The Influence of 1 wt.% Cr on the Corrosion Resistance of Low-Alloy Steel in Marine Environments
Abstract
1. Introduction
2. Materials and Methods
3. Result
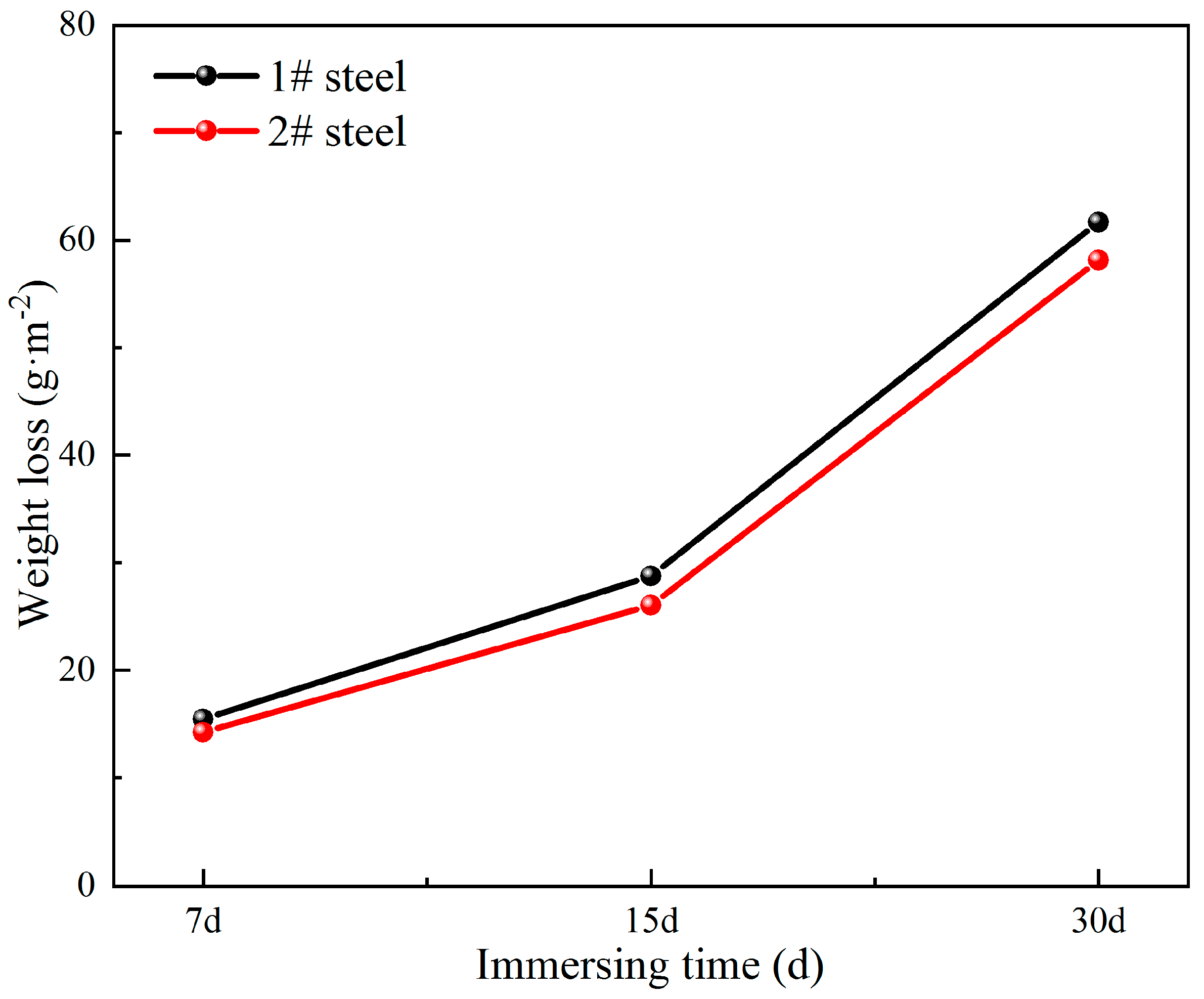
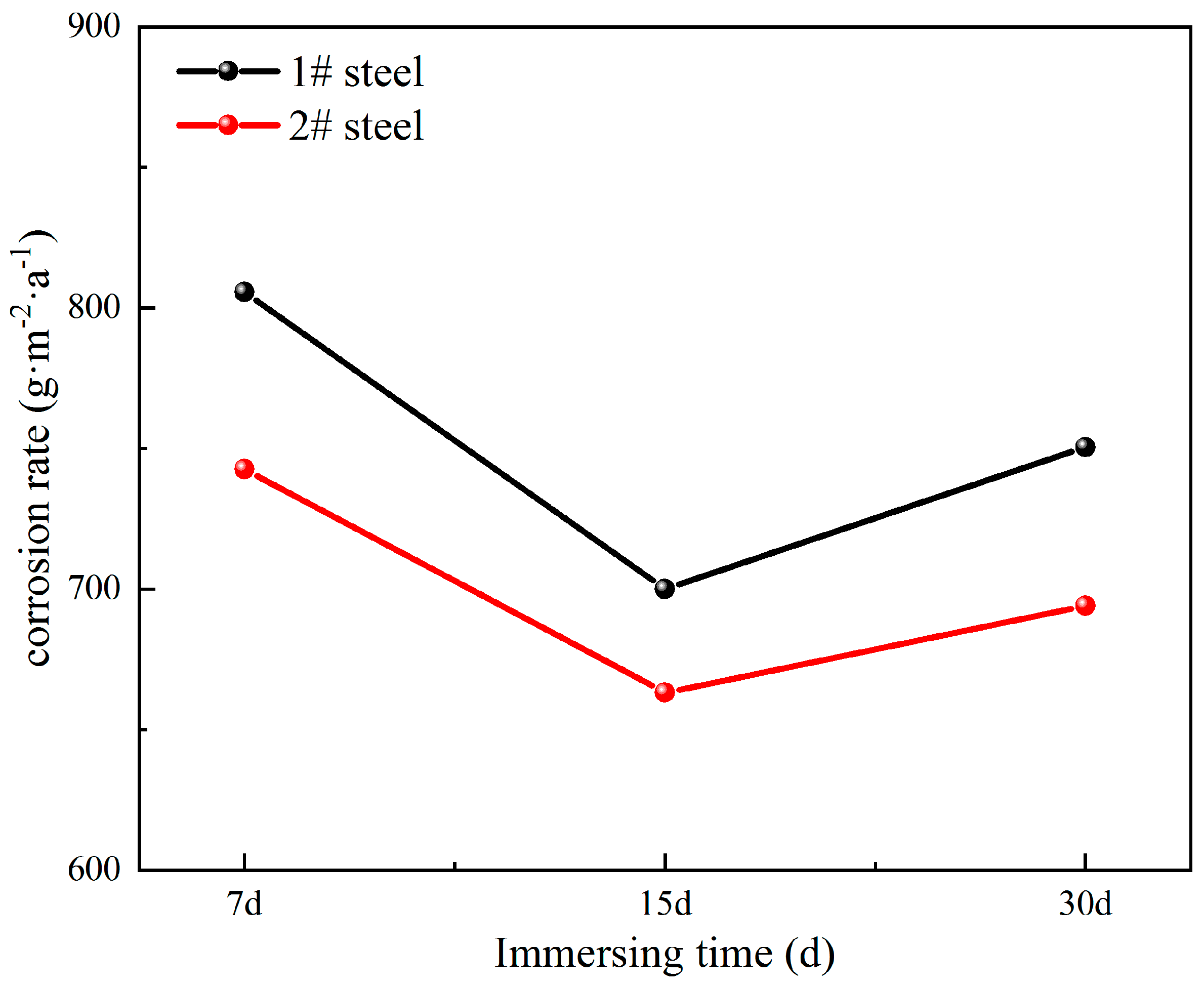
3.1. Corrosion Rate Analysis
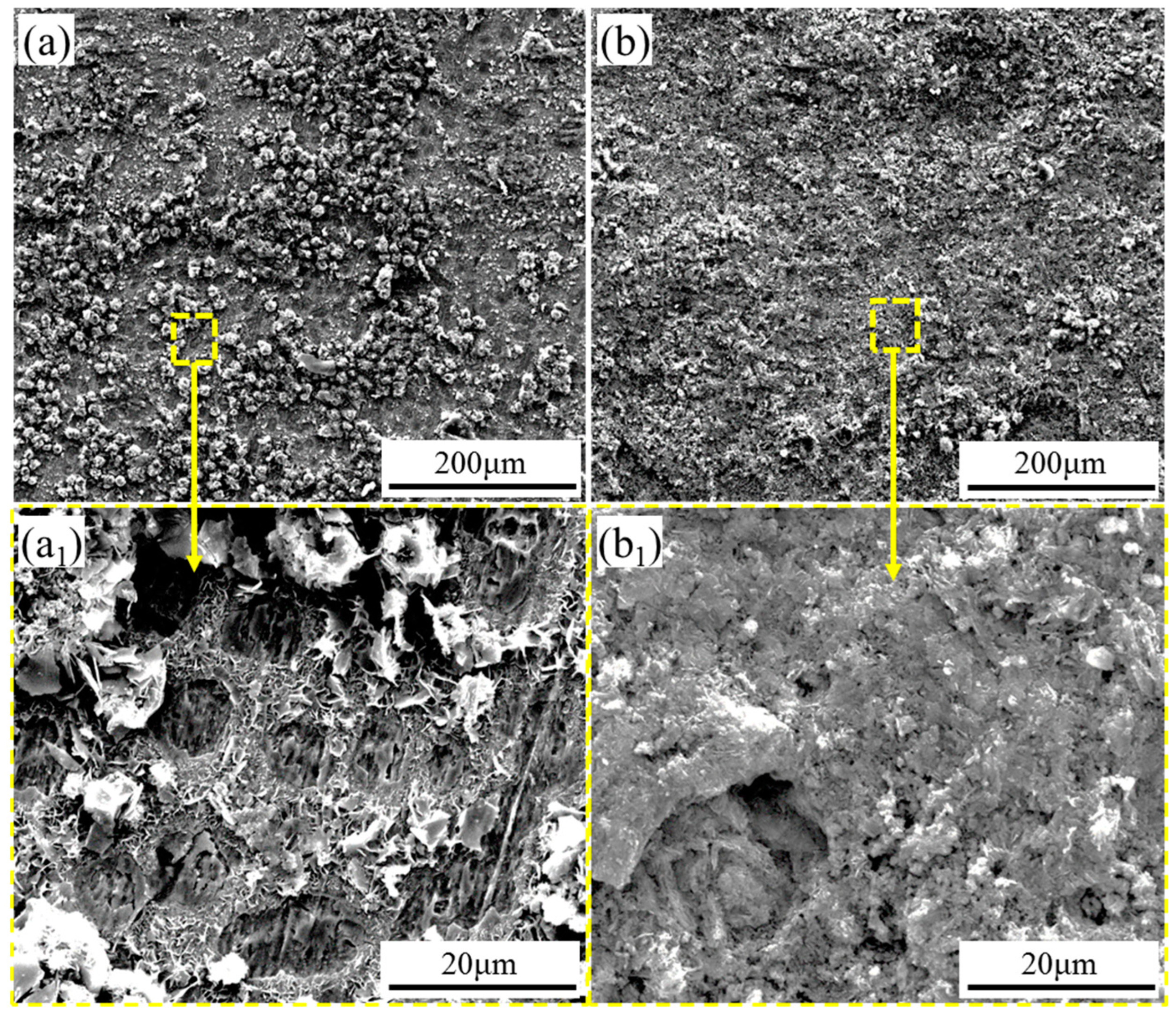
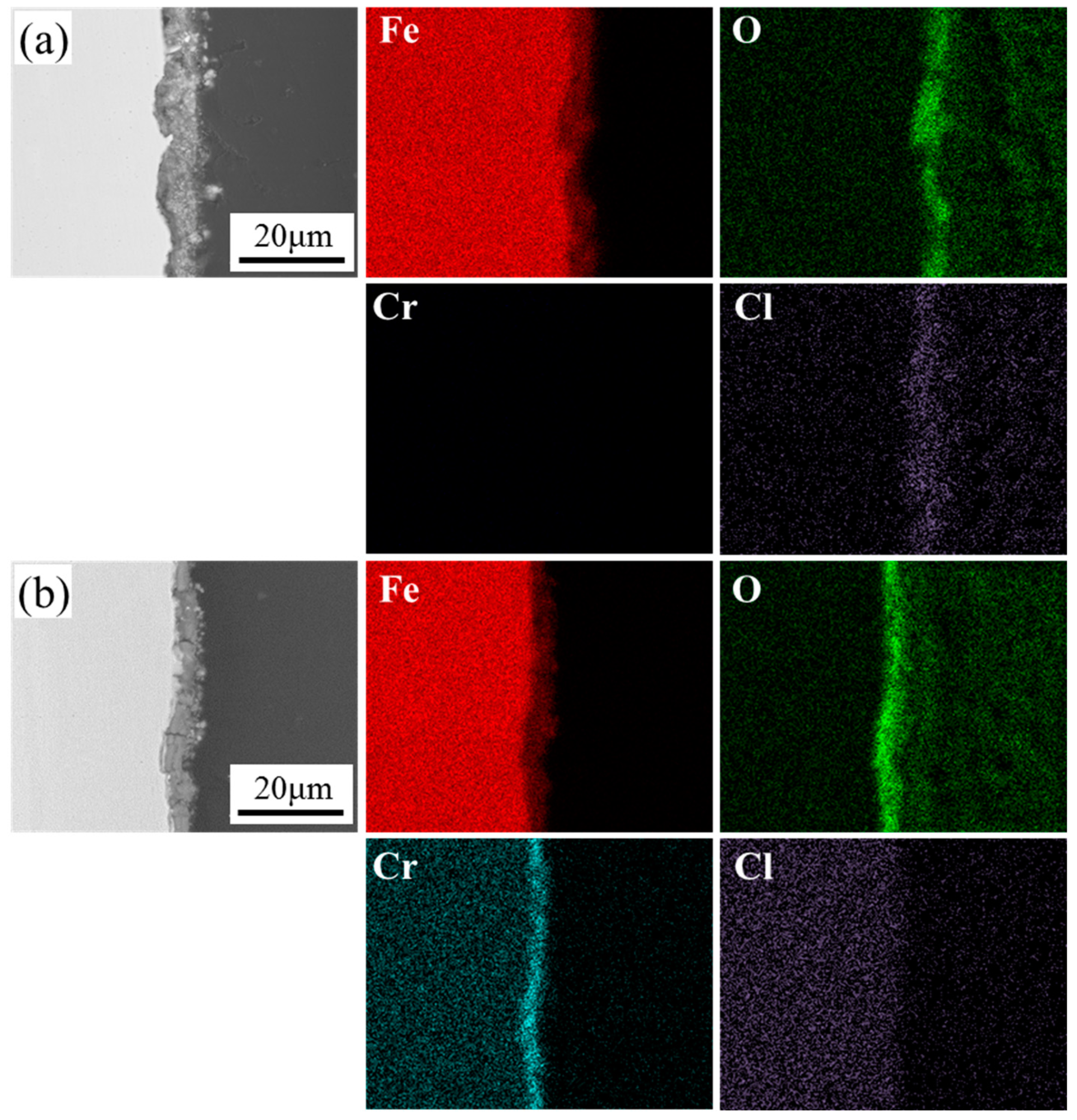
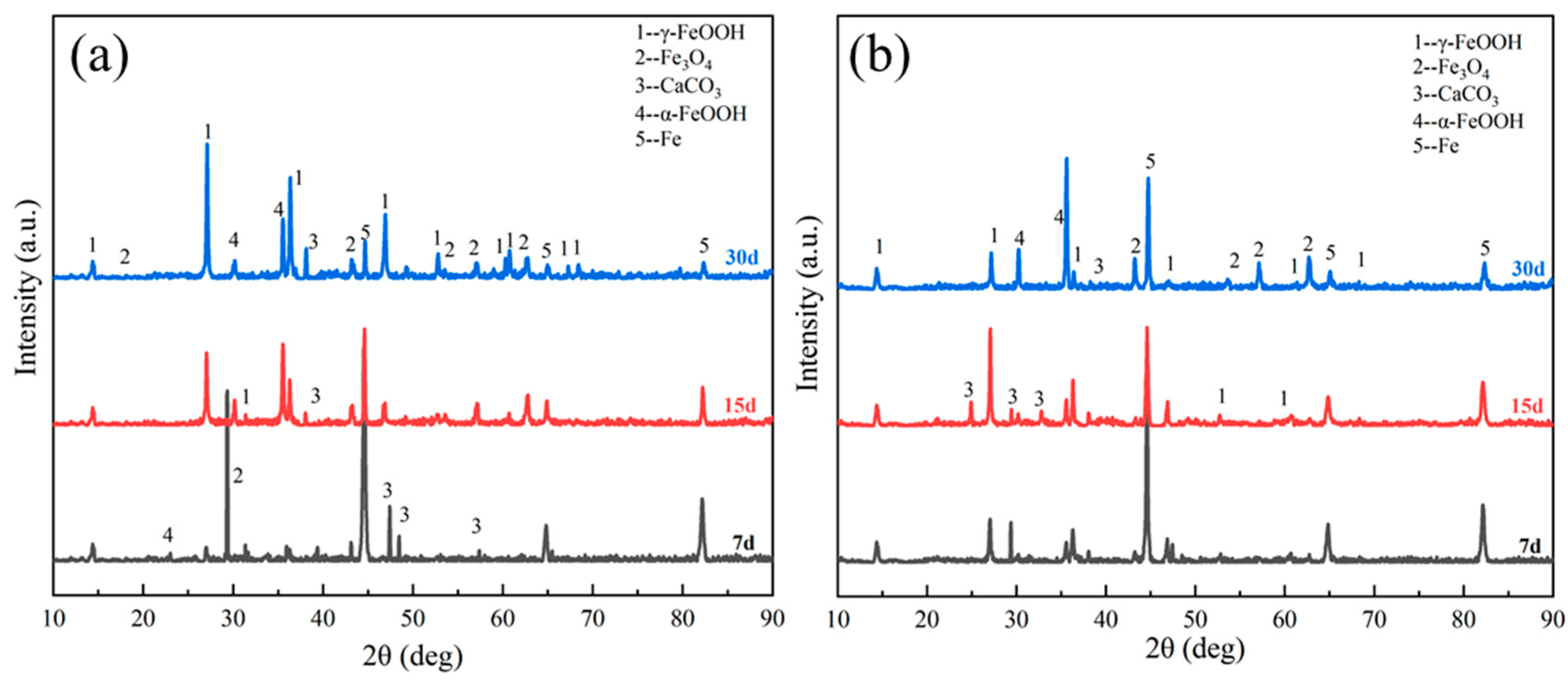
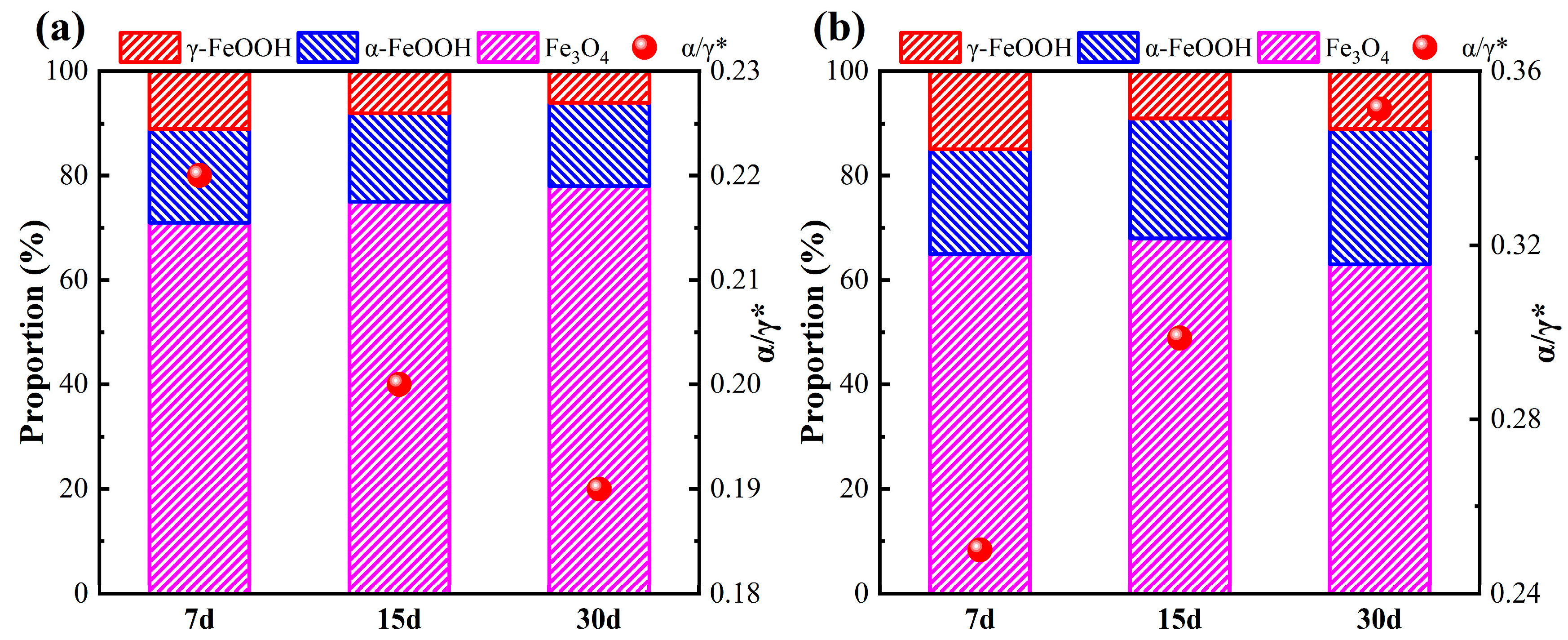
3.2. Evolution of Corrosion Products
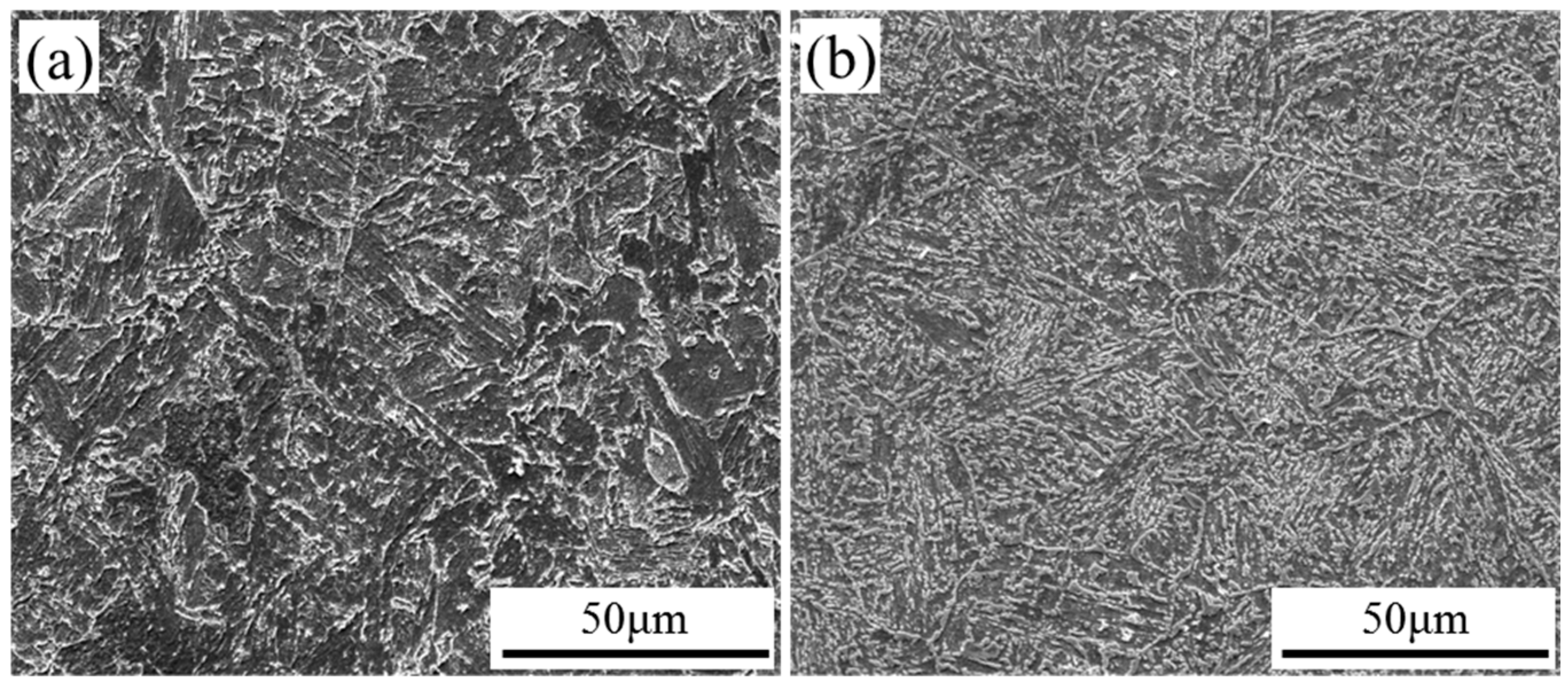
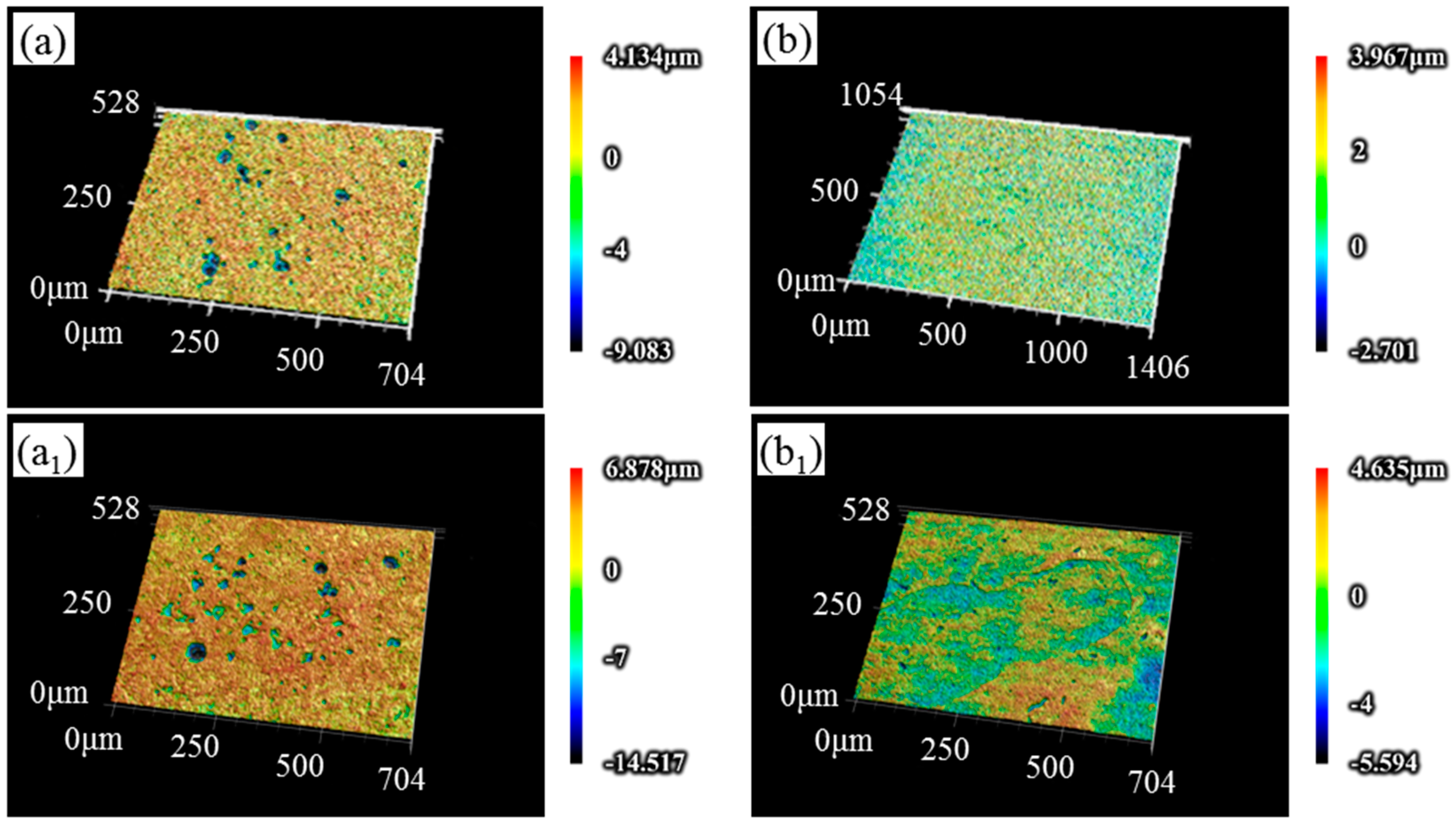
3.3. Corrosion Morphology Analysis
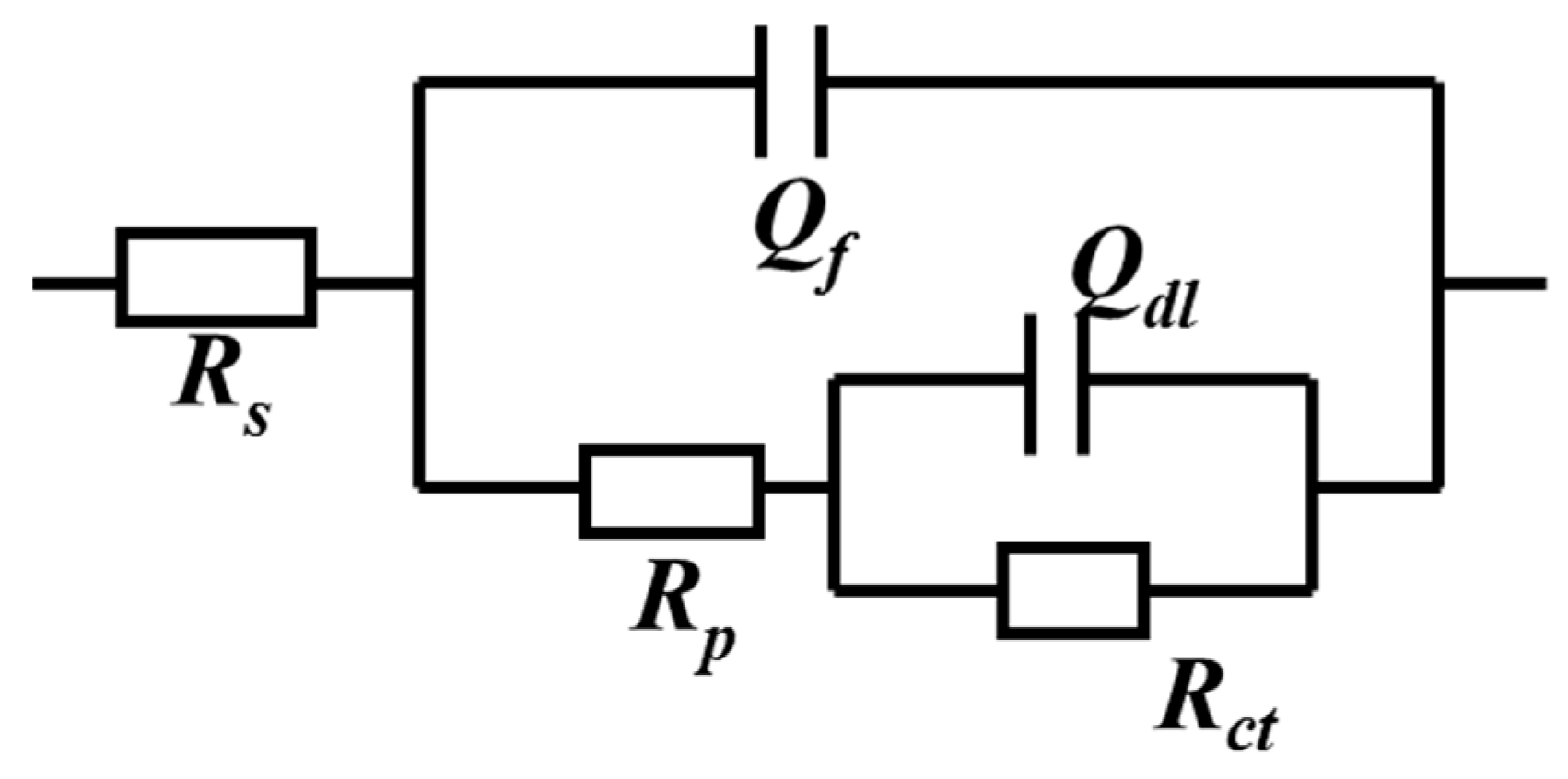
3.4. Electrochemical Analysis
4. Discussion
5. Conclusions
- (1)
- The addition of Cr can change the microstructure, promoting the transformation from granular bainite to lath bainite;
- (2)
- The addition of Cr can effectively reduce the corrosion rate of low-alloy structural steel in the marine environment;
- (3)
- The addition of Cr can promote the formation of stable α-FeOOH in the rust layer, improve the loose and porous structure of the corrosion product layer, promote the densification of the rust layer, and effectively hinder the infiltration of corrosive ions;
- (4)
