The Potential of Duplex Stainless Steel Processed by Laser Powder Bed Fusion for Biomedical Applications: A Review
Abstract
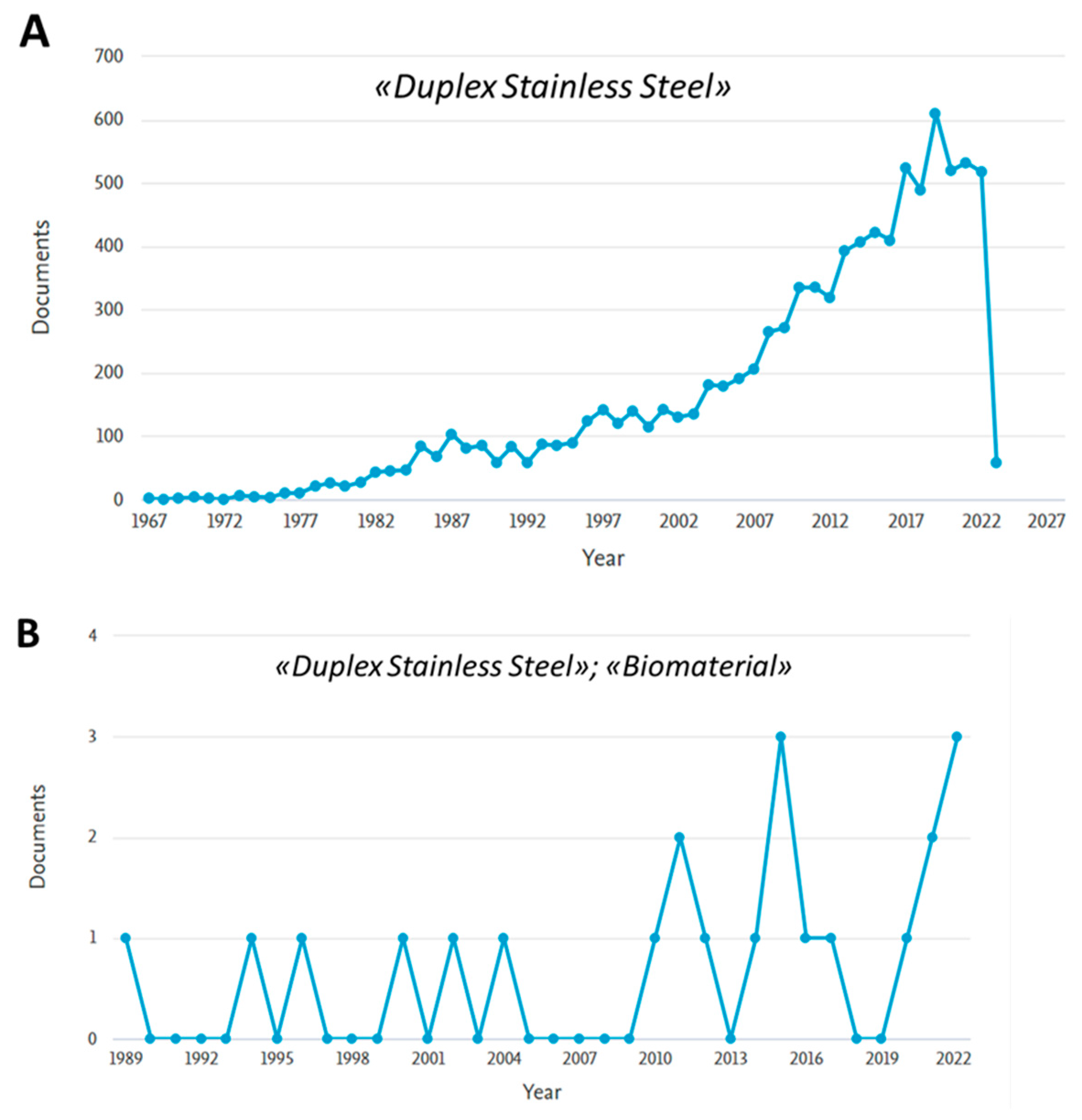
1. Introduction
2. DSS for Biomedical Applications
2.1. In Vitro Performances
- (1)
- The human body fluids comprising minerals, ionic salts, hydrolytic enzymes, proteins, nucleic acids, gases, carbonates, and lipids, thus representing an extremely tough environment;
- (2)
- The demanding loading conditions.
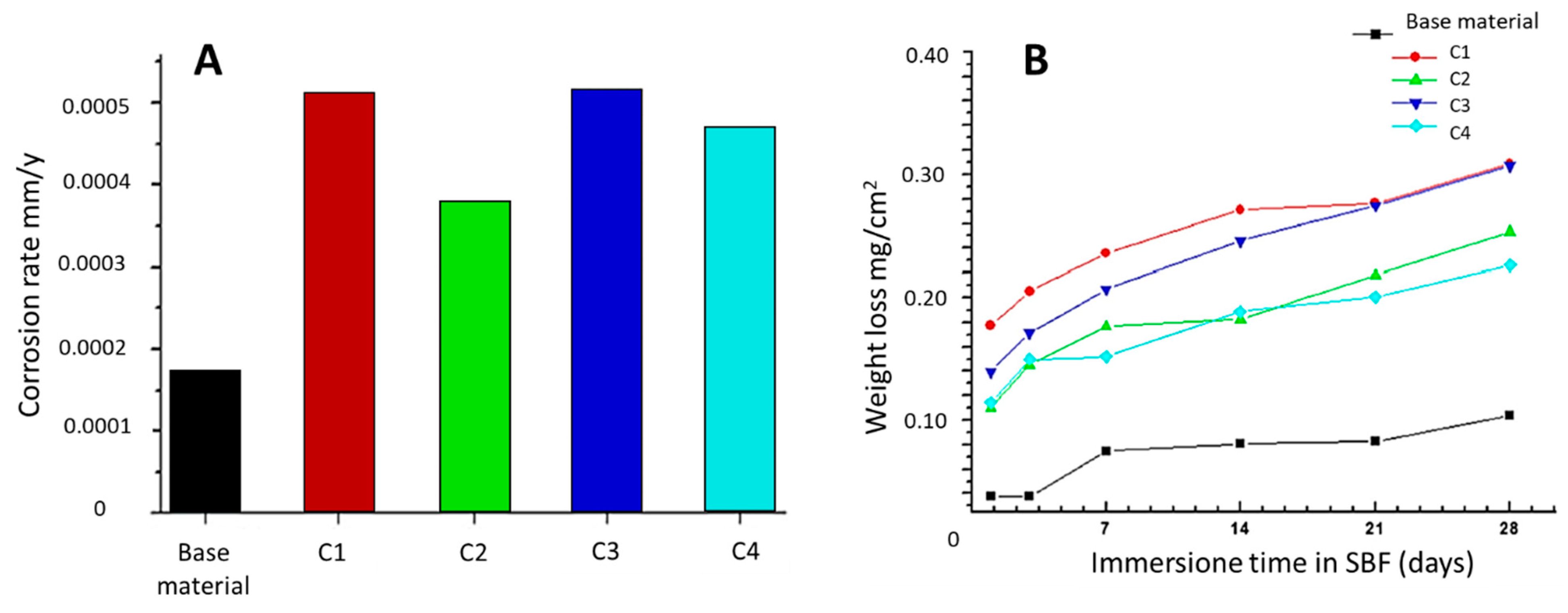
2.1.1. Corrosion Behavior
| Stainless Steels | Degradation Test | Mechanical Behavior | Magnetic Behavior | Reference |
|---|---|---|---|---|
| The resistance of the tested steels to localized corrosion in artificial physiological solution is as follows: 23Cr-4Ni < AISI 316L < ASTM F138 < 22Cr-5Ni-3Mo < 27Cr-31Ni-3.5Mo < 25Cr-7Ni-4Mo-N | − | − | [6] |
| 25Cr-7Ni-4Mo-0.28N DSS shows fatigue limits remarkably higher than ASTM F138 ASS | − | [28] | ||
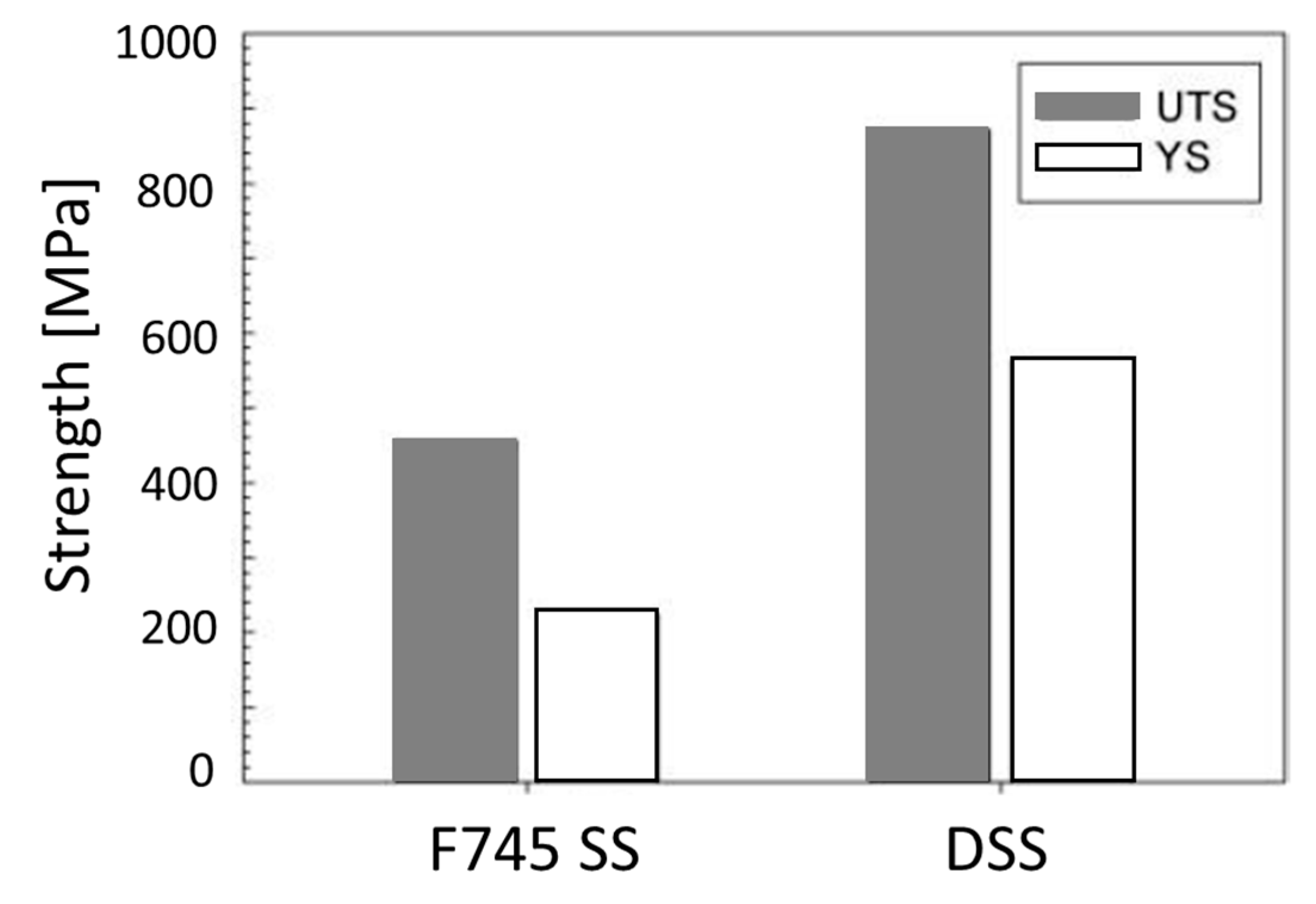
| 25Cr-7Ni-4Mo-N DSS suffers lower susceptibility to localised corrosion in body simulated media than F745 SS | UTS and YS values for the DSS were 870 and 560 MPa, while they were 460 and 220 MPa for the F745 SS, respectively; while F745 SS shows higher elongation (30%) than DSS (20%) | DSS shows higher magnetic saturation and remanence, whereas F745 SS has a higher coercive force | [5] |
| SAF 2507 exhibits higher corrosion resistance in artificial saliva than SAF 2205, both before and after heat treatment | [17] | ||
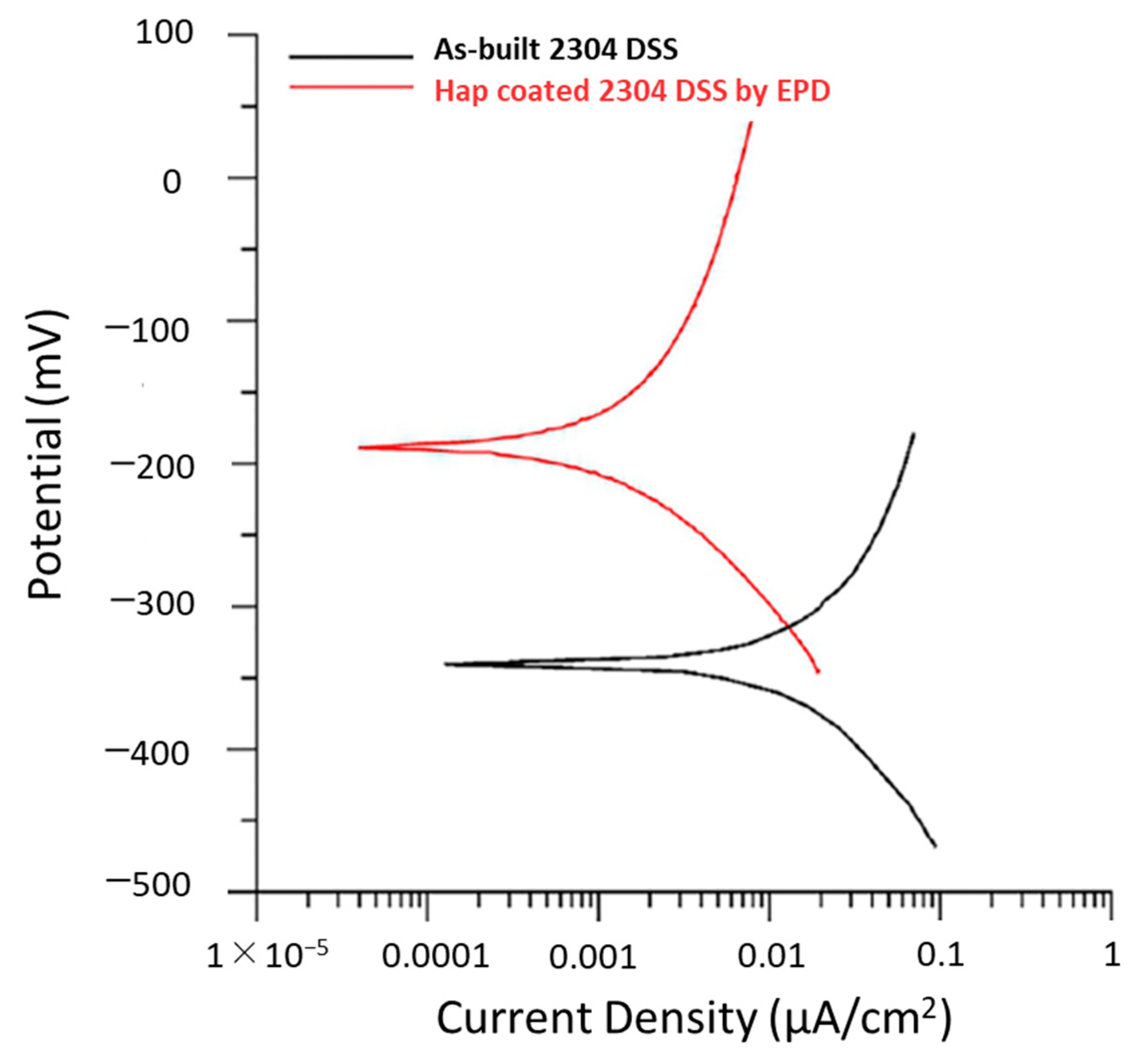
| 2205 DSS coated with hydroxyapatite/titania (Hap/TiO2) nanocompositeusing EPD process | Coating deposited (Hap/TiO2 nanocomposite) exhibits improved pitting corrosion resistance when immersed in Ringer’s physiological solution with respect to uncoated 2205 DSS | [16] | ||
| 2304 DSS coated with hydroxyapatite (Hap) through EPD process | The Hap coating on the 2304 DSS has higher corrosion resistance in SBF than the uncoated samples | [7] | ||
| AISI 2205 DSS | Very low corrosion rates due to very low weight losses were detected in all samples kept in SBF at different times | [31] | ||
| The general corrosion resistance of DSS is slightly inferior to that of austenitic SS; however, DSS does not show any signs of pitting when exposed to synthetic biofluids, and exhibits excellent resistance to localised corrosion | [27] | ||
| 2205 DSS treated with Electrical discharge machining (EDM) | The surface treatment by EDM significantly enhances the corrosion resistance and biological performances of 2205 DSS | [15] | ||
| 2205 has a longer passivation range than 316L in a sodium chloride solution | [32] |
2.1.2. Mechanical Behavior
2.1.3. Magnetic Behaviour
2.2. In Vivo Experiments
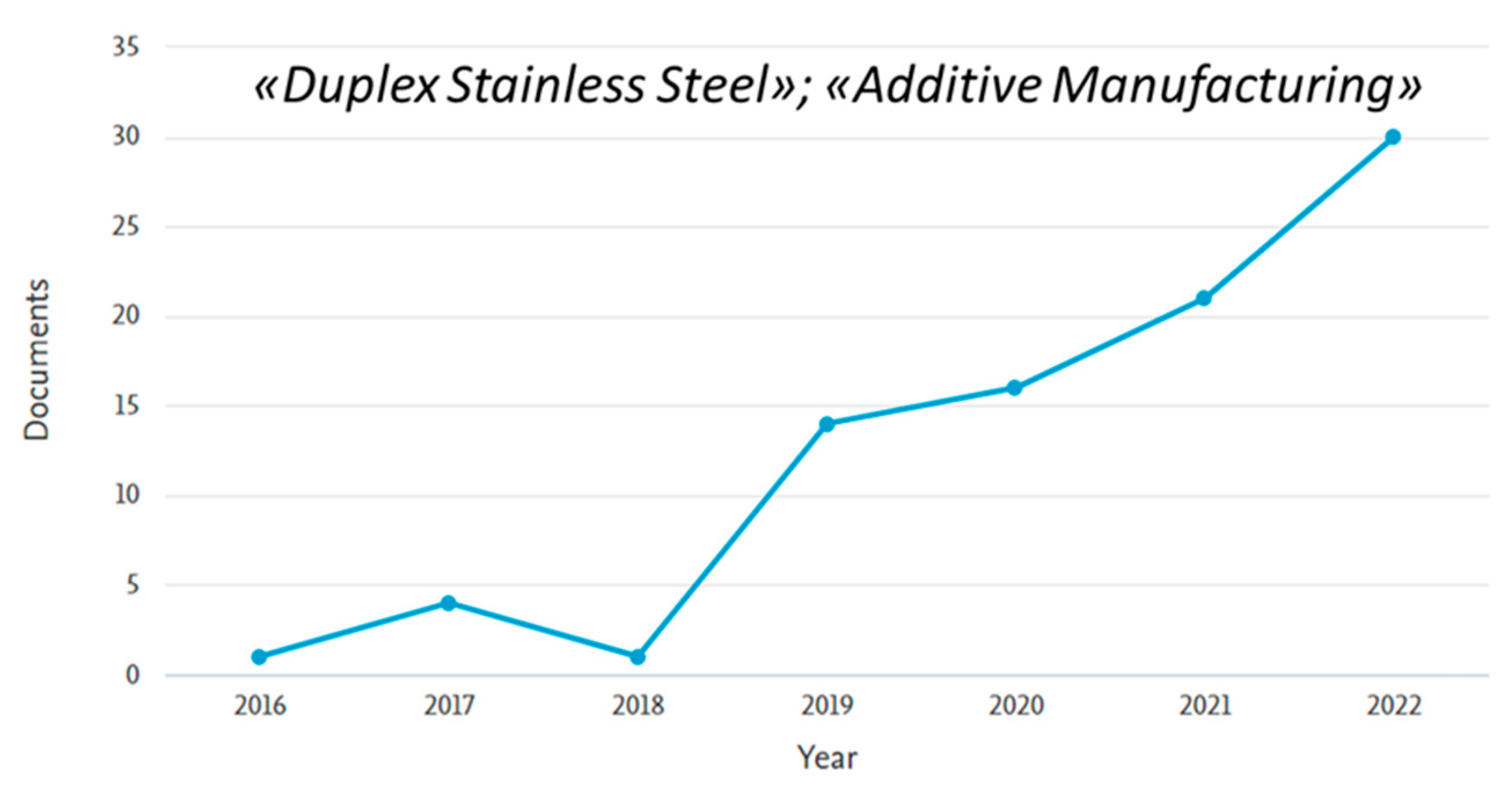
3. Laser Powder Bed Fusion (LPBF) of DSS
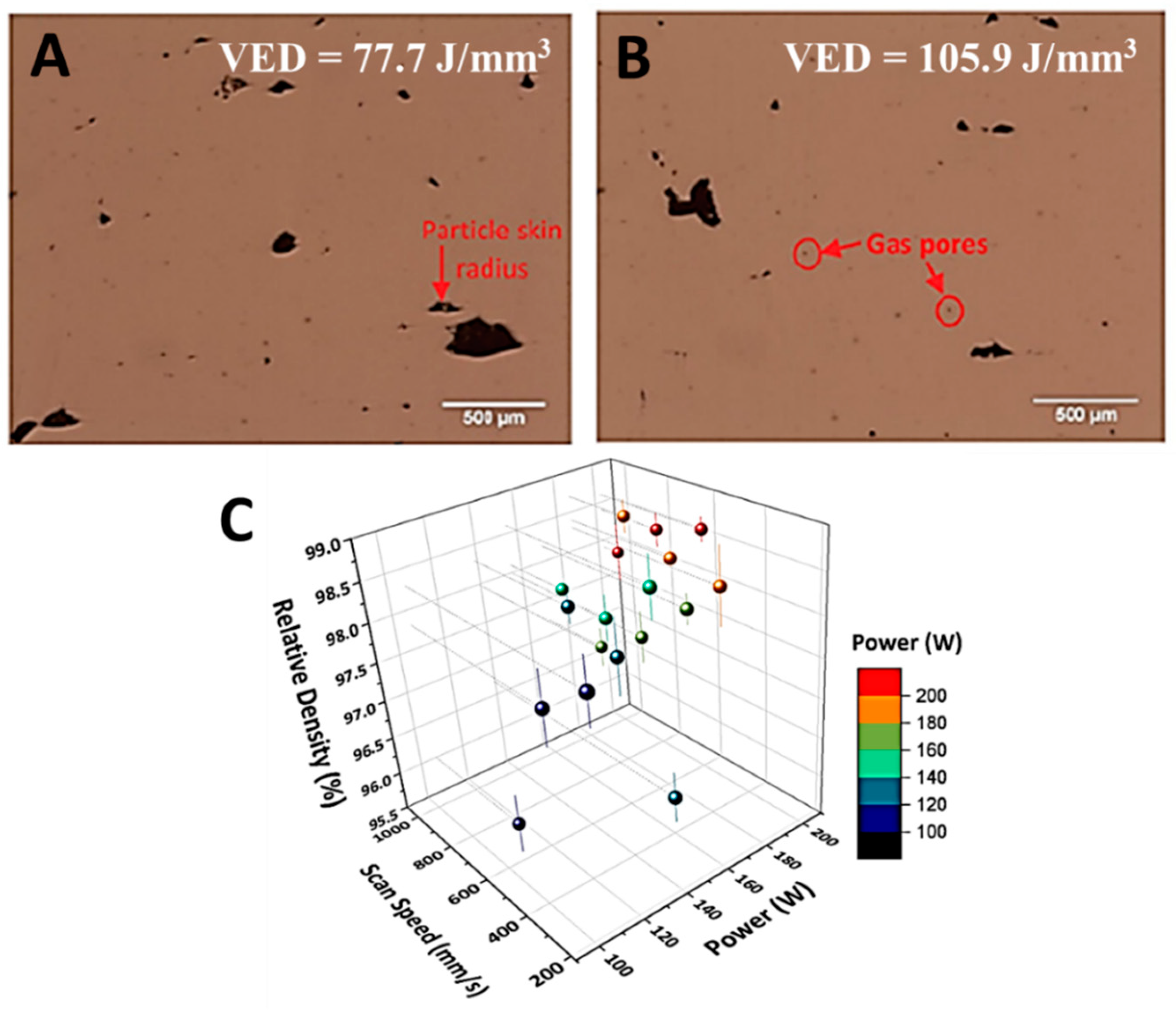
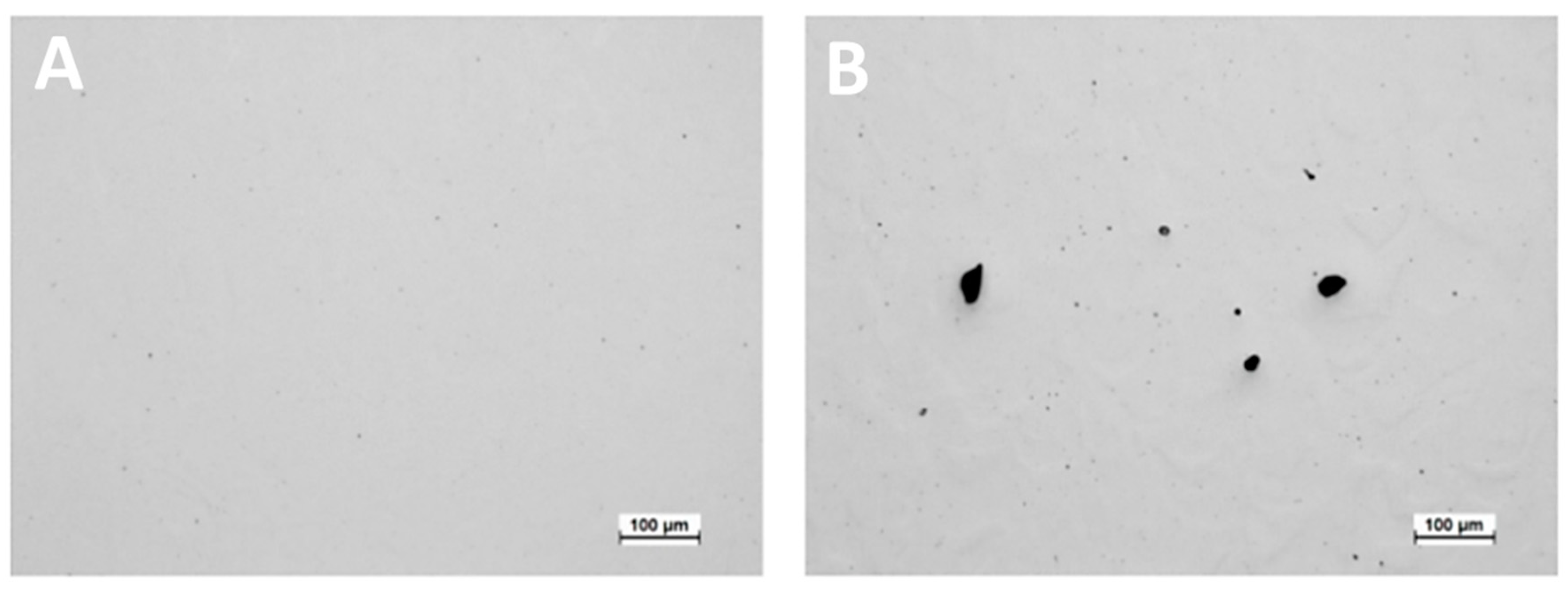
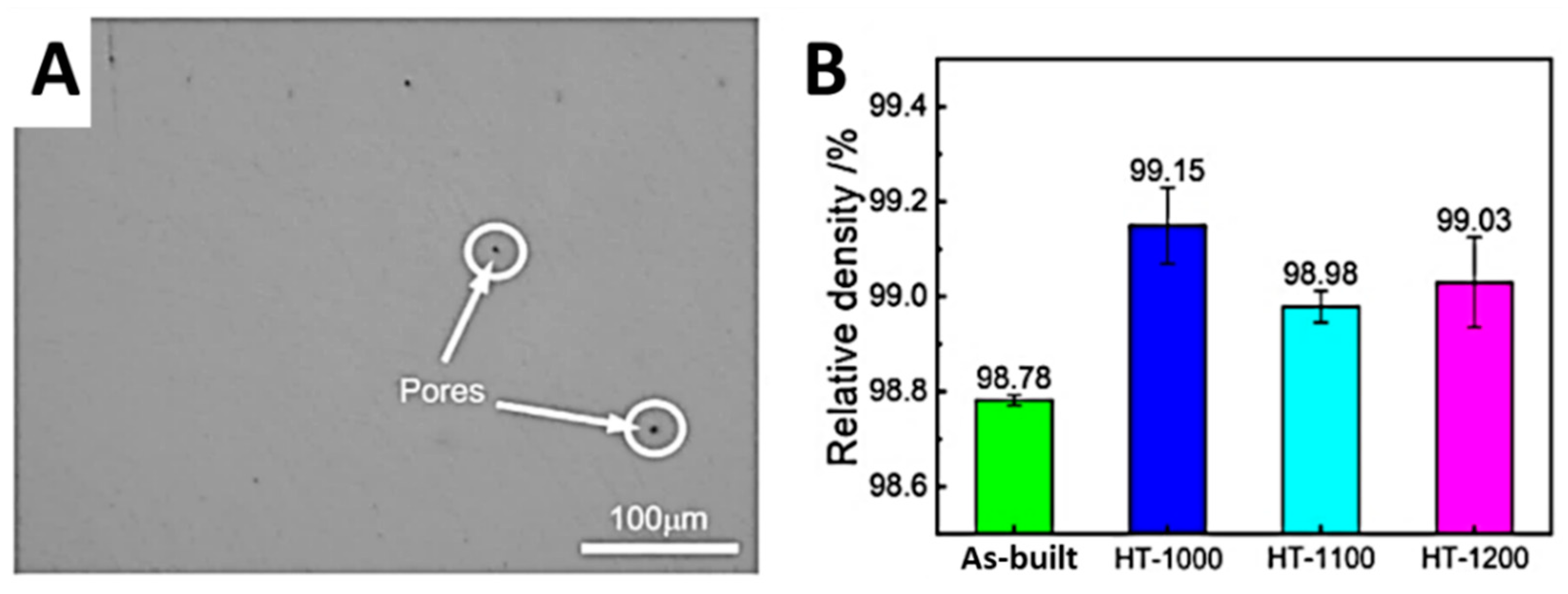
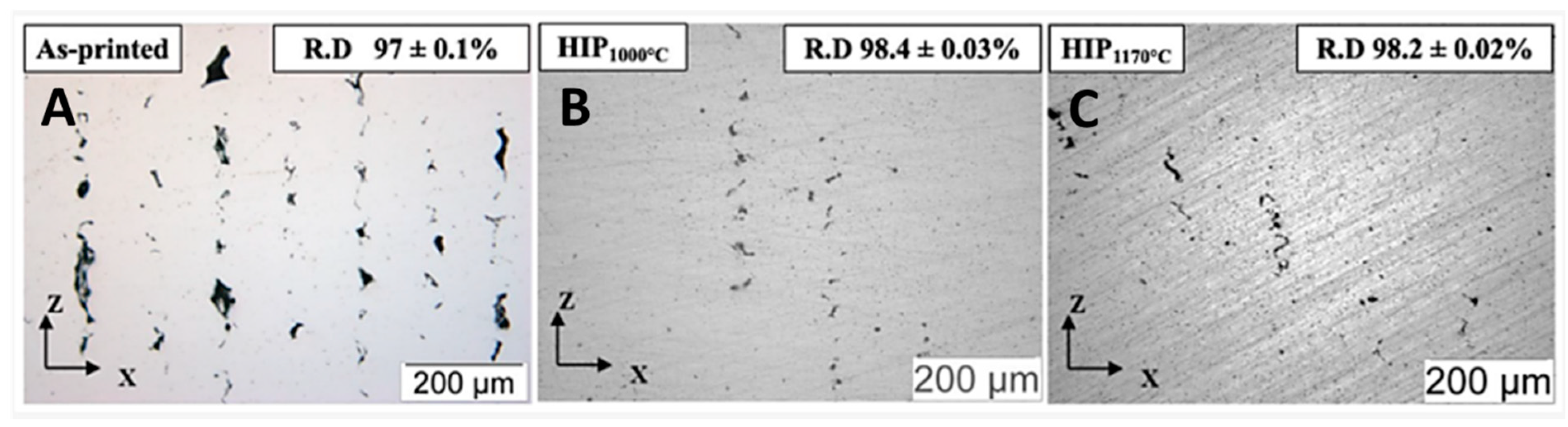
