Effect of CaO/Al2O3 Ratio on Physical Properties of Lime-Alumina-Based Mould Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
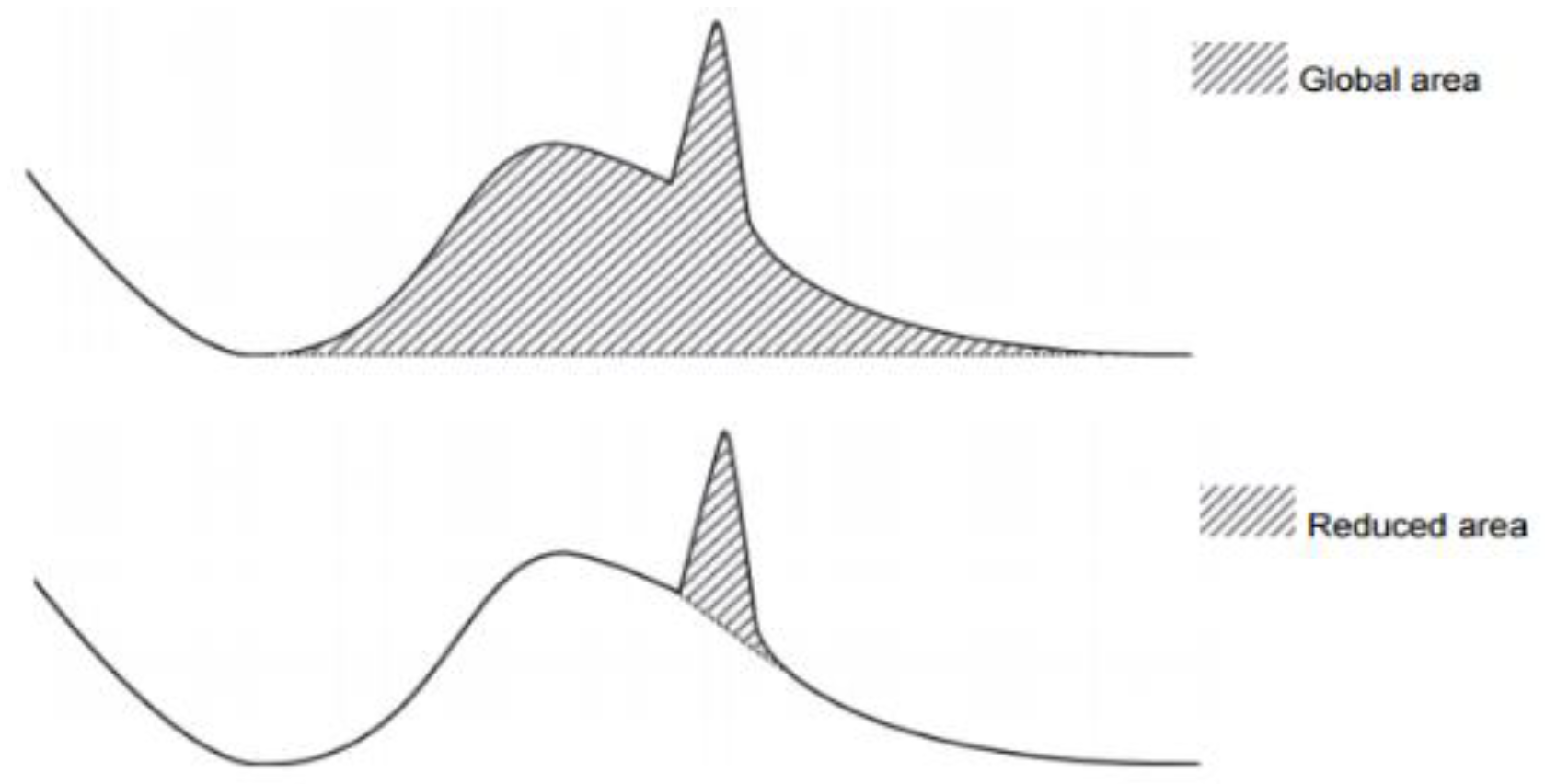
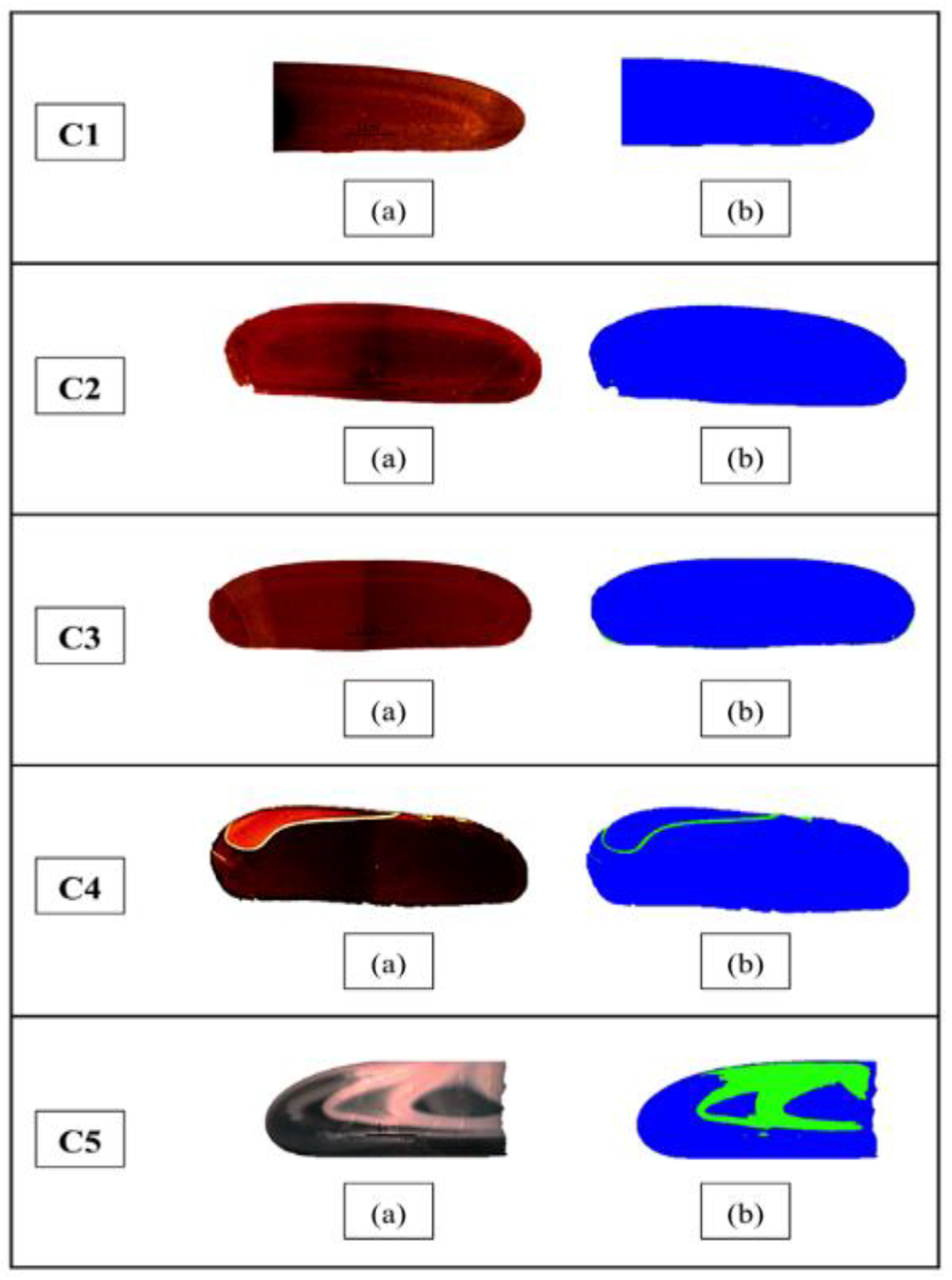
2.2. Methods for Measuring Crystallinity
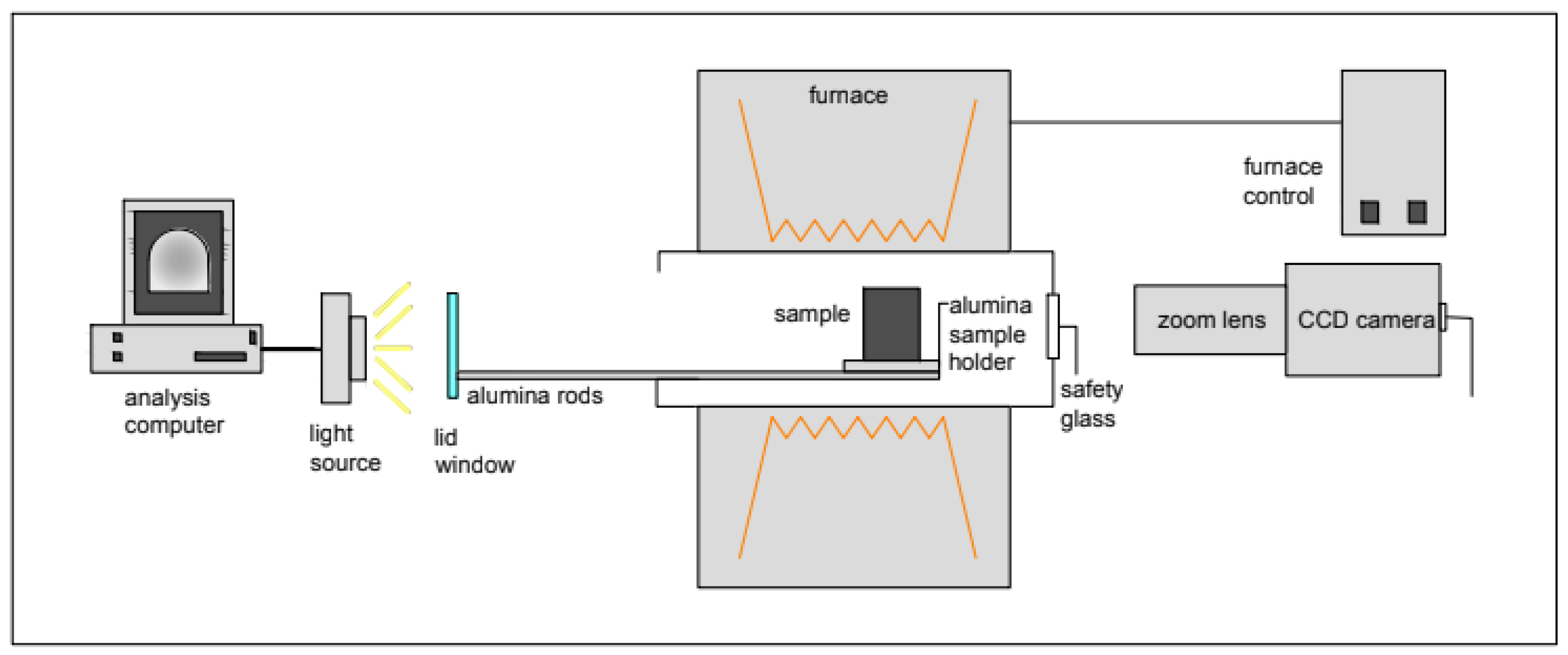
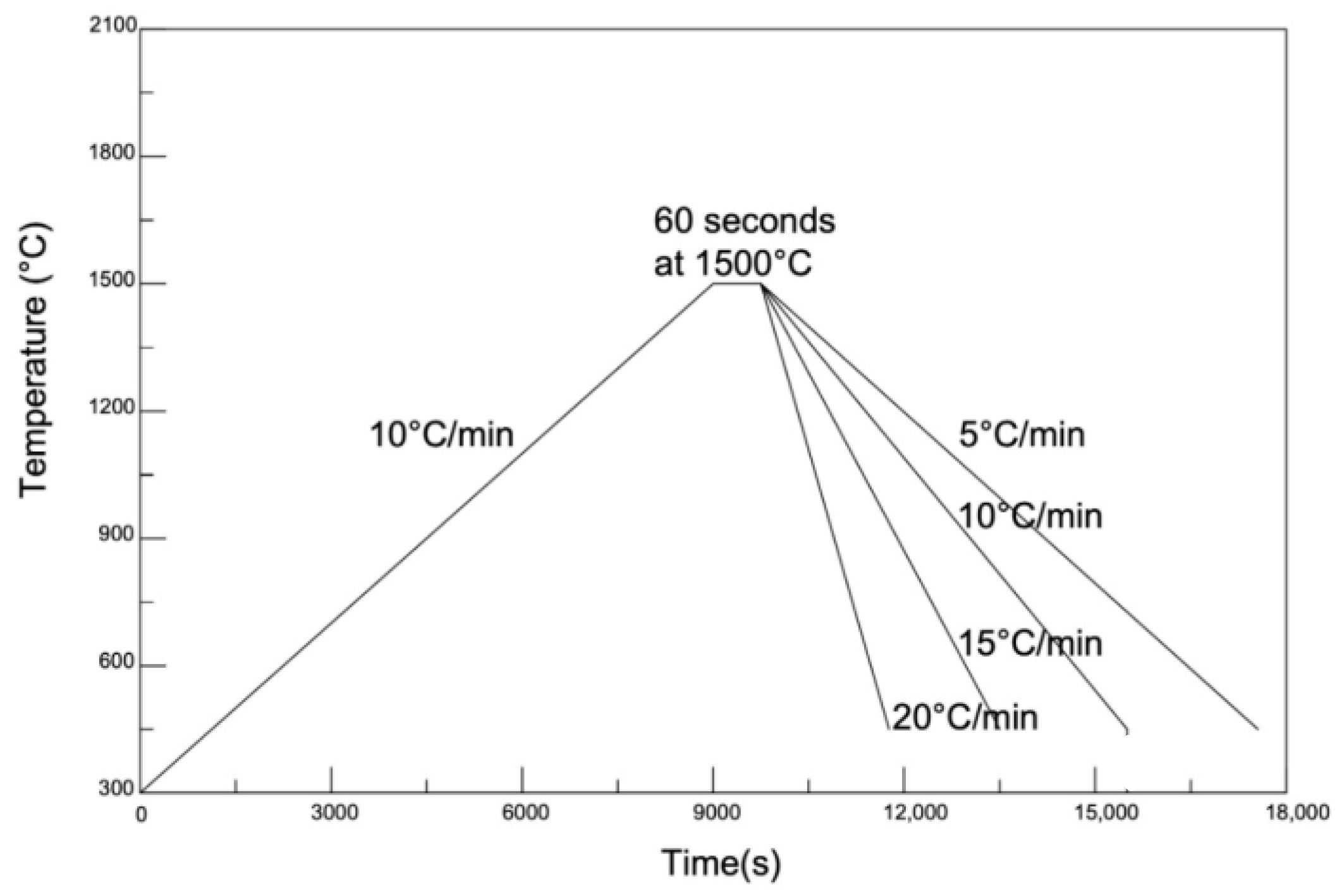
2.3. Methods for Examination of Melting and Crystallisation Behaviour
2.4. Methods for Measuring Viscosity
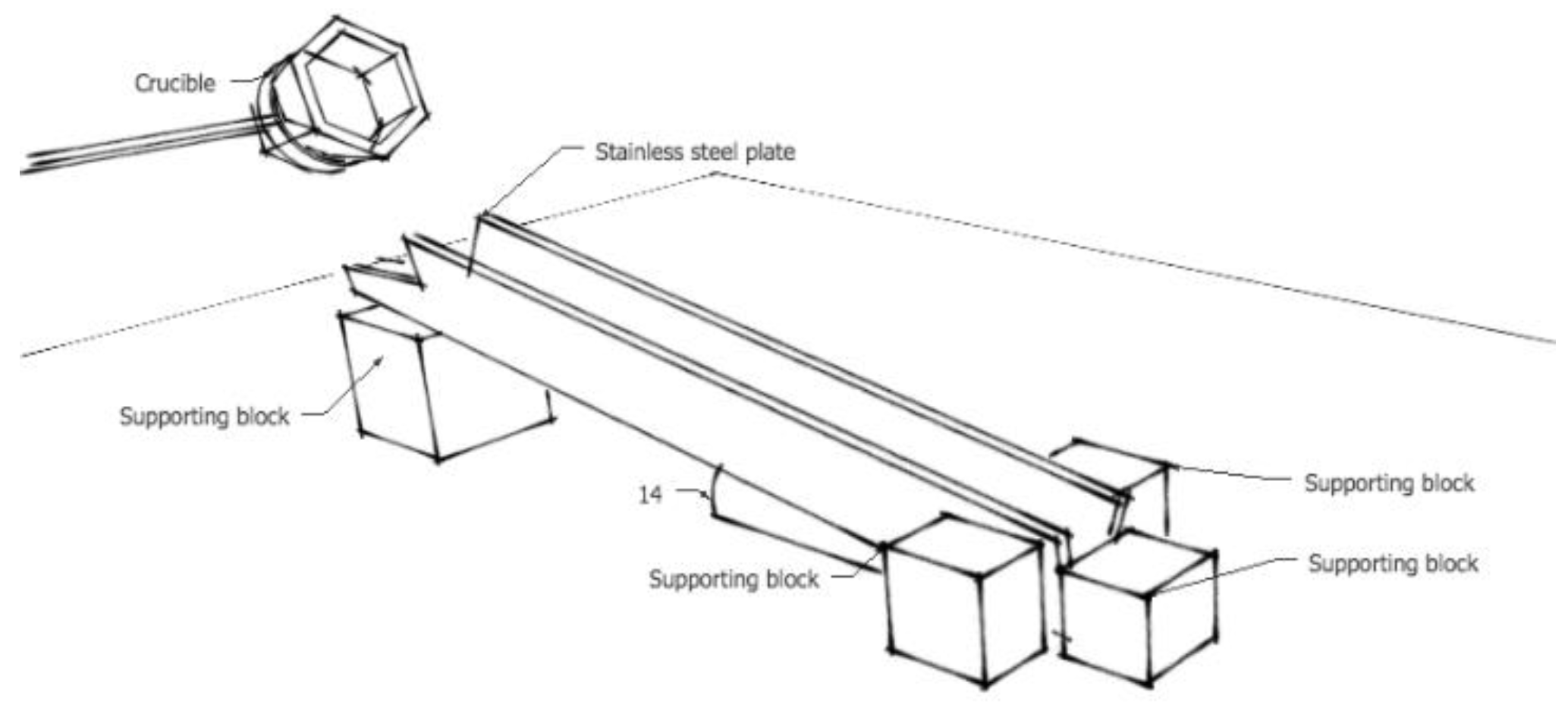
2.4.1. Inclined Plane Test (IPT)
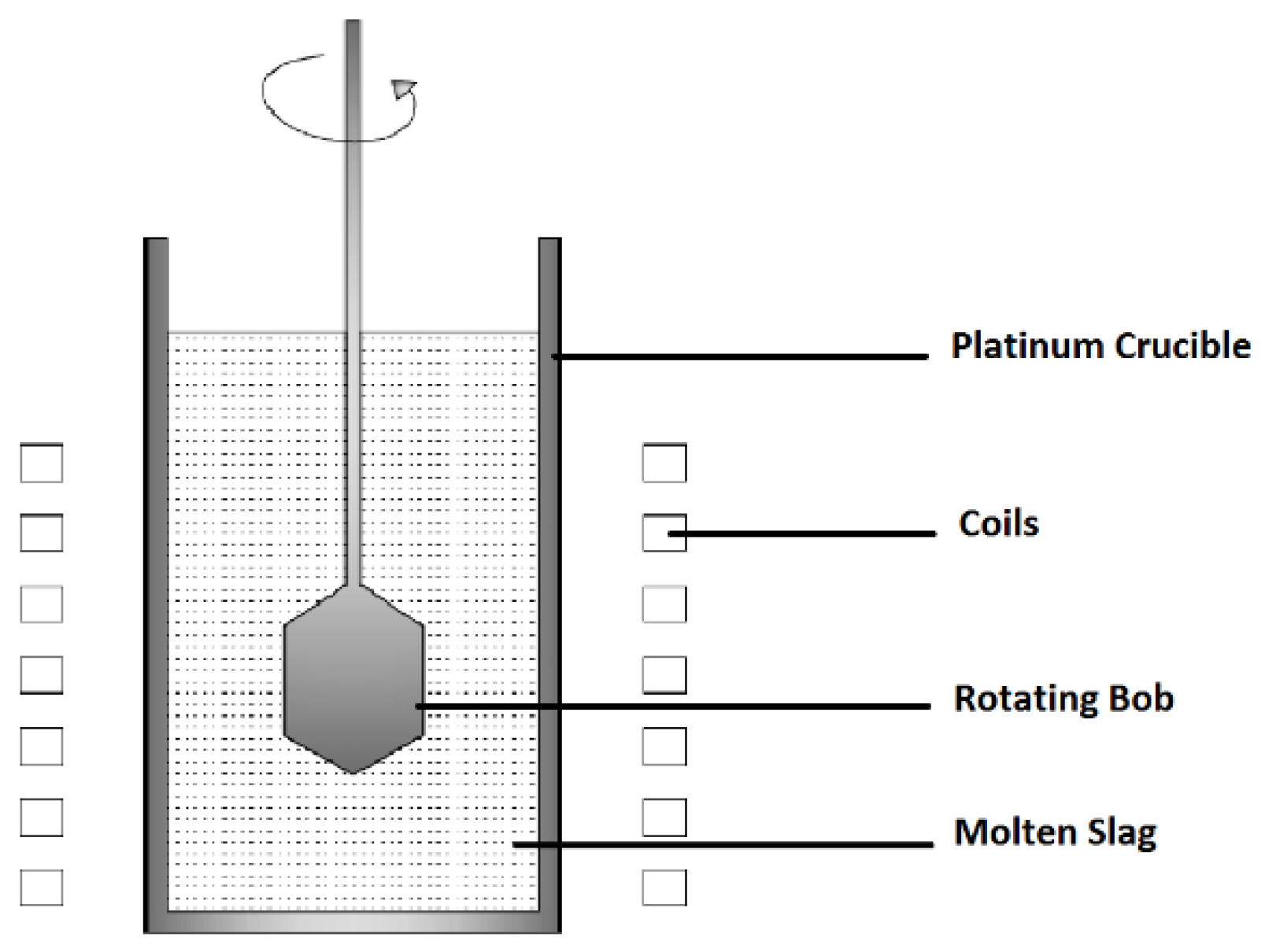
2.4.2. Rotational Viscometry
2.4.3. FactSage
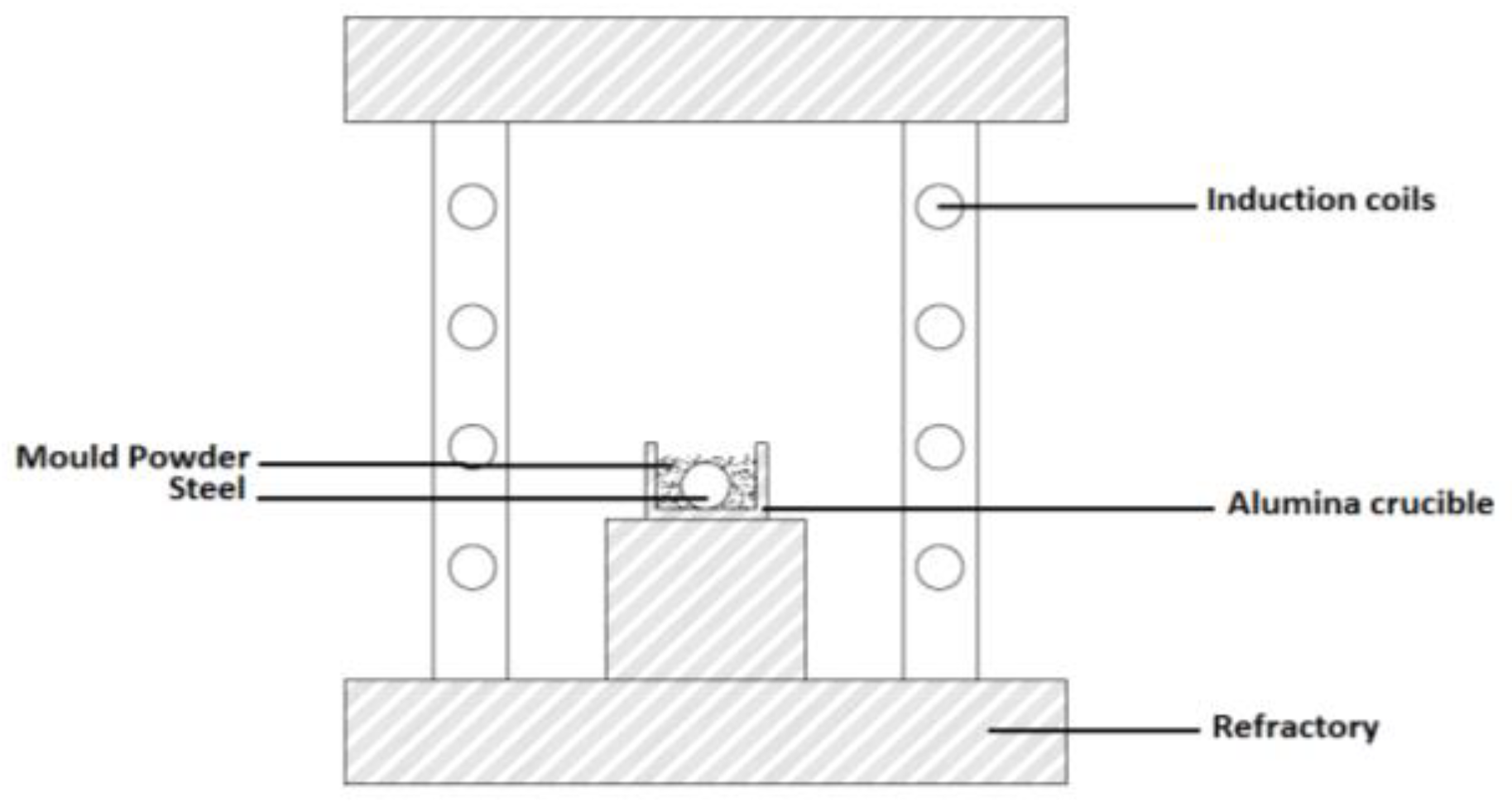
2.5. Examination of Steel-Slag Interaction
3. Results and Discussion
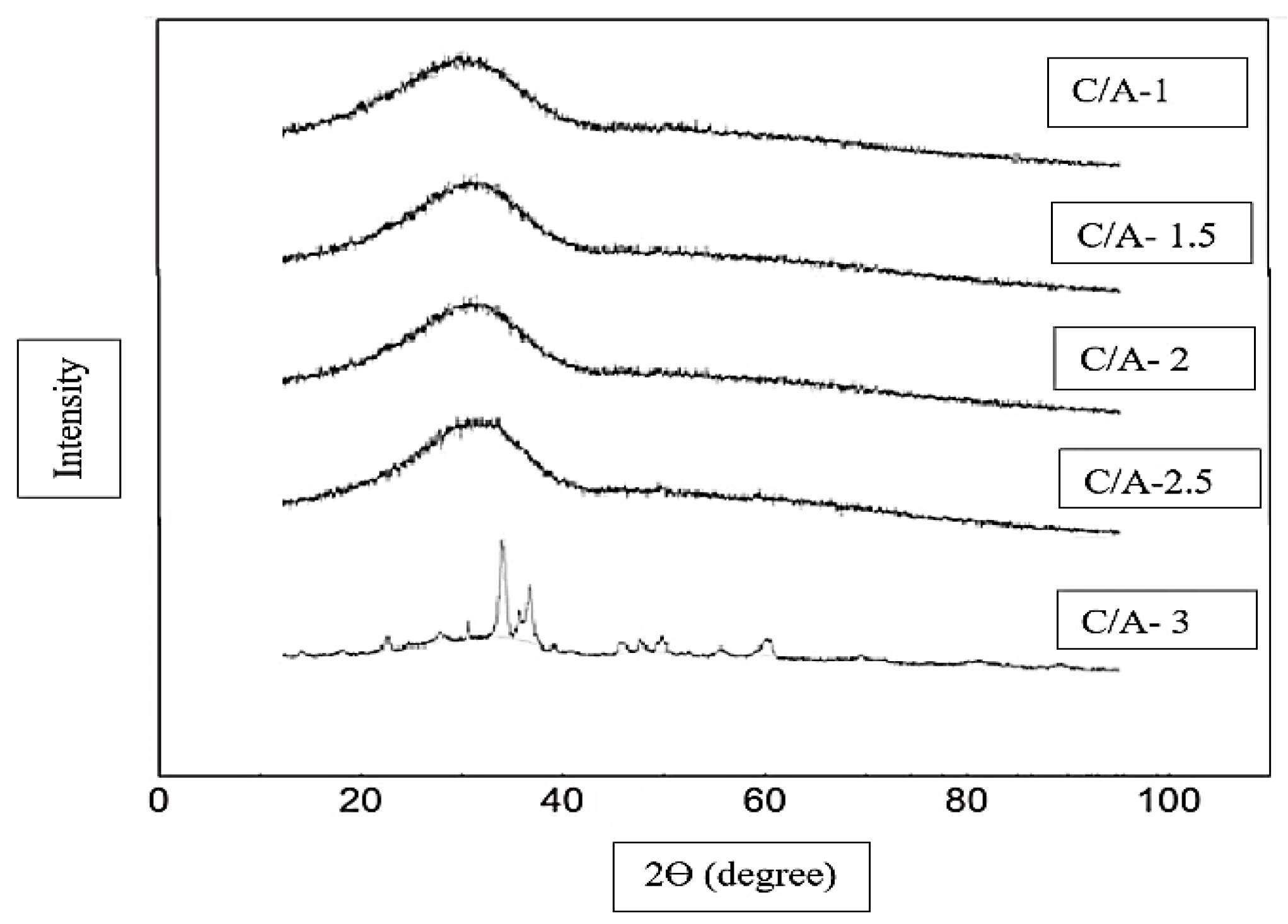
3.1. Effect of CaO/Al2O3 Ratio on the Crystallinity of Mould Powder
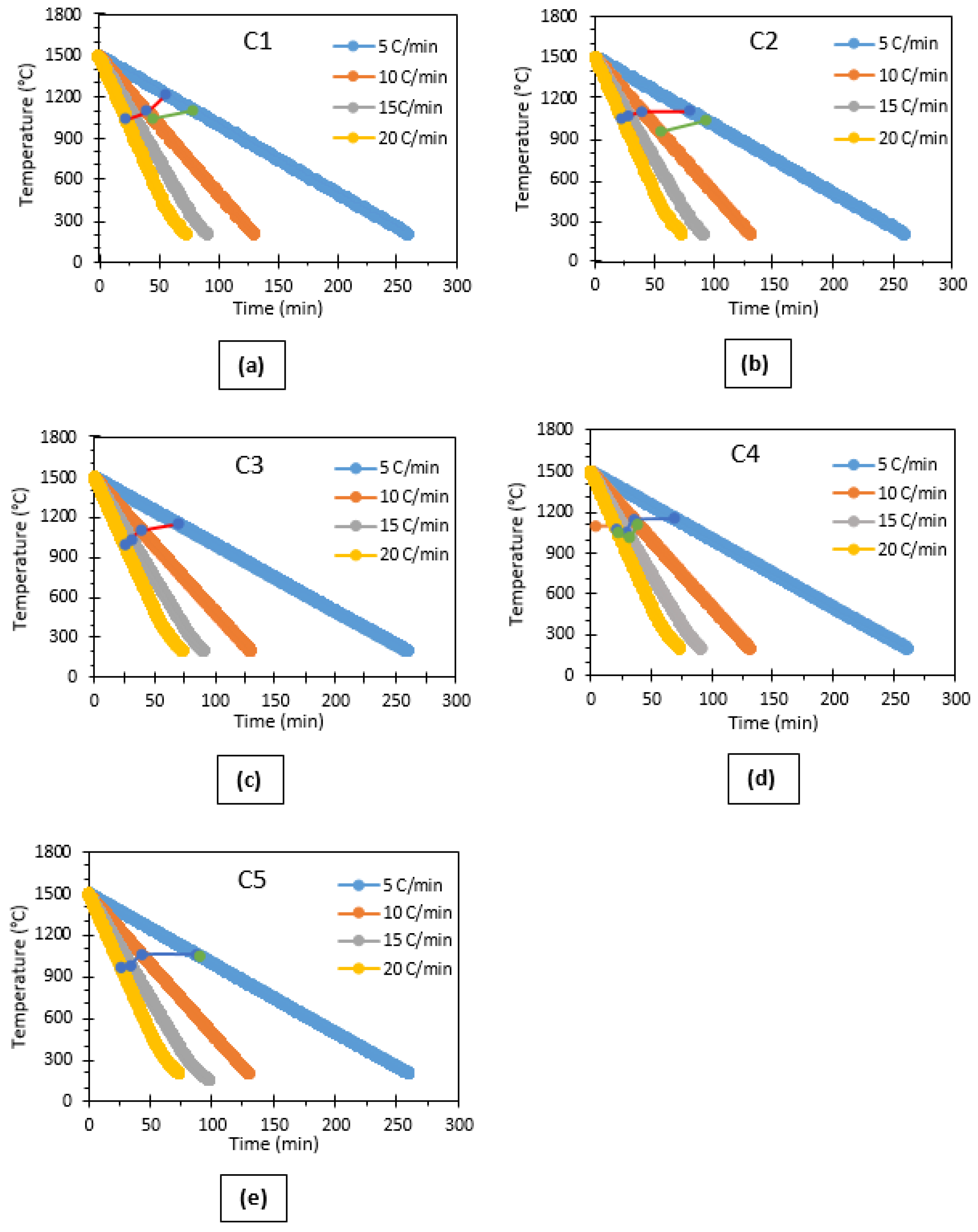
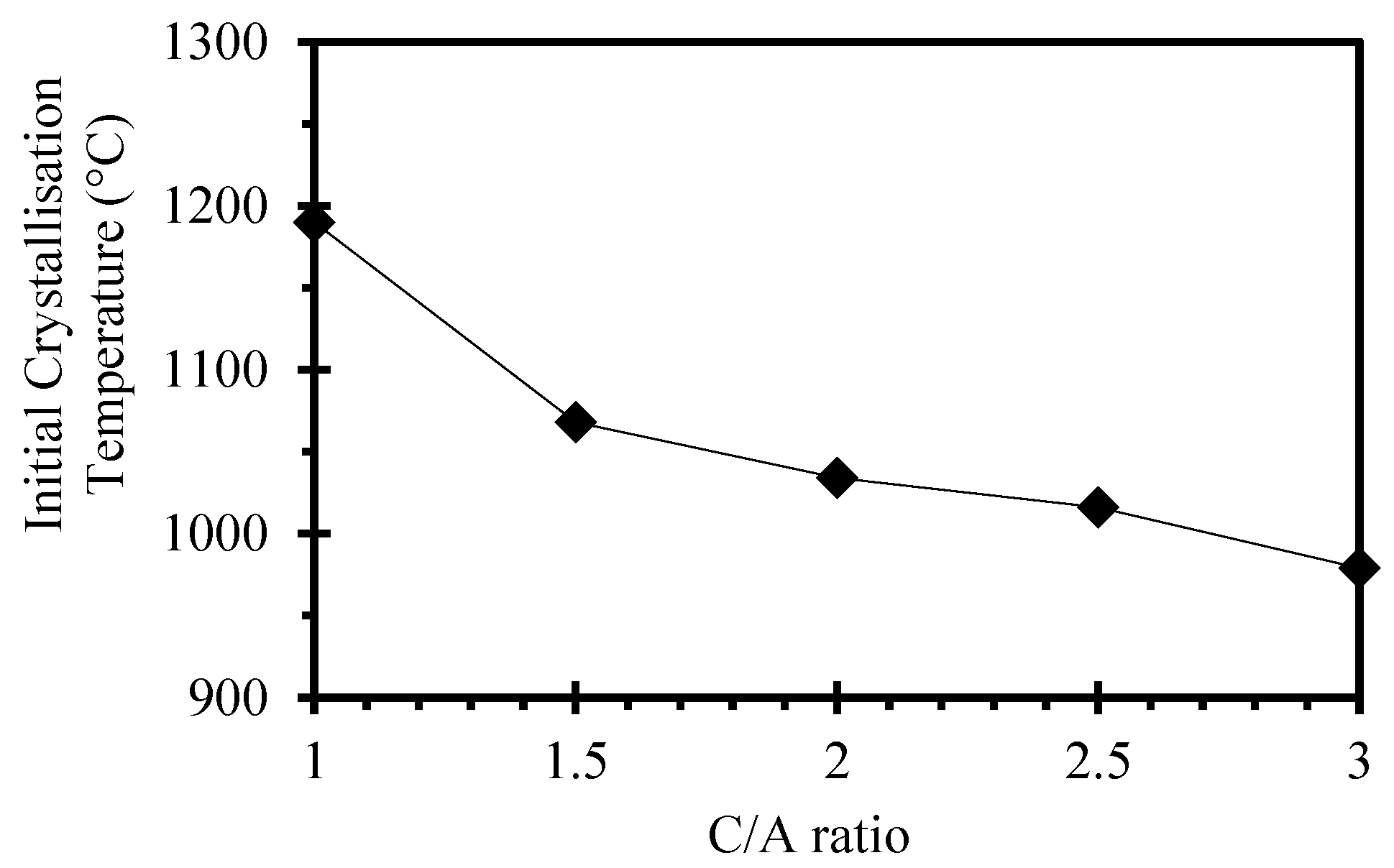
3.2. Effects of CaO/Al2O3 Ratio on the Crystallisation Behaviour of the Mould Flux
- Cooling rates in STA measurements are lower than those in the % crystallinity measurements.
- The higher amount of mould powder used in the % crystallisation tests could increase the natural convection which is beneficial for crystallisation.
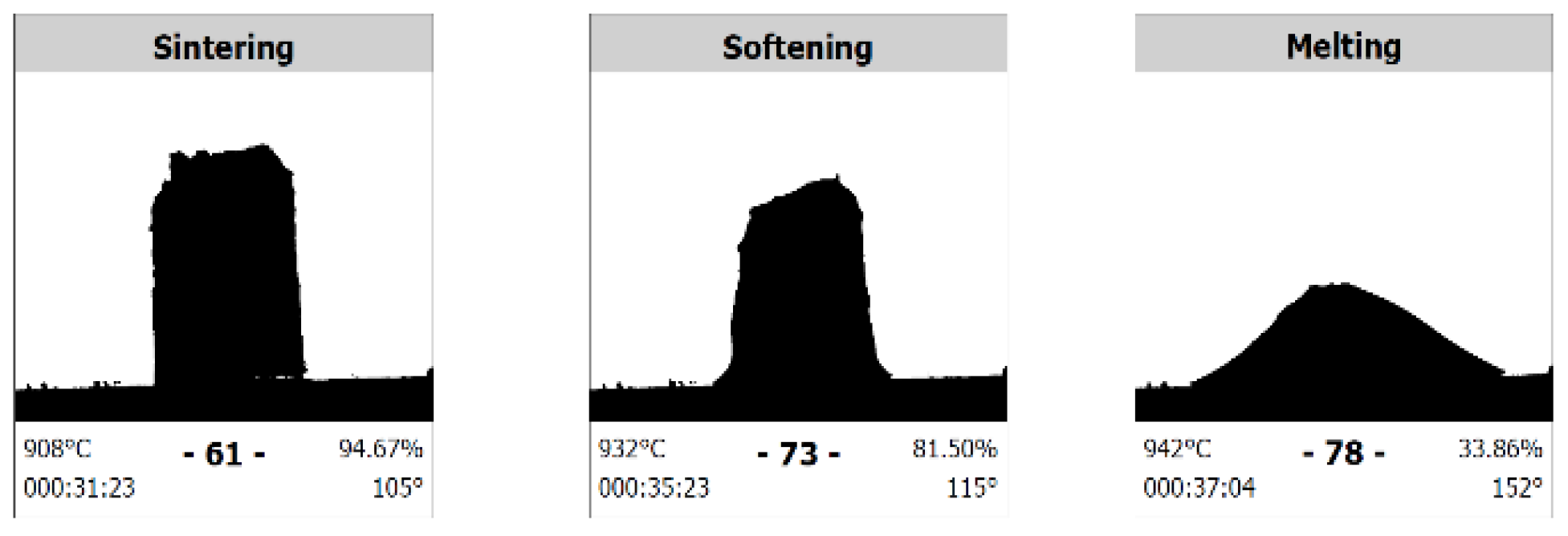
3.3. Effect of CaO/Al2O3 Ratio on Melting Behaviour
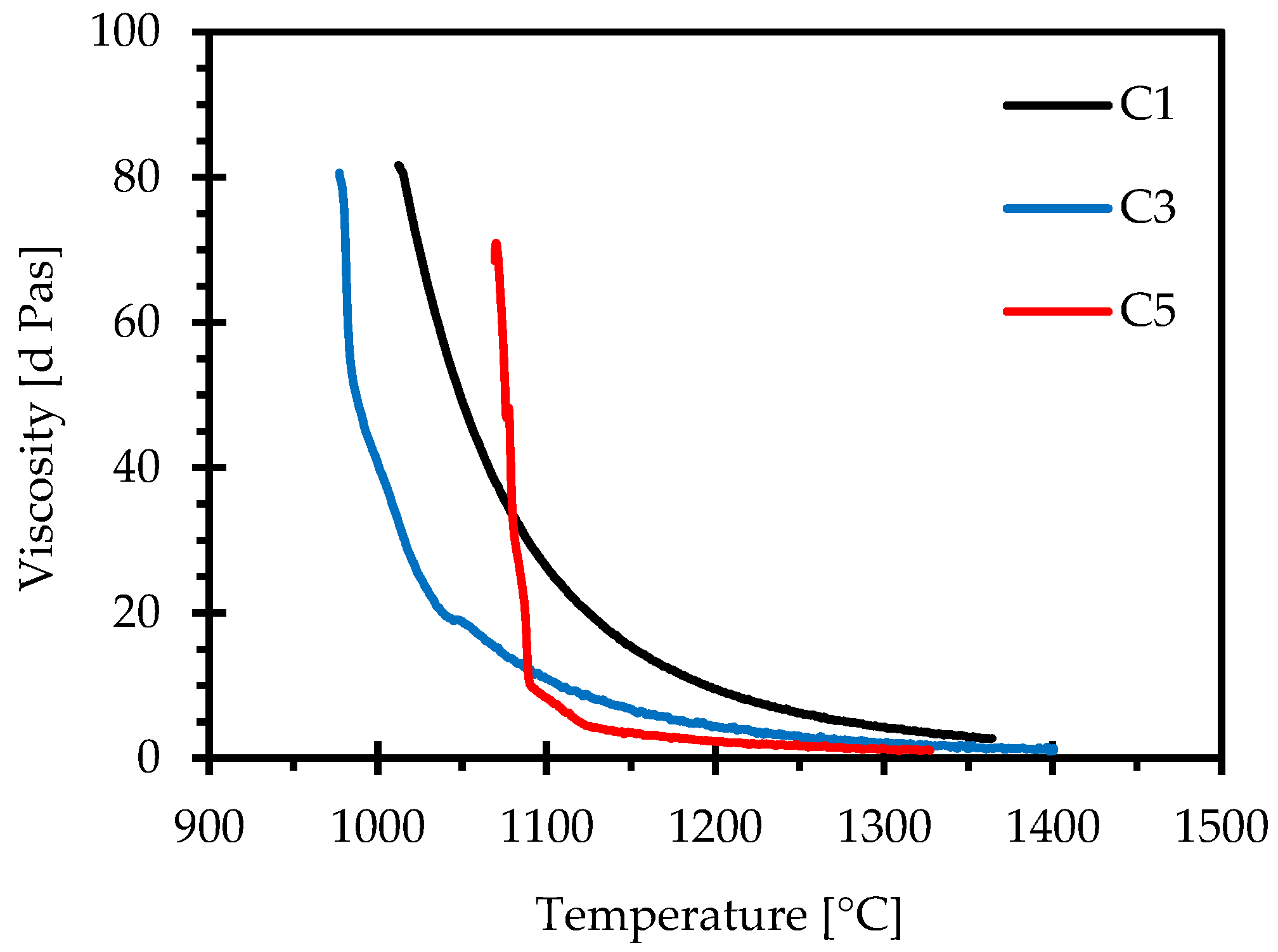
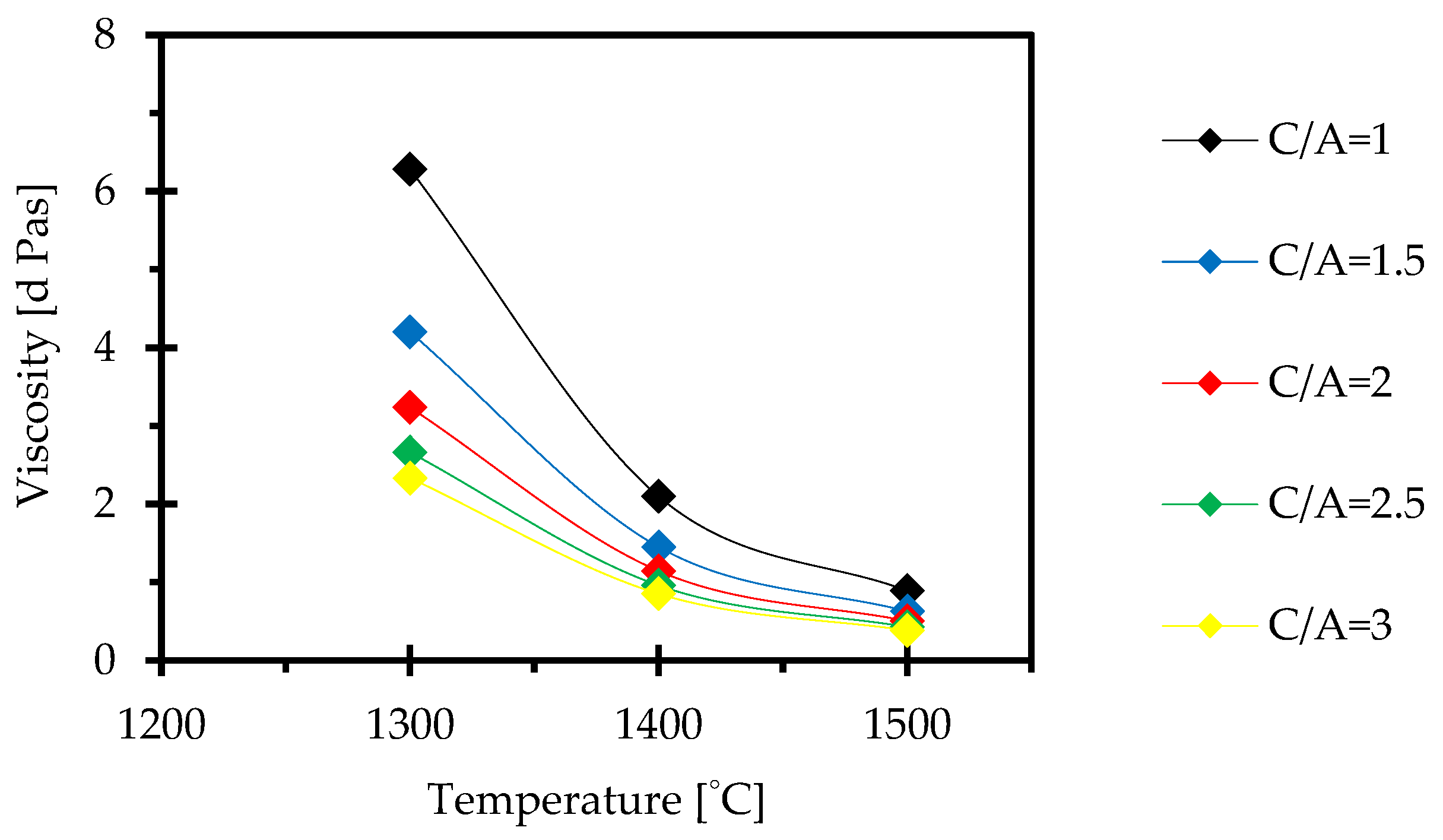
3.4. Effect of CaO/Al2O3 Ratio on Viscosity
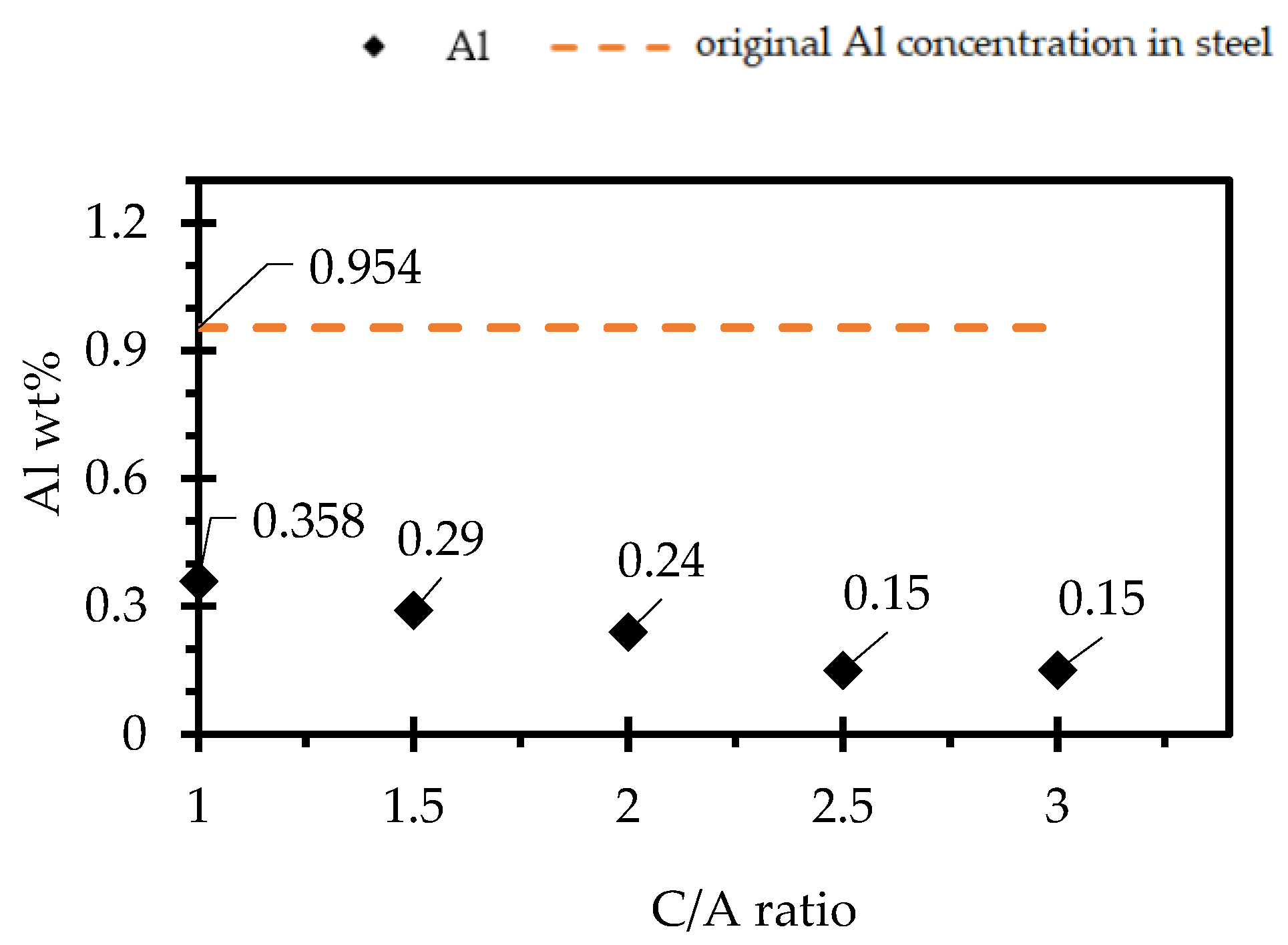
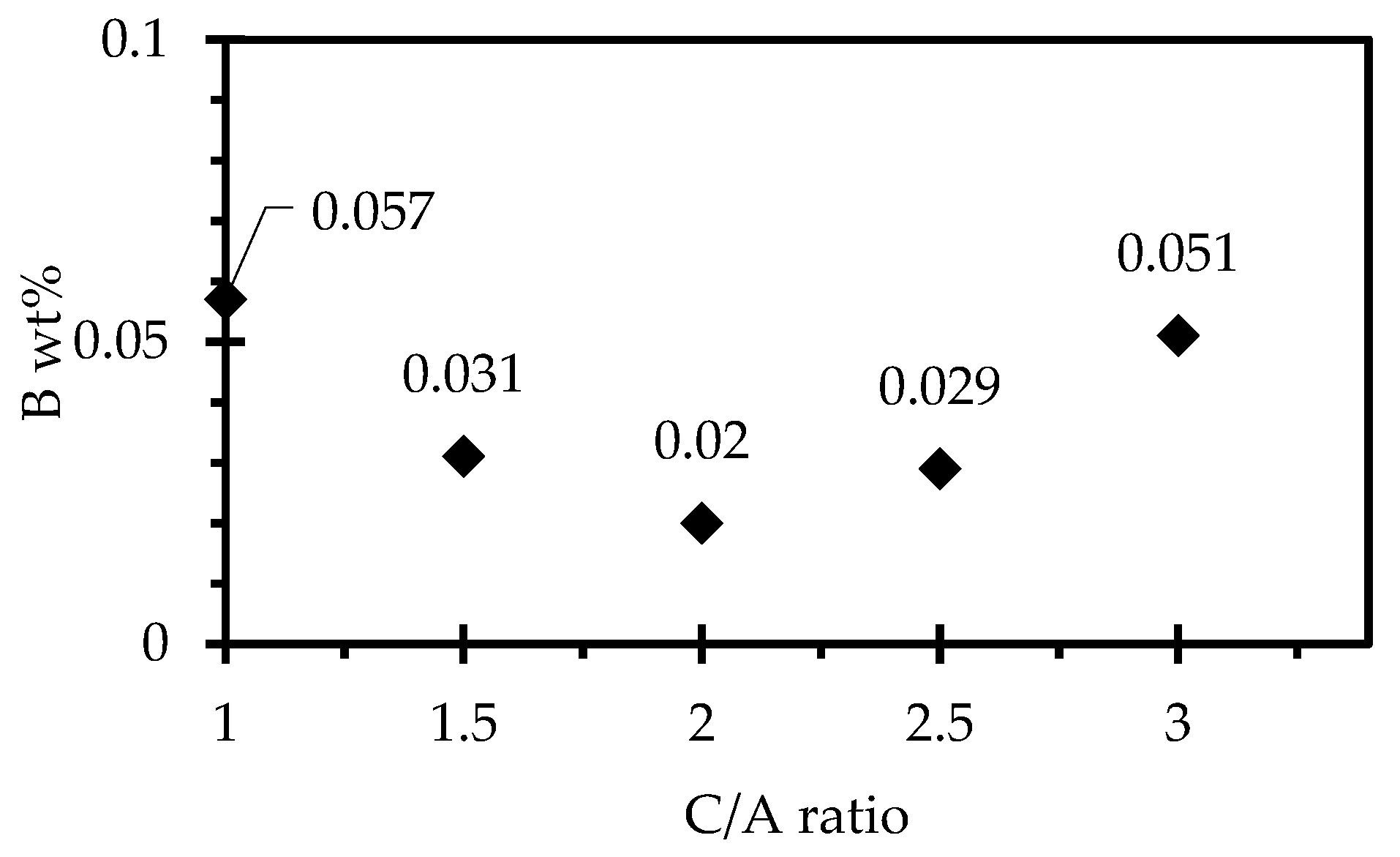
