Abstract
MXenes possess unique features that are useful for broader industrial development. However, although many different compositions of MXenes have been discovered, little research has been conducted on the optimal synthesis strategy for producing the best MXenes yield. Therefore, substantial work is performed on the synthesis’ structure and property relationship for direct methanol fuel cell (DMFC) applications since MXenes have been successfully hybridised with rice husk ash (RHA). In this study, to produce titanium-based MXene, Ti3C2 nanopowders are added to the rice husk ash matrix to synthesise hybrid RHA/MXene composites (R-MX). Using different weight percentages of MXene hybridised with rice husk ash (2 wt. % R-MX, 4 wt. % R-MX and 6 wt. % R-MX), different electrochemical properties are obtained. Meanwhile, electrochemical analysis is undertaken to investigate the methanol oxidation performance using Linear Sweep Voltammetry (LSV). The highest percentage of the R-MX hybrid composite, 6 wt. % MXene, showed the lowest Tafel slope (148 mV/dec) and the highest ionic exchange current density in the same Tafel analysis. Moreover, the incorporation of MXene into RHA produced good results from the chronoamperometry analysis (CA), with the highest percentage of the hybrid composite, R-6MX, showing the highest retention rate of 97.28%. Meanwhile, the Nyquist plot analysis showed an increasing semicircle arc diameter at the lower-frequency region, implying a lower interfacial charge resistance upon the addition of MXene into RHA. This outcome corresponded to the CA and LSV analysis findings, R-6MX showed a remarkable performance in terms of having the highest peak current density of 0.9454 mA/cm2 and retention rate of 97.28%. Both of these values show that hybrid R-6MX was able to maintain a high current for the entire duration. The current is maintained in a stable form for some time, proving that R-6MX was the most stable, with a minimal corrosion reaction and tolerance in a methanol medium. The results from this study enabled an evaluation of the possibility of utilising low-cost, green RHA material for fuel cell applications to promote sustainability. The novelty of this work is that a cheap source of silica-based RHA, a type of waste material, is incorporated with MXene through hybridisation processes.
1. Introduction
Green technology refers to the use of facile and environmentally benign processes and products that have minimum impact on nature. The full utilization of green technology or also commonly known as clean technology is that it helps to minimize pollution and conserve natural resources, in addition to promoting economic well-being and sustainability. By reducing the utilization of other harmful and polluting resources, green technology can help to reduce the damage to mother nature. Overall, green technology is considered to be an essential component of a sustainable future. Due to the involvement of human activities in carrying out developmental projects, environmental deterioration has been a worrying issue. Because of the constant rise in energy demand, the sustainable development of reliable and stable energy sources is the primary concern in green technology. Other than solar and wind energy, the fuel cell is the most enticing source of renewable energy. Due to the non-renewable fossil fuels’ impact on worsening the environment, fuel cells have drawn a lot of attention. Fuel cells are very reliable and promising because so long as fuel is continually provided, it can produce an energy output that is steady and consistent.
Direct Methanol Fuel Cell (DMFC) is a type of fuel cell that uses methanol as its fuel and produces electricity through an electrochemical process. In DMFC, methanol is directly utilised inside the anode chamber of the fuel cell. This is where the catalytic reaction resulted in movement of electrons and protons. The electrons are then used to power the external circuit, while the protons travelled across a proton exchange membrane to the cathode chamber, where they combined with oxygen to form water as the process by-product [1]. The use of the direct methanol fuel cell (DMFC) as a safe power source has garnered academic attention due to its low emissions. DMFCs can operate at low temperatures, are safe and easy to use. DMFCs have high energy density, making them a good replacement to non-replenishable fuel cells which will usually end in landfills after one cycle of use. This aspect of DMFCs is attractive, especially given the growing issue of environmental pollution from electronic waste. Methanol is a liquid fuel that is stable and easy to operate, making DMFCs safer to use compared to other types of fuel cells. As DMFC operates at a considerably low temperature, the risk of thermal runway is very minimal, hence, making DMFCs more reliable. DMFCs also produce relatively low emissions compared to other types of fuel cells, as they only emit water and carbon dioxide [2].
A fuel cell’s performance is greatly affected by its catalyst support materials [3]. Silica-based materials have been researched for their ability to act as anode catalyst support materials in DMFCs [4]. Among all silica-based materials, rice husk ash (RHA) stands out due to its unique and effective performance in fundamental and applied research. In this paper, porous-structured silica nanoparticles sourced from rice husk ash (RHA) will be hybridised with MXene and act as an anode catalyst support material in DMFCs. RHA, with its porous-structured silica nanoparticles, was chosen because it was expected to be ideal as a support, given its large specific surface area, high durability, mechanical and thermal stability, excellent dispersibility in aqueous media and high resistance to corrosion [5,6]. RHA composed of a high percentage of silica, making it a suitable material for use as a compound in electrodes or in the electrolyte of energy storage applications such as batteries and fuel cells. Moreover, rice husks are abundantly available and are considered as an agricultural waste material, thus making RHA a low-cost and appealing addition in electrochemical applications [7]. Using RHA in electrochemical applications can also reduce the environmental impact caused by the disposal of rice husks as waste. RHA can be ground into a small nanoparticle size with a high surface area to turn it into an effective material for use in catalysts and in the formation of electrodes. In addition to that, RHA is also chemically stable and does not deteriorate easily [8]. RHA can also improve the electrochemical properties of the electrodes in batteries and fuel cells, increasing their efficiency and performance.
MXene, the main component in this research, is also known as 2D transition metal carbide and nitride which is derived from the layered carbides (MAX) phase through etching and exfoliation of the aluminium phase. MXene, in general, has high electrical conductivity, which makes it useful for electrochemical applications such as energy storage and electrocatalysis. The large surface area resulting from the etching and exfoliation of its layers makes it useful for applications such as electrocatalysts, where a large surface area is needed to increase the rate of catalytic reactions [9]. MXene is highly stable in aqueous and non-aqueous electrolytes, which makes it a good candidate for electrochemical applications such as batteries and supercapacitors [10]. Meanwhile, due to its 2D-layered structure, MXene offers an effective mechanical improvement due to its large surface area. Individual MXene layers are very thin and small in size, measuring less than one nanometre, so MXene may be easily combined with various other materials such as RHA [11]. MXene can be made from inexpensive and abundant materials for some of its components, opening a possibility for it to be a cost-effective option for many electrochemical applications. MXene can be functionalized with a variety of chemical groups, making it versatile for a wide range of electrochemical applications [12]. In various research studies, MXene has exhibited good performance as an electrode electrocatalyst in different energy storage applications. In some cases, MXene showed electrochemical improvements, possibly due to its unique characteristic of having terminated functional groups on top of its multi-layered structure [13]. These physical and chemical properties help to affect the structure and properties of the catalyst. Overall, MXene’s unique combination of properties, including high conductivity, large surface area, high stability, low cost, versatility and environmental friendliness, make it a promising material for many electrochemical applications.
Some of the drawbacks that can be found in DMFC are the methanol crossover issue and stunted activity catalysts. Meanwhile, in some reports, it is found that DMFC exhibits a decrease in ionic conductivity at high temperatures. On top of that, the expensive cost of platinum (Pt) has been the main concern of developing DMFC for commercialization [14]. In some other research, graphene oxide and a sulfonated derivative were added to a Nafion membrane, resulting in improved proton conductivity, and thus, solving the methanol crossover issues [15]. In another study, to solve the expensive cost of Pt, a minimal amount of Pt was used and it was then coated with layers of graphene [16]. On the other hand, improved catalyst activity was recorded when mesoporous carbon was added as a support in DMFC, thus, less Pt was required [17]. The permeation of methanol across the electrolyte membrane might hinder the process of the methanol oxidation reaction and disrupt its kinetics. In some cases, fuel efficiency reductions are reported when mixed potential occurs during the process of methanol diffusion from anode to cathode through the membrane. Due to this, not only is lower cell potential observed, but it also wastes fuel [18]. In another report, when they supplied DMFC with a high concentration of (above 1 M) methanol solution, a discernible decrease in total cell potential was observed due to the same issue of methanol crossover [19]. To solve this methanol crossover issue, novel modifications to the proton exchange membrane can be conducted. Furthermore, using different catalyst-loading at the anode and cathode can also help in resolving the same issue [20].
In the current study, the hybridisation of RHA and MXene as an anode catalyst support material was investigated for the first time. While both RHA and MXene have displayed great potential in different study areas, they are rarely hybridised and explored as an anode catalyst support material. To date, no investigations have been published on the hybridisation of RHA and MXene to be employed as an anode catalyst support material. The performance of the hybrid composite underwent extensive characterisation analysis to observe its microstructural aspects before and after the hybridisation process. Moreover, a series of electrochemical analyses comprising, for example, LSV, were carried out to investigate the electrochemical performance of the hybrid as an anode catalyst.
2. Materials and Methods
2.1. Hybrid RHA/MXene Preparation
Multi-layered Ti3C2 MXene was prepared by selectively etching the Al layer from its corresponding MAX phase (Ti3AlC2) using the LiF-HCl acidic etching process, as described in the literature [21]. Briefly, MAX phase was added slowly into an acid etchant solution containing 15 (±3) mL of 12 M hydrochloric acid, HCl containing pre-dissolved LiF, under constant stirring at 35 (±2) °C for 24 h. The resulting mixture was then washed with deionised water using the centrifugation method repetitively until it was neutral and had a pH of around 7 (±0.5). The mixture then underwent vacuum oven-drying to produce MXene in powder form. The RHA powder was first processed using the alkali-extraction method to remove any impurities before being added to 1.5 M NaOH solution and mixed through constant stirring for 1 h under heat treatment. The solution was then titrated against 1.5 M hydrochloric acid, HCL, until it became a neutral solution with a pH of around 7 (±0.5). The etched MXene was then slowly dispersed into the processed RHA solution under continuous stirring at 35 (±2) °C for 24 h. The resulting mixture was then dried in a vacuum oven at 70 (±2) °C for another 24 h to obtain hybrid RHA/MXene in powder form.
