Study on Growth Mechanism and Characteristics of Zirconium Alloy Micro-Arc Oxidation Film
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
3.1. Growth of MAO Coating
3.2. Surface Morphology of MAO Coating
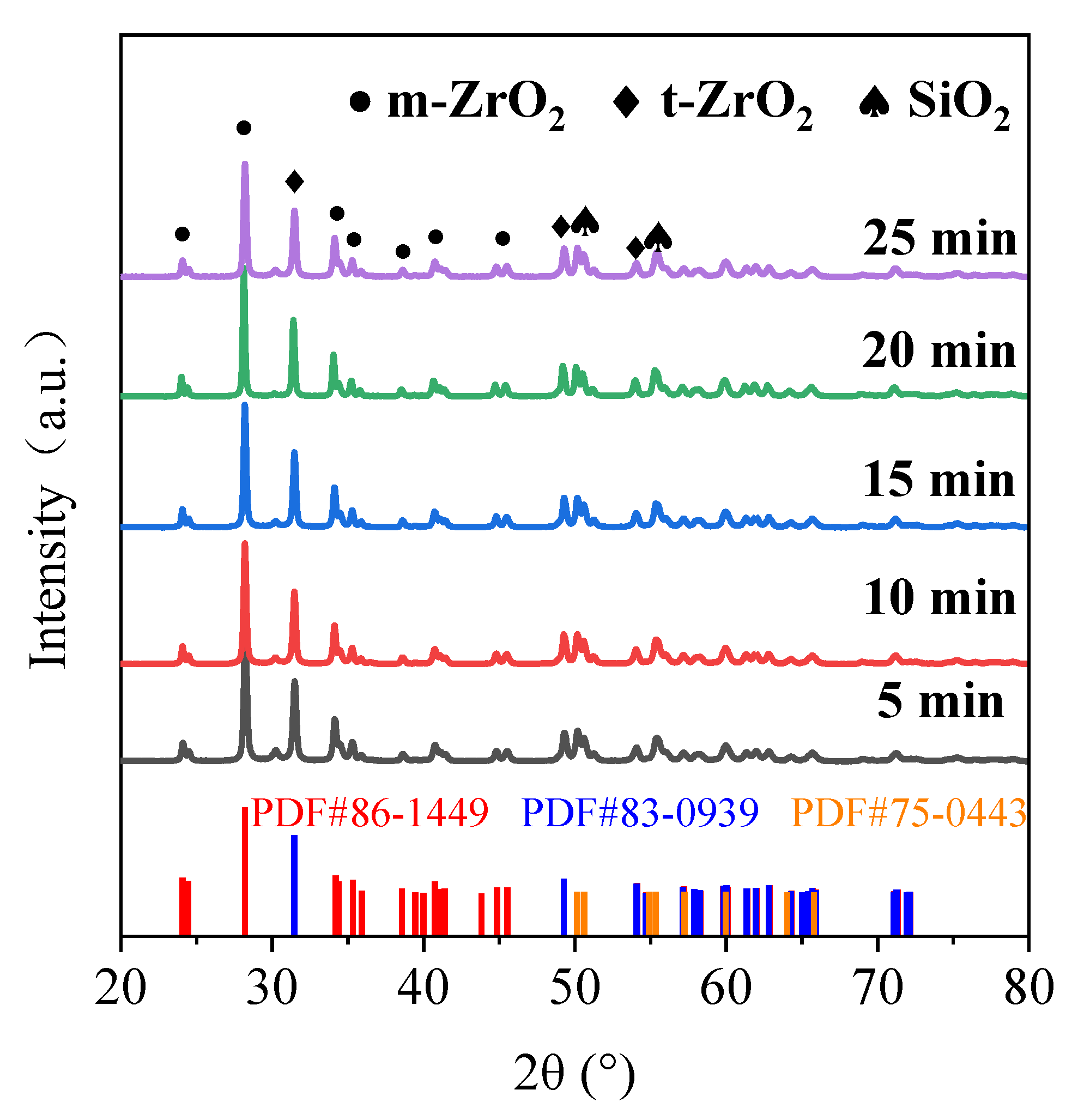
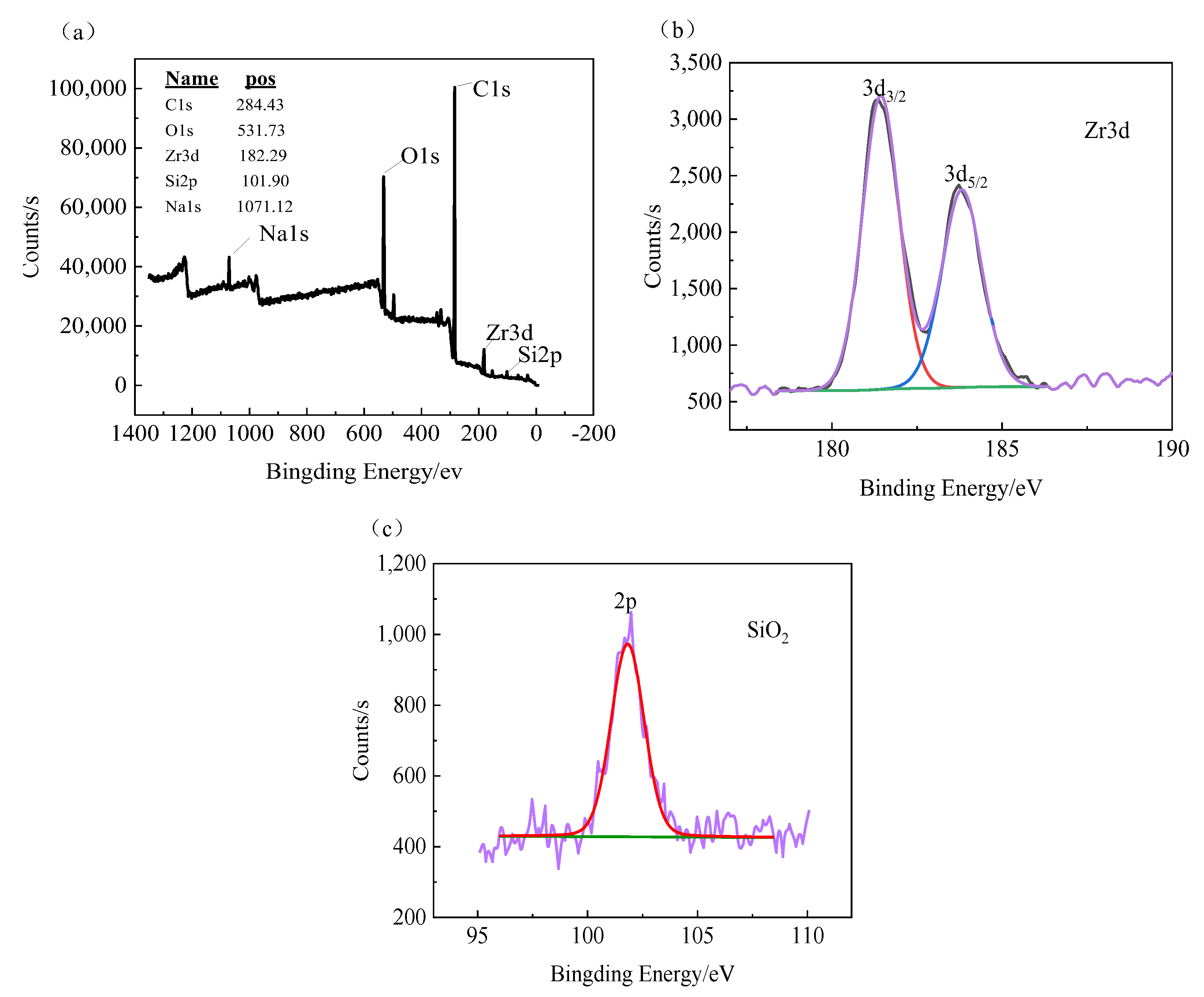
3.3. Composition of MAO Coating
3.4. Properties of MAO Coating
4. Conclusions
- (1)
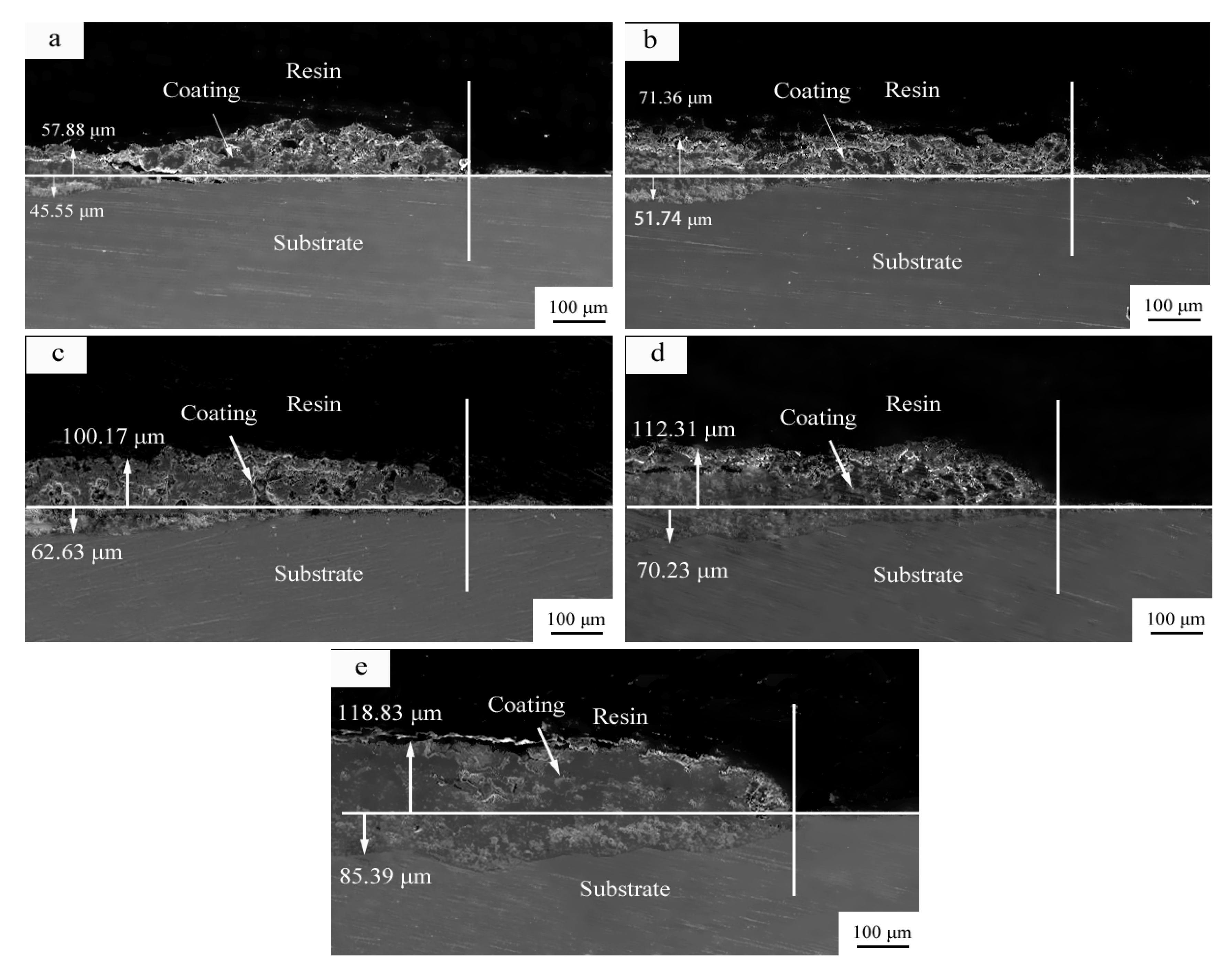
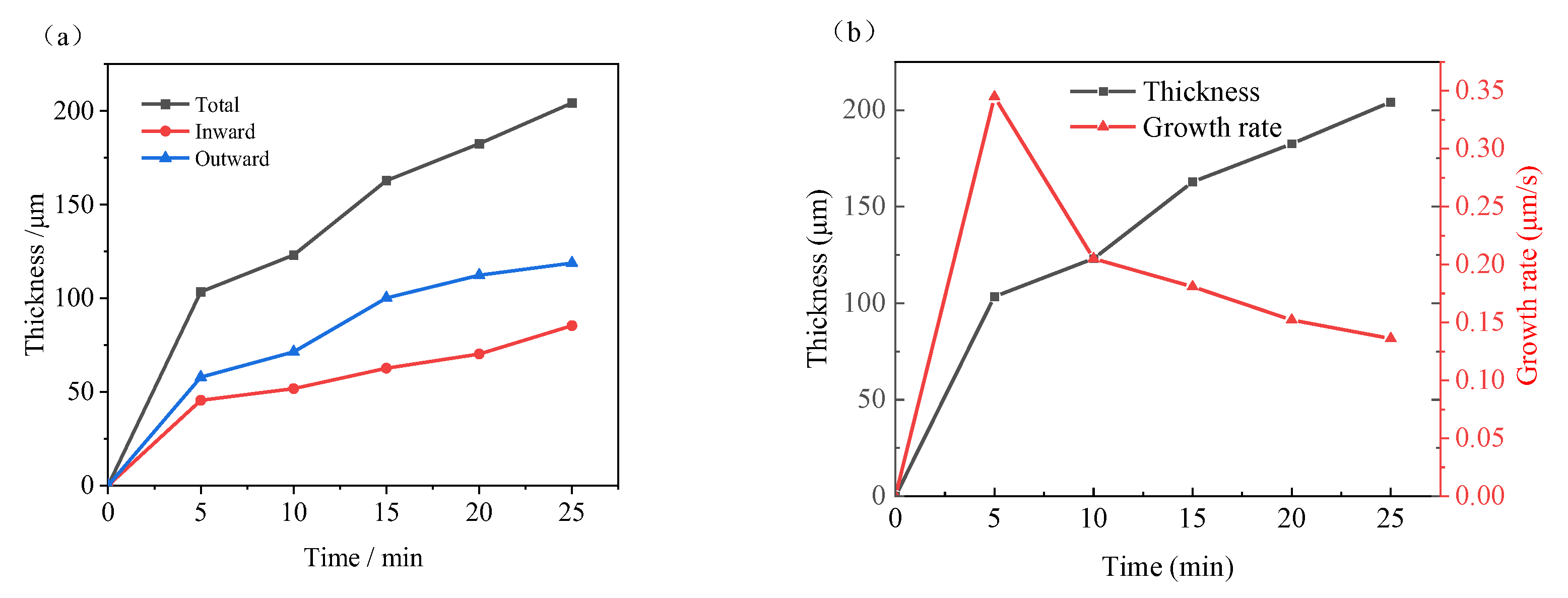
