The Correlation among the Atomic Structure, Electronic Valence Band and Properties of Zr-Cu-Al-Ag Bulk Metallic Glasses
Abstract
1. Introduction
2. Methodology
2.1. Molecular Dynamic Simulations
2.2. Experiments
3. Results and Discussion
3.1. The Structure and GFA of Zr50Cu44.5−xAl5.5Agx (x = 0, 1.5, 3 at.%) BMGs
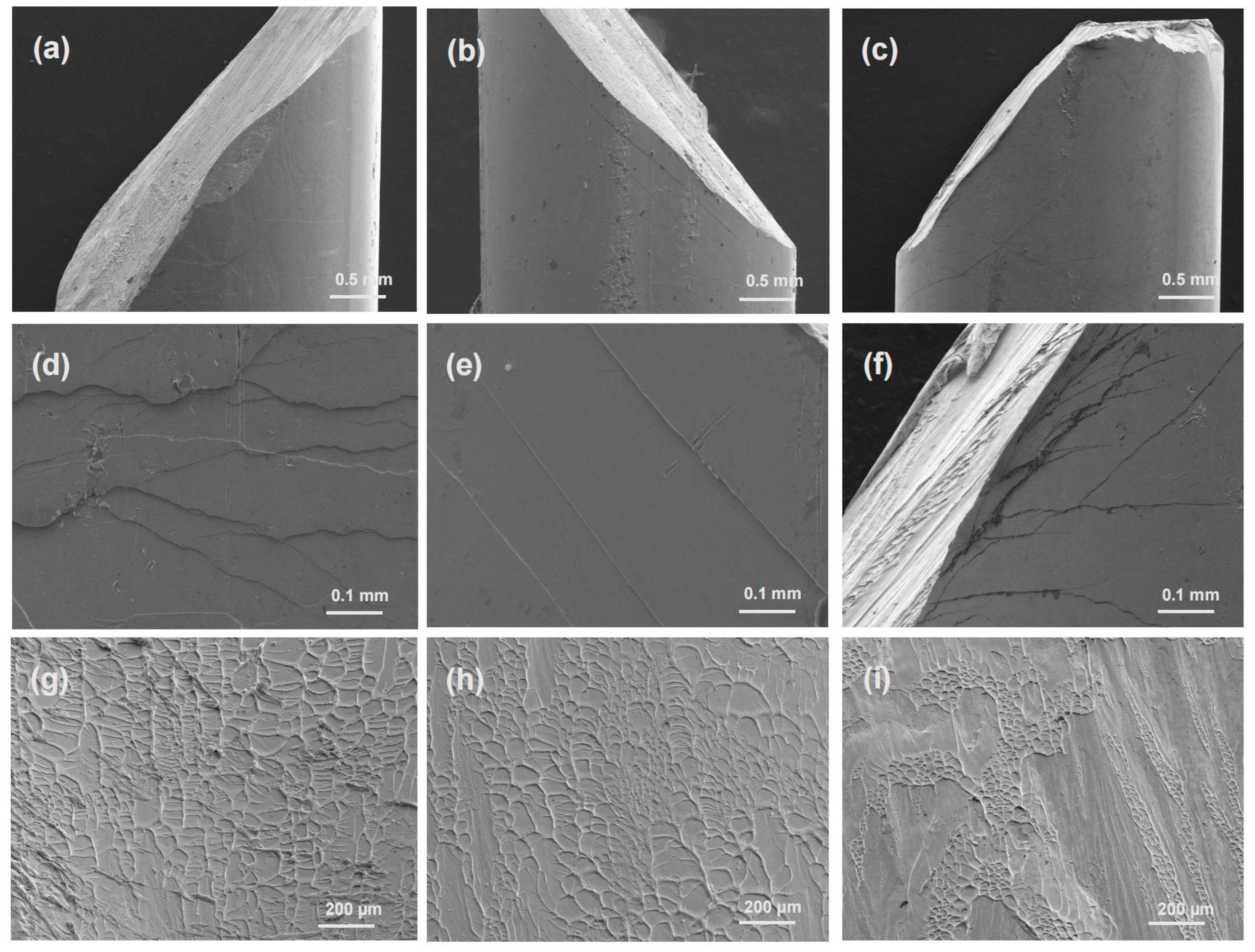
3.2. Mechanical Properties of the Zr50Cu44.5−xAl5.5Agx (x = 0, 1.5, 3 at.%) BMGs
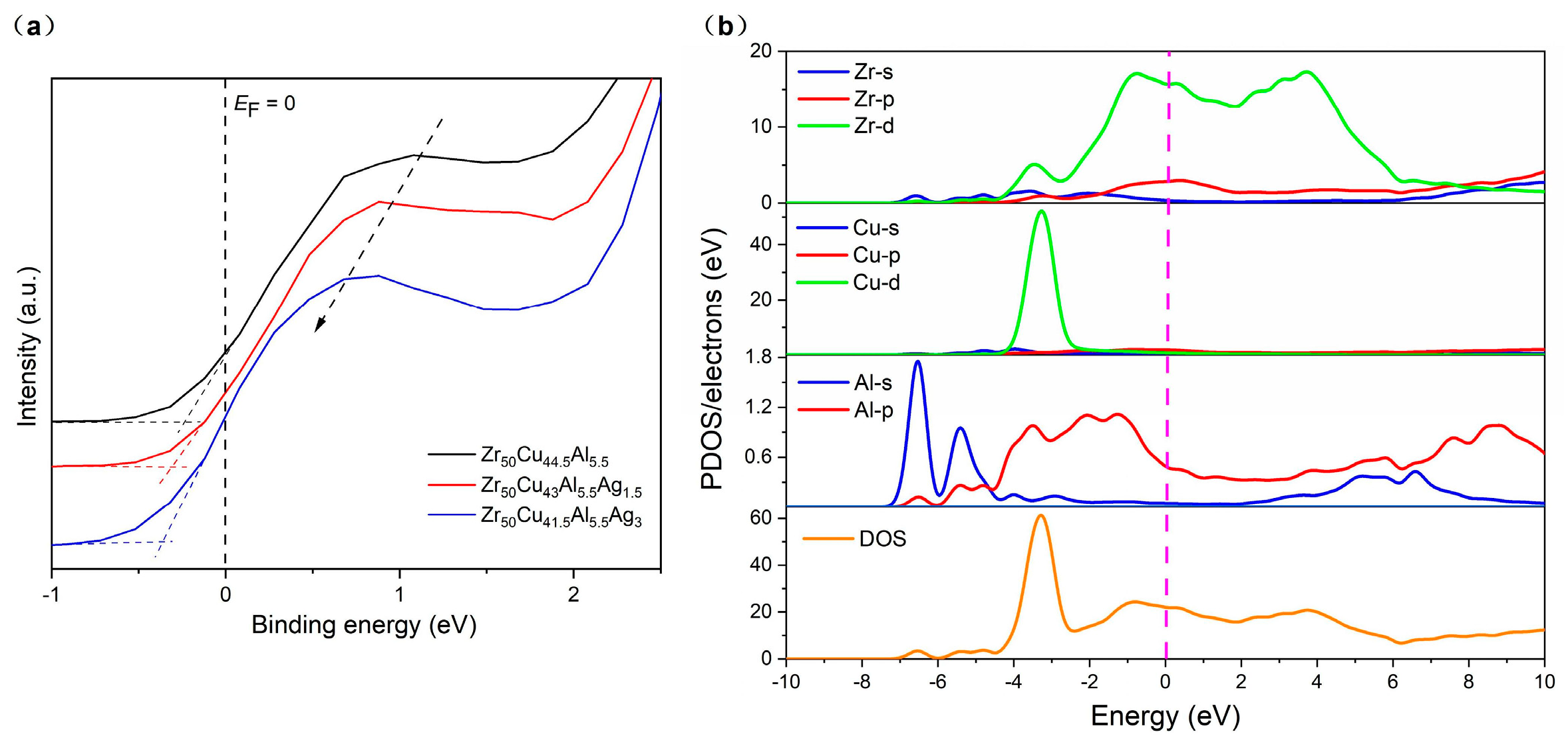
3.3. Relationship between the Properties and Valence Band Positions of Zr50Cu44.5−xAl5.5Agx (x = 0, 1.5, 3 at.%) BMGs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Greer, A.L. Metallic Glasses. Science 1995, 267, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Telford, M. The case for bulk metallic glass. Mater. Today 2004, 7, 36–43. [Google Scholar] [CrossRef]
- Wang, W.H. Bulk Metallic Glasses with Functional Physical Properties. Adv. Mater. 2009, 21, 4524–4544. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, T.; Zhou, J.; Zhou, X.; Hao, Q.; Qiao, J. The improvement of the plasticity of a Zr–Ni–Al bulk metallic glass by static quenching. Mater. Sci. Eng. A 2022, 851, 143624. [Google Scholar] [CrossRef]
