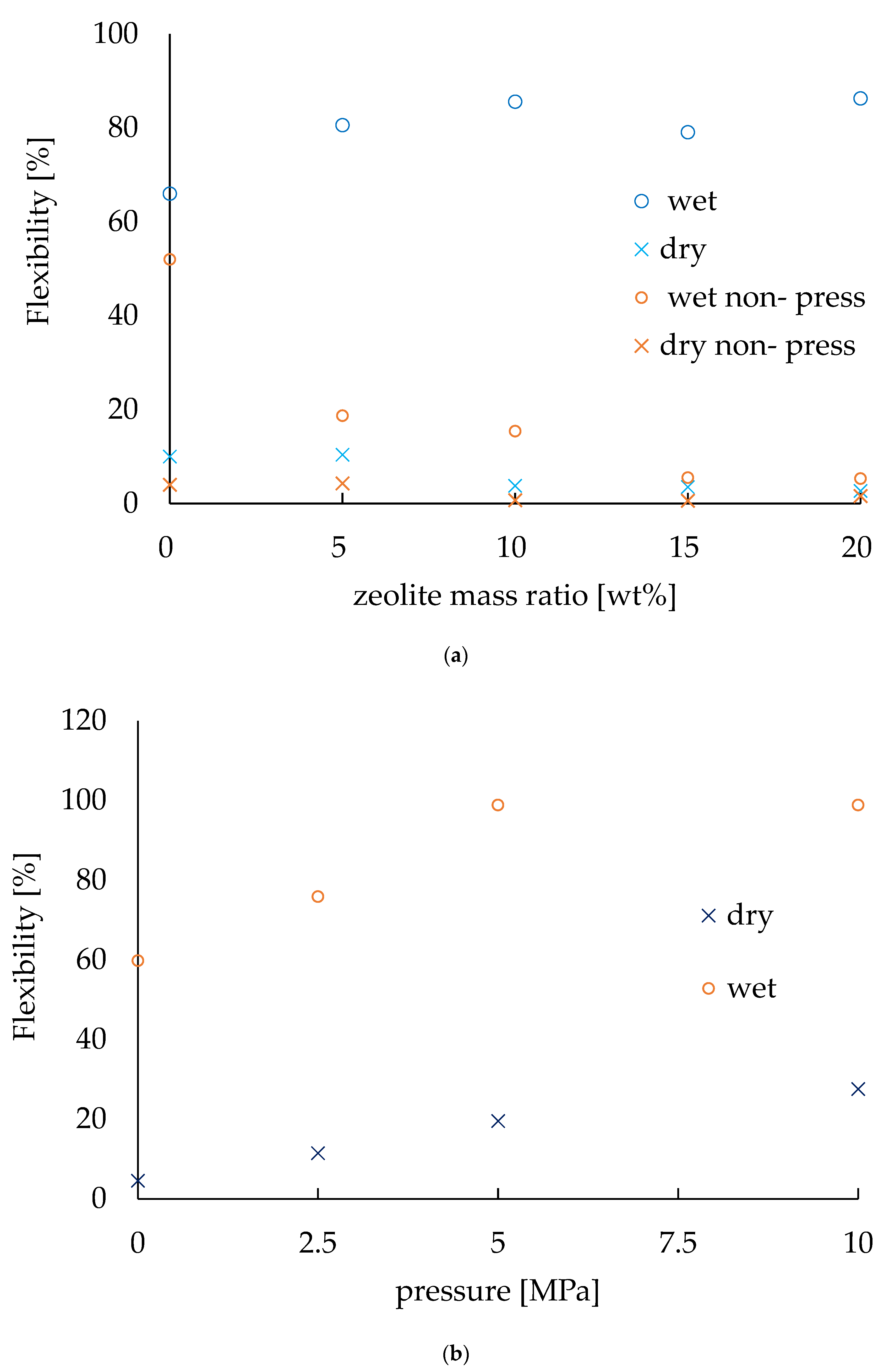Abstract
The Fukushima Daiichi nuclear power plant accident happened after the devastating earthquake in the Pacific coastal area of Japan on 11 March 2011. After the accident, radioactive materials spread out over a wide area in Japan. Radioactive materials were retained on soil surfaces, causing environmental problems. Among the radioactive materials, cesium (137Cs) has a long half-life of 30.2 years, and it remains near the surface soil; therefore, it is necessary to remove soil contaminated by 137Cs. The contaminated soil layer of inhabited areas in Fukushima was already removed before April 2020. However, the remediation method of Cs with other radioactive materials needs further study, as the large quantity of contaminated soil is not easy to preserve. Electrokinetic (EK) treatment is one of the soil remediation technologies that utilizes EK phenomena at the interface between contaminated soils by transferring ions from the soil. We have developed a new type of EK method in which a cathode is placed on the surface of the soil and an anode is place inside the soil. By applying DC voltage in between the electrodes, the Cs ions can be removed from the contaminated soil. The removed Cs ions are gathered near to the cathode, and if the cathode can adsorb the Cs ions, then only the cathode needs to be preserved, solving the problem of storing a large amount of soil. We have been working to prepare a new type of cathode that can be effective in adsorbing Cs ions and at the same time easier to store and handle. We used natural zeolite and rice husk charcoal (kuntan) to prepare this electrode, which showed good potential for adsorbing Cs ions. The electrode showed flexibility, which is helpful for storing it in the same way as pasture rolls. However, the experiments were conducted in the laboratory with non-radioactive Cs; field experiments and observations are needed for practical applications of this method, as well as the new electrodes.
1. Introduction
The accident at the Fukushima Daiichi nuclear power plant happened after the devastating earthquake on 11 March 2011 in the coastal area of Northern Japan, and spread radioactive materials (i.e., 131I, 137Cs, 134Cs, 90Sr, etc.) over a wide area [1,2,3,4]. Both 134Cs and 131I have a relatively short half-life of 2.06 years and 8.04 days, respectively, and show little impact on the environment [5]. The half-life of 90Sr is about 28.9 years, and it is highly reactive with aqueous solutions, so the deposition in the atmosphere is at the same level as before the Fukushima nuclear accident [4]. Kinase et al., (2020) estimated that it would take another 30 years for 137Cs to reach the same level of concentration in the atmosphere as before the accident. In addition, 137Cs is highly adsorbed in soil, so radioactive Cs does not reach deep into the soil [1,6]. Furthermore, 137Cs has a long half-life of about 30.2 years [1,2,6] and remains in the environment for a long time. Therefore, the remediation of soil contaminated with 137Cs is a major challenge [7,8]. Zhidkin et al. (2020) measured almost 89% of remaining Cs near a depth of ~25 cm from the surface soil in the Tulu region of Russia. Shcheglov et al. (2014) published their comparison data of 137Cs in vast pine forest areas and soil profiles in the years 1986 and 2011. They found that around 80% of remaining 137Cs were adsorbed within ~4 cm of the soil layer [9]. Yamasaki et al., (2016) discussed the adsorption of 137Cs in clay areas of sea soil where they found that more than 96% of 137Cs adsorbed in the soil layers of the sea as Cs is tightly bound to various sizes of soil particles [10]. Thus, it is clear that 137Cs is a clear threat to life in radioactive polluted areas.
Around the affected areas of Japan, a large amount of surface soil was removed and stored in an interim storage facility. The amount of removed soil was estimated at 14 × 106 m3 and the removal of the soil was performed before April 2018 [5] and the soil was stored before April 2022 [11]. The removal of soil polluted with radioactive materials and storing it for an indefinite number of years is definitely a dangerous and difficult job [6,8]. Moreover, there needs to be a lot of places to store the removed soil. Hence, effective methods are needed to purify the soil of pollutants, so that only the pollutants, not the soil, can be stored.