- The MAO film exhibits bidirectional growth, both inward and outward. The fastest growth occurs in the first 5 min, with a thickness increase of 103.43 μm. From 10 to 15 min, the growth rate of the film slows down, with a thickness increase of only 39.76 μm. Overall, the film grows outward by 118.83 μm and inward by 85.39 μm during 0–25 min, with an average growth rate of 0.079 μm/s outward and 0.057 μm/s inward. The outward growth rate is always higher than the inward growth rate.
- (2)
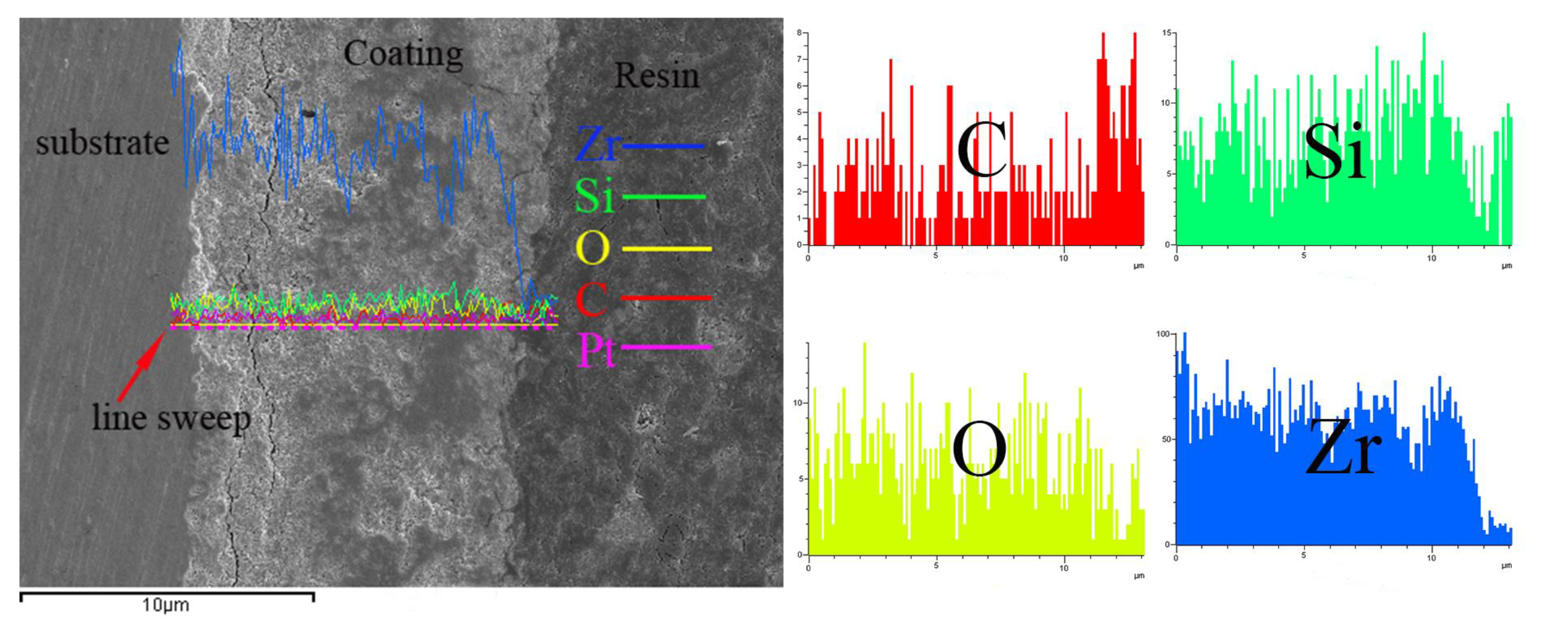
- The MAO film on the zirconium alloy mainly consists of a monoclinic phase (m-ZrO2), a tetragonal phase (t-ZrO2), and a small amount of SiO2, with a higher content of monoclinic phase. The binding energies of Zr are 185.67 eV (3d3/2) and 181.36 eV (3d5/2). The film is mainly composed of Zr, O, and Si, with Zr evenly distributed in the film and a lower Si content closer to the substrate.