- Lan, S.; Zhu, L.; Wu, Z.; Gu, L.; Zhang, Q.; Kong, H.; Liu, J.; Song, R.; Liu, S.; Sha, G.; et al. A medium-range structure motif linking amorphous and crystalline states. Nat. Mater. 2021, 20, 1347–1352. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Zhu, F.; Yuan, Y.; Chang, D.J.; Kim, D.S.; Pham, M.; Rana, A.; Tian, X.; Yao, Y.; et al. Determining the three-dimensional atomic structure of an amorphous solid. Nature 2021, 592, 60–64. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, J.; He, W.; Peng, P. Lowest-energy structural and electronic properties of CunZr13−n (n = 3–10) clusters in metallic glasses via CALYPSO search and density functional theory calculations. J. Mol. Liq. 2021, 343, 117603. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Xian, G.; Zhou, L.-L.; Tian, Z.-A.; Chen, Q.; Mo, Y.-F.; Liu, R.-S.; Gao, T.-H.; Xie, Q.; He, M. Influence of cluster correlation on nanoclusters in Fe-Ni amorphous alloys. J. Alloys Compd. 2021, 891, 161953. [Google Scholar] [CrossRef]
- Kim, H.-K.; Ahn, J.-P.; Lee, B.-J.; Park, K.-W.; Lee, J.-C. Role of atomic-scale chemical heterogeneities in improving the plasticity of Cu-Zr-Ag bulk amorphous alloys. Acta Mater. 2018, 157, 209–217. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Q.; Zhou, H.; Hu, W.; Xu, T.; Zheng, L.; Nutor, R.; Wang, X.; Cao, Q.; Zhang, D.; et al. Tailoring microstructure of metallic glass for delocalized plasticity by pressure annealing: Forward and inverse studies. Acta Mater. 