2.2. Electrode Preparation
To form electrocatalyst ink, 5 (±2) mg of the hybrid composite powder was mixed with 30 µL of 5 wt. % Nafion, 130 (±5) µL of DI water and 130 (±5) µL of isopropyl alcohol. The mixture in the container was stirred using an ultrasonic bath for 2 h to ensure it was properly dispersed. Then, 1.6 (±0.3) µL of the mixed ink was pipetted onto a glassy carbon electrode (GCE) before being left overnight at room temperature to dry before it was tested.
2.3. Electrochemical Measurement
An electrochemical measurement of the hybrid RHA and MXene electrocatalysts was carried out using a Gamry Potentiostat (Model: Interface 1010) electrochemical workstation. Electrochemical analysis was used to investigate the methanol oxidation performance. Linear Sweep Voltammetry (LSV), Electrochemical Impedance Spectroscopy (EIS) and chronoamperometry (CA) were also performed. Three electrode setups were arranged such that the glassy carbon electrode (GCE) with an area of 0.0707 (±0.03) cm−2 was the working electrode, Ag/AgCl was the reference electrode and a Pt rod was the counter electrode. The electrolyte solution used was 1.0 M CH3OH and 0.5 M H2SO4. LSV analysis was undertaken at a 50 mVs−1 scan rate, and the range of potentials was from 0 to 1.0 V vs. Ag/AgCl at room temperature. EIS analysis was carried out with a frequency range from 0.01 Hz to 1 MHz. CA analysis was carried out at −0.2 V for 3600 s.
3. Results
The synthesised hybrid RHA/MXene composites were analysed through structural characterisation and electrochemical analysis to determine their significance as an electro-catalyst in the DMFC application. To further confirm the effective dispersion of both materials within the hybrid composite, EDX analysis was carried out. In addition, XRD analysis was utilised to examine the chemical structure of the samples.
3.1. Hybrid Composites Structural Characterisation
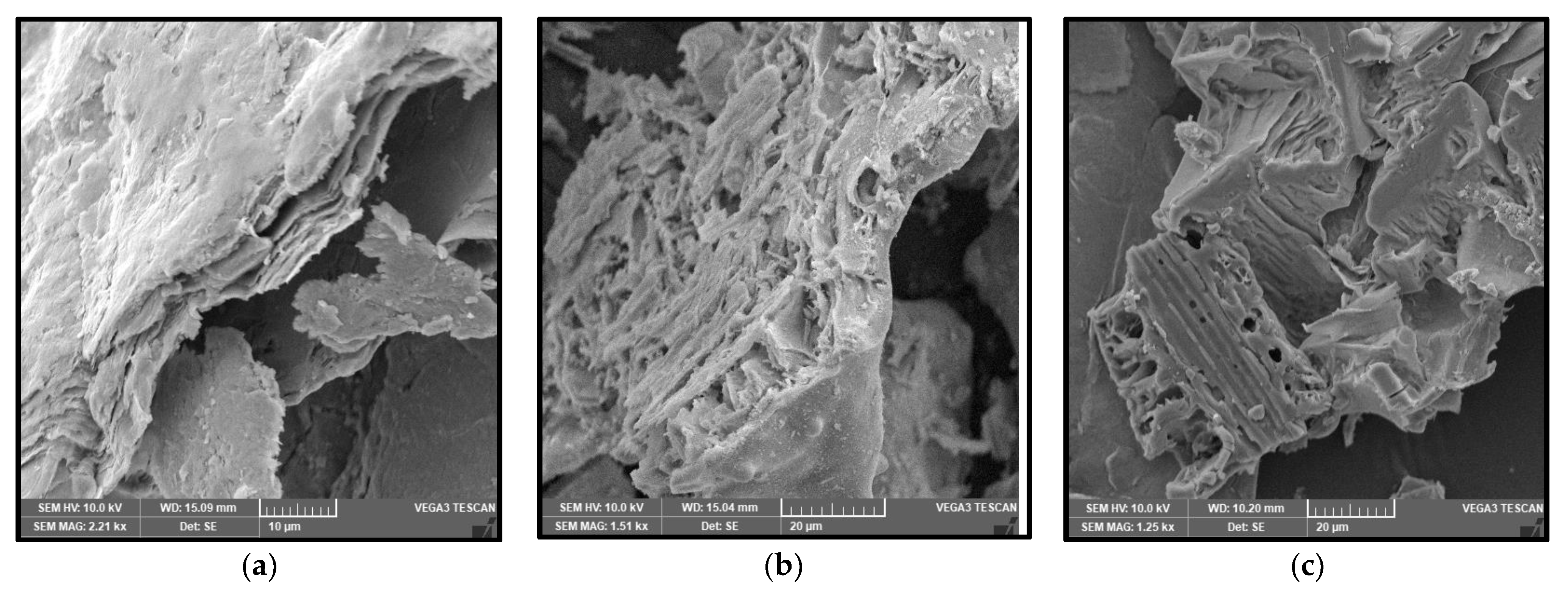
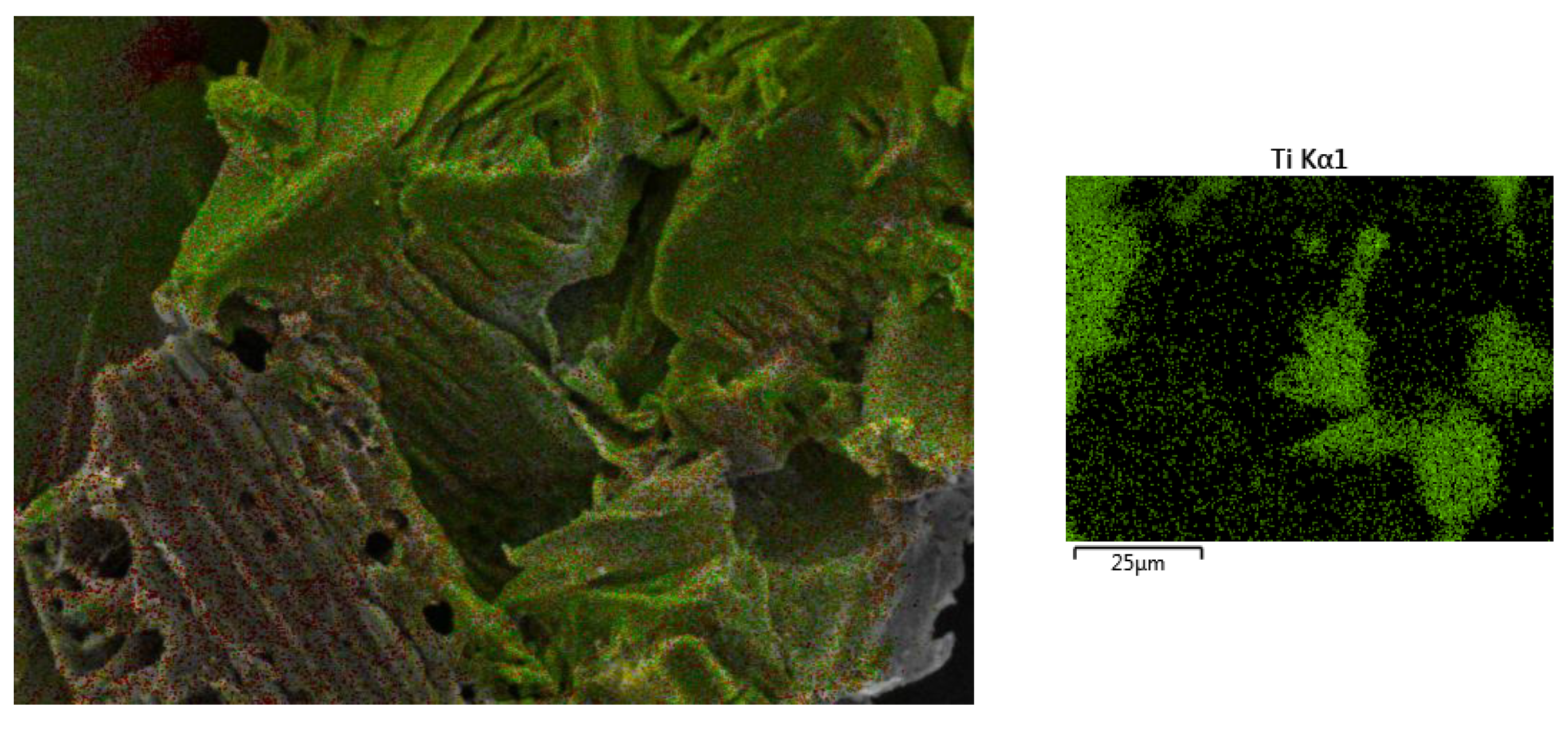
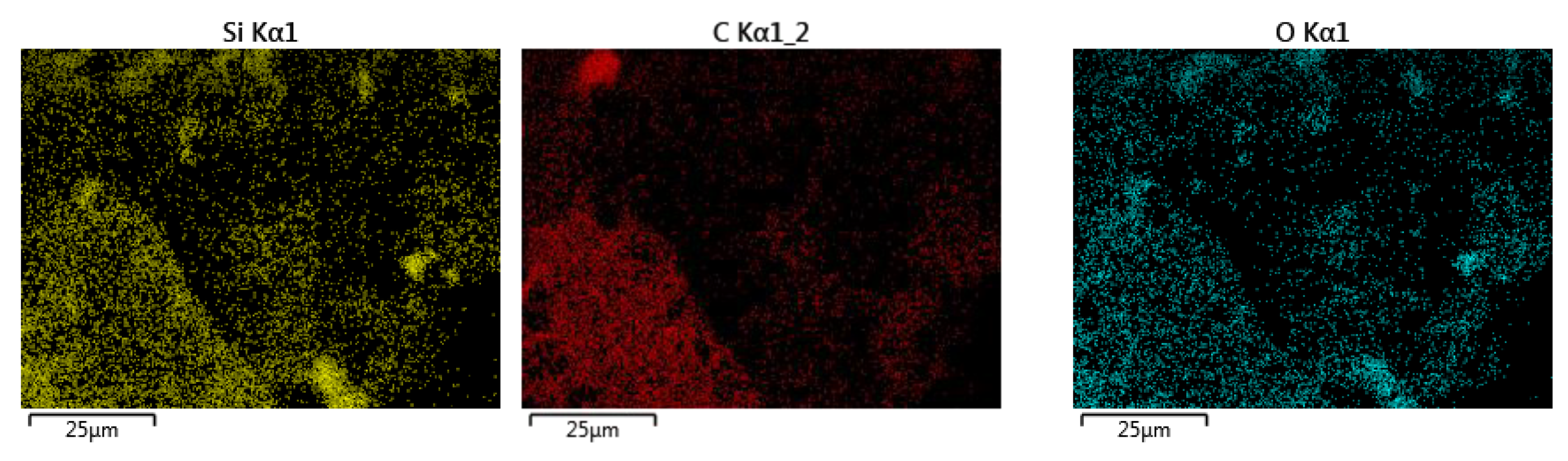
Characterisation using Scanning Electron Microscopy (SEM) was carried out to identify the success of the MXene etching from its MAX phase. Al layers were removed from the precursor MAX phase, leaving behind the multi-layered Ti3C2. One distinctive proof of successful MXene synthesis is the shape of the accordion-like layered structure of the synthesised products. SEM under high magnification revealed that multiple layers of MXenes had been successfully produced after the Al layers had been removed from them. With the Al layer removed, the interlayer distance increased, with the sheets being spread apart from each other because of the Al etching using LiF-HCl as the etchant. It can be observed from Figure 1a that the MXenes lamination has been successful, based on the noticeable separation between the layers of MXene. This finding is in agreement with the literature [22], whereby the in situ LiF-HCl was able to etch the Al layer from the precursor MAX phase, leaving behind the 2D-layered nano MXene, the layers of which had a noticeable laminated accordion-like structure. The size of the individual MXene layers was less than 1 µm, with a separation between the interlayer of the synthesised MXene. In Figure 1b, the RHA microstructure under SEM analysis can be seen. RHA is a porous substance and the microscopy image clearly shows the nano-sized porous structure of the RHA. RHA is mainly comprised of silica structures, which are highly porous as a result of the organic compound volatilisation during the preparation stage of the RHA compound [23]. As shown in Figure 1c, the synthesised hybrid RHA/MXene, hereafter referred to as R-MX, was analysed using SEM microscopy. The structure of the composite was shown to be composed of both porous and multi-layered structures, indicating the successful binding of both RHA and MXene in the hybridisation process. Energy Dispersive X-ray spectroscopy (EDX) was carried out to analyse the elemental composition of the synthesised hybrid R-MX composite. Firstly, the presence of certain chemical components in both MXene (Ti3C2) and RHA (SiO2) can be seen in the elemental mapping spectroscopy, as shown in Figure 2. Both RHA and MXene were well dispersed throughout the hybrid composite structures, indicating the successful homogeneity of both components during the hybridisation process [24].
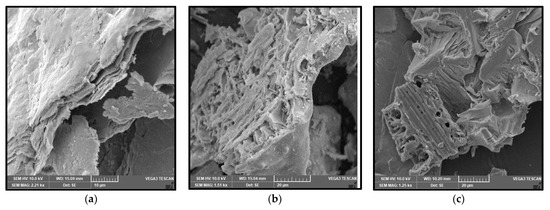
Figure 1.
(a) Multilayer structure of MXene; (b) porous structure of RHA; (c) hybrid R-MX composites under SEM characterisation.
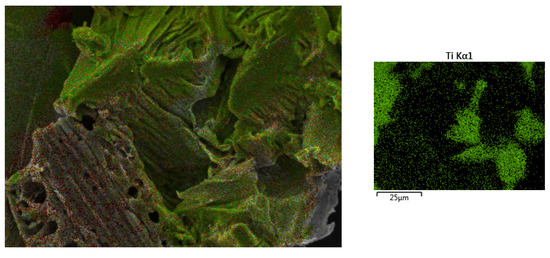
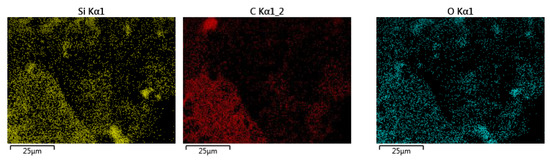
Figure 2.
EDX elemental mapping of the R-MX composite.
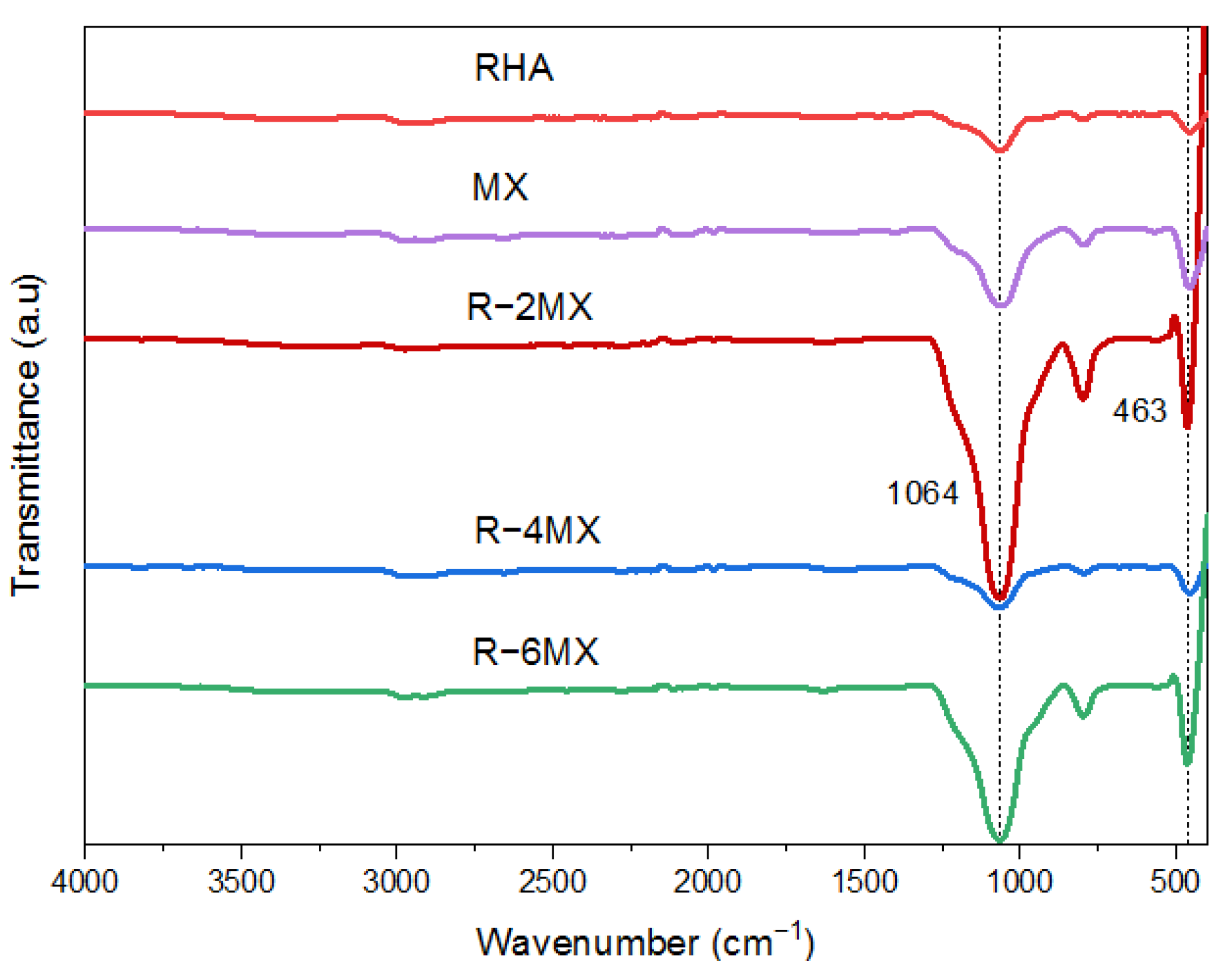
Further chemical characterisation of the hybrid composites was undertaken using Fourier Transform Infrared Spectroscopy (FTIR) to identify the chemical bonding of the particles. The FTIR analysis was carried out within a wavelength range from 4000 cm−1 to 400 cm−1, as shown in Figure 3. The spectrum obtained represents the molecular identification of the samples. The IR spectrum of the delaminated MX had a vibrational mode at 463 and 1064 cm−1, which was attributed to the -OH functional group out-of-plane vibrations of MXene [25]. The small peaks observed in the RHA IR spectrum started to increase as MXene was hybridised into the RHA matrix. Obvious increases in intensity occurred in R-2MX and R-6MX at 463 and 1064 cm−1, respectively. This finding showed that a synergistic quantity of MXene had been incorporated successfully into the RHA matrix. The IR absorption spectra of R-2MX, R-4MX and R-6MX hybrids had broad peaks, corresponding to vibrational bands at 1064 cm−1. These were attributed to the OH/H2O adsorbed on the RHA surface since these bands were observed to be low in the case of delaminated MX flakes.