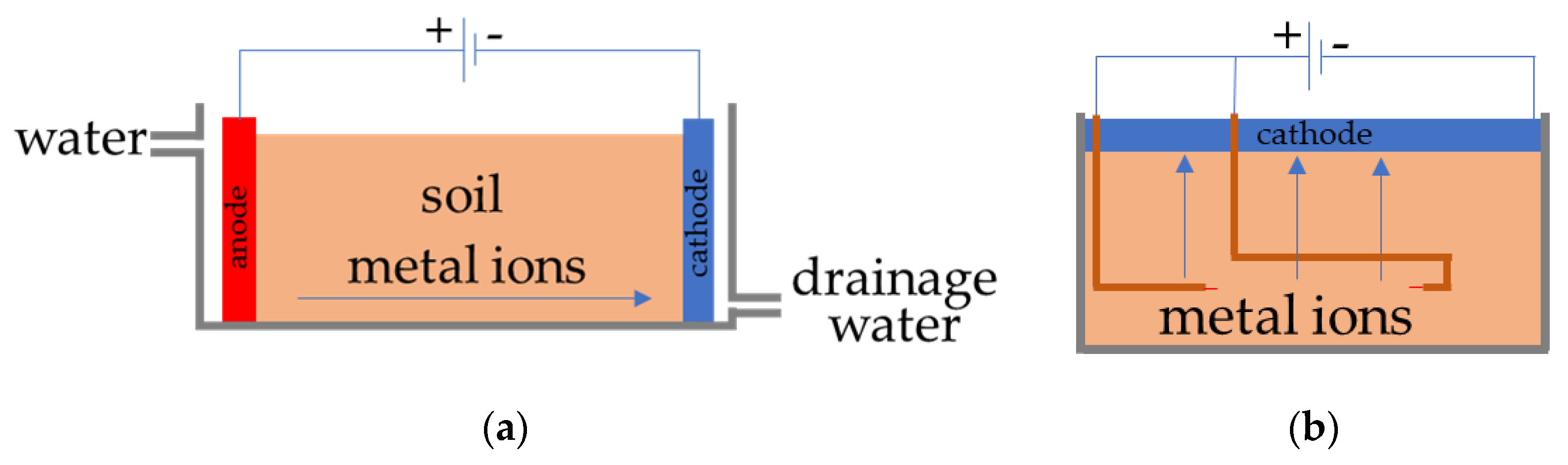
Electrokinetic (EK) treatment is one of the technologies for the remediation of contaminated soil [12,13,14,15,16]. This method deals with transferring the ions in soil using electrokinetic phenomena. Studies have been conducted on the recovery of Cs-contaminated soil by EK treatment, and the effectiveness of the treatment has been reported [13,14,15,16]. In EK treatment, electrodes are inserted into the soil and a DC voltage is applied between the electrodes. During the treatment, water is supplied from the anode side to cathode. The positive ions of metallic pollutants move to the cathode side from the anode side through the soil and thus, the soil is purified. This method can be applied in situ, almost unattended, except for the installation and recovery of the treatment equipment, and without the excavation of contaminated soil. However, it would be difficult to apply this method extensively because of the risk of secondary contamination from the wastewater generated during the EK process [8,12].
We have developed a new type of EK method to solve these drawbacks of EK treatment for soil remediation (Figure 1a conventional, Figure 1b new EK method). In this new method, a flat electrode is laid on the surface soil as cathode, and rod-type electrodes are inserted into the soil as anode electrodes [17,18]. This treatment process is called the FEM-EK (flat electrode method electrokinetic) process; it is easier to install in vast areas and it can even be installed in mountainous areas. The electric power consumption of this method was very low (i.e., 6.72 Wh) where the experiments were conducted for 7 days [17]. The cathode electrode should be able to adsorb metal ions that have been transferred to the soil surface by the EK treatment. Moreover, if the cathode electrodes are flexible enough to bend, then they can be installed on rough surfaces and after the treatment process is performed, only the cathode electrodes with the pollutants adsorbed in them need to be stored, and not the soil. Therefore, flexible ion adsorption electrodes have been fabricated for use as cathodes in the FEM-EK process [17]. Some attempts have been taken to fabricate this special type of electrode in the past by our research group, which showed the ability of Cs adsorption, but the flexibility of the electrodes was low [19,20]. However, the electrode lacks water resistance, and it was found to be difficult to maintain its shape when it was exposed to marshy soil for a certain period (around 10 days) and emitted the charcoal powder from it. Therefore, the fabrication of electrodes is necessary in order to achieve the remediation of pollutant soil using the FEM-EK process.
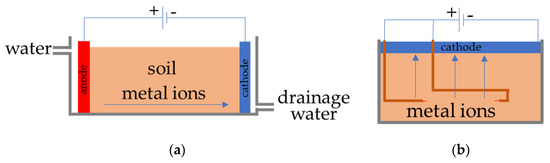
Figure 1.
Diagram of electrokinetic (EK) remediation. (a) Conventional EK treatment. (b) FEM-EK treatment.
The purpose of this study is to improve the cathode electrode for the achievement of a better performance in FEM-EK treatment. We have used the burnt charcoal of rice husks (kuntan) as conductive materials and natural zeolite to ensure the Cs adsorption quality of the electrode. Moreover, we found that applying extra pressure to the electrode during the manufacturing process made the electrodes more flexible compared to previous reports [19,20].
2. Materials and Methods
2.1. Fabrication of the Electrode
The basic fabrication method is same as described in previous papers [19,20], but a new pressing process has been added to improve the properties of the electrodes. The main material used was burnt rice husk charcoal (kuntan) for the conductive and adsorbent material. Zeolite was also used as an adsorbent material. Abaca (plant tissues) and starch glue were used to prepare the electrode. The charcoal was dried at 75 °C for 12 h using a constant-temperature dryer (STAC P-50K, manufactured by AS ONE, Osaka, Japan). Zeolite was ground with a mixer (SKS-H, TIGER, Osaka, Japan) and sieved (SKH-01, AS ONE, Osaka, Japan) to adjust the particle size to 53 µm or less. The amount of material used in one electrode fabrication was 9.1 g of charcoal, 1.0 g of abaca, and an arbitrary amount of zeolite. The process is described below.
- (1)
- 2.0 g of starch glue, zeolite, and abaca were added to 150 mL of warm water and stirred at about 1000 rpm using a stirrer (SMT-102, AS ONE, Osaka, Japan).
- (2)
- Kuntan is added and the mixture is stirred at 500 rpm using the stirrer.
