The Plasma Electrolytic Oxidation of Aluminum Using Microsecond-Range DC Pulsing
Abstract
:1. Introduction
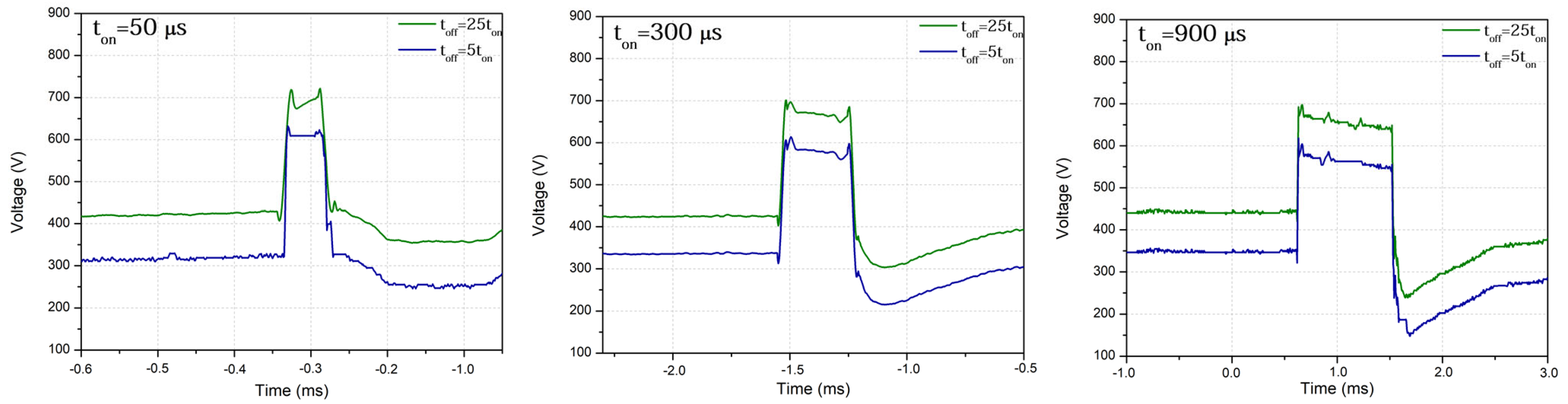
2. Materials and Methods
3. Results and Discussion
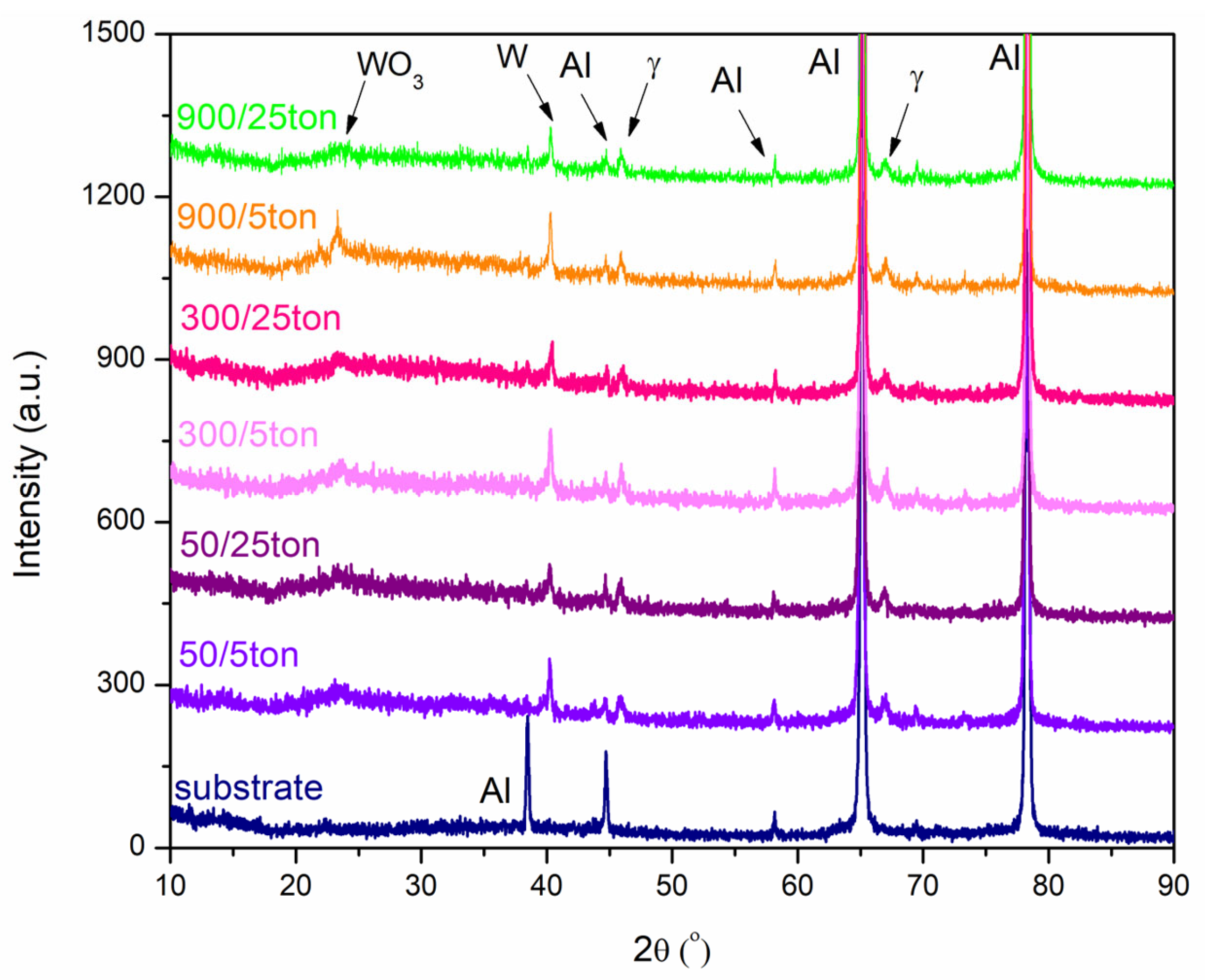
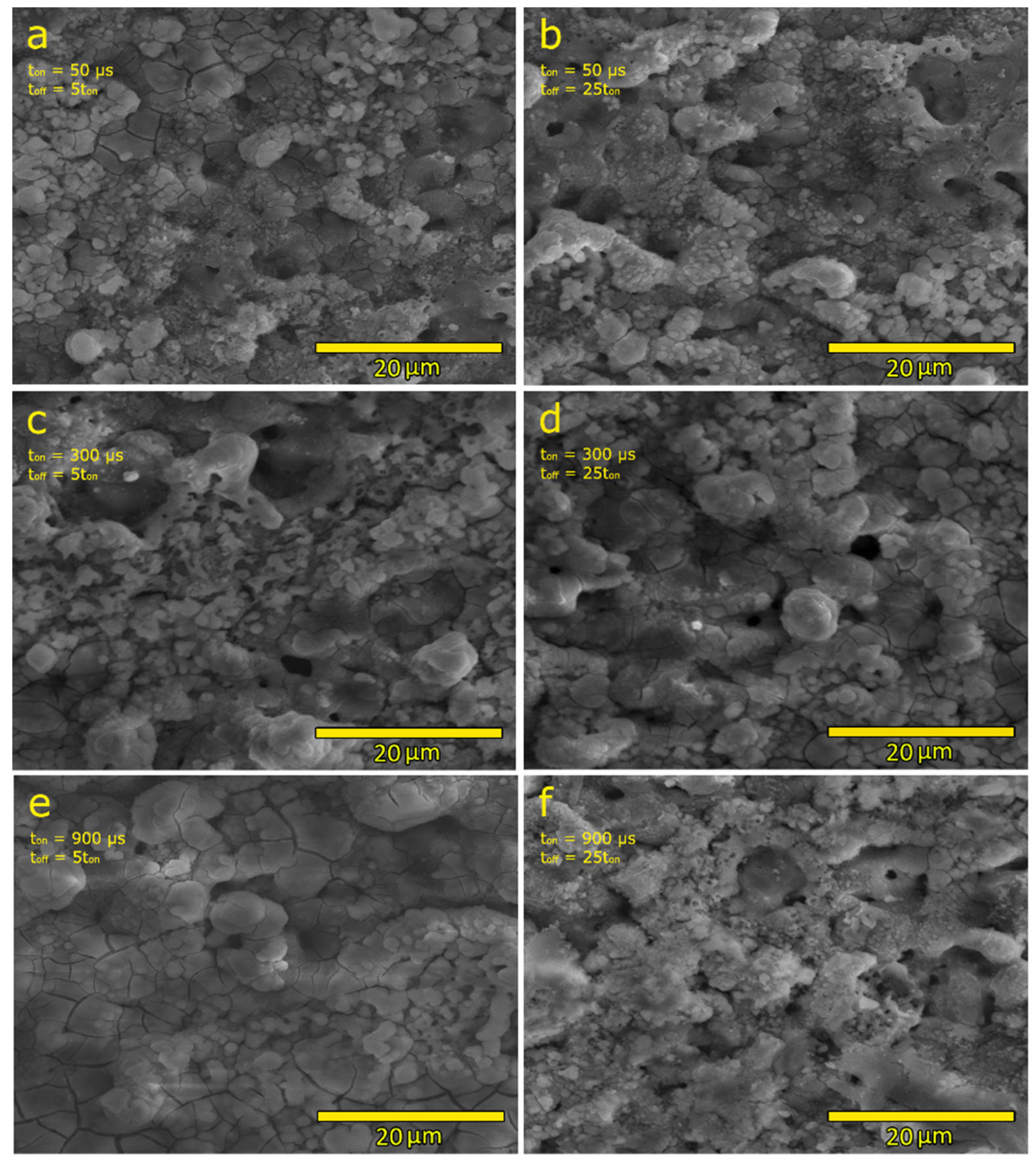
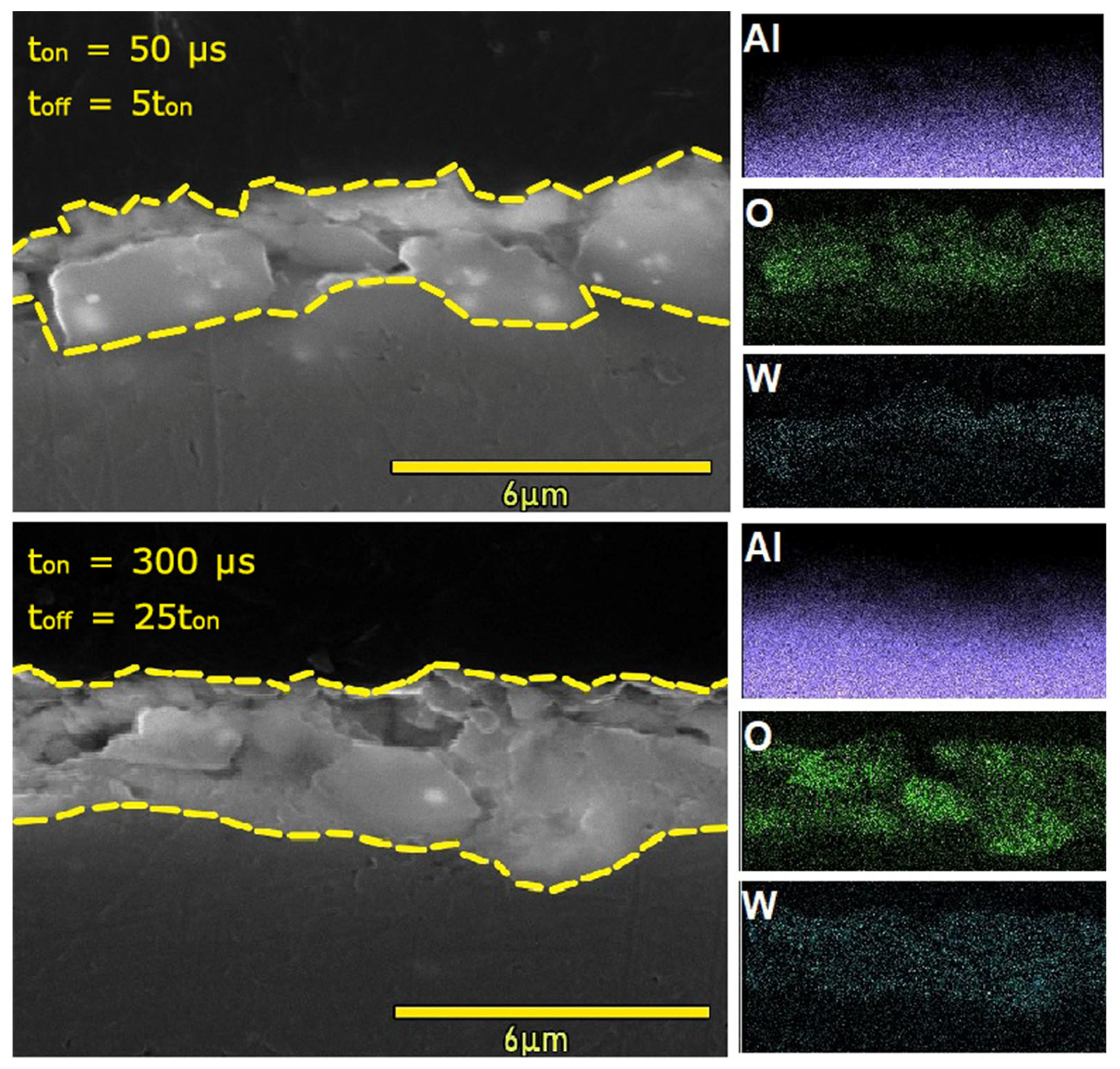
3.1. Phase Composition, Surface Morphology and Chemical Composition of the PEO Coatings
3.2. Photocatalytic Activity and Photoluminescence of the PEO Coatings
4. Conclusions
- (a)
- Changing the pulse length (ton = 50 μs, 300 μs, 900 μs) and duty cycle (toff = 5 ton, 25 ton) does not have a considerable influence on the phase and chemical composition of formed coatings. However, a higher level of crystallization in the formed PEO coatings is observed for higher duty cycle values. The formed oxide coatings contain species originating both from the substrate and the electrolyte solution, but their composition does not change dramatically when changing the processing conditions in the investigated range of electrical parameters;
- (b)
- Processing conditions play an important role in the morphology of obtained coatings, especially in their thickness. For coatings with pulse lengths of 50 μs and 300 μs, the thickness increases with the lowering duty cycle, while for the coatings with a pulse length of 900 μs, an opposite trend is observed. Conversely, the porosity of the 900 μs pulse samples increases with the lowering duty cycle, while for the other two pulse lengths, the exact opposite trend is observed. The PEO coatings formed with 50 µs pulses (regardless of the duty cycle value) show higher roughness than the coatings formed with pulse lengths of 300 µs and 900 µs.
- (c)
- The photocatalytic activity and photoluminescence of formed PEO coatings are dependent on both their morphological properties and their chemical composition. The highest photoactivity is observed for the coatings with ton = 300 μs and toff = 25 ton, which coincides with the maximum PL intensity. However, comparable photocatalytic activity and photoluminescence are observed for the coating formed with ton = 50 μs and toff = 25 ton, which requires about six times less energy for PEO processing.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yerokhin, A.L.; Nie, X.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Shoesmith, D.W.; Liu, X.Y.; Rohani, S. Effect of duty cycle and applied current frequency on plasma electrolytic oxidation (PEO) coating behavior. Surf. Coat. Technol. 2013, 226, 100–107. [Google Scholar] [CrossRef]
- de Lima, R.N.; Vitoriano, J.d.O.; Ferreira, M., Jr.; Alves Junior, C. Plasma species and coating compositions in aluminum treated by PEO using shot square pulse. Mater. Res. 2020, 23, e2019044. [Google Scholar]
- Bertuccioli, C.; Garzoni, A.; Martini, C.; Morri, A.; Rondelli, G. Plasma electrolytic oxidation (PEO) layers from silicate/phosphate baths on Ti-6Al-4V for biomedical components: Influence of deposition conditions and surface finishing on dry sliding behaviour. Coatings 2019, 9, 614. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, K.-H.; Kim, S.-J. Characterization of ceramic oxide coatings prepared by plasma electrolytic oxidation using pulsed direct current with different duty ratio and frequency. Appl. Surf. Sci. 2020, 516, 146049. [Google Scholar] [CrossRef]
- Mojsilović, K.; Jovović, J.; Stojadinović, S.; Vasilić, R. Micro-second range pulsed DC plasma electrolytic oxidation on Ti and Nb. Solid State Sci. 2022, 133, 107018. [Google Scholar] [CrossRef]
