Static Crystallization, an Alternative Methodology for Synthesis of High-Purity Aluminum
Abstract
:1. Introduction
1.1. Fractional Crystallization
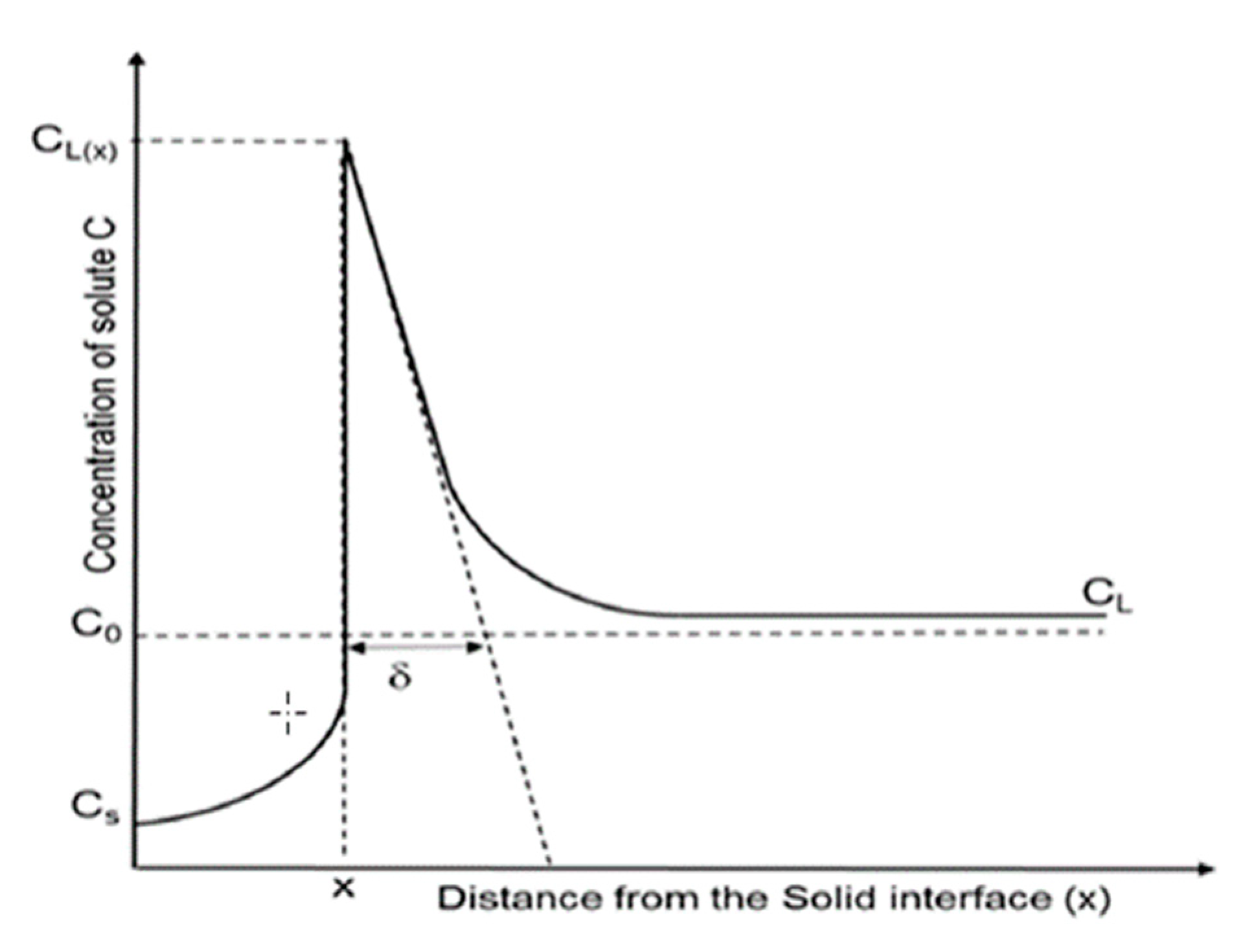
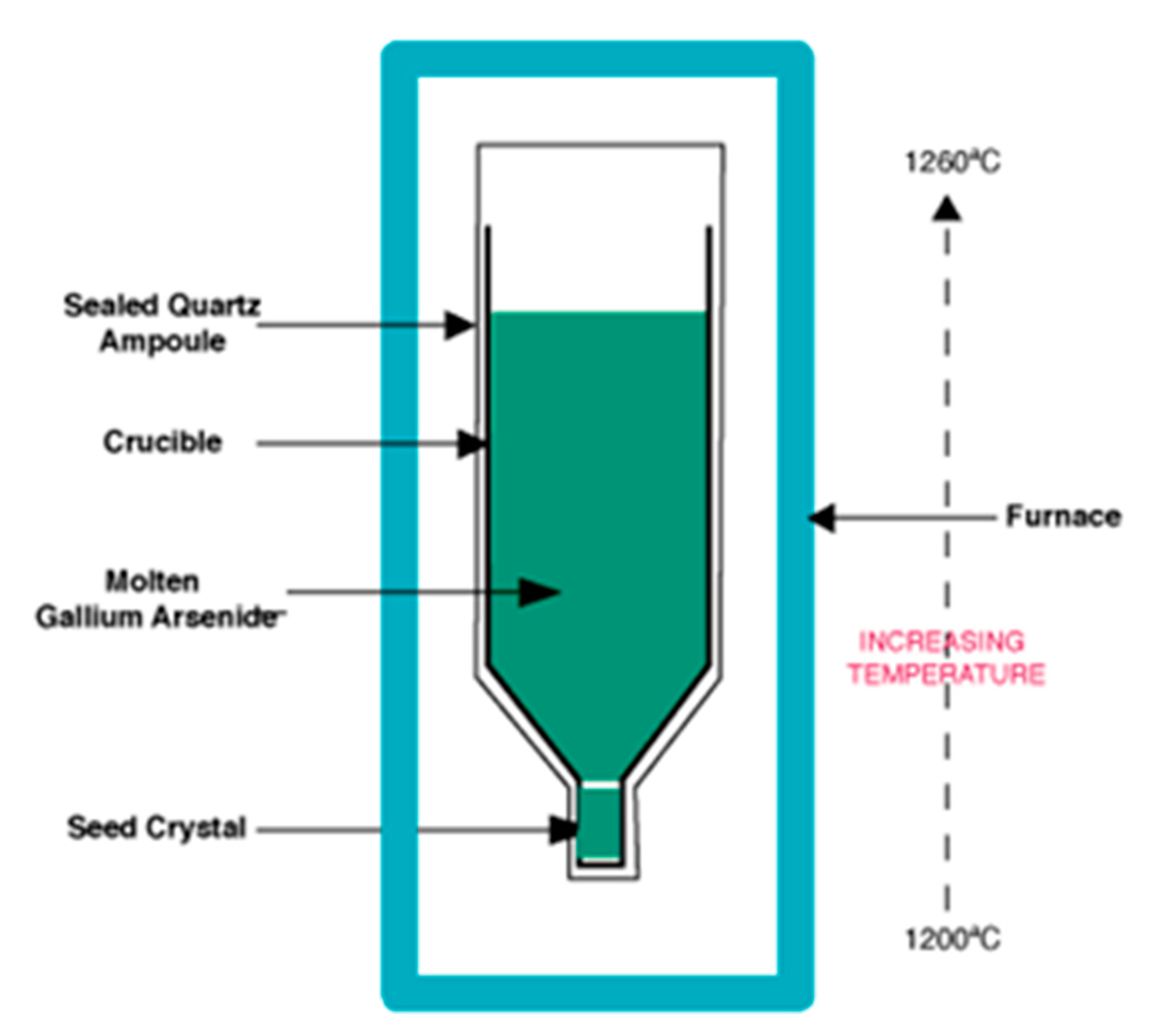
1.2. Vertical Gradient Freeze as a Prototype for Static Crystallization
2. Materials and Methods
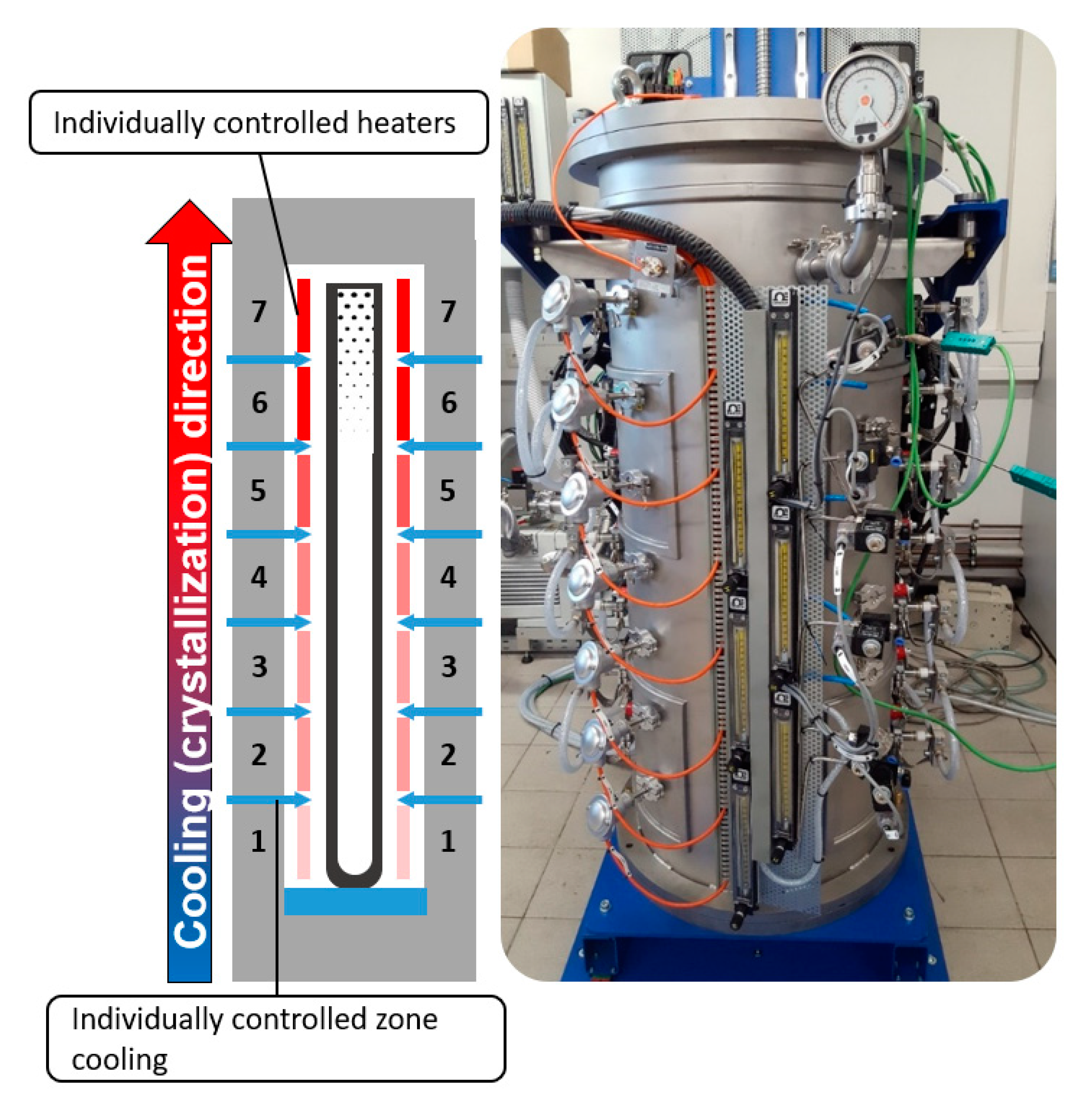
2.1. Static Crystallization Furnace
2.2. Initial Material
2.3. Experimental Methodology
2.4. Sample Preparation and Analysis
3. Results and Discussion
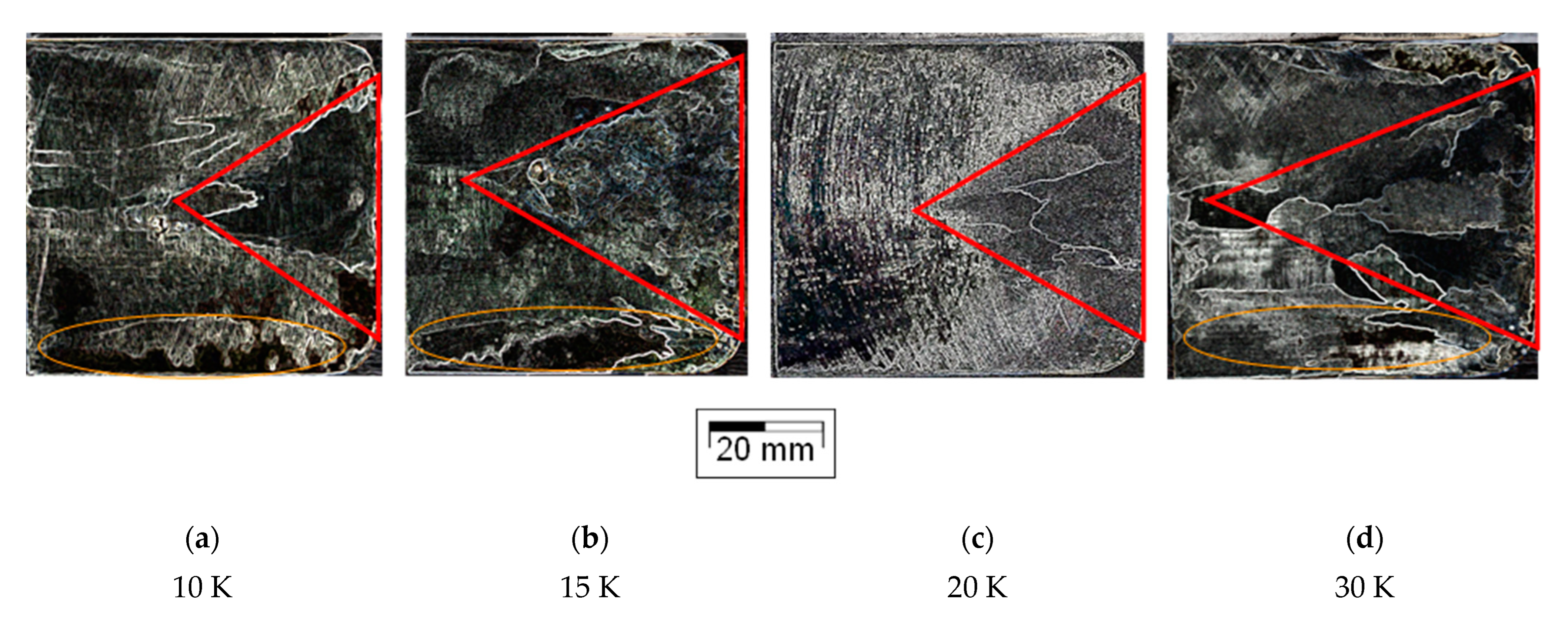
3.1. Macrographic Analysis
3.2. Chemical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tanaka, I.; Hoshikawa, H.; Megumi, T. Refining Technology and Low Temperature Properties for High Purity Aluminium; Sumitomo Chemical Co., Ltd.: Tokyo, Japan, 2013; pp. 1–12. [Google Scholar]
- Zhang, J.; Sun, B.; He, B. Principle and control of new-style purification equipment of 5N high purity aluminum. Chin. J. Mech. Eng. 2006, 42, 64. [Google Scholar] [CrossRef]
