Influence of Heat Treatment and High-Pressure Torsion on Phase Transformations in TiZrHfMoCr High-Entropy Alloy
Abstract
1. Introduction
2. Experimental Section
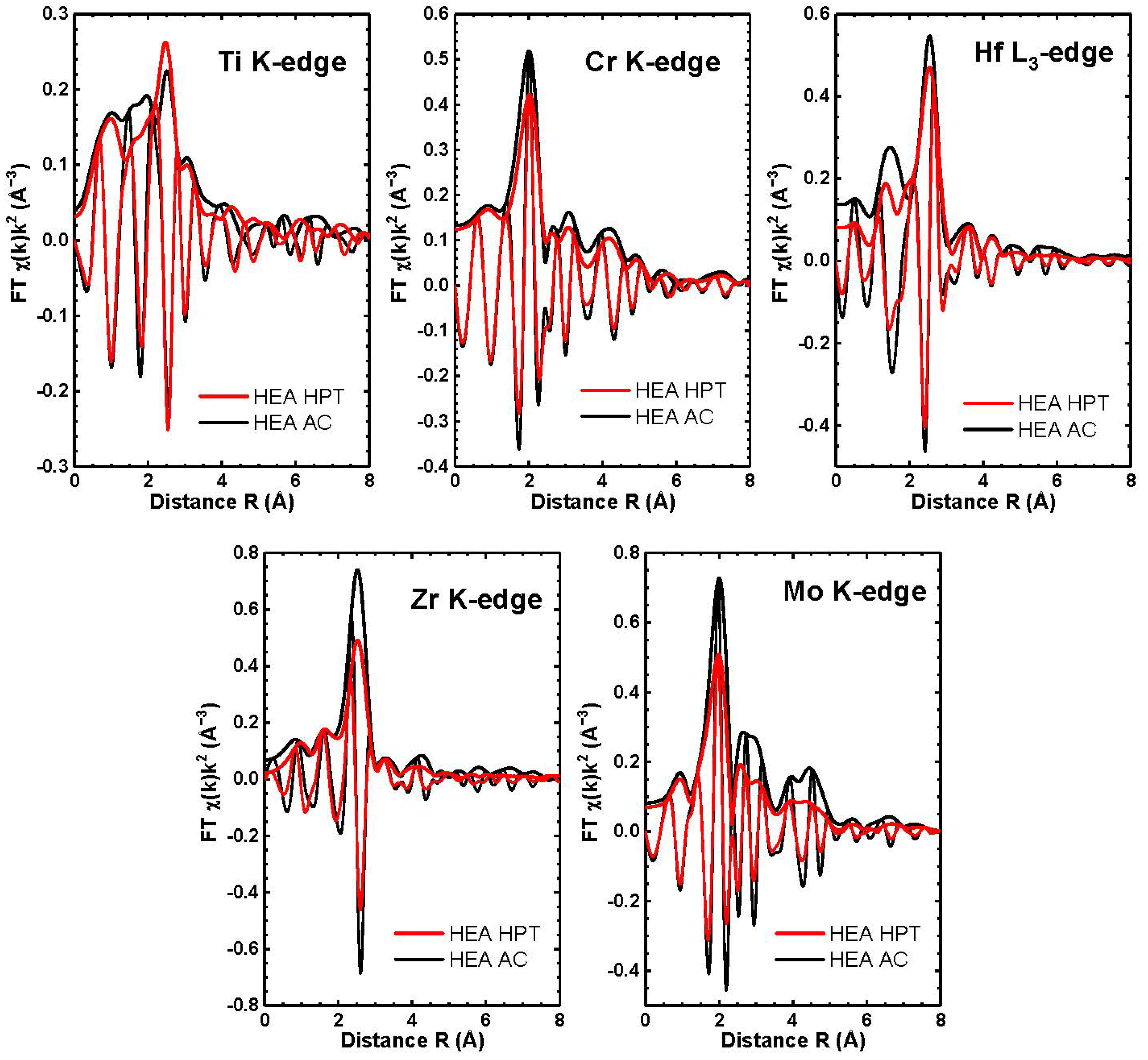
3. Results Together with Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Straumal, B.B.; Klinger, L.; Kuzmin, A.; Lopez, G.A.; Korneva, A.; Straumal, A.B.; Vershinin, N.; Gornakova, A.S. High Entropy Alloys Coatings Deposited by Laser Cladding: A Review of Grain Boundary Wetting Phenomena. Coatings 2022, 12, 343. [Google Scholar] [CrossRef]
- Straumal, B.; Korneva, A.; Kuzmin, A.; Klinger, L.; Lopez, G.A.; Vershinin, N.; Straumal, A.; Gornakova, A. High Entropy Alloys for Energy Conversion and Storage: A Review of Grain Boundary Wetting Phenomena. Energies 2022, 15, 7130. [Google Scholar] [CrossRef]
- Straumal, B.; Rabkin, E.; Lopez, G.A.; Korneva, A.; Kuzmin, A.; Gornakova, A.; Straumal, A.; Baretzky, B. Grain Boundary Wetting Phenomena in High Entropy Alloys Containing Nitrides, Carbides, Borides, Silicides, and Hydrogen: A Review. Crystals 2021, 11, 1540. [Google Scholar] [CrossRef]
- Amiri, A.; Shahbazian-Yassar, R. Recent progress of high-entropy materials for energy storage and conversion. J. Mater. Chem. A 2021, 9, 782–823. [Google Scholar] [CrossRef]
- Liu, H.; Syama, L.; Zhang, L.; Lee, C.; Liu, C.; Dai, Z.; Yan, Q. High-entropy alloys and compounds for electrocatalytic energy conversion applications. Susmat 2021, 1, 482–505. [Google Scholar] [CrossRef]
- Gao, P.-H.; Fu, R.-T.; Chen, B.-Y.; Zeng, S.-C.; Zhang, B.; Yang, Z.; Guo, Y.-C.; Liang, M.-X.; Li, J.-P.; Lu, Y.-Q.; et al. Corrosion Resistance of CoCrFeNiMn High Entropy Alloy Coating Prepared through Plasma Transfer Arc Claddings. Metals 2021, 11, 1876. [Google Scholar] [CrossRef]
- Wang, M.; Lu, Y.; Zhang, G.; Cui, H.; Xu, D.; Wei, N.; Li, T. A novel high-entropy alloy composite coating with core-shell structures prepared by plasma cladding. Vacuum 2021, 184, 109905. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Y.; Feng, X.; Tian, Z.; Song, R. Thermal barrier coatings with high-entropy oxide as a top coat. Ceram. Int. 2022, 48, 1349–1359. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Yan, S.; Yu, G.; Chen, J.; He, J.; Yin, F. Microstructure evolution and mechanical properties of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings under post-annealing. J. Alloys Compd. 2021, 872, 159607. [Google Scholar] [CrossRef]
- Xue, M.; Mao, X.; Lv, Y.; Chi, Y.; Yang, Y.; He, J.; Dong, Y. Comparison of Micro-nano FeCoNiCrAl and FeCoNiCrMn Coatings Prepared from Mechanical Alloyed High-entropy Alloy Powders. J. Therm. Spray Technol. 2021, 30, 1666–1678. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Zhu, S.; Yu, Y.; Wang, Z.; Zhang, X.; Lu, B. Microstructural characteristics and enhanced wear resistance of nanoscale Al2O3/13 wt%TiO2-reinforced CoCrFeMnNi high entropy coatings. Surf. Coat. Technol. 2021, 412, 127019. [Google Scholar] [CrossRef]
- Xiao, J.-K.; Li, T.-T.; Wu, Y.-Q.; Chen, J.; Zhang, C. Microstructure and Tribological Properties of Plasma-Sprayed CoCrFeNi-based High-Entropy Alloy Coatings Under Dry and Oil-Lubricated Sliding Conditions. J. Therm. Spray Technol. 2021, 30, 926–936. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Zhang, B.; Yu, Y.; Wang, Z.; Zhang, X.; Lu, B. Microstructure and Properties of Al2O3-13wt.%TiO2-Reinforced CoCrFeMnNi High-Entropy Alloy Composite Coatings Prepared by Plasma Spraying. J. Therm. Spray Technol. 2021, 30, 772–786. [Google Scholar] [CrossRef]
- Ma, X.; Ruggiero, P.; Bhattacharya, R.; Senkov, O.N.; Rai, A.K. Evaluation of New High Entropy Alloy as Thermal Sprayed Bondcoat in Thermal Barrier Coatings. J. Therm. Spray Technol. 2022, 31, 1011–1020. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, M.; Wang, G.; Yang, X.; Wang, S. Wear and Corrosion Resistance Analysis of FeCoNiTiAlx High-Entropy Alloy Coatings Prepared by Laser Cladding. Coatings 2021, 11, 155. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.; Jin, G.; Liu, Y.; Zhang, Y.; Fang, Y. In-situ synthesis of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings by laser cladding: Alloy design and microstructure evolution. Surf. Coat. Technol. 2021, 405, 126728. [Google Scholar] [CrossRef]
- Qiu, X. Microstructure and corrosion properties of Al2CrFeCo CuNiTi high entropy alloys prepared by additive manufacturing. J. Alloys Compd. 2021, 887, 161422. [Google Scholar] [CrossRef]
- Hussien, M.; Walton, K.; Vishnyakov, V. Synthesis and Corrosion Resistance of FeMnNiAlC10 Multi-Principal Element Compound. Materials 2021, 14, 6356. [Google Scholar] [CrossRef]
- Rao, S.G.; Shu, R.; Boyd, R.; le Febvrier, A.; Eklund, P. The effects of copper addition on phase composition in (CrFeCo)1−yNy multicomponent thin films. Appl. Surf. Sci. 2022, 572, 151315. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Z.; Yang, W.; Zhang, P.; Lu, Y.; Pu, J. Microstructure and corrosion behavior of AlCrTiV-X (X = Cu, Mo, CuMo) high-entropy alloy films in 3.5 wt.% NaCl solution. Surf. Interfaces 2021, 27, 101558. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Chung, C.-H. Tribological and Mechanical Properties of Multicomponent CrVTiNbZr(N) Coatings. Coatings 2021, 11, 41. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Bagdasaryan, A.; Horodeck, P.; Tarelnyk, V.; Buranich, V.; Amekura, H.; Okubo, N.; Ishikawa, N.; Beresnev, V. Positron annihilation studies of defect structure of (TiZrHfNbV)N nitride coatings under Xe14+ 200 MeV ion irradiation. Mater. Lett. 2021, 303, 130548. [Google Scholar] [CrossRef]