- The improvement effect of 1 wt.% Cr on the rust layer is limited, as the corrosion product layer still has obvious cracks. Further research is needed to better optimize the corrosion product layer structure and inhibit the corrosion of low-alloy steel in the marine environment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Southwell, C.; Forgeson, B.; Alexander, A. Corrosion of Metals in Tropical Environments (Part 2—Atmospheric Corrosion of Ten Structural Steels). Corrosion 1958, 14, 55–59. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Chen, L.; Zhang, T.; Fan, Y.; Zhao, Y.; Li, S.; Yang, W.; Banthukul, W. Optimize Ni, Cu, Mo element of low Cr-steel rebars in tropical marine atmosphere environment through two years of corrosion monitoring. Cem. Concr. Compos. 2022, 125, 104317. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, S.; Jiang, C.; Yang, J.J.; Sha, G. Formation of high-temperature inner oxide scale on low alloy steels: Segregation, partitioning and transformation reactions. Corros. Sci. 2022, 195, 109980. [Google Scholar] [CrossRef]
- Sun, Y.P.; Wei, X.; Dong, J.H.; Chen, N.; Zhao, H.Y.; Ren, Q.Y.; Ke, W. Understanding the role of alloyed Ni and Cu on improving corrosion resistance of low alloy steel in the simulated Beishan groundwater. J. Mater. Sci. Technol. 2022, 130, 124–135. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhai, Y.; Qiao, Y.; Zheng, C.; Wang, D.; Shi, X.; Lu, H.; Liu, C. Corrosion behavior of low alloy steel used for new pipeline exposed to H2S-saturated solution. Int. J. Hydrog. Energy 2022, 47, 33000–33013. [Google Scholar] [CrossRef]
