Separation of Cobalt, Samarium, Iron, and Copper in the Leaching Solution of Scrap Magnets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
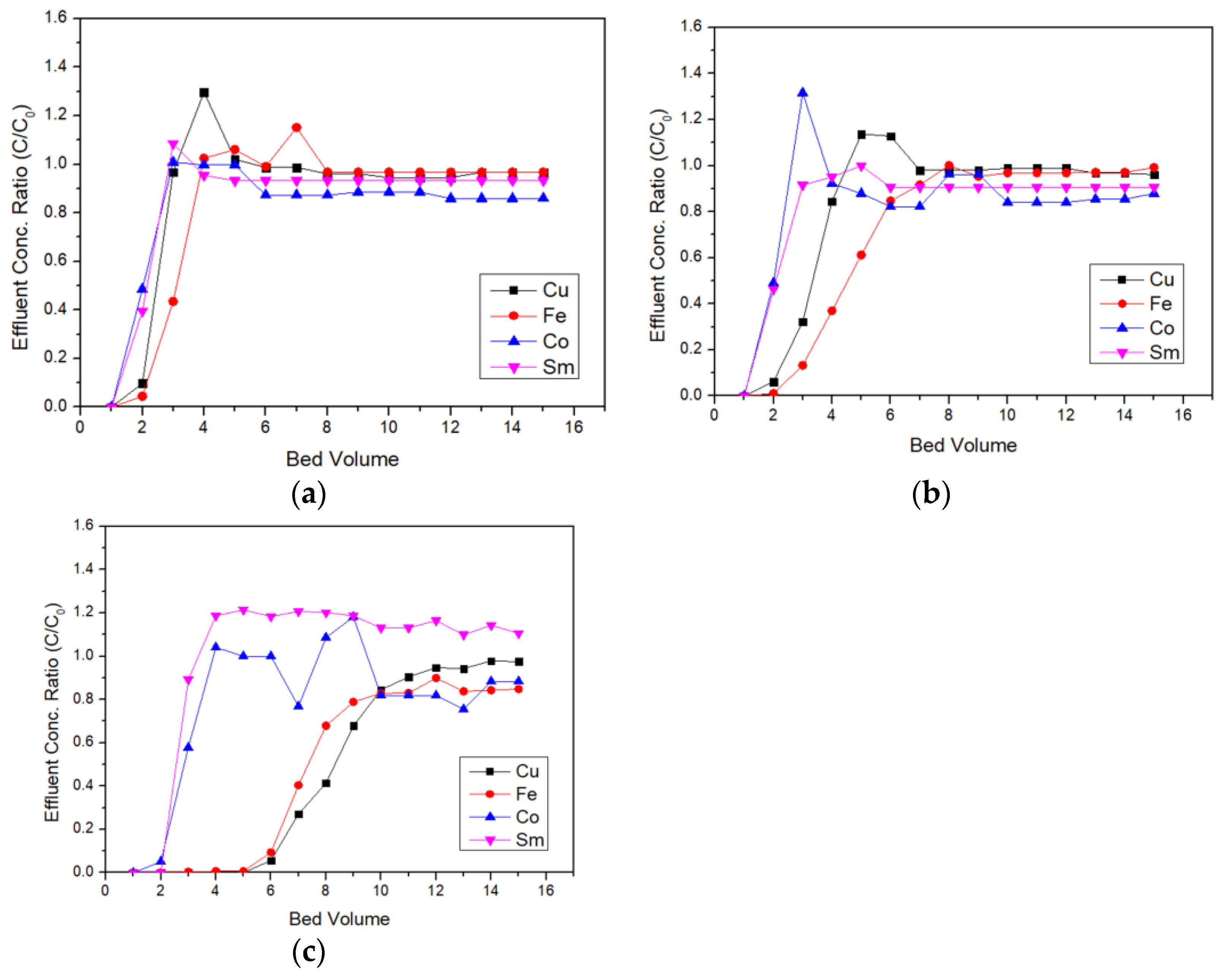
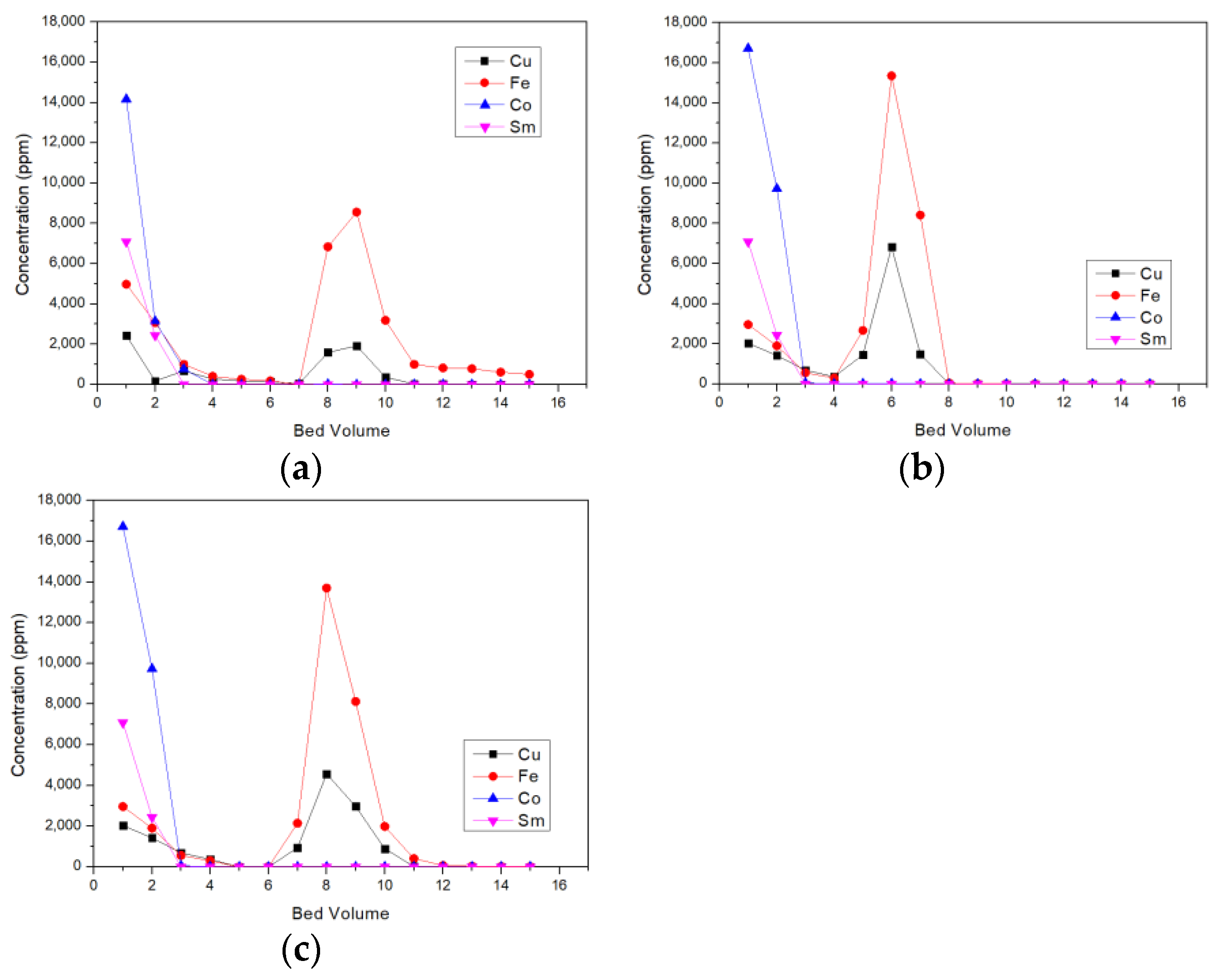
2.2. Resin and Column Experiments
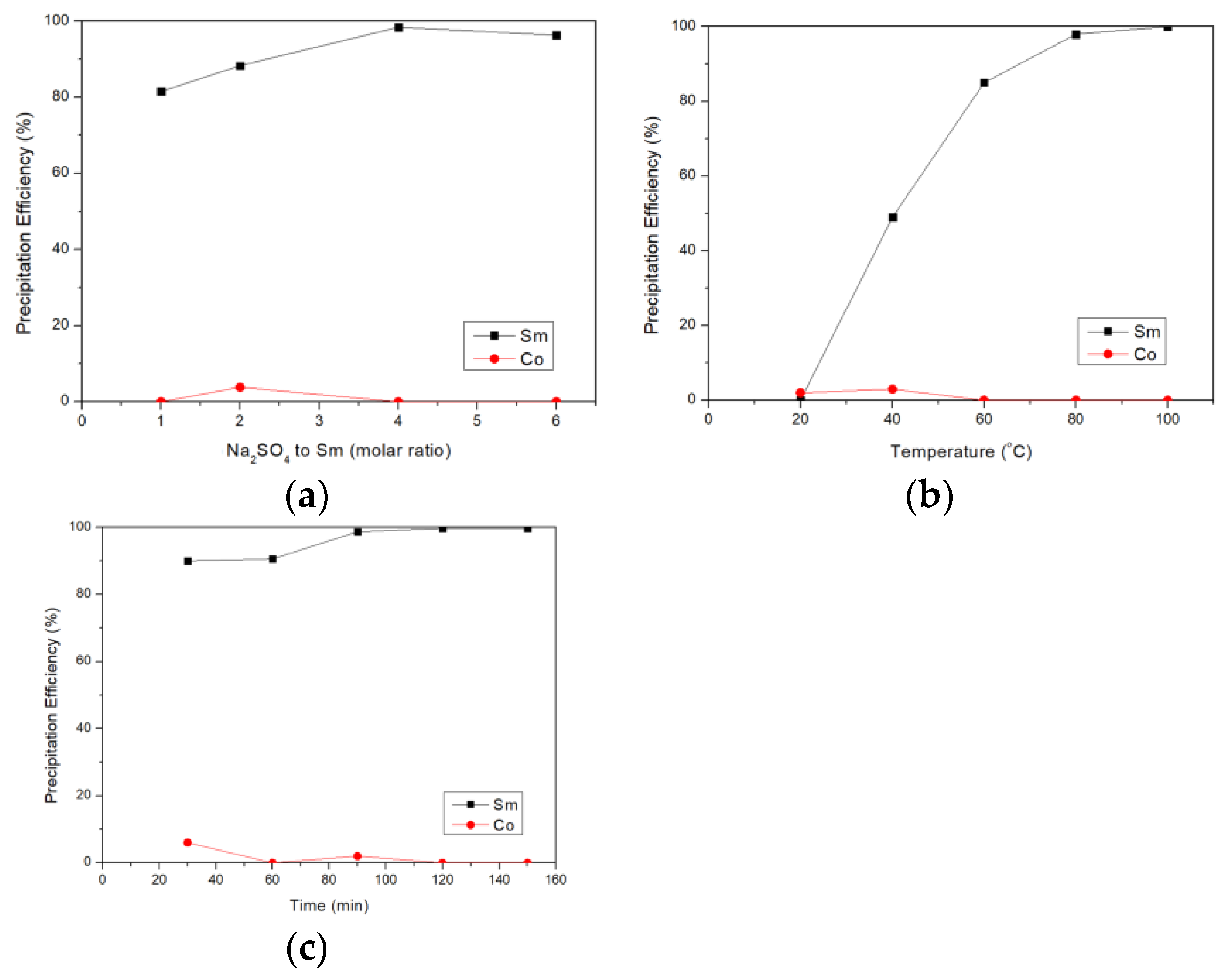
2.3. Precipitation Separation Experiment
2.4. Analytical Methods
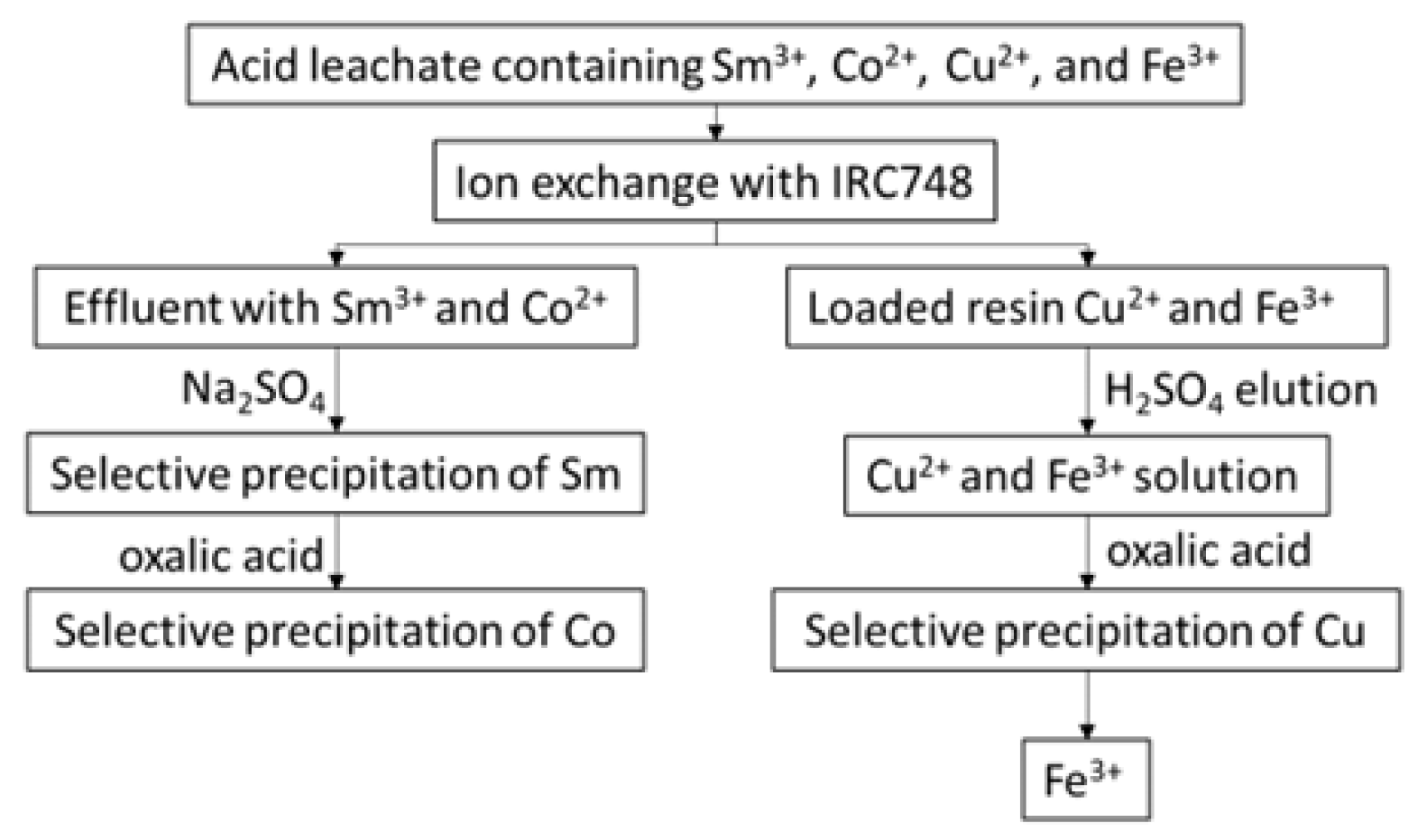
2.5. Separation Process
3. Results and Discussion
3.1. Ion-Exchange
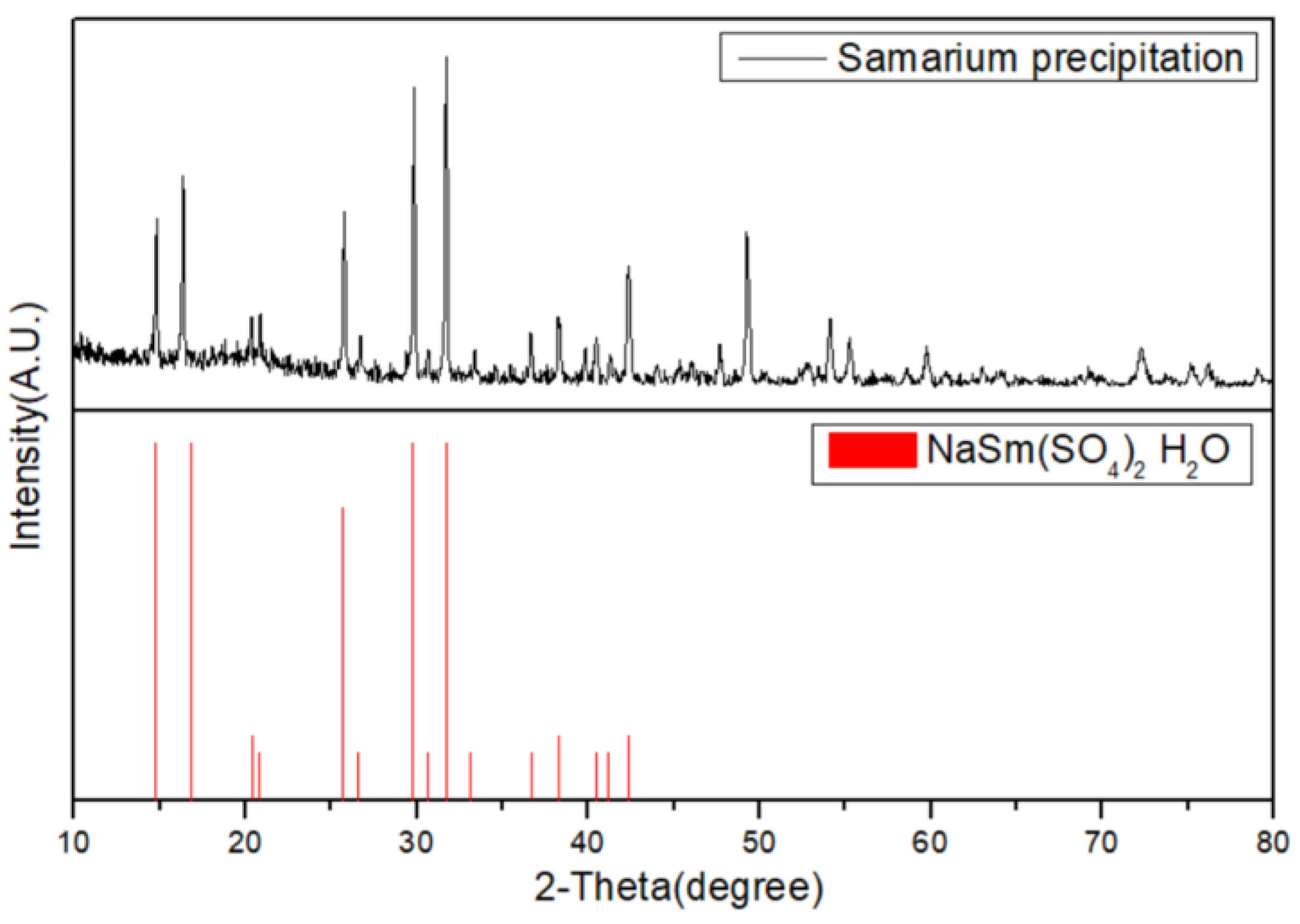
3.2. Selective Precipitation of Sm by Na2SO4
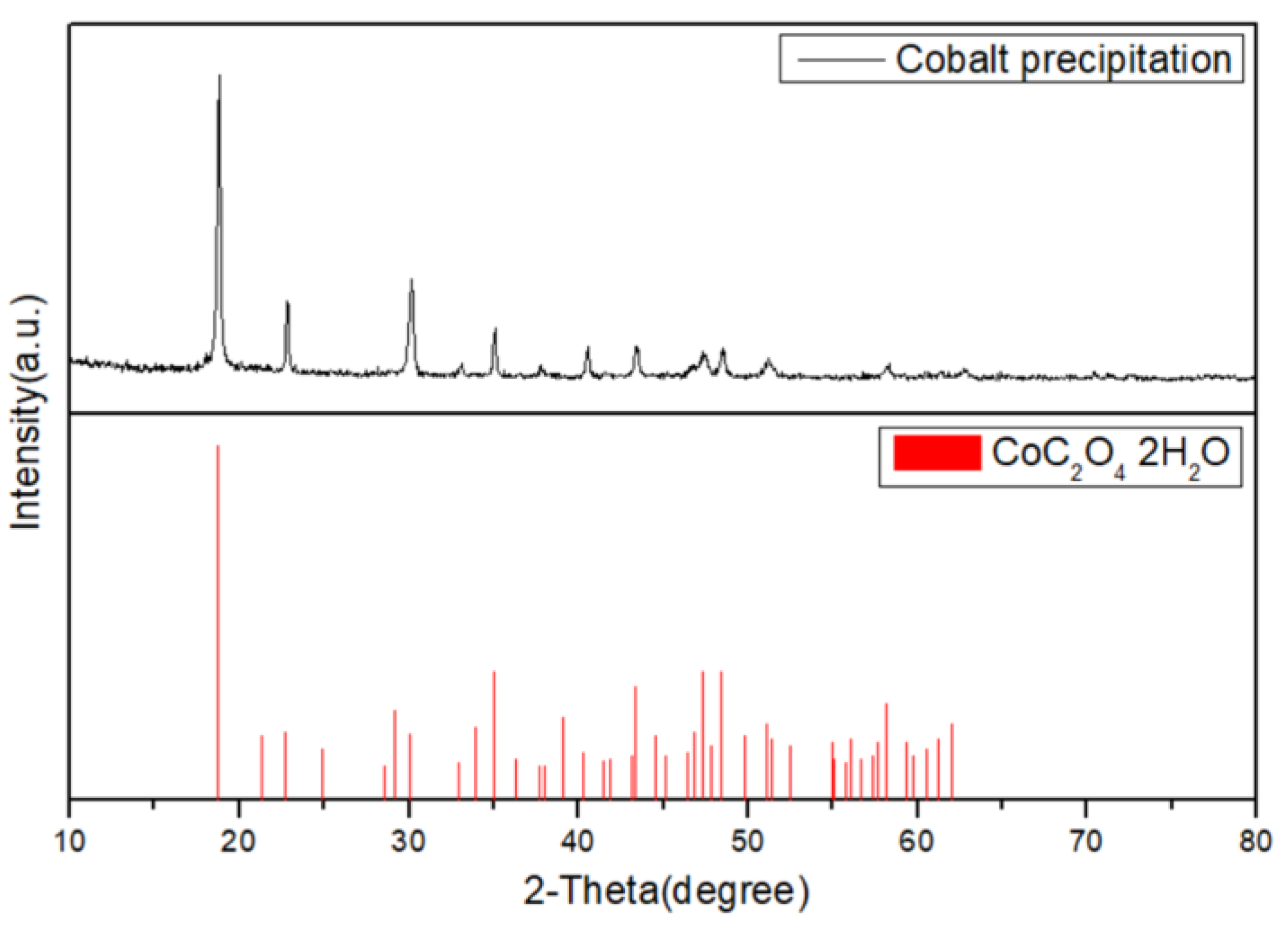
3.3. Selective Precipitation of Co by Oxalate
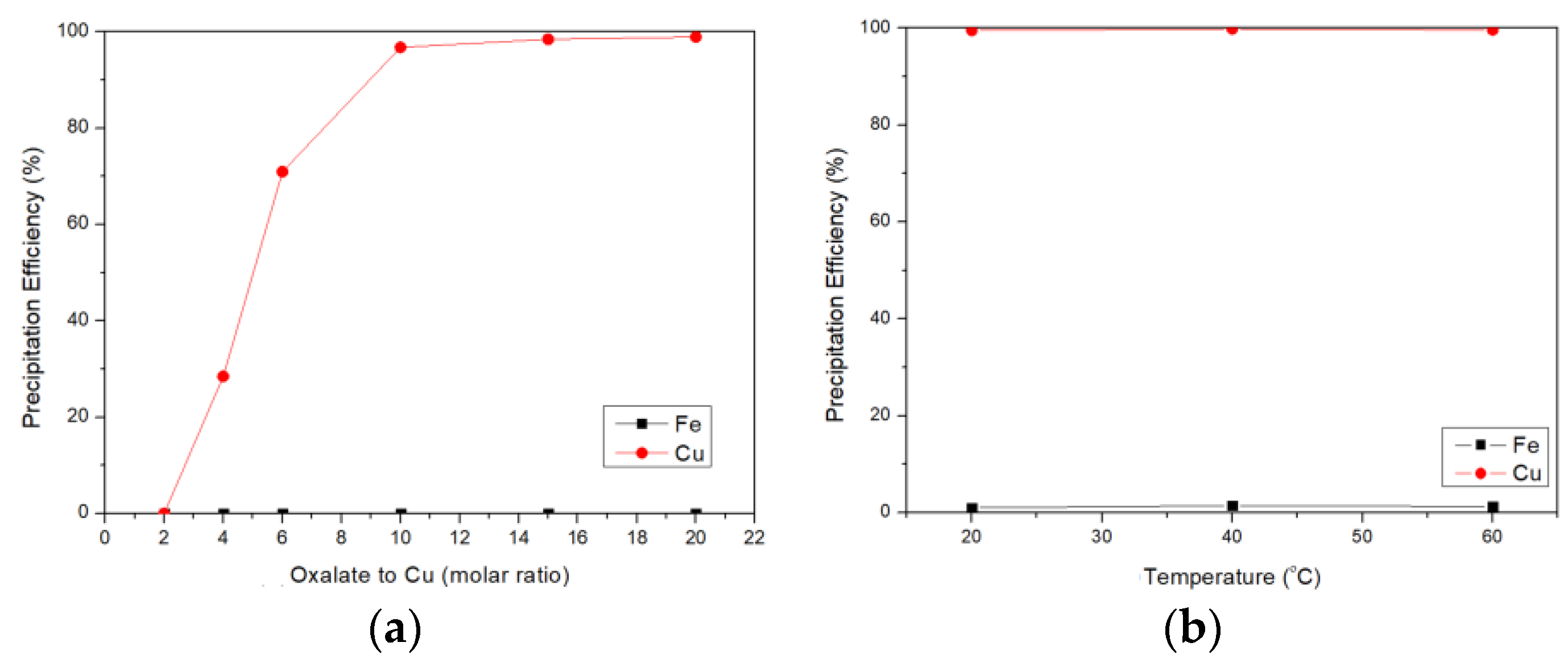
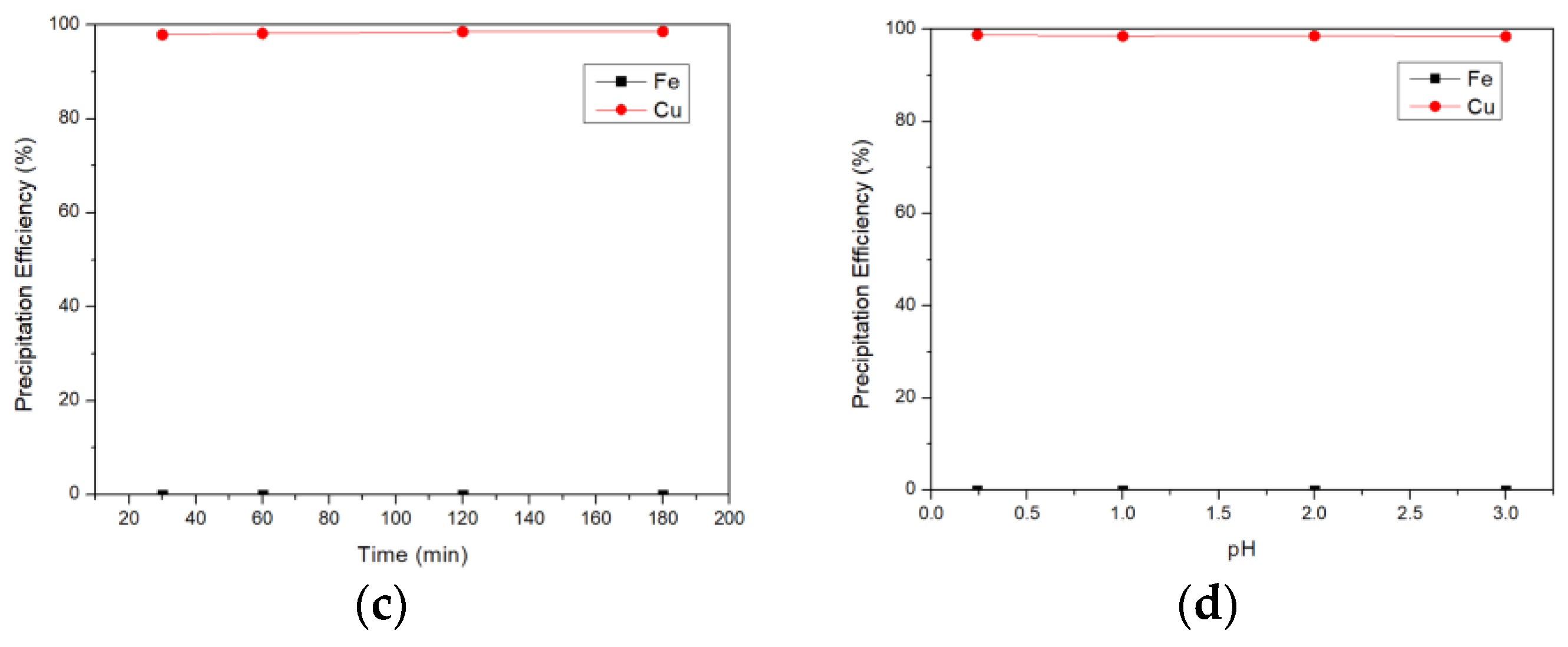
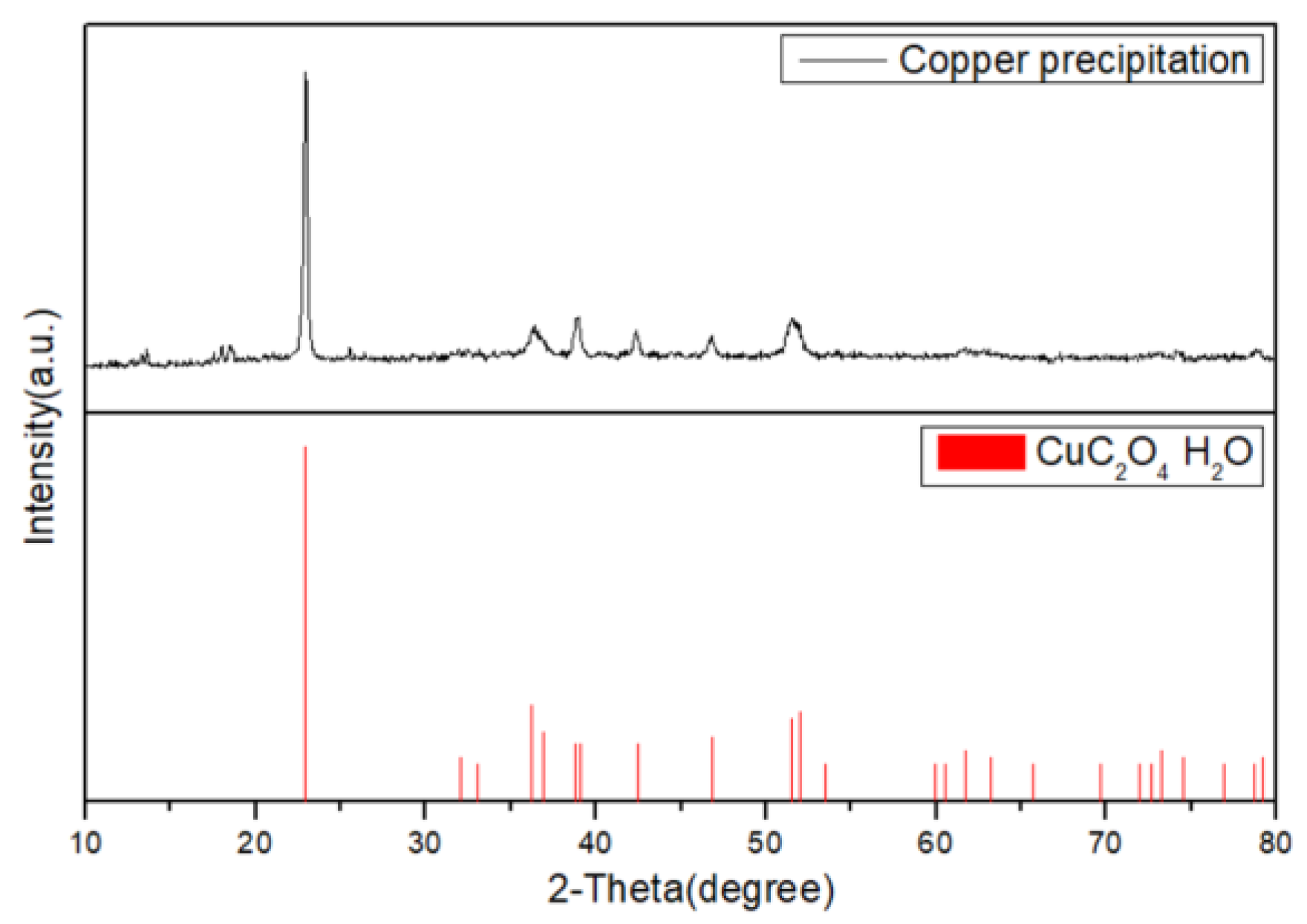
3.4. Selective Precipitation of Cu by Oxalate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, H.O.; Rim, K.T. Worker Safety in the Rare Earth Elements Recycling Process From the Review of Toxicity and Issues. Saf. Health Work. 2019, 10, 409–419. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en (accessed on 28 August 2022).