- Frutuoso, F.G.S.d.O.; Vitoriano, J.d.O.; Alves-Junior, C. Controlling plasma electrolytic oxidation of titanium using current pulses compatible with the duration of microdischarges. Results Mater. 2023, 15, 100310. [Google Scholar] [CrossRef]
- Hryniewicz, T. Plasma electrolytic oxidation of metals and alloys. Metals 2018, 8, 1058. [Google Scholar] [CrossRef]
- Martin, J.; Nomine, A.; Ntomprougkidis, V.; Migot, S.; Bruyere, S.; Soldera, F.; Belmonte, T.; Henrion, G. Formation of a metastable nanostructured mullite during plasma electrolytic oxidation of aluminium in “soft” regime condition. Mater. Des. 2019, 180, 107977. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Toulabifard, A.; Hakimizad, A.; Di Franco, F.; Raeissi, K.; Santamaria, M. Synergistic effect of W incorporation and pulsed current mode on wear and tribocorrosion resistance of coatings grown by plasma electrolytic oxidation on 7075 Al alloy. Mater. Res. Express 2019, 6, 106502. [Google Scholar] [CrossRef]
- Bajat, J.R.; Vasilić, R.; Stojadinović, S.; Mišković-Stanković, V. Corrosion stability of oxide coatings formed by plasma electrolytic oxidation of aluminum: Optimization of process time. Corrosion 2013, 69, 693–702. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Rudnev, V.S.; Andenko, N.A.; Kaidalova, T.A.; Panin, E.S.; Gordienko, P.S. Anodic-spark oxidation of aluminum alloy in tungstate electrlytes. Russ. J. Appl. Chem. 2002, 75, 573–578. [Google Scholar] [CrossRef]
