Enhanced Precipitation of Gibbsite from Sodium Aluminate Solution by Adding Agglomerated Active Al(OH)3 Seed
Abstract
:1. Introduction
- Aluminum sulphate reacts with sodium aluminate to form aluminum hydroxide and sodium sulphate:
- The neutralization of free caustic also takes place:
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.3. Analysis
3. Results and Discussion
3.1. Active Seed Preparation
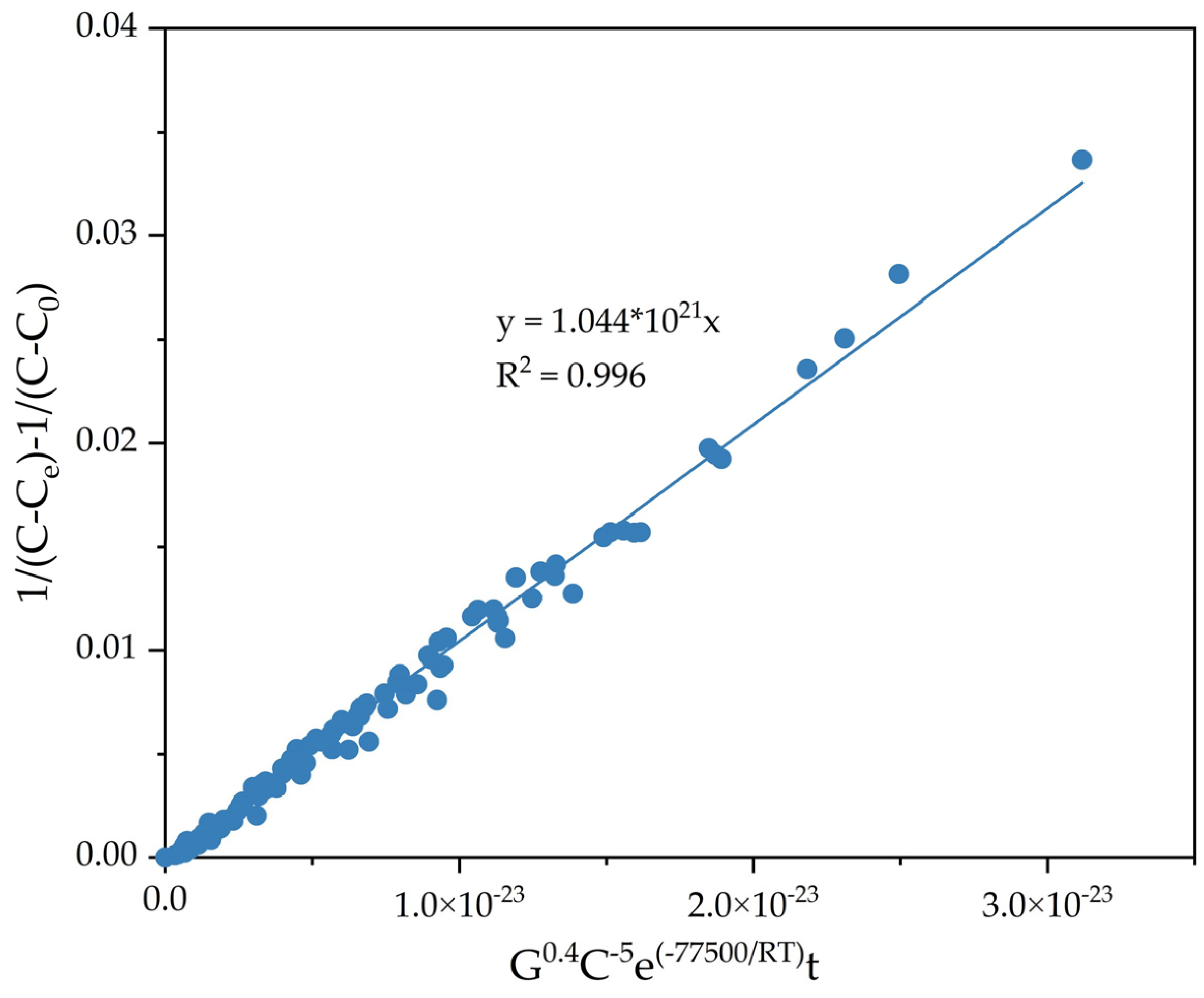
3.2. Kinetics of Precipitation from Sodium Aluminate Solution Using Coarse Active Seed
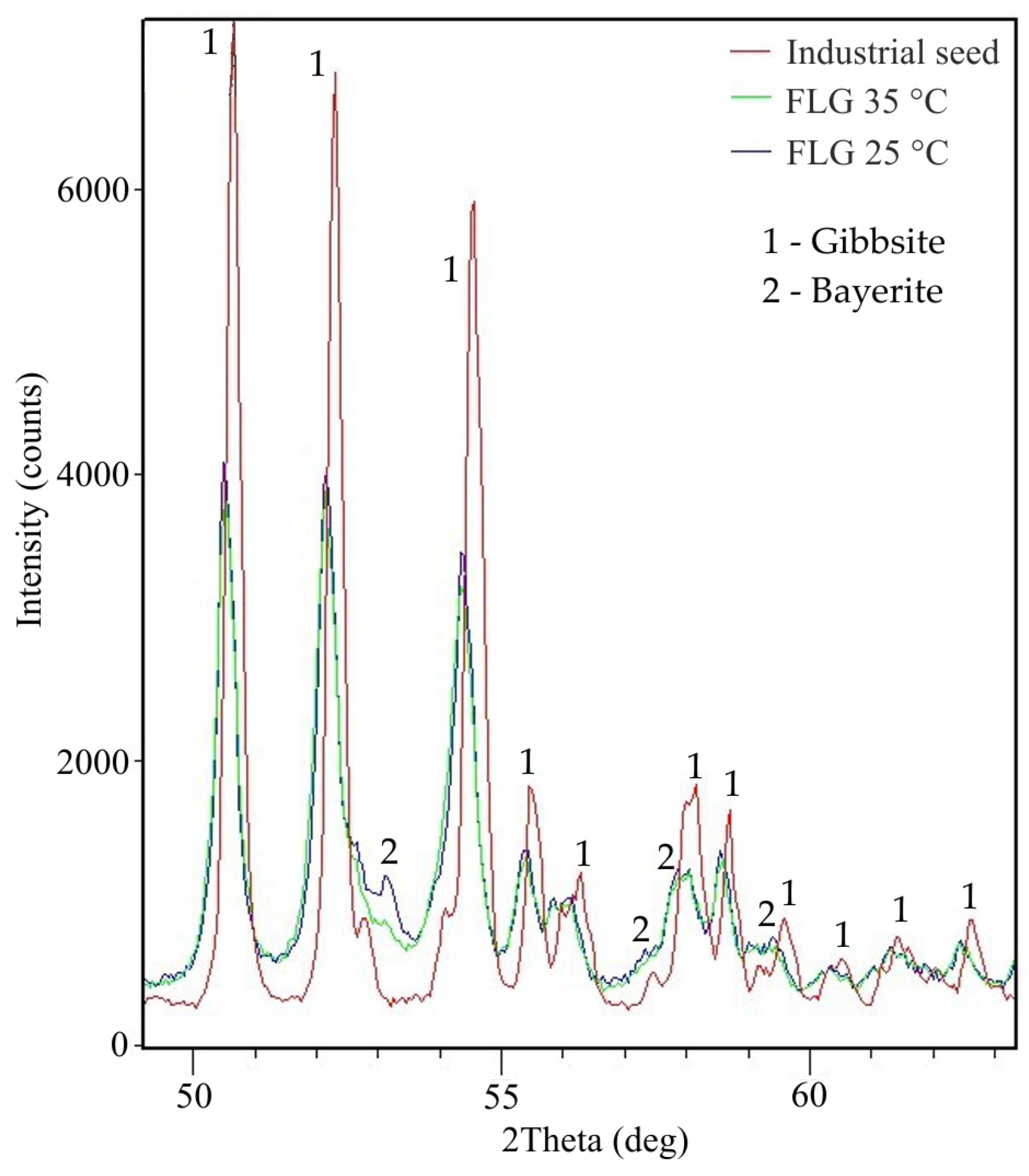
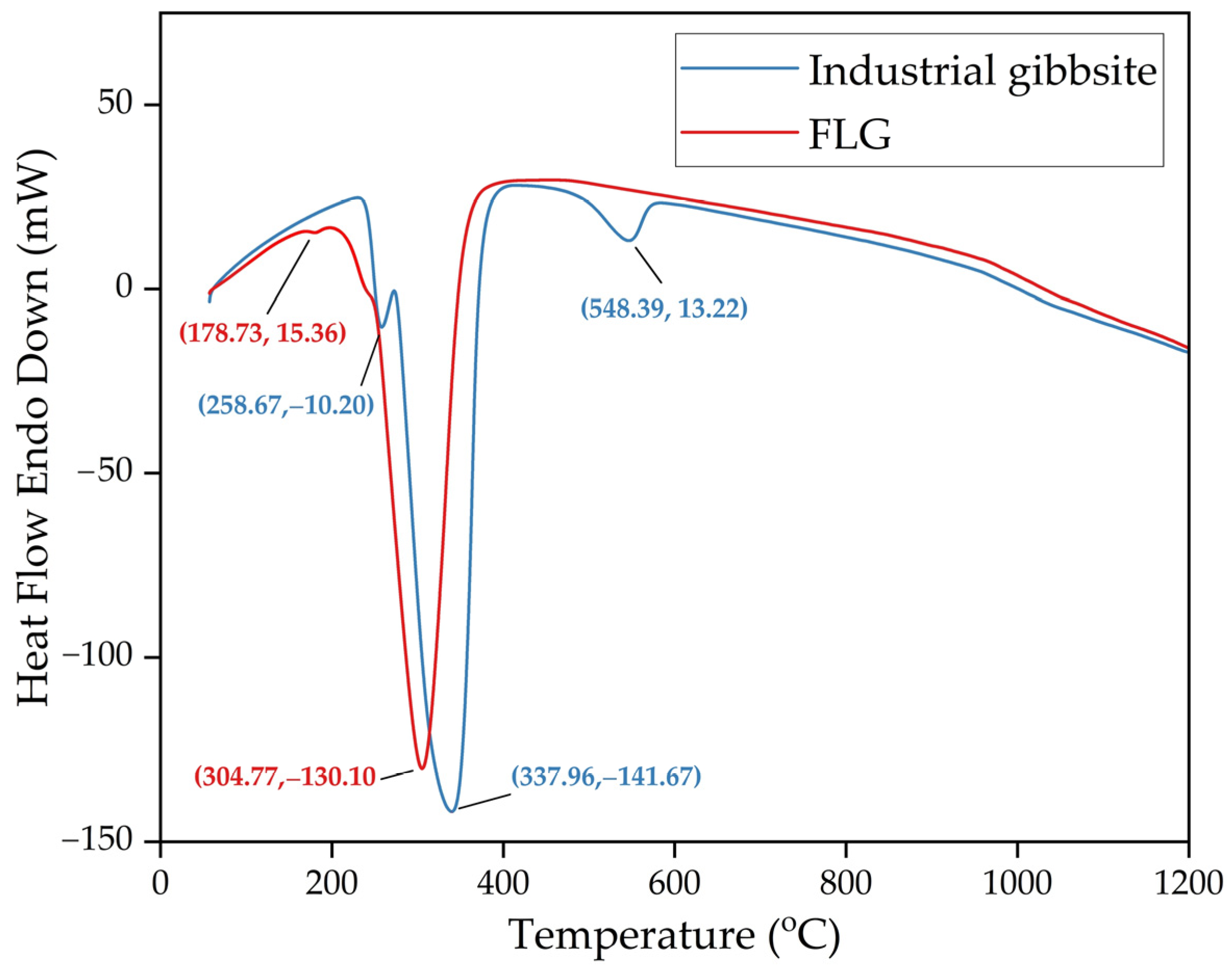
3.3. Solid Product Characterization
4. Conclusions
- The effect of temperature and time on the FLG precipitation indicates that the temperature increase from 20 to 35 °C leads to an increase in the precipitation rate from 40% to 60%. It was found that the maximum precipitation rate was obtained at 35 °C, which is in agreement with the literature data on kinetics of the precipitation of gibbsite from sodium aluminate solution.
- The increase in the amount of seed from 10 g L−1 to 40 g L−1 helps to increase the precipitation rate from 42.9% to 54.8%.
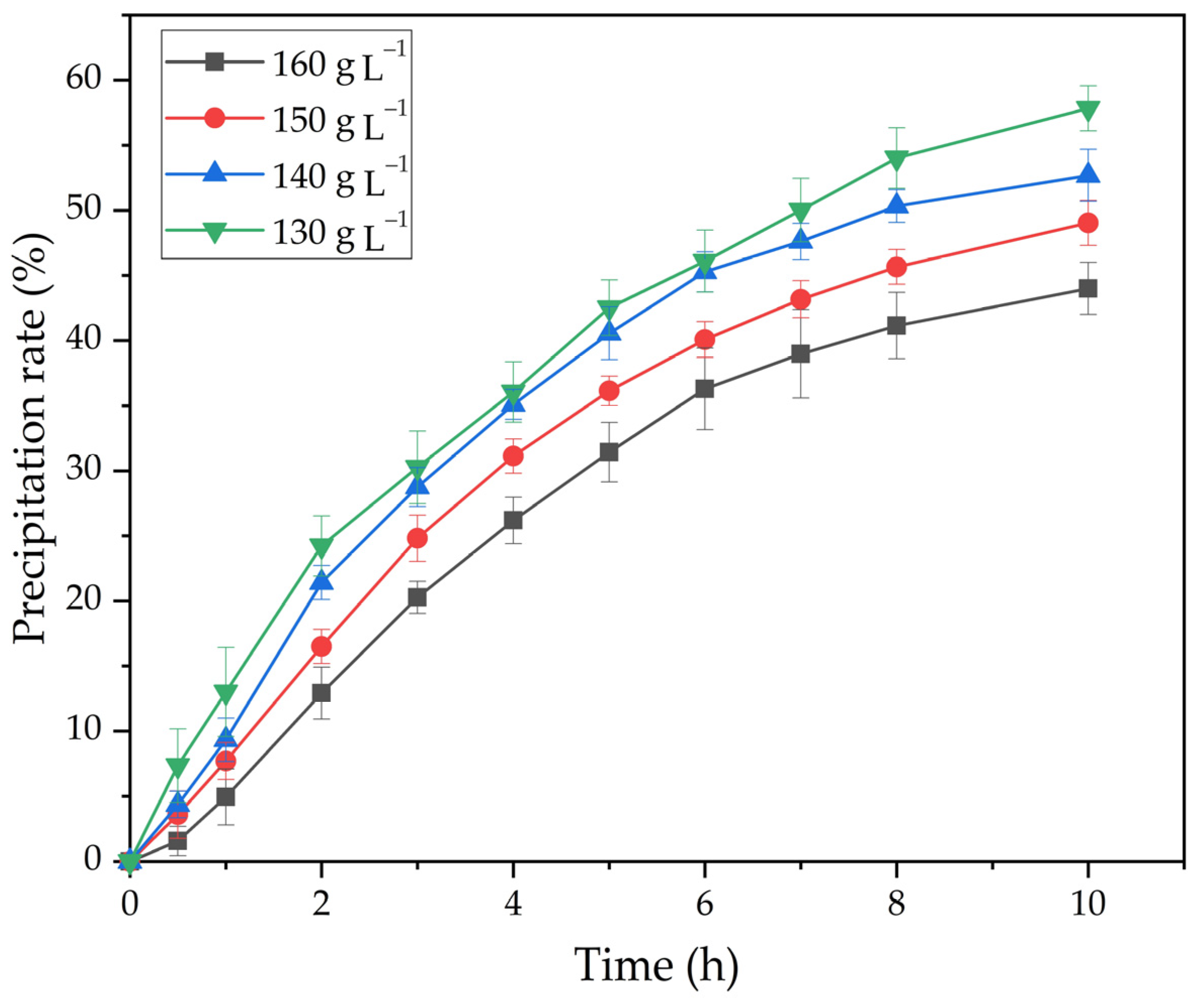
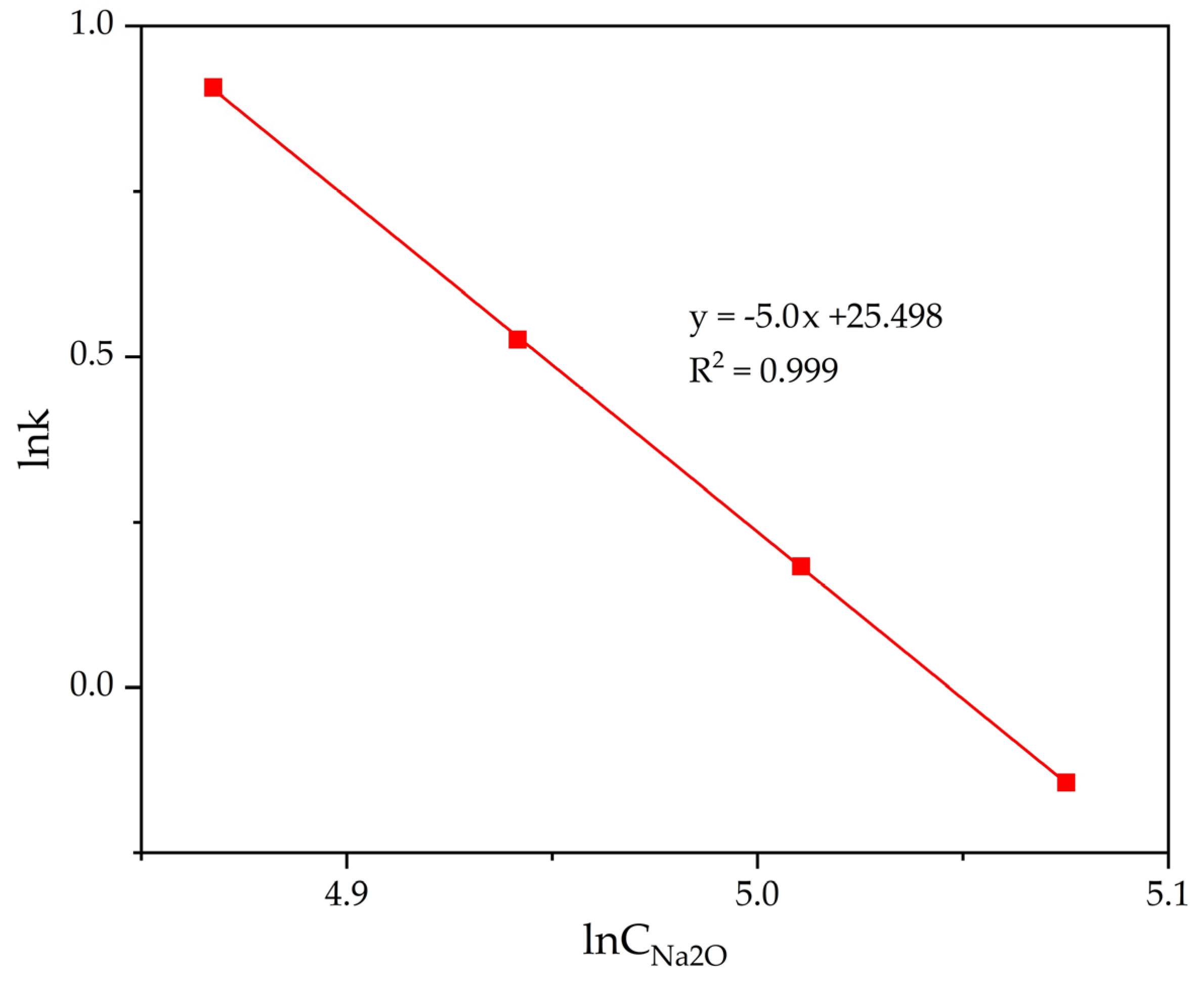