- Ustinov, A.; Demchenkov, S.; Melnychenko, T.; Skorodzievskii, V.; Polishchuk, S. Effect of structure of high entropy CrFeCoNiCu alloys produced by EB PVD on their strength and dissipative properties. J. Alloys Compd. 2021, 887, 161408. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Mazilkin, A.; Straumal, B.; Kilmametov, A.; Straumal, P.; Baretzky, B. Phase Transformations Induced by Severe Plastic Deformation. Mater. Trans. 2019, 60, 1489–1499. [Google Scholar] [CrossRef]
- Gubicza, J.; Heczel, A.; Kawasaki, M.; Han, J.-K.; Zhao, Y.; Xue, Y.; Huang, S.; Lábár, J.L. Evolution of microstructure and hardness in Hf25Nb25Ti25Zr25 high-entropy alloy during high-pressure torsion. J. Alloys Compd. 2019, 788, 318–328. [Google Scholar] [CrossRef]
- Čížek, J.; Haušild, P.; Cieslar, M.; Melikhova, O.; Vlasák, T.; Janeček, M.; Král, R.; Harcuba, P.; Lukáč, F.; Zýka, J.; et al. Strength enhancement of high entropy alloy HfNbTaTiZr by severe plastic deformation. J. Alloys Compd. 2018, 768, 924–937. [Google Scholar] [CrossRef]
- Lukáč, F.; Dudr, M.; Čížek, J.; Harcuba, P.; Vlasák, T.; Janeček, M.; Kuriplach, J.; Moon, J.; Kim, H.S.; Zýka, J.; et al. Defects in High Entropy Alloy HfNbTaTiZr Prepared by High Pressure Torsion. Acta Phys. Pol. A 2018, 134, 891–894. [Google Scholar] [CrossRef]
- Chandan, A.; Hung, P.; Kishore, K.; Kawasaki, M.; Chakraborty, J.; Gubicza, J. On prominent TRIP effect and non-basal slip in a TWIP high entropy alloy during high-pressure torsion processing. Mater. Charact. 2021, 178, 111284. [Google Scholar] [CrossRef]
- Kilmametov, A.; Kulagin, R.; Mazilkin, A.; Seils, S.; Boll, T.; Heilmaier, M.; Hahn, H. High-pressure torsion driven mechanical alloying of CoCrFeMnNi high entropy alloy. Scr. Mater. 2019, 158, 29–33. [Google Scholar] [CrossRef]
- Shahmir, H.; Nili-Ahmadabadi, M.; Shafiee, A.; Andrzejczuk, M.; Lewandowska, M.; Langdon, T.G. Effect of Ti on phase stability and strengthening mechanisms of a nanocrystalline CoCrFeMnNi high-entropy alloy. Mater. Sci. Eng. A 2018, 725, 196–206. [Google Scholar] [CrossRef]
- Shahmir, H.; Nili-Ahmadabadi, M.; Shafiee, A.; Langdon, T.G. Effect of a minor titanium addition on the superplastic properties of a CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2018, 718, 468–476. [Google Scholar] [CrossRef]
- Protasova, S.G.; Straumal, B.; Mazilkin, A.A.; Stakhanova, S.V.; Straumal, P.B.; Baretzky, B. Increase of Fe solubility in ZnO induced by the grain boundary adsorption. J. Mater. Sci. 2014, 49, 4490–4498. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Stakhanova, S.V.; Straumal, P.B.; Bulatov, M.F.; Schütz, G.; Tietze, T.; Goering, E.; Baretzky, B. Grain boundaries as a source of ferromagnetism and increased solubility of Ni in nanograined ZnO. Rev. Adv. Mater. Sci. 2015, 41, 61–71. [Google Scholar]
- Mazilkin, A.; Straumal, B.; Kilmametov, A.; Boll, T.; Baretzky, B.; Kogtenkova, O.; Korneva, A.; Zięba, P. Competition for impurity atoms between defects and solid solution during high pressure torsion. Scr. Mater. 2019, 173, 46–50. [Google Scholar] [CrossRef]
- Ivanisenko, Y.; Sauvage, X.; Mazilkin, A.; Kilmametov, A.; Beach, J.A.; Straumal, B.B. Bulk Nanocrystalline Ferrite Stabilized through Grain Boundary Carbon Segregation. Adv. Eng. Mater. 2018, 20, 1800443. [Google Scholar] [CrossRef]
- Nagase, T.; Iijima, Y.; Matsugaki, A.; Ameyama, K.; Nakano, T. Design and fabrication of Ti-Zr-Hf-Cr-Mo and Ti-Zr-Hf-Co-Cr-Mo high-entropy alloys as metallic biomaterials. Mater. Sci. Eng. C 2020, 107, 110322. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef]
- Nagase, T.; Mizuuchi, K.; Nakano, T. Solidification Microstructures of the Ingots Obtained by Arc Melting and Cold Crucible Levitation Melting in TiNbTaZr Medium-Entropy Alloy and TiNbTaZrX (X = V, Mo, W) High-Entropy Alloys. Entropy 2019, 21, 483. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Hori, T.; Nakano, T. Microstructure of equiatomic and non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. J. Alloys Compd. 2018, 753, 412–421. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Smekhova, A.; Kuzmin, A.; Siemensmeyer, K.; Luo, C.; Chen, K.; Radu, F.; Weschke, E.; Reinholz, U.; Buzanich, A.G.; Yusenko, K.V. Al-driven peculiarities of local coordination and magnetic properties in single-phase Alx-CrFeCoNi high-entropy alloys. Nano Res. 2022, 15, 4845–4858. [Google Scholar] [CrossRef]
- Welter, E.; Chernikov, R.; Herrmann, M.; Nemausat, R. A beamline for bulk sample X-ray absorption spectroscopy at the high brilliance storage ring PETRA III. AIP Conf. Proc. 2019, 2054, 040002. [Google Scholar] [CrossRef]
- Kalinko, A. XAESA v0.06. 2022. Available online: https://github.com/aklnk/xaesa (accessed on 12 December 2022).