- (3)
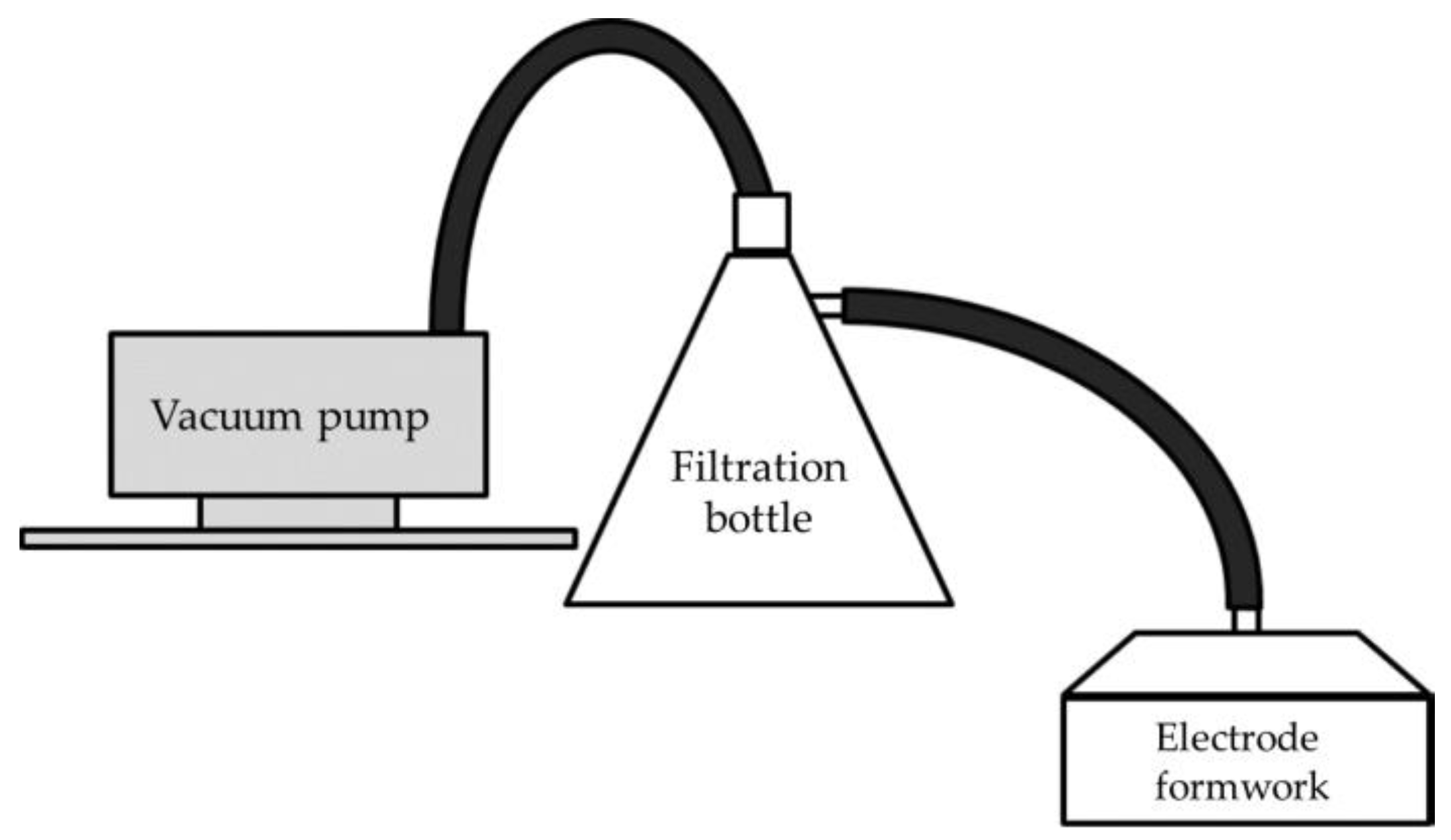
- Water is aspirated using a suction-type electrode fabrication device to dehydrate and mold the mixture (Figure 2).
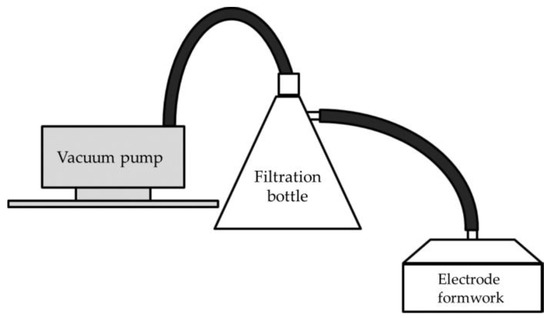 Figure 2. Schematic diagram of the electrode manufacturing apparatus (redrawn from Hatakeyama et al., 2017).
Figure 2. Schematic diagram of the electrode manufacturing apparatus (redrawn from Hatakeyama et al., 2017). - (4)
- The dehydrated electrodes are placed in a constant temperature dryer and dried at 75 °C for 12 h.
- (5)
- The dried electrode is softened by soaking it in about 10 mL of water and placed in a press frame.
- (6)
- Pressing is performed at an arbitrary pressure and time using a heat press machine (H300-01K, manufactured by AS ONE, Osaka, Japan).
- (7)
- Again, the pressed electrodes are placed in the constant temperature dryer and dried at 75 °C for 12 h.
Starch paste dissolved in warm water was used to make the warm water viscous in order to uniformly disperse the zeolite on abaca. After the rice husk charcoal (kuntan) was added, the rotation speed was decreased to prevent the charcoal from being crushed.
2.2. Flexibility Evaluation
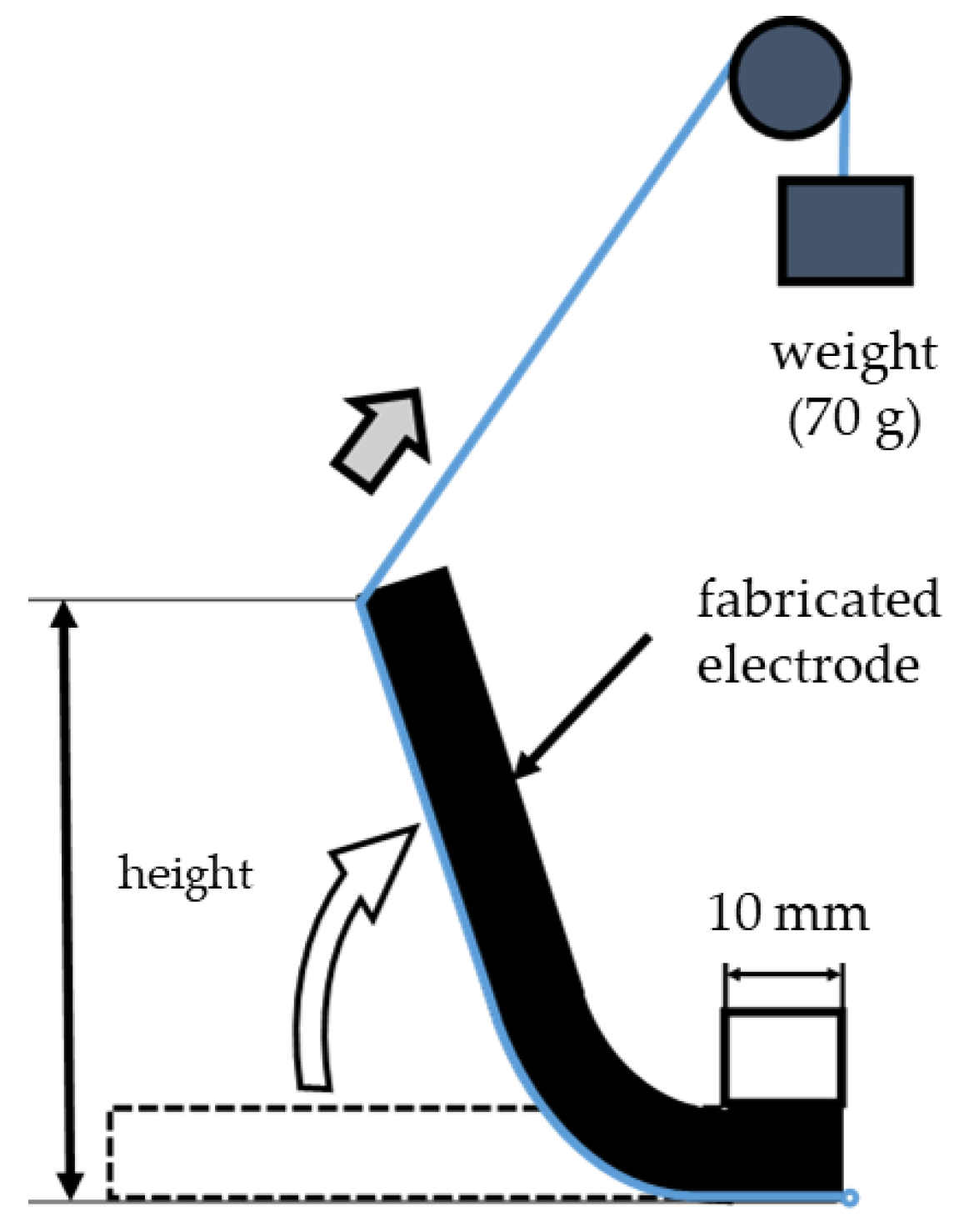
An evaluation method for flexibility was devised by referring to test examples of stress and the bending strength of the specimens and mechanical analysis methods for biofunctions, in accordance with the characteristics of the materials [21]. The dimensions of the electrodes used were 97 mm (length) and 68 mm (width), and the thickness varied with the pressing pressure. As shown in Figure 3, a 10 mm portion of the 97 mm length of the electrode was fixed, and a string with a 70 g weight was passed under the electrode. The electrode was lifted by releasing the weight naturally. The height of the lifted electrode was recorded using a height gauge (HDS-C, manufactured by Mitutoyo, Kanagawa, Japan). The maximum flexibility of the electrode was 100% when the lifted height was 87 mm (i.e., 97–10 mm). Thus, the flexibility (%) was calculated from the degree of the lifted height of the electrodes. It can be understood that in nature, the electrodes would be wetted with natural moisture during the FEM-EK treatment, so the measurements were performed in a dry state and wet state by adding tap water. Again, the change in flexibility was also evaluated with the amount of zeolite.
