Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials
Abstract
1. Introduction
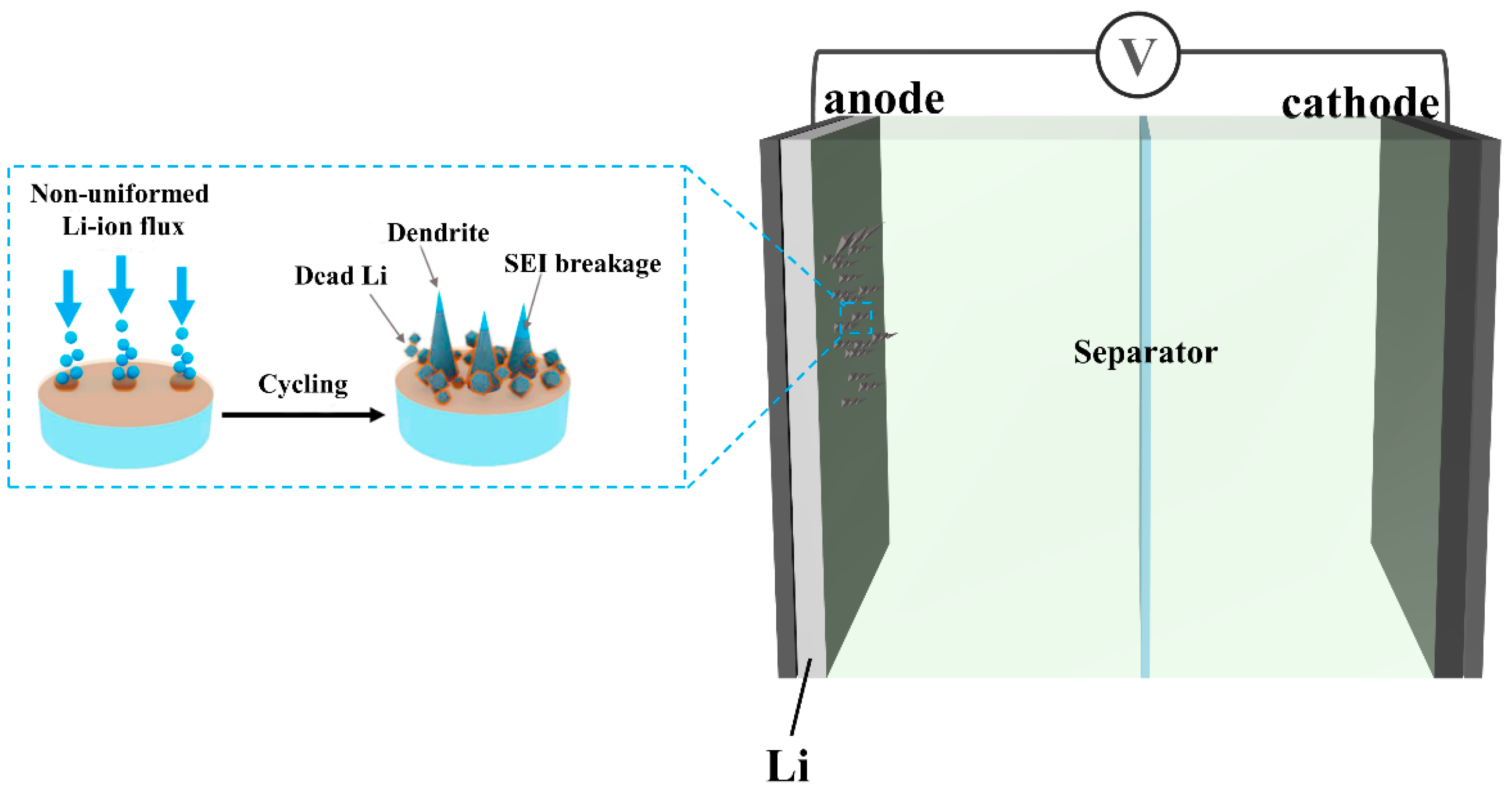
2. Metal Electrode
3. Abnormal Metal Electrode
3.1. Foamed Metal Battery
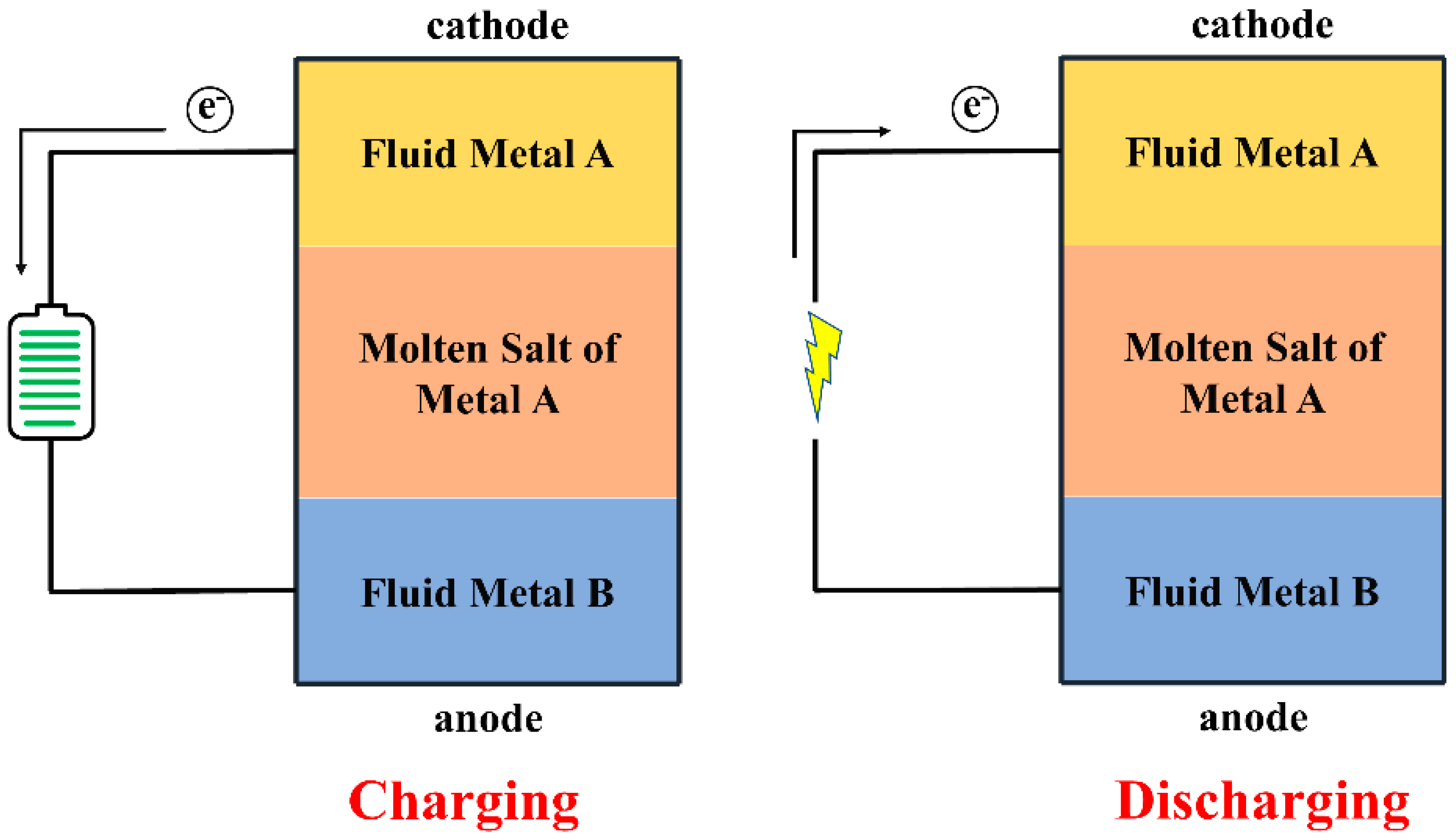
3.2. Liquid Metal Battery
4. Metallic Compound Electrode Materials
4.1. Metallic Oxide Electrode Materials
4.2. Metallic Nitride Electrode Materials
4.3. Metallic Sulfide Electrode Materials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luis, A.; Frank, W.; de León Carlos, P. 3D Porous Metal Electrodes: Fabrication, Characterisation and Use. Curr. Opin. Electrochem. 2019, 16, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Chong, Y.; Zhang, Q. Review on Li Deposition in Working Batteries: From Nucleation to Early Growth. Adv. Mater. 2021, 33, 2004128. [Google Scholar] [CrossRef]
- Deepak, K.; Kuhar, R.S.; Dinesh, S.K. Progress and prospects of sodium-sulfur batteries: A Review. Solid State Ion. 2017, 312, 8–16. [Google Scholar] [CrossRef]
- James, P.; Divya, S.; Damian, G.; Neeraj, S. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Wajid, A.; et al. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, 2000730. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.; Li, F. The Rechargeable Aluminum Battery: Opportunities and Challenges. Angew. Chem. 2019, 131, 11978–11996. [Google Scholar] [CrossRef]
- Rana, M.; Fuminori, M. Magnesium batteries: Current state of the art, issues and future perspectives. Beilstein J. Nanotechnol. 2014, 5, 1291–1311. [Google Scholar] [CrossRef]
- Elena Arroyo-de Dompablo, M.; Ponrouch, A.; Johansson, P.; Rosa Palacin, M. Achievements, Challenges, and Prospects of Calcium Batteries. Chem. Rev. 2019, 120, 6331–6357. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Dylan, K.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Tianpin, J.W.; Khalil, A. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Lu, Z.; Li, W.; Long, Y.; Liang, J.; Liang, Q.; Wu, S.; Tao, Y.; Weng, Z.; Lv, W.; Yang, Q. Constructing a High-Strength Solid Electrolyte Layer by In Vivo Alloying with Aluminum for an Ultrahigh-Rate Lithium Metal Anode. Adv. Funct. Mater. 2020, 30, 1907343. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.; Shen, X.; Zhang, X.; Chen, X.; Cheng, X.; Chong, Y.; Zhao, C.; Zhang, Q. Coralloid Carbon Fiber-Based Composite Lithium Anode for Robust Lithium Metal Batteries. Joule 2018, 2, 764–777. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, G.; Liu, X.; Guo, B.; Xu, G.; Huang, Z.; Wu, M.; Liu, H.; Dou, S.; Wu, C. Dendrite-Free Sodium Metal Anodes Enabled by a Sodium Benzenedithiolate-Rich Protection Layer. Angew. Chem. Int. Ed. 2020, 59, 1916716. [Google Scholar] [CrossRef]
