Abstract
During the iron ore sintering process, its SiO2 content considerably affects the sinter quality. In this study, the effect of SiO2 content on iron ore sintering indexes was studied, with mineral composition and microstructure analyses. It was shown that the strength and reducibility of sintering products improved when SiO2 content increased. The sinter yield and tumbler index increased from 69.57% and 58.67% to 74.02% and 62.32%, respectively, with SiO2 content increasing from 3.92% to 5.12%. Moreover, the reduction disintegration index (RDI+3.15) increased from 66.50% to 68.28%. There was a quadratic function relationship between SiO2 content (x) and RDI+3.15 (y): y = −0.4841x2 + 5.8932x + 50.8189. Maintaining the SiO2 content in the range of 4.52% to 5.12% would promote the formation of compound calcium ferrite (SFCA) and silicate as the main binding phase which determined the quality of sintering products.
1. Introduction
China is the world’s largest steel producer and consumer, and the output of crude steel in China has exceeded one billion tons/year in recent years. The blast furnace process is the dominant ironmaking route to supply the feedstocks for steelmaking [1,2,3,4]. For BF ironmaking, more than 75% of its iron-containing burden is iron ore sinter, which is produced by sintering a mixture of iron ore fines with the additions of flux (e.g., limestone), solid fuel (e.g., coke breeze), and return fines (recycled sinter with a particle size smaller than 5 mm) [5,6,7,8].
The main chemical components of sinter are FexOy, CaO, SiO2, MgO, and Al2O3. According to Kasai E et al., CaO separated from MgO due to the decomposition of dolomite tends to react with hematite and forms a melt at low temperatures [9]. Hayashi M et al. researched the effect of MgO on the wettability and permeability of the calcium ferrite liquid phase. MgO addition made the soft melting zone in the sinter become thinner and the permeability of the blast furnace is improved [10,11,12]. The equilibrium phase composition of sinter was affected by A12O3 content, the relative proportion and chemical composition of the minerals changed depending on different levels of A12O3. The amount of calcium ferrite in the sinter increased with A12O3 content [13,14,15]. In general, SiO2 mainly participates in the formation of the liquid phase (calcium ferrite and compound calcium ferrite), depending on the sintering temperature and atmosphere, eventually affecting sinter quality. With high SiO2 content, more silicate phases can be formed and melted in calcium ferrite to form compound calcium ferrite (SFCA) and silicate melts [16,17,18,19].
The main binding phase in the sinter was compound calcium ferrite (complex silico-ferrites of calcium) which determined sinter strength and reducibility. Calcium ferrite, i.e., CaO·Fe2O3 (CF), does not react with CaO and Fe2O3 until SFCA begins to form at temperatures around 1050 °C [20,21]. These previous studies could provide a guide for evaluating the effect of SiO2 on sinter quality [22,23,24]. However, there is no in-depth study of the formation process of SFCA with SiO2 participation and the change in sintering quality with different SiO2 contents.
In this study, the effect of silicon content on chemical components, reduction disintegration indexes, and microstructures of sintering products from the sintering machine were first explored. The consolidation property and sintering property of sintering products with different silicon contents were then studied through mini-sintering experiments and sintering pot tests. The mineral composition and microstructures of different sintering products were examined. The results are expected to offer a useful guide for improving the sintering of iron ore with low silicon dioxide content.
2. Materials and Methods
2.1. Materials
In order to adjust SiO2 content and basicity exactly, the briquettes were made with chemical reagents of Fe2O3, SiO2, CaO, MgO, and Al2O3 (Macklin, Shanghai, China). Table 1 presents the chemical components of green briquettes. The SiO2 content increased from 4% to 5.5%, while the Fe2O3 content decreased from 82.9% to 81.4%. Meanwhile, the CaO, MgO, and Al2O3 contents remained at 9.5%, 1.6%, and 2.0%, respectively.

Table 1.
Chemical components of the green briquettes (wt. %).
In this investigation, iron ore blend (iron concentrates, iron ore fines, and some iron-bearing dust), fluxes (limestone, burnt lime, and dolomite), coke breeze, and return fines (sinter < 5 mm in grain size) for sintering were provided by Angang Steel Co. Ltd. in Anshan, China. The main chemical compositions of the main raw materials are shown in Table 2. It can be seen from Table 2 that the total iron grade of the iron ore blend was 60.26% and the SiO2 content was only 3.95%. The SiO2 content of dolomite was 5.59%. The CaO contents of limestone and burnt lime were 48.87% and 74.27%, respectively. In the benchmark test, 81.60% iron ore blend, 4.9% limestone, 4.0% dolomite, 5.0% burnt lime, 4.5% coke breeze, and 10% return fines were mixed for sintering. For the resulting mixture, its TFe, SiO2, and CaO contents were 56.18 wt. %, 4.52 wt. %, and 9.03 wt. %, respectively.

