Effects of Micro-Arc Oxidation Surface Treatment on the Corrosion Resistance of Ti-6Al-4V Electron-Beam-Welded Joints
Abstract
1. Introduction
2. Experimental Details
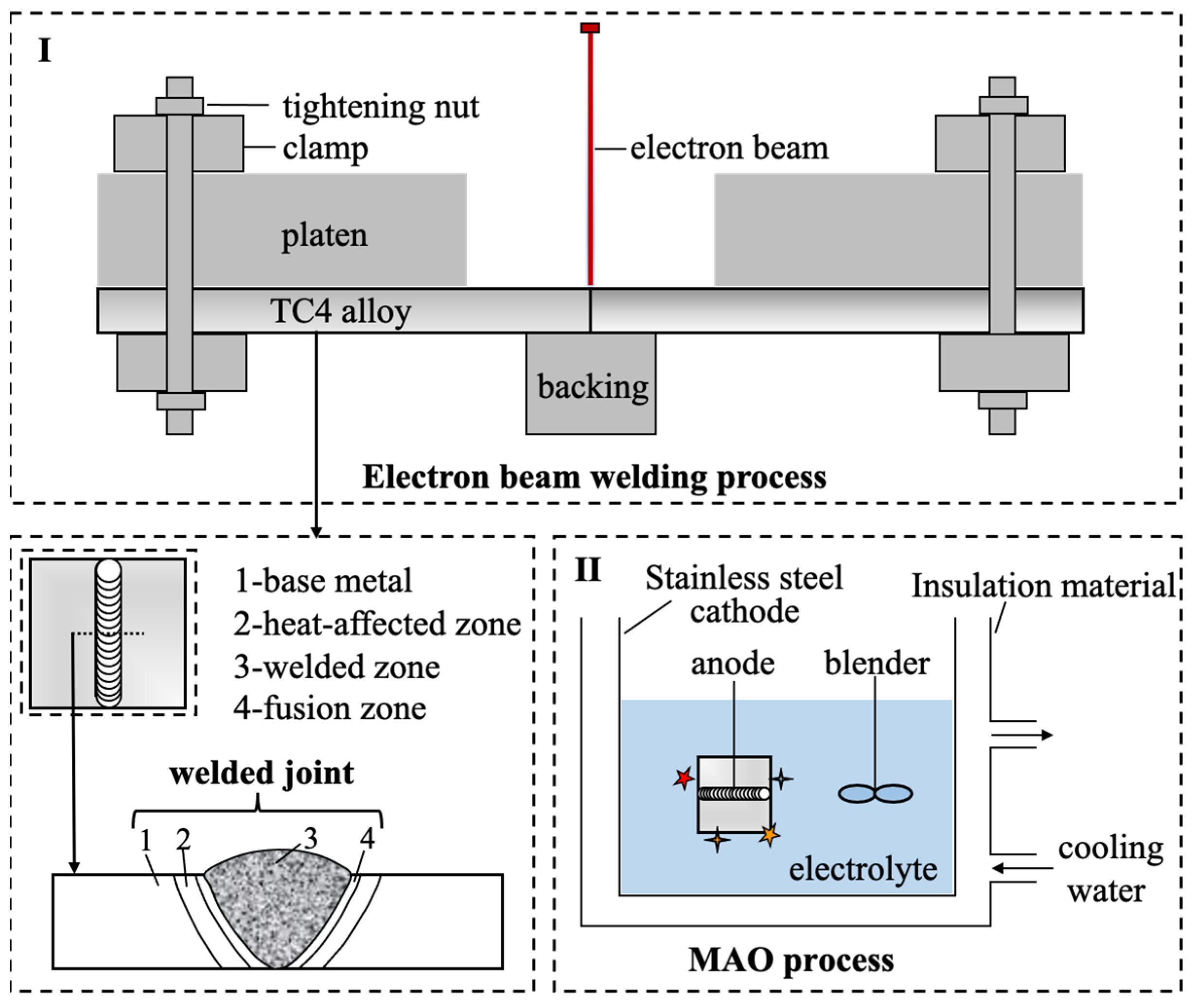
2.1. Preparation of Electron-Beam-Welded Ti-6Al-4V Joints
2.2. Preparation of MAO Coatings
2.3. Characterization
2.4. Electrochemical Assessment
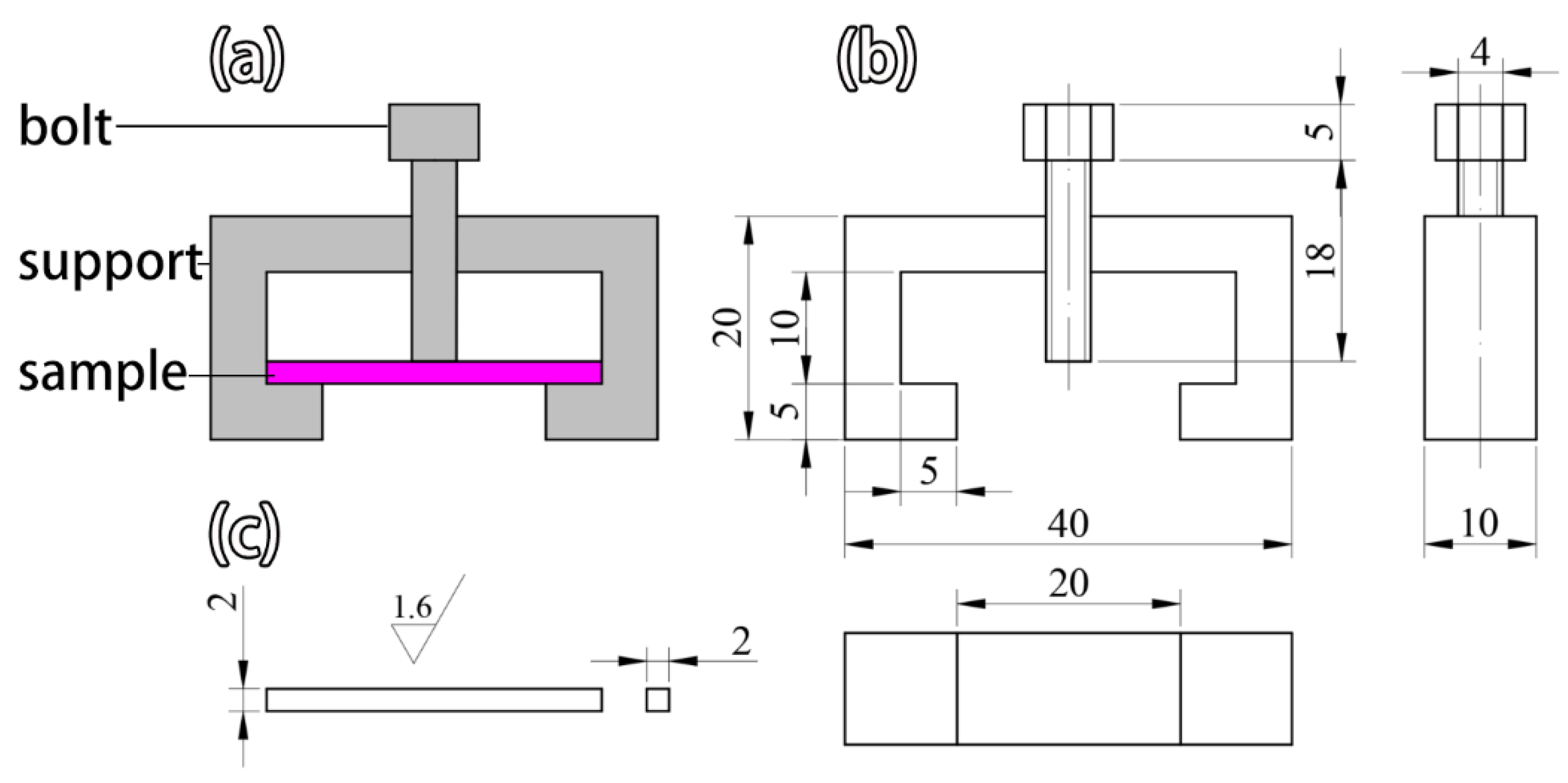
2.5. Stress Corrosion Evaluation
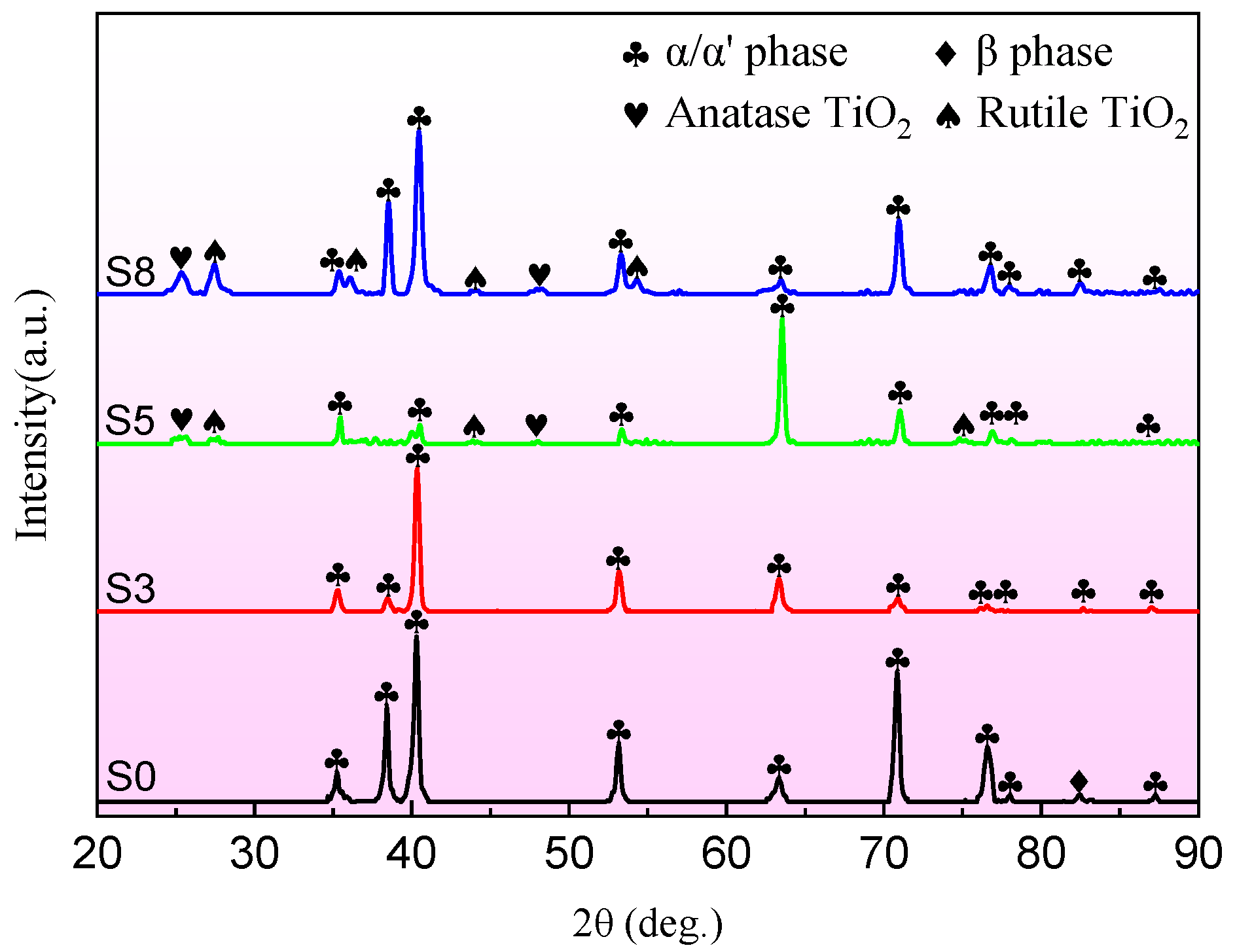
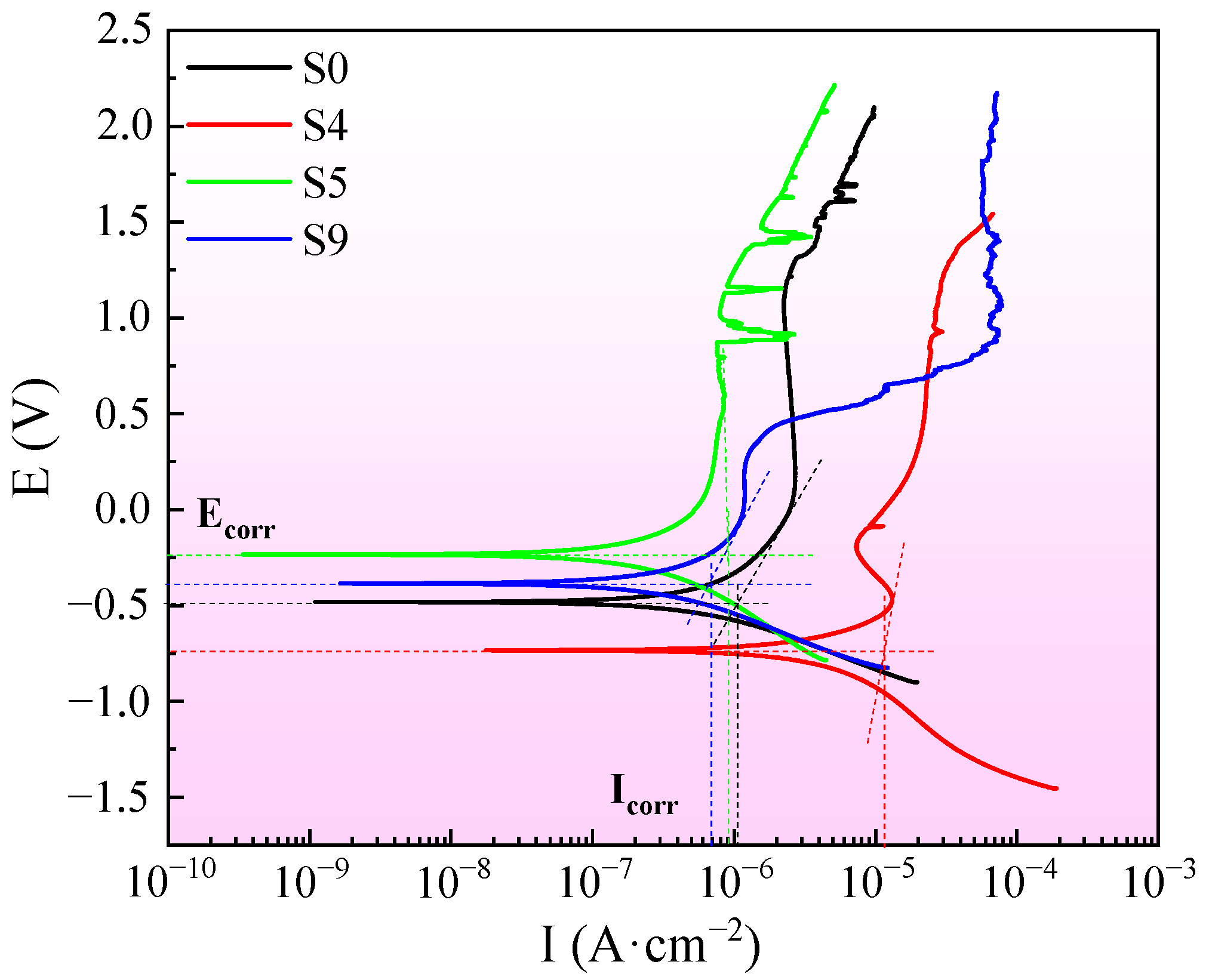
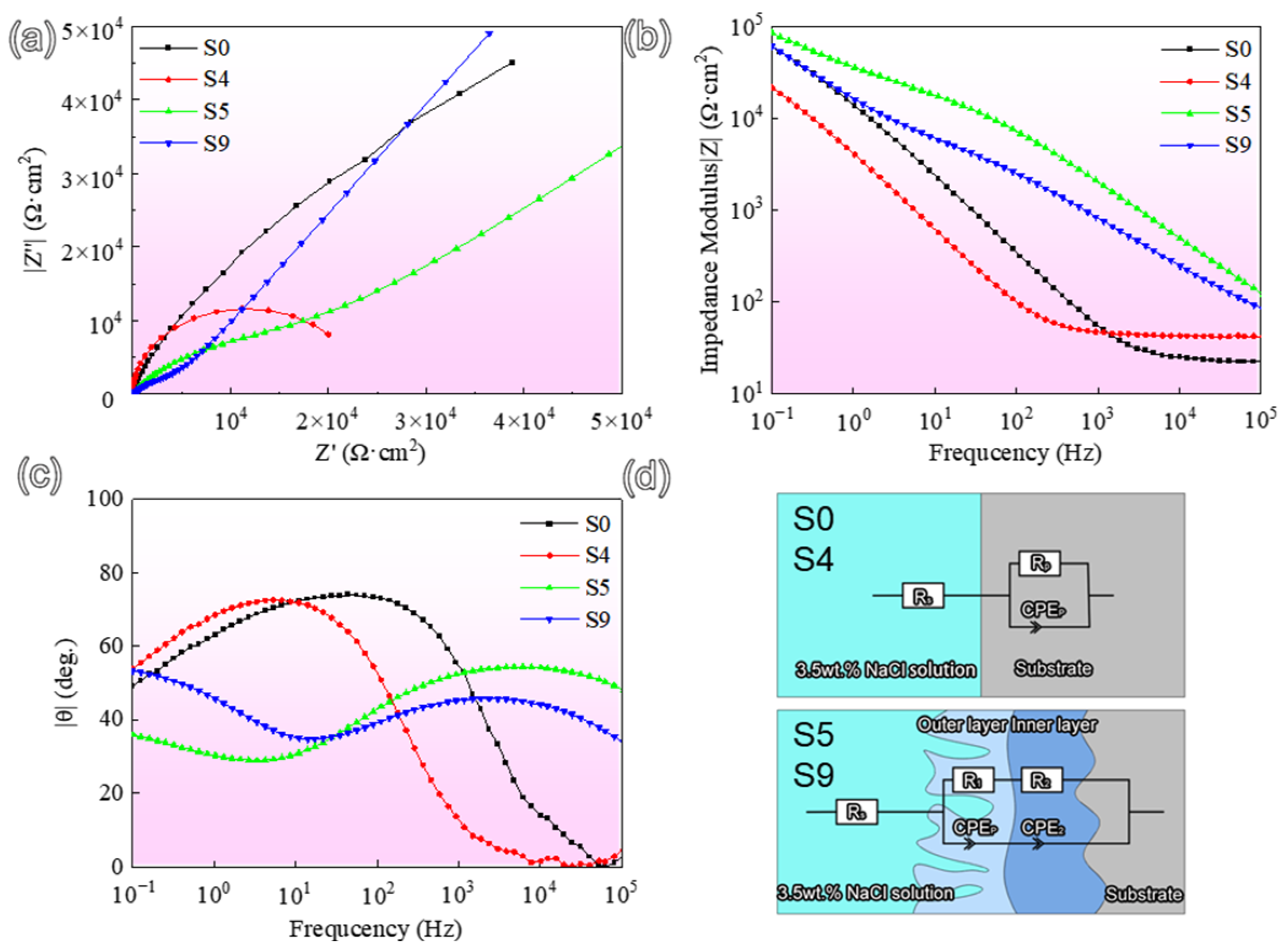
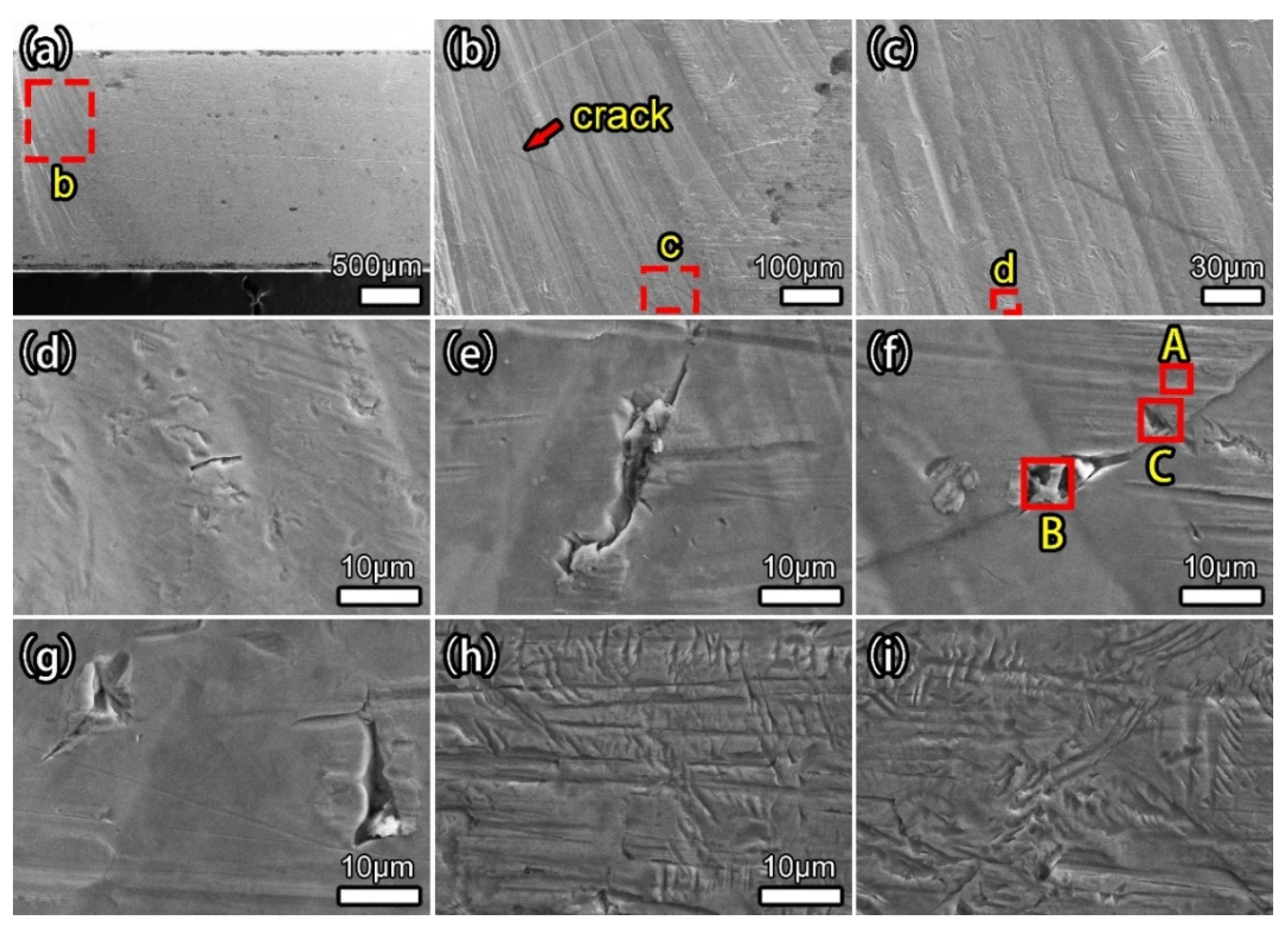
3. Results and Discussion
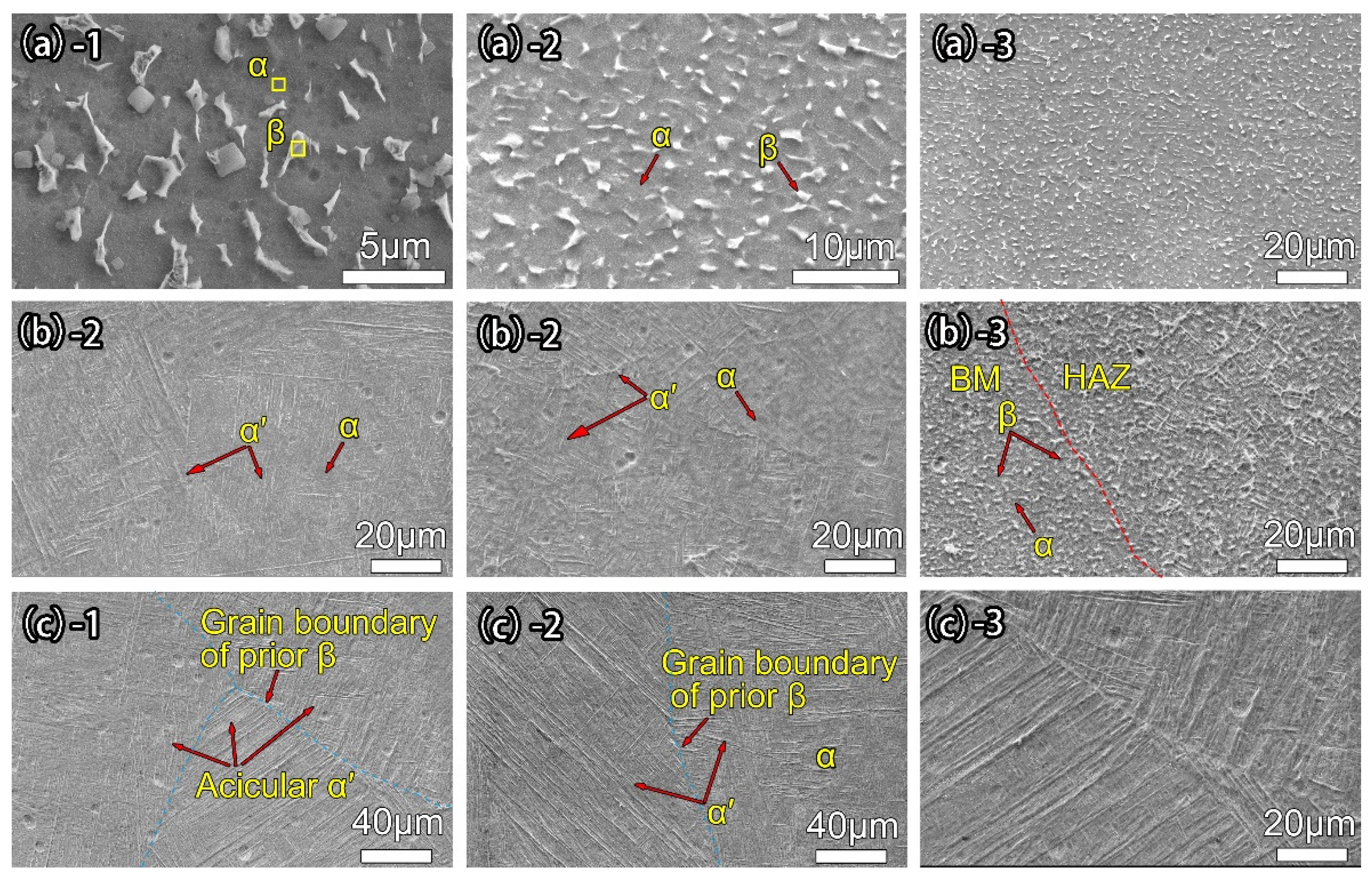
3.1. Ti-6Al-4V Joint Morphology
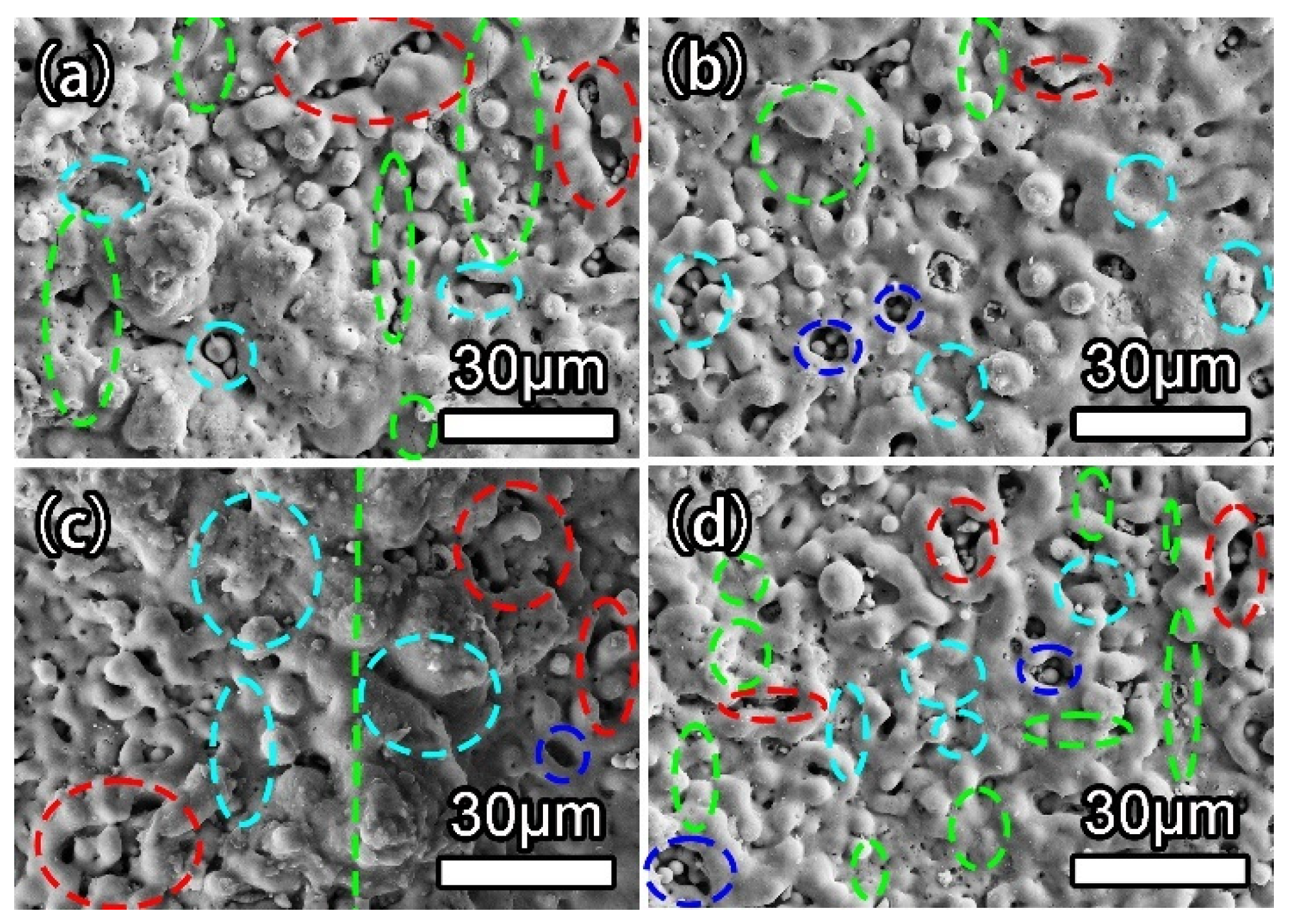
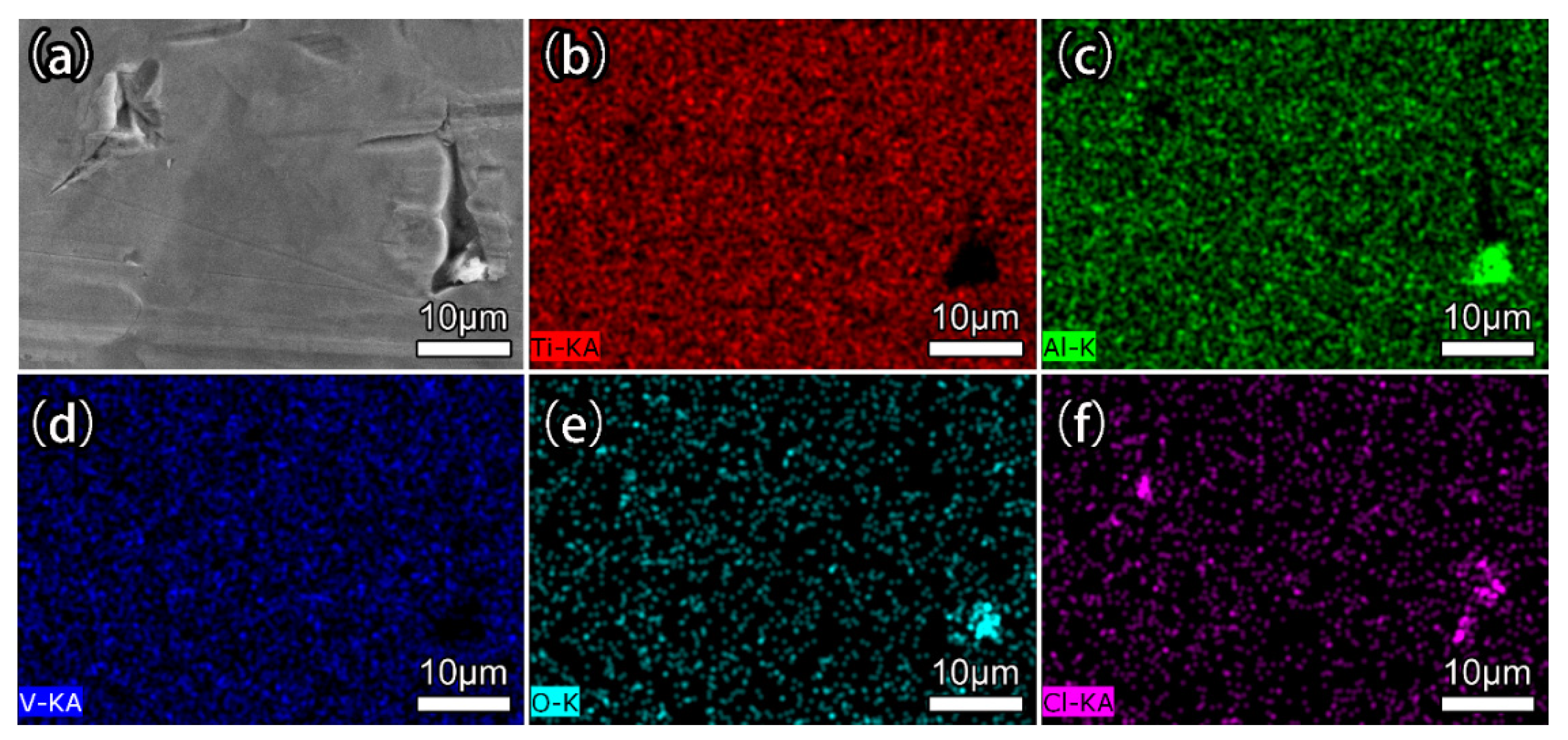
3.2. Coating Morphology and Structure
3.3. Electrochemical Corrosion
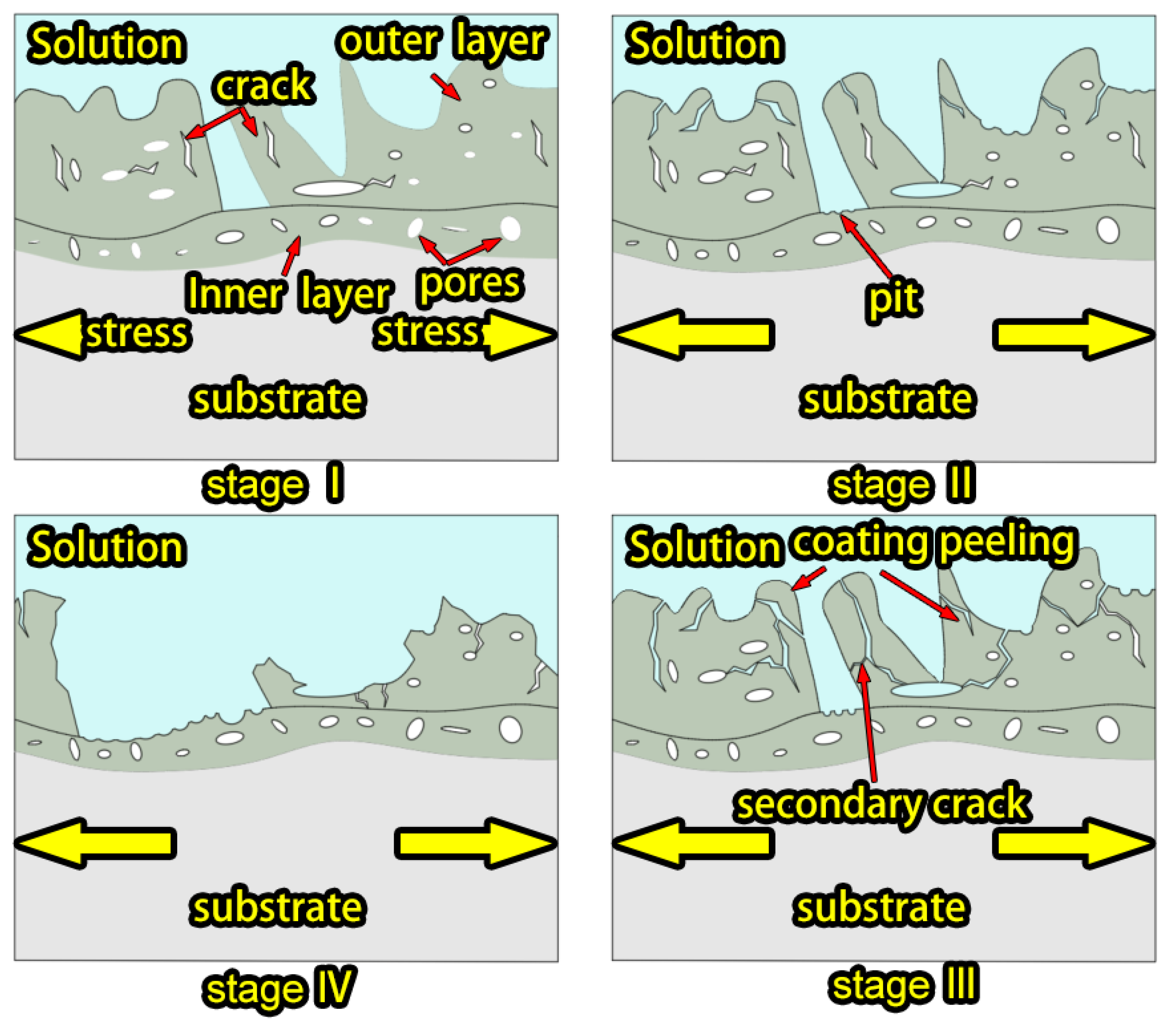
3.4. Stress Corrosion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, G.Q.; Zhang, G.; Yin, Q.X.; Zhang, B.G.; Feng, J.C. Microstructure evolution of electron beam welded joints of Ti-43Al-9V-0.3Y and Ti-6Al-4V alloys. Mater. Lett. 2018, 233, 336–339. [Google Scholar] [CrossRef]
- Li, J.N.; Li, J.S.; Qi, W.J.; Liu, K.G. Characterization and mechanical properties of thick TC4 titanium alloy sheets welded joint by vacuum EBW. Vacuum 2019, 168, 108812. [Google Scholar] [CrossRef]