- Zhi, S.; Sun, J.; Sun, Y.; Cui, Y. A Highly Reversible Room-Temperature Sodium Metal Anode. ACS Cent. Sci. 2015, 1, 449–455. [Google Scholar] [CrossRef]
- Lukas, S.; Ilie, H.; Martin, W.; Stefan, F. An Electrolyte for Reversible Cycling of Na Metal and Na Intercalation Compounds. ChemSusChem 2016, 10, 1601222. [Google Scholar] [CrossRef]
- Andrew, B.; Faezeh, M.; Ruhamah, Y.; Douglas, M.; Maria, F.; Patrick, H. Extensive Sodium Metal Plating and Stripping in a Highly Concentrated Inorganic-Organic Ionic Liquid Electrolyte through Surface Pretreatment. ChemElectroChem 2017, 4, 1600784. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Dong, J.; Chun, L.K.; Qin, L.; Lei, Y.; Li, B.; Zhai, D.; Wu, Y.; Kang, F. Artificial Solid-Electrolyte Interphase Enabled High-Capacity and Stable Cycling Potassium Metal Batteries. Adv. Energy Mater. 2019, 9, 1902697. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Huang, Q.; Chen, Y.; Guo, L. Simultaneous defect regulation by p-n type co-substitution in a Na3V2(PO4)3/C cathode for high performance sodium ion batteries. Dalton Trans. 2022, 51, 10943–10955. [Google Scholar] [CrossRef]
- Xiao, N.; Zheng, J.; Gerald, G.; Luke, S.; Wu, Y. Anchoring an Artificial Protective Layer to Stabilize Potassium Metal Anode in Rechargeable K-O2 Batteries. ACS Appl. Mater. Interfaces 2019, 11, 16571–16577. [Google Scholar] [CrossRef]
- Yang, Q.; Ding, Y.; He, G. An Amalgam Route to Stabilize Potassium Metal Anodes over Wide Temperature Range. Chem. Commun. 2020, 56, 3512–3515. [Google Scholar] [CrossRef]
- Shougo, H.; Seok, L.; Jang, L.; Kensuke, T.; Yi, C. Avoiding Short Circuits From Zinc Metal Dendrites in Anode by Backside-Plating Configuration. Nat. Commun. 2016, 7, 11801. [Google Scholar] [CrossRef]
- Muñoz-Torrero, D.; Leung, P.; García-Quismondo, E.; Ventosa, E.; Anderson, M.; Palma, J.; Marcilla, R. Investigation of different anode materials for aluminium rechargeable batteries. J. Power Sources 2018, 374, 77–83. [Google Scholar] [CrossRef]
- Gao, T.; Hou, S.; Huynh, K.; Wang, F.; Nico, E.; Fan, X.; Fudong, H.; Luo, C.; Mao, M.; Li, X.; et al. Existence of Solid Electrolyte Interphase in Mg Batteries: Mg/S Chemistry as an Example. ACS Appl. Mater. Interfaces 2018, 10, 14767–14776. [Google Scholar] [CrossRef] [PubMed]