- Xu, X.X.; Zhang, T.Y.; Wu, W.; Jiang, S.; Yang, J.W.; Liu, Z.Y. Optimizing the resistance of Ni-advanced weathering steel to marine atmospheric corrosion with the addition of Al or Mo. Constr. Build. Mater. 2021, 279, 122341. [Google Scholar] [CrossRef]
- Konishi, H.; Yamashita, M.; Uchida, H.; Jun, M. Characterization of rust layer formed on Fe, Fe-Ni and Fe-Cr alloys exposed to Cl-rich environment by Cl and Fe K-Edge XANES measurements. Mater. Trans. 2005, 46, 329–336. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Ishii, Y.; Okada, T.; Kimura, M.; Kihira, H. Magnetic property based characterization of rust on weathering steels. Corros. Sci. 2005, 47, 2477–2491. [Google Scholar] [CrossRef]
- Kamimura, T.; Stratmann, M. The influence of chromium on the atmospheric corrosion of steel. Corros. Sci. 2001, 43, 429–447. [Google Scholar] [CrossRef]
- Yuan, R.; Wu, H.B.; Gu, Y. Effect of alloyed Cr on corrosion behavior of low-alloy steel in wet atmosphere. Mater. Corros.-Werkst. Korros. 2022, 73, 918–931. [Google Scholar] [CrossRef]
- Yang, X.J.; Yang, Y.; Sun, M.H.; Jia, J.H.; Cheng, X.Q.; Pei, Z.B.; Li, Q.; Xu, D.; Xiao, K.; Li, X.G. A new understanding of the effect of Cr on the corrosion resistance evolution of weathering steel based on big data technology. J. Mater. Sci. Technol. 