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES analysis of oxides at the nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef]
- Kilmametov, A.; Ivanisenko, Y.; Straumal, B.; Mazilkin, A.; Gornakova, A.; Kriegel, M.; Fabrichnaya, O.; Rafaja, D.; Hahn, H. Transformations of α′ martensite in Ti–Fe alloys under high pressure torsion. Scr. Mater. 2017, 136, 46–49. [Google Scholar] [CrossRef]
- Kilmametov, A.; Ivanisenko, Y.; Mazilkin, A.; Straumal, B.; Gornakova, A.; Fabrichnaya, O.; Kriegel, M.; Rafaja, D.; Hahn, H. The α → ω and β → ω phase transformations in Ti–Fe alloys under high-pressure torsion. Acta Mater. 2018, 144, 337–351. [Google Scholar] [CrossRef]
- Kilmametov, A.R.; Ivanisenko, Y.; Straumal, B.B.; Gornakova, A.S.; Mazilkin, A.A.; Hahn, H. The α → ω Transformation in Titanium-Cobalt Alloys under High-Pressure Torsion. Metals 2018, 8, 1. [Google Scholar] [CrossRef]
- Straumal, B.B.; Korneva, A.; Kilmametov, A.R.; Lityńska-Dobrzyńska, L.; Gornakova, A.S.; Chulist, R.; Karpov, M.I.; Zięba, P. Structural and Mechanical Properties of Ti–Co Alloys Treated by High Pressure Torsion. Materials 2019, 12, 426. [Google Scholar] [CrossRef]
- Korneva, A.; Straumal, B.; Chulist, R.; Kilmametov, A.; Bała, P.; Cios, G.; Schell, N.; Zięba, P. Grain refinement of intermetallic compounds in the Cu–Sn system under high pressure torsion. Mater. Lett. 2016, 179, 12–15. [Google Scholar] [CrossRef]
- Korneva, A.; Straumal, B.; Kilmametov, A.; Lityńska-Dobrzyńska, L.; Cios, G.; Bała, P.; Zięba, P. Effect of high pressure torsion on microstructure of Cu–Sn alloys with different content of Hume Rothery phase. Mater. Charact. 2016, 118, 411–416. [Google Scholar] [CrossRef]