2021, 220, 117282. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.Z.; Hao, S.G.; Kramer, M.J.; Ho, K.M. Structural heterogeneity and medium-range order in ZrxCu100−x metallic glasses. Phys. Rev. B 2009, 80, 184201. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Duan, H. Study of the effects of metalloid elements (P, C, B) on Fe-based amorphous alloys by ab initio molecular dynamics simulations. J. Appl. Phys. 2015, 117, 104901. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Li, H.; Liu, H.; Mo, J.; Lan, S.; Li, M.; Wang, X.-L.; Eckert, J.; Huo, J. Inheritance factor on the physical properties in metallic glasses. Mater. Futures 2022, 1, 035601. [Google Scholar] [CrossRef]
- Wang, W.H. Roles of minor additions in formation and properties of bulk metallic glasses. Prog. Mater. Sci. 2007, 52, 540–596. [Google Scholar] [CrossRef]
- Zeng, Y.Q.; Yu, J.S.; Tian, Y.; Hirata, A.; Fujita, T.; Zhang, X.H.; Nishiyama, N.; Kato, H.; Jiang, J.Q.; Inoue, A.; et al. Improving glass forming ability of off-eutectic metallic glass formers by manipulating primary crystallization reactions. Acta Mater. 2020, 200, 710–719. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Zhu, Z.; Wang, H.; Li, Y.; Kato, H.; Zhang, H. Effect of substituting elements on thermal stability and glass-forming ability of an Al-based Al–Ni–Er metallic glass. J. Alloys Compd. 2017, 707, 97–101. [Google Scholar] [CrossRef]