Table 2.
Chemical composition of raw materials for sintering (wt. %).
2.2. Methods
The consolidation properties of mixtures of the raw materials with different silicon contents were studied through mini-sintering experiments which characterize the changes of briquettes of the mixtures under given conditions that simulate those in sintering pot tests. The green briquettes and the schematic diagram of the roasting equipment are shown in Figure 1. Different chemical reagents were weighted in a certain proportion and mixed gently, and 4.0 g of the blend was pressed to form green briquettes (diameter 15 mm, height 12–14 mm) in a steel die at a pressure of 5 MPa for 1 min using a hydraulic press machine. The briquettes were preheated in the low-temperature zone at about 500 °C and then roasted in the zone of 1250 °C for 10 min in the tube furnace (BoYunTong Instrument Technology Co. Ltd., Nanjing, China). After cooling, the roasted briquettes were used for compressive strength measurement. Meanwhile, the heights and diameters of the green briquettes and roasted briquettes were measured to calculate the volume shrinkages of the briquettes.
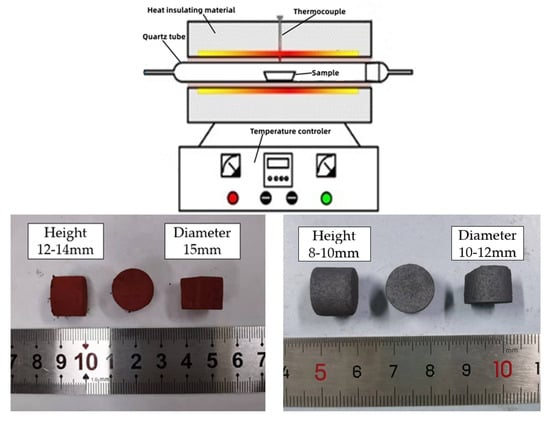
Figure 1.
Schematic of the roasting equipment and photos of the green briquettes and roasted briquettes.
The variations of properties of the sinter with silicon content were determined through sintering pot tests which could simulate the industrial-scale sinter-making process. A laboratory-scale sintering pot with a height of 900 mm and a diameter of 200 mm was used, as shown in Figure 2. The sintering process included proportioning, mixing, granulating, charging, igniting, sintering, cooling, crushing, sieving, and testing [5,6,25,26]. Firstly, the raw materials were blended. In this process, lime slacking was carried out with 80% extra water. Before lime was added, all the other matched materials were blended together. The blended stack was then pitted in the middle and the proportioned lime was put in the pit. After slacking water was added, the lime was covered with the blended mix. After slacking time of 5 min, the slacked lime was mixed with other ores and then the mix was watered and further mixed. The main purpose of secondary mixing was granulation. This process was conducted in a Φ600 × 1400 mm drum mixer with a rotary speed of 15 r/min which was independently developed by Central South University. After granulation for 5 min, the mix was discharged in a plate.
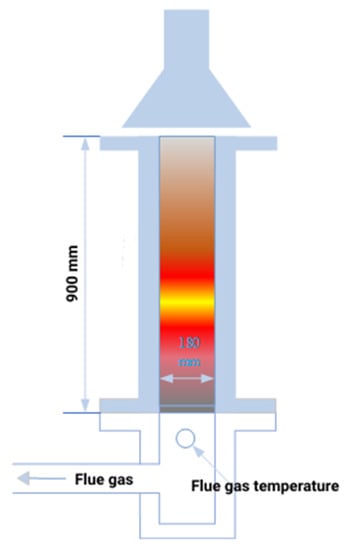
Figure 2.
Schematic of the apparatus for sintering pot tests.