- Ostermann, F. Anwendungstechnologie Aluminium; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–180. [Google Scholar]
- Kondo, M.; Maeda, H.; Mizuguchi, M.J.J. The production of high-purity aluminum in Japan. JOM 1999, 42, 36–37. [Google Scholar] [CrossRef]
- Curtolo, D.C.; Friedrich, S.; Bellin, D.; Nayak, G.S.; Friedrich, B.J.M. Definition of a first process window for purification of aluminum via “Cooled Finger” crystallization technique. Metals 2017, 7, 341. [Google Scholar] [CrossRef]
- Yan, X.Y.; Fray, D.J. Molten salt electrolysis for sustainable metals extraction and materials processing—A review. In Electrolysis: Theory, Types and Applications; Nova Science Publishers: New York, NY, USA, 2010; pp. 255–302. [Google Scholar]
- Pfann, W.G. Zone Melting: This technique offers unique advantages in purification and in control of composition in various substances. Science 1962, 135, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Bertazzoli, R.; Garcia, A. Experimental impurity segregation and numerical analysis based on variable solute distribution coefficients during multi-pass zone refining of aluminum. J. Cryst. Growth 2008, 310, 1274–1280. [Google Scholar] [CrossRef]
- Widiatmo, J.V.; Harada, K.; Yamazawa, K.; Arai, M. Estimation of impurity effect in aluminium fixed-point cells based on thermal analysis. Metrologia 2006, 43, 561. [Google Scholar] [CrossRef]
- Barthel, J.; Buhirg, E.; Al, E. Kristallisation aus Schmelzen; Verlag für Grundstoffindustrie: Leipzig, Germany, 1982; p. 356. [Google Scholar]
- Burton, J.; Prim, R.; Slichter, W. The distribution of solute in crystals grown from the melt. Part I. Theoretical. J. Chem. Phys. 1953, 21, 1987–1991. [Google Scholar] [CrossRef]
- Burton, J.; Prim, R.; Slichter, W. The distribution of solute in crystals grown from the melt. Part II. Experimental. J. Chem. Phys. 1953, 21, 1991–1996. [Google Scholar] [CrossRef]