3.1. Relative Density and Defects
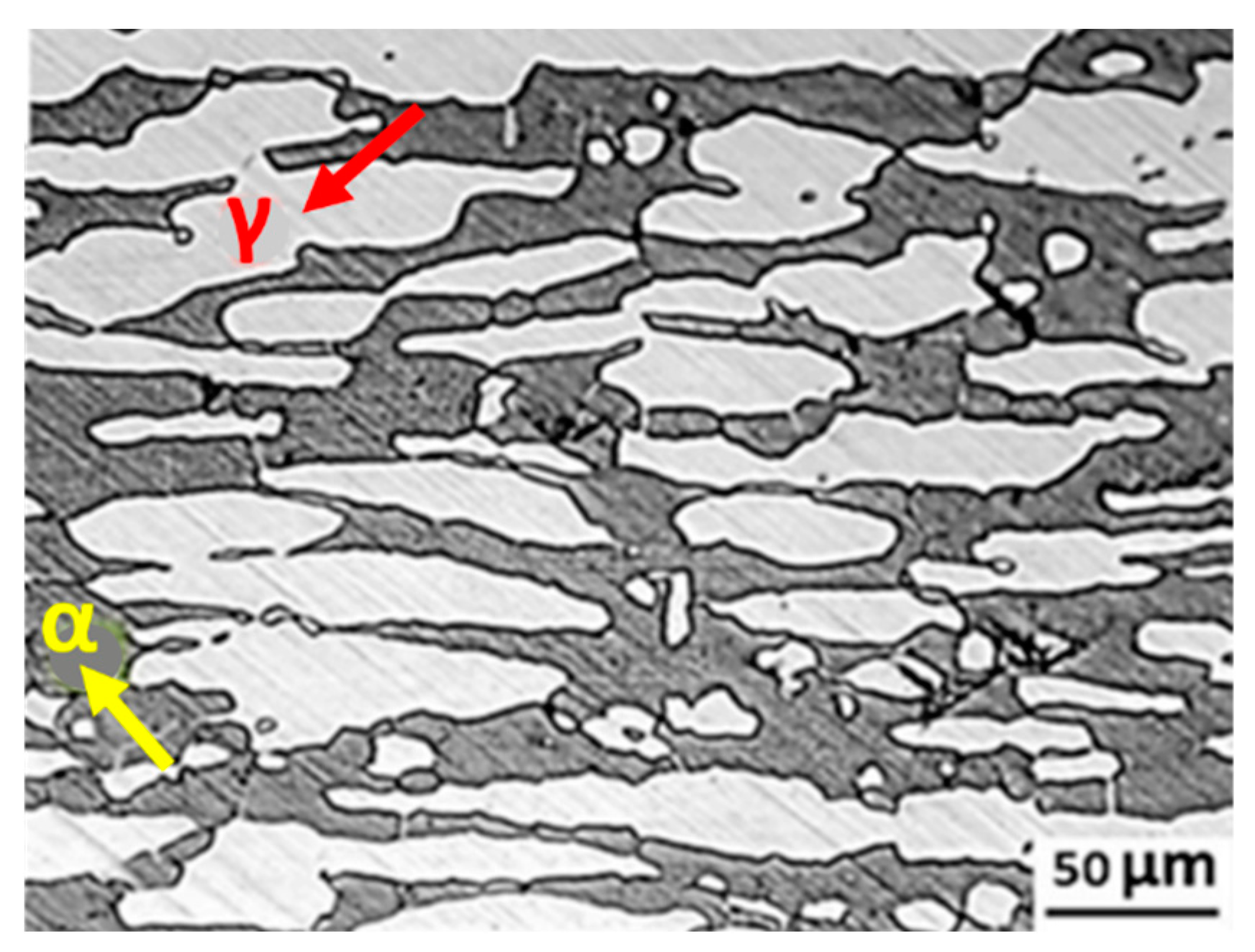
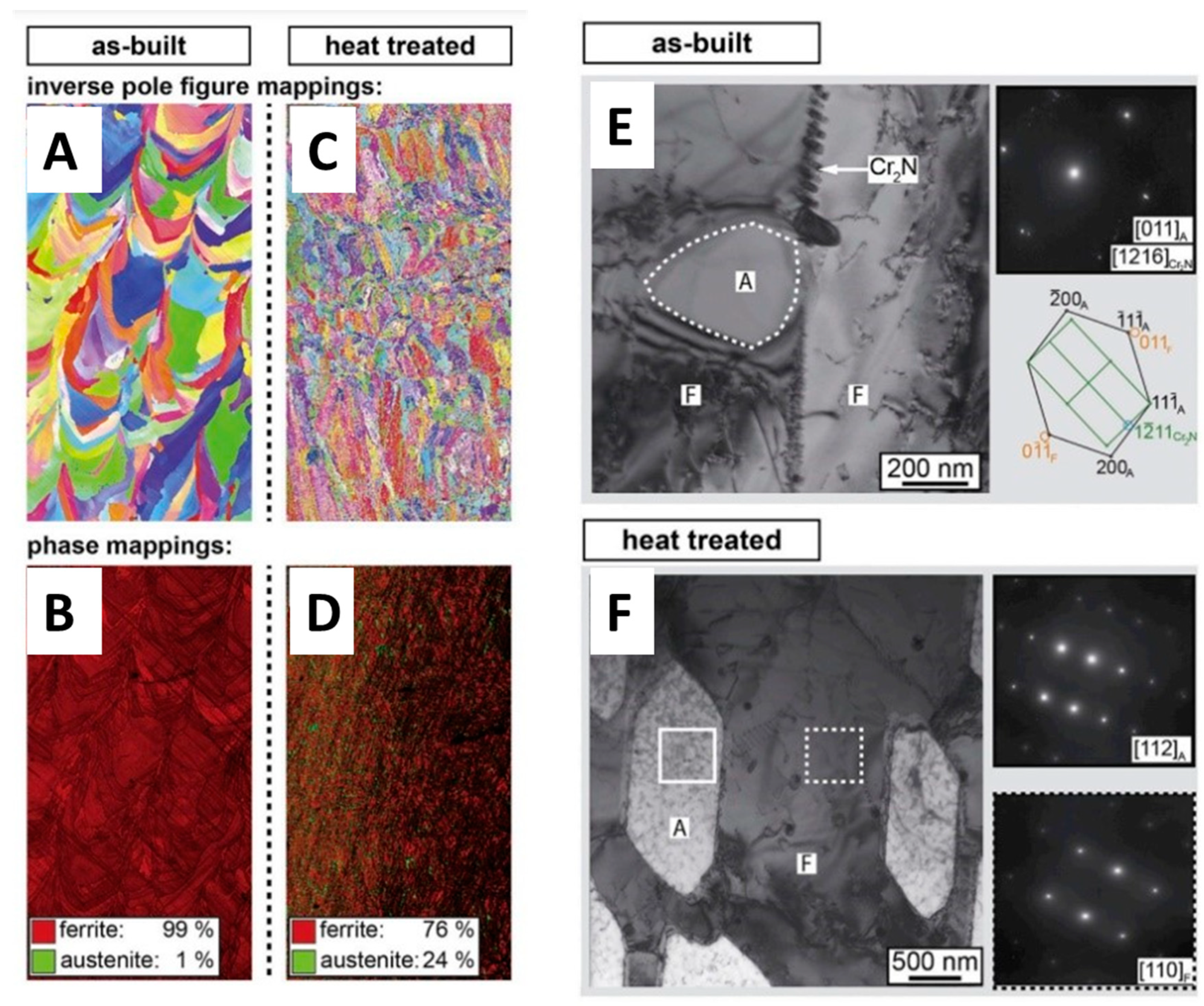
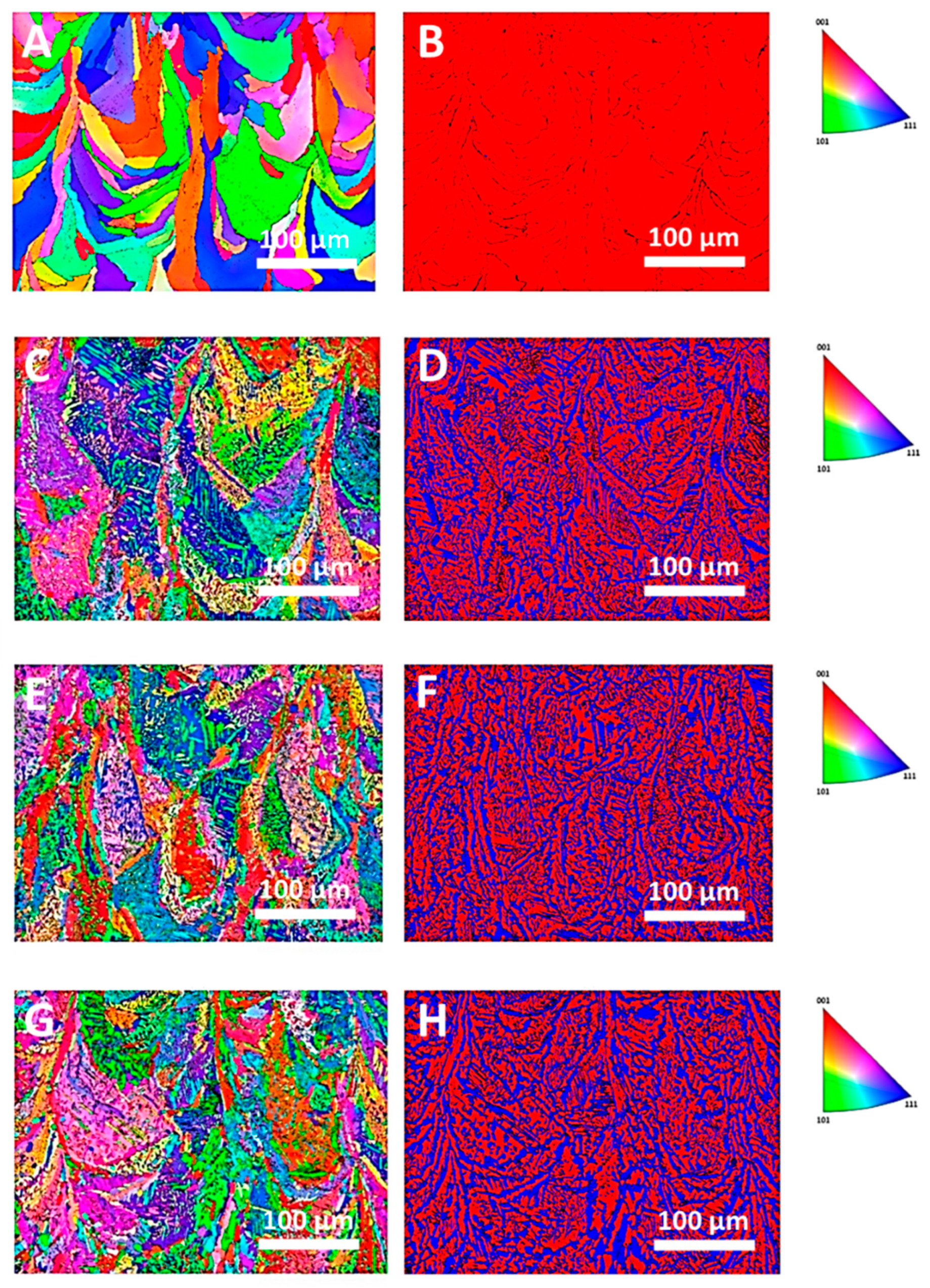
3.2. Microstructure Evolution
3.3. Evolution of Mechanical Properties
3.3.1. Hardness
3.3.2. Tensile Properties
3.3.3. Fatigue Behaviour
3.4. Corrosion Resistance
3.5. Magnetic Properties
4. Conclusions
- -
- Residual porosity and lack of fusion of the L-PBF processed duplex stainless steels (DSS) are controlled by the volumetric energy density (VED), in order to obtain full dense components;
- -
- The arrangement of the austenite-ferrite phases is balanced upon the subsequent heat-treatment of as-built samples;
- -
- The hardness and tensile strength of L-PBF DSS exceeds that of conventionally produced steels, also showing superior corrosion and magnetic properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López, D.A.; Durán, A.; Ceré, S. Electrochemical characterization of AISI 316L stainless steel in contact with simulated body fluid under infection conditions. J. Mater. Sci. Mater. Med. 2008, 19, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium implants: Prospects and challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Roman, A.M.; Geantă, V.; Cimpoeșu, R.; Munteanu, C.; Lohan, N.M.; Zegan, G.; Cernei, E.R.; Ionită, I.; Cimpoes, N.; Ioanid, N. In-vitro analysis of FeMn-Si smart biodegradable alloy. Materials 2022, 15, 568. [Google Scholar] [CrossRef] [PubMed]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Gregorutti, R.W.; Grau, J.E.; Sives, F.; Elsner, C.I. Mechanical, electrochemical and magnetic behaviour of duplex stainless steel for biomedical applications. Mater. Sci. Technol. 2015, 31, 1818–1824. [Google Scholar] [CrossRef]
- Cigada, A.; Rondelli, G.; Vicentini, B.; Giacomazzi, M.; Roos, A. Duplex stainless steels for osteosynthesis devices. J. Biomed. Mater. Res. 1989, 23, 1087–1095. [Google Scholar] [CrossRef]
- Hammood, A.S. Biomineralization of 2304 duplex stainless steel with surface modification by electrophoretic deposition. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800019896215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, A.; Yin, B.; Wen, P. Additive manufacturing of duplex stainless steels-a critical review. J. Manuf. Process. 2022, 73, 496–517. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.H.; Wu, C.B.; Zhao, Y.; Zu, G.Q.; Zhu, W.W.; Ran, X. A short review on the role of alloying elements in duplex stainless steels. Tungsten 2022, 1–21. [Google Scholar] [CrossRef]
- Kain, V. Stress corrosion cracking (SCC) in stainless steels. In Stress Corrosion Cracking; Woodhead Publishing: Sawston, Cambridge, UK, 2011; pp. 199–244. [Google Scholar] [CrossRef]
- Charles, J. Duplex families and applications: A review. Part 1: From duplex pioneers up to 1991. In Stainless SteelWorld; KCI group: Sparks, MD, USA, 2015; pp. 1–5. [Google Scholar]
- Freitas, G.C.L.D.; da Fonseca, G.S.; Moreira, L.P.; Leite, D.N.F. Phase transformations of the duplex stainless steel UNS S31803 under non-isothermal conditions. J. Mater. Res. Technol. 2021, 11, 1847–1851. [Google Scholar] [CrossRef]
