Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking
Abstract
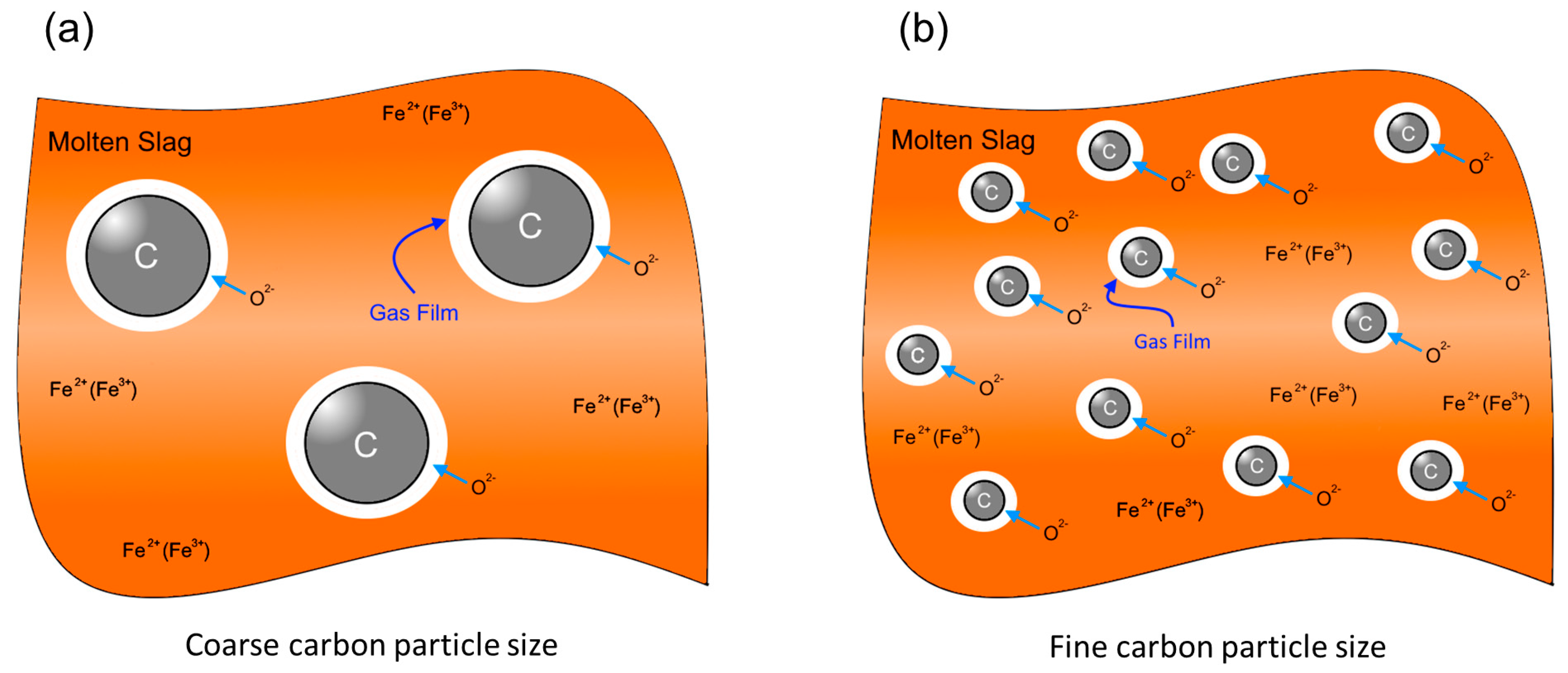
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scanning Electron Microscope Analysis
2.3. TGA Reactivity Analysis
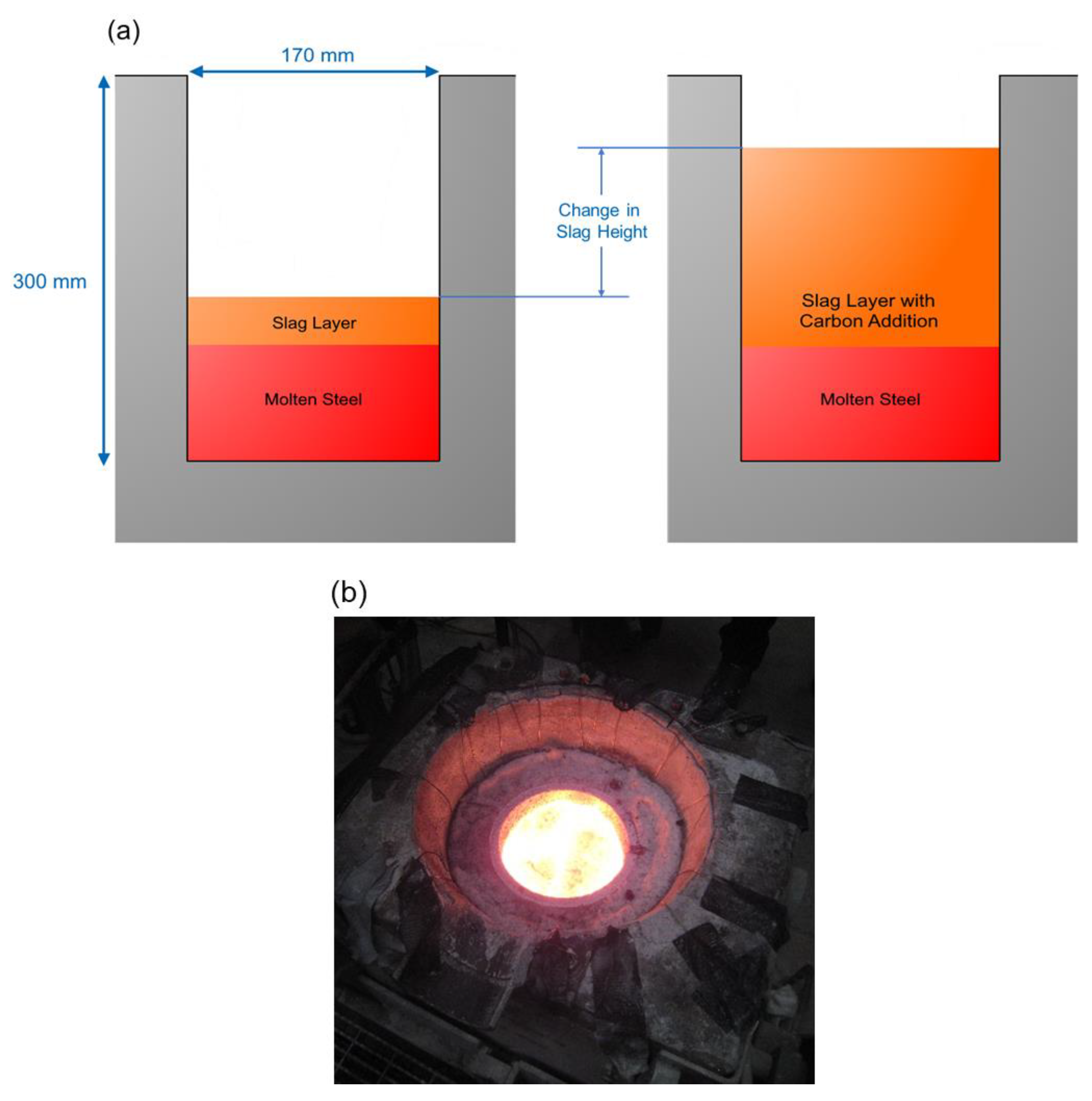
2.4. Experimental Set-Up and Method
3. Results and Discussions
3.1. Surface Morphology Analysis
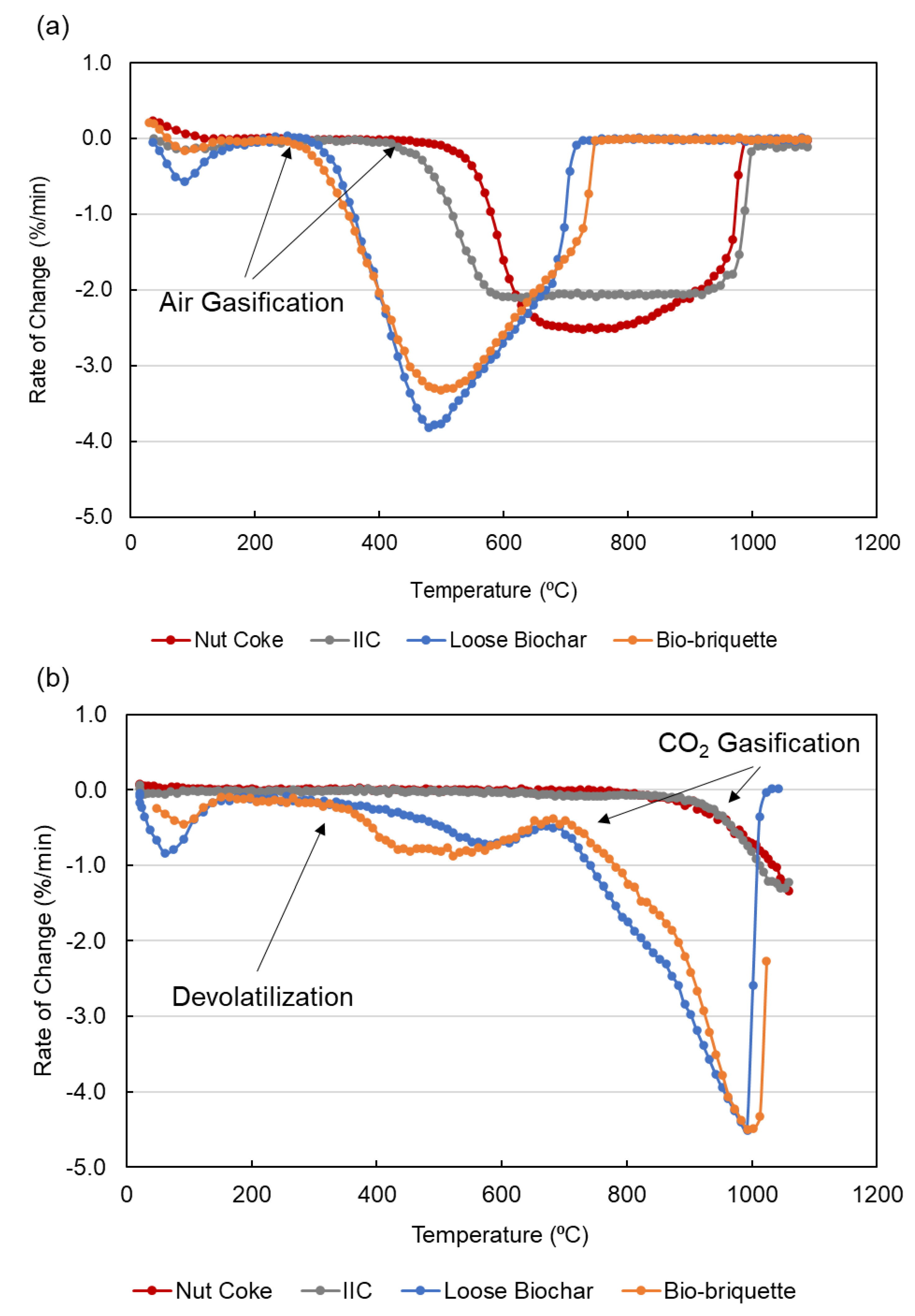
3.2. Reactivity with Air and CO2
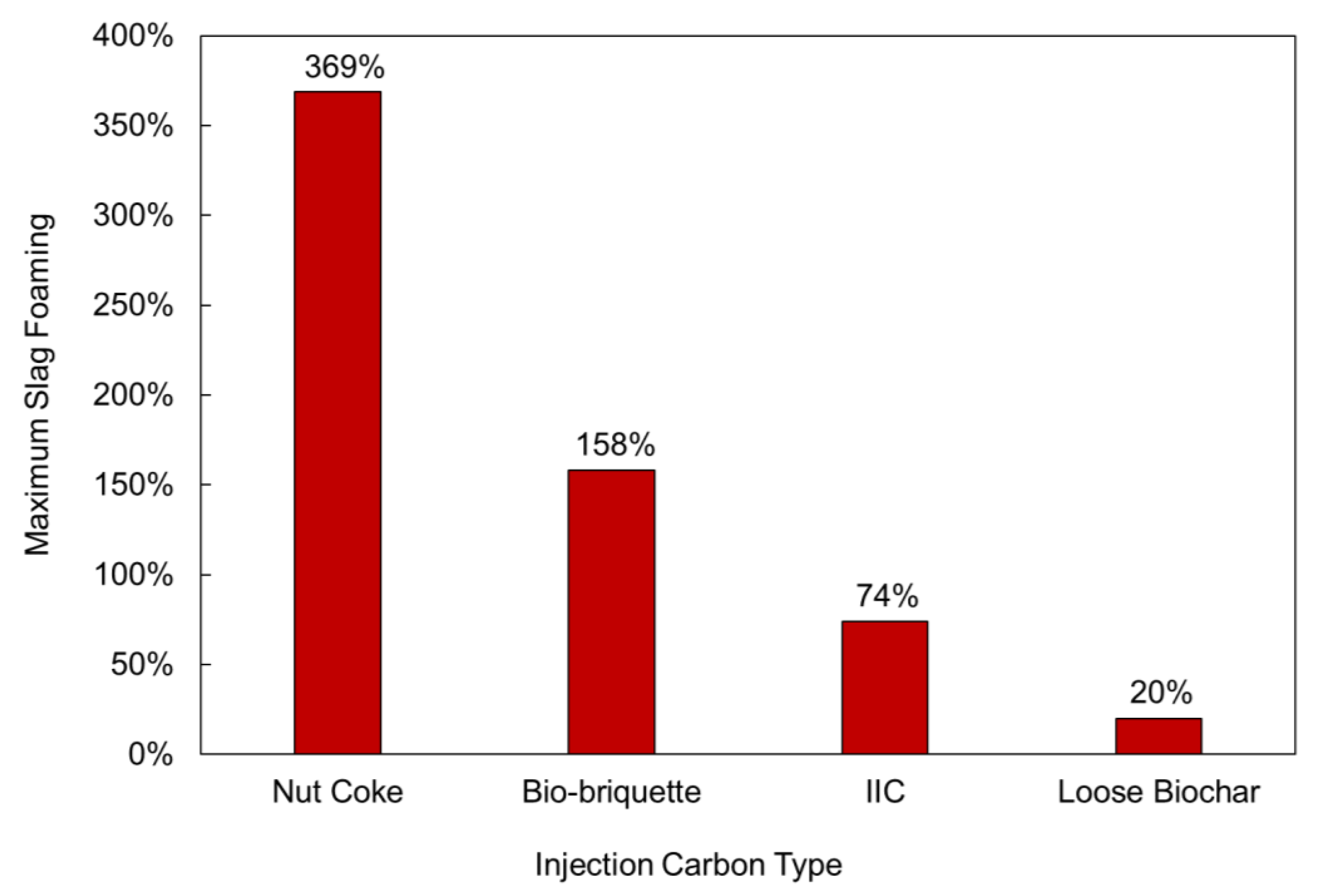
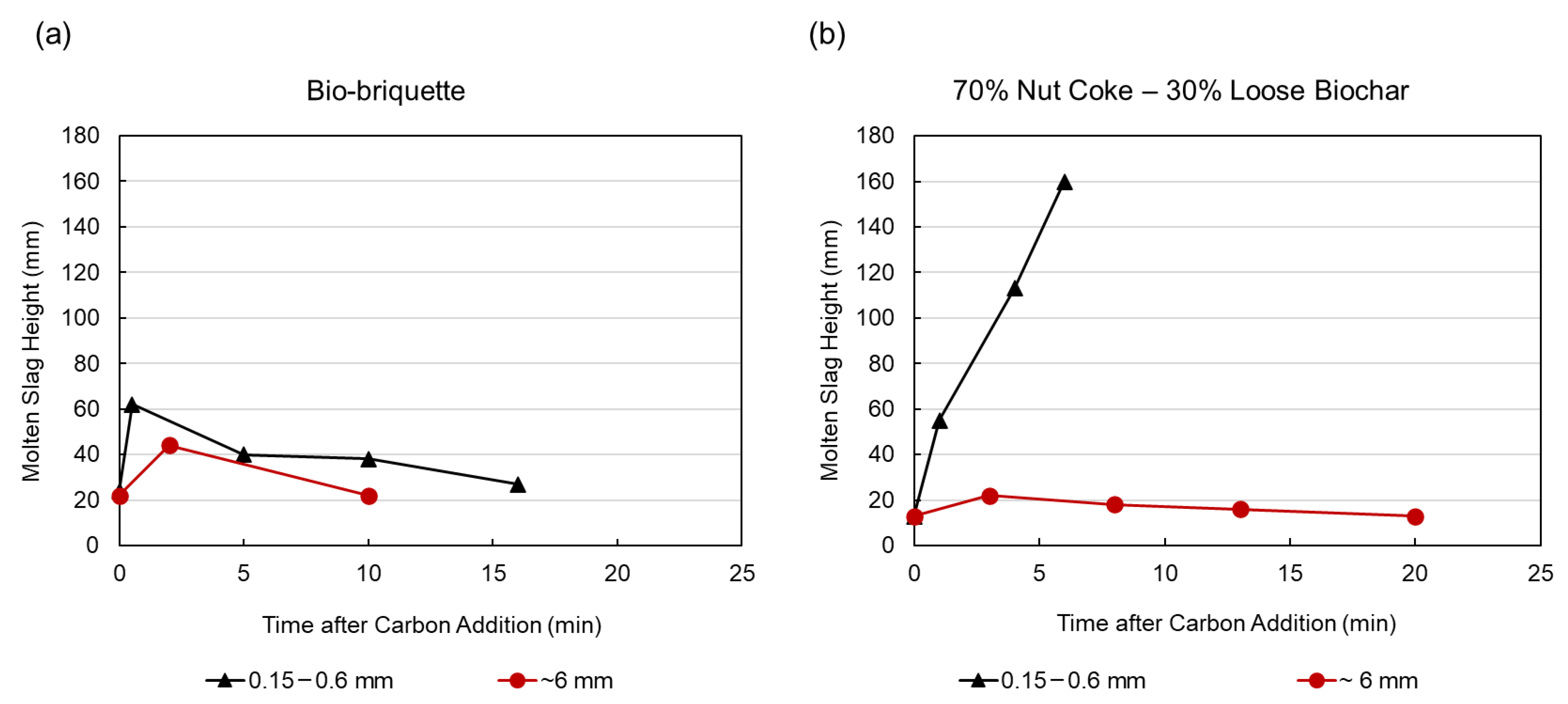
3.3. Slag Foaming Measurements
3.4. Ranking for Optimal Steelmaking Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Coal Information; IEA Publications: Paris, France, 2015. [Google Scholar]
- International Energy Agency. Energy Statistics of Non-OECD Countries 2015; IEA Publications: Paris, France, 2015. [Google Scholar]
- Daehn, K.; Basuhi, R.; Gregory, J.; Berlinger, M.; Somjit, V.; Olivetti, E.A. Innovations to Decarbonize Materials Industries. Nat. Rev. Mater. 2022, 7, 275–294. [Google Scholar] [CrossRef]
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef] [PubMed]