Then, 1 kg of sinter chucks with diameters of 10–16 mm were charged on the grate at the bottom of the sintering pot as a hearth layer. The sintering feed was placed into the sinter pot. Natural gas was used as fuel for ignition. The suction pressure during ignition was 5000 Pa and the ignition temperature was 1100 °C. After igniting for 2 min, the gas flow was cut off to end the flame. The hood was left above the pot for insulation for 1 min. After insulation, the hood was moved away and the suction pressure was increased to 12 kPa to begin sintering. The temperature of the exhaust gas was recorded to monitor the sintering process. The burnt-though point (BTP) was determined at the top temperature point. The sintering time was counted from the beginning of ignition to BTP. After BTP, the suction pressure was decreased to 5 kPa to cool the sinter cake. After cooling for 3 min, the sinter cake was discharged, sieved, and sampled for subsequent characterization.
The typical sintering indexes, including sinter yield (Y, %), tumbler index (TI, %), and productivity (P, t·m−2·h−1), were measured to evaluate the sinter quality. The vertical sintering speed is the height of the sinter bed divided by sintering time (mm/min). The sinter yield is the mass of the sinter > 5 mm after screening divided by the total sinter mass after subtracting the hearth layer mass of 1 kg (%). The tumbler index is the mass of the sinter > 6.3 mm divided by the original mass of 7.5 kg put into a tumbler for 8 min (%), based on the methodology outlined in ISO3271. Productivity is the production capacity that sintering machines could reach at a specific area and time (t·m−2·h−1) [5,6]. The reduction disintegration index (RDI) is an important metallurgical property of the finished sinter. The RDI was tested according to the Chinese government standards of GB/T 13242-2017. The RDI+6.3 and RDI+3.15 were calculated according to the following equation:
where: is the mass of the sinter before tumbling (g), is the mass of the sinter > 6.3 mm after tumbling (g), is the mass of the sinter (>3.15 mm~<6.3 mm) after tumbling (g).
The chemical components of the sintering products were analyzed by X-ray fluorescence (XRF, Axios Max, Alemlo, The Netherlands). The changes in the phases and microstructures in each case were studied using modern microcosmic detecting equipment such as an X-ray diffractometer (XRD, D8 ADVANCE, Karlsruhe, Germany), an optical microscope (DMI5000 M, Leica, Wetzlar, Germany), and a scanning electron microscope (JSM-7900F, JEOL, Akishima, Tokyo, Japan) equipped with an EDAX energy dispersive X-ray spectroscopy (EDS) detector.
3. Results
3.1. The Brief Analyzation of Finished Sinter
Table 3 shows the chemical components of sinters obtained from a sintering machine in a sintering plant. The total iron grade of each sinter was higher than 58%. The SiO2 content varied from 4.27% to 4.92%. At the same time, the binary basicity (R, R = CaO/SiO2) changed from 2.11 to 1.83. Moreover, the Al2O3 contents differed from each other. Previous studies have shown that both SiO2 and Al2O3 contents affected mineral composition and metallurgical properties. The effect of SiO2 content on the reduction disintegration indexes of the sinter is shown in Figure 3. When the SiO2 content was 4.27%, RDI+6.3 and RDI+3.15 were 28.2% and 58.03%, respectively. Both increased to 36.39% and 63.93% with a SiO2 content of 4.92%. In other words, they were increased by 26.9% and 10.2%, respectively. It indicated that the SiO2 content was positively related to RDI+3.15, whose increase would be beneficial for blast furnace production [22,25,26].

Table 3.
Chemical components of the sinter from the sintering machine (wt. %).
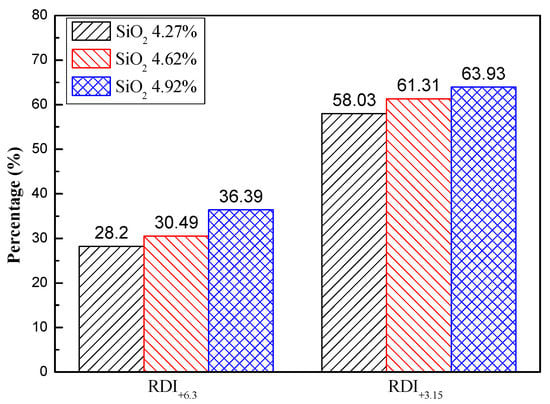
Figure 3.
Reduction disintegration indexes of the finished sinter with different SiO2 contents.
The mineral phases of the above three sinter samples are carried out under X-ray diffraction and the results are shown in Figure 4. As can be seen from Figure 4, there are five identical mineral phases for the three kinds of sinter because of the similar raw materials used, mainly including hematite, magnetite, calcium ferrite, magnesium ferrite, and calcium silicate. In order to further study the mineral distribution and morphological characteristics, the microstructures of the three representative samples, which were analyzed under the optical microscope, are shown in Figure 5.