- Tiller, W.; Jackson, K.; Rutter, J.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Metall. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Ostrogorsky, A.G.; Glicksman, M.E. Segregation and component distribution. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 995–1047. [Google Scholar]
- Aleks, G.O.; Martin, E.G. Segregation and Component Distribution. In Handbook of Crystal Growth: Bulk Crystal Growth, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 1000–1018. ISBN 9780444633064. [Google Scholar] [CrossRef]
- Viardin, A.; Böttger, B.; Apel, M. Phase field assisted analysis of a solidification based metal refinement process. Mater. Theory 2022, 6, 1–22. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification; Trans Tech Publications: Zürich, Switzerland, 1992; pp. 46–48. [Google Scholar]
- Ecklebe, S.; Woittennek, F.; Winkler, J. Control of the vertical gradient freeze crystal growth process via backstepping. IFAC-PapersOnLine 2020, 53, 7758–7764. [Google Scholar] [CrossRef]
- Wang, X.; Ma, N.; Bliss, D.; Iseler, G. Solute segregation during modified vertical gradient freezing of alloyed compound semiconductor crystals with magnetic and electric fields. Int. J. Heat Mass Transf. 2006, 49, 3429–3438. [Google Scholar] [CrossRef]
- Birkmann, B.; Rasp, M.; Stenzenberger, J.; Müller, G. Growth of 3″ and 4″ gallium arsenide crystals by the vertical gradient freeze (VGF) method. J. Cryst. Growth 2000, 211, 157–162. [Google Scholar] [CrossRef]
- Monberg, E.; Gault, W.; Simchock, F.; Dominguez, F. Vertical gradient freeze growth of large diameter, low defect density indium phosphide. J. Cryst. Growth 1987, 83, 174–183. [Google Scholar] [CrossRef]
- Brice, J.C.; Rudolph, P. Crystal Growth. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007; pp. 559–560. [Google Scholar]
- Wafer Technology Ltd. Available online: http://www.wafertech.co.uk/growth.htm (accessed on 27 November 2022).