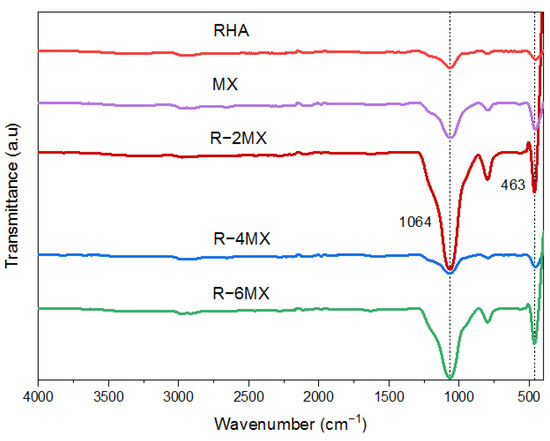
Figure 3.
FTIR analysis of different weight percentages of R-MX composites.
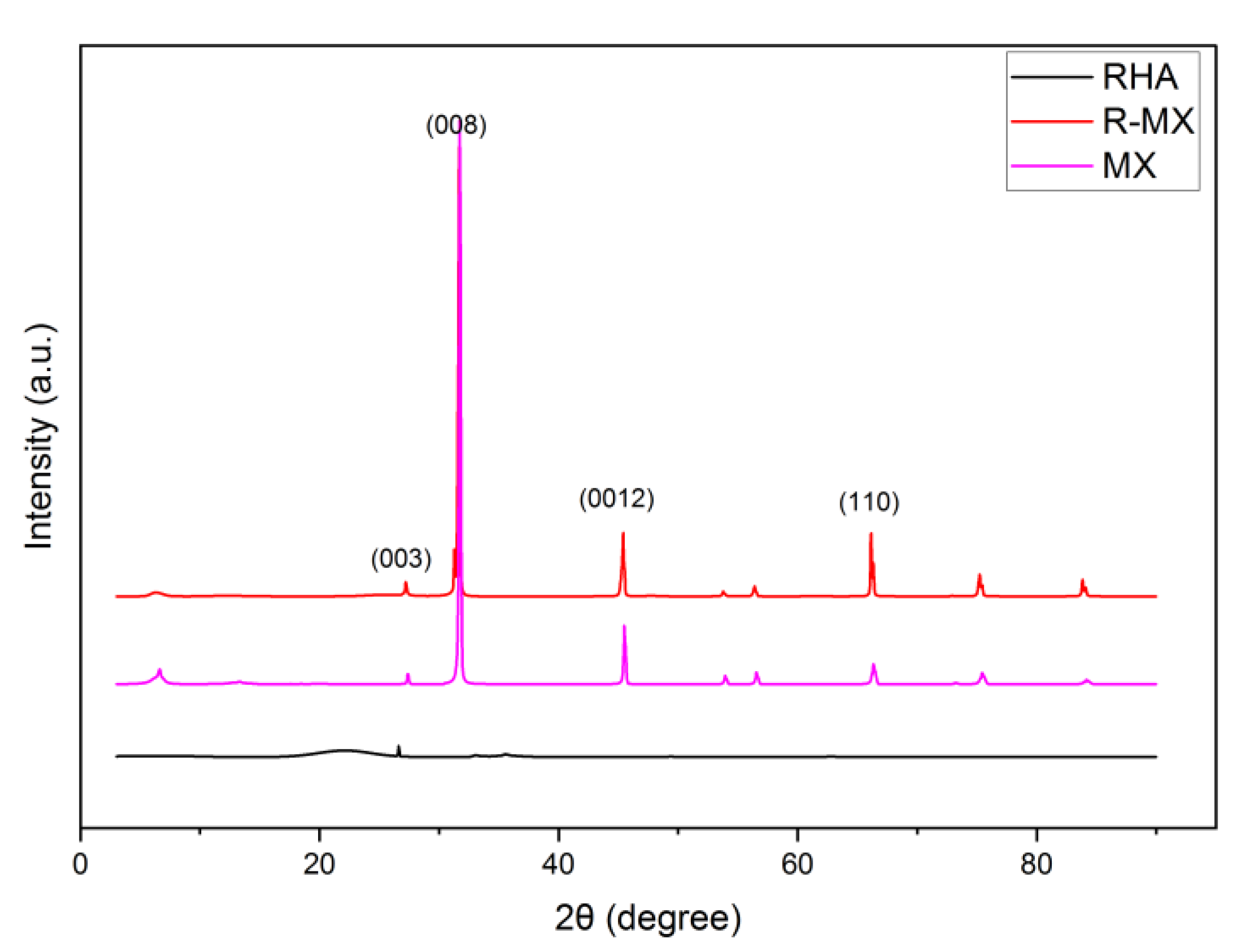
XRD analysis was carried out to assess the chemical structure of the plain RHA and hybrid R-MX, which can be seen in Figure 4. In the diffractogram of RHA, the peak rises at 28.22° (2θ), representing the leading quartz silica phase (003) of the RHA belonging to the tridymite phase of silica (JCPDS no. 16–0152). The RHA diffraction peaks show some crystalline patterns. Meanwhile, amorphous silica peaks are visible in the slight bump between 20° and 30° (2θ) [26]. For the R-MX diffraction pattern, obvious peaks can be observed at angles of 2θ ≈ 32.75°, 42.6° and 63.52°. These angles of 2θ represent the crystal faces of (008), (0012) and (110) of the MXene chemical structure, respectively [27]. The highest peak can be observed at 2θ ≈ 32.75°, which corresponds to the (008) plane. This might be due to the successful removal of the Al layers from the MAX phase, leaving the synthesised MXene (Ti3C2). The effect of incorporating MXene during the hybridisation process of the R-MX composites was indicated in the broadening and rising peaks of the XRD pattern moving on to a high angle of diffraction. Two rising peaks at 2θ ≈ 42.6° and 63.52°, representing the (0012) and (110) planes, might occur due to the increased interplanar spacing between the layers of MXene and, in addition, the porous structure of RHA. These would result in an expansion of the unit cell volume [28].
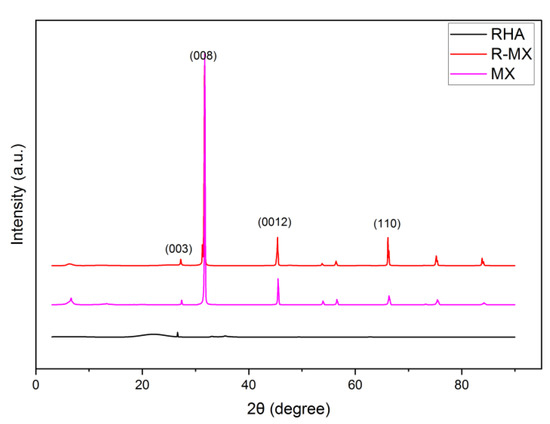
Figure 4.
XRD analysis of plain RHA and R-MX composites (JCPDS no. 16–0152).
