Influence of the Morphology of Eutectoid Steels on Corrosion Resistance in NaCl Aqueous Medium with and without CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization of the Wires
2.3. Electrochemical Tests
2.4. Raman Spectroscopy
3. Results
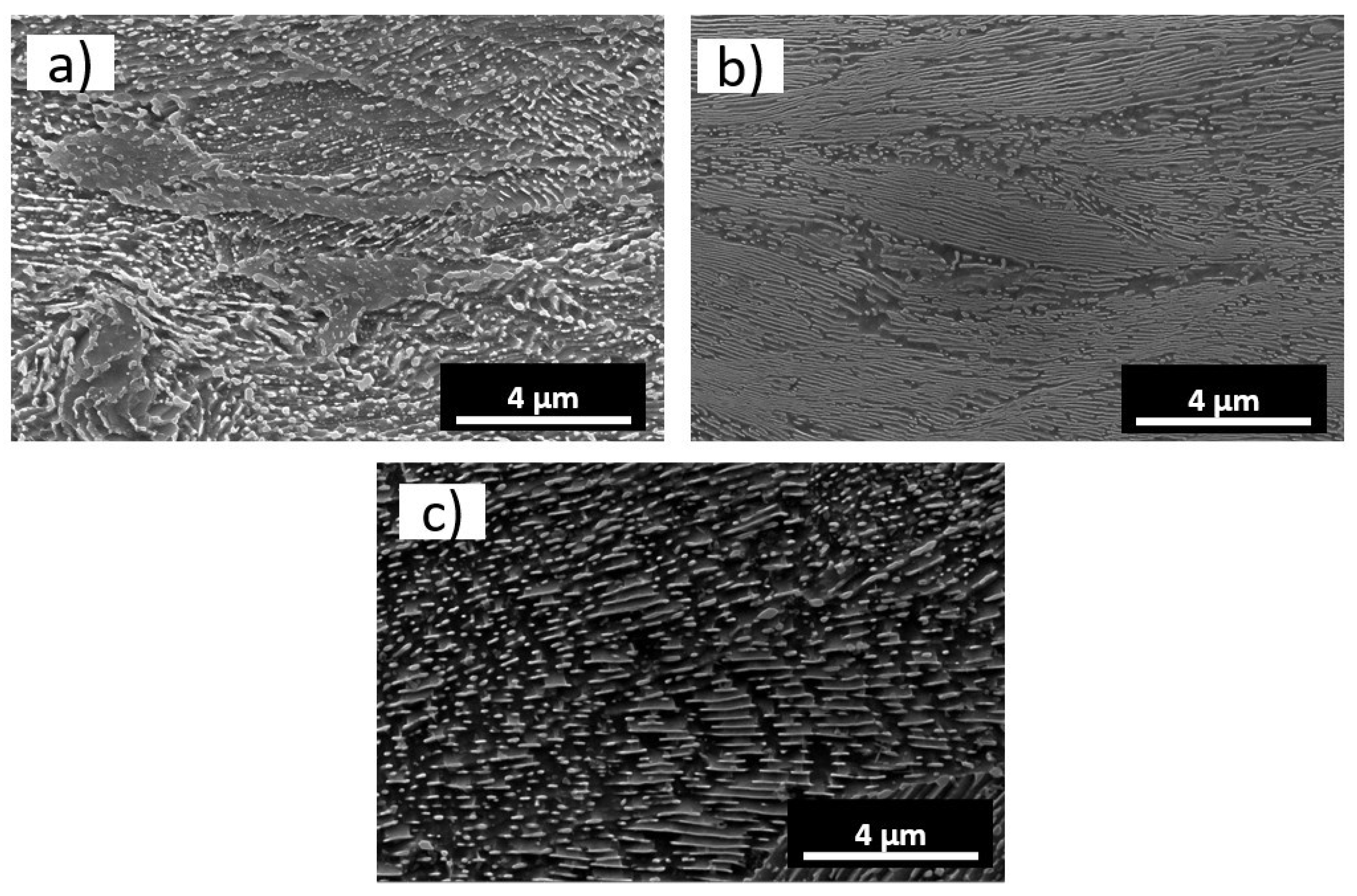
3.1. Characterization of the Cementite of the Wires
3.2. Electrochemical Tests
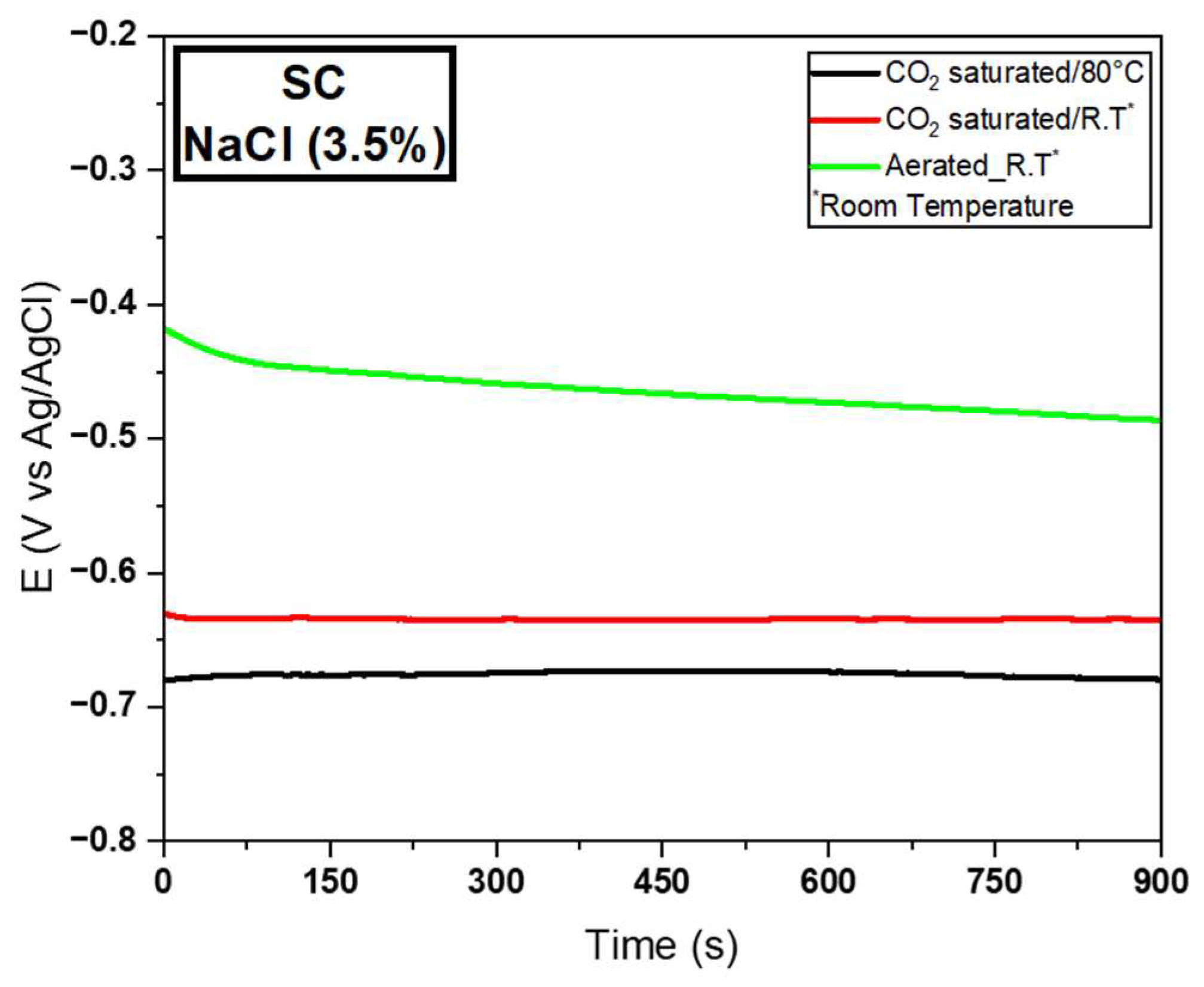
3.2.1. Electrochemical Tests for the SC Wire