- Vinayan, B.P.; Zhao-Karger, Z.; Diemant, T.; Chakravadhanula, V.S.K.; Schwarzburger, N.I.; Cambaz, M.A.; Behm, R.J.; Kübel, C.; Fichtner, M. Performance study of magnesium–sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 2015, 8, 3269–3306. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Arumugam, M. Performance Enhancement and Mechanistic Studies of Magnesium-Sulfur (Mg-S) Cells with an Advanced Cathode Structure. ACS Energy Lett. 2016, 1, 431–437. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Michael, L.; Joël, T.; Tsutomu, M.; Takurou, M.; Jeong-Hyeok, I. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Nam, J.; Jun, N.; Young, K.; Woon, Y.; Seungchan, R.; Hyuk, I.S. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cell. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Gao, F.; Xu, B.; Wang, Q.; Cai, F.; He, S.; Zhang, M.; Wang, Q. Potentiostatic deposition of CoNi2S4 nanosheet arrays on nickel foam: Effect of depostion time on the morphology and pseudocapacitive performance. J. Mater. Sci. 2016, 51, 10641–10651. [Google Scholar] [CrossRef]
- Saeed, S.; Rahim, M.; Elham, A. One-step fabrication of electrochemically reduced graphene oxide/nickel oxide composite for binder-free supercapacitors. Int. J. Hydrog. Energy 2016, 41, 17496–17505. [Google Scholar] [CrossRef]
- Rizwan, A.; Jang-Hoon, H.; In-Hyuck, S. Enhancement of the compressive strength of highly porous Al2O3 foam through crack healing and improvement of the surface condition by dip-coating. Ceram. Int. 2014, 40, 3679–3685. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, X.; Song, Y.; Duan, L. ElectroDeposition for Foamed Zinc Material from Zinc Sulfate Solution. Mater. Sci. Forum 2007, 561, 1669–1672. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Dou, H.; Wei, G.; Wang, X. Enhanced rate capability of nanostructured three-dimensional graphene/Ni3S2 composite for supercapacitor electrode. Ceram. Int. 2016, 42, 9858–9865. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Navanietha, R.; Ammaiyappan, S.; Jonathan, W.; Leung, L.P.; Michael; K.H.; Berchmans, S. Effect of composites based Nickel foam anode in microbial fuel cell using Acetobacter aceti and Gluconoabater roesus as a biocatalysts. Bioresour. Technol. 2016, 217, 113–120. [Google Scholar] [CrossRef]
- Prasun, K.; Chandrasekhar, K.; Archana, K.; Ezhaveni, S.; Soo, K.B. Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies 2018, 11, 343. [Google Scholar] [CrossRef]
- Gerard, D.; Bennetto; Jeremy, M.; Sibel, R.; John, S.; Christopher, T. Electron-transfer coupling in microbial fuel cells performance of fuel cells containing selected microorganism-mediator-substrate combinations. J. Chem. Technol. Biotechnol. Biotechnol. 2008, 34, 13–27. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.; He, D.; Han, K.; Zhao, H.; Qu, X. High Performance Antimony-Bismuth-Tin Positive Electrode for Liquid Metal Battery. Chem. Mater. 2018, 30, 8739–8746. [Google Scholar] [CrossRef]
