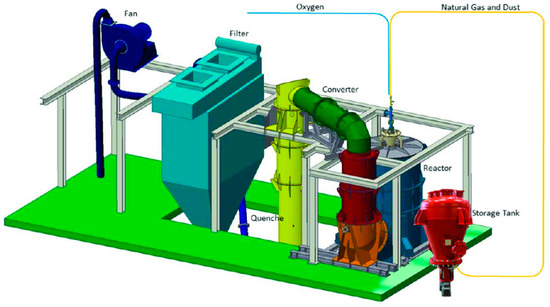
Figure 2.
Three-dimensional sketch of the RecoDust pilot plant.
The primary explosion protection of the transmitting vessel is done by inerting with nitrogen. In the process, the transmitting vessel is purged with nitrogen until the oxygen concentration is below the limiting oxygen concentration of 10%. After conveying and before opening the transmitting vessel, the inerting is performed again by nitrogen purging until the methane concentration is below 4%. Due to the high metallization degree of the BOF dust (10–35%), natural gas is also a good option as a conveying gas apart from nitrogen. If oxygen would be used, a potential exothermic oxidation reaction of the metallic iron could occur (local hot spots being a safety-related handling problem). The occurring chemical reaction is:
xFemet. + y/2O2 ↔ FexOy
The conditions of the executed smelting campaign K12 (six trials K12-1 to K12-6 were done) are shown in Table 2. All six trials were carried out with a Flash-Reactor temperature of 1700 °C. The air excess ratio of the burner is 0.7 in the first four trials K12-1 to K12-4 and 0.8 in the last two trials, K12-5 and K12-6. One trial has a total feed of up to 250 kg.

Table 2.
Experimental program and conditions of the smelting campaign K12.
When the dosing of the dust feed is done, both products, the iron-rich RDS and the zinc-rich CZO, are collected, and samples are taken for wet chemical analysis.
2.3. Evaluation of the Products of the RecoDust Process: The RDS and the CZO
Due to the low zinc content of the RDS (<0.5%), process internal recycling at the sinter plant or the blast furnace (depending on the grain size distribution) is possible. If the RDS is used at the sinter plant, the effects of its usage there can be pretested in laboratory scale. These tests were operated in a so-called lab-scale sinter pot (~200 kg material input). Before operating the sinter pot, the RecoDust slag was mixed to a common sinter mixture with an amount of 3.29 wt.% and sinter pot tests were carried out. Afterwards, RDI tests according to ISO 4696-1 [21] of the finished sinter products are conducted and the mixture with RDS was compared with a mixture without it. The sample designation is shown in Table 3; those with RDS in the mixture are designated as MRDS1 and MRDS2 and those without RDS in the mixture as BMA1 and BMA2.

Table 3.
Designation of the samples for the low-temperature reduction disintegration, data from [22].
The RDI test consists of two steps. The first step, the low-temperature reduction disintegration, is carried out in a vertical reduction furnace, shown in Figure 3, at a temperature of 500 °C and a gas composition of 58% nitrogen, 20% carbon monoxide, 20% carbon dioxide, and 2% hydrogen. Each sample has a weight of at least 2 kg and a grain size between 10 and 12.5 mm. In the second step, the sample from the reduction tube is placed into the tumble drum, which rotates for a total of 300 revolutions at a rate of 30 revolutions per minute. The tumbled samples are sieved at 6.30 mm, 3.15 mm, and 500 µm. The used tumble drum is shown in Figure 4.
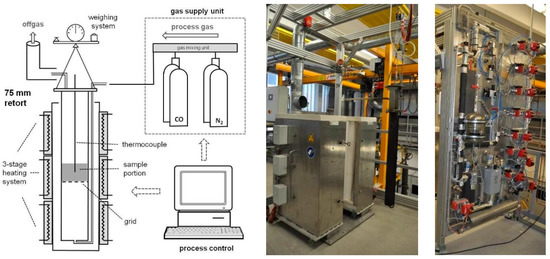
Figure 3.
Vertical reduction furnace (Montanuniversitaet Leoben, Chair of Ferrous Metallurgy), reproduced from [23].