3.5. Effect of CaO/Al2O3 Ratio on the Steel-Slag Interaction
4. Conclusions
- According to the X-ray Diffraction results, the powder with a C/A ratio of 3 has relatively high crystallinity in comparison with the other C-series mould powders, which was also confirmed by metallographic determination measurements. In addition, CCT test results indicate that an increase in C/A ratio decreases the initial crystallisation temperature of these mould powders at a cooling rate of 15 °C/min. This demonstrates that the percentage of crystallinity of C-series mould powders as obtained by X-ray Diffraction are not in accordance with the CCT test results.
- The results of the HSM tests indicated that the melting temperature of lime-alumina-based mould powders increased when the C/A ratio ranged from 1 to 3. However, these results are not consistent with the literature data for conventional mould powders.
- Viscosity measurements with the IPT technique show that the viscosity sharply decreases and then gradually stabilises with an increase in the C/A ratio. The rotating bob viscometer analysis of C1, C3 and C5 mould powders showed that the results were in line with the above findings, which lends to the credibility of this study. A good correlation between the measured and the calculated viscosities was observed for these mould powders.
- The results from the steel/slag interaction tests show that the degree of steel/slag interaction decreases with an increase in Al2O3 content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.H.; Liao, L.; Zheng, K.; Hu, H.H.; Wang, F.M.; Zhang, Z.T. Effect of B2O3 addition on viscosity of mould powder slag containing low silica content. Ironmak. Steelmak. 2014, 41, 67–74. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, W.; Zhou, L.; Lu, B.; Kang, Y.B. Effects of MnO on crystallisation, melting, and heat transfer of CaO-Al2O3 based mold flux used for high Al-TRIP steel casting. Metall. Mater. Trans. B 2014, 45, 1510–1519. [Google Scholar] [CrossRef]
- Shi, C.B.; Seo, M.D.; Cho, J.W.; Ki, S.H. Crystallisation characteristics of CaO-Al2O3 based mould flux and their effects on in-mold performance during high-aluminium TRIP steels continuous casting. Metall. Mater. Trans. B 2014, 45, 1081–1097. [Google Scholar] [CrossRef]
- Cho, J.W.; Blazek, K.; Frazee, M.; Yin, H.; Park, J.H.; Moon, S.W. Assessment of CaO-Al2O3 based mold flux system for high aluminium TRIP casting. ISIJ Int. 2013, 53, 62–70. [Google Scholar] [CrossRef]
- Fu, X.J.; Wen, G.H.; Tang, P.; Liu, Q.; Zhou, Z.Y. Effects of CaO/Al2O3 ratio on crystallisation behaviour of CaO-Al2O3 based mould fluxes for high Al TRIP steel. Ironmak. Steelmak. 2014, 41, 341–342. [Google Scholar] [CrossRef]
- Blazek, K.; Yin, H.; Skoczylas, G.; McClymonds, M.; Frazee, M. Development and evaluation of lime-alumina based mold powders for casting high aluminium TRIP steel grades. Iron Steel Technol. 2011, 8, 232–249. [Google Scholar]
- Jiang, B.; Wang, W.; Sohn, I.; Wei, J.; Zhou, L.; Lu, B. A kinetic study of the effect of ZrO2 and CaO/Al2O3 ratios on the crystallisation behaviour of a CaO-Al2O3-based slag system. Metall. Mater. Trans. B 2014, 45, 1057–1067. [Google Scholar] [CrossRef]