- Relatively high alkali concentrations were found to inhibit the precipitation of FLG. An increase in the Na2Ok concentration from 130 to 160 g L−1 leads to a decrease in the precipitation rate from 58.3 to 44.0%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loginova, I.V.; Shoppert, A.A.; Chaikin, L.I. Extraction of Rare-Earth Metals During the Systematic Processing of Diaspore-Boehmite Bauxites. Metallurgist 2016, 60, 198–203. [Google Scholar] [CrossRef]
- Loginova, I.V.; Shoppert, A.A.; Chaikin, L.I. Effect of Adding Sintering Furnace Electrostatic Precipitator Dust on Combined Leaching of Bauxites and Cakes. Metallurgist 2015, 59, 698–704. [Google Scholar] [CrossRef]
- Hond, R.D.; Hiralal, I.; Rijkeboer, A. Alumina Yield in the Bayer Process Past, Present and Prospects. In Essential Readings in Light Metals; Donaldson, D., Raahauge, B.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 528–533. ISBN 978-1-118-64786-8. [Google Scholar]
- Chen, Q.; Yin, J.; Yin, Z. Effect of Mechanically Activated Seeds on the Agglomeration Process of Supersaturated Sodium Aluminate Liquors. Light Met. 2007, 175, 157–161. [Google Scholar]
- Zeng, J.-S.; Yin, Z.-L.; Chen, Q.-Y. Decomposition Enhancement of Seeded Sodium Aluminate Liquor by Activated Seed. Zhongguo Youse Jinshu Xuebao Chin. J. Nonferr. Met. 2008, 18, 361–365. [Google Scholar]
- Zeng, J.; Yin, Z.; Chen, Q. Intensification of Precipitation of Gibbsite from Seeded Caustic Sodium Aluminate Liquor by Seed Activation and Addition of Crown Ether. Hydrometallurgy 2007, 89, 107–116. [Google Scholar] [CrossRef]