- Straumal, B.; Kilmametov, A.; Baretzky, B.; Kogtenkova, O.; Straumal, P.B.; Lityńska-Dobrzyńska, L.; Chulist, R.; Korneva, A.; Zięba, P. High pressure torsion of Cu–Ag and Cu–Sn alloys: Limits for solubility and dissolution. Acta Mater. 2020, 195, 184–198. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kilmametov, A.R.; Korneva, A.; Mazilkin, A.A.; Straumal, P.B.; Zięba, P.; Baretzky, B. Phase transitions in Cu-based alloys under high pressure torsion. J. Alloys Compd. 2017, 707, 20–26. [Google Scholar] [CrossRef]
- Murdock, J.; Lundy, T.; Stansbury, E. Diffusion of Ti44 and V48 in titanium. Acta Met. 1964, 12, 1033–1039. [Google Scholar] [CrossRef]
- Köhler, U.; Herzig, C. On the Anomalous Self-Diffusion in B.C.C. Titanium. Phys. Status Solidi B 1987, 144, 243–251. [Google Scholar] [CrossRef]
- Hood, G.; Schultz, R. Tracer diffusion in α-Zr. Acta Met. 1974, 22, 459–464. [Google Scholar] [CrossRef]
- Horváth, J.; Dyment, F.; Mehrer, H. Anomalous self-diffusion in a single crystal of α-zirconium. J. Nucl. Mater. 1984, 126, 206–214. [Google Scholar] [CrossRef]
- Kidson, G.; McGurn, J. Self-diffusion in body-centered cubic zirconium. Can. J. Phys. 1961, 39, 1146–1157. [Google Scholar] [CrossRef]
- Federer, J.I.; Lundy, I.S. Diffusion of Zr and Cb in beta zirconium. Trans. Metall. Soc. AIME 1963, 227, 592–598. [Google Scholar]
- Herzig, C.; Eckseler, H. On the Anomalous Self-Diffusion in ß-Zirconium: Temperature Dependence of the Isotope Effect. Int. J. Mater. Res. 1979, 70, 215–223. [Google Scholar] [CrossRef]