- Lu, B.; Chen, K.; Wang, W.; Jiang, B. Effects of Li2O and Na2O on crystallisation behaviour of lime-alumina based mold flux for Casting High-Al Steels. Metall. Mater. Trans. B 2014, 45, 1496–1509. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Yang, Y.; Lippold, C.; McLean, A. Effect of CaO/Al2O3 ratio on viscosity and crystallisation behaviour of mould flux for high Alnon-magnetic steel. Ironmak. Steelmak. 2015, 42, 698–704. [Google Scholar] [CrossRef]
- Seyrek, M.; Thackray, R. Investigation on Viscosity of CaO-Al2O3 Based Mould Fluxes for the Continuous Casting of High-Al Steels. Mater. Proc. 2021, 3, 13. [Google Scholar] [CrossRef]
- Mills, K.C.; Courtney, L.; Fox, A.B.; Harris, B. The use of thermal analysis in the determination of the crystalline fraction of slag films. Thermochim. Acta 2002, 391, 175–184. [Google Scholar] [CrossRef]
- DIFFRAC. EVA User Manual; Bruker AXS GmbH: Karlsruhe, Germany, 2013. [Google Scholar]
- Dey, A.; Riaz, S. Viscosity measurement of mould fluxes using inclined plane test and development of mathematical model. Ironmak. Steelmak. 2013, 39, 391–397. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, W.; Lu, B. Effects of B2O3 and BaO on the crystallisation behaviour of CaO-Al2O3 based mould flux for casting high-Al steels. Metall. Mater. Trans. B 2015, 46, 873–881. [Google Scholar] [CrossRef]