- Su, M.L.; Li, J.N.; Liu, K.G.; Qi, W.J.; Weng, F.; Zhang, Y.B.; Li, J.S. Mechanical property and characterization of TA1 titanium alloy sheets welded by vacuum electron beam welding. Vacuum 2019, 159, 315–318. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Q.; Kong, J.; Peng, Y.; Xiang, Y.; Luo, T.Y.; Wang, K.H.; Zhu, J. Effect of beam offset on the characteristics of copper/304stainless steel electron beam welding. Vacuum 2016, 128, 205–212. [Google Scholar] [CrossRef]
- Sandhya, V.; Naga Phani Sastry, M.; Hemachandra Reddy, K. Influence of Welding Speed, Voltage, and Beam current on the microstructure and mechanical properties of Electron Beam-Welded Titanium radial joints. Mater. Today Proc. 2022, 64, 442–447. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Nishimura, T.; Min, X.H.; Tsuzaki, M. The Effect of Microstructure on Corrosion of Molybdenum-Bearing Titanium Alloys in High Chloride and Acidic Solution at High Temperature. Mater. Trans. 2009, 50, 2545–2551. [Google Scholar] [CrossRef]
- Shamir, M.; Junaid, M.; Khan, F.N.; Taimoor, A.A.; Baig, M.N. A comparative study of electrochemical corrosion behavior in Laser and TIG welded Ti–5Al–2.5Sn alloy. J. Mater. Res. Technol. 2019, 8, 87–98. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Liu, L.; Zhang, B.; Wang, F.H. A primary study of the effect of hydrostatic pressure on stress corrosion cracking of Ti-6Al-4V alloy in 3.5% NaCl solution. Corros. Sci. 2020, 165, 108402. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Abdollah Saboori, M.R.; Khalaj, G.; Ghareh Shiran, M.K. Microstructural Evolutions and its Impact on the Corrosion Behavior of Explosively Welded Al/Cu Bimetal. Metals 2020, 10, 634. [Google Scholar] [CrossRef]
- Shargh, S.F.; Saadat, A.; Najafi, A.; Gharehshiran, M.R.K.; Khalaj, G. Investigating the effect of post weld heat treatment on corrosion properties of explosive bonded interface of AA5083/AA1050/SS 321 tubes. Mater. Res. Express 2020, 7, 036529. [Google Scholar] [CrossRef]
- Fernández-López, P.; Alves, S.A.; Azpitarte, I.; San-José, J.T.; Bayón, R. Corrosion and tribocorrosion protection of novel PEO coatings on a secondary cast Al-Si alloy: Influence of polishing and sol-gel sealing. Corros. Sci. 2022, 207, 110548. [Google Scholar] [CrossRef]
- Quintero, D.; Galvis, O.; Calderón, J.A.; Gómez, M.A.; Castaño, J.G.; Echeverría, F.; Habazaki, H. Control of the physical properties of anodic coatings obtained by plasma electrolytic oxidation on Ti6Al4V alloy. Surf. Coat. Technol. 2015, 283, 210–222. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Montazeri, M.; Baradaran, A. Preparation of ceramic coating on Ti substrate by plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cai, G.Y.; Lv, Y.; Wu, Y.L.; Dong, Z.H. Growth mechanism of titania on titanium substrate during the early stage of plasma electrolytic oxidation. Surf. Coat. Technol. 2020, 400, 126202. [Google Scholar] [CrossRef]