- (3)
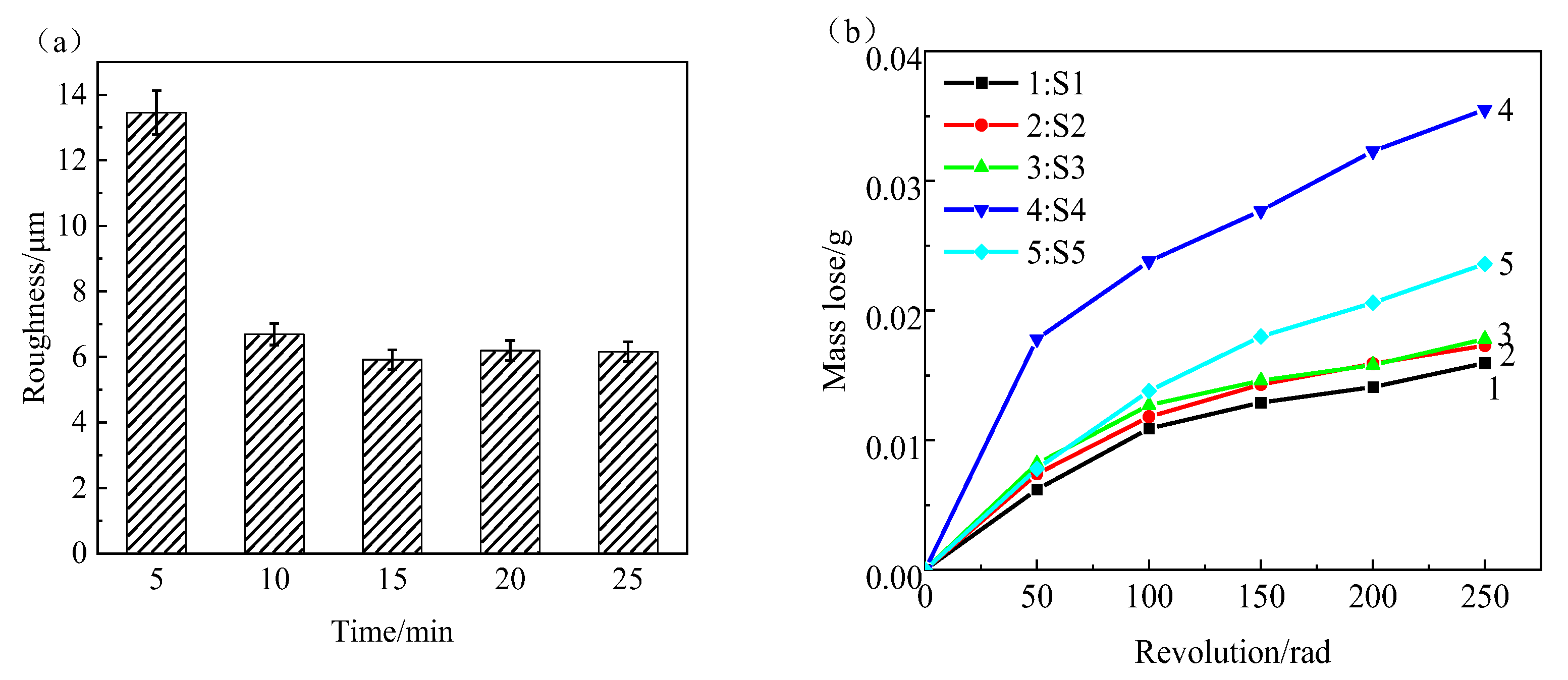
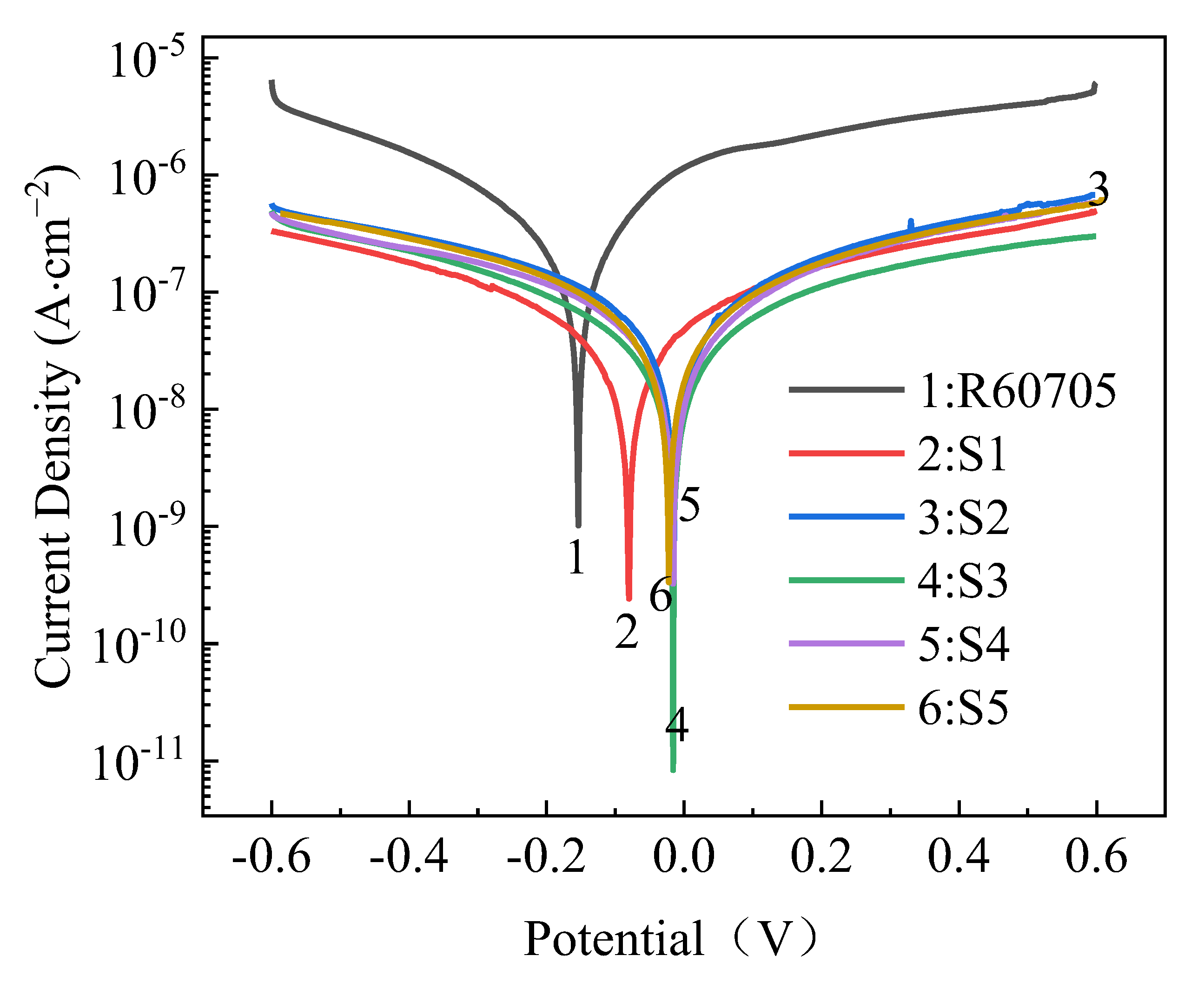
- The roughness of the film initially decreases and then increases with increasing oxidation time. The lowest roughness is observed at 15 min, with a Ra value of 5.917 μm. The increase in roughness after 15 min is not significant, and the effect of oxidation time on roughness diminishes. The loss of film thickness due to wear is similar at 5, 10, and 15 min of oxidation time, indicating similar wear resistance. At 20 min, the film shows the highest loss of thickness and the poorest wear resistance, while at 25 min, the loss of film thickness decreases and wear resistance improves. The corrosion resistance of the MAO film shows a trend of initially increasing and then decreasing with oxidation time, with the minimum corrosion current density observed at 15 min, which is the optimal time for obtaining the best corrosion resistance. Based on comprehensive analysis, the optimal oxidation time for preparing the zirconium alloy micro-arc oxidation film is 15 min.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Mu, L.; Zhuang, M.; Li, M.; Ma, Z. Effect of Pulse Frequency on Phase Composition and Properties of Amorphous Zirconium Alloy Micro-arc Oxidation Coating. J. Phys. Conf. Ser. 2022, 2174, 012043. [Google Scholar] [CrossRef]
- Durdu, S.; Aktug, S.L.; Aktas, S.; Yalcin, E.; Cavusoglu, K.; Altinkok, A.; Usta, M. Characterization and in vitro properties of anti-bacterial Ag-based bioceramic coatings formed on zirconium by micro-arc oxidation and thermal evaporation. Surf. Coat. Technol. 2017, 331, 107–115. [Google Scholar] [CrossRef]