- Lu, B.; Wang, W.; Li, J.; Zhao, H.; Huang, D. Effect of basicity and B2O3 on the crystallisation and heat transfer behaviours of low fluorine mold flux for casting medium carbon steels. Metall. Mater. Trans. B 2013, 44, 365–376. [Google Scholar] [CrossRef]
- Gao, J.; Wen, G.; Sun, Q.; Tang, P.; Liu, Q. The influence of Na2O on the solidification and crystallisation behaviour of CaO-SiO2-Al2O3 based mould flux. Metall. Mater. Trans. B 2015, 46, 1850–1859. [Google Scholar] [CrossRef]
- Wang, H.; Tang, P.; Wen, G.H. Effect of Na2O on crystallisation behaviour and heat transfer of high Al steel mould fluxes. Ironmak. Steelmak. 2011, 38, 369–373. [Google Scholar] [CrossRef]
- Mills, K.C. Structure and properties of slags used in the continuous casting of steel: Part 2 Specialist mould powders. ISIJ Int. 2016, 56, 14–23. [Google Scholar] [CrossRef]
- Li, Z.; Thackray, R.; Mills, K.C. A test to determine crystallinity of mould fluxes. In VII International Conference on Molten Slags Fluxes and Salts; The South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2004. [Google Scholar]
- Thombre, D.B. Estimation of glass-forming ability and glass stability of Lithium-Borosilicate glasses. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 2, 124–132. [Google Scholar]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Mills, K.C.; Carl-Ake, D. The Casting Powders Book, 1st ed.; Springer: Cham, Switzerland, 2017; p. 318. [Google Scholar]
- Zanotto, E.D.; Coutinho, F.A.B. How many non-crystalline solids can be made from all the elements of the periodic table? J. Non-Cryst. Solids 2004, 1, 285–288. [Google Scholar] [CrossRef]