- Holappa, L. A General Vision for Reduction of Energy Consumption and CO2 Emissions from the Steel Industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- International Energy Agency. Iron and Steel Technology Roadmap Towards More Sustainable Steelmaking Part of the Energy Technology Perspectives Series; IEA Publications: Paris, France, 2020. [Google Scholar]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Min, D.J.; Han, J.W.; Chung, W.S. A Study of the Reduction Rate of FeO in Slag by Solid Carbon. Metall. Mater. Trans. B 1999, 30, 215–221. [Google Scholar] [CrossRef]
- Sarma, B.; Cramb, A.W.; Fruehan, R.J. Reduction of FeO in Smelting Slags by Solid Carbon: Experimental Results. Metall. Mater. Trans. B 1996, 27, 717–730. [Google Scholar] [CrossRef]
- Baracchini, G.; Bianco, L.; Cirilli, F.; Echterhof, T.; Griessacher, T.; Marcos, M.; Mirabile, D.; Reichel, T.; Rekersdrees, T.; Sommerauer, H. Biochar for a Sustainable EAF Steel Production (GREENEAF2); Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Moriconi, A.; Moriconi, E.; Marcos, M.; Demus, T.; Echterhof, T.; Pfeifer, H.; Beiler, C.; et al. Sustainable EAF Steel Production (GREENEAF); Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Kieush, L.; Schenk, J.; Koveria, A.; Rantitsch, G.; Hrubiak, A.; Hopfinger, H. Utilization of Renewable Carbon in Electric Arc Furnace-Based Steel Production: Comparative Evaluation of Properties of Conventional and Non-Conventional Carbon-Bearing Sources. Metals 2023, 13, 722. [Google Scholar] [CrossRef]
- Fidalgo, B.; Berrueco, C.; Millan, M. Chars from Agricultural Wastes as Greener Fuels for Electric Arc Furnaces. J. Anal. Appl. Pyrolysis 2015, 113, 274–280. [Google Scholar] [CrossRef]
- Huang, X.; Ng, K.W.; Giroux, L.; Duchesne, M.; Li, D.; Todoschuk, T. Interaction Behavior of Biogenic Material with Electric Arc Furnace Slag. Fuels 2021, 2, 420–436. [Google Scholar] [CrossRef]
- Demus, T.; Reichel, T.; Schulten, M.; Echterhof, T.; Pfeifer, H. Increasing the Sustainability of Steel Production in the Electric Arc Furnace by Substituting Fossil Coal with Biochar Agglomerates. Ironmak. Steelmak. 2016, 43, 564–570. [Google Scholar] [CrossRef]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Di Sante, L.; Moriconi, A.; Moriconi, E.; Agorio, M.M.; Pfeifer, H.; Echterhof, T.; Demus, T. Sustainable