- Sun, L.; Ma, Y.; An, L.; Wang, X.; Gao, W. Comparative Study on Anti-corrosion Electrochemical Response of Micro-arc Oxidation Coatings with Different Thicknesses under High and Low Voltage. Surf. Technol. 2021, 50, 366–374. [Google Scholar]
- Shi, L.; Qiu, C.; Liu, H. Review on Hydrogen Absorption Cracking Behavior of Zirconium Alloy Surface of Nuclear Fuel Cladding. Mech. Eng. 2022, 3, 47–48+51. [Google Scholar]
- Wei, K.; Xue, W.; Qu, Y. Advance in Microarc Oxidation Surface Treatment on Zr. Surf. Technol. 2019, 48, 11–23. [Google Scholar]
- Nahum, E.Z.; Lugovskoy, S.; Lugovskoy, A.; Kazanski, B.; Sobolev, A. The study of hydroxyapatite growth kinetics on CP-Ti and Ti65Zr treated by Plasma electrolytic oxidation process. J. Mater. Res. Technol. 2023, 24, 2169–2186. [Google Scholar] [CrossRef]
- Meiyi, Y.B.L.M.Y.; Jianian, Z.B.S. In Situ Impedance Characteristics of Zirconium Alloy Corrosion In High Temperature And Pressure Water Environment. Acta Metall. Sin. 2010, 46, 946–950. [Google Scholar]
- Zhang, Z.; He, F.; Huang, C.; Song, Z.; Yang, J.; Wang, X. Effect of Fe3+ and F on black micro-arc oxidation ceramic coating of magnesium alloy. Int. J. Appl. Ceram. Technol. 2022, 19, 2203–2212. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Du, J.; Zhang, J.; Ji, S. Oxide Coatings on Zirconium Alloy Deposited by AC Microarc Oxidation. Rare Met. Mater. Eng. 2005, 34, 1330–1333. [Google Scholar]