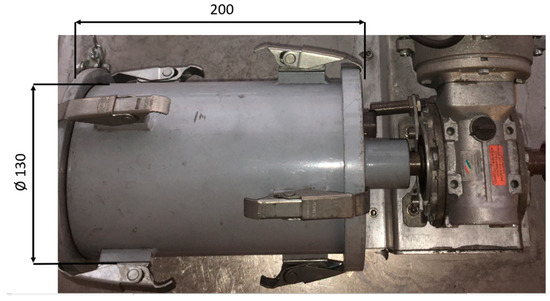
Figure 4.
Tumble drum (Chair of Ferrous Metallurgy, Montanuniversitaet Leoben).
CZO, the second product of the RecoDust process, is treated by a so-called double-step leaching process to decrease the chlorine content of the CZO as a result of its harmful behavior in a zinc electrolysis since the aim is to achieve a product quality that can be in principle used as secondary raw material in the primary zinc industry (no search regarding the current demand on secondary raw materials in the zinc industry was performed during this study). The decrease of chlorine in this case is more important than fluoride since the chlorine content in the feedstock is five times higher.
The applied leaching process consists of two steps and was supported by Chair of Nonferrous Metallurgy, Montanuniversitaet Leoben.
In the first step, the dust is leached at 60–70 °C with pH~10 and a solid/liquid ratio of 250 g/under addition of soda (Na2CO3). The alkaline metals and chlorine are thereby leached with a yield of approx. 90%. The addition of soda is necessary for a precipitation of dissolved zinc (reaction 1) as a hardly soluble carbonate.
ZnCl2(aq) + Na2CO3 ↔ ZnCO3(s) + 2NaCl (reaction 1)
A solid–liquid separation of the filter cake prepares the material for the second step, in which fresh water is heated up to 50–60 °C at a pH~10 and a solid/liquid ratio of 500 g/L. By adding soda, the halogen content is further reduced. The flowsheet of the double-step soda leaching is shown in Figure 5.
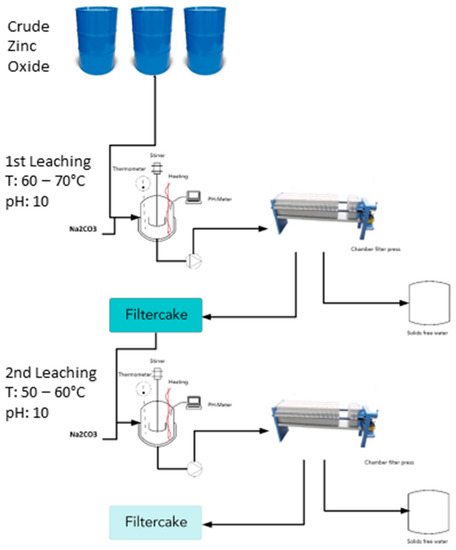
Figure 5.
Flowsheet of the double-step leaching treatment of the CZO (Montanuniversitaet Leoben, Chair of Nonferrous Metallurgy).
3. Results
3.1. Feed Material Characterization
The results of the chemical analysis are shown in Table 4. The iron content of the dust feed is about 50 wt.%. The metallization degree of the sample is calculated with the following formula [24]:
The reduction degree of iron is calculated with [24]:
Oxygen (O) and total iron (Fetot) must be given in Mol.
The basicity B2 is calculated with
The basicity B4 is calculated with

Table 4.
Chemical composition of the dust feed.
Table 4.
Chemical composition of the dust feed.
| Species | Dust Feed [wt.%] |
|---|---|
| CaO | 8.45 |
| Fetot | 50.05 |
| Femet | 20.78 |
| FeRed | 31.06 |
| Zn | 14.60 |
| B2 | 6.40 |
| B4 | 7.01 |
| SiO2 | 1.32 |
| Al2O3 | 0.11 |
| MgO | 1.57 |
| Cl | 0.06 |
| F | 0.01 |
3.2. Chemical Analysis of the RecoDust Slag and the Crude Zinc Oxide
As mentioned before, a uniform dosing rate is necessary for a high dezincification rate. The target dosing rate during the first four trials was 140 kg/h and 170 kg/h in trials K12-5 and K12-6. In Figure 6, the dosing rates of the six trials K12-1 to K12-6 are shown. The worst dosing rate occurs in trial K12-1 with a dosing of rate up to 830 kg/h. During trial K12-4, the target dosing rate of about 140 kg/h could be achieved for almost the full duration of the process. Trials K12-5 and K12-6 had a stop during the trial due to technical problems, so they are split into two parts. After the break of trial K12-5, the dosing rate was much lower (125–160 kg/h) than the target dosing rate of 170 kg/h. In contrast, the dosing rate during trial K12-6 after the stop started higher than the target dosing rate with more than 250 kg/h and ended at about 188 kg/h.