- Wang, T.; Hou, Q.; Zhang, L. Enhanced heterogeneity and plasticity in a Zr-Cu-Al bulk metallic glass with micro-addition of oxygen. Mater. Sci. Eng. A 2021, 831, 142222. [Google Scholar] [CrossRef]
- Pan, J.; Chan, K.; Chen, Q.; Li, N.; Guo, S.; Liu, L. The effect of microalloying on mechanical properties in CuZrAl bulk metallic glass. J. Alloys Compd. 2010, 504, S74–S77. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Sordelet, D.J.; Kramer, M.J. Using atomistic computer simulations to analyze X-ray diffraction data from metallic glasses. J. Appl. Phys. 2007, 102, 043501. [Google Scholar] [CrossRef]
- Zhou, X.; Wadley, H.; Johnson, R.; Larson, D.; Tabat, N.; Cerezo, A.; Petford-Long, A.; Smith, G.; Clifton, P.; Martens, R.; et al. Atomic scale structure of sputtered metal multilayers. Acta Mater. 2001, 49, 4005–4015. [Google Scholar] [CrossRef]
- Baskes, M.I. Modified embedded-atom potentials for cubic materials and impurities. Phys. Rev. B 1992, 46, 2727–2742. [Google Scholar] [CrossRef]
- Lee, B.-J.; Baskes, M.I. Second nearest-neighbor modified embedded-atom-method potential. Phys. Rev. B 2000, 62, 8564–8567. [Google Scholar] [CrossRef]