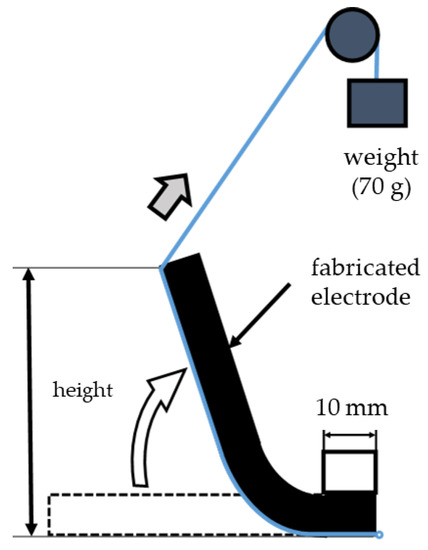
Figure 3.
Schematic diagram of flexibility measurements (redrawn from Hatakeyama et al., 2017).
2.3. Evaluation of Conductivity
To measure conductivity, acrylic plates were used to hold the electrode sample, and two copper plates were used to sandwich the sample while measuring the conductivity of the electrodes under various conditions. One of the copper plate electrodes (ground electrode) was sufficiently large for the sample, and the upper electrode (source voltage) was a circular electrode with a diameter of 20 mm. A digital ultra-high resistance/micro ammeter (R8340/8340A, ADVANTEST, Tokyo, Japan) was used to apply voltage, and the resistance was measured when 100 V was applied to the electrode in the dry state and 10 V in the wet state. The conductivity was calculated from the measured resistance of the sample electrodes and the area of the upper copper plate using the following Equation (1).
Here, σ is the electrical conductivity [S/m], R is the measurement resistance (Ω), L is the distance between the electrodes (i.e., the thickness of the electrode) (m), and S is the area of the electrodes (m2).
Tap water was dropped onto the center of the dry sample. The conductivity of the tap water was measured using a conductivity meter (B-771, HORIBA, Kyoto, Japan) and the value was 6900 µS/m. Considering the time required for the water to permeate the electrodes, measurements were carried out after three minutes of dropping water. The voltage application time was as short as possible to prevent a conductivity change due to electrolysis during the measurement.
Experiments were conducted to investigate the relationship between the zeolite mass ratio and the conductivity of the electrodes. Electrodes with zeolite mass ratios of 0, 5, 10, and 15 wt.% were pressed at 2.5 MPa for 1 min and then the tap water was dripped in amounts of 5 mL and 10 mL. Again, the resistance of these electrodes was also measured as previously described. The conductivity was calculated from the measured resistance using Equation (1).
2.4. Evaluation of Cs Adsorption
Cs adsorption experiments were conducted for fabricated electrodes cut into 2 cm in length and width. A 500 ppb Cs solution of 150 mL was prepared using a non-radioactive Cs standard solution (133Cs, 1000 mg/L, Wako Pure Chemical Industries, Ltd., Osaka, Japan) and distilled water. The electrode was fixed in the Cs solution by a copper wire for 48 h. To prevent the evaporation of the solution, the container was sealed with plastic wrap and stored in a refrigerator. After, 48 h, the electrode was removed from the Cs solution, and the solution was centrifuged at 3000 rpm for 10 min using a centrifuge (H-19FMR, Kokusan, Kyoto, Japan) to remove impurities such as pieces of kuntan. The ICP mass spectrometer (Agilent7500series, Agilent Technologies, Santa Clara, CA, USA) was used to evaluate the adsorption of Cs in the electrodes. The decrease in the concentration of cesium solution after 48 h, was divided by the mass of the electrode to calculate the amount of adsorption per unit mass [mg/kg].
3. Results and Discussion
3.1. Fabrication of Electrodes
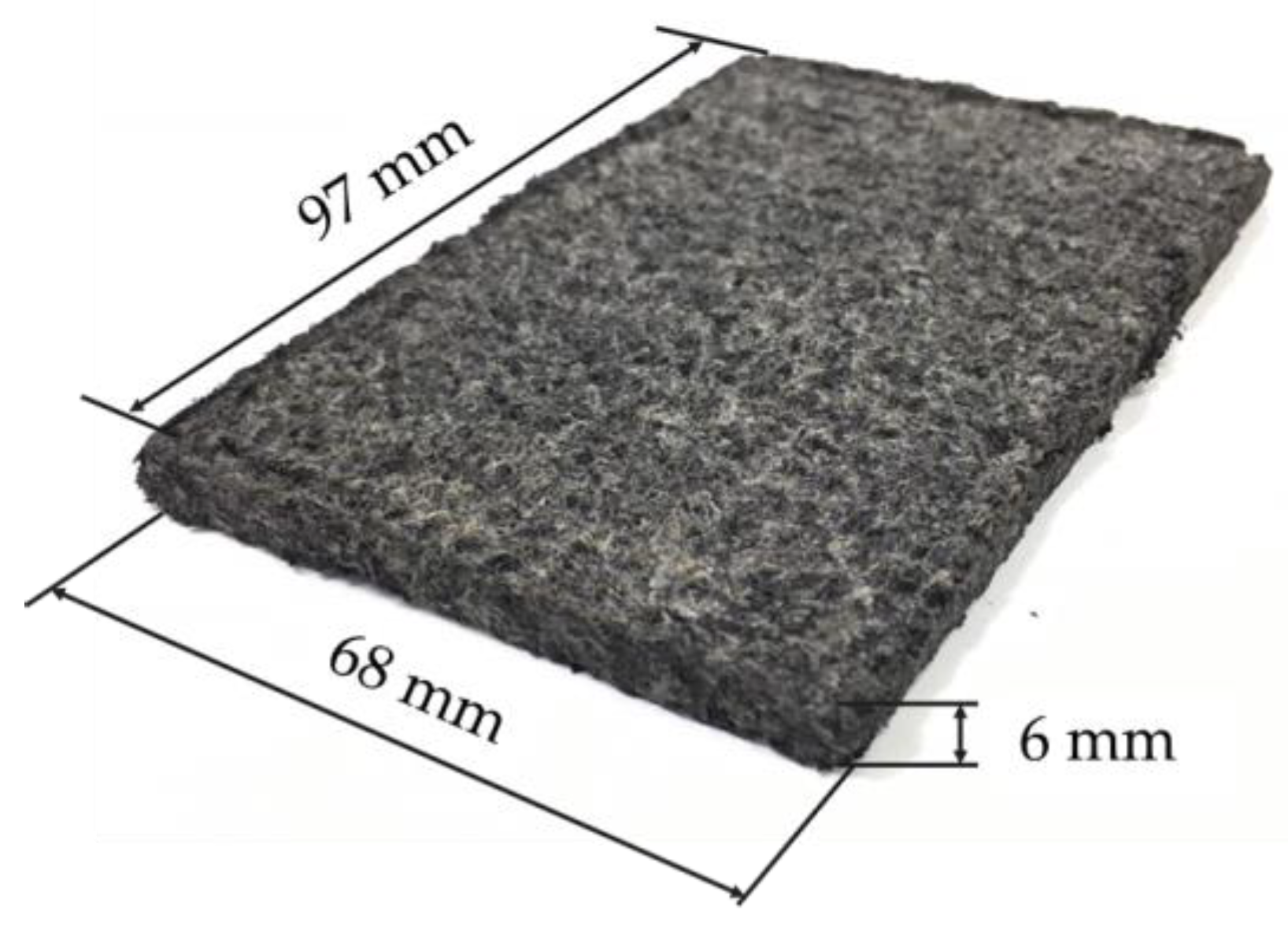
An example of an electrode made by pressing at 2.5 MPa for 1 min without adding zeolite is shown in Figure 4. The dimensions were 97 mm in length, 68 mm in width, and approximately 6 mm in thickness. The thickness of the electrode without pressing was approximately 10 mm, thus the thickness decreased to approximately 6 mm due to pressing.