- Fonseca, G.S.D.; Mendes, P.S.N.; Silva, A.C.M. Sigma phase: Nucleation and growth. Metals 2019, 9, 34. [Google Scholar] [CrossRef]
- Biezma, M.V.; Martin, U.; Linhardt, P.; Ress, J.; Rodríguez, C.; Bastidas, D.M. Non-destructive techniques for the detection of sigma phase in duplex stainless steel: A comprehensive review. Eng. Fail. Anal. 2021, 122, 105227. [Google Scholar] [CrossRef]
- Mahajan, A.; Sidhu, S.S.; Devgan, S. Examination of hemocompatibility and corrosion resistance of electrical discharge-treated duplex stainless steel (DSS-2205) for biomedical applications. Appl. Phys. A 2020, 126, 737. [Google Scholar] [CrossRef]
- Hammood, A.S.; Naser, M.S.; Radeef, Z.S. Electrophoretic Deposition of Nanocomposite Hydroxyapatite/Titania Coating on 2205 Duplex Stainless Steel Substrate. Jom 2021, 73, 524–533. [Google Scholar] [CrossRef]
- Hammood, A.S.; Noor, A.F.; Alkhafagy, M.T. Evaluation of corrosion behavior in artificial saliva of 2507 and 2205 duplex stainless steel for orthodontic wires before and after heat treatment. J. Mater. Sci. Mater. Med. 2017, 28, 187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, M.; Chohan, J.S. The role of additive manufacturing for biomedical applications: A critical review. J. Manuf. Process. 2021, 64, 828–850. [Google Scholar] [CrossRef]
- Cook, D.; Gervasi, V.; Rizza, R.; Kamara, S.; Liu, X.C. Additive fabrication of custom pedorthoses for clubfoot correction. Rapid Prototyp. J. 2010, 16, 189–193. [Google Scholar] [CrossRef]
- Han, C.; Fang, Q.; Shi, Y.; Tor, S.B.; Chua, C.K.; Zhou, K. Recent advances on high-entropy alloys for 3D printing. Adv. Mater. 2020, 32, 1903855. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Deng, G.; Deng, C.; Bai, Y.; Yang, Y.; Wu, W.; Chen, J.; Liu, Y.; Wang, Y.; et al. Recent progress on additive manufacturing of multi-material structures with laser powder bed fusion. Virtual Phys. Prototyp. 2022, 17, 329–365. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, A.; Tripathi, O.; Srivastava, S.; Singh, B.; Awasthi, A.; Rajput, S.K.; Sonia, P.; Singhal, P.; Saxena, K.K. Powder bed fusion process in additive manufacturing: An overview. Mater. Today Proc. 2020, 26, 3058–3070. [Google Scholar] [CrossRef]
- Yang, N.; Gong, Y.; Chen, H.; Li, W.; Zhou, C.; Zhou, R.; Shao, H. Personalized Artificial Tibia Bone Structure Design and Processing Based on Laser Powder Bed Fusion. Machines 2022, 10, 205. [Google Scholar] [CrossRef]
- Munsch, M. Laser additive manufacturing of customized prosthetics and implants for biomedical applications. In Laser Additive Manufacturing; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 399–420. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.P.; Singamneni, S. Magnetic characterization of selective laser-melted Saf 2507 duplex stainless steel. Jom 2017, 69, 569–574. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Marikkannu, C.; Srinivasan, P.B.; Muthupandi, V. Corrosion behaviour of Ti6Al4V and duplex stainless steel (UNS31803) in synthetic bio-fluids. Anti-Corrosion Methods Mater. 2002, 49, 33–37. [Google Scholar] [CrossRef]