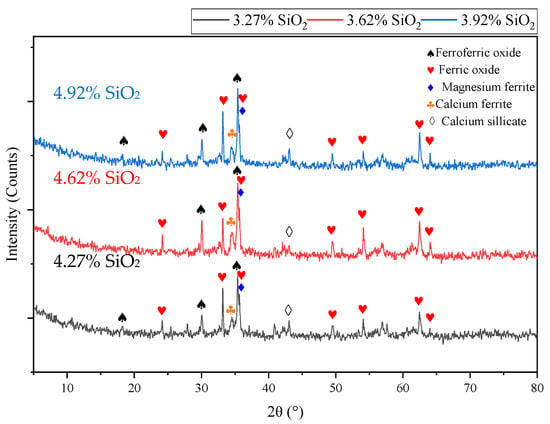
Figure 4.
Mineral phase compositions of finished sinters with different SiO2 contents.
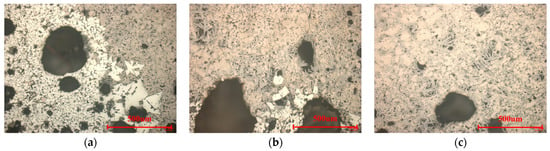
Figure 5.
Microstructure of finished sinters with different silicon dioxide contents. ((a)—4.27% SiO2, (b)—4.62% SiO2, (c)—4.92% SiO2).
In Figure 5, the microstructure of the sinter changes obviously with the increase in SiO2 content. When the SiO2 content of the sinter was 4.27%, there were two remarkably different areas (Figure 5a). The bright white area was made up of most secondary hematite, and the light blue area consisted of compound calcium ferrite, magnetite, and little calcium silicate. By this time, the compound calcium ferrite was columnar. The previous study shows that SiO2 reacts with the primary phase of CaO·Fe2O3 to form 2CaO·Fe2O3 at approximately 1000 °C, and then 2CaO·Fe2O3 reacts with Fe2O3 and CaO·Fe2O3 to form compound calcium ferrite at about 1100 °C. Therefore, the low content of SiO2 was bad for liquid phase formation. While increasing SiO2 content to 4.62%, the bright white area decreased remarkably (Figure 5b). The light blue area enlarged, resulting in an increase in SFCA and Fe2O3. At this point, most of the compound calcium ferrite changed to fine needle-like. This is because the high content of MgO and Al2O3 led to the increase of liquid viscosity and difficulty in calcium ferrite precipitation, which made it more needle-like without further growth. Finally, the bright white area of secondary hematite accumulation in the sinter disappeared with the increase of SiO2 content to 4.92% (Figure 5c). In light blue and pale brown areas, the needle calcium ferrite of good crystallization was closely connected with the fused magnetite, and more calcium silicate acted as the second bonding phase, which made the sinter structure compact and homogeneous.
3.2. Effects of SiO2 Content on the Consolidation Property of the Sinter
As shown in Table 1 and Figure 6, the CaO content remained at 9.5%, so the basicity of the briquettes declined from 2.38 to 1.73. The compressive strength of the roasted briquettes is also illustrated in Figure 6. The compressive strength improved with increasing SiO2 content. The value was only 8.01 × 103 N when the SiO2 content in the briquettes was the lowest, 4.0%. While increasing SiO2 content to 4.5% and 5%, the compressive strength significantly increased to 9.32 KN3 and 11.6 KN, respectively. The strength continued to be enhanced when SiO2 content exceeded 5%, but the total iron content was lower. It was obvious that an increase in SiO2 content has an important influence on compressive strength enhancement, even if the basicity declines gradually when the mixture has enough CaO. It was found that the quantity of calcium ferrite increased with the increase of SiO2 content under optimal conditions, including a roasting temperature of 1250–1280 °C and a time of 13 min [27]. Figure 7 shows the height, diameter, and volume shrinkage of the roasted briquettes. The volume shrinkages increased gradually with the increase in SiO2 content, and the reason was that more SiO2 led to a more liquid phase and less porosity.
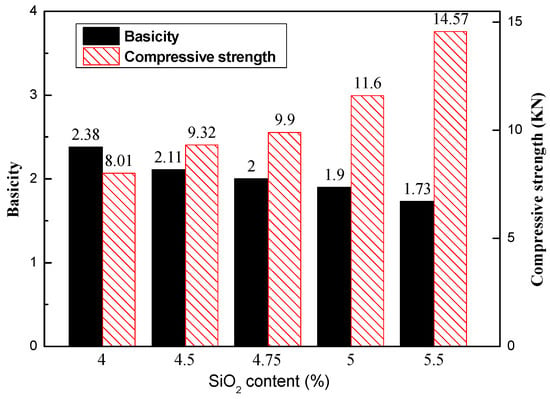
Figure 6.