| Element | k | Element | k |
|---|---|---|---|
| Fe | 0.018–0.053 | Ti | 7–11 |
| Cu | 0.15–0.153 | Si | 0.082–0.12 |
| Ag | 0.2–0.3 | K | 0.56 |
| Au | 0.18 | Zr | 2.3–3 |
| Zn | 0.35–0.47 | Pb | 0.0007 |
| Ni | 0.004–0.09 | P | <0.01 |
| Mn | 0.55–0.9 | Sc | 0.9 |
| Mg | 0.29–0.5 | Sb | 0.09 |
| Ca | 0.06–0.08 | V | 3.3–4.3 |
| Cr | 1.8 | Na | 0.013 |
| Elements | Melt 1 (wt.%) | Melt 2 (wt.%) | Melt 3 (wt.%) | Average (wt.%) |
|---|---|---|---|---|
| Al | 99.7414 | 99.7947 | 99.7737 | 99.770 |
| Si | 0.1160 | 0.0908 | 0.1040 | 0.104 |
| Fe | 0.0426 | 0.0324 | 0.0333 | 0.036 |
| Pb | 0.0469 | 0.0475 | 0.0502 | 0.048 |
| Trial | Cooling Rate (K/h) | Temperature Gradient (K) |
|---|---|---|
| 1 | 10 | 20 |
| 2 | 20 | 20 |
| 3 | 50 | 20 |
| 4 | 10 | 10 |
| 5 | 10 | 15 |
| 6 | 10 | 30 |
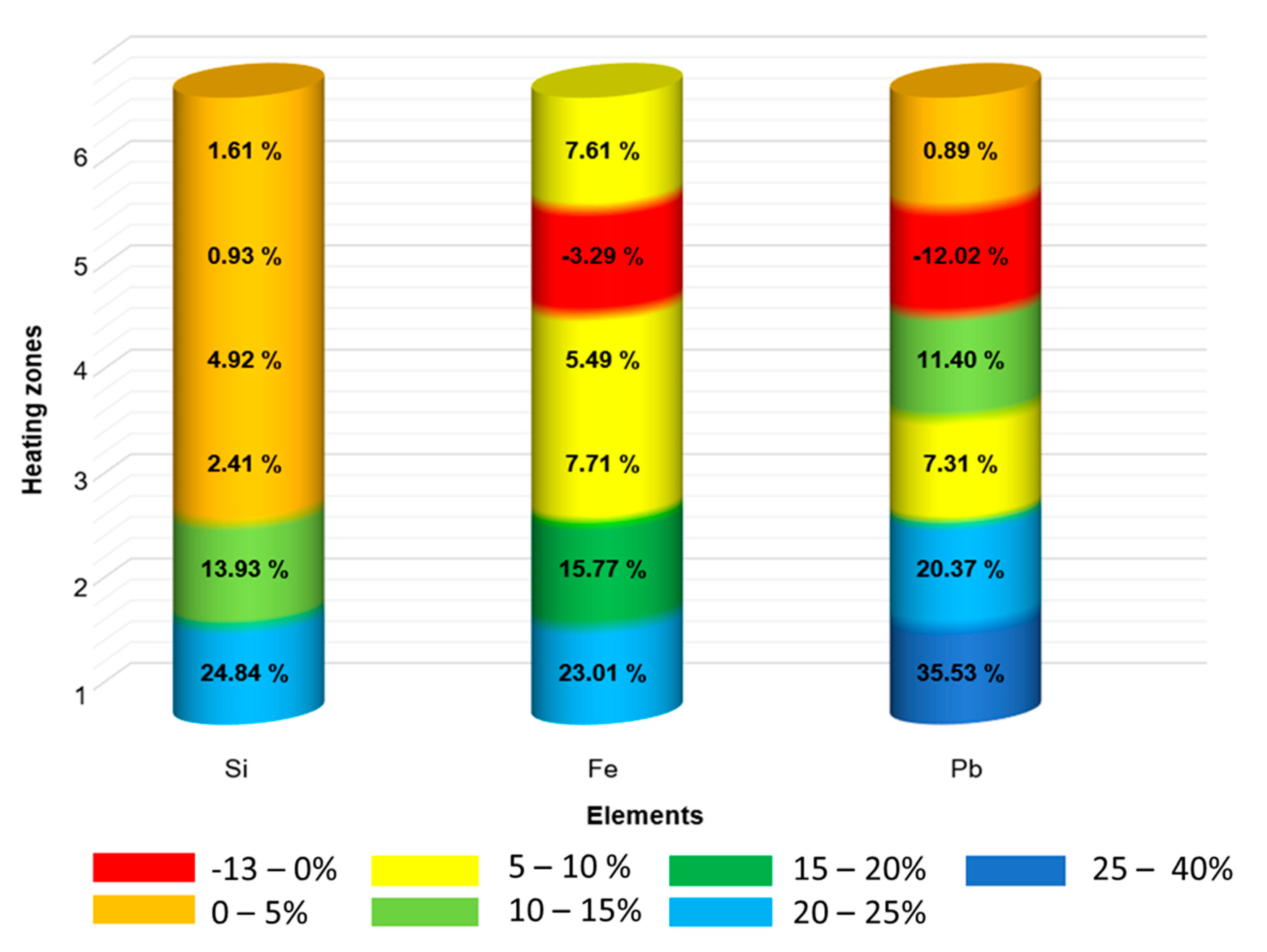
| Impurities | Highest Purification Factor | C0 (wt.%) | Cs (wt.%) |
|---|---|---|---|
| Si | 24.84% | 0.104 | 0.078 |
| Fe | 23.01% | 0.036 | 0.028 |