3.2. Electrochemical Performance Analysis
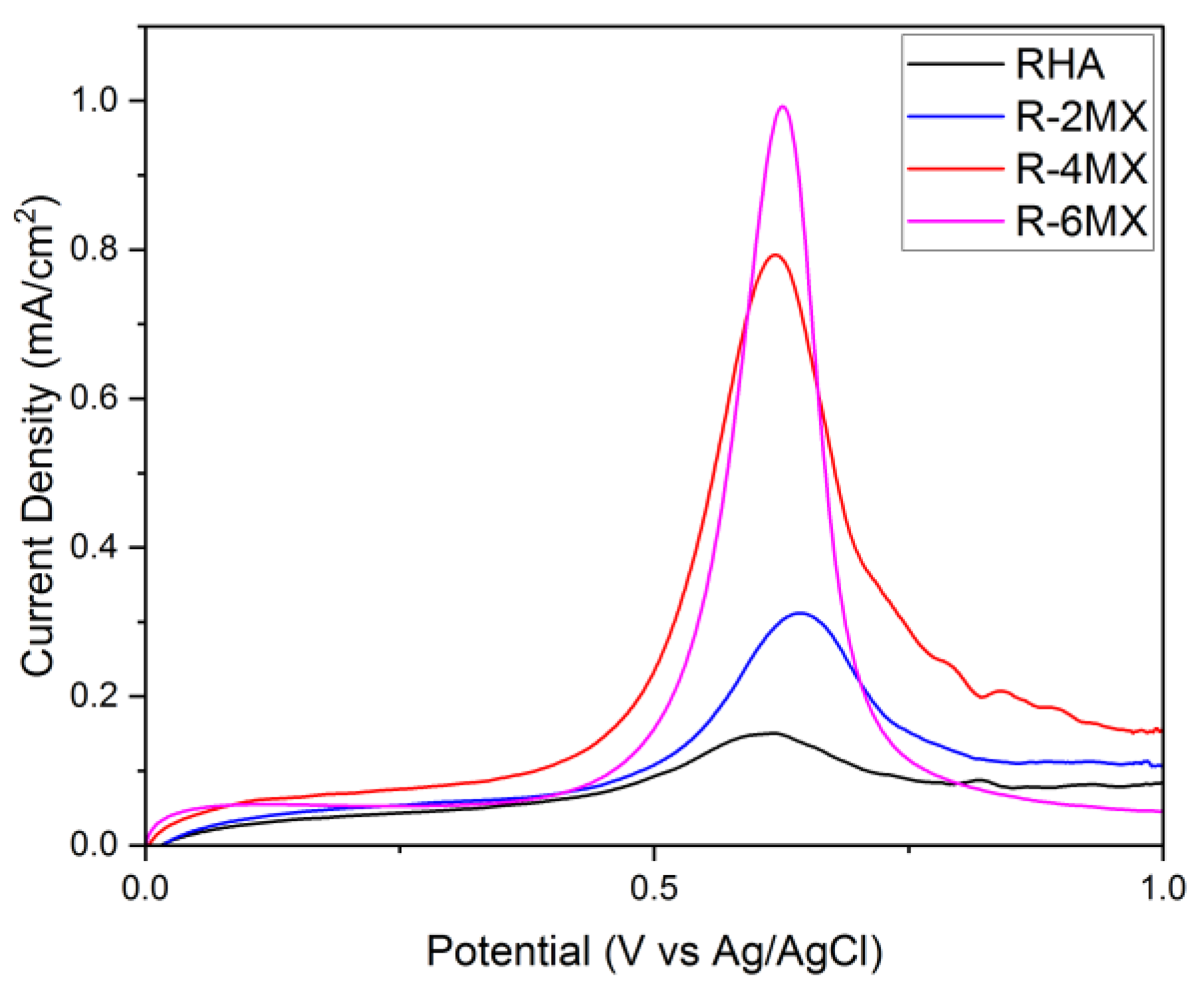
Figure 5 shows the linear sweep voltammetry results for plain RHA and hybrid RHA and MXene, that is, R-MX with different compositions of different MXene percentages. As the above results illustrate, an obvious increase in peaks occurred upon modifying the RHA hybridisation with different compositions of MXene. Upon the addition of MXene, R-2MX, R-4MX and R-6MX had their peak increases. These occurred in that particular order, with 6 wt. % of RHA-MXene having the highest peak of all the composites. This finding was likely due to the presence of MXene in the nanoporous RHA, which assisted in increasing the microscopic surface area, as well as improving the catalytic effect by enhancing the electron transfer rate [29].
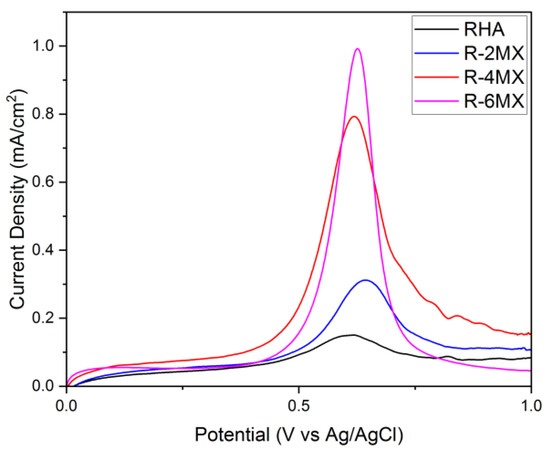
Figure 5.
LSV analysis, current density against the potential of different composites of R-MX.
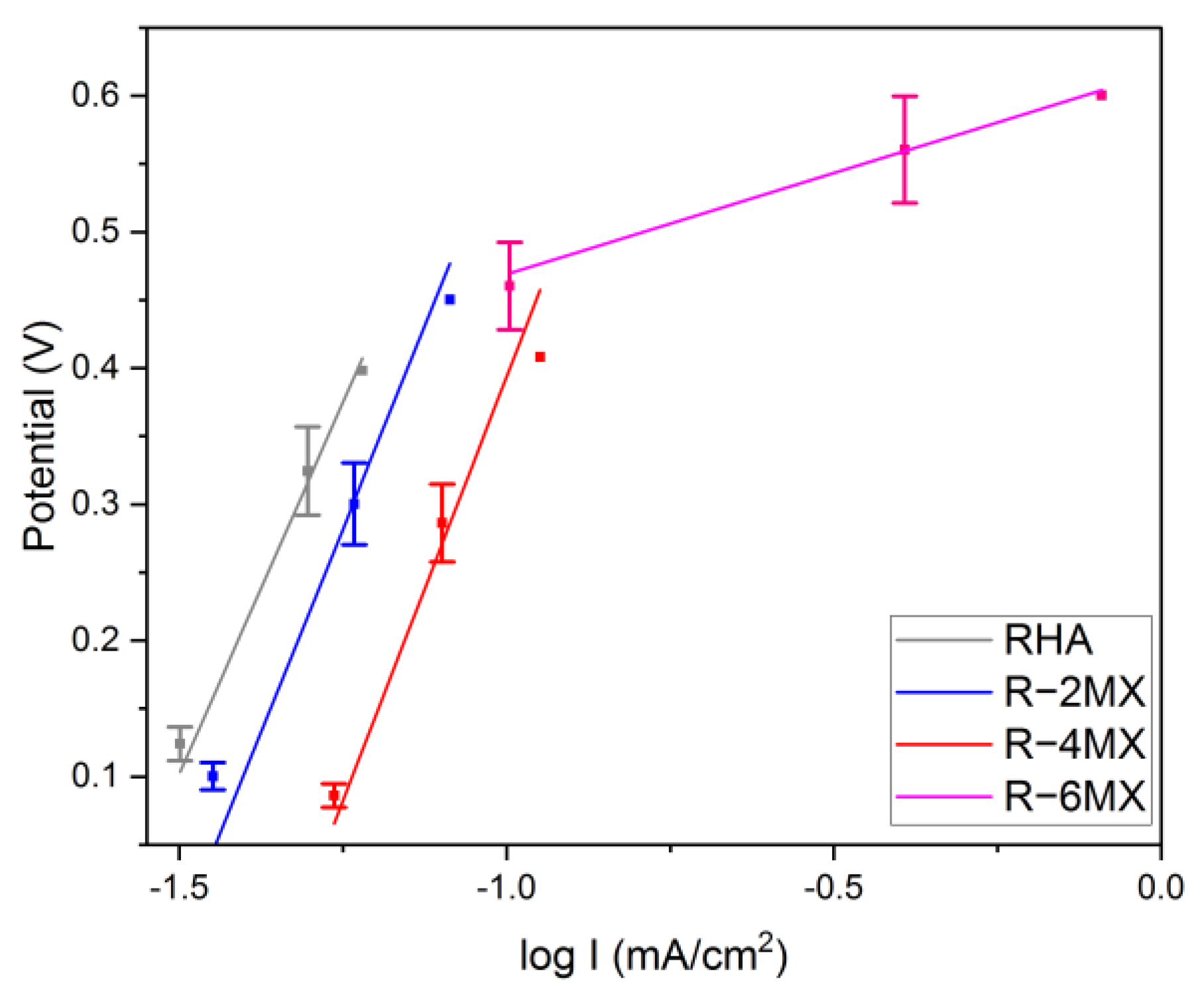
Having obtained the LSV results, further data were derived to perform the Tafel analysis. In this analysis, the electrochemical oxidation reaction rate was derived in correlation to the overpotential. The analysis was carried out using the Tafel equation, as follows:
in which η represents the overpotential in the system. Meanwhile, ‘b’ represents the Tafel slope, I is the current density and ‘a’ represents the ion exchange current density [30]. The slopes obtained enabled the analysis of the anodic kinetics of the plain RHA, R-2MX, R-4MX and R-6MX electrocatalysts’ reactions to the methanol oxidation reaction (MOR). The Tafel analysis derived from the LSV plot is presented in Table 1. In comparison to the other electrocatalyst composites, R-6MX had the lowest slope (148 mV/dec). The progressive slope obtained shows the fast kinetics of the ionic charge transfer in the DMFC system. This shows that the highest percentage of R/MX hybrid could produce the highest catalytic activity and result in the onset of a potential shift to the negative potential [31].
η = b log(I) + a,

Table 1.
Tafel slope analysis of different weight percentages of RHA and MXene electrocatalysts.
When the value of η = 0, the value to which the Tafel line was extrapolated, as shown in Figure 6, enabled the ion exchange current density, ‘a’, to be obtained. The ion exchange current density measures the activeness of the electrocatalyst towards the electrochemical reaction in the system. Upon the hybridisation of R-MX, with the increased weight percentage of MXene, the electrocatalysts increased their ionic exchange current density value. Of all the electrocatalysts, R-6MX had the highest ionic exchange current density, which might be attributed to the incorporation of MXene into the RHA matrix, leading to the dilution of catalytic surface sites.