- Douglas, K.; Tom, W. Fluid Mechanics of Liquid Metal Batteries. Appl. Mech. Rev. 2017, 70, 31. [Google Scholar] [CrossRef]
- Nore, H.W.; Cappanera, C.; Jean-Luc, L.G. Tayler instability in liquid metal colums and liquid metal batteries. J. Fluid Mech. 2015, 771, 79–114. [Google Scholar] [CrossRef]
- Ning, X.; Satyajit, P.; Brice, C.; Huayi; Paul, B.; Donald, S. Self-healing Li-Bi Liquid Metal Battery for Grid-scale energy Storage. J. Power Sources 2014, 275, 370–376. [Google Scholar] [CrossRef]
- Li, H.; Yin, H.; Wang, K.; Cheng, S.; Jiang, K.; Donald, S. Liquid Metal Electrodes for Energy Storage Batteries. Adv. Energy Mater. 2016, 6, 239–248. [Google Scholar] [CrossRef]
- Stephen, H.N.M.; James, L.; Cameron, L.; Susan, O.; Kunlei, L. Cathode candidates for zinc-based thermal-electrochemical energy storage. Int. J. Energy Res. 2015, 40, 393–399. [Google Scholar] [CrossRef]
- Viktor, N.; Joze, M.; Marko, H.; Aljana, P.; Miran, G. Preparation and Electrochemical Characterization of Aluminium Liquid Battery Cells with Two Different Electrolytes (NaCl-BaCl2-AlF3-NaF and LiF-AlF3-BaF2). Acta Chim. Slov. 2015, 62, 796–804. [Google Scholar] [CrossRef]
- David, B.; Hojong, K.; Aislinn, S.; Donald, S. Magnesium-Antimony Liquid Metal Battery for Stationary Energy Storage. J. Am. Chem. Soc. 2012, 134, 1895–1897. [Google Scholar] [CrossRef]
- Hojong, K.; Dane, B.; Takanari, O.; Donald, S. Calcium–bismuth electrodes for large-scale energy storage (liquid metal batteries). J. Power Sources 2013, 241, 239–248. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Zhao, C.; Hu, Y.; Magda, T.; Li, H.; Huang, X.; Chen, L. Recent advances of electrode materials for low-cost sodium-ion batteries towards practical application for grid energy storage. Energy Storage Mater. 2017, 7, 130–151. [Google Scholar] [CrossRef]
- Jocelyn, N.; Sophie, P.; Hojong, K.; Brian, S.; Donald, S. Thermodynamic properties of calcium–magnesium alloys determined by emf measurements. Electrochim. Acta 2013, 91, 293–301. [Google Scholar] [CrossRef]
- Margaret, K.; Jocelyn, N.; Donald, S. Electrochemical Determination of the Thermodynamic Properties of Lithium-Antimony Alloys. J. Electrochem. Soc. 2015, 162, A421–A425. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Zhang, K.; Yuan, J.; Li, J.; Zhu, D.; Kiyoshi, O.; Qin, L. Comparison of Reduction Products from Graphite Oxide with that from Graphene Oxide for Anode Application in Lithium-ion Batteries and Sodium-ion Batteries. Nanoscale 2017, 9, 2585–2595. [Google Scholar] [CrossRef]
- Ramzi, N.; Guofeng, Z.; Jiming, S. Facile and low-cost synthesis of cobalt-doped MnO2 decorated with graphene oxide for high performance 2.3 V aqueous asymmetric supercapacitors. Electrochim. Acta 2020, 345, 136198. [Google Scholar] [CrossRef]