- Quan, H.; Gao, Y.; Wang, W. Tungsten oxide-based visible light-driven photocatalysts: Crystal and electronic structures and strategies for photocatalytic efficiency enhancement. Inorg. Chem. Front. 2020, 7, 817–838. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Salleh, W.N.W.; Aziz, F.; Jye, L.W.; Yusof, N.; Ismail, A.F. Review on tungsten trioxide as a photocatalysts for degradation of recalcitrant pollutants. J. Clean. Prod. 2021, 309, 127438. [Google Scholar] [CrossRef]
- Tian, J.; Shang, K.; Xue, X.; Yang, L. Degradation of methyl orange waste water by electrochemical oxidation method. J. Phys. Conf. Ser. 2013, 418, 012134. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R.; Radić, N.; Tadić, N.; Stefanov, P.; Grbić, B. The formation of tungsten doped Al2O3/ZnO coatings on aluminum by plasma electrolytic oxidation and their application in photocatalysis. Appl. Surf. Sci. 2016, 377, 37–43. [Google Scholar] [CrossRef]
- Thompson, G.E.; Skeldon, P.; Shimizu, K.; Wood, G.C. The composition of barrier-type anodic films formed on aluminium in molybdate and tungstate electrolytes. Phil. Trans. R. Soc. Lond. A 1995, 350, 143–168. [Google Scholar]
- Mojsilović, K.; Tadić, N.; Lačnjevac, U.; Stojadinović, S.; Vasilić, R. Characterization of Al-W oxide coatings on aluminum formed by pulsed direct current plasma electrolytic oxidation at ultra-low duty cycles. Surf. Coat. Technol. 2021, 411, 126982. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Wang, Y.K.; Li, B.S.; Han, G.R. The effects of Na2WO4 concentration on the properties of microarc oxidation coatings on aluminum alloy. Mater. Lett. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Karbasi, M.; Nikoomanzari, E.; Hosseini, R.; Bahramian, H.; Chaharmahali, R.; Giannakis, S.; Kaseem, M.; Fattah-alhosseini, A. A review on plasma electrolytic oxidation coatings for organic pollutant degradation: How to prepare them and what to expect of them? J. Environ. Chem. Eng. 2023, 11, 110027. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Zhang, X.; Wang, L.; Zou, J.J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef]