2022, 104, 67–80. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.O.; Jeong, Y.J.; Lee, S.G.; Choi, J.K.; Kim, S.J. Long-Term Corrosion Behavior of Strong and Ductile High Mn-Low Cr Steel in Acidic Aqueous Environments. Materials 2022, 15, 1746. [Google Scholar] [CrossRef]
- Gupta, K.K.; Haratian, S.; Ambat, R. On CO2 corrosion resistance of low carbon steels in the formation water chemistry: The impact of Cr content as an alloying element. In Proceedings of the 18th Nordic Corrosion Congres, Turku, Finland, 31 May–2 June 2022. [Google Scholar]
- Łukaszczyk, A.; Banaś, J.; Pisarek, M.; Seyeux, A.; Marcus, P.; Światowska, J. Effect of Cr Content on Corrosion Resistance of Low-Cr Alloy Steels Studied by Surface and Electrochemical Techniques. Electrochem 2021, 2, 546–562. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Yang, F.L.; Chen, H.D.; Liang, N.N.; Wu, Y.Y.; Zhang, J.C.; Ma, H.; Klu, E.E.; Gao, B.; et al. Corrosion behavior and mechanism of Cr-Mo alloyed steel: Role of ferrite/bainite duplex microstructure. J. Alloys Compd. 2019, 809, 151787. [Google Scholar] [CrossRef]
- Zhang, S.H.; Bian, T.T.; Mou, L.M.; Yan, X.Y.; Zhang, J.L.; Zhang, Y.Z.; Liu, B.S. Alloy design employing Ni and Mo low alloying for 3Cr steel with enhanced corrosion resistance in CO2 environments. J. Mater. Res. Technol. 2023, 24, 1304–1321. [Google Scholar]