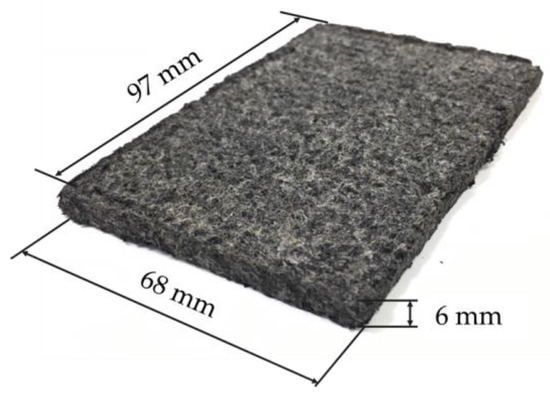
Figure 4.
An example of an electrode fabricated with 2.5 MPa pressure.
3.2. Flexibility
The flexibility [%] was calculated by dividing the lifted height of the electrode by 87 mm, which is the maximum height at which the electrode can be lifted as described before. Figure 5 shows the flexibility of the electrodes with various zeolite amounts in different conditions. The zeolite amount was chosen as 0, 5, 10, 15, and 20 wt.% in mass ratio of the electrodes to fabricate the electrodes. The pressing pressure was 2.5 MPa and the pressing time was 1 min for all the electrodes. Then, the flexibility was measured for both a wet and dry system; the results are plotted in Figure 5a. In order to evaluate the pressing effect in manufacturing electrodes, another set of electrodes were prepared with the same conditions but without any pressing and their flexibility properties were also plotted on the same graph. In the dry state, the maximum flexibility was approximately 10% without any zeolite and a decrease in flexibility was observed as the zeolite mass ratio increased. This was due to the zeolite inhibiting the flexibleness of the abaca of the electrodes. On the other hand, in wet conditions, the flexibility was about 80% regardless of the change in zeolite mass ratio. Thus, for the wet conditions, the flexibility was about eight times higher compared to that of the dry conditions. In addition, the high flexibility of the electrodes in the wet state enabled them to adhere to the soil, making it possible to conduct the EK treatment effectively. Moreover, it also enabled them to be rolled and collected after EK treatment. The flexibility of the electrodes fabricated without any pressing showed the same trends, though the values of flexibility were clearly smaller compared to those electrodes made with the pressing effect. Again, the flexibility was 4.0% (dry state) and 12.7% (wet conditions) [20], so, it is clear that this new method has improved the flexibility of the electrodes 2.5 times in dry conditions and almost 6.3 times in wet conditions.
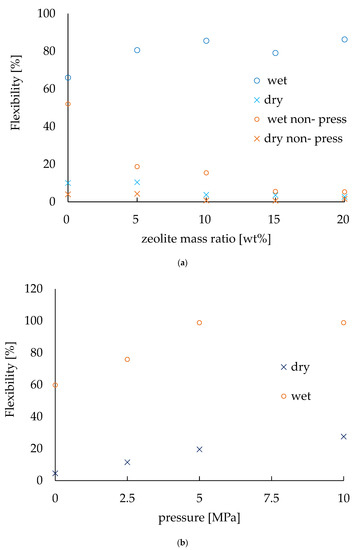
Figure 5.
Evaluation of flexibility. (a) Flexibility varies with the amount of zeolite. (b) Flexibility depends on the pressure applied during fabrication.
To investigate how the pressing effect resulted in an increase in the flexibility of the electrodes, similar experiments were conducted on the basis of pressing pressure. The electrodes were prepared with no zeolite so that the pressing effect could be evaluated without any complexity. The results are shown in Figure 5b. For both the wet and dry state, the flexibility of the electrodes tended to increase with increasing pressure of pressing. The values of the slope of pressing pressure were 2.45 in dry conditions and 3.95 in wet conditions.
From these experiments, the flexibility of the non-pressed electrode was found to be decreasing as the zeolite mass ratio increased, even in wet conditions. It indicates that the higher flexibility of the pressed electrode was not due to wetting, but to the effect of pressing. These results confirm that pressing improves the flexibility of the electrodes.