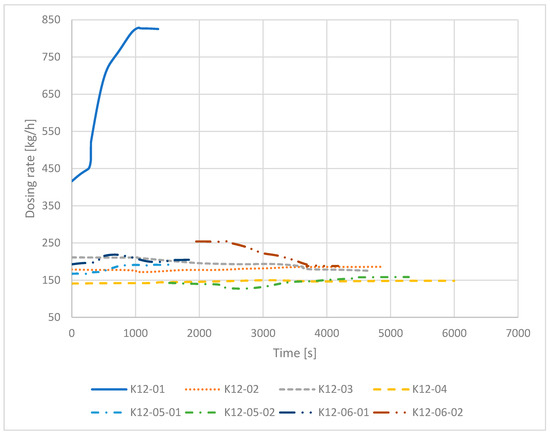
Figure 6.
Dosing rate of trials K13-01 to K13-06 over the feeding time.
3.2.1. Chemical Analysis of the RDS
Figure 7 shows the most important chemical parameters, which are the content of Fetot, Al2O3, and zinc derived from the chemical analyses. Furthermore, the calculated FeRed is shown. The zinc content of the RDS in trial K12-1 shows a significantly higher value with a proportion ~4 wt.% due to the high dosing rate of up to 830 kg/h. Trials K12-2 to K12-4 show a decreasing zinc content, from 0.54 to 0.41 wt.%, probably because of the lowest dosing rate in trial K12-4. Trial K12-5 was carried out with an air excess ratio of 0.8 and shows a further decrease in the zinc content to 0.3 wt.-%. Trial K12-6 has a higher zinc content, with a value of 0.87 wt.%, which is most likely a result of the lower dosing rate in trial K12-5 and the higher dosing rate in trial K12-6.
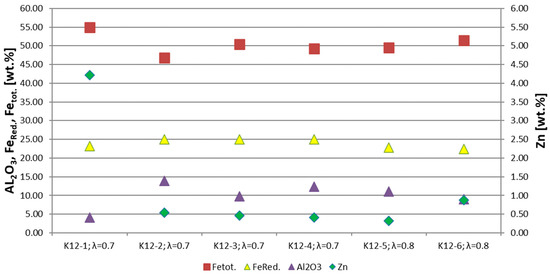
Figure 7.
Most important chemical parameters of the RDS of 6 test trials.
The Al2O3 content is an important indicator for the wear of refractory material. The RDS in trial K12-1 has an Al2O3 content of 4 wt.%, which is clearly below that of 9 to 14 wt.% in trials K12-2 to K12-6. This is because the higher dosing rate leading to a shorter residence time of the RDS in the Flash-Reactor until the tapping starts. The iron reduction degree shows no correlation with the air excess ratio and is between 15 and 20% in all six trials. The total iron content of the RDS in trial K12-1 shows the highest value with ~55 wt.% due to the lower thinning effect of the refractory. Trials K12-2 to K12-6 have total iron contents of 47 to 53 wt.%.
Thinning effects with remaining RDS from previous trials should be mentioned; for example, in case trial K12-2, the remaining RDS from trial K12-1 has a higher zinc content of more than four wt.%.
3.2.2. Chemical Analysis of the Crude Zinc Oxide
The chemical parameters of the CZO are shown in Figure 8, the most important one being the ZnO content. The CZO of trial K12-1 has the highest ZnO content with 83 wt.%, which is most likely based on the dosing rate of more than 800 kg/h and the different conveying conditions. In trials K12-2 to K12-6, the ZnO content lies between 65 and 80 wt.%. Based on this, there is no correlation between the air excess ratio and the ZnO content of the RDS. Trial K12-1 shows the lowest total iron content with ~5 wt.%. In the other trials, the total iron content lies between 9 and 18 wt.%. Another important parameter for the quality of the CZO is the chlorine content, which decreases from 1.05 wt.% in trial K12-1 to 0.4 wt.% in trial K12-6. In summary, a too-high dosing rate during K12-1 led to higher contents of volatile components, such as zinc, and chlorine and lower contents of non-volatile components, such as Fetot.