- Li, J.-P.; Yin, Z.-L.; Lü, B.-L.; Chen, Q.-Y. Effects of L-Leucine Additives on Seeded Precipitation of Sodium Aluminate Solution. Zhongguo Youse Jinshu Xuebao Chin. J. Nonferr. Met. 2010, 20, 1855–1860. [Google Scholar]
- Liu, G.; Wu, G.; Chen, W.; Li, X.; Peng, Z.; Zhou, Q.; Qi, T. Increasing Precipitation Rate from Sodium Aluminate Solution by Adding Active Seed and Ammonia. Hydrometallurgy 2018, 176, 253–259. [Google Scholar] [CrossRef]
- Stephenson, J.L.; Kapraun, C. Dynamic Modeling of Yield and Particle Size Distribution in Continuous Bayer Precipitation. In Essential Readings in Light Metals; Donaldson, D., Raahauge, B.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 891–897. ISBN 978-3-319-48574-4. [Google Scholar]
- Zhang, B.; Pan, X.; Yu, H.; Tu, G.; Bi, S. Effect of Organic Impurity on Seed Precipitation in Sodium Aluminate Solution. In Light Metals 2018; Martin, O., Ed.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–47. ISBN 978-3-319-72283-2. [Google Scholar]
- Pan, X.; Yu, H.; Tu, G.; Bi, S. Effects of Precipitation Activity of Desilication Products (DSPs) on Stability of Sodium Aluminate Solution. Hydrometallurgy 2016, 165, 261–269. [Google Scholar] [CrossRef]
- Sweegers, C.; de Coninck, H.C.; Meekes, H.; van Enckevort, W.J.P.; Hiralal, I.D.K.; Rijkeboer, A. Morphology, Evolution and Other Characteristics of Gibbsite Crystals Grown from Pure and Impure Aqueous Sodium Aluminate Solutions. J. Cryst. Growth 2001, 233, 567–582. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Qi, T.; Li, X.; Zhou, Q.; Peng, Z. Two-Stage Process for Precipitating Coarse Boehmite from Sodium Aluminate Solution. JOM 2017, 69, 1888–1893. [Google Scholar] [CrossRef]
- Valeev, D.; Shoppert, A.; Mikhailova, A.; Kondratiev, A. Acid and Acid-Alkali Treatment Methods of Al-Chloride Solution Obtained by the Leaching of Coal Fly Ash to Produce Sandy Grade Alumina. Metals 2020, 10, 585. [Google Scholar] [CrossRef]
- Lee, S.O.; Jung, K.H.; Oh, C.J.; Lee, Y.H.; Tran, T.; Kim, M.J. Precipitation of Fine Aluminium Hydroxide from Bayer Liquors. Hydrometallurgy 2009, 98, 156–161. [Google Scholar] [CrossRef]
- Bhattacharya, I.N.; Pradhan, J.K.; Gochhayat, P.K.; Das, S.C. Factors Controlling Precipitation of Finer Size Alumina Trihydrate. Int. J. Miner. Process. 2002, 65, 109–124. [Google Scholar] [CrossRef]
- Ye, P.-H.; Qi, T.-G.; Zhou, Q.-S.; Wu, X.-P.; Peng, Z.-H.; Liu, G.-H.; Li, X.-B. Particles Evolution during Seeded Precipitation of Sodium Aluminate Solution by Adding Active Seed. Zhongguo Youse Jinshu Xuebao Chin. J. Nonferr. Met. 2020, 30, 1172–1181. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.; Du, H.; Xu, H.; Wang, S.; Zhang, Y. Improved Precipitation of Gibbsite from Sodium Aluminate Solution by Adding Methanol. Hydrometallurgy 2009, 98, 38–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.; Zhang, Y.; Xu, H.; Zhang, Y. Additives Effects on Crystallization and Morphology in a Novel Caustic Aluminate Solution Decomposition Process. Front. Chem. Eng. China 2009, 3, 88–92. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, Y.; Bi, S.; Lǚ, Z.; Yang, Y.; Li, B. Kinetics of Crystallization in Sodium Aluminate Liquors. In Proceedings of the TMS Light Metals, Charlotte, NC, USA, 14–18 March 2004; pp. 71–75. [Google Scholar]
- Misra, C.; White, E.T. Mathematical Model of the Bayer Precipitation Process for Alumina Production. In Proceedings of the CHEMECA’70, Melbourne & Sydney, Australia, 19–26 August 1970; Butterworth Co., (Aust.) Ltd.: Chatswood, Australia, 1970; Volume 7, pp. 52–76. [Google Scholar]
- Li, J.; Addai-Mensah, J.; Thilagam, A.; Gerson, A.R. Growth Mechanisms and Kinetics of Gibbsite Crystallization: Experimental and Quantum Chemical Study. Cryst. Growth Des. 2012, 12, 3096–3103. [Google Scholar] [CrossRef]
- Xue, J.; Liu, C.; Luo, M.; Lin, M.; Jiang, Y.; Li, P.; Yu, J.; Rohani, S. Secondary Nucleation and Growth Kinetics of Aluminum Hydroxide Crystallization from Potassium Aluminate Solution. J. Cryst. Growth 2019, 507, 232–240. [Google Scholar] [CrossRef]
- Li, J.; Prestidge, C.A.; Addai-Mensah, J. Secondary Nucleation of Gibbsite Crystals from Synthetic Bayer Liquors: Effect of Alkali Metal Ions. J. Cryst. Growth 2000, 219, 451–464. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Zhou, Q.; Liu, G.; Peng, Z. Concentration Variation of Aluminate Ions during the Seeded Precipitation Process of Gibbsite from Sodium Aluminate Solution. Hydrometallurgy 2011, 106, 93–98. [Google Scholar] [CrossRef]