Electric Arc Furnace Steel Production: GreenEAF. In Proceedings of the European Electric Steelmaking Conference (EEC), Graz, Austria, 25–28 September 2012. [Google Scholar]
- Demus, T.; Echterhof, T.; Pfeifer, H. Replacement of Fossil Carbon with Biogenic Residues in the Electric Steelmaking Process. In Proceedings of the International Workshop EAF Perspectives on Automation; Materials, Energy & Environment, Milan, Italy, 29–30 March 2012. [Google Scholar]
- Ji, F.Z.; Barati, M.; Coley, K.; Irons, G.A. Kinetics of Coal Injection into Iron Oxide Containing Slags. Can. Metall. Q. 2005, 44, 85–94. [Google Scholar] [CrossRef]
- Corbari, R.; Matsuura, H.; Halder, S.; Walker, M.; Fruehan, R.J. Foaming and the Rate of the Carbon-Iron Oxide Reaction in Slag. Metall. Mater. Trans. B 2009, 40, 940–948. [Google Scholar] [CrossRef]
- Rodriguez, H.H.; Conejo, A.N.; Morales, R.D. Theoretical Analysis of the Interfacial Phenomena during the Injection of Carbon Particles into EAF Slags. Steel Res. 2001, 72, 298–303. [Google Scholar] [CrossRef]