- Azmat, S.; Jan, T.; Ilyas, S.Z.; Hassan, A.; Habib, I.; Mahmood, Q.; Mahmood, A. Solar light triggered photocatalytic performance of WO3 nanostructures; waste water treatment. Mater. Res. Express 2018, 5, 115025. [Google Scholar] [CrossRef]
- Cheng, O.; Zhang, G.K. Enhanced photocatalytic performance of tungsten-based photocatalysts for degradation of volatile organic compounds: A review. Tungsten 2020, 2, 240–250. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Vasilić, R.; Petković, M.; Stefanov, P.; Zeković, L.; Grbić, B. Photocatalytic properties of TiO2/WO3 coatings formed by plasma electrolytic oxidation of titanium in 12-tungstosilicic acid. Appl. Catal. B Environ. 2012, 126, 334–341. [Google Scholar] [CrossRef]
- Pancielejko, A.; Rzepnikowska, M.; Zaleska-Medynska, A.; Łuczak, J.; Mazierski, P. Enhanced Visible Light Active WO3 Thin films toward air purification: Effect of the Synthesis Conditions. Materials 2020, 13, 3506. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhnong, X.X.; He, C.L.; Zhang, B.; Cvelbar, U.; Ostrikov, K. Solvent-dependent structures and photoluminescence of WO3-x nanomaterials grown in nonaqueous solutions. J. Alloys Compd. 2021, 854, 157249. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, R.; Li, H.; Wang, L. Formation, detection, and function of oxygen vacancy in metal oxides for solar energy conversion. Adv. Funct. Mater. 2022, 32, 2109503. [Google Scholar] [CrossRef]

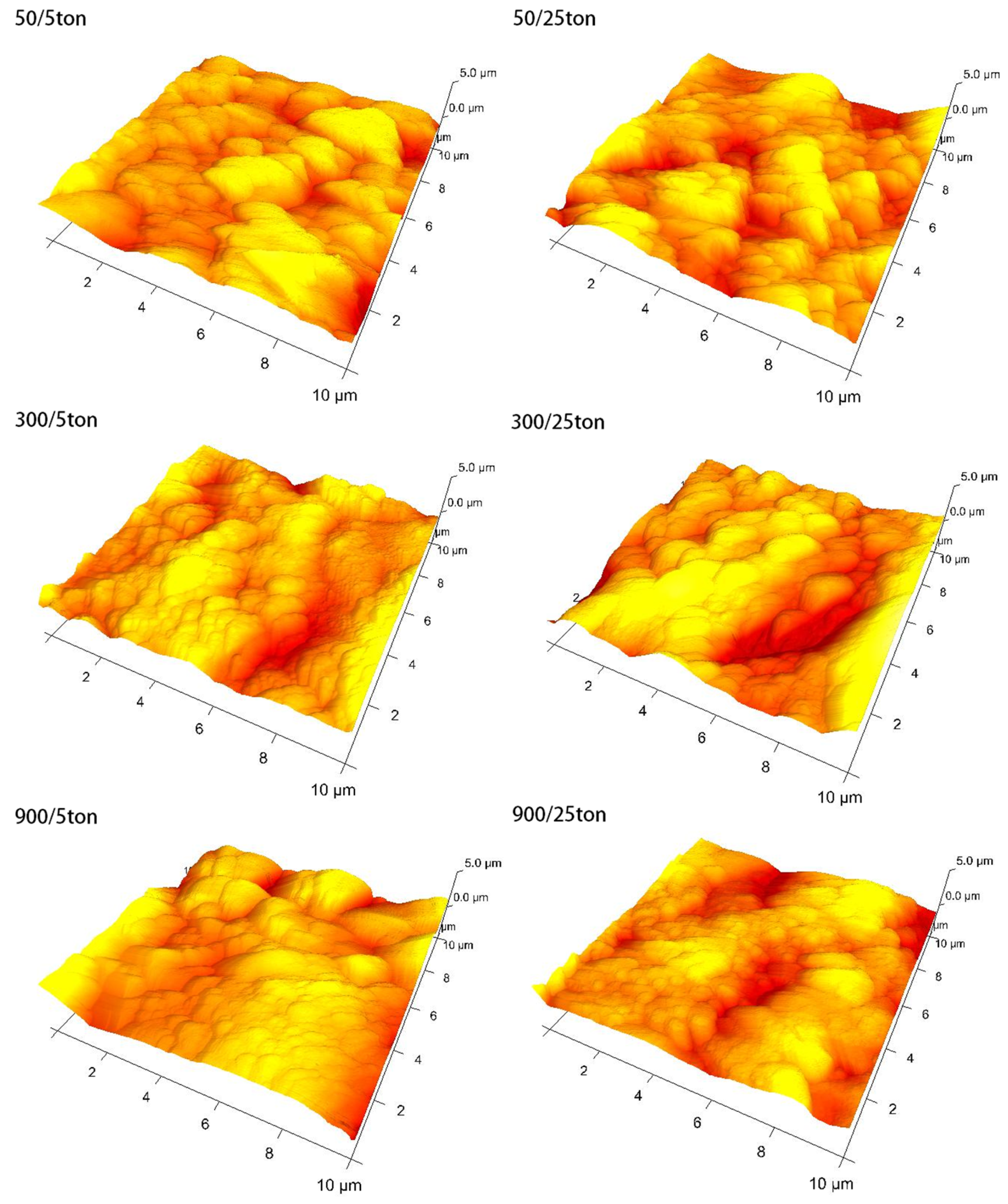
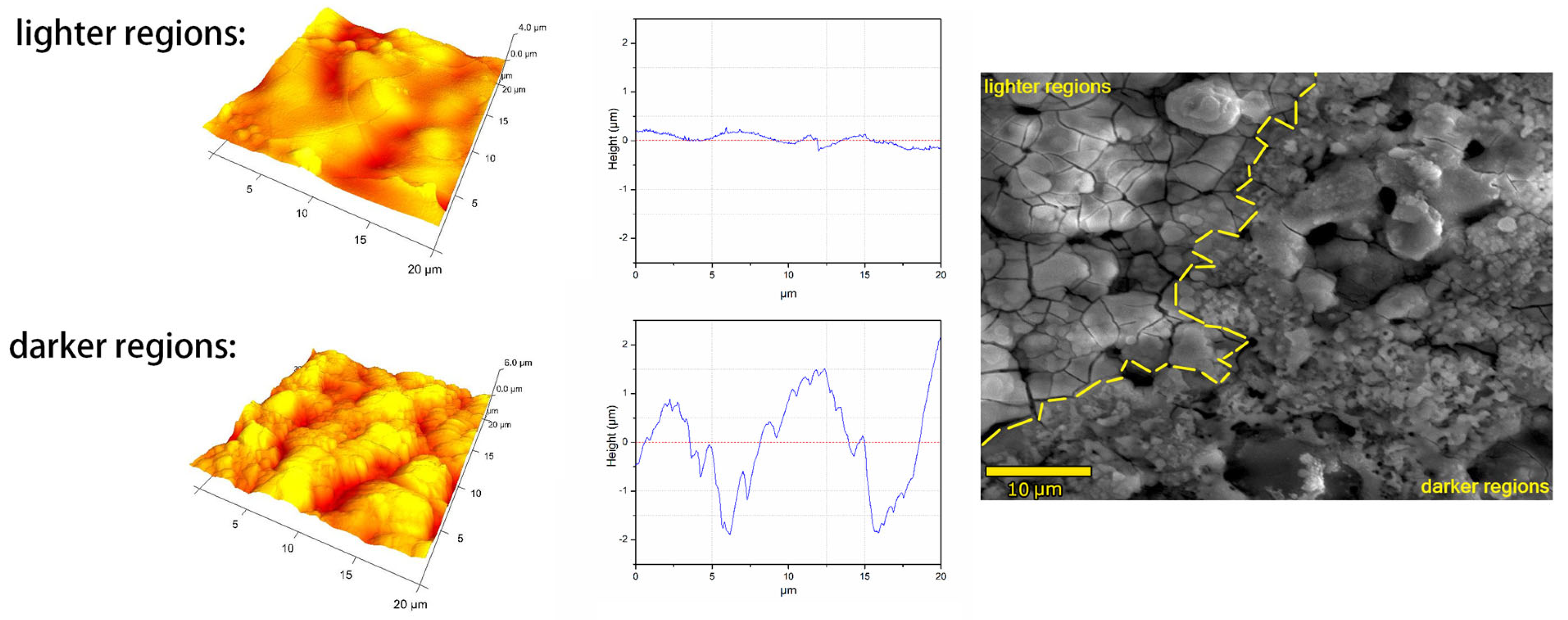

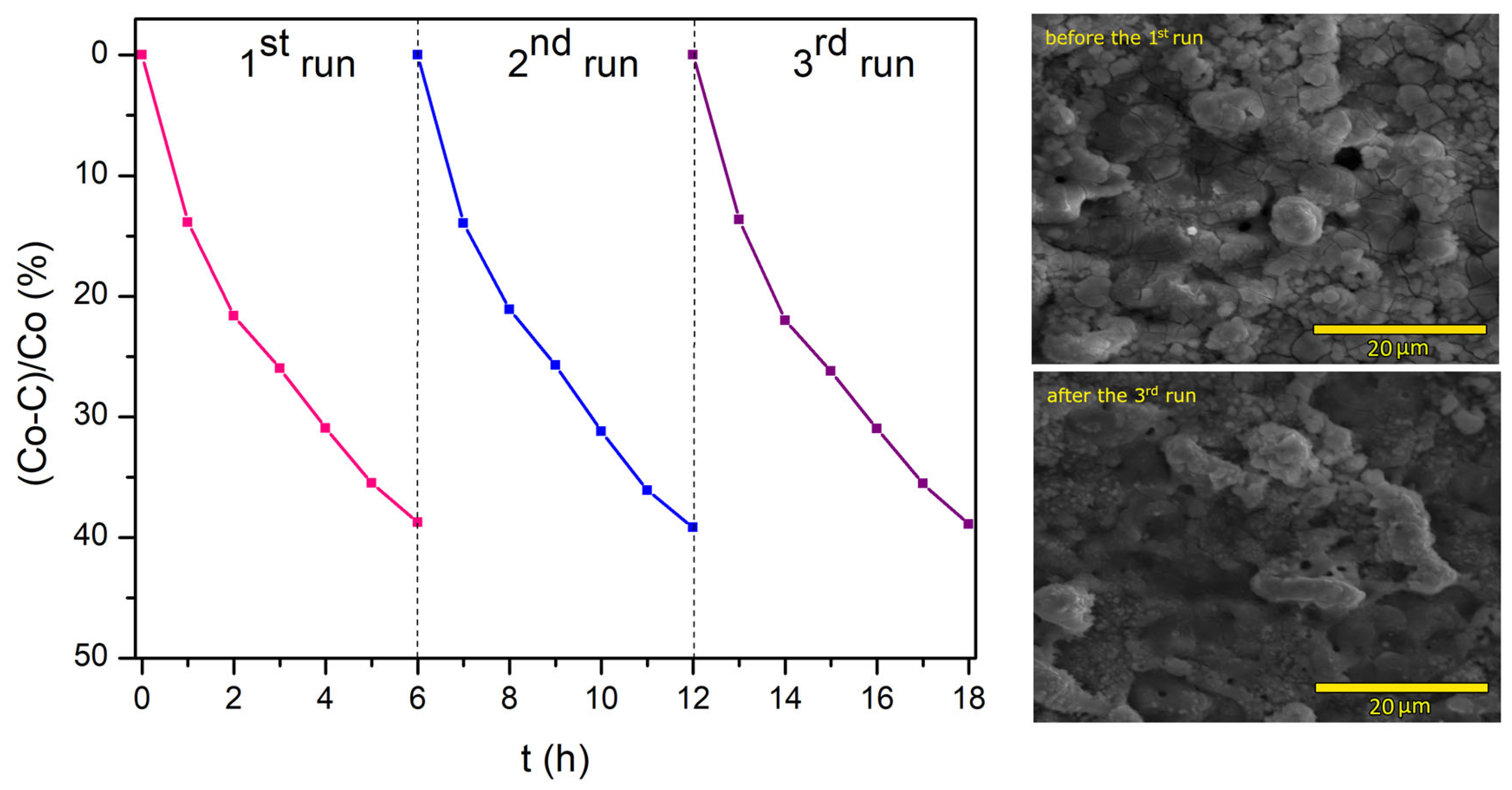
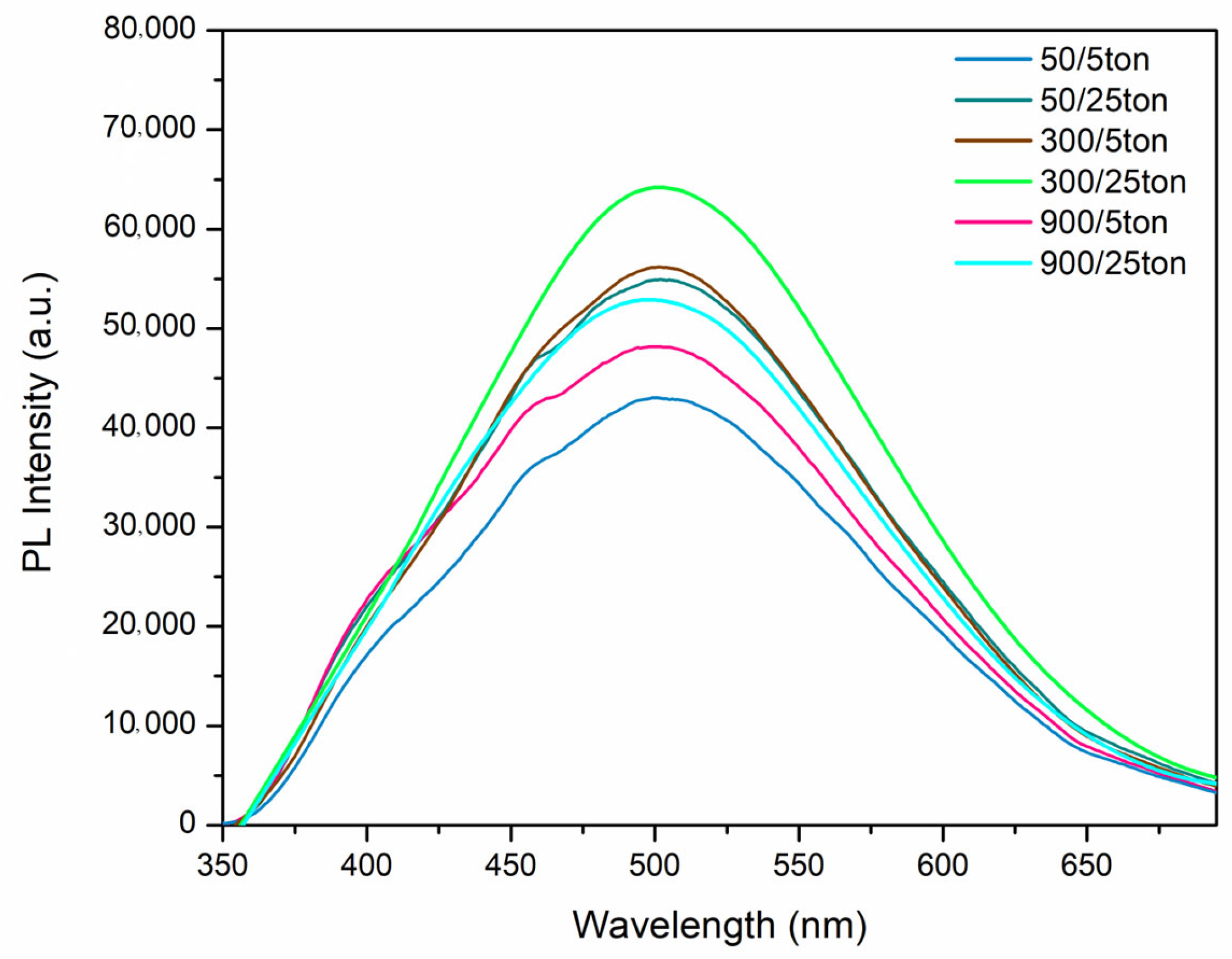
| Sample Name (ton/toff) | ton (µs) | toff (μs) | Dt |