Compressive strength of roasted briquettes with different SiO2 contents.
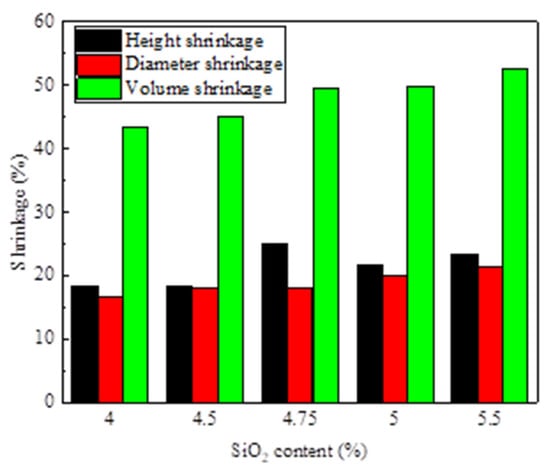
Figure 7.
Height, diameter, and volume shrinkage of roasted briquettes.
3.3. Research on Sintering Property with Different Silicon Content
3.3.1. Effect on Sintering Indexes and the Reduction Disintegration Index
In sinter pot trials, the effect of SiO2 content on sintering indexes is shown in Figure 8. Obviously, the sinter yield, tumbler index, and productivity increased with SiO2 content. Meanwhile, solid fuel consumption decreased gradually. The yield and tumbler index increased from 69.57% and 58.67% to 74.02% and 62.32%, respectively. As the SiO2 content increased from 3.92% to 4.82%, productivity grew from 1.55 to 1.68 t·m−2·h−1, with a decrease in solid fuel consumption from 62.15% to 60.58%. Furthermore, increasing the SiO2 ratio to 5.12% led to a slight decrease in productivity with an increase in solid fuel consumption. However, other studies have shown that the productivity and tumbler index of the sinter are improved when the silicon content rose from 5.04% to 5.38%. Therefore, in this case, either a too-high or too-low SiO2 content was harmful to sintering.
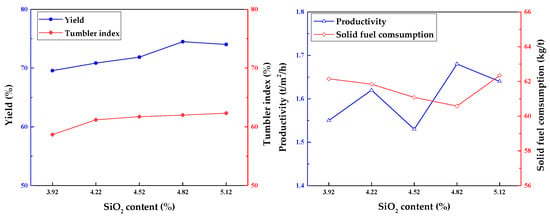
Figure 8.
Effect of SiO2 content on sintering indexes.
The effect of SiO2 content on the RDI of the sinter from the sinter pot trial has been studied and the results are shown in Figure 9. There were slight decreases in the RDI+6.3 value but a perceptible increase in the RDI+3.15 value from 66.50% to 68.28% with increasing SiO2 content. Meanwhile, further increasing the SiO2 content led to a slight decline in RDI−0.5. According to Yan et al., the reduction disintegration index gradually increased from 75.95% to 87.21% when the SiO2 content increased from 4.8% to 5.7%. It revealed that increasing the SiO2 proportion (3.92–5.7%) contributed to a better reduction disintegration index of the sinter produced with different raw materials.
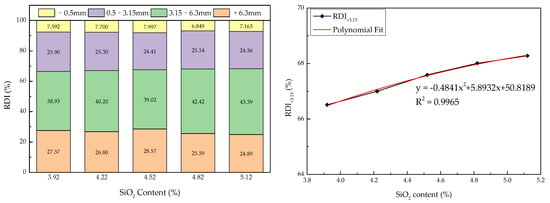
Figure 9.
Reduction disintegration indexes of sintering products with different silicon dioxide contents.
Furthermore, using curve polynomial fitting analysis, the relationship between the reduction disintegration index and SiO2 content could be determined. There was a quadratic function relationship between SiO2 content (x) and the RDI+3.15 of sinter (y): y = −0.4841x2 + 5.8932x + 50.8189 (R2 = 0.9965). For the sinter, a high RDI+3.15 and a lower RDI−0.5 are advantageous to blast furnace ironmaking. Therefore, sinter products need to maintain a sufficient content of silicon dioxide (4.5–5.0%).