- Cigada, A.; Amici, F.; Cavallini, M.; De Santis, G.; Gatti, A.M.; Giacomazzi, M.; Rondelli, G.; Roos, A.; Vicentini, B.; Zaffe, D. Characterization of a high performance duplex stainless steel for orthopedic applications. In Engineering in Medicine and Biology Society. In Proceedings of the Twelfth Annual International Conference of the IEEE, Philadelphia, PA, USA, 1–4 November 1990; pp. 2082–2084. [CrossRef]
- Barbosa, M.A. Chapter 10 Corrosion of Metallic Implants. Handbook of biomaterial properties; Springer: New York, NY, USA, 2016; pp. 509–548. [Google Scholar]
- Pan, J.; Karlen, C.; Ulfvin, C. Electrochemical study of resistance to localized corrosion of stainless steels for biomaterial applications. J. Electrochem. Soc. 2000, 147, 1021. [Google Scholar] [CrossRef]
- Köse, C. Characterization of weld seam surface and corrosion behavior of laser-beam-welded AISI 2205 duplex stainless steel in simulated body fluid. J. Mater. Sci. 2020, 55, 17232–17254. [Google Scholar] [CrossRef]
- Platt, J.A.; Guzman, A.; Zuccari, A.; Thornburg, D.W.; Rhodes, B.F.; Oshida, Y.; Moore, B.K. Corrosion behavior of 2205 duplex stainless steel. Am. J. Orthod. Dentofac. Orthop. 1997, 112, 69–79. [Google Scholar] [CrossRef]
- Cigada, A.L.B.E.R.T.O.; Santis, G.D.; Gatti, A.M.; Roos, A.; Zaffe, D. In vivo behavior of a high performance duplex stainless steel. J. Appl. Biomater. 1993, 4, 39–46. [Google Scholar] [CrossRef]
- Park, B.H.; Koo, B.S.; Kim, Y.K.; Kim, M.K. The induction of hyperthermia in rabbit liver by means of duplex stainless steel thermoseeds. Korean J. Radiol. 2022, 3, 98–104. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Zhang, W. Additive manufacturing of metallic components–process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Davidson, K.; Singamneni, S. Selective laser melting of duplex stainless steel powders: An investigation. Mater. Manuf. Process. 2016, 31, 1543–1555. [Google Scholar] [CrossRef]
- Bertoli, U.S.; Wolfer, A.J.; Matthews, M.J.; Delplanque, J.P.R.; Schoenung, J.M. On the limitations of volumetric energy density as a design parameter for selective laser melting. Mater. Des. 2017, 113, 331–340. [Google Scholar] [CrossRef]
- Zhao, C.; Parab, N.D.; Li, X.; Fezzaa, K.; Tan, W.; Rollett, A.D.; Sun, T. Critical instability at moving keyhole tip generates porosity in laser melting. Science 2020, 370, 1080–1086. [Google Scholar] [CrossRef]
- du Plessis, A. Effects of process parameters on porosity in laser powder bed fusion revealed by X-ray tomography. Addit. Manuf. 2019, 30, 100871. [Google Scholar] [CrossRef]
- Knezović, N.; Garašić, I.; Jurić, I. Influence of the interlayer temperature on structure and properties of wire and arc additive manufactured duplex stainless steel product. Materials 2020, 13, 5795. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.P.; Singamneni, S.B. Metallographic evaluation of duplex stainless steel powders processed by selective laser melting. Rapid Prototyp. J. 2017. 23, 23,1146–1163. [CrossRef]