- Manuraj, M.; Jomiya, C.; Narayanan, U.; Raghavan, R. Heterostructured MoS2-RuO2 nanocomposite: A promising electrode material for supercapacitors. J. Alloy. Compd. 2020, 836, 155420. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Ramachandran; Ravi; Vaishnavi, G.; Ramesh, G.; Sakunthala. MnFe₂O₄ Nanoparticles as an Efficient Electrode for Energy Storage Applications. J. Nanosci. Nanotechnol. 2020, 20, 96–105. [Google Scholar] [CrossRef]
- Iftikhar, H.; Saad, M.; Awais, A.; Nadir, A.; Wail, A.Z. Uniform growth of Zn-Mn-Co ternary oxide nanoneedles for high-performance energy-storage applications. J. Electroanal. Chem. 2019, 835, 262–272. [Google Scholar] [CrossRef]
- Huang, M.L.; Gu, C.; Ge, X.; Wang, X.; Tu, J. NiO nanoflakes grown on porous graphene frameworks as advanced electrochemical pseudocapacitor materials. J. Power Sources 2014, 259, 98–105. [Google Scholar] [CrossRef]
- Sivalingam, R.; Karuppasamy; Arumugam, S.; Hyun-Seok, K.; Hemraj, Y.; Soo, K.H. Core shell nanostructured of Co3O4@RuO2 assembled on nitrogen-doped graphene sheets electrode for an efficient supercapacitor application. J. Alloys Compd. 2021, 877, 160297. [Google Scholar] [CrossRef]
- Li, S.; Duan, Y.; Teng, Y.; Fan, N.; Huo, Y. MOF-derived tremelliform Co3O4/NiO/Mn2O3 with excellent capacitive performance. Appl. Surf. Sci. 2019, 478, 247–254. [Google Scholar] [CrossRef]
- Md, A.; Sangaraju, S. Sulfonated graphene oxide-decorated block copolymer as a proton-exchange membrane: Improving the ion selectivity for all-vanadium redox flow batteries. J. Mater. Chem. A 2018, 6, 17740–17750. [Google Scholar] [CrossRef]
- So, K.; KapSeung, Y.; Bo-Hye, K. Enhanced electrical capacitance of heteroatom-decorated nanoporous carbon nanofiber composites containing graphene. Electrochim. Acta 2014, 137, 781–788. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Xia, C.; Wang, W.; Tian, F. Electrochemical capacitance of polypyrrole–titanium nitride and polypyrrole–titania nanotube hybrids. New J. Chem. 2014, 38, 1284–1293. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, L.; Wang, X.; Zhao, G.; Hou, P.; Xu, X. Design of Multilayered Porous Aluminum Nitride for Supercapacitor Applications. Energy Fuels 2021, 35, 12628–12636. [Google Scholar] [CrossRef]
- Han, X.; Tao, K.; Wang, D.; Han, L. Design of porous cobalt sulfide nanosheets array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2017, 10, 2765–3741. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Chang, H.; Wei, T.; Qi, S.; Li, Y.; Zhu, Y. Approaching High-Performance Electrode Materials of ZnCo2S4 nanoparticle Wrapped carbon nanotubes for Supercapacitors. J. Mater. 2020, 7, 563–576. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Q.; Zheng, K.; Qin, J.; Zhou, D.; Wang, J. Impact of lithium salts on the combustion characteristics of electrolyte under diverse pressures. Energies 2020, 13, 5373. [Google Scholar] [CrossRef]