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Sinha, M.K.; Pramanik, S.; Kumari, A.; Sahu, S.K.; Prasad, L.B.; Jha, M.K.; Pandey, B.D. Recovery of value added products of Sm and Co from waste SmCo magnet by hydrometallurgical route. Sep. Purif. Technol. 2017, 179, 1–12. [Google Scholar] [CrossRef]
- Batchu, N.K.; Binnemans, K. Effect of the diluent on the solvent extraction of neodymium(III) by bis(2-ethylhexyl)phosphoric acid (D2EHPA). Hydrometallurgy 2018, 177, 146–151. [Google Scholar] [CrossRef]
- Gorzin, H.; Ghaemi, A.; Hemmati, A.; Maleki, A. Studies on effective interaction parameters in extraction of Pr and Nd using Aliquat 336 from NdFeB magnet-leaching solution: Multiple response optimizations by desirability function. J. Mol. Liq. 2020, 324, 115123. [Google Scholar] [CrossRef]
- Jilin, X.; Zhengxian, H.; Junming, L.; Zhenchen, Z. Corrosion Behavior of Sintered NdFeB Magnets in Different Acidic Solutions. Rare Met. Mater. Eng. 2015, 44, 786–790. [Google Scholar] [CrossRef]
- Lai, W.; Liu, M.; Li, C.; Suo, H.; Yue, M. Recovery of a composite powder from NdFeB slurry by co-precipitation. Hydrometallurgy 2014, 150, 27–33. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, N.; Li, Y.; Wu, P.; Dang, Z.; Ke, Y. Efficient recovery of rare earth elements from discarded NdFeB magnets. Process Saf. Environ. Prot. 2019, 124, 317–325. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Wang, J.; Wang, H.; Peng, C.; Wilson, B.P.; Lundström, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets. Miner. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- Liu, Q.; Tu, T.; Guo, H.; Cheng, H.; Wang, X. High-efficiency simultaneous extraction of rare earth elements and iron from NdFeB waste by oxalic acid leaching. J. Rare Earths 2020, 39, 323–330. [Google Scholar] [CrossRef]