- Celtek, M.; Sengul, S.; Domekeli, U. Glass formation and structural properties of Zr50Cu50−xAlx bulk metallic glasses investigated by molecular dynamics simulations. Intermetallics 2017, 84, 62–73. [Google Scholar] [CrossRef]
- Celtek, M.; Sengul, S.; Domekeli, U.; Guder, V. Dynamical and structural properties of metallic liquid and glass Zr48Cu36Ag8Al8 alloy studied by molecular dynamics simulation. J. Non-Cryst. Solids 2021, 566, 120890. [Google Scholar] [CrossRef]
- Çeltek, M.; Şengül, S.; Dömekeli, U. Hızlı Soğutma Sürecinde Dörtlü Zr48Cu36Ag8Al8 İri Hacimli Metalik Camının Atomik Yapısının Gelişimi. Süleyman Demirel Üniversitesi Fen Bilim. Enstitüsü Derg. 2019, 23, 954–962. [Google Scholar] [CrossRef]
- Weiss, E.J.; Ward, L.; Oberdorfer, C.; Withrow, T.; Riegner, D.C.; Agrawal, A.; Windl, W. Rapid production of accurate embedded-atom method potentials for metal alloys. arXiv 2022, arXiv:2208.02223. [Google Scholar]
- Cheng, Y.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Hirata, A.; Guan, P.; Fujita, T.; Hirotsu, Y.; Inoue, A.; Yavari, A.R.; Sakurai, T.; Chen, M. Direct observation of local atomic order in a metallic glass. Nat. Mater. 2010, 10, 28–33. [Google Scholar] [CrossRef]
- Luo, W.K.; Sheng, H.W.; Ma, E. Pair correlation functions and structural building schemes in amorphous alloys. Appl. Phys. Lett. 2006, 89, 131927. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Hui, X.; Gao, R.; Chen, G.; Shang, S.-L.; Wang, Y.; Liu, Z.-K. Short-to-medium-range order in Mg65Cu25Y10 metallic glass. Phys. Lett. A 2008, 372, 3078–3084. [Google Scholar] [CrossRef]