- Li, Y.; Ratchev, I.P. Rate of Interfacial Reaction between Molten CaO-SiO2-Al2O3-FexO and CO-CO2. Metall. Mater. Trans. B 2002, 33, 651–660. [Google Scholar] [CrossRef]
- Fun, F. Rates and Mechanisms of FeO Reduction from Slags. Metall. Trans. 1970, 1, 2537–2541. [Google Scholar] [CrossRef]
- Huang, X.A.; Ng, K.W.; Giroux, L.; Duchesne, M. Carbonaceous Material Properties and Their Interactions with Slag During Electric Arc Furnace Steelmaking. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2019, 50, 1387–1398. [Google Scholar] [CrossRef]

| Methods | Coke | IIC | Loose Biochar | Bio- Briquette | ||
|---|---|---|---|---|---|---|
| Proximate, wt.% (db) | FC | ASTM D7582 | 88.90 | 91.31 | 87.78 | 67.72 |
| VM | ISO 562 | 0.86 | 6.03 | 6.01 | 31.16 | |
| Ash | ASTM D7582 | 10.24 | 2.66 | 6.21 | 1.12 | |
| Ultimate, wt.% (db) | C | ASTM D5373 | 87.4 | 86.1 | 89.2 | 79.2 |
| H | ASTM D5373 | 0.26 | 1.97 | 0.85 | 4.31 | |
| N | ASTM D5373 | 1.29 | 1.66 | 0.51 | 0.11 | |
| S | ASTM D4239 | 0.80 | 5.10 | 0 | 0 | |
| O (by diff) | In-house | 0.01 | 2.52 | 3.19 | 15.25 | |
| Ash Composition (db, as wt.% of ash) | SiO2 | ASTM D4326 | 49.98 | 52.03 | 41.56 | 5.83 |
| Al2O3 | 29.94 | 27.93 | 7.34 | 1.85 | ||