- Jia, Y.; Lin, X.; Zou, X. Research & Development History, Status and Prospect of Zirconium Alloys. Mater. China 2022, 41, 354–370. [Google Scholar]
- Hu, D.; Tao, L.; Gao, G. Effect of voltage on properties of micro-arc oxidation coating formed on Zr-4 alloy from a Na2SiO3–(NaPO3)6–K2ZrF6 electrolyte. Electroplat. Finish. 2021, 40, 1352–1358. [Google Scholar]
- Lin, Q.; Luan, L.; Cheng, S. Surface oxidation modification of R60705 Zr alloy. Heat Treat. Met. 2013, 38, 104–107. [Google Scholar]
- Wang, W.; Cui, W.; Xiao, Z.; Qin, G. The improved corrosion and wear properties of Ti-Zr based alloys with oxide coating in simulated seawater environment. Surf. Coat. Technol. 2022, 439, 128415. [Google Scholar] [CrossRef]
- Wang, S.; Guo, F.; Liu, L. Effect of Process Parameters on Thickness and Morphology of Micro-Arc Oxidation Ceramic Coating of Zirconium Alloy. Rare Met. Mater. Eng. 2008, 1466–1470. [Google Scholar]
- Yan, Y.; Han, Y.; Li, D.; Huang, J.; Lian, Q. Effect of NaAlO2 concentrations on microstructure and corrosion resistance of Al2O3/ZrO2 coatings formed on zirconium by micro-arc oxidation. Appl. Surf. Sci. 2010, 256, 6359–6366. [Google Scholar] [CrossRef]
- Xue, W.; Deng, Z.; Lai, Y.; Zhang, T. Growth Regularity of Ceramic Film in Microarc Oxidation on Cast Magnesium Alloy. Trans. Met. Heat Treat Ment. 1998, 3, 43–46. [Google Scholar]
- Wang, P.; Li, J.; Guo, Y.; Yang, Z. Growth process and corrosion resistance of ceramic coatings of micro-arc oxidation on Mg-Gd-Y magnesium alloys. J. Rare Earths 2010, 28, 798–802. [Google Scholar] [CrossRef]