- Nisar, S.S.; Arun, S.; Choe, H.C. Plasma electrolytic oxidation coatings on femtosecond laser-treated Ti-6Al-4V alloy for bio-implant use. Surf. Coat. Technol. 2023, 464, 129553. [Google Scholar] [CrossRef]
- Wu, G.L.; Li, L.; Sun, M.; Wang, Y.; Luo, F.; Zhang, Q.L.; Liu, R.; Chen, Z.J.; Yao, J.H. Microstructural evolution and biological properties of PEO coating on SLM-prepared NiTi alloy. Surf. Coat. Technol. 2023, 452, 129065. [Google Scholar] [CrossRef]
- Liu, J.A.; Li, S.H.; Han, Z.W.; Cao, R.Z. Improved corrosion resistance of friction stir welded magnesium alloy with micro-arc oxidation/electroless plating duplex coating. Mater. Chem. Phys. 2021, 257, 123753. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, L.L. Improving Corrosion Resistance of Friction Stir Welding Joint of 7075 Aluminum Alloy by Micro-arc Oxidation. J. Mater. Sci. Technol. 2014, 30, 1251–1254. [Google Scholar] [CrossRef]
- Kamal Jayaraj, R.; Sree Sabari, S.; Prasanna Teja, K. Enhancing the corrosion resistance of stir zone of friction stir welded AZ31b magnesium alloy using micro arc oxidation coatings. Mater. Today Proc. 2019, 15, 68–75. [Google Scholar] [CrossRef]