- Nigon, G.N.; Isgor, O.B.; Pasebani, S. The effect of annealing on the selective laser melting of 2205 duplex stainless steel: Microstructure, grain orientation, and manufacturing challenges. Opt. Laser Technol. 2021, 134, 106643. [Google Scholar] [CrossRef]
- Papula, S.; Song, M.; Pateras, A.; Chen, X.B.; Brandt, M.; Easton, M.; Hänninen, H. Selective laser melting of duplex stainless steel 2205: Effect of post-processing heat treatment on microstructure, mechanical properties, and corrosion resistance. Materials 2019, 12, 2468. [Google Scholar] [CrossRef]
- Xiang, H.; Zhao, W.; Lu, Y. Effect of solution temperature on microstructure and mechanical properties of selective laser melted Fe–22Cr–5Ni-0.26 N duplex stainless steel. J. Mater. Res. Technol. 2022, 19, 1379–1389. [Google Scholar] [CrossRef]
- Bartlett, A.A. Demonstration of a crystalline phase change in a solid. Phys. Teach. 1975, 13, 545–547. [Google Scholar] [CrossRef]
- Arumugham Akilan, A.; Enneti, R.K.; Balla, V.K.; Atre, S.V. Effects of Hot Isostatic Pressing on the Properties of Laser-Powder Bed Fusion Fabricated Water Atomized 25Cr7Ni Stainless Steel. Lubricants 2022, 10, 340. [Google Scholar] [CrossRef]
- Hengsbach, F.; Koppa, P.; Duschik, K.; Holzweissig, M.J.; Burns, M.; Nellesen, J.; Schaper, M. Duplex stainless steel fabricated by selective laser melting-Microstructural and mechanical properties. Mater. Des. 2017, 133, 136–142. [Google Scholar] [CrossRef]
- Murkute, P.; Pasebani, S.; Burkan Isgor, O. Metallurgical and electrochemical properties of super duplex stainless steel clads on low carbon steel substrate produced with laser powder bed fusion. Sci. Rep. 2020, 10, 10162. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Jiang, Z.H.; Zhang, Z.R.; Yang, Y. Effect of grain size on mechanical properties of nickel-free high nitrogen austenitic stainless steel. J. Iron Steel Res. Int. 2009, 16, 58–61. [Google Scholar] [CrossRef]
- Mukherjee, T.; DebRoy, T. A digital twin for rapid qualification of 3D printed metallic components. Appl. Mater. Today 2019, 14, 59–65. [Google Scholar] [CrossRef]
- Wei, H.L.; Mukherjee, T.; Zhang, W.; Zuback, J.S.; Knapp, G.L.; De, A.; DebRoy, T. Mechanistic models for additive manufacturing of metallic components. Prog. Mater. Sci. 2021, 116, 100703. [Google Scholar] [CrossRef]
- Saeidi, K.; Kevetkova, L.; Lofaj, F.; Shen, Z. Novel ferritic stainless steel formed by laser melting from duplex stainless steel powder with advanced mechanical properties and high ductility. Mater. Sci. Eng. A 2016, 665, 59–65. [Google Scholar] [CrossRef]
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in additive manufacturing: A review of their microstructure and properties. Mater. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Mahale, R.S.; Rajendrachari, S.; Vasanth, S.; Krishna, H.; Kapanigowda, N.S.; Chikkegowda, S.P.; Patil, A. Technology and challenges in additive manufacturing of duplex stainless steels. Biointerface Res. Appl. Chem. 2022, 12, 1110–1119. [Google Scholar] [CrossRef]