- Sharda, S.; Sharma, P.; Sharma, V. A study of thermal stability and crystallisation kinetics of SbSeGe glassy alloys. In ICMAEM-2017; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Inoue, A.; Shen, B.; Takeuchi, A. Fabrication, properties and applications of bulk glassy alloys in late transition metal- based systems. Mater. Sci. Eng. A 2006, 441, 18–25. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Glass-forming ability of alloys. J. Non-Cryst. Solids 1993, 156–158, 473–480. [Google Scholar] [CrossRef]
- Hruby, A. Evaluation of glass-forming tendency by means of DTA. Czechoslov. J. Phys. B 1972, 22, 1187–1193. [Google Scholar] [CrossRef]
- Yu, X.; Wen, G.H.; Tang, P.; Wang, H. Investigation on viscosity of mould fluxes during continuous casting of aluminium containing TRIP steel. Ironmak. Steelmak. 2013, 36, 623–630. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.; Jiang, M. Properties investigation of CaO–Al2O3–SiO2–Li2O–B2O3–Ce2O3 mould flux with different w(CaO)/w(Al2O3) for heat-resistant steel continuous casting. Can. Metall. Q. 2017, 56, 212–220. [Google Scholar] [CrossRef]
- McMillan, P.F.; Petuskey, W.T.; Cote, B.; Massiot, D.; Landron, C.; Coutures, J.P. A structural investigation of CaO-A12O3 glasses via 27A1 MAS-NMR. J. Non-Cryst. Solids 1996, 195, 261–271. [Google Scholar] [CrossRef]