- Sun, W.J.; Wang, S.L.; Wu, M.; Hong, M.; Chen, Y.H.; Xin, J.J.; Zhang, P.; Qin, Y.B.; Fang, N.W. Revealing tensile behaviors and fracture mechanism of Ti–6Al–4V titanium alloy electron-beam-welded joints using microstructure evolution and in situ tension observation. Mater. Sci. Eng. A 2021, 824, 141811. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.L.; Ma, W.J.; Wang, Y.; Yang, J.G.; Liu, S.F.; Tang, H.P. Corrosion and wear properties of micro-arc oxidation treated Ti6Al4V alloy prepared by selective electron beam melting. Trans. Nonferrous Met. Soc. China 2020, 30, 2132–2142. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wu, X.Q.; Xue, Z.G.; Matykina, E.; Skeldon, P.; Thompson, G.E. Microstructure, corrosion and wear performance of plasma electrolytic oxidation coatings formed on Ti–6Al–4V alloy in silicate-hexametaphosphate electrolyte. Surf. Coat. Technol. 2013, 217, 129–139. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Kim, J.; Park, H.W. High performance corrosion and wear resistant Ti-6Al-4V alloy by the hybrid treatment method. Appl. Surf. Sci. 2020, 504, 144388. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Zhang, Y.P.; Hu, J.L.; Hou, B.; Wang, H.Y.; Dong, C.; Zhou, Y. The role of microstructure on corrosion fatigue behavior of thick-plate Ti–6Al–4V joint via vacuum electron beam welding. Vacuum 2020, 182, 109714. [Google Scholar] [CrossRef]
- Sun, Z.C.; Li, X.S.; Wu, H.L.; Yang, H. Morphology evolution and growth mechanism of the secondary Widmanstatten α phase in the TA15 Ti-alloy. Mater. Charact. 2016, 118, 167–174. [Google Scholar] [CrossRef]