| Fe2O3 | 8.62 | 6.08 | 14.69 | 11.27 | ||
| P2O5 | 0.59 | 0.270 | 0.87 | 1.460 | ||
| CaO | 3.17 | 0.98 | 13.02 | 34.04 | ||
| MgO | 0.94 | 0.72 | 3.12 | 5.31 | ||
| Na2O | 0.2 | 0.67 | 1.91 | 0.20 | ||
| K2O | 1.82 | 2.41 | 7.33 | 10.56 | ||
| Basicity | (Fe2O3 + P2O5 + CaO + MgO + Na2O + K2O)/(SiO2 + Al2O3) | 0.185 | 0.136 | 0.819 | 7.992 | |
| HHV | (MJ/kg) | ISO 1928 | 29.68 | - | 31.2 | 31.3 |
| Carbon Type | C | O | S |
|---|---|---|---|
| Nut Coke | 92.5 | 5.5 | 0.5 |
| IIC | 88.8 | 5.5 | 5.5 |
| Loose Biochar | 82.2 | 17.0 | - |
| Bio-Briquette | 77.6 | 22.0 | - |
| Carbon Samples | Air Reaction Temperature (°C) | CO2 Reaction Temperature (°C) |
|---|---|---|
| Nut Coke | 502 | 879 |
| IIC | 426 | 898 |
| Loose Biochar | 303 | 672 |
| Bio-briquette | 274 | 682 |
| Nut Coke | IIC | Loose Biochar | Bio-Briquette | |
|---|---|---|---|---|
| %Ash (db) | 10.24 | 2.66 | 6.21 | 1.12 |
| %VM (db) | 0.86 | 6.03 | 6.01 | 31.16 |
| %C (db) | 87.4 | 86.1 | 89.2 | 79.2 |
| HHV (MJ/kg) | 29.68 | - | 31.2 | 31.3 |
| CaO | 3.17 | 0.98 | 13.02 | 34.04 |
| Na2O | 0.2 | 0.67 | 1.91 | 0.20 |
| P2O5 | 0.59 | 0.270 | 0.87 | 1.460 |
| K2O | 1.82 | 2.41 | 7.33 | 10.56 |
| Air Reactivity | 502 | 426 | 303 | 293 |
| Density (kg/m3) | ~1000 | - | ~200 | ~400 |
| Surface Morphology | Rough | Spherical without sharp edge | Cell structure with smooth surface | Rough |
| Tensiometer Interaction | Continuous gas bubble formation | - | Not fully melted | Limited gas formation |
| Max. Slag Foaming | 369% | 74% | 20% | 158% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiGiovanni, C.; Li, D.; Ng, K.W.; Huang, X. Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking. Metals 2023, 13, 1003. https://doi.org/10.3390/met13061003
DiGiovanni C, Li D, Ng KW, Huang X. Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking. Metals. 2023; 13(6):1003. https://doi.org/10.3390/met13061003
Chicago/Turabian StyleDiGiovanni, Christopher, Delin Li, Ka Wing Ng, and Xianai Huang. 2023. "Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking" Metals 13, no. 6: 1003. https://doi.org/10.3390/met13061003
APA StyleDiGiovanni, C., Li, D., Ng, K. W., & Huang, X. (2023). Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking. Metals, 13(6), 1003. https://doi.org/10.3390/met13061003