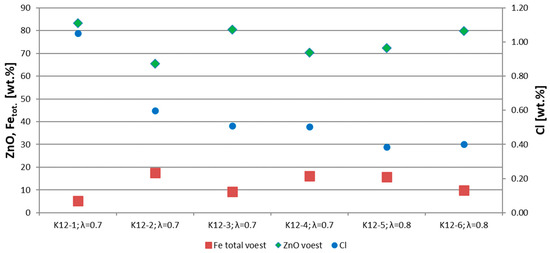
Figure 8.
Chemical composition of trials K12-1 to K12-6.
3.2.3. RDI Tests with the RDS
The results of the RDI tests are shown in Figure 9. The RDI + 6.3 (the figure “6.3” in RDI + 6.3 means the share of material remaining after sieving of 6.3 mm; RDI test is described in Section 2.3) is between 71 and 75% for all samples, the RDI − 3.15 (again, the figure “−3.15” means the share of material being with particle sizes smaller than 3.15 mm after sieving) is between 10–12%, and the RDS − 0.05 is between 2.3–2.99%. The RDI + 6.3 of every sample is between 71.1 and 74.6%. Samples MRDS1 and MRDS2 have an RDI + 6.3 of 73.71 and 74.4%, in the middle of the other samples. The RDI − 3.15 values of samples MRDS1 and MRDS2 (11.85 and 10.49%) are also in the middle of all samples. Regarding the value RDI − 0.05, there is also no significant deviation of the samples with RDS compared to the samples without RDS [22]. To sum up, the addition of RDS to the sinter pot mixture has no influence on the RDI values +6.3, −3.15, and −0.05 and can therefore be considered as being suitable regarding the mechanical stability of the final sinter product.
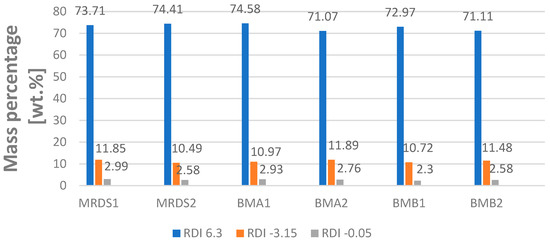
Figure 9.
RDI content of the mixture with 3.3 wt.% RDS in comparison to the mixture without RDS, data from [22].
3.2.4. Soda Leaching
The results of both soda leaching trials are shown in Table 5. The most important parameter is the removal of chlorine. The iron and zinc content of every product is also shown in Table 5. The feed has a chlorine concentration of 3790 mg/kg, which equals to 0.379 wt.%. After the first washing step, the concentration of chlorine decreased to 282 (VK1/1) and 283 mg/kg (VK2/1) and to 212 (VK1/2) and 211 mg/kg (VK2/2) after the second washing step, respectively. The concentration of zinc increased from 59.5 wt.% in the feed to 61.7 or 61.5 wt.% after the second washing step. The concentration of iron decreased from 12.9 wt.% in the feed to 12.2 wt.% after the second washing step. The results showed the suitability to upgrade the quality of the CZO being an important indicator that RecoDust produces a zinc-enriched material being potentially useable in zinc industry.

Table 5.
Content of zinc, total iron, and chlorine of the feed, after the first and second washing steps of both samples.
4. Discussion and Conclusions
In the current paper, a method for the efficient selective recovery of iron and zinc from steel mill dusts is presented and experimentally demonstrated. To recycle the iron-rich fraction of the RecoDust process, which is the RDS, a certain purity must be reached with special focus on the zinc content to be as low as possible. Due to the new pneumatic conveying system of the RecoDust pilot plant, a high zinc separation rate (“dezincification”) of more than 98% could be observed; however, the zinc separation efficiency strongly depends on achieving the target dosing rate. In the trials shown here, the zinc content of the RDS equaling to 0.4 wt.% demonstrated the ability that RecoDust can produce an Fe-rich product with enough quality for an internal recycling as secondary iron source. The 0.4 wt.% zinc is much lower than that of the commissioning phase of the pneumatic conveying system (when starting the pneumatic conveying system, zinc content of 3.1 wt.% was obtained in the RDS) [25]. This low zinc content now being obtained in the RDS is due to the stable uniform dust dosing rate managed by the pneumatic conveying system. The lowest RDS zinc content was achieved in trial K12-5 using an air excess ratio of 0.8. Trial K12-6, which also had an air excess ratio of 0.8, shows a higher zinc content in the RDS due to two reasons. On the one hand, the dosing rate was higher than the target dosing rate. On the other hand, the air excess ratio was higher (0.8 instead of 0.7). These results show that, by achieving the target dosing rate, a higher air excess ratio can lead to the same dezincification and makes the RecoDust process more efficient. This high dezincification of the dust feed is necessary to reuse the RDS as an iron ore substitute. To understand the influence of RDS in the sinter mixture, some sinter pot and subsequent RDI tests were conducted and compared to a standard sinter mixture without RDS. The values of RDI + 6.3, RDI − 3.15, and RDI − 0.05 show no significant deviation between the mixture with RDS and a standard sinter without RDS. This test should be repeated to obtain a deeper understanding of the RDS influence on the final sinter.