- Qin, T.; Lin, X.; Yu, J.; Wang, M.; Guo, P.F.; Li, J.Q.; Zhang, Y.F.; Liu, J.R.; Zhang, S.L.; Huang, W.D. Performance of different microstructure on electrochemical behaviors of laser solid formed Ti–6Al–4V alloy in NaCl solution. Corros. Sci. 2021, 185, 109392. [Google Scholar] [CrossRef]
- Seo, D.I.; Lee, J.B. Corrosion Characteristics of Additive-Manufactured Ti-6Al-4V Using Microdroplet Cell and Critical Pitting Temperature Techniques. J. Electrochem. Soc. 2019, 166, C428–C433. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, S.M.; Samuel Lim, C.V.; Zhou, X.G.; Chen, X.B.; Hinton, B.R.W.; Boyer, R.R.; Williams, J.C.; Wu, X.H. The mechanism of aqueous stress-corrosion cracking of α + β titanium alloys. Corros. Sci. 2017, 125, 29–39. [Google Scholar] [CrossRef]
- Dong, Y.C.; Huang, S.; Wang, Y.Y.; Zhang, B.; Alexandrov, I.V.; Chang, H.; Dan, Z.H.; Ma, L.; Zhou, L. Stress corrosion cracking of TC4 ELI alloy with different microstructure in 3.5% NaCl solution. Mater. Charact. 2022, 194, 112357. [Google Scholar] [CrossRef]
- Ao, N.; Liu, D.X.; Zhang, X.H.; Liu, C.S. Enhanced fatigue performance of modified plasma electrolytic oxidation coated Ti-6Al-4V alloy: Effect of residual stress and gradient nanostructure. Appl. Surf. Sci. 2019, 489, 595–607. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, Y.M.; Cao, G.; Chen, C.L.; Zhu, Y.X.