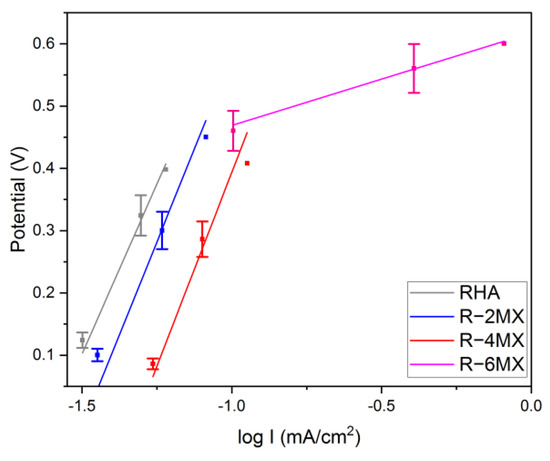
Figure 6.
Tafel plot obtained from LSV analysis.
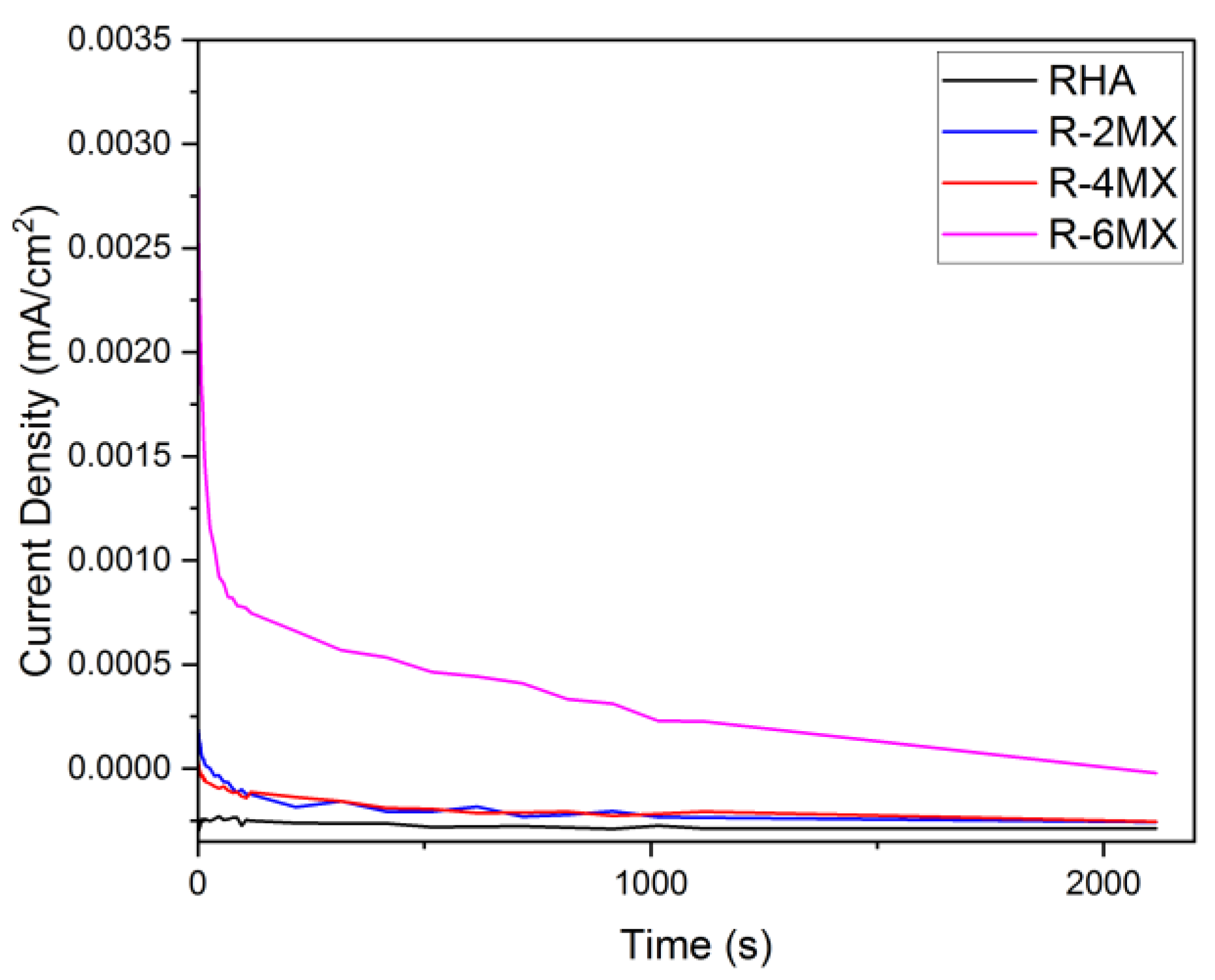
Figure 7 shows the chronoamperometry (CA) curves for the plain RHA and R-MX hybrid electrocatalysts with different weight percentages. CA analysis was carried out at −0.2 V for 3600 s, and the electrolyte solution used was 1.0 M CH3OH and 0.5 M H2SO4 at room temperature conditions. From the CA analysis, the poisoning tolerance, catalytic stability and chemical reactions with regard to the MOR were obtained. Based on the CA analysis, the retention rates for the different weight percentage compositions of the electrocatalysts are presented in Table 2, with the standard deviation of all the values being 3.049. The highest retention rate was observed in the R-6MX electrocatalyst, which had a retention value of 97.28%. R-6MX maintained the highest initial and steady current densities over the entire period. The other electrocatalysts experienced gradual decreases in their currents over certain periods, but R-6MX was able to maintain a high current for the entire duration. The current was maintained in a stable form for some time, proving that R-6MX was the most stable, with a minimal corrosion reaction and tolerance in a methanol medium. The decaying catalytic current density values of all the electrocatalysts might have occurred due to the low potential applied and the inability to completely perform an oxidation reaction against the formation of the intermediate species [32]. Furthermore, the addition of MXene to RHA was demonstrated to have synergic effects, lowering the poisoning rate and promoting the stability of the electrocatalysts. This might have occurred due to the combination of the porous RHA structure and the layered MXene structure enhancing the total surface area and further improving the electrocatalysts’ active reaction site.
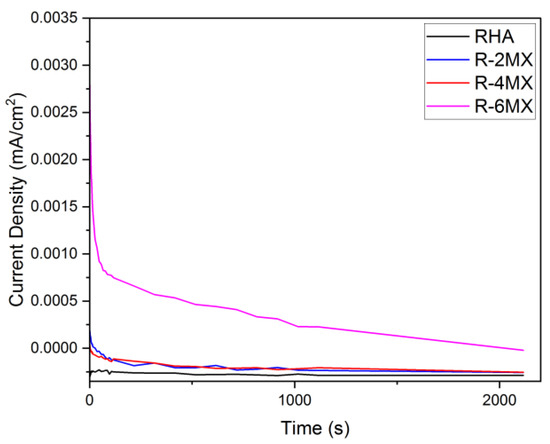
Figure 7.
CA plot of RHA and MXene with different weight percentages of composites.

Table 2.
Retention rates of electrocatalysts obtained through CA analysis.
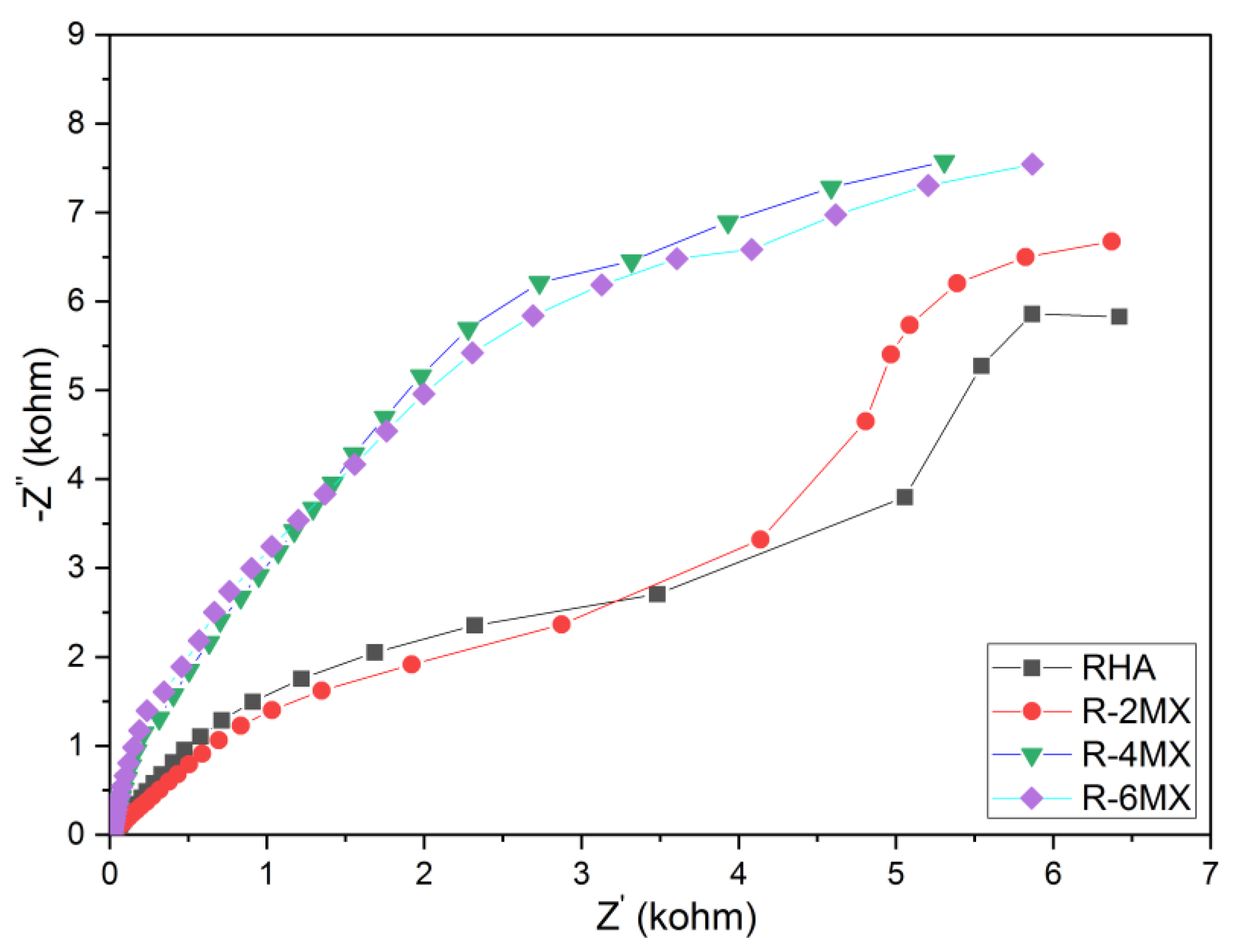
Electrochemical impedance spectroscopy (EIS) was utilized in order to qualitatively analyse the electrochemical kinetics of the hybridized R-MX composite. Through the EIS analysis carried out, Nyquist diagram were obtained and plotted. The charge transfer kinetics upon the hybridisation of R-MX was examined through Nyquist plots of the EIS analysis, as shown in Figure 8. Nyquist plots were obtained by plotting the imaginary against the real impedance at different frequencies. In comparison with the plain RHA, 2 wt. %, 4 wt. % and 6 wt. % of the RHA-MXene hybrid composite produced significantly larger diameters of the semicircle in the high-medium frequency region. The slope of the straight line in the low-frequency region highlighted the significantly improved charge transfer kinetics upon the incorporation of the MXene/RHA nanosheets [33]. As the plot demonstrates, the resistance in the electron transport decreased with the increasing MXene weight percentages. Compared to the plain RHA, all the R-2MX, R-4MX and R-6MX curves showed increasing vertical rises in the low-frequency region. The semicircle arc in the high-frequency area was the largest with R-4MX, implying the lowest interfacial charge resistance. The decreasing resistance observed upon increasing the MXene weight percentage corresponded to the pattern demonstrated in the CA analysis, in which the retention rates increased due to the lower interfacial charge resistance.