- Makarova, I.; Ryl, J.; Sun, Z.; Kurilo, I.; Górnicka, K.; Laatikainen, M.; Repo, E. One-step recovery of REE oxalates in electro-leaching of spent NdFeB magnets. Sep. Purif. Technol. 2020, 251, 117362. [Google Scholar] [CrossRef]
- Makarova, I.; Soboleva, E.; Osipenko, M.; Kurilo, I.; Laatikainen, M.; Repo, E. Electrochemical leaching of rare-earth elements from spent NdFeB magnets. Hydrometallurgy 2020, 192, 105264. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Aktan, E.; Borra, C.R.; Blanpain, B.; Van Gerven, T.; Guo, M. Recycling of NdFeB magnets using nitration, calcination and water leaching for REE recovery. Hydrometallurgy 2017, 167, 115–123. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Riaño, S.; Binnemans, K. Alkali baking and solvometallurgical leaching of NdFeB magnets. Hydrometallurgy 2020, 191, 105213. [Google Scholar] [CrossRef]
- Padhan, E.; Nayak, A.K.; Sarangi, K. Recovery of neodymium and dysprosium from NdFeB magnet swarf. Hydrometallurgy 2017, 174, 210–215. [Google Scholar] [CrossRef]
- Padhan, E.; Sarangi, K. Recovery of Nd and Pr from NdFeB magnet leachates with bi-functional ionic liquids based on Aliquat 336 and Cyanex 272. Hydrometallurgy 2017, 167, 134–140. [Google Scholar] [CrossRef]
- Tunsu, C. 8-Hydrometallurgy in the recycling of spent NdFeB permanent magnets. In Waste Electrical and Electronic Equipment Recycling; Vegliò, F., Birloaga, I., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 175–211. [Google Scholar] [CrossRef]
- Van Loy, S.; Önal, M.A.R.; Binnemans, K.; Van Gerven, T. Recovery of valuable metals from NdFeB magnets by mechanochemically assisted ferric sulfate leaching. Hydrometallurgy 2020, 191, 105154. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, C.; Wang, Y.; Zhao, Z.; Li, B. Recycling of ultrafine NdFeB waste by the selective precipitation of rare earth and the electrodeposition of iron in hydrofluoric acid. Sep. Purif. Technol. 2020, 230, 115870. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Y.L.; Yu, H.Y.; Zhang, H.; Zhong, X.C.; Liu, Z.W. Synthesis of hard magnetic NdFeB composite particles by recycling the waste using microwave assisted auto-combustion and reduction method. Waste Manag. 2019, 87, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Pradhan, S.; Mishra, S. Efficiency of Aliquat 336 for hydrometallurgical separation of Sm (III) and Co (II) from nitrate medium. Miner. Eng. 2019, 139, 105872. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S.; Acharya, M.R. Hydrometallurgical route for recovery and separation of samarium (III) and cobalt (II) from simulated waste solution using tri-n-octyl phosphine oxide—A novel pathway for synthesis of samarium and cobalt oxides nanoparticles. J. Alloy. Compd. 2020, 815, 152423. [Google Scholar] [CrossRef]
- Orefice, M.; Audoor, H.; Li, Z.; Binnemans, K. Solvometallurgical route for the recovery of Sm, Co, Cu and Fe from SmCo permanent magnets. Sep. Purif. Technol. 2019, 219, 281–289. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Selective sorption of nickel and cobalt from sulphate solutions using chelating resins. International J. Miner. Process. 2004, 74, 359–371. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, A.; Zhang, D.; Zhang, X.; Yang, T. Sulfuric acid leaching of SmCo alloy waste and separation of samarium from cobalt. Hydrometallurgy 2017, 174, 66–70. [Google Scholar] [CrossRef]
- Soare, L.C.; Lemaitre, J.; Bowen, P.; Hofmann, H. A thermodynamic model for the precipitation of nanostructured copper oxalates. J. Cryst. Growth 2006, 289, 278–285. [Google Scholar] [CrossRef]

| Element | Sm | Co | Fe | Cu |
|---|---|---|---|---|
| wt % | 21.94 | 48.04 | 16.96 | 4.63 |
| Element | Sm | Co | Fe | Cu |
|---|---|---|---|---|
| Concentration (ppm) | 13,618 | 26,762 | 7584 | 2695 |
| Sample | Na | Sm | S | O |
|---|---|---|---|---|
| NaSm(SO4)2 | 12.08% | 13.23% | 21.57% | 53.12% |
| Sample | Co | O | C |
|---|---|---|---|
| CoC2O4 | 12.88% | 57.55% | 28.90% |