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Zou, Y.C.; Ouyang, J.H.; et al. High temperature oxidation and hot corrosion behaviors of PEO and PEO/polysilazane preceramic-based dual-layer coatings on Ti6Al4V alloy. Corros. Sci. 2023, 216, 111076. [Google Scholar] [CrossRef]



| Ti | Al | V | Fe | O | C | N | H | |
|---|---|---|---|---|---|---|---|---|
| Ti-6Al-4V | Bal | 6.2 | 4.1 | 0.3 | 0.2 | 0.1 | 0.05 | 0.015 |
| Sample | Sample Description | EBW | MAO |
|---|---|---|---|
| S0 | base metal (BM) | No | No |
| S1 | heat-affected zone (HAZ) | Yes | No |
| S2 | fusion zone (FZ) | Yes | No |
| S3 | welded zone (WZ) | Yes | No |
| S4 | welded joint (WJ) | Yes | No |
| S5 | BM treated by MAO | No | Yes |
| S6 | HAZ treated by MAO | Yes | Yes |
| S7 | FZ treated by MAO | Yes | Yes |
| S8 | WZ treated by MAO | Yes | Yes |
| S9 | WJ treated by MAO | Yes | Yes |
| Samples | Ecorr/V | Icorr/A·cm−2 | βa/mV·dec−1 | βc/mV·dec−1 |
|---|---|---|---|---|
| S0 | −0.482 | 1.08 × 10−6 | 1227.5 | 384.64 |
| S4 | −0.733 | 1.14 × 10−5 | 812.69 | 1204.3 |
| S5 | −0.234 | 9.33 × 10−7 | 3278.4 | 910.65 |
| S9 | −0.463 | 6.76 × 10−7 | 864.44 | 450.31 |
| Parameter | S0 | S4 | S5 | S9 |
|---|---|---|---|---|
| Rs (Ω · cm2) | 21.85 | 42.51 | 29.02 | 31.81 |
| CPEp/1(Ω−1sncm−2) | 1.45 × 10−5 | 5.09 × 10−5 | 1.63 × 10−6 | 8.17 × 10−6 |
| np/1 | 0.83 | 0.85 | 0.65 | 0.57 |
| Rp/1 (kΩ · cm2) | 100.37 | 45.63 | 19.13 | 8.49 |
| CPE2(Ω−1sncm−2) | 1.41 × 10−5 | 1.42 × 10−5 | ||