- Chan, K.W.; Tjong, S.C. Effect of secondary phase precipitation on the corrosion behavior of duplex stainless steels. Materials 2014, 7, 5268–5304. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.; Jiang, Y.; Sun, T.; Xu, J.; Li, J. Effect of thermal cycles on the corrosion and mechanical properties of UNS S31803 duplex stainless steel. Corros. Sci. 2009, 51, 2969–2975. [Google Scholar] [CrossRef]
- Verma, J.; Taiwade, R.V. Effect of welding processes and conditions on the microstructure, mechanical properties and corrosion resistance of duplex stainless steel weldments—A review. J. Manuf. Process. 2017, 25, 134–152. [Google Scholar] [CrossRef]
- Atamert, S.; King, J.E. Sigma-phase formation and its prevention in duplex stainless steels. J. Mater. Sci. Lett. 1993, 12, 1144–1147. [Google Scholar] [CrossRef]
- Kokawa, H.; Tomita, M.; Kuwana, T. Effect of nitrogen on impact toughness of duplex stainless steel weld metal. Weld. Int. 1995, 9, 217–224. [Google Scholar] [CrossRef]
- Saeidi, K.; Alvi, S.; Lofaj, F.; Petkov, V.I.; Akhtar, F. Advanced mechanical strength in post heat treated SLM 2507 at room and high temperature promoted by hard/ductile sigma precipitates. Metals 2019, 9, 199. [Google Scholar] [CrossRef]
- Souza, E.C.D.; Rossitti, S.M.; Fortulan, C.A.; Rollo, J.M.D.D.A. Influence of ferrite phase content on the electrochemical properties of duplex stainless steels. Mater. Res. 2016, 20, 21–29. [Google Scholar] [CrossRef]
- Hwang, H.; Park, Y. Effects of heat treatment on the phase ratio and corrosion resistance of duplex stainless steel. Mater. Trans. 2009, 50, 1548–1552. [Google Scholar] [CrossRef]
- Badji, R.; Bouabdallah, M.; Bacroix, B.; Kahloun, C.; Belkessa, B.; Maza, H. Phase transformation and mechanical behavior in annealed 2205 duplex stainless steel welds. Mater. Charact. 2008, 59, 447–453. [Google Scholar] [CrossRef]
- Yilmaz, H.; Williams, C.J.; Derby, B. Size effects on strength and plasticity of ferrite and austenite pillars in a duplex stainless steel. Mater. Sci. Eng. A 2020, 793, 139883. [Google Scholar] [CrossRef]
- Fischer, T.; Kuhn, B.; Rieck, D.; Schulz, A.; Trieglaff, R.; Wilms, M.B. Fatigue Cracking of Additively Manufactured Materials—Process and Material Perspectives. Appl. Sci. 2020, 10, 5556. [Google Scholar] [CrossRef]
- Molaei, R.; Fatemi, A. Fatigue design with additive manufactured metals: Issues to consider and perspective for future research. Procedia Eng. 2018, 213, 5–16. [Google Scholar] [CrossRef]
- Kunz, J.; Boontanom, A.; Herzog, S.; Suwanpinij, P.; Kaletsch, A.; Broeckmann, C. Influence of hot isostatic pressing post-treatment on the microstructure and mechanical behavior of standard and super duplex stainless steel produced by laser powder bed fusion. Mater. Sci. Eng. A 2020, 794, 139806. [Google Scholar] [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Effect of grain size on the corrosion resistance of low carbon steel. Mater. Res. Express 2020, 7, 016522. [Google Scholar] [CrossRef]