- Chen, L.J.; Dong, B.J.; Liu, W.; Wu, F.; Li, H.; Zhang, T.Y. Failure analysis of corrosion products formed during CO2 pre-corrosion of X70 and 3Cr steels: Effect of oxygen contamination. Eng. Fail. Anal. 2022, 140, 106529. [Google Scholar] [CrossRef]
- Kashima, K.; Sugae, K.; Kamimura, T.; Miyuki, H.; Kudo, T. Effect of Chromium Contents on Atmospheric Corrosion of Steel in Chloride Environment. J. Jpn. Inst. Met. Mater. 2013, 77, 107–113. [Google Scholar] [CrossRef]
- Xu, X.X.; Wu, W.; Li, N.N.; Zhang, L.L.; Wang, Y.; Liu, Z.Y.; Li, X.G. Effect of 0.1 wt% Nb on the microstructure and corrosion fatigue performance of high strength steels. Corros. Sci. 2023, 219, 111242. [Google Scholar] [CrossRef]
- Ma, H.C.; Liu, Z.Y.; Du, C.W.; Wang, H.R.; Li, X.G.; Zhang, D.W.; Cui, Z.Y. Stress corrosion cracking of E690 steel as a welded joint in a simulated marine atmosphere containing sulphur dioxide. Corros. Sci. 2015, 100, 627–641. [Google Scholar] [CrossRef]
- Xu, X.X.; Liu, Z.Y.; Zhao, T.L.; Cui, Q.Q.; Zhang, T.Y.; Li, X.G. Corrosion fatigue behavior of Fe-16Mn-0.6C-1.68Al twinning-induced plasticity steel in simulated seawater. Corros. Sci. 2021, 182, 109282. [Google Scholar]