The goal to create a CZO product purity, which could be in principle returned to the primary zinc industry, has been fulfilled. Although the high Fetot content in the CZO has a negative impact on the possible revenue, it must be considered that the CZO comes from a pilot plant. Additionally, an optimized burner and Flash-Reactor geometry should make it possible to further reduce the total iron content in the CZO. To remove chlorine of the CZO, a double-step soda leaching was applied successfully.
With the results described in this paper, the possibility of treating steel mill dusts with possible recovery routes for the RDS and the CZO are described. In summary, the RecoDust process has several advantages over the state-of-the-art processes, which have no requirement for a pre-treatment, a compact plant size, and the possibility of a selective zinc and iron recovery. Furthermore, the dezincification rate with this feed material is about 98%. Another challenge for future research is to demonstrate the flexibility of RecoDust in terms of treating different feed materials. Apart from dusts with varying zinc contents, the mixture of BOF and EAF dusts will also be investigated. From the current point of view, a large spectrum of dusts (BOF, EAF, BOF-EAF mixture) should be processable with RecoDust to derive secondary iron- and zinc-rich products with enough purity for a further internal recycling (RDS to be returned into the sinter plant or BF) or external recycling (CZO to zinc industry).
Author Contributions
Conceptualization, W.R.; methodology, W.R. and L.C.; investigation, W.R.; methodology, W.R. and L.C.; writing—original draft preparation, W.R.; writing—review and editing, W.R., J.R., H.R., N.M., D.K., A.A. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the funding support of K1-MET GmbH, metallurgical competence center. The research program of the K1-MET competence center is supported by COMET (Competence Center for Excellent Technologies), the Austrian program for competence centers. COMET is funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation, and Technology, the Federal Ministry for Labor and Economy, the Federal States of Upper Austria, Tyrol, and Styria as well as the Styrian Business Promotion Agency (SFG) and the Standortagentur Tyrol. Furthermore, Upper Austrian Research GmbH gives continuous support. Besides the public funding from COMET, this research project is partially financed by the scientific partner Montanuniversitaet Leoben and the industrial partner Novolipetsk Steel.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef]
- Rieger, J.; Colla, V.; Matino, I.; Branca, T.A.; Stubbe, G.; Panizza, A.; Brondi, C.; Falsafi, M.; Hage, J.; Wang, X.; et al. Residue Valorization in the Iron and Steel Industries: Sustainable Solutions for a Cleaner and More Competitive Future Europe. Metals 2021, 11, 1202. [Google Scholar] [CrossRef]
- International Lead & Zinc Study Group. Available online: https://www.ilzsg.org/static/statistics.aspx?from=1 (accessed on 4 February 2022).