- Shang, F.; Chen, X.; Wang, Z.; Ji, Z.; Ming, F.; Ren, S.; Qu, X. The microstructure, mechanical properties, and corrosion resistance of UNS S32707 hyper-duplex stainless steel processed by selective laser melting. Metals 2019, 9, 1012. [Google Scholar] [CrossRef]
- Jiang, D.; Birbilis, N.; Hutchinson, C.R.; Brameld, M. On the microstructure and electrochemical properties of additively manufactured duplex stainless steels produced using laser-powder bed fusion. Corrosion 2020, 76, 871–883. [Google Scholar] [CrossRef]





| Stainless Steels | Experimental Study | Reference |
|---|---|---|
| Crevice corrosion investigation of implant on rabbit and sheep femurs and of Endomedullary nails (Ender type) in patient femurs for about 6 months, to set up an alternative to the employ of conventional austenitic SS | [28] |
| Cylindrical pin (2 mm diameter, 6 mm length) implantation in rabbit for 6 and 12 months to find an alternative with respect to the ASTM F138 austenitic SS | [33] | |
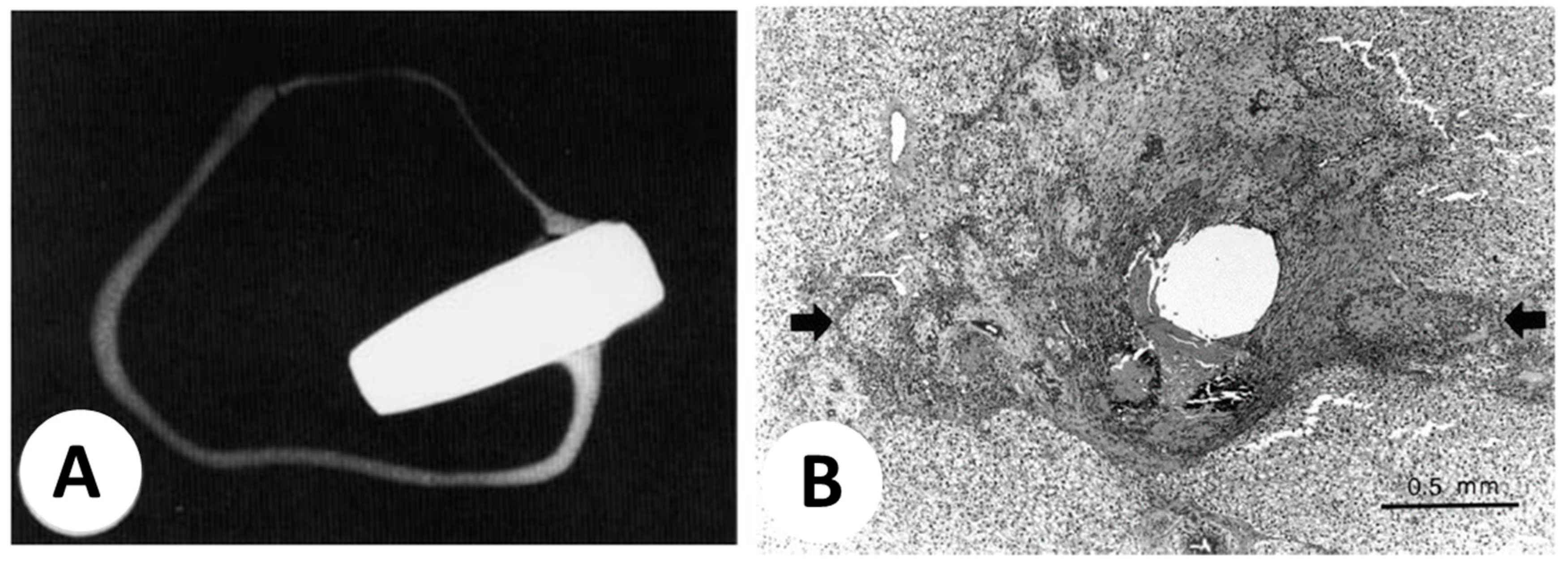
| Thermoseed (0.23 mm diameter, either L-shaped and I-shaped) insertion in the liver of New Zealand white rabbits for inducing heating by magnetic field exposition to cause hyperthermia | [34] |
| DSS | Reference | VED (J/mm3) | Sample | Microstructure | ||
|---|---|---|---|---|---|---|
| Austenite Percentage (vol%) | Ferrite Percentage (vol%) | Secondary Phases | ||||
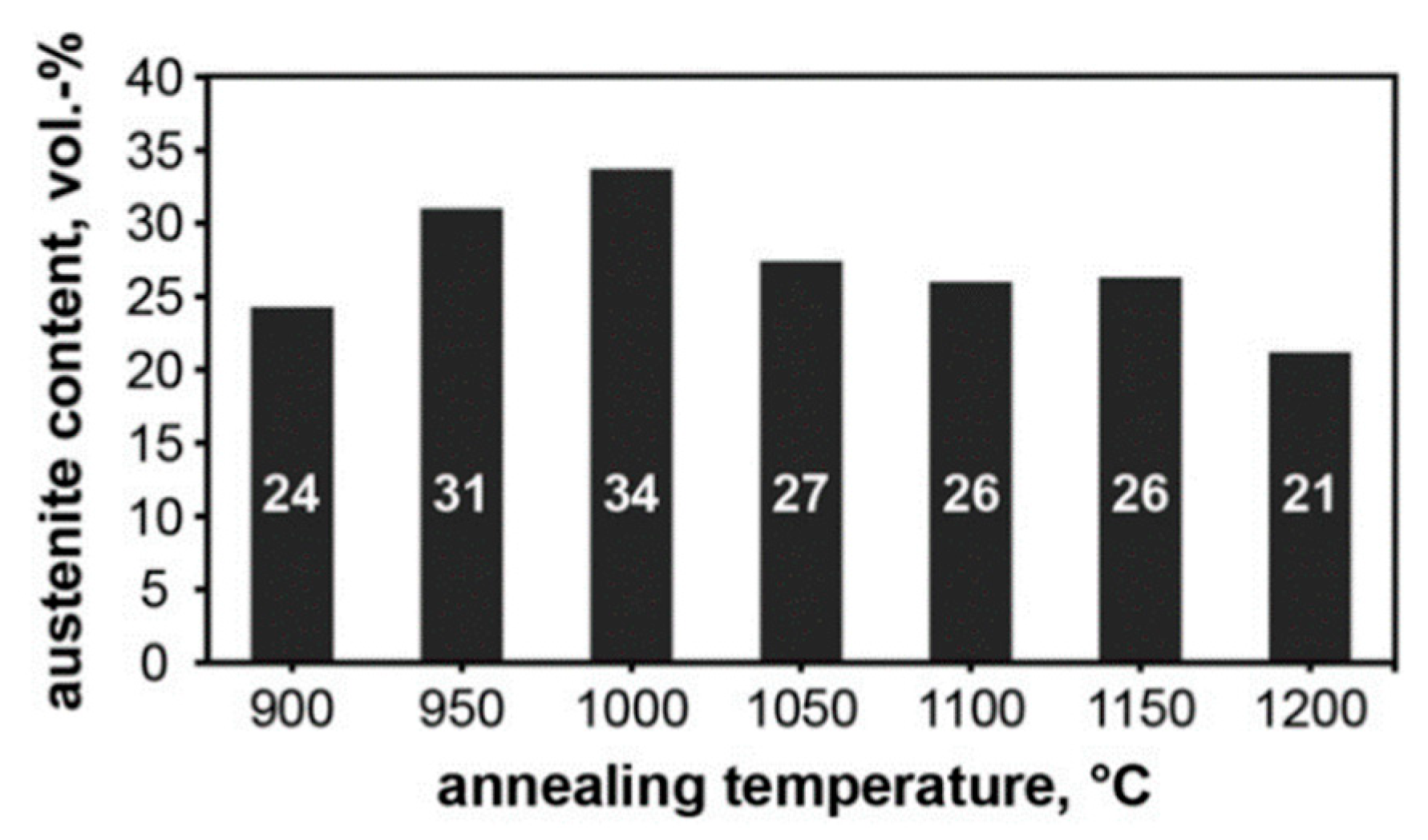
| 2205 | [47] | 59 | as built HT 900 °C HT 1000 °C HT 1200 °C | 1 24 34 21 | 99 76 66 79 | Nitrides, γ2 |
| 2205 | [42] | 156 | as built HT 1100 °C | 0.7 48.3 | 99.3 0.7 | − |
| 2507 | [52] | 127 | as built | 22 ÷ 25 | 75/78 | Nitrides |
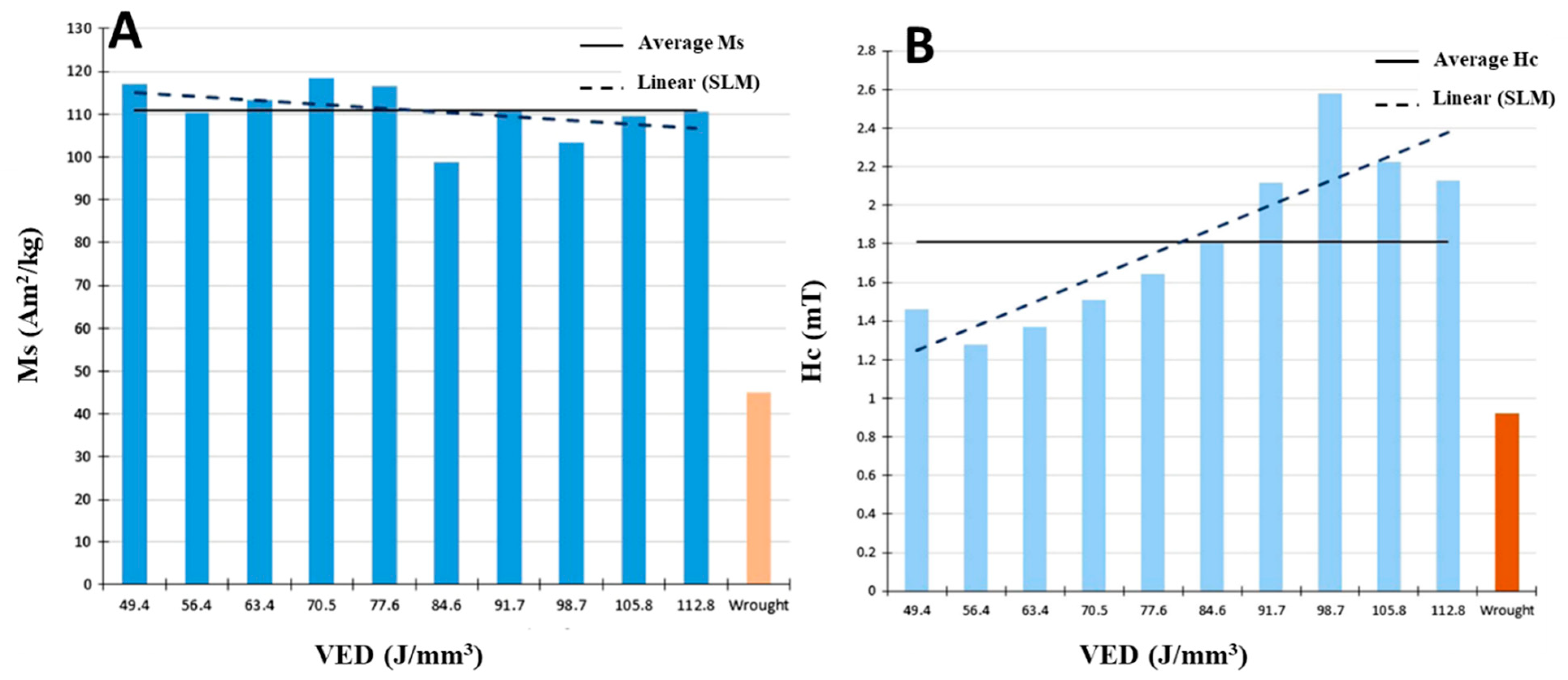
| 2507 | [41] | 91.7–141 | as built | 31.2 | 68.8 | Nitrides |
| 2507 | [26] | 84.6 112.8 | as built | 32.89 8.3 | 67.11 91.7 | − |
| 2507 | [60] | 133 | HT 1200 °C | 71 | 13 | σ |
| 2507 | [48] | 1333.33–133.33 | as built | 1.1–10.7 | 88.9–98.7 | − |
| 2205 | [43] | − | HT 950 °C/5 min HT 1000 °C/5 min HT 1050 °C/5 min HT 1100 °C/5 min | 40 43 43 43 | 60 57 57 57 | − |
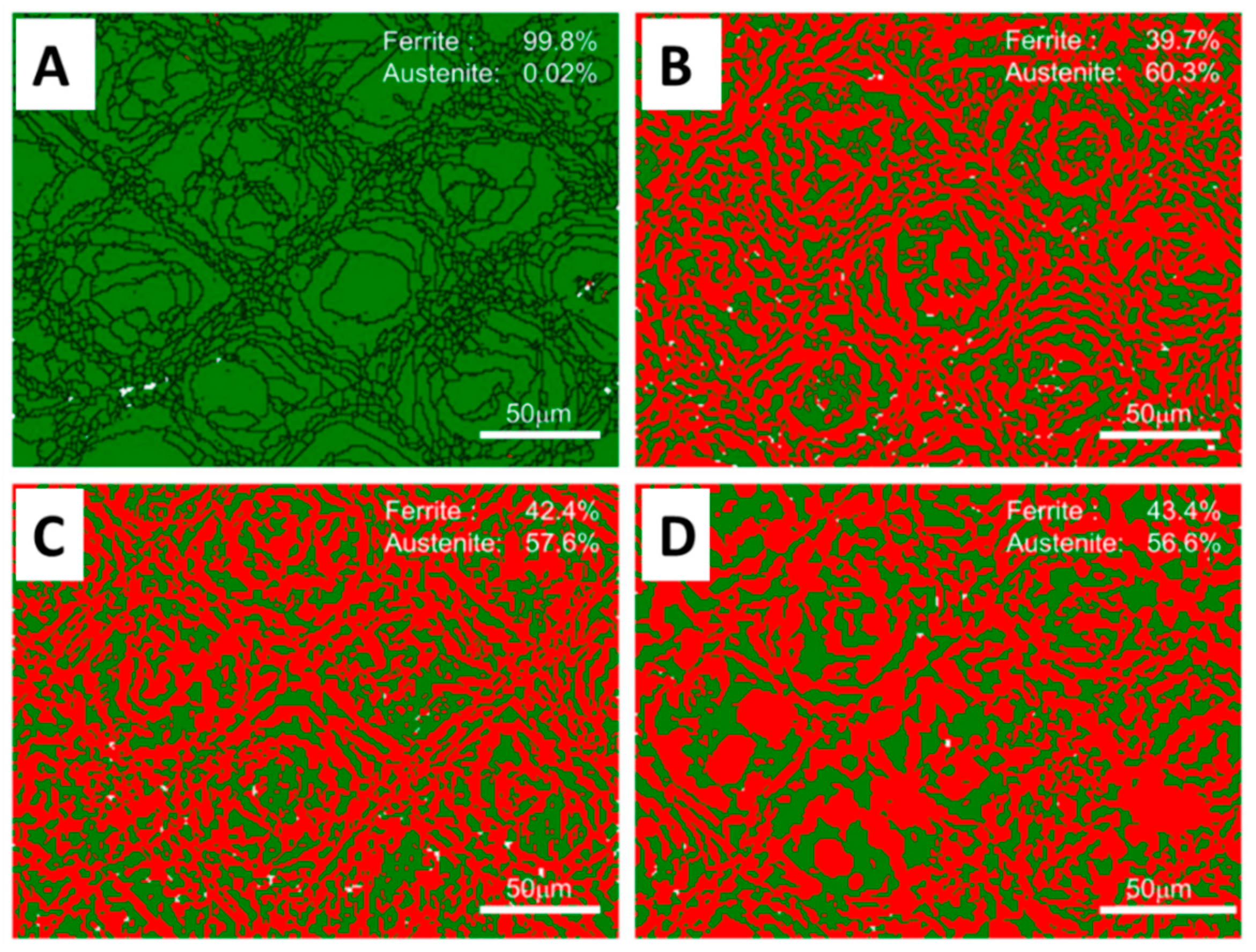
| Fe-22Cr-5Ni-0.26N | [44] | 79 | as built HT 1000 °C HT 1100 °C, HT 1200 °C | 0.02 60.3 57.6 56.6 | 99.8 39.7 42.4 43.4 | − |
| Sample | As Built | Annealed 950/5 | Annealed 1000/5 | Annealed 1050/5 | Annealed 1100/5 | Annealed 1000/60 | Annealed 1050/60 | Cold-Rolled 2205 DSS |
|---|---|---|---|---|---|---|---|---|
| Hardness (HV) | 336.6 ± 6.4 | 279.9 ± 10.3 | 279.8 ± 11.2 | 270.5 ± 8.8 | 255.2 ± 4.2 | 262.6 ± 9.6 | 265.0 ± 8.5 | 247.0 ± 1.5 |
| Sample | As-Built | Annealed 950°/5 | Annealed 1000°/5 | Annealed 1050°/5 | Annealed 1100°/5 | Annealed 1000°/60 | Annealed 1050°/60 | Ref. Alloy 2205 |
|---|---|---|---|---|---|---|---|---|
| Yield strength (MPa) | 950.0 ± 9.2 | 560.8 ± 3.3 | 549.0 ± 4.9 | 535.5 ± 9.2 | 524.0 ± 2.8 | 531.7 ± 2.5 | 517.1 ± 2.9 | 450–500 |