- Wu, W.; Cheng, X.Q.; Zhao, J.B.; Li, X.G. Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives. Corros. Sci. 2020, 165, 108416. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.Y.; Liu, Z.Y.; Liu, C.; Li, X.G. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Jia, J.H.; Wu, W.; Cheng, X.Q.; Zhao, J.B. Ni-advanced weathering steels in Maldives for two years: Corrosion results of tropical marine field test. Constr. Build. Mater. 2020, 245, 118463. [Google Scholar] [CrossRef]
- Jia, J.H.; Liu, Z.Y.; Li, X.G.; Du, C.W.; Li, W. Comparative study on the stress corrosion cracking of a new Ni-advanced high strength steel prepared by TMCP, direct quenching, and quenching & tempering. Mater. Sci. Eng. 2021, 825, 141854. [Google Scholar]
- Li, J.K.; Fu, B.G.; Dong, T.S.; Li, G.L.; Liu, Y.Y.; Liu, J.G.; Wang, C.Y.; Cui, W. A new nano-scale rhombic precipitate in the SiC ceramic manufactured by milling-sintering process. Mater. Charact. 2023, 198, 112731. [Google Scholar] [CrossRef]
- Xu, X.X.; Cheng, H.L.; Wu, W.; Liu, Z.Y.; Li, X.G. Stress corrosion cracking behavior and mechanism of Fe-Mn-Al-C-Ni high specific strength steel in the marine atmospheric environment. Corros. Sci. 2021, 191, 109760. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Liu, W.; Zhu, Y.C.; Zhang, B.L.; Li, S.Z.; Lu, M.X. Effect of Small Content of Chromium on Wet-Dry Acid Corrosion Behavior of Low Alloy Steel. Acta Metall. Sin. 2017, 30, 164–175. [Google Scholar] [CrossRef]