- Xue, Y.; Hao, X.; Liu, X.; Zhang, N. Recovery of Zinc and Iron from Steel Mill Dust—An Overview of Available Technologies. Materials 2022, 15, 4127. [Google Scholar] [CrossRef] [PubMed]
- Nedar, L. Dust formation in a BOF converter. Steel Res. 1996, 67, 320–327. [Google Scholar] [CrossRef]
- Grudinsky, P.I.; Zinoveev, D.V.; Dyubanov, V.G.; Kozlov, P.A. State of the Art and Prospect for Recycling of Waelz Slag from Electric Arc Furnace Dust Processing. Inorg. Mater. Appl. Res. 2019, 10, 1220–1226. [Google Scholar] [CrossRef]
- von Billerbeck, E.; Ruh, A.; Kim, D.-S. Verarbeitung von Filterstäuben aus der Elektrostahlerzeugung im Wälzprozess. In Mineralische Nebenprodukte und Abfälle–Flaschen, Schlacken, Stäube und Baurestmassen; TK Verlag: Neuruppin, Germany, 2014; pp. 387–398. [Google Scholar]
- Kikuchi, S.; Kobayashi, I.; Tsuge, O.; Toduka, K. Features of FASMTET Process. Kobelco Technol. Rev. 2010, 29, 77–84. [Google Scholar] [CrossRef]
- Suetens, T.; Klaasen, B.; van Acker, K.; Blanpain, B. Comparison of electric arc furnace dust treatment technologies using exergy efficiency. J. Clean. Prod. 2014, 65, 152–167. [Google Scholar] [CrossRef]
- Nakayama, T.; Taniishi, H. Dust recycling technology for electric arc furnace using RHF. Nippon. Steel Eng. Tech. Rev. 2011, 2, 25–29. [Google Scholar]
- Lemperle, M.; Jennes, R.; Cappel, J. Oxycup Furnace Operation at Tisco. In Proceedings of the Technical Contiribution to the 6th International Congress on the Science and Technology of Ironmaking—ICSTI/42nd ABM Ironmaking Seminar % 13th ABM Iron Ore Symposium, Rio de Janeiro, Brazil, 14–18 October 2012. [Google Scholar]
- Sassen, K.-J.; Hillmann, C. The dk Process—A Solution for the By-Products of the European Steel Industry. In Proceedings of the 21st International Conference on Metallurgy and Materials, Brno, Czech Republic, 23–25 May 2012. [Google Scholar]
- Sassen, K.-J.; Hillmann, C. Verwertung eisenhaltiger Reststoffe zu Roheisen und Zinkkonzentrat. Miner. Nebenprodukte Und Abfälle 2015, 2, 431–446. [Google Scholar]
- Stewart, D.J.; Barron, A.R. Pyrometallurgical removal of zinc from basic oxygen steelmaking dust—A review of best available technology. Resour. Conserv. Recycl. 2020, 157, 104746. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.-Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Zhang, X.; Wang, P.; She, X. Numerical simulation of the direct reduction of pellets in a rotary hearth furnace for zinc-containing metallurgical dust treatment. Int. J. Min. Met. Mater 2013, 20, 636–644. [Google Scholar] [CrossRef]
- Holtzer, M.; Kmita, A.; Roczniak, A. The Recycling of Materials Containing Iron and Zinc in the OxyCup Process. Arch. Foundry Eng. 2015, 1, 126–130. [Google Scholar]
- Hillmann, C.; Sassen, K.J. Processing of zinc-bearing BOF dusts in a blast furnace. World Iron Steel 2013, 5, 8–9. [Google Scholar]
- Antrekowtisch, J. Recycling und Rohstoffe; TK-Verl: Neuruppin, Germany, 2015; ISBN 978-3-944310-20-6. [Google Scholar]
- Reiter, W.; Lasser, M.; Rieger, J.; Raupenstrauch, H.; Tappeiner, T. Der RecoDust-Prozess—Behandlung von zinkhaltigen Stahlwerksstäuben und weiteren Nebenprodukten aus integrierten Hüttenwerken. BHM Berg—Und Hüttenmännische Mon. 2020, 165, 297–301. [Google Scholar] [CrossRef]
- Iron Ores for Blast Furnace Feedstocks. Determination of the Reducibility by the Rate of Reduction Index; BSI British Standards: London, UK, 2007.
- Reiter, W. Verwertungsmöglichkeiten der RecoDust-Schlacke; University of Leoben: Leoben, Austria, 2020. [Google Scholar]
- Spreitzer, D. Niedertemperaturkornzerfall nach, ISO 4696-1, Internal Report. 2017.
- Dobner, W.; Bosch, H. Bestimmung des Reduktionsgrades von Eisenschwamm und vorreduziertem Erz. Arch. Für Das Eisenhüttenwesen 1979, 50, 385–388. [Google Scholar] [CrossRef]
- Reiter, W.; Rieger, J.; Lasser, M.; Raupenstrauch, H.; Tappeiner, T. The RecoDust Process—Upscale of a Pilot Plant. Steel Res. Int. 2020, 91, 2000191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).