3.3. Evaluation of Conductivity
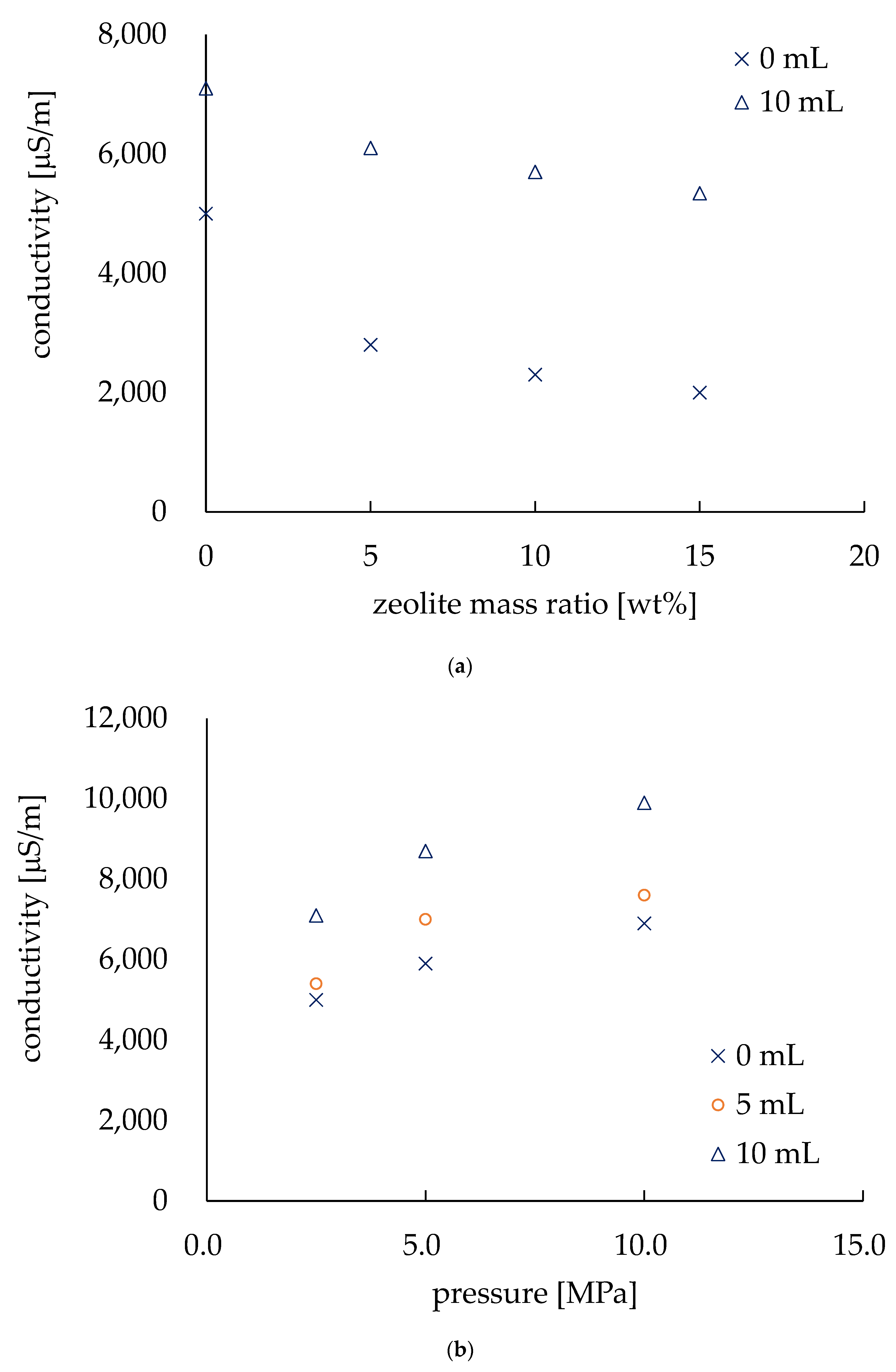
Figure 6a shows the relationship between the zeolite amount and the conductivity of the electrodes. The conductivity of the electrodes was measured in both dry and wet conditions (i.e., dripping 10 mL of tap water onto the electrode). The horizontal axis shows the amount of zeolite in terms of the mass ratio (wt.%) of the electrodes, and the values of conductivity (µS/m) are on the vertical axis. The pressure was the same (i.e., 2.5 MPa) for the electrodes in this experiment. In the dry state, the highest value of conductivity was found at 5000 µS/m, and it started to decrease as the zeolite mass ratio was increased. Zeolite is a dielectric material [8,22], thus an increase in zeolite in the electrodes decreased the conductivity of the electrodes. However, in wet conditions, the conductivity also decreased with an increase in zeolite, but due to the conductivity of tap water (6900 µS/m), a decrease in conductivity reduction was observed. We observed the effect of pressing during fabrication by changing the pressing pressure by 2.5, 5.0, and 10.0 MPa. These electrodes were made with no zeolite. An increase in conductivity was found for the electrodes in both dry and wet conditions due to the pressing pressure. The pressed electrode (in dry conditions) was approximately 6.25 times more conductive than the non-pressed electrode, as the conductivity of the electrode was approximately 800 µS/m fabricated without pressing pressure [20]. This indicates that the conductivity can be improved by pressing while fabricating the electrodes. Thus, an improvement in conductivity by pressing was confirmed. Since the previous studies have reported that EK treatment can be performed by electrodes with a conductivity as low as 10 µS/m [23], our electrodes will doubtless be eligible for EK treatment.
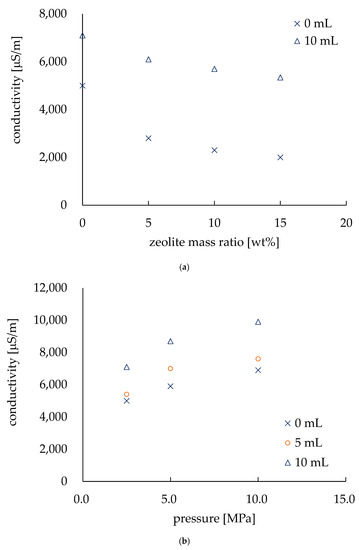
Figure 6.
Conductivity evaluation of the electrodes. (a) Conductivity varies with the amount of zeolite. (b) Conductivity depends on the pressure applied during fabrication.
3.4. Cs Adsorption
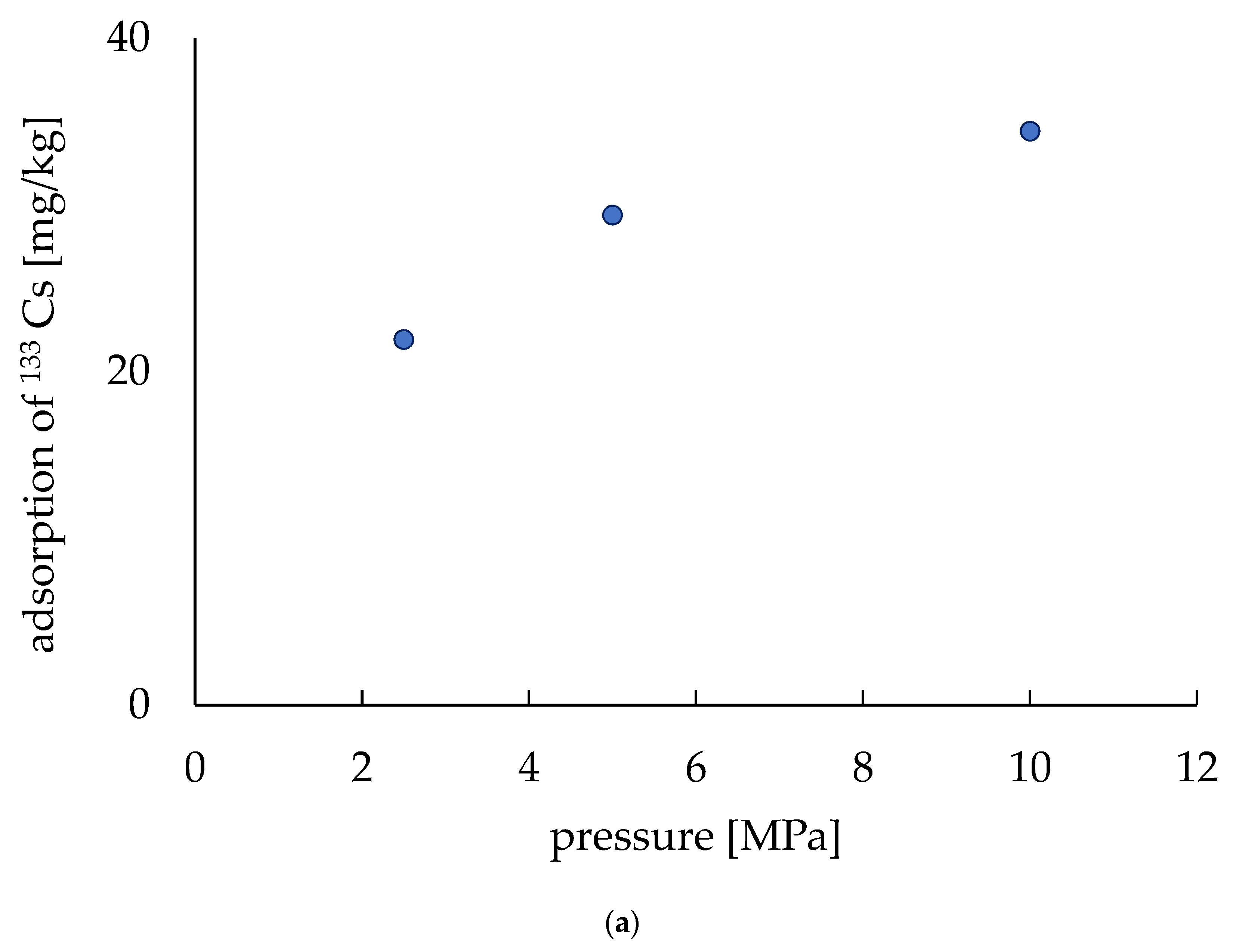
The electrodes were made with three different pressing pressures (2.5, 5.0, 10.0 MPa) and their Cs adsorption capability was evaluated. Due to our understanding of the effect of pressing on fabricating these electrodes, no extra adsorption material was added to the electrodes (i.e., no zeolite was added). Figure 7a shows the relation of pressing pressure to the Cs adsorption capability, where the adsorption capability was shown by the per unit mass (mg/kg) of the electrodes. The amount of adsorption per unit mass was low at 2.5 MPa (21.9 mg/kg) and it increased to 29.4 mg/kg for 5 MPa. It increased further when the pressure was 10 MPa to 34.4 mg/kg. The adsorption of 133Cs was found to be 16.6 mg/kg without any pressing of the electrodes according to a previous report [20]. These results indicate that a higher adsorption rate can be obtained by pressing at a higher pressure. Furthermore, as zeolite is a good adsorbent, it can be understood that adding zeolite will make the electrodes more adsorbent.