- Dong, H.; Ma, Y.; Guo, H.; Zhang, Y.; Hao, Y. Multi-dimensional investigation on coating growth of micro arc oxidation on AZ91D magnesium alloys. Chin. J. Nonferrous Met. 2015, 25, 690–696. [Google Scholar]
- Yang, W.; Jiang, B.L.; Shi, H.Y. Formation and growth mechanism of microarc oxidation coating on LY12 aluminium alloy. Chin. J. Nonferrous Met. 2010, 20, 1949–1954. [Google Scholar]
- Wang, Z.X.; Zhang, Z.Y.; Lv, W.G.; Gan, J.J.; Lu, S. Micro-arc oxidation mechanism of ZK60 magnesium alloy prepared under two-step voltage-increasing mode. J. Jiangsu Univ. Sci. Technol. 2019, 33, 14–20. [Google Scholar]
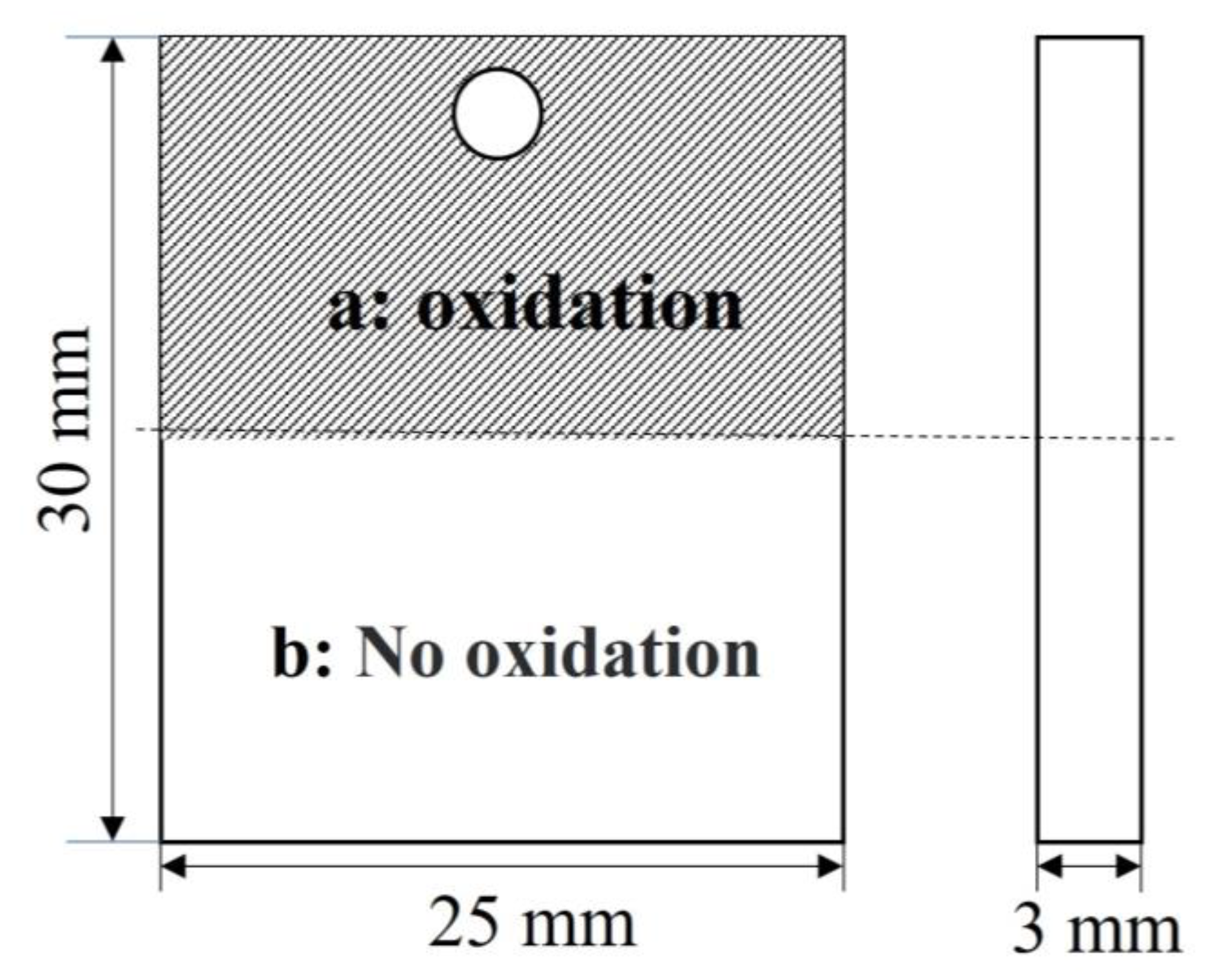


| Specimen Codes | Oxidation Time (min) | Forward/Negative Voltage (V) | Frequency (Hz) | Duty Cycle (%) |
|---|---|---|---|---|
| S1 | 5 | 350/140 | 200 | 50 |
| S2 | 10 | |||
| S3 | 15 | |||
| S4 | 20 | |||
| S5 | 25 |
| Element | Zr | O | Si | C | Pt |
|---|---|---|---|---|---|
| wt% | 48.73 | 17.14 | 14.32 | 10.16 | 9.65 |
| at% | 21.71 | 41.28 | 9.11 | 19.57 | 8.33 |
| Specimen Codes | Ecoor (V) | Jcorr (A·cm−2) | βa (mV/dec) | βc (mV/dec) | Rp (kΩ·cm2) |
|---|---|---|---|---|---|
| R60705 | −0.169 | 2.237 × 10−7 | 485 | 432 | 444 |
| S1 | −0.072 | 9.659 × 10−8 | 527 | 494 | 1147 |
| S2 | −0.027 | 3.6 × 10−8 | 726 | 462 | 3409 |
| S3 | −0.016 | 8.876 × 10−9 | 481 | 422 | 11,010 |
| S4 | −0.013 | 1.649 × 10−8 | 488 | 538 | 6746 |