3.3.2. Analysis of Phases and Microstructure Changes in Sinter Products
Table 4 presents the chemical component changes of the sintering products with the increase in SiO2 content and Figure 10 shows corresponding mineral compositions as analyzed by XRD. In Table 4, the CaO content changed from 8.74% to 9.21% and the Al2O3 contents were all higher than 1.92%. The basicity of the sinter similarly declined from 2.23 to 1.78. As shown in Figure 10, the main mineral phases of sintering products included hematite, magnetite, calcium ferrite, and silicate. The calcium ferrite content increased, whereas the magnetite and silicate contents decreased. The results suggested that the products had similar phase compositions.

Table 4.
Chemical components of sinters from sintering pot tests (mass %).
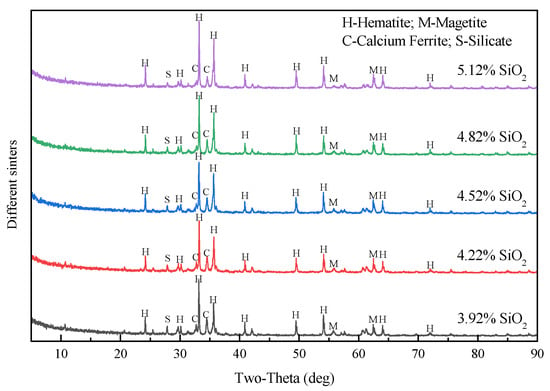
Figure 10.
Mineral compositions of sinters with different SiO2 content.
The sinter obtained from the sintering pot test with a SiO2 content of 4.82% was selected for SEM-EDS analysis. Figure 11 presents the elemental determination of the main minerals. Both spots A and C denote calcium ferrite. B, D, E, and F represent magnetite, silicate, hematite or unmelted iron ore particles, and residual calcium oxide, respectively. The primary minerals were calcium ferrite, hematite, magnetite, and silicate, which were the same as those in the XRD analysis. The binding phases were mainly calcium ferrite, especially SFCA in the forms of needle and strip.
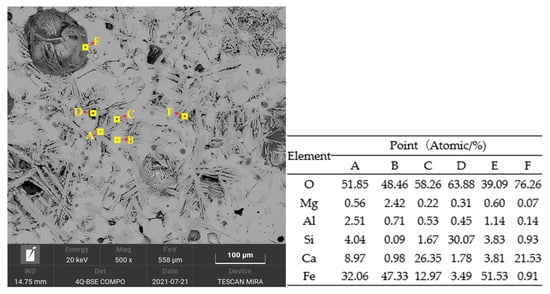
Figure 11.
SEM image and EDS analysis result of the sinter with silicon dioxide of 4.82%. A, C—Compound calcium ferrite (SFCA), B—Magnetite, D—Silicate, E—Hematite, and F—Calcium oxide.
The microstructure is also a critical factor for the properties of sinter products. As shown in Figure 12, there was less compound calcium ferrite (SFCA) in the sinter with the SiO2 content of 3.9%, and there were numerous unmelted hematite and magnetite particles. After the SiO2 content increased to 4.2%, some compound calcium ferrite in the form of a strip formed and bound the solid particles of hematite and magnetite to a whole. As the SiO2 content increased to 4.5% and 4.8%, more SFCA was generated. Its structure was very dense and homogeneous, enabling high mechanical strength and excellent quality for the sinter product. By further increasing the SiO2 content to 5.1%, some siliceous mineral particles existed independently and their structure became relatively loose and inhomogeneous. Therefore, sufficient silicon dioxide is needed to produce enough SFCA as the main binding phase to ensure the mechanical strength and performance of the sinter. However, excessive SiO2 is disadvantageous to ore mineralization, reducing sinter quality.
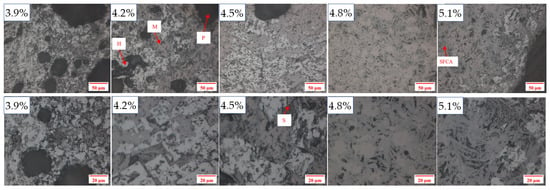
Figure 12.
Microstructures of sintering products with different silicon dioxide contents. (H—hematite, M—magnetite, S—silicate, P—pore, and SFCA—compound calcium ferrite).
4. Conclusions
In this study, the effect of silica content on iron ore sintering was investigated in detail.