- Liu, W.M.; Liu, J.; Pan, H.B.; Cao, F.B.; Wu, Z.J.; Lv, H.H.; Xu, Z.Y. Synergisic effect of Mn, Cu, P with Cr content on the corrosion behavior of weathering steel as a train under the simulated industrial atmosphere. J. Alloys Compd. 2020, 834, 155095. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Xu, Y.; Liu, Z. Effects of Cr, Ni and Cu on the corrosion behavior of low carbon microalloying steel in a Cl− containing environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Sun, M.H.; Pang, Y.J.; Du, C.W.; Li, X.G.; Wu, Y.M. Optimization of Mo on the corrosion resistance of Cr-advanced weathering steel designed for tropical marine atmosphere. Constr. Build. Mater. 2021, 302, 124346. [Google Scholar]
- Sun, M.H.; Du, C.W.; Liu, Z.Y.; Liu, C.; Li, X.G.; Wu, Y.M. Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere. Corros. Sci. 2021, 186, 109427. [Google Scholar] [CrossRef]


| Steel | C | Si | Mn | P | S | Ni | Cu | Cr |
|---|---|---|---|---|---|---|---|---|
| 1# | 0.044 | 0.52 | 1.22 | 0.0089 | 0.0021 | 0.98 | 0.31 | - |
| 2# | 0.039 | 0.46 | 1.30 | 0.0085 | 0.0019 | 1.02 | 0.31 | 0.97 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | H3BO3 | SrCl2 | NaF |
|---|---|---|---|---|---|---|---|---|---|
| 24.53 | 5.20 | 4.09 | 1.16 | 0.695 | 0.201 | 0.101 | 0.027 | 0.025 | 0.003 |
| Rs (Ω·cm2) | Qf × 10−4 (Ω cm−2sn1) | n1 | Rp (Ω·cm2) | Qdl × 10−4 (Ω cm−2sn2) | n2 | Rct (Ω·cm2) | χ2 × 10−4 | |