- Li, X.; Yan, L.; Zhao, D.; Zhou, Q.; Liu, G.; Peng, Z.; Yang, S.; Qi, T. Relationship between Al(OH)3 Solubility and Particle Size in Synthetic Bayer Liquors. Trans. Nonferr. Met. Soc. China 2013, 23, 1472–1479. [Google Scholar] [CrossRef]
- Sonthalia, R.; Behara, P.; Kumaresan, T.; Thakre, S. Review on Alumina Trihydrate Precipitation Mechanisms and Effect of Bayer Impurities on Hydrate Particle Growth Rate. Int. J. Miner. Process. 2013, 125, 137–148. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, X.; Zhou, J. Particle Distribution Model of Gibbsite Precipitation Process in Alumina Production. Chem. Eng. Process. Process Intensif. 2011, 50, 741–746. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Li, Y.-Q.; Fu, M.-H.; Liu, B.; Li, L. Concentration and Mass Ratio Model of Gibbsite Precipitation Process in Alumina Production. Zhongnan Daxue Xuebao Ziran Kexue Ban J. Cent. South Univ. Sci. Technol. 2011, 42, 1543–1548. [Google Scholar]
- Mirzaei, H.; Noaparast, M.; Abdollahi, H. Modeling and Optimizing Aluminum Hydroxide Precipitation Process in Industrial Scale; Case Study: Iran Alumina Plant. J. Min. Environ. 2021, 12, 235–251. [Google Scholar] [CrossRef]