| Tensile strength (MPa) | 1071.3 ± 6.9 | 868.7 ± 2.3 | 848.0 ± 1.0 | 836.7 ± 1.1 | 813.0 ± 0.6 | 824.4 ± 1.8 | 812.1 ± 4.1 | 600–655 |
| Uniform elongation (%) | 7.0 ± 1.3 | 22.9 ± 0.4 | 23.9 ± 1.2 | 24.4 ± 1.1 | 24.1 ± 0.4 | 25.2 ± 0.9 | 25.3 ± 0.9 | >25 |
| Elongation to fracture (%) | 16.0 ± 1.1 | 39.7 ± 1.6 | 45.8 ± 2.6 | 42.1 ± 3.5 | 39.7 ± 3.4 | 43.4 ± 2.5 | 41.8 ± 1.5 | − |
| Sample | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| As-built | 826 ± 32 | 872 ± 33 | 11 ± 2 |
| Heat treated | 465 ± 3 | 622 ± 19 | 21.3 ± 1.4 |
| Wrought (ASTM A790) | 655 (448) | 882 (621–1103) | 25(25) |
| DSS | Reference | VED (J/mm3) | Sample | Hardness (HV) | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|---|---|---|---|
| 2205 | [47] | 59 | as built HT 900 °C HT 1000 °C HT 1200 °C | − | − | 940 720 ÷ 760 | 12 28 (HT 1000 °C) |
| 2205 | [42] | 156 | wrought as built HT 1100 °C | 267 419 258 | 655 826 465 | 882 872 622 | 25 11 21.3 |
| 2507 | [52] | 127 | as built | 450 | 1214 | 1321 | >45 |
| 2507 | [41] | 91.7–141 | as built | 408.4 (91.7 J/mm3) | − | − | − |
| 2507 | [26] | 84.6–112.8 | as built | 447.8 (VED = 49.4 J/mm3) ÷ decreasing | − | − | − |
| 2507 | [60] | 133 | HT 1200 °C | 400 | 686 | 920 | 1.8 |
| 2205 | [43] | − | ref. 2205 (from DSS producer) as built HT 950 °C/5 min HT 1000 °C/5 min HT 1050 °C/5 min HT 1100 °C/5 min | − | 450 ÷ 500 950 560.8 549 535.5 524 | 600 ÷ 655 1071.3 868.7 848 836.7 813 | >25 7 22.9 23.9 24.4 24.1 |
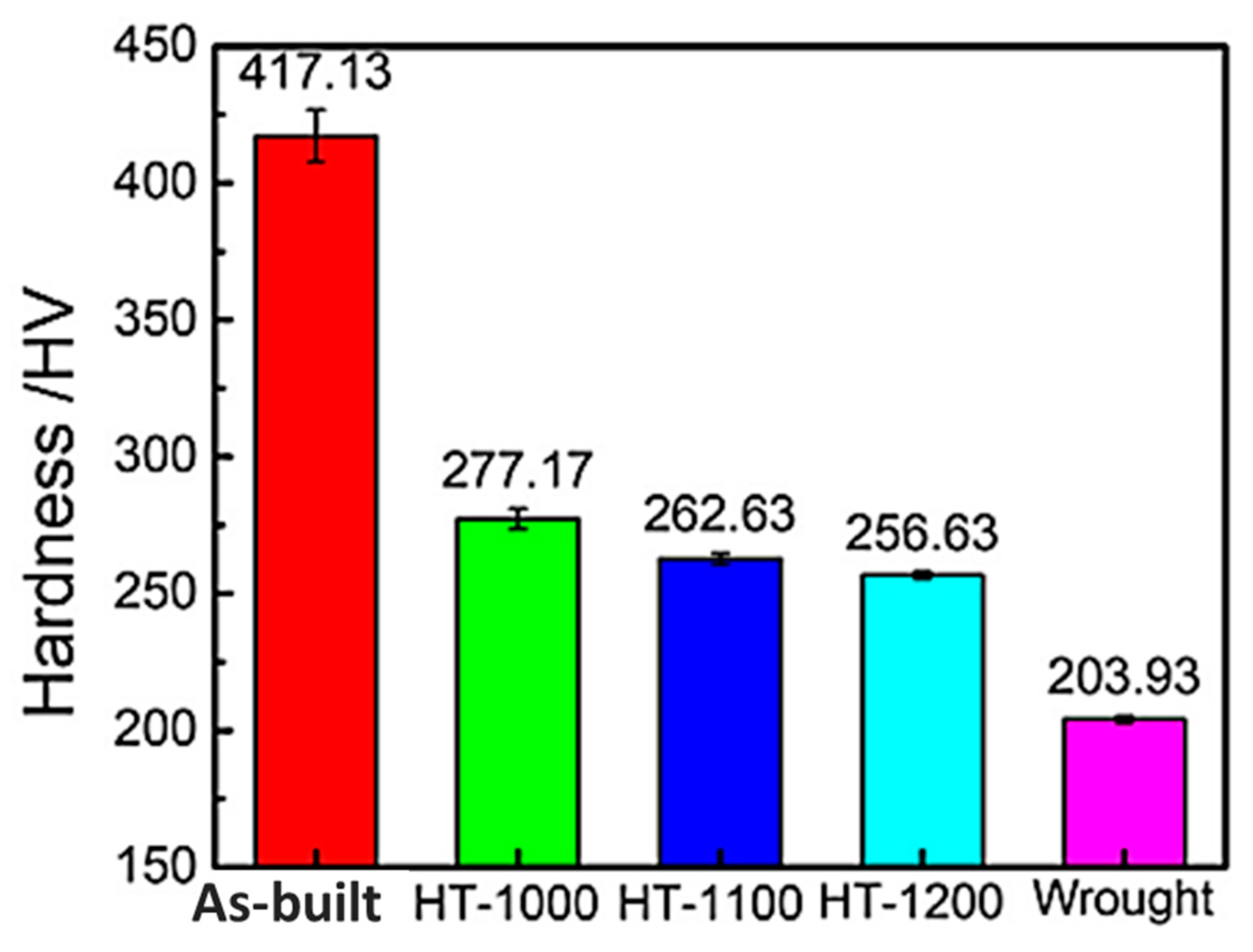
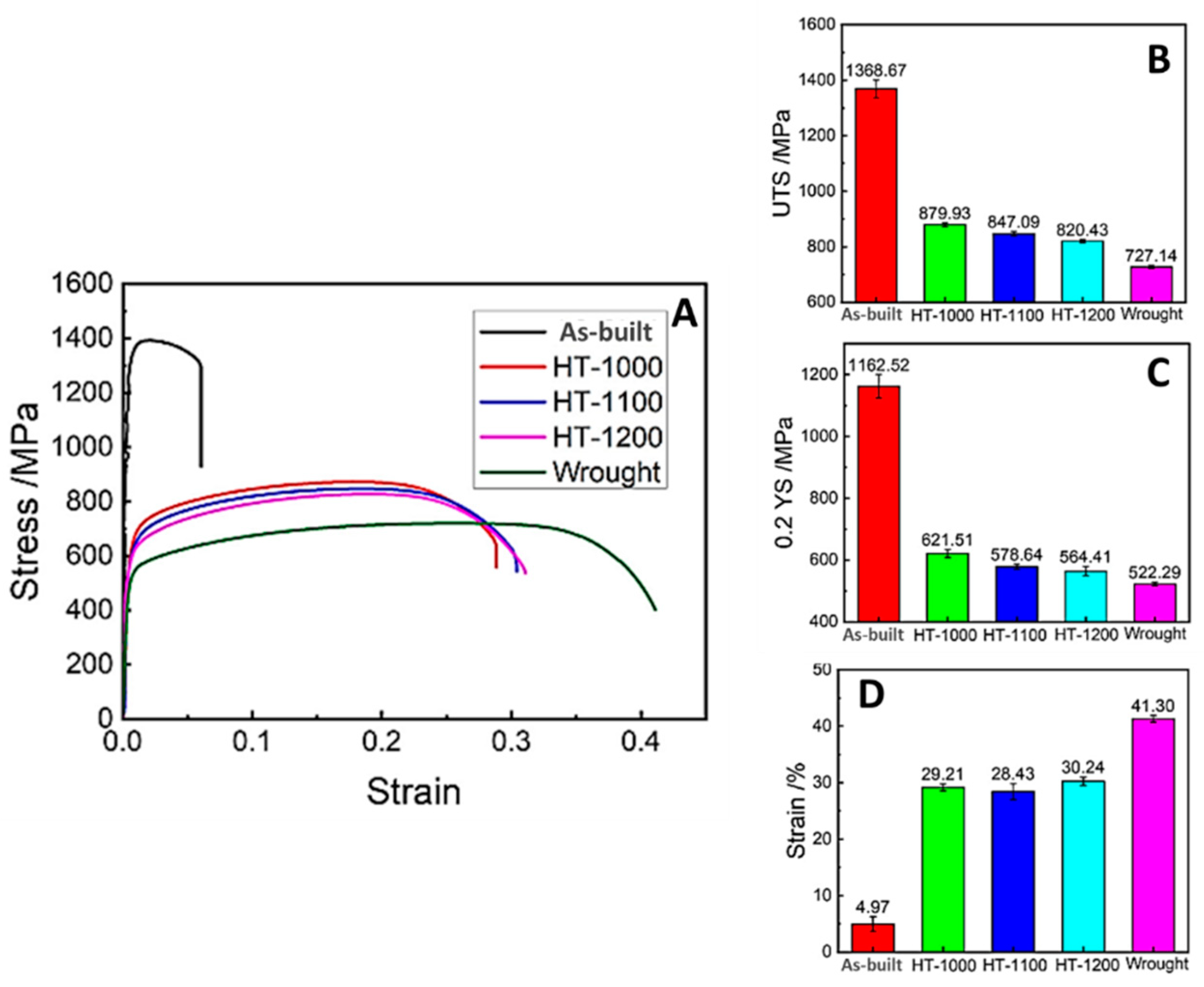
| Fe-22Cr-5Ni-0.26N | [44] | 79 | wrought as built HT 1000 °C HT 1100 °C HT 1200 °C | 203.93 417.13 277.17 262.63 256.63 | 522.29 1162.52 621.51 578.64 564.41 | 727.14 1368.67 879.93 847.09 820.43 | 41.30 4.97 29.21 28.43 30.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, M.L.; Santoni, A.; Santecchia, E.; Spigarelli, S.; Fiori, F.; Mengucci, P.; Cabibbo, M. The Potential of Duplex Stainless Steel Processed by Laser Powder Bed Fusion for Biomedical Applications: A Review. Metals 2023, 13, 949. https://doi.org/10.3390/met13050949
Gatto ML, Santoni A, Santecchia E, Spigarelli S, Fiori F, Mengucci P, Cabibbo M. The Potential of Duplex Stainless Steel Processed by Laser Powder Bed Fusion for Biomedical Applications: A Review. Metals. 2023; 13(5):949. https://doi.org/10.3390/met13050949
Chicago/Turabian StyleGatto, Maria Laura, Alberto Santoni, Eleonora Santecchia, Stefano Spigarelli, Fabrizio Fiori, Paolo Mengucci, and Marcello Cabibbo. 2023. "The Potential of Duplex Stainless Steel Processed by Laser Powder Bed Fusion for Biomedical Applications: A Review" Metals 13, no. 5: 949. https://doi.org/10.3390/met13050949
APA StyleGatto, M. L., Santoni, A., Santecchia, E., Spigarelli, S., Fiori, F., Mengucci, P., & Cabibbo, M. (2023). The Potential of Duplex Stainless Steel Processed by Laser Powder Bed Fusion for Biomedical Applications: A Review. Metals, 13(5), 949. https://doi.org/10.3390/met13050949