| n2 | 0.45 | 0.66 | ||
| R2 (MΩ · cm2) | 1.95 × 107 | 1.39 × 107 |
| Elements | A | B | C |
|---|---|---|---|
| Ti | 90.74 | 75.65 | 76.19 |
| Al | 6.14 | 7.49 | 4.65 |
| V | 1.87 | 2.29 | 0.89 |
| O | 1.25 | 12.91 | 14.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wu, P.; Mei, J.; Yu, Z.; Yang, J.; He, Y.; Li, H.; Lv, C.; Ren, S.; Xu, J.; et al. Effects of Micro-Arc Oxidation Surface Treatment on the Corrosion Resistance of Ti-6Al-4V Electron-Beam-Welded Joints. Metals 2023, 13, 1161. https://doi.org/10.3390/met13071161
Ma Y, Wu P, Mei J, Yu Z, Yang J, He Y, Li H, Lv C, Ren S, Xu J, et al. Effects of Micro-Arc Oxidation Surface Treatment on the Corrosion Resistance of Ti-6Al-4V Electron-Beam-Welded Joints. Metals. 2023; 13(7):1161. https://doi.org/10.3390/met13071161
Chicago/Turabian StyleMa, Yinghe, Peng Wu, Jinhui Mei, Zhen Yu, Jianguo Yang, Yanming He, Huaxin Li, Chuanyang Lv, Sendong Ren, Jianping Xu, and et al. 2023. "Effects of Micro-Arc Oxidation Surface Treatment on the Corrosion Resistance of Ti-6Al-4V Electron-Beam-Welded Joints" Metals 13, no. 7: 1161. https://doi.org/10.3390/met13071161
APA StyleMa, Y., Wu, P., Mei, J., Yu, Z., Yang, J., He, Y., Li, H., Lv, C., Ren, S., Xu, J., Cai, Z., & Chu, P. K. (2023). Effects of Micro-Arc Oxidation Surface Treatment on the Corrosion Resistance of Ti-6Al-4V Electron-Beam-Welded Joints. Metals, 13(7), 1161. https://doi.org/10.3390/met13071161