- Zhang, S.; Chong, K.; Zhang, Z.; Gao, Y.; Cao, Y.; Wu, D.; Qian, Z.; Zhao, G.; Zou, Y. An ab initio simulation and experimental studies of the glass-forming ability and properties of Al86Ni(14−x)Zrx (x = 1∼7) alloys. J. Non-Cryst. Solids 2022, 586, 121566. [Google Scholar] [CrossRef]
- Guo, G. Quasi-Icosahedral Clusters in Zr-Based Metallic Glasses. Metals 2020, 10, 1135. [Google Scholar] [CrossRef]
- Mauro, N.A.; Wessels, V.; Bendert, J.C.; Klein, S.; Gangopadhyay, A.K.; Kramer, M.J.; Hao, S.G.; Rustan, G.E.; Kreyssig, A.; Goldman, A.I.; et al. Short- and medium-range order in Zr80Pt20 liquids. Phys. Rev. B 2011, 83, 184109. [Google Scholar] [CrossRef]
- Lad, K. Correlation between atomic-level structure, packing efficiency and glass-forming ability in Cu–Zr metallic glasses. J. Non-Cryst. Solids 2014, 404, 55–60. [Google Scholar] [CrossRef]
- Löffler, J.F. Bulk metallic glasses. Intermetallics 2003, 11, 529–540. [Google Scholar] [CrossRef]
- Guo, S.; Li, N.; Zhang, C.; Liu, L. Enhancement of plasticity of Fe-based bulk metallic glass by Ni substitution for Fe. J. Alloys Compd. 2010, 504, S78–S81. [Google Scholar] [CrossRef]