- Loginova, I.V.; Shoppert, A.A. Preparation of Active Aluminum Hydroxide and Its Use for Production of Finely Dispersed Alumina. Russ. J. Non-Ferr. Met. 2014, 55, 234–237. [Google Scholar] [CrossRef]
- Vernon, C.F.; Brown, M.J.; Lau, D.; Zieba, M.P. Mechanistic Investigation of Gibbsite Growth. In Proceedings of the 6th International Alumina Quality Workshop, Brisbane, Australia, 8–13 September 2002; pp. 33–39. [Google Scholar]
- Vernon, C.; Loh, J.; Lau, D.; Stanley, A. Soda Incorporation During Hydrate Precipitation. In Essential Readings in Light Metals; Donaldson, D., Raahauge, B.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 602–607. ISBN 978-3-319-48574-4. [Google Scholar]
- Li, X.; Feng, G.; Zhou, Q.; Peng, Z.; Liu, G. Phenomena in Late Period of Seeded Precipitation of Sodium Aluminate Solution. Trans. Nonferr. Met. Soc. China 2006, 16, 947–950. [Google Scholar] [CrossRef]

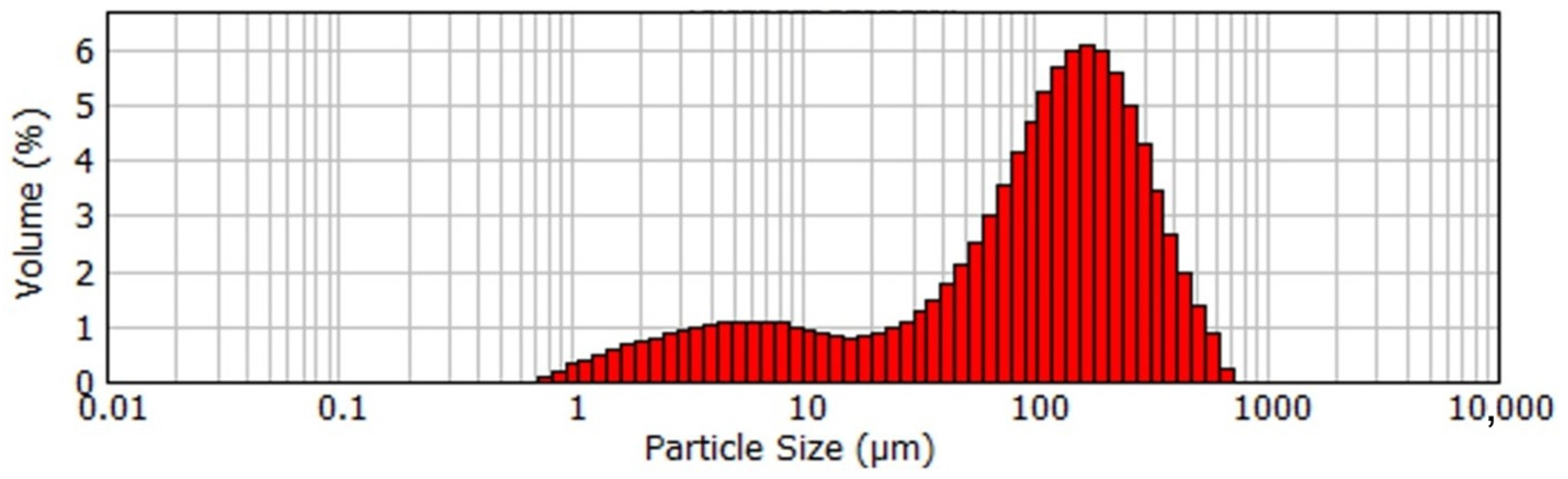
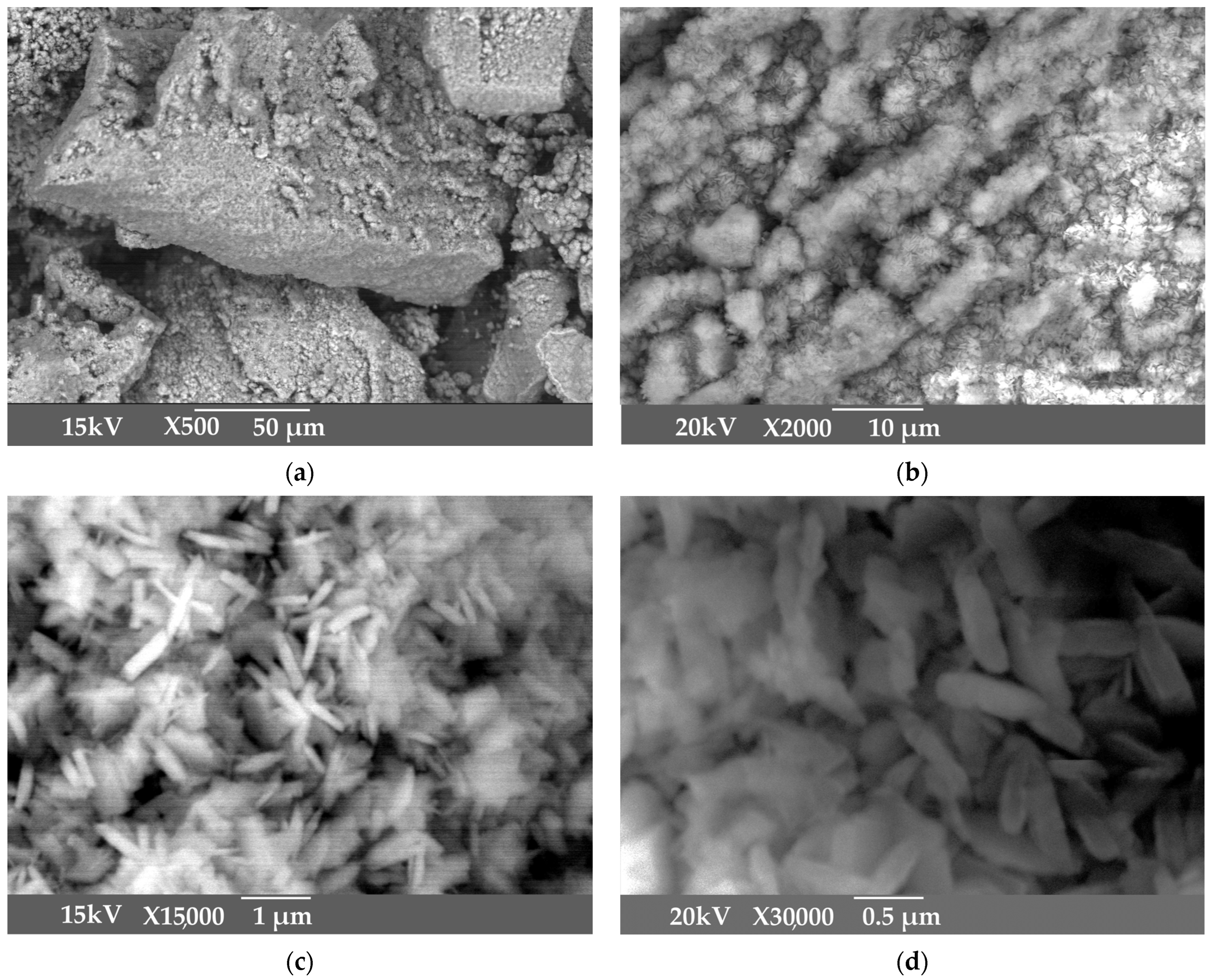
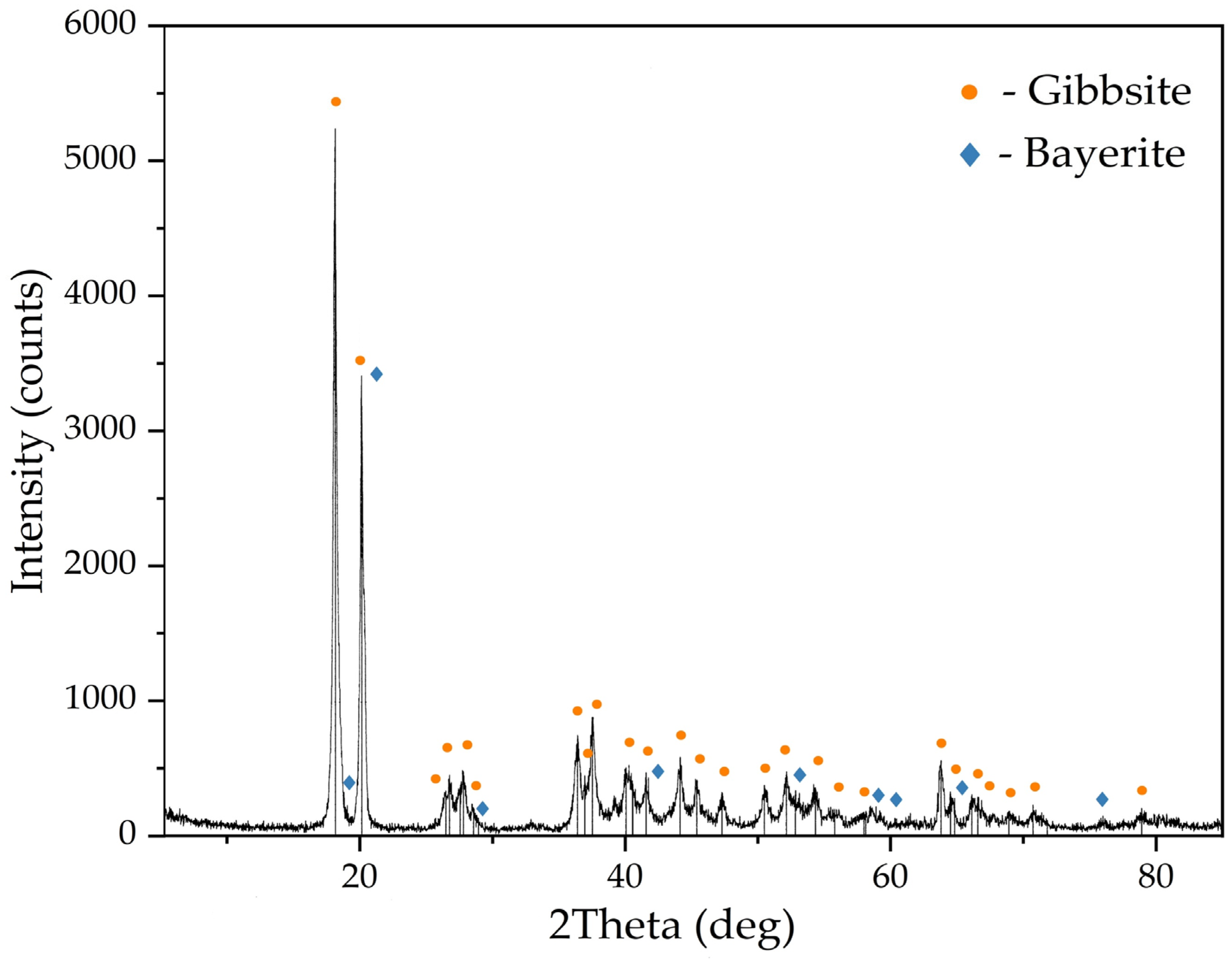
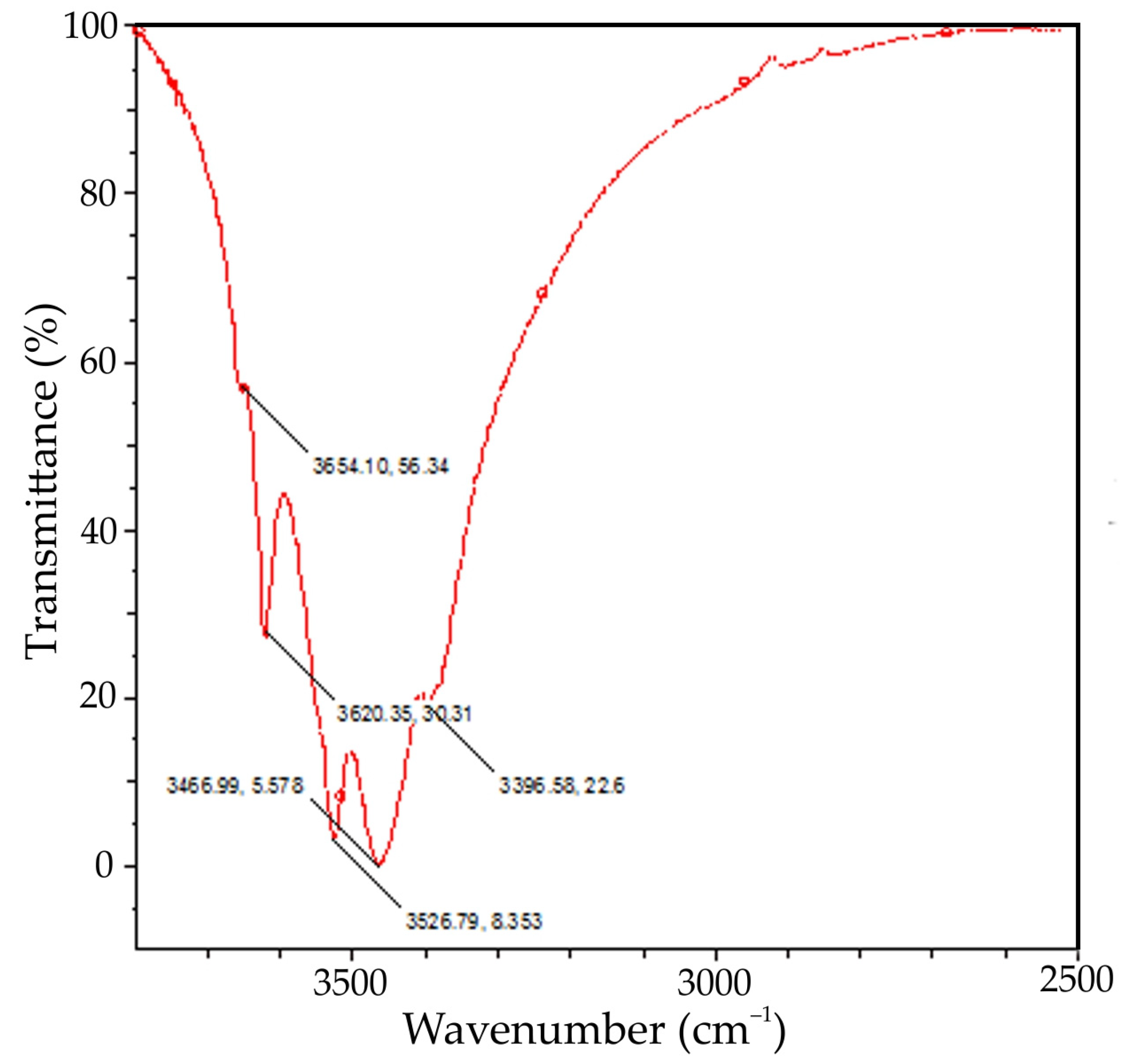
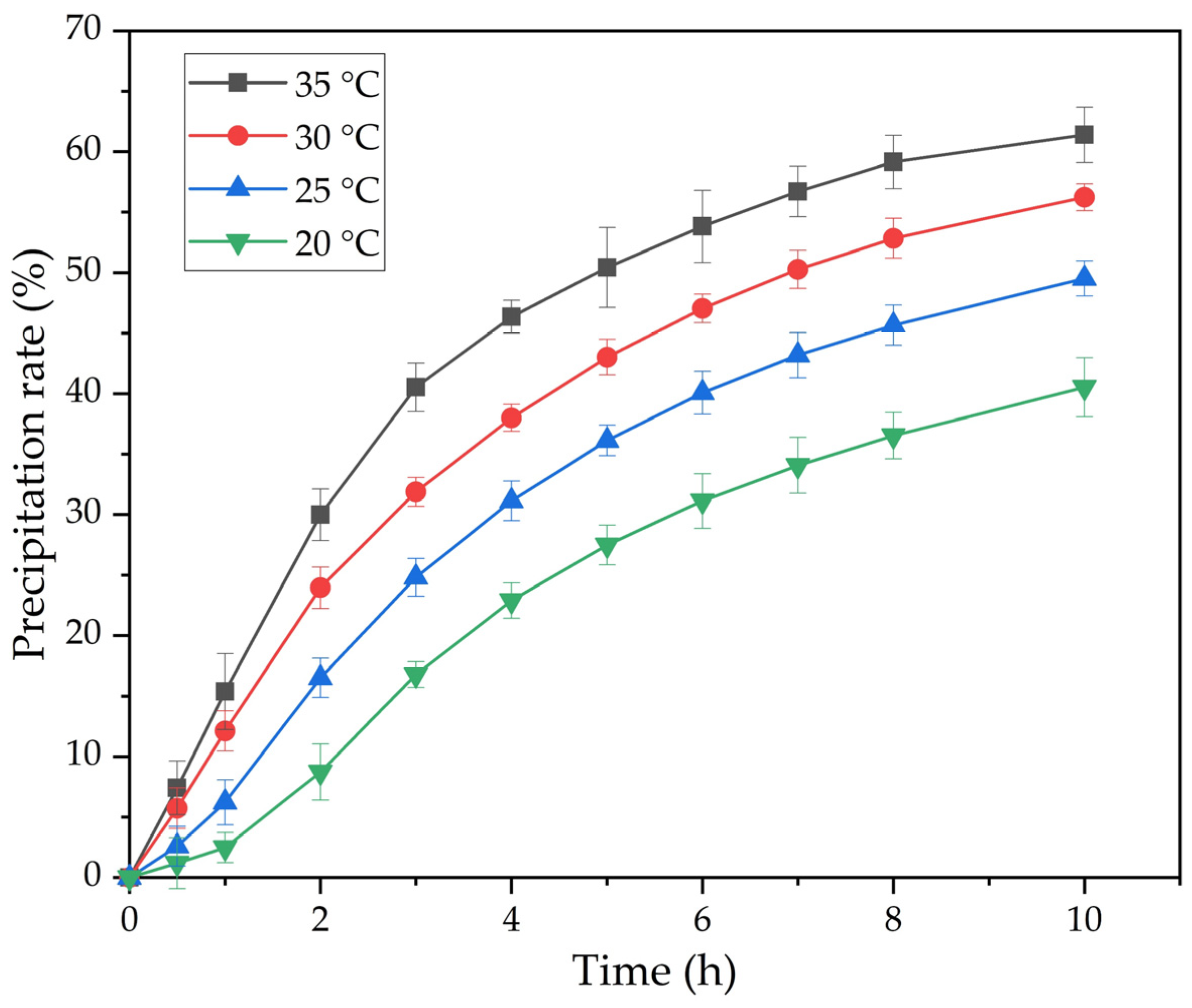

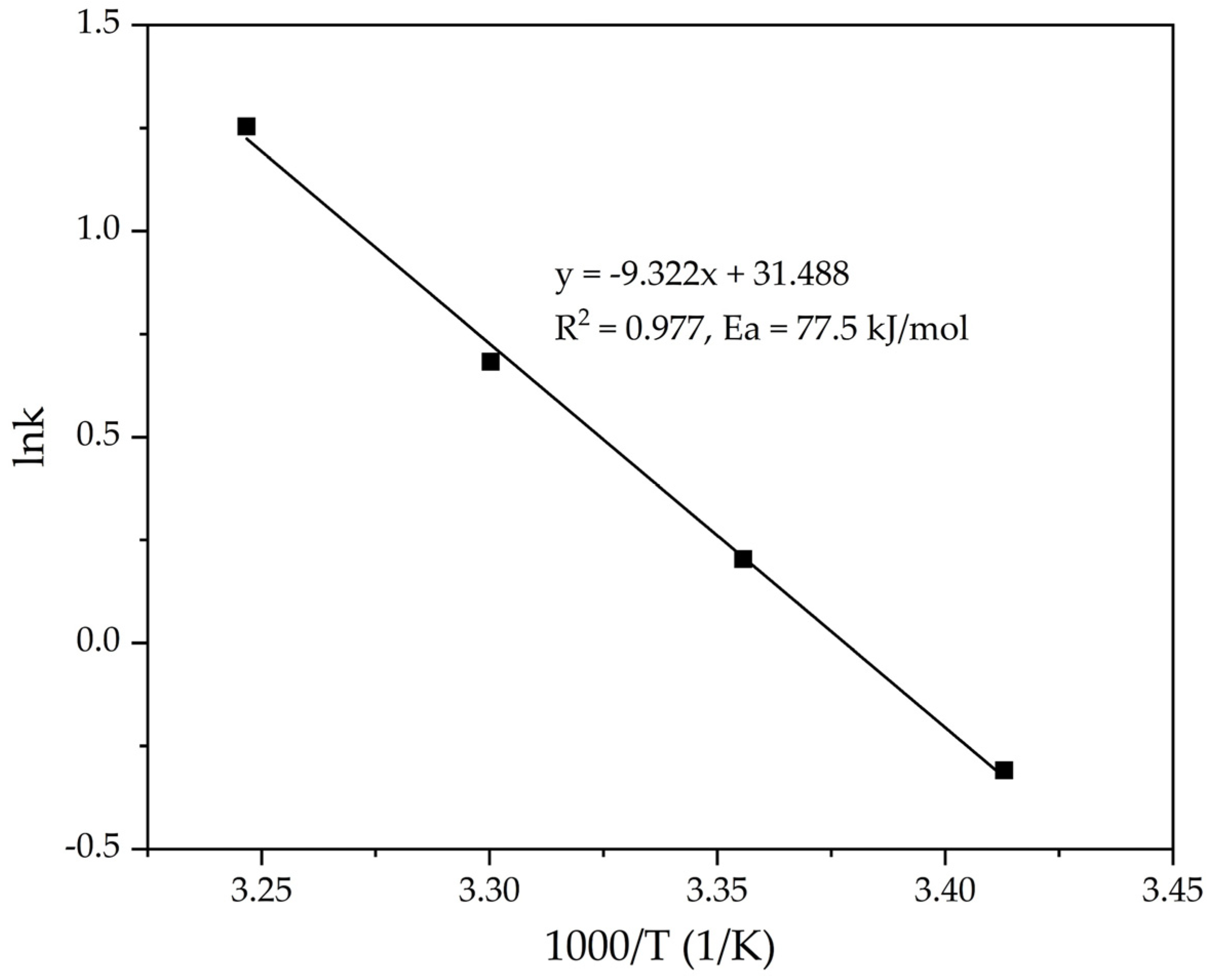
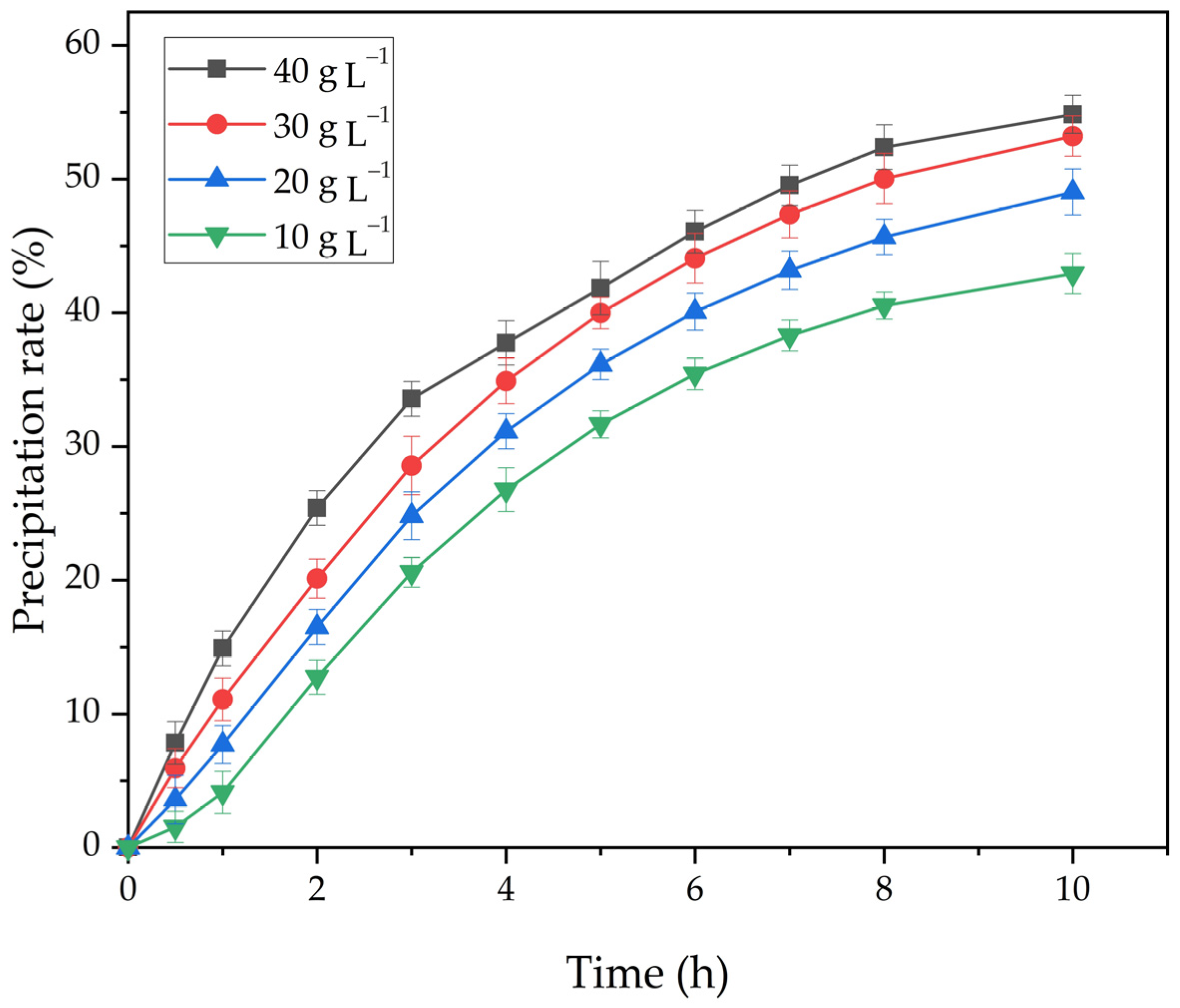
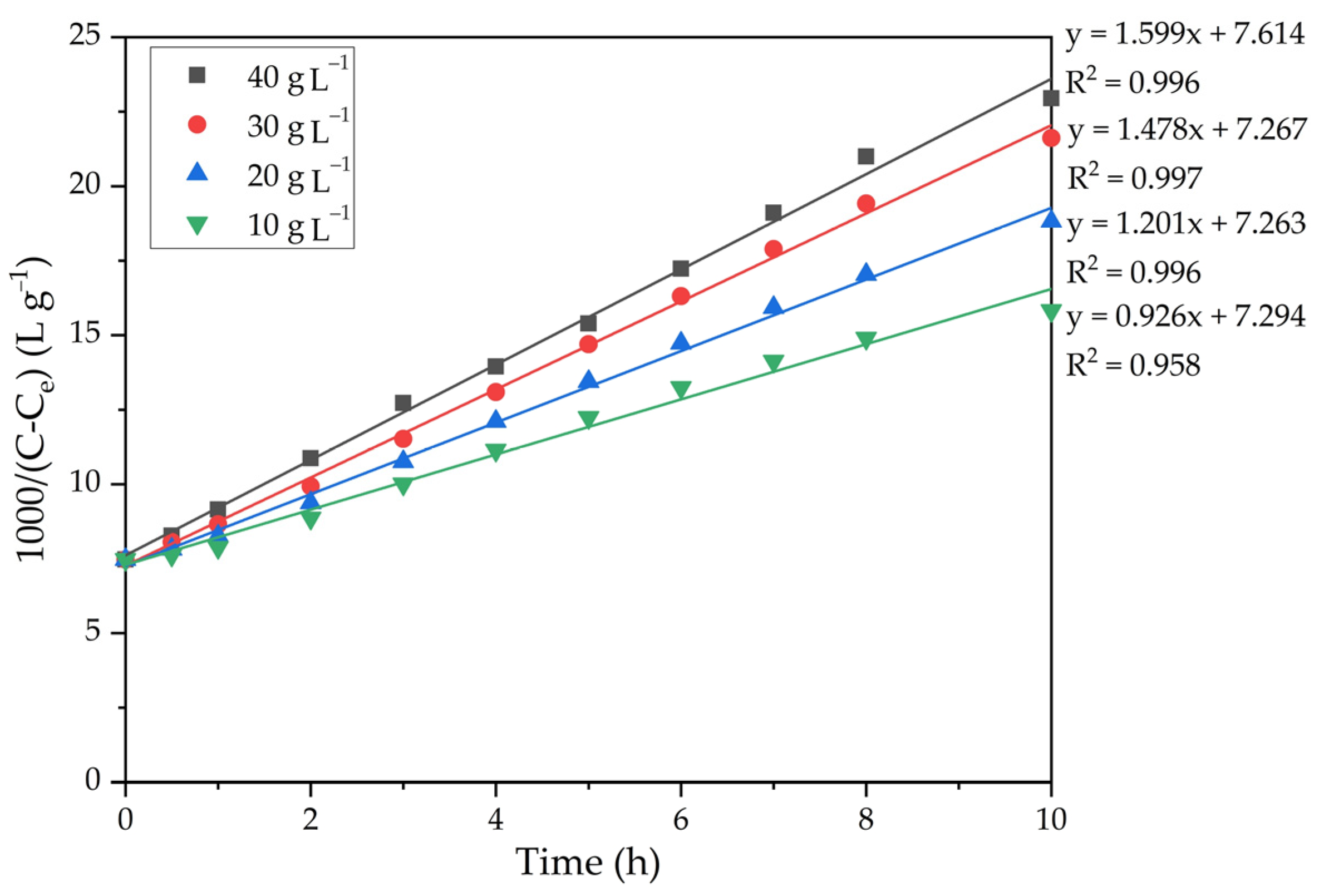


| T, K | 293 | 298 | 303 | 308 |
|---|---|---|---|---|
| 1000/T, K−1 | 3.413 | 3.356 | 3.301 | 3.247 |
| K | 3.796 | 3.477 | 3.147 | 2.545 |
| lnK | −0.310 | 0.203 | 0.683 | 1.253 |
| G, g L−1 | 10 | 20 | 30 |
|---|---|---|---|
| lnG | 2.303 | 2.996 | 3.401 |