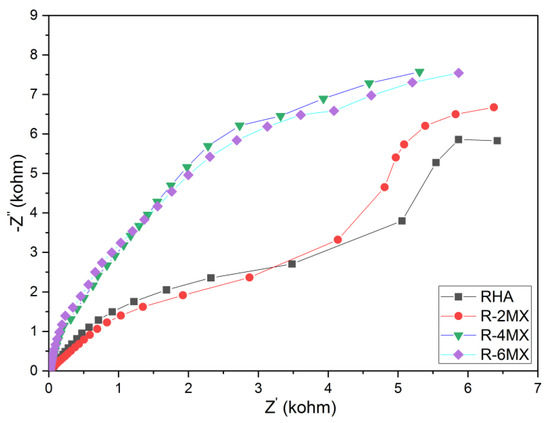
Figure 8.
Nyquist plot of plain RHA and different compositions of R-MX.
When Nyquist plots at high frequency domain are analyzed, it is suggested that both RHA and R-2MX samples have characterized a porous electrode behavior. This indicates that their electrochemical behavior is prevalent with both planar and porous electrode [34,35]. At low frequency domain, these examined samples depict slopes at 45 degrees indicating a possible charge and transport/diffusion in the electrochemical mechanism. On the other hand, from the high up to low frequency domains, both the R-4MX and R-6MX samples have depicted a similar predominant capacitive behavior.
The electrochemical performance comparison between some electrocatalysts were presented in Table 3. Different electrocatalysts used in fuel cell applications with various scan rates are listed below. The peak current density represents the current performance of the fuel cell. High current density enables a high power density performance, which means that a large amount of power can be generated or transmitted over a small area. High current density can lead to increased efficiency in electrochemical systems, opening up the possibilities in lowering the operating costs and increasing the performance of electrochemical devices. High current density can be beneficial in battery and energy storage systems, as it allows for a higher charge and discharge rate. On top of that, the high current density can bring about improvement in the kinetic reliability of the electrochemical system. Other than peak current density, the retention rate is another comparison parameter used to compare the electrochemical performance of the different electrocatalysts. The retention rate represents the reliability of an electrochemical system in terms of its stability after a period of time of usage. The value of the retention rate describes the corrosion rate and poisoning tolerance of the electrocatalyst in the methanol medium. Over time, there will be declination in catalytic activity with continuous use, most likely because of the poisoning of the electrocatalyst due to the prolonged methanol oxidation reaction. Hence, a high value of the retention rate of electrocatalysts shows good reliability and stability in the electrochemical system. A high retention rate means that the current was maintained in stable form for some time, proving that the electrocatalyst was the most stable, with a minimal corrosion reaction and tolerance in a methanol medium.

Table 3.
Electrochemical performance comparison between several electrocatalysts to R-6MX.
As listed in the table, R-6MX showed a remarkable performance in terms of having the highest peak current density of 0.9454 mA/cm2 and retention rate of 97.28%. Both of these values show that hybrid R-6MX was able to maintain high current for the entire duration. The current was maintained in a stable form for some time, proving that R-6MX was the most stable, with a minimal corrosion reaction and tolerance in a methanol medium. The incorporation of MXene into RHA was observed to have synergic effects, lowering the poisoning rate and promoting the stability of the electrocatalysts. This might have occurred due to the combination of the porous RHA structure and the layered MXene structure enhancing the total surface area and further improving the electrocatalysts’ active reaction site. In comparison to the other results from the literature listed in Table 3, the as-prepared R-6MX delivers an excellent electrochemical performance, which gives rise to the potential for this material to be utilized as a new and attractive active material for the application as an anode catalyst support material.
4. Conclusions
This study focuses on the synthesis of novel MXene-RHA nanohybrids with valuable energy functionalities as electrodes. To sum up, the addition of MXene into RHA, with its remarkable characteristics following the hybridisation processes, brought an improvement in the porosity of the hybrid composites, further enhancing the interfacial surface, ion diffusion ability and electrochemical performance. It also highlighted the exceptional porous structure and large surface area of RHA, which plays a role as the matrix for the hybrid composite. It was inferred that the successful hybridisation between RHA and MXene could also have been obtained due to the exceptional physiochemical characteristics of hydrophilicity, the small thickness, and the large surface area of MXene [40].
To sum it all, the explored MXene-RHA nanohybrids show the following exceptional advantages as an anode electrode catalyst:
- Cheap and environmentally friendly source of material;
- High electrochemical performance in electrocatalytic activity;
- High retention rate and low oxidation poisoning rate;
- Minimal corrosion reaction and tolerance in a methanol medium.
The advantages could be related to the high utilization of MXene and RHA nanohybrids as well as the favourable performance of RHA, containing a high percentage of silica as an electrocatalyst due to its high surface area and porous structure. Furthermore, the synergistic effect upon the successful hybridization of MXene-RHA nanohybrids also plays a vital role in the methanol oxidation reaction performance. All the aforementioned characteristics should encourage further research on the use of efficient electrode materials in DFMCs, while researchers should consider employing RHA as the hybridisation matrix. In the future, different MXenes such as Ti2C could be employed in the hybridisation process with RHA, which would enable a comparison with the current study. Other potential cheap waste materials such as sugarcane bagasse could also be employed as the hybridisation matrix.