- Conner, R.; Rosakis, A.; Johnson, W.; Owen, D. Fracture toughness determination for a beryllium-bearing bulk metallic glass. Scr. Mater. 1997, 37, 1373–1378. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Ji, M.; Wang, C.; Ho, K.; Russell, A.; Mudryk, Y.; Becker, A.; Larson, J. Influence of the electronic structure on the ductile behavior of B2 CsCl-type AB intermetallics. Acta Mater. 2009, 57, 5876–5881. [Google Scholar] [CrossRef]
- Wu, N.C.; Zuo, L.; Wang, J.Q.; Ma, E. Designing aluminum-rich bulk metallic glasses via electronic-structure-guided microalloying. Acta Mater. 2016, 108, 143–151. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.C.; Xiang, J.F.; Xi, X.K.; Wang, W.H. NMR Signature of Evolution of Ductile-to-Brittle Transition in Bulk Metallic Glasses. Phys. Rev. Lett. 2011, 107, 236403. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Tian, Z.-A.; Liu, R.-S.; Gao, T.-H.; Guo, X.-T.; Mo, Y.-F.; Xie, Q. Icosahedron-forming ability of MgZn alloys studied by molecular dynamics simulations. J. Alloys Compd. 2017, 700, 61–66. [Google Scholar] [CrossRef]
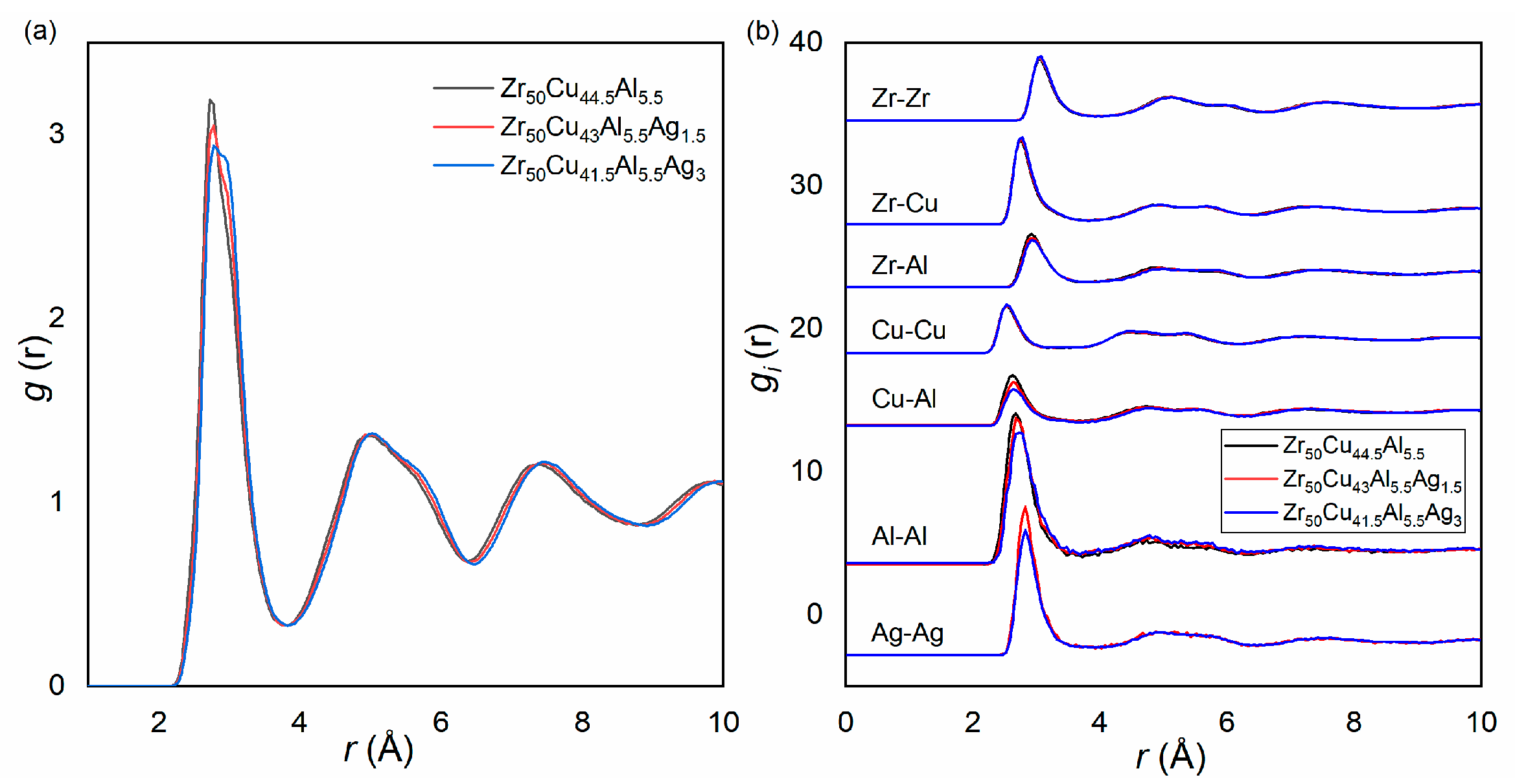
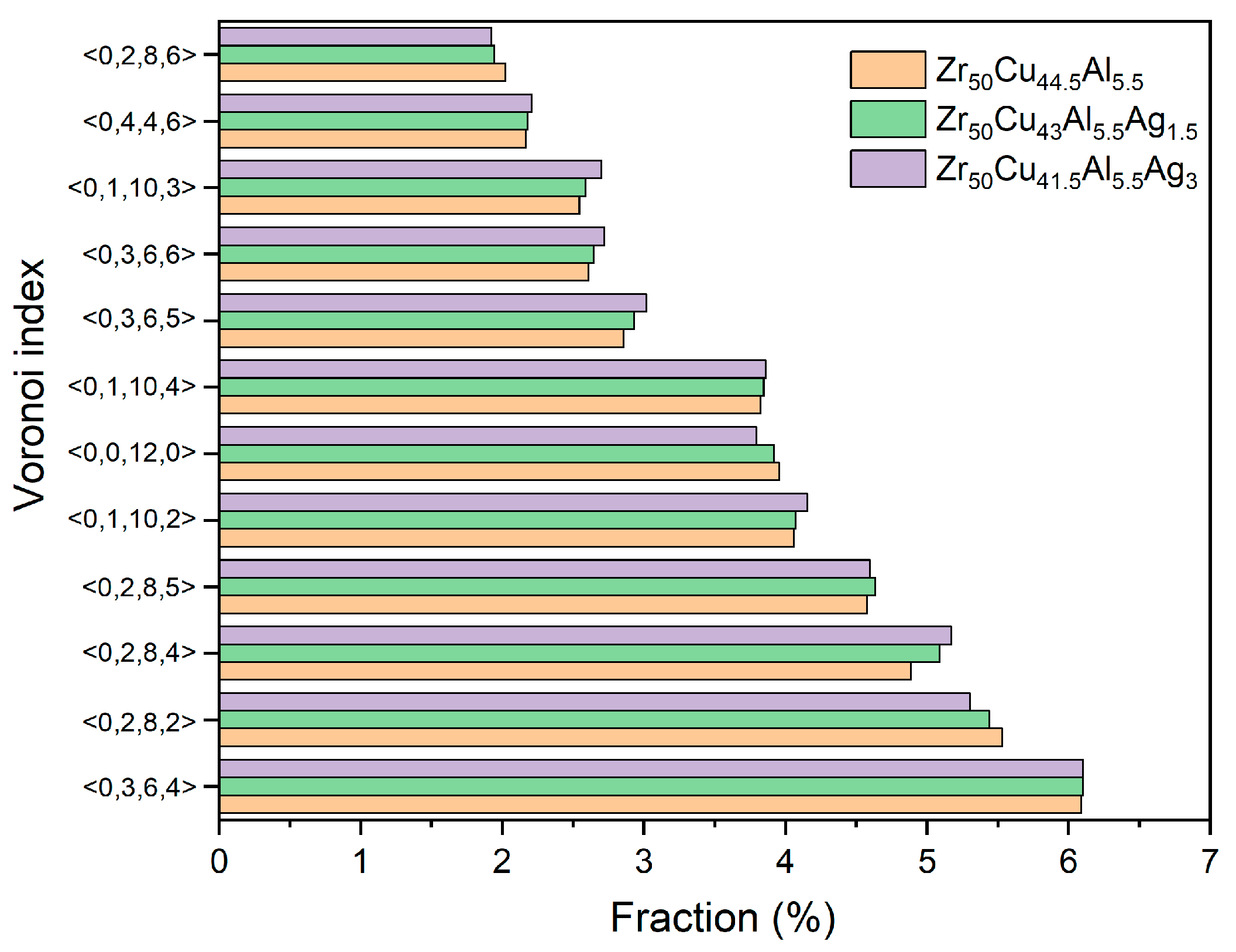

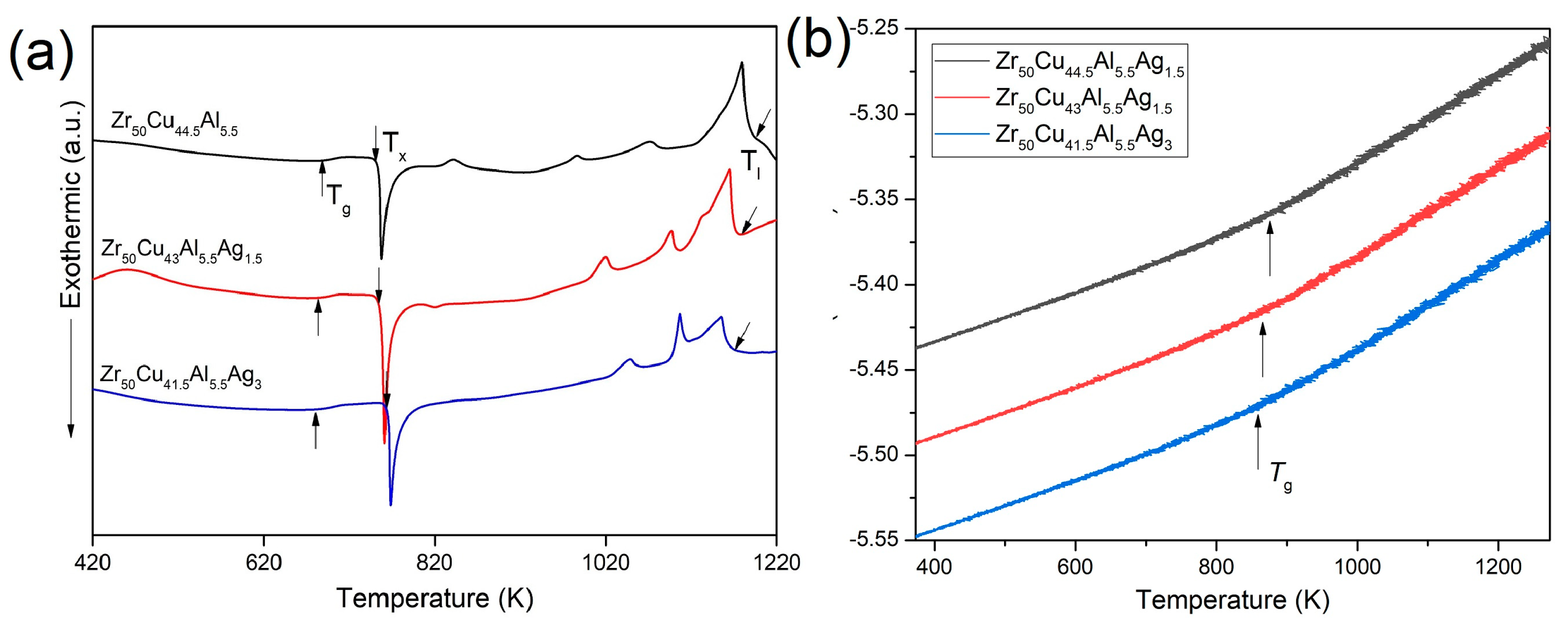
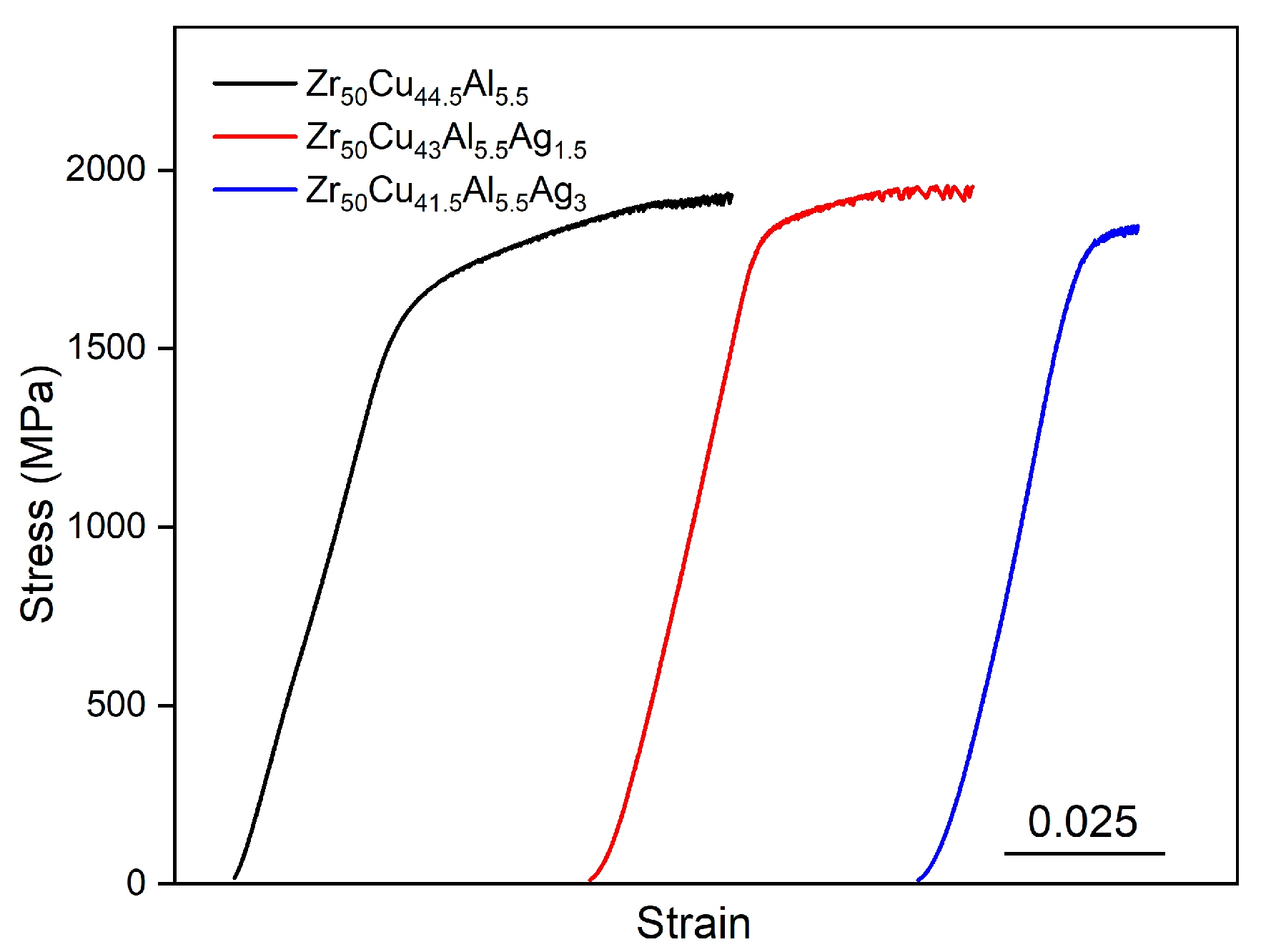
| Zr50Cu44.5Al5.5 | Zr50Cu43Al5.5Ag1.5 | Zr50Cu41.5Al5.5Ag3 | |
|---|---|---|---|
| Dmax (mm) | 2.5 | 3.0 | 5.0 |
| Tg (K) | 687 | 683 | 680 |