- When the mixture of raw materials for sintering had enough CaO, the increase in SiO2 content significantly affected the compressive strength and volume shrinkage of the roasted briquette, even if the basicity declined gradually. The SiO2 content should be not less than 4.5% to obtain good consolidation properties.
- The RDI+3.15 of sinter from the sintering machine and sintering pot tests were both affected by SiO2 content, which increased from 58.03% and 66.50% to 63.93% and 68.28%, respectively, along with an increase in SiO2 content. There was a quadratic function relationship between SiO2 content (x) and the RDI+3.15 (y): y = −0.4841x2 + 5.8932x + 50.8189. Meanwhile, maintaining sufficient silicon oxide was beneficial to the iron ore sintering process.
- The main mineral phases of sintering products were compound calcium ferrite, hematite, magnetite, and calcium silicate. The distribution and morphological characteristics of different mineral phases changed remarkably with SiO2 content variation. As a consequence, sufficient silicon oxide was needed to form enough compound calcium ferrite, which was the main binding phase that ensured the mechanical strength and metallurgical properties of the sintering products.
Author Contributions
Conceptualization, J.Z.; Data curation, J.L., W.J. and J.Z.; Investigation, W.J., D.C., Q.Z. and L.X.; Methodology, Q.Z. and Y.J.; Resources, J.L. and X.M.; Software, D.C. and H.Z.; Writing—original draft, J.L. and C.L.; Writing—review & editing, Q.Z. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 52274290) and the Hunan Provincial Natural Science Foundation of China (Grant No. 2021JJ40776).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that supports the findings of this study are available within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, C.; Huang, J.B.; Wang, M.; Song, Y. Energy efficiency in China’s iron and steel industry: Evidence and policy implications. J. Clean. Prod. 2018, 177, 837–845. [Google Scholar] [CrossRef]
- Kimura, M.; Obayashi, I.; Takeichi, Y.; Murao, R.; Hiraoka, Y. Non-empirical identification of trigger sites in heterogeneous processes using persistent homology. Sci. Rep. 2018, 8, 3553. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.Q.; Zhang, Y.B.; Li, G.H.; Zhong, Q.; Luo, J.; Su, Z.J.; Rao, M.J.; Peng, Z.W.; Jiang, T. Effective preparation of blast furnace burdens from superfine iron concentrates by composite agglomeration process. Ironmak. Steelmak. 2020, 47, 908–914. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Zhang, J.; Zhang, Y.; Niu, L.; Cheng, Q. Study of stand-support sintering to achieve high oxygen potential in iron ore sintering to enhance productivity and reduce CO content in exhaust gas. J. Clean. Prod. 2020, 252, 119855. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, G.H.; Liu, H.B.; Jiang, T. An efficient method for iron ore sintering with high-bed layer: Double-layer sintering. J. Iron Steel Res. Int. 2021, 28, 1366–1374. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Xue, Y.X.; Pan, J.; Yang, C.C.; Guo, Z.Q.; Tian, H.Y.; Huang, X. An investigation into the distinctive sintering performance and consolidation mechanism of limonitic laterite ore. Powder Technol. 2020, 367, 616–631. [Google Scholar] [CrossRef]
- Webster, N.A.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Effect of Oxygen Partial Pressure on the Formation Mechanisms of Complex Ca-rich Ferrites. ISIJ Int. 2013, 53, 774–781. [Google Scholar] [CrossRef]
- Jiang, T. Principle and Technology of Agglomeration of Iron Ores; Central South University Press: Changsha, China, 2015; pp. 1–36. [Google Scholar]
- Kasai, E.; Sakano, Y.; Kawaguchi, T.; Nakamura, T. Influence of properties of fluxing materials on the flow of melt formed in the sintering process. ISIJ Int. 2000, 40, 857–862. [Google Scholar] [CrossRef]