- Pruthi, D.D.; Agarwala, R.P. Solute and solvent diffusion in Zr-V alloys. Philos. Mag. A 1982, 46, 841–848. [Google Scholar] [CrossRef]
- Davis, B.; McMullen, W. Bulk self-diffusion of Hf181 in monocrystalline alpha hafnium-2.1% zirconium. Acta Met. 1972, 20, 593–599. [Google Scholar] [CrossRef]
- Winslow, E.R.; Lundy, I.S. Self-diffusion in hafnium. Trans. Metall. Soc. AIME 1965, 233, 1790–1796. [Google Scholar]
- Herzig, C.; Manke, L.; Bussman, W. Bulk self-diffusion in hafnium. In Proceedings of the Yamada Vth Conference on Point Defects and Defect Interactions in Metals, Kyoto, Japan, 16–20 November 1981; Takamura, J.I., Doyama, M., Kiritani, M., Eds.; University of Tokyo Press: Tokyo, Japan, 1982; pp. 578–584. [Google Scholar]
- Bronfin, M.B.; Bokshtein, S.Z.; Zhukhovitsky, A.A. Determination of diffusion coefficient using the shift of activity curve. Zavod. Lab. 1960, 26, 828–830. [Google Scholar]
- Maier, K.; Mehrer, H.; Rein, G. Self-Diffusion in Molybdenum. Int. J. Mater. Res. 1979, 70, 271–276. [Google Scholar] [CrossRef]
- Mundy, J.N.; Tse, C.W.; McFall, W.D. Isotope effect in chromium self-diffusion. Phys. Rev. B 1976, 13, 2349–2357. [Google Scholar] [CrossRef]
- Mundy, J.N.; Hoff, H.A.; Pelleg, J.; Rothman, S.J.; Nowicki, L.J.; Schmidt, F.A. Self-diffusion in chromium. Phys. Rev. B 1981, 24, 658–665. [Google Scholar] [CrossRef]
- Straumal, B.; Valiev, R.; Kogtenkova, O.; Zieba, P.; Czeppe, T.; Bielanska, E.; Faryna, M. Thermal evolution and grain boundary phase transformations in severely deformed nanograined Al–Zn alloys. Acta Mater. 2008, 56, 6123–6131. [Google Scholar] [CrossRef]