Author Contributions
Conceptualisation, M.K.N., A.H.M.A., R.S, N.A. and K.H.T.; methodology, M.K.N., R.S, N.A. and K.H.T.; validation, M.K.N. and N.A.; formal analysis, M.K.N.; investigation, M.K.N.; resources, A.H.M.A., R.S. and N.A.A.; data curation, R.S, N.A. and K.H.T.; writing—original draft preparation, M.K.N.; writing—review and editing, A.H.M.A., R.S. and K.H.T.; visualisation, R.S, N.A. and K.H.T.; supervision, A.H.M.A., R.S. and N.A.A.; project administration, A.H.M.A. and R.S.; funding acquisition, A.H.M.A. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Research Management Center of Universiti Putra Malaysia (UPM/GP-IPB/2020/9688700) and Research Centre for Nano-Materials and Energy Technology (RCNMET), Sunway University.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank University Putra Malaysia for the financial support, as well as the researchers from the Research Centre for Nano-Materials and Energy Technology (RCNMET), Sunway University for their support in providing the materials, testing facilities, and advice in the course of completing this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramli, Z.A.C.; Shaari, N.; Saharuddin, T.S.T. Progress and major BARRIERS of nanocatalyst development in direct methanol fuel cell: A review. Int. J. Hydrogen Energy 2022, 47, 22114–22146. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct methanol fuel cells system–A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- de Sá, M.H.; Pinto, A.M.F.R.; Oliveira, V.B. Passive direct methanol fuel cells as a sustainable alternative to batteries in hearing aid devices—An overview. Int. J. Hydrogen Energy 2022, 47, 16552–16567. [Google Scholar] [CrossRef]
- Sarfraz, A.; Ali, A.; Ahmad, B.; Mushtaq, M.N.; Ahmad, M.A.; Raza, A.H.; Akbar, M.; Raza, R.; Syväjärvi, M. Catalytic Effect of Silicon Carbide on the Composite Anode of Fuel Cells. ACS Appl. Energy Mater. 2021, 4, 6436–6444. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrogen Energy 2017, 42, 10238–10247. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, T.S.; Xu, J.; Lin, Z.; Yan, X. Impact of pore size of ordered mesoporous carbon FDU-15-supported platinum catalysts on oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 3325–3334. [Google Scholar] [CrossRef]
- Daniel-Mkpume, C.C.; Aigbodion, V.S.; Obikwelu, D.O.N. Electrochemical analysis and microstructure of value-added functional Zn-ZnO-rice husk ash composite coating of mild steel. Chem. Data Collect. 2021, 35, 100767. [Google Scholar] [CrossRef]
- Temeche, E.; Zhang, X.; Laine, R.M. Electrochemical Performance of LixSiON Polymer Electrolytes Derived from an Agriculture Waste Product, Rice Hull Ash. ACS Appl. Polym. Mater. 2021, 3, 2144–2152. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.H.; Lee, W.-Y. An electrochemical sensor for capsaicin based on two-dimensional titanium carbide (MXene)-doped titania-Nafion composite film. Microchem. J. 2023, 185, 108216. [Google Scholar] [CrossRef]
- Bhat, A.; Anwer, S.; Bhat, K.S.; Mohideen, M.I.H.; Liao, K.; Qurashi, A. Prospects challenges and stability of 2D MXenes for clean energy conversion and storage applications. NPJ 2D Mater. Appl. 2021, 5, 61. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, P.; Sati, D.C.; Neffati, R.; Sharma, P. Recent advances in two-dimensional MXenes for power and smart energy systems. J. Energy Storage 2022, 50, 104604. [Google Scholar] [CrossRef]
- Mozafari, M.; Soroush, M. Surface functionalization of MXenes. Mater. Adv. 2021, 2, 7277–7307. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Cheng, R.; Yang, J.; Cui, C.; Zhang, C.; Wang, X. MXene-coated silk-derived carbon cloth toward flexible electrode for supercapacitor application. J. Energy Chem. 2018, 27, 161–166. [Google Scholar] [CrossRef]
- Ye, F.; Wang, Z.; Xu, C.; Yuan, M.; Liu, P.; Yang, W.; Liu, G. Mechanism and kinetic study of pulse electrodeposition process of Pt/C catalysts for fuel cells. Renew. Energy 2020, 145, 514–520. [Google Scholar] [CrossRef]
- Simari, C.; Stallworth, P.; Peng, J.; Coppola, L.; Greenbaum, S.; Nicotera, I. Graphene oxide and sulfonated-derivative: Proton transport properties and electrochemical behavior of Nafion-based nanocomposites. Electrochim. Acta 2019, 297, 240–249. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Radu, T.; Biris, A.R.; Dan, M.; Voica, C.; Watanabe, F.; Biris, A.S.; Vulcu, A. Platinum nanoparticles coated by graphene layers: A low-metal loading catalyst for methanol oxidation in alkaline media. J. Energy Chem. 2020, 40, 81–88. [Google Scholar] [CrossRef]
- Li, X.; Lv, Y.; Pan, D. Pt catalysts supported on lignin-based carbon dots for methanol electro-oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 110–118. [Google Scholar] [CrossRef]
- Karaoglan, M.U.; Ince, A.C.; Glüsen, A.; Colpan, C.O.; Müller, M.; Stolten, D.; Kuralay, N.S. Comparison of single-cell testing, short-stack testing and mathematical modeling methods for a direct methanol fuel cell. Int. J. Hydrogen Energy 2021, 46, 4844–4856. [Google Scholar] [CrossRef]
- Mathew, A.S.; Naigil, B.; George, E.; Benny, E.; Baby, R. Design, fabrication and testing of a direct methanol fuel cell stack. Mater. Today Proc. 2022, 58, 400–406. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. Approaches to overcome the barrier issues of passive direct methanol fuel cell—Review. Renew. Sus. Energy Rev. 2017, 67, 1087–1104. [Google Scholar] [CrossRef]
- Shuck, C.E.; Gogotsi, Y. Taking MXenes from the lab to commercial products. J. Chem. Eng. 2020, 401, 125786. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Morsin, M.; Ahmad, M.K.; Nayan, N.; Tee, K.S. Synthesis, characterization and antifungal property of Ti3C2Tx MXene nanosheets. Ceram. Int. 2020, 46, 20306–20312. [Google Scholar] [CrossRef]
- Vieira, A.P.; Filho, R.D.T.; Tavares, L.M.; Cordeiro, G.C. Effect of particle size, porous structure and content of rice husk ash on the hydration process and compressive strength evolution of concrete. Constr Build Mater. 2020, 236, 117553. [Google Scholar] [CrossRef]
- Alnoor, H.; Elsukova, A.; Palisaitis, J.; Persson, I.; Tseng, E.N.; Lu, J.; Hultman, L.; Persson, P.O.Å. Exploring MXenes and their MAX phase precursors by electron microscopy. Mater. Today Adv. 2021, 9, 100123. [Google Scholar] [CrossRef]
- Grzegórska, A.; Wysocka, I.; Głuchowski, P.; Ryl, J.; Karczewski, J.; Zielińska-Jurek, A. Novel composite of Zn/Ti-layered double hydroxide coupled with MXene for the efficient photocatalytic degradation of pharmaceuticals. Chemosphere 2022, 308, 136191. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, J.; Singh, S.K.; Mustakim, S.M.; Patel, A.; Das, S.K.; Behera, U. Characterization and utilization of rice husk ash (RHA) in fly ash—Blast furnace slag based geopolymer concrete for sustainable future. Mater. Today Proc. 2020, 33, 5162–5167. [Google Scholar] [CrossRef]
- Ghosh, A.; Pal, H.; Das, T.; Chatterjee, S.; Das, A. Synthesis and Characterization of MXene from MAX phase. Mater. Today Proc. 2022, 58, 714–716. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Iqbal, M.Z.; Akinwande, D.; Rizwan, S. Ti3 C2-MXene/Bismuth Ferrite Nanohybrids for Efficient Degradation of Organic Dyes and Colorless Pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef] [PubMed]
- Montella, C.; Tezyk, V.; Effori, E.; Laurencin, J.; Siebert, E. Linear sweep and cyclic voltammetry of porous mixed conducting oxygen electrode: Formal study of insertion, diffusion and chemical reaction model. Solid State Ion. 2021, 359, 115485. [Google Scholar] [CrossRef]
- Ai, Z.; Chang, B.; Xu, C.; Huang, B.; Wu, Y.; Hao, X.; Shao, Y. Interface engineering in the BNNS@Ti3C2 intercalation structure for enhanced electrocatalytic hydrogen evolution. New J. Chem. 2019, 43, 8613–8619. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Wong, W.Y.; Mohd Zainoodin, A.; Lim, K.L.; Kamarudin, S.K. NiPd Supported on Mesostructured Silica Nanoparticle as Efficient Anode Electrocatalyst for Methanol Electrooxidation in Alkaline Media. Catalysts 2020, 10, 1235. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.; Wu, M.; Shi, Z.; Lin, Z.; Kang, X.; Chen, S. PdAg@Pd core-shell nanotubes: Superior catalytic performance towards electrochemical oxidation of formic acid and methanol. J. Power Sources 2018, 398, 201–208. [Google Scholar] [CrossRef]
- Lee, S.; Jin, X.; Kim, I.Y.; Gu, T.H.; Choi, J.W.; Nahm, S.; Hwang, S.J. Superior additive of exfoliated RuO2 nanosheet for optimizing the electrode performance of metal oxide over graphene. J. Phys. Chem. C 2016, 120, 11786–11796. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multi-Coatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS Investigation of the Corrosion Behavior of Steel Bars Embedded into Modified Concretes with Eggshell Contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Han, L.; Cui, P.; He, H.; Liu, H.; Peng, Z.; Yang, J. A seed-mediated approach to the morphology-controlled synthesis of bimetallic copper–platinum alloy nanoparticles with enhanced electrocatalytic performance for the methanol oxidation reaction. J. Power Sources 2015, 286, 488–494. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Zainoodin, A.M.; Pa’ad, K.M.; Lim, K.L. Investigation of palladium-mesostructured silica nanoparticles (Pd-MSN) as anode electrocatalyst for alkaline direct methanol fuel cell. Chem. Phys. Lett. 2021, 785, 139125. [Google Scholar] [CrossRef]
- Jiang, C.; Zhan, B.; Li, C.; Huang, W.; Dong, X. Synthesis of three-dimensional self-standing graphene/Ni(OH)2 composites for high-performance supercapacitors. RSC Adv. 2014, 4, 18080–18085. [Google Scholar] [CrossRef]
- Ishak, N.A.I.M.; Kamarudin, S.K.; Timmiati, S.N.; Karim, N.A.; Basri, S. Biogenic platinum from agricultural wastes extract for improved methanol oxidation reaction in direct methanol fuel cell. J. Adv. Res. 2021, 28, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shin, S.-J.; Kim, N.; Kang, B.; Piao, H.; Choy, J.-H.; Kim, H.; Hwang, S.-J. Superior role of MXene nanosheet as hybridization matrix over graphene in enhancing interfacial electronic coupling and functionalities of metal oxide. Nano Energy 2018, 53, 841–848. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).