- Webster, N.A.; Pownceby, M.I.; Pattel, R.; Manuel, J.R.; Kimpton, J.A. Fundamentals of silico-ferrite of calcium and aluminium (SFCA) iron ore sinter bonding phase formation: Effects of basicity and magnesium on crystallisation during cooling. ISIJ Int. 2019, 59, 263–267. [Google Scholar] [CrossRef]
- Kimura, H.; Endo, S.; Yajima, K.; Tsukihashi, F. Effect of oxygen partial pressure on liquidus for the CaO-SiO2-FeOx system at 1573 K. ISIJ Int. 2004, 44, 2040–2045. [Google Scholar] [CrossRef]
- Xu, L.P.; Liu, H.B.; Cheng, D.; Zhong, Q.; Rao, M.J.; Li, G.H. Insight into mechanisms of CaCl2 for improving reduction disintegration of iron ore sinter: An experimental and DFT investigation. J. Mol. Liq. 2022, 366, 120334. [Google Scholar] [CrossRef]
- Li, L.S.; Liu, J.B.; Wu, X.R.; Ren, X.; Bing, W.B.; Wu, L.S. In fluence of Al2O3 on equilibrium sinter phase in N2 atmpsphere. ISIJ Int. 2010, 50, 327–329. [Google Scholar] [CrossRef]
- Kalenga, M.K.; Garbers-Craig, A.M. Investigation into how the magnesia, silica, and alumina contents of iron ore sinter influence its mineralogy and properties. J. South. Afr. I Min. Metall. 2010, 110, 447–456. [Google Scholar]
- Machida, S.; Nushiro, K.; Ichikawa, K.; Noda, H.; Sakai, H. Experimental evaluation of chemical compostion and viscosity of melts formed during iron ore sintering. Tetsu-to-Hagane 2006, 92, 755–762. [Google Scholar] [CrossRef]
- Long, H.M.; Wu, X.J.; Chun, T.J. Assimilation behavior of calcium ferrite and calcium diferrite with sintered Al2O3 and MgO. Metall. Mater. Trans. B. 2016, 47, 2830–2836. [Google Scholar] [CrossRef]
- Yu, B.; Lv, X.W.; Xiang, S.L.; Bai, C.G.; Yin, J.Q. Wetting behavior of calcium ferrite melts on sintered mgO. ISIJ Int. 2015, 55, 1558–1564. [Google Scholar] [CrossRef]
- Yu, B.; Lv, X.W.; Xiang, S.L.; Bai, C.G.; Yin, J.Q. Wetting Behavior of Al2O3 Substrate by Calcium Ferrite Series Melts. ISIJ Int. 2015, 55, 483–490. [Google Scholar] [CrossRef]
- Nakashima, K.; Saito, N.; Shinozaki, S.; Tanaka, R.; Maeda, T.; Shimizu, M.; Mori, K. Wetting and penetration behavior of calcium ferrite melts to sintered hematite. ISIJ Int. 2004, 44, 2052–2056. [Google Scholar] [CrossRef]
- Jeon, J.; Jung, S.; Sasaki, Y. Formation of calcium ferrites under controlled oxygen potentials at 1273 K. ISIJ Int. 2010, 50, 1064–1070. [Google Scholar] [CrossRef]
- Ding, X.; Guo, X.M. The sintering characteristics of mixing SiO2 with calciumferrite at 1473 K (1200 °C). Metall. Mater. Trans. B 2015, 46, 1742–1750. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, L.P.; Zhong, Q.; Liu, C.; Liu, H.B.; Rao, M.J.; Peng, Z.W.; Li, G.H. Efficient preparation of blast furnace burdens from titanomagnetite concentrate by composite agglomeration process. JOM 2021, 73, 326–333. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Patrick, T.R.C. Stability of SFC (silico-ferrite of calcium): Solid solution limits, thermal stability and selected phase relationships within the Fe2O3–CaO–SiO2 (FCS) system. Eur. J. Miner. 2000, 12, 455–468. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Pownceby, M.I.; Madsen, I.C.; Christensen, A.N. Reaction sequences in the formation of silico-ferrite of calcium and aluminum in iron ore sinter. Metall. Mater. Trans. B 2004, 35, 929–936. [Google Scholar] [CrossRef]
- Li, G.H.; Liu, C.; Rao, M.J.; Fan, Z.Y.; You, Z.X.; Zhang, Y.B.; Jiang, T. Behavior of SO2 in the process of flue gas circulation sintering (FGCS) for iron ores. ISIJ Int. 2014, 54, 37–42. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, Z.W.; Peng, Z.W.; Rao, M.J.; Zhang, Y.B.; Li, G.H. Preparation of BF burden from titanomagnetite concentrate by composite agglomeration process (CAP). ISIJ Int. 2015, 55, 1599–1607. [Google Scholar] [CrossRef]
- Fan, X.H.; Meng, J.; Chen, X.L.; Zhuang, J.M.; Li, Y.; Yuan, L.S. Influence factors of calcium ferrite formation in iron ore sintering. J. Cent. South Univ. 2008, 39, 1125–1131. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).