- Straumal, B.; Klinger, L.; Shvindlerman, L. The influence of pressure on indium diffusion along single tin-germanium interphase boundaries. Scr. Met. 1983, 17, 275–279. [Google Scholar] [CrossRef]
- Molodov, D.; Straumal, B.; Shvindlerman, L. The effect of pressure on migration of 001 tilt grain boundaries in tin bicrystals. Scr. Met. 1984, 18, 207–211. [Google Scholar] [CrossRef]
- Molodov, D.; Swiderski, J.; Gottstein, G.; Lojkowski, W.; Shvindlerman, L. Effect of pressure on grain boundary migration in aluminium bicrystals. Acta Met. Mater. 1994, 42, 3397–3407. [Google Scholar] [CrossRef]
- Muller, J.E.; Jepsen, O.; Andersen, O.K.; Wilkins, J.W. Systematic Structure in the K-Edge Photoabsorption Spectra of the4dTransition Metals: Theory. Phys. Rev. Lett. 1978, 40, 720–722. [Google Scholar] [CrossRef]
- Müller, J.; Jepsen, O.; Wilkins, J. X-ray absorption spectra: K-edges of 3d transition metals, L-edges of 3d and 4d metals, and M-edges of palladium. Solid State Commun. 1982, 42, 365–368. [Google Scholar] [CrossRef]
- Keski-Rahkonen, O.; Krause, M.O. Total and partial atomic-level widths. At. Data Nucl. Data Tables 1974, 14, 139–146. [Google Scholar] [CrossRef]
- Qi, B.; Perez, I.; Ansari, P.H.; Lu, F.; Croft, M. L2 and L3 measurements of transition-metal 5d orbital occupancy, spin-orbit effects, and chemical bonding. Phys. Rev. B 1987, 36, 2972–2975. [Google Scholar] [CrossRef]

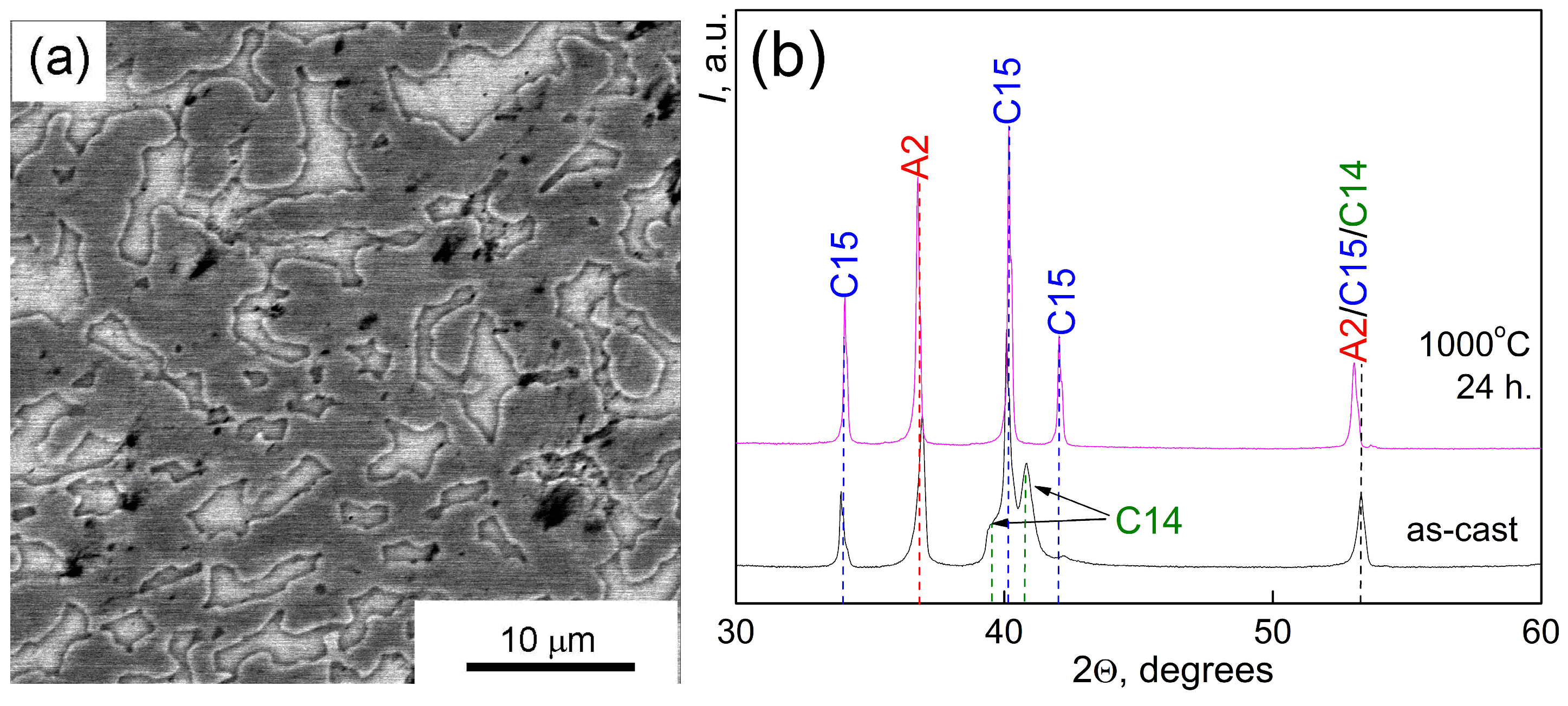