|---|---|---|---|
| 50/5ton | 50 | 250 | 16.66 |
| 50/25ton | 50 | 1250 | 3.85 |
| 300/5ton | 300 | 1500 | 16.66 |
| 300/25ton | 300 | 7500 | 3.85 |
| 900/5ton | 900 | 4500 | 16.66 |
| 900/25ton | 900 | 22,500 | 3.85 |
| Element | Atomic Fraction (%) | |||||
|---|---|---|---|---|---|---|
| 50/5ton | 50/25ton | 300/5ton | 300/25ton | 900/5ton | 900/25ton | |
| O | 64.34 | 67.53 | 70.88 | 71.25 | 75.32 | 64.94 |
| Al | 29.89 | 25.22 | 21.32 | 20.85 | 17.47 | 28.15 |
| W | 5.77 | 7.25 | 7.79 | 7.90 | 7.22 | 6.92 |
| Sample Name | Thickness (µm) | Porosity (%) | Roughness (nm) |
|---|---|---|---|
| 50/5ton | 1.7 ± 0.2 | 9.84 ± 0.15 | 368 ± 18 |
| 50/25ton | 2.9 ± 0.2 | 6.43 ± 0.12 | 495 ± 9 |
| 300/5ton | 2.8 ± 0.4 | 10.61 ± 0.19 | 317 ± 7 |
| 300/25ton | 3.7 ± 0.3 | 9.41 ± 0.13 | 419 ± 16 |
| 900/5ton | 3.1 ± 0.1 | 8.59 ± 0.13 | 319 ± 14 |
| 900/25ton | 2.1 ± 0.3 | 11.13 ± 0.18 | 421 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojsilović, K.; Stojadinović, S.; Vasilić, R. The Plasma Electrolytic Oxidation of Aluminum Using Microsecond-Range DC Pulsing. Metals 2023, 13, 1931. https://doi.org/10.3390/met13121931
Mojsilović K, Stojadinović S, Vasilić R. The Plasma Electrolytic Oxidation of Aluminum Using Microsecond-Range DC Pulsing. Metals. 2023; 13(12):1931. https://doi.org/10.3390/met13121931
Chicago/Turabian StyleMojsilović, Kristina, Stevan Stojadinović, and Rastko Vasilić. 2023. "The Plasma Electrolytic Oxidation of Aluminum Using Microsecond-Range DC Pulsing" Metals 13, no. 12: 1931. https://doi.org/10.3390/met13121931
APA StyleMojsilović, K., Stojadinović, S., & Vasilić, R. (2023). The Plasma Electrolytic Oxidation of Aluminum Using Microsecond-Range DC Pulsing. Metals, 13(12), 1931. https://doi.org/10.3390/met13121931