| Sample | Cu | C | O |
|---|---|---|---|
| CuC2O4 | 14.40% | 28.10% | 51.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-Z.; Hsieh, Y.-H.; Tang, Y.-C.; Shen, Y.-H. Separation of Cobalt, Samarium, Iron, and Copper in the Leaching Solution of Scrap Magnets. Metals 2023, 13, 90. https://doi.org/10.3390/met13010090
Wang J-Z, Hsieh Y-H, Tang Y-C, Shen Y-H. Separation of Cobalt, Samarium, Iron, and Copper in the Leaching Solution of Scrap Magnets. Metals. 2023; 13(1):90. https://doi.org/10.3390/met13010090
Chicago/Turabian StyleWang, Jian-Zhi, Yi-Hsun Hsieh, Yi-Chin Tang, and Yun-Hwei Shen. 2023. "Separation of Cobalt, Samarium, Iron, and Copper in the Leaching Solution of Scrap Magnets" Metals 13, no. 1: 90. https://doi.org/10.3390/met13010090
APA StyleWang, J.-Z., Hsieh, Y.-H., Tang, Y.-C., & Shen, Y.-H. (2023). Separation of Cobalt, Samarium, Iron, and Copper in the Leaching Solution of Scrap Magnets. Metals, 13(1), 90. https://doi.org/10.3390/met13010090