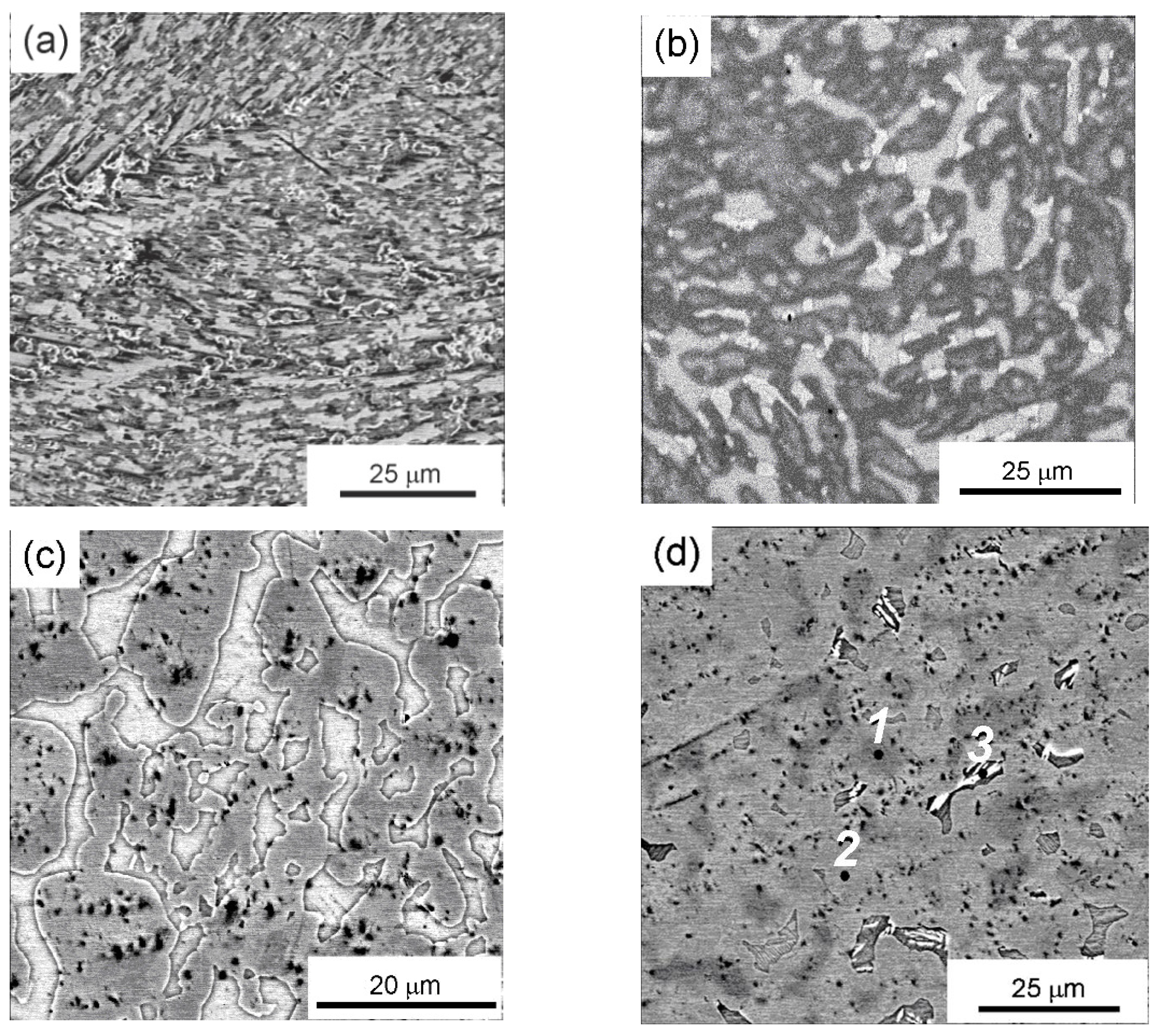

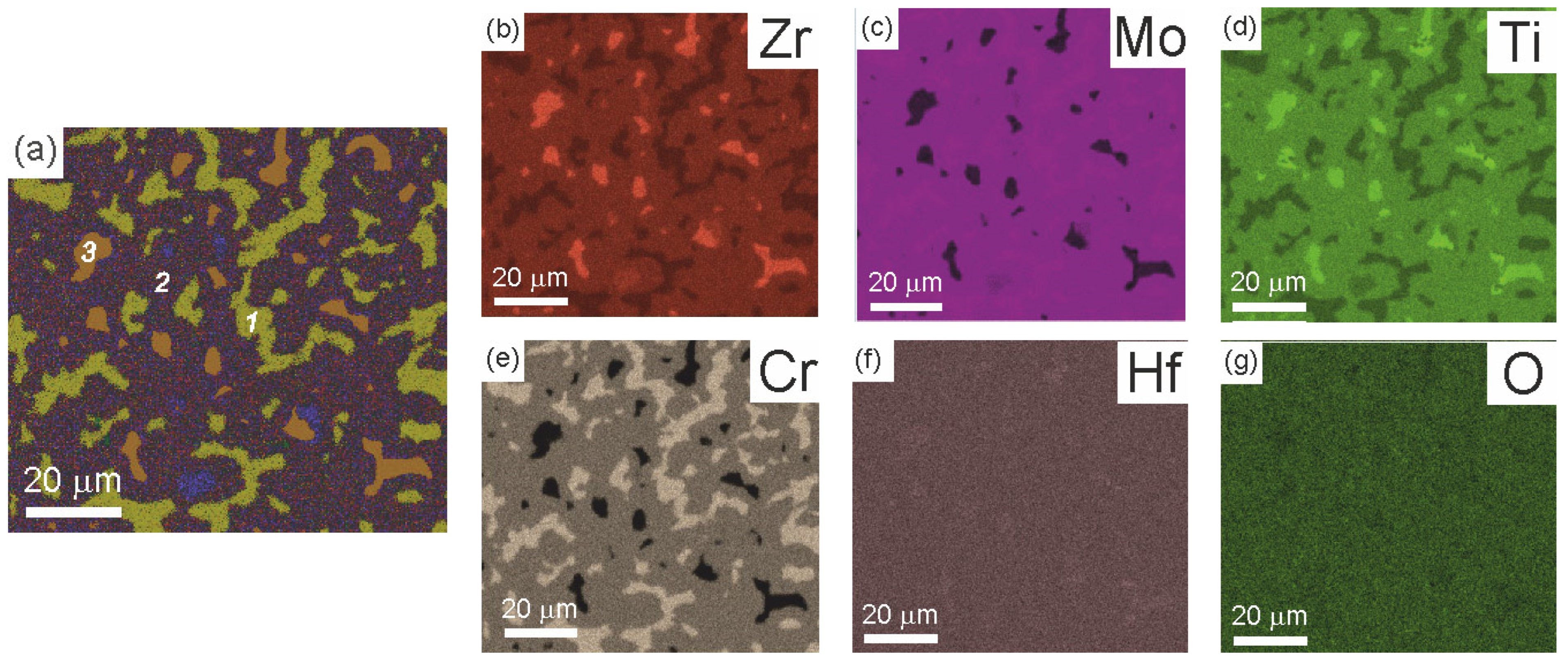
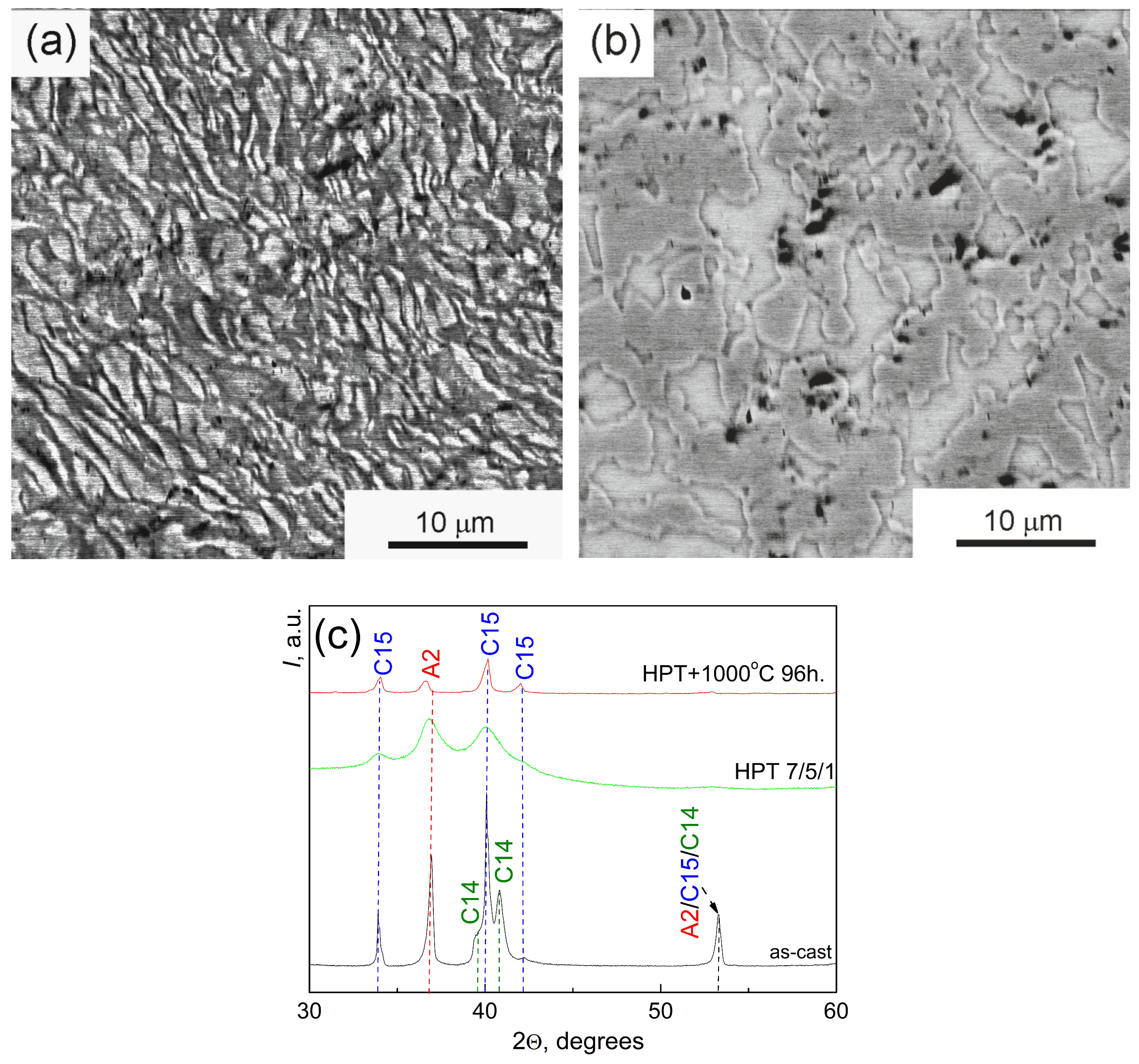
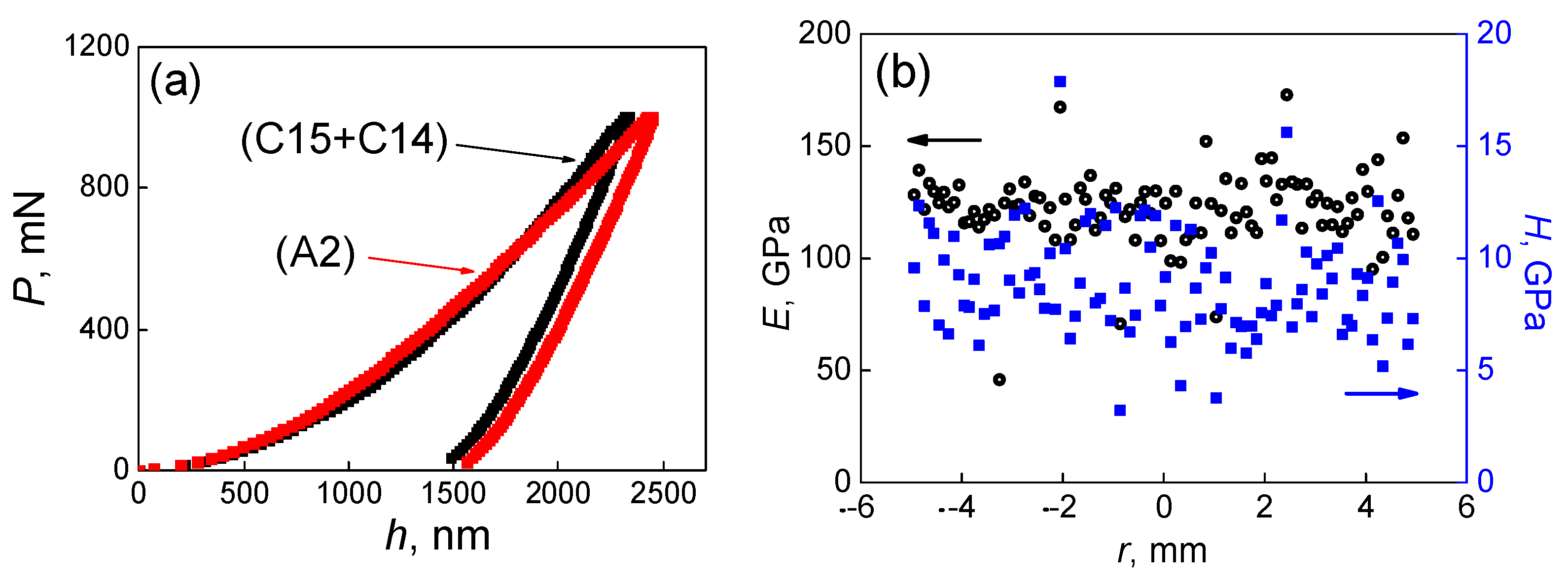
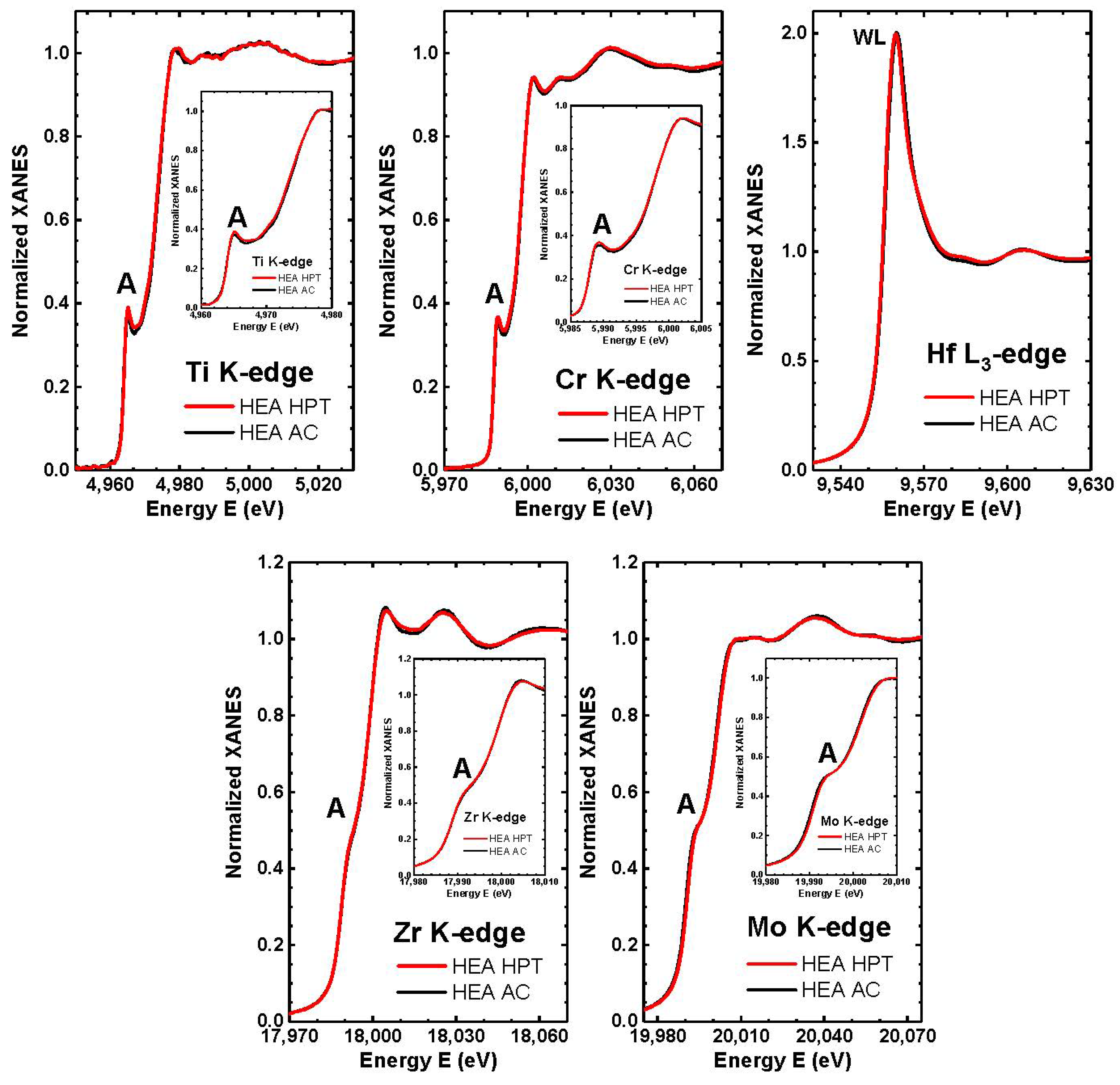
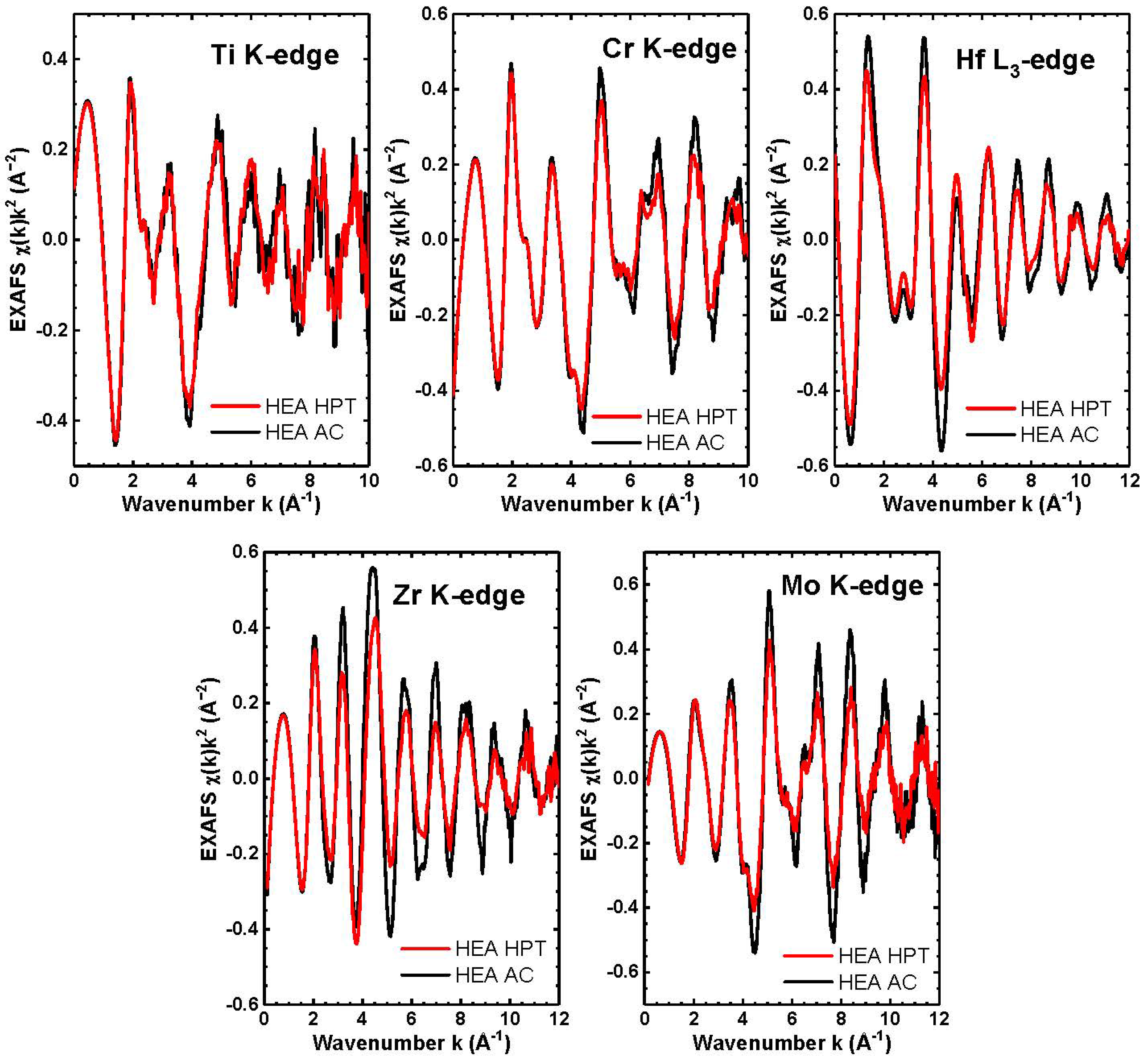

| Point | Phase | Ti | Cr | Zr | Mo | Hf |
|---|---|---|---|---|---|---|
| 1 | (Hf)cub (A2) | 6.3 ± 0.1 | 12.8 ± 0.1 | 13.0 ± 0.2 | 29.4 ± 0.3 | 38.5 ± 0.3 |
| 2 | (Mo,Cr)2Zr (C15) | 12.4 ± 1.9 | 10.3 ± 1.1 | 24.9 ± 1.6 | 15.1 ± 2.1 | 38.6 ± 0.6 |
| 3 | Cr2Zr (C14) | 14.6 ± 0.9 | 7.9 ± 0.9 | 28.2 ± 1.2 | 11.1 ± 0.9 | 39.5 ± 0.6 |
| Average composition | 11.3 | 9.9 | 22.1 | 17.8 | 38.9 | |
| Phase | Initial Alloy | 1000 °C 24 h | ||
|---|---|---|---|---|
| a, c, nm | V, % | a, nm | V, % | |
| (A2) | 0.3435 | 35 | 0.3452 | 30 |
| (C15) | 0.7451 | 45 | 0.7436 | 70 |
| (C14) | 0.5249; 0.8656 | 20 | - | - |
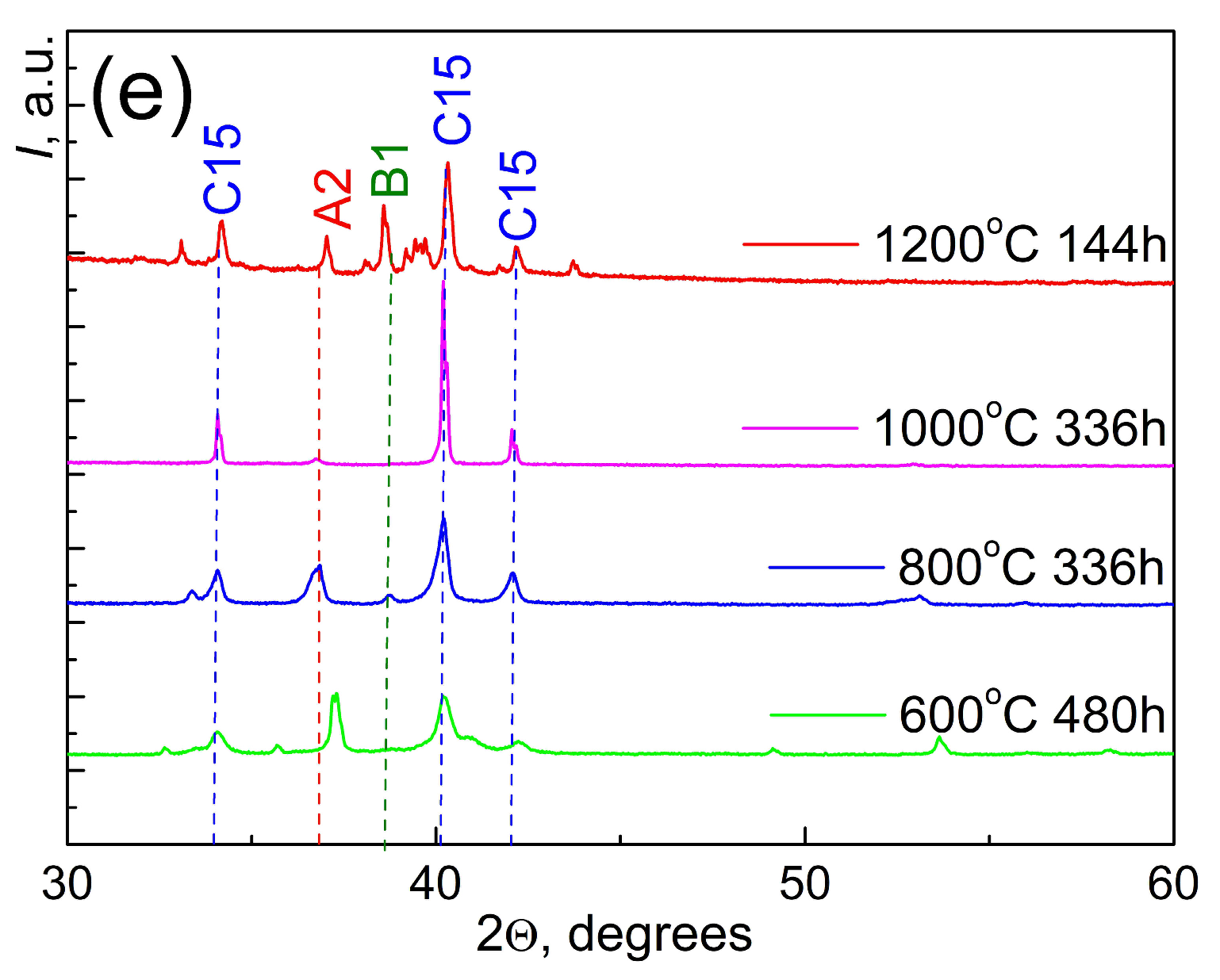
| Phase | 600 °C 480 h | 800 °C 336 h | 1000 °C 336 h | 1200 °C 144 h | ||||
|---|---|---|---|---|---|---|---|---|
| a, c, нм | V, % | a, нм | V, % | a, нм | V, % | a, нм | V, % | |
| (A2) | 0.3417 | 45 | 0.3445 | 25 | 0.3464 | 2 | 0.3429 | 5 |
| (C15) | 0.7424 | 45 | 0.7431 | 70 | 0.7453 | 98 | 0.7416 | 90 |
| (C15-2) | - | - | - | - | - | - | 0.7621 | 3 |
| (B1) | - | - | 0.4642 | 5 | - | - | 0.4656 | 2 |
| (C36) | 0.5021; 0.6438 | 10 | - | - | - | - | ||
| Point | Phase | Ti | Cr | Zr | Mo | Hf |
|---|---|---|---|---|---|---|
| 1 | (A2) | 8.18 | 19.15 | 14.16 | 24.73 | 33.78 |
| 2 | (C15) | 12.70 | 12.03 | 20.77 | 20.08 | 34.42 |
| 3 | (B1) | 18.35 | 1.26 | 37.95 | 5.15 | 37.29 |