|---|---|---|---|---|---|---|---|---|
| 1#—7 d | 113.7 | 8.64 | 0.74 | 205.8 | 3.61 | 0.69 | 715 | 3.48 |
| 1#—15 d | 106.5 | 6.58 | 0.72 | 262 | 5.14 | 0.72 | 980 | 1.42 |
| 1#—30 d | 95.22 | 9.12 | 0.64 | 615 | 6.23 | 1 | 1978 | 2.26 |
| 2#—7 d | 92.88 | 1.25 | 0.74 | 221.2 | 4.71 | 0.75 | 825.1 | 4.20 |
| 2#—15 d | 130.4 | 4.56 | 0.76 | 261.8 | 2.37 | 0.77 | 1341 | 3.08 |
| 2#—30 d | 128.6 | 3.46 | 0.87 | 720.7 | 8.01 | 0.64 | 2881 | 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, N.; Chen, H.; Xu, X. The Influence of 1 wt.% Cr on the Corrosion Resistance of Low-Alloy Steel in Marine Environments. Metals 2023, 13, 1050. https://doi.org/10.3390/met13061050
Gao J, Wang N, Chen H, Xu X. The Influence of 1 wt.% Cr on the Corrosion Resistance of Low-Alloy Steel in Marine Environments. Metals. 2023; 13(6):1050. https://doi.org/10.3390/met13061050
Chicago/Turabian StyleGao, Jianzhuo, Ningxi Wang, Hui Chen, and Xuexu Xu. 2023. "The Influence of 1 wt.% Cr on the Corrosion Resistance of Low-Alloy Steel in Marine Environments" Metals 13, no. 6: 1050. https://doi.org/10.3390/met13061050
APA StyleGao, J., Wang, N., Chen, H., & Xu, X. (2023). The Influence of 1 wt.% Cr on the Corrosion Resistance of Low-Alloy Steel in Marine Environments. Metals, 13(6), 1050. https://doi.org/10.3390/met13061050