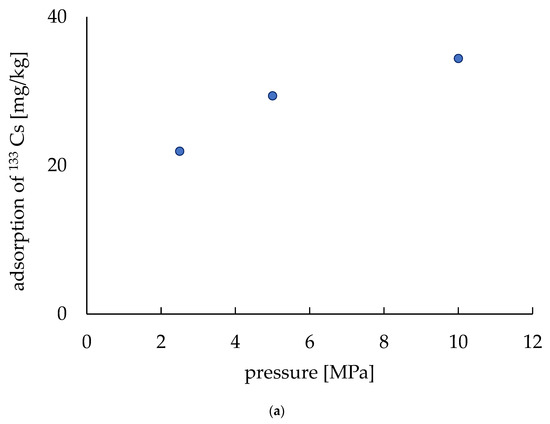
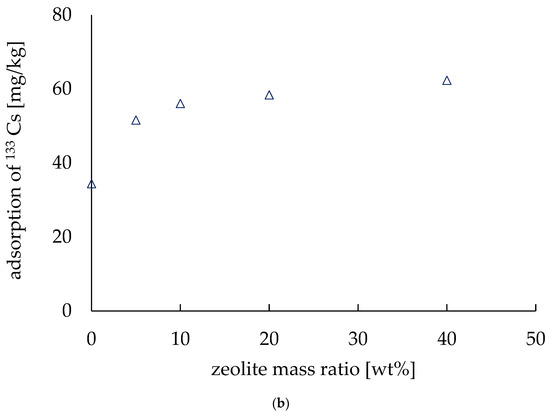
Figure 7.
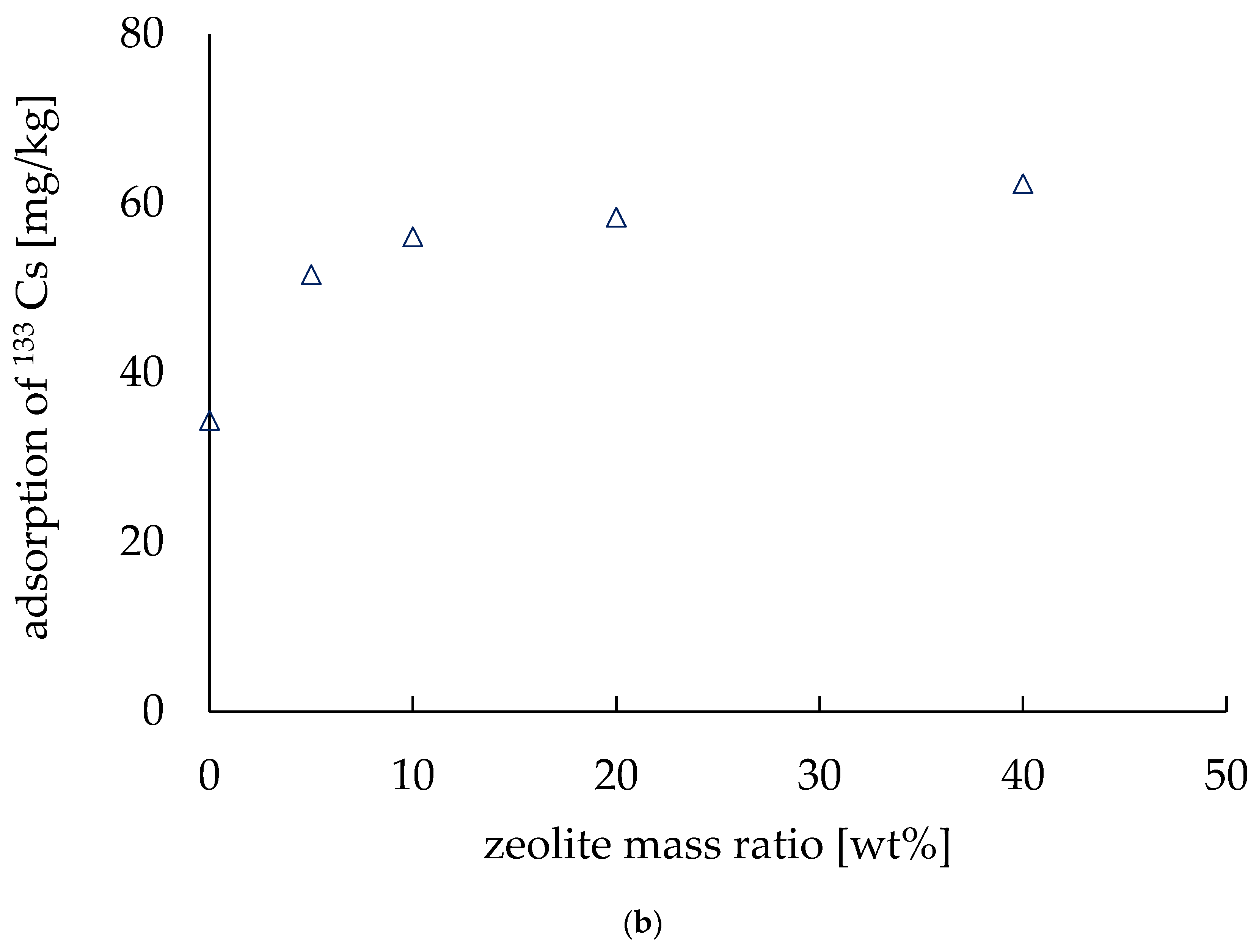
Adsorption of 133Cs. (a) Adsorption depends on the pressure applied during fabrication. (b) Adsorption varies with the amount of zeolite.
Figure 7b shows how the Cs adsorption ability of the electrodes increased with the amount of zeolite. The horizontal axis shows the zeolite mass ratio (wt.%) and the adsorption amount per unit mass (mg/kg) is shown as the vertical axis. The results show that the amount of adsorption per unit mass increases as the zeolite mass ratio increases. This confirms that it is possible to increase the amount of Cs adsorbed from a solution by using zeolite. When the zeolite mass ratio was 40 wt.%, the amount of Cs adsorbed was 62.3 mg/kg, which indicates that approximately 90% of the cesium of the solution was adsorbed. Munthali et al. (2015) also found that more than 90% of Cs ions were adsorbed by zeolite from different electrolyte solutions in their research [22]. Moreover, Kumar et al. (2021) studied the effect of high pressure on synthesized zeolite, and they found no structural phase transition before 11.4 GPa [24]. Again, when the zeolite mass ratio was 0 wt.%, the adsorption amount was 34.4 mg/kg, which is equivalent to 50% of the Cs of Cs solution, indicating that the addition of zeolite improved the adsorption amount of Cs. It is, however, worth mentioning that adding zeolite made the electrodes brittle, and thus the conductivity was decreased, as we discussed in the previous section. The electrode with a zeolite mass ratio of 40 wt.% exhibited a high adsorption capacity, but after 48 h in the Cs solution, the electrode lost its shape and became very brittle. Therefore, it is difficult to increase the amount of zeolite when preparing electrodes.
4. Conclusions
In this study, cathode electrodes were developed using environmentally friendly materials including burnt rice husk charcoal (kuntan), zeolite, abaca, and starch glue, in order to achieve flexibility and a high adsorbing capacity of Cs ions for FEM-EK treatment. We used pressing on the electrodes while fabricating them and it improved the flexibility and conductivity, as well as the Cs adsorbing capability of the electrodes. The results are summarized as follows.
- (1)
- Although zeolite is a good adsorbent, due to its dielectric properties and brittle nature, it is not easy to use in EK treatment as a material for fabricating electrodes, but we have used zeolite with other conductive material (kuntan, burnt rice husk charcoal) and prepared cathode electrodes which can be fully flexible and conductive and Cs adsorbent. Thus, this electrode can be used in FEM-EK treatment in order to purify polluted soil.