| lnK | −0.077 | 0.183 | 0.390 |
| CNa2O, g dm–3 | 130 | 140 | 150 |
|---|---|---|---|
| lnCNa2O | 4.868 | 4.942 | 5.011 |
| lnK | 0.907 | 0.526 | 0.183 |
| Fraction, µm | –45 | 45–63 | 63–125 | 125–200 | 200–315 | +315 | d50 |
|---|---|---|---|---|---|---|---|
| FLG 35 °C | 100.0 | - | - | - | - | - | 1.5 |
| FLG 25 °C | 24.7 | 6.1 | 22.4 | 23.7 | 14.9 | 8.2 | 115.5 |
| Industrial seed | 13.5 | 19.2 | 62.5 | 4.0 | 1.0 | - | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoppert, A.; Valeev, D.; Alekseev, K.; Loginova, I. Enhanced Precipitation of Gibbsite from Sodium Aluminate Solution by Adding Agglomerated Active Al(OH)3 Seed. Metals 2023, 13, 193. https://doi.org/10.3390/met13020193
Shoppert A, Valeev D, Alekseev K, Loginova I. Enhanced Precipitation of Gibbsite from Sodium Aluminate Solution by Adding Agglomerated Active Al(OH)3 Seed. Metals. 2023; 13(2):193. https://doi.org/10.3390/met13020193
Chicago/Turabian StyleShoppert, Andrei, Dmitry Valeev, Konstantin Alekseev, and Irina Loginova. 2023. "Enhanced Precipitation of Gibbsite from Sodium Aluminate Solution by Adding Agglomerated Active Al(OH)3 Seed" Metals 13, no. 2: 193. https://doi.org/10.3390/met13020193
APA StyleShoppert, A., Valeev, D., Alekseev, K., & Loginova, I. (2023). Enhanced Precipitation of Gibbsite from Sodium Aluminate Solution by Adding Agglomerated Active Al(OH)3 Seed. Metals, 13(2), 193. https://doi.org/10.3390/met13020193