- Poe, B.T.; McMillan, P.F.; Cote, B.; Massiot, D.; Coutures, J.P. Structure and dynamics in calcium aluminate liquids: High-temperature 27Al NMR and Raman spectroscopy. J. Am. Ceram. Soc. 1994, 77, 1832–1838. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, G.; Tang, P.; Sridhar, S. The influence of Al2O3/SiO2 ratio on the viscosity of mould fluxes. ISIJ Int. 2008, 48, 739–746. [Google Scholar] [CrossRef]
- Yan, W.; McLean, A.; Yang, Y.; Chen, W.; Barati, M. Evaluation of mould flux for continuous casting of high-aluminium steel. In Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts (MOLTEN16), Seattle, WA, USA, 22–25 May 2016. [Google Scholar]
- Kim, G.H.; Sohn, I. Role of B2O3 on the viscosity and structure in the CaO-Al2O3-Na2O-based system. Metall. Mater. Trans. B 2014, 45, 86–95. [Google Scholar] [CrossRef]
- Fox, A.B.; Mills, K.C.; Lever, D.; Bezerra, C.; Valadares, C.; Unamuno, I.; Laraudogoitia, J.J.; Gisby, J. Development of fluoride-free fluxes for billet casting. ISIJ Int. 2005, 45, 1051–1058. [Google Scholar] [CrossRef]
- Rudnitzki, J.; Shepherd, R.; Balichev, E.; Karrasch, S.; Krüger, F. Investigation of Al2O3 pick-up in mould slag during continuous casting of Al containing steels. Eur. Contin. Cast. Conf. 2014, 23, 26. [Google Scholar]
- Kim, M.S.; Lee, S.W.; Cho, J.W.; Park, M.S.; Lee, H.G.; Kang, Y.B. A reaction between high Mn-high Al steel and CaO-SiO2-type molten mould flux: Part I. Composition evolution in molten mould flux. Metall. Mater. Trans. B 2013, 44, 299–308. [Google Scholar] [CrossRef]





| Powder | C/A | SiO2 | CaO | Al2O3 | Li2O | Na2O | B2O3 |
|---|---|---|---|---|---|---|---|
| C1 | 1 | 10 | 30 | 30 | 5 | 10 | 15 |