| Pb | 35.53% | 0.048 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotenbruck, M.; Curtolo, D.C.; Friedrich, S.; Müller, C.J.; Rademacher, N.; Friedrich, B. Static Crystallization, an Alternative Methodology for Synthesis of High-Purity Aluminum. Metals 2023, 13, 280. https://doi.org/10.3390/met13020280
Gotenbruck M, Curtolo DC, Friedrich S, Müller CJ, Rademacher N, Friedrich B. Static Crystallization, an Alternative Methodology for Synthesis of High-Purity Aluminum. Metals. 2023; 13(2):280. https://doi.org/10.3390/met13020280
Chicago/Turabian StyleGotenbruck, Michaela, Danilo C. Curtolo, Semiramis Friedrich, Clemens J. Müller, Nico Rademacher, and Bernd Friedrich. 2023. "Static Crystallization, an Alternative Methodology for Synthesis of High-Purity Aluminum" Metals 13, no. 2: 280. https://doi.org/10.3390/met13020280
APA StyleGotenbruck, M., Curtolo, D. C., Friedrich, S., Müller, C. J., Rademacher, N., & Friedrich, B. (2023). Static Crystallization, an Alternative Methodology for Synthesis of High-Purity Aluminum. Metals, 13(2), 280. https://doi.org/10.3390/met13020280