- Qin, J.; Liu, C.; Huang, Q. Simulation on fire emergency evacuation in special subway station based on Pathfinder. Case Stud. Therm. Eng. 2020, 21, 100677. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase change materials application in battery thermal management system: A review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef]
- Xu, D.; Huang, G.; Guo, L.; Chen, Y.; Ding, C.; Liu, C. Enhancement of catalytic combustion and thermolysis for treating polyethylene plastic waste. Adv. Compos. Hybrid Mater. 2022, 5, 113–129. [Google Scholar] [CrossRef]
- Li, D.; Hu, J.; Wang, C.; Guo, L.; Zhou, J. Metal-organic framework-induced edge-riched growth of layered Bi2Se3 towards ultrafast Na-ion storage. J. Power Sources 2023, 555, 232387. [Google Scholar] [CrossRef]
- Xiao, T.; Li, J.; Zhuang, X.; Zhang, W.; Wang, S.; Chen, X.; Xiang, P.; Jiang, L.; Tan, X. Wide potential window and high specific capacitance triggered via rough NiCo2S4 nanorod arrays with open top for symmetric supercapacitors. Electrochim. Acta 2018, 269, 397–404. [Google Scholar] [CrossRef]

| Metal | Redox Potential (V vs. NHE) | Mass Specific Capacity (mA·h/g) | Capacity Density (mA·h/cm3) | Melting Point (°C) | Crustal Abundance (ppm) |
|---|---|---|---|---|---|
| Li | −3.045 | 3860 | 2061 | 108 | 18 |
| Na | −2.714 | 1166 | 1128 | 97.72 | 22,700 |
| K | −2.928 | 685 | 591 | 63.65 | 21,000 |
| Mg | −2.372 | 2204 | 3835 | 648 | 27,640 |
| Ca | −2.868 | 1337 | 2072 | 842 | 46,660 |
| Zn | −0.762 | 819 | 5851 | 419 | 70 |
| Al | −1.662 | 2979 | 8043 | 660 | 83,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Wang, S.; Chen, Y.; Liu, C.; Li, Q. Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials. Metals 2023, 13, 703. https://doi.org/10.3390/met13040703
Huang Q, Wang S, Chen Y, Liu C, Li Q. Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials. Metals. 2023; 13(4):703. https://doi.org/10.3390/met13040703
Chicago/Turabian StyleHuang, Que, Silong Wang, Yanjun Chen, Changcheng Liu, and Qiyue Li. 2023. "Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials" Metals 13, no. 4: 703. https://doi.org/10.3390/met13040703
APA StyleHuang, Q., Wang, S., Chen, Y., Liu, C., & Li, Q. (2023). Brief Introduction on Manufacturing and Characterization of Metallic Electrode and Corresponding Modified Materials. Metals, 13(4), 703. https://doi.org/10.3390/met13040703