| Phase | As-Cast Alloy | High-Pressure Torsion | High Pressure Torsion + 1000 °C 96 h | |||
|---|---|---|---|---|---|---|
| a, c, nm | V, % | a, нм | V, % | a, нм | V, % | |
| (A2) | 0.3435 | 35 | 0.3460 | 60 | 0.3461 | 40 |
| (C15) | 0.7451 | 45 | 0.7451 | 40 | 0.7435 | 60 |
| (C14) | 0.5249; 0.8656 | 20 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornakova, A.; Straumal, B.; Kuzmin, A.; Tyurin, A.; Chernyaeva, E.; Druzhinin, A.; Afonikova, N.; Davdian, G. Influence of Heat Treatment and High-Pressure Torsion on Phase Transformations in TiZrHfMoCr High-Entropy Alloy. Metals 2023, 13, 1030. https://doi.org/10.3390/met13061030
Gornakova A, Straumal B, Kuzmin A, Tyurin A, Chernyaeva E, Druzhinin A, Afonikova N, Davdian G. Influence of Heat Treatment and High-Pressure Torsion on Phase Transformations in TiZrHfMoCr High-Entropy Alloy. Metals. 2023; 13(6):1030. https://doi.org/10.3390/met13061030
Chicago/Turabian StyleGornakova, Alena, Boris Straumal, Alexei Kuzmin, Alexander Tyurin, Elena Chernyaeva, Alexander Druzhinin, Natalia Afonikova, and Gregory Davdian. 2023. "Influence of Heat Treatment and High-Pressure Torsion on Phase Transformations in TiZrHfMoCr High-Entropy Alloy" Metals 13, no. 6: 1030. https://doi.org/10.3390/met13061030
APA StyleGornakova, A., Straumal, B., Kuzmin, A., Tyurin, A., Chernyaeva, E., Druzhinin, A., Afonikova, N., & Davdian, G. (2023). Influence of Heat Treatment and High-Pressure Torsion on Phase Transformations in TiZrHfMoCr High-Entropy Alloy. Metals, 13(6), 1030. https://doi.org/10.3390/met13061030