| S5 | −0.024 | 4.536 × 10−8 | 509 | 513 | 2448 |
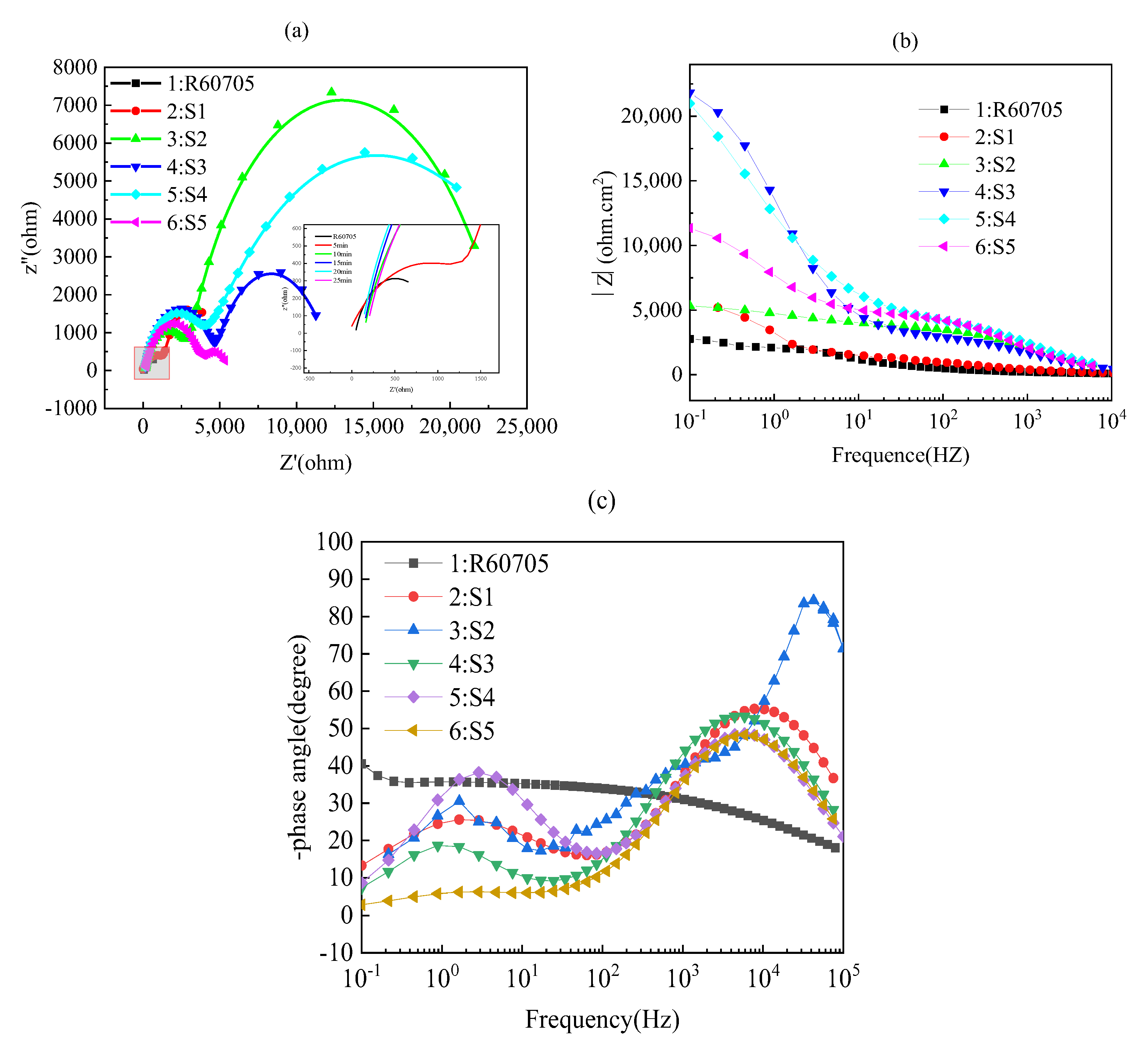
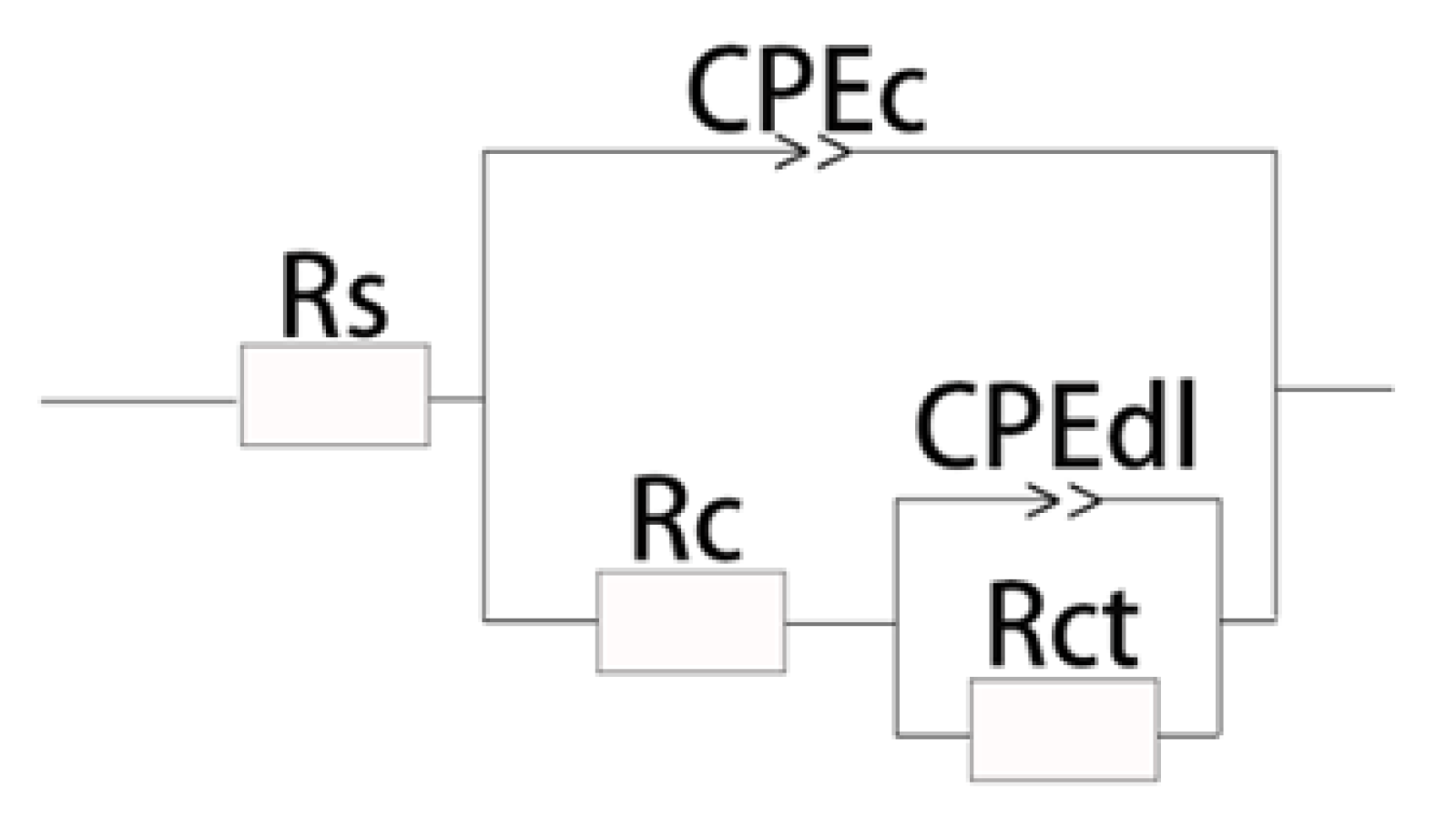
| Specimens Code | Rs (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | R (Ω·cm2) | Error (%) |
|---|---|---|---|---|---|
| R60705 | 103.2 | − | 2976 | 3.08 × 103 | 0.66809 |
| 5 | 109.6 | 3941 | 1229 | 5.28 × 103 | 2.4234 |
| 10 | 132.3 | 1539 | 3648 | 5.32 × 103 | 3.4268 |
| 15 | 150.22 | 4645 | 17,018 | 2.18 × 104 | 1.1838 |
| 20 | 153.1 | 2900 | 17,839 | 2.09 × 104 | 4.1792 |
| 25 | 165 | 3578 | 7157 | 1.09 × 104 | 1.8697 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Lv, K.; Du, Z.; Chen, W.; Ji, P.; Wang, W.; Pang, Z. Study on Growth Mechanism and Characteristics of Zirconium Alloy Micro-Arc Oxidation Film. Metals 2023, 13, 935. https://doi.org/10.3390/met13050935
Wang M, Lv K, Du Z, Chen W, Ji P, Wang W, Pang Z. Study on Growth Mechanism and Characteristics of Zirconium Alloy Micro-Arc Oxidation Film. Metals. 2023; 13(5):935. https://doi.org/10.3390/met13050935
Chicago/Turabian StyleWang, Mingli, Kai Lv, Zhaoxin Du, Weidong Chen, Pengfei Ji, Wei Wang, and Zhi Pang. 2023. "Study on Growth Mechanism and Characteristics of Zirconium Alloy Micro-Arc Oxidation Film" Metals 13, no. 5: 935. https://doi.org/10.3390/met13050935
APA StyleWang, M., Lv, K., Du, Z., Chen, W., Ji, P., Wang, W., & Pang, Z. (2023). Study on Growth Mechanism and Characteristics of Zirconium Alloy Micro-Arc Oxidation Film. Metals, 13(5), 935. https://doi.org/10.3390/met13050935