- (2)
- Zeolite works as an adsorbent of Cs but adding zeolite to the electrodes showed a decline in conductivity and flexibility. The FEM-EK treatment is proposed for the treatment of a vast polluted area in conditions where the cathode electrodes will be exposed to the natural conditions of rain, moisture, etc. Considering this point, we evaluated the conductivity and flexibility of the fabricated electrodes by dripping with water and found an improvement in these characteristics, even after the addition of zeolite.
- (3)
- Considering our results from this work, it is suggested that the appropriate zeolite mass ratio is ~15 wt.% of an electrode, or even less than that amount might be helpful for adsorbing Cs.
As described above, a pressing process was added to the electrode fabrication method for the FEM-EK treatment, and its performance was evaluated. The improved flexibility and conductivity enabled an increase in the zeolite mass ratio, which was thought to hinder its performance, and led to an increase in the adsorption capacity. However, the practical application of these electrodes in FEM-EK treatment should be considered, which will be our next focus of work.
Author Contributions
Conceptualization, M.K., Y.S. and S.O.; methodology, A.K., M.K., S.O., H.K., M.T. and S.K.; software, A.K., M.K., N.O. and N.Y.; validation, A.K., M.K., H.K., Y.S., T.S. and M.S.; formal analysis, A.K., M.K. and H.K.; investigation, A.K., M.K. and H.K.; resources, M.K., Y.S. and T.S.; data curation, A.K., M.K., H.K. and N.O.; writing—original draft preparation, A.K. and M.K.; writing—review and editing, A.K., M.K. and N.Y.; visualization, A.K. and M.K.; supervision, M.K. and N.Y.; project administration, M.K.; funding acquisition, M.K., S.K. and N.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by a Grant-in-Aid for Scientific Research (C) 18K11690, Grant-in-Aid for Scientific Research (B) 19H02121, and Grant-in-Aid for Scientific Research (C) 22K12423, from the Japanese Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akai, J.; Nomura, N.; Matsushita, S.; Kudo, H.; Fukuhara, H.; Matsuoka, S.; Matsumoto, J. Mineralogical and geomicrobial examination of soil contamination by radioactive Cs due to 2011 Fukushima Daiichi Nuclear Power Plant accident. Phys. Chem. Earth 2013, 58, 57–60. [Google Scholar] [CrossRef]
- Konoplev, A. Fukushima and Chernobyl: Similarities and Differences of Radiocesium Behavior in the Soil–Water Environment. Toxic 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, G.M.; Chierici, A.; De Souza, S.O.; D’Errico, F.; Iannotti, A.; Malizia, A. Effects of Ionizing Radiation on Flora Ten Years after the Fukushima Dai-ichi Disaster. Plants 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Kinase, T.; Adachi, K.; Sekiyama, T.T.; Kajino, M.; Igarashi, Y. Temporal Variations of 90Sr and 137Cs in Atmospheric Depositions after the Fukushima Daiichi Nuclear Power Plant Accident with Long-term Observations. Sci. Rep. 2020, 10, 21627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.H.; Lee, H.; Kim, Y. Distributions of Radiocesium and Plutonium in the Korean Seas and North Pacific after the Fukushima Accident, 2011–2014. J. Mar. Sci. Eng. 2022, 10, 1541. [Google Scholar] [CrossRef]
- Annual Report on the Environment in Japan 2018. Available online: https://www.env.go.jp/en/wpaper/2018/index.html (accessed on 26 December 2022).
- Zhidkin, A.P.; Shamshurina, E.N.; Golosov, V.N.; Komissarov, M.A.; Ivanova, N.N.; Ivanov, M.M. Detailed study of post-Chernobyl Cs-137 redistribution in the soils of a small agricultural catchment (Tula region, Russia). J. Environ. Radioact. 2020, 223, 106386. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. (Ed.) Countermeasures for Radioactive Contamination after the Great East Japan Earthquake—From Radiation Basics to Environmental Impact Assessment, Decontamination Technology and its Initiatives; NTS Publishing Company: Tokyo, Japan, 2012; pp. 67–105. ISBN 978-4-86469-030-0. [Google Scholar]
- Shcheglov, I.A.; Tsvetnova, O.B.; Klyashtorin, A. The fate of Cs-137 in forest soils of Russian Federation and Ukraine contaminated due to the Chernobyl accident. J. Geochem. Explor. 2014, 142, 75–81. [Google Scholar] [CrossRef]
- Yamasaki, S.; Imoto, J.; Furuki, G.; Ochiai, A.; Ohnuki, T.; Sueki, K.; Nanba, K.; Ewing, R.C.; Utsunomiya, S. Radioactive Cs in the estuary Sediments near Fukushima Daiichi Nuclear Power Plant. Sci. Total Environ. 2016, 551, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Annual Report on the Environment in Japan 2020. Available online: https://www.env.go.jp/en/wpaper/2020/index.html (accessed on 26 December 2022).
- IAEA Annual Report for 2005. Available online: https://www.iaea.org/publications/reports/annual-report-2005 (accessed on 26 December 2022).
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Cai, Z.P.; Chen, D.R.; Fang, Z.Q.; Xu, M.Q.; Li, W.S. Enhanced electrokinetic remediation of copper-contaminated soils near a mine tailing using the approaching-anode technique. J. Environ. Eng. 2016, 142, 04015079. [Google Scholar] [CrossRef]
- Zhou, D.; Deng, C.; Cang, L.; Alshawabkeh, A.N. Electrokinetic remediation of a Cu-Zn contaminated red soil by controlling the voltage and conditioning catholyte pH. Chemosphere 2005, 61, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, L.; Mishchuk, N.; Kovalchuk, V. Basic principles and problems in decontamination of natural disperse systems. The electrokinetic treatment of soils. Adv. Colloid Interface Sci. 2022, 310, 102798. [Google Scholar] [CrossRef] [PubMed]
- Kishida, T.; Kabir, M.; Suzuki, M.; Nakajima, S. Electrokinetic remediation of cesium from contaminated soil by using FEM-EK process. J. Inst. Electrostat. Jpn. 2017, 41, 51–56. (In Japanese) [Google Scholar]
- Kabir, M.; Kumagai, A.; Sato, Y.; Sato, M.; Kumagai, S.; Yoshimura, N. FEM-EK Process: A New Remediation Method of Contaminated Soil. In Proceedings of the Ninth International Conference on Materials Engineering for Resources, Akita, Japan, 21–22 October 2021. AB–2. [Google Scholar]
- Hatakeyama, Y.; Kabir, M.; Suzuki, M.; Nakajima, S. Development of flexible ion adsorption electrode by using the burnt rice husk (kuntan) and zeolite. J. Inst. Electrostat. Jpn. 2017, 41, 111–116. (In Japanese) [Google Scholar]
- Kabir, M.; Hatakeyama, Y.; Nakajima, S. Manufacturing Method of Cathode Electrode for FEM-EK Process to Adsorb Cesium (Cs) Ion. Nat. Environ. Pollut. Technol. 2018, 17, 237–241. [Google Scholar]
- Horin, S.; Owashi, M.; Kasai, Y. Study of the Softness of Human Hair (1) Development of the Softness Measurement by Means of the Bending Length. J. Soc. Cosmet. Chem. Jpn. 1974, 9, 36–38. (In Japanese) [Google Scholar]
- Munthali, N.W.; Johan, E.; Aono, H.; Matsue, N. Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J. Asian Ceram. Soc. 2015, 3, 245–250. [Google Scholar] [CrossRef]
- Suzuki, M.; Shoji, T.; Yoshimura, N. Research on heavy metal recovery using the electrokinetic phenomenon. IEEE Trans. Elect. Electron. Eng. 2006, 1, 1–7. [Google Scholar]
- Kumar, M.M.; Irshad, K.A.; Jena, H. Removal of Cs+ and Sr2+ ions from simulated radioactive waste solutions using Zeolite-A synthesized from kaolin and their structural stability at high pressures. Microporous Mesoporous Mater. 2021, 312, 110773. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).