| C2 | 1.5 | 10 | 36 | 24 | 5 | 10 | 15 |
| C3 | 2 | 10 | 40 | 20 | 5 | 10 | 15 |
| C4 | 2.5 | 10 | 43 | 17 | 5 | 10 | 15 |
| C5 | 3 | 10 | 45 | 15 | 5 | 10 | 15 |
| Composition (wt.%) | Fe | Mn | Al | Si | Gd |
|---|---|---|---|---|---|
| Steel | 96.672 | 1.345 | 0.954 | 0.667 | 0.287 |
| Powder | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|
| NBO/T ratio | 0.9 | 1.3 | 1.7 | 1.9 | 2.2 |
| Samples | Tg (°C) | Tliq (°C) | Tx (°C) | KT | KH | Tx − Tg |
|---|---|---|---|---|---|---|
| C1 | 700.2 | 1218 | 1190 | 0.57 | 17.5 | 498.8 |
| C2 | 694.6 | 1223 | 1068 | 0.57 | 2.4 | 373.4 |
| C3 | 691.1 | 1229 | 1034 | 0.56 | 1.8 | 342.9 |
| C4 | 688.6 | 1232 | 1016 | 0.56 | 1.5 | 327.4 |
| C5 | 686.9 | 1234 | 979 | 0.56 | 1.1 | 292.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyrek, M.; Thackray, R. Effect of CaO/Al2O3 Ratio on Physical Properties of Lime-Alumina-Based Mould Powders. Metals 2023, 13, 719. https://doi.org/10.3390/met13040719
Seyrek M, Thackray R. Effect of CaO/Al2O3 Ratio on Physical Properties of Lime-Alumina-Based Mould Powders. Metals. 2023; 13(4):719. https://doi.org/10.3390/met13040719
Chicago/Turabian StyleSeyrek, Mustafa, and Richard Thackray. 2023. "Effect of CaO/Al2O3 Ratio on Physical Properties of Lime-Alumina-Based Mould Powders" Metals 13, no. 4: 719. https://doi.org/10.3390/met13040719
APA StyleSeyrek, M., & Thackray, R. (2023). Effect of CaO/Al2O3 Ratio on Physical Properties of Lime-Alumina-Based Mould Powders. Metals, 13(4), 719. https://doi.org/10.3390/met13040719