| Tx (K) | 743 | 749 | 758 |
| Tl (K) | 1196 | 1178 | 1171 |
| ΔT = Tx − Tg (K) | 56 | 66 | 78 |
| γ = Tx/(Tg + Tl) | 0.395 | 0.402 | 0.410 |
| x (at.%) | Yield Strength (MPa) | Fracture Strength (MPa) | Plastic Strain (%) |
|---|---|---|---|
| 0 | 1437 | 1914 | 4.6 |
| 1.5 | 1707 | 1952 | 3.2 |
| 3 | 1565 | 1842 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopur, P.; Chen, W.; Zhou, Y.; Zhou, J.; Wang, T. The Correlation among the Atomic Structure, Electronic Valence Band and Properties of Zr-Cu-Al-Ag Bulk Metallic Glasses. Metals 2023, 13, 1181. https://doi.org/10.3390/met13071181
Hopur P, Chen W, Zhou Y, Zhou J, Wang T. The Correlation among the Atomic Structure, Electronic Valence Band and Properties of Zr-Cu-Al-Ag Bulk Metallic Glasses. Metals. 2023; 13(7):1181. https://doi.org/10.3390/met13071181
Chicago/Turabian StyleHopur, Parida, Wenqi Chen, Yulong Zhou, Jialu Zhou, and Tuo Wang. 2023. "The Correlation among the Atomic Structure, Electronic Valence Band and Properties of Zr-Cu-Al-Ag Bulk Metallic Glasses" Metals 13, no. 7: 1181. https://doi.org/10.3390/met13071181
APA StyleHopur, P., Chen, W., Zhou, Y., Zhou, J., & Wang, T. (2023). The Correlation among the Atomic Structure, Electronic Valence Band and Properties of Zr-Cu-Al-Ag Bulk Metallic Glasses. Metals, 13(7), 1181. https://doi.org/10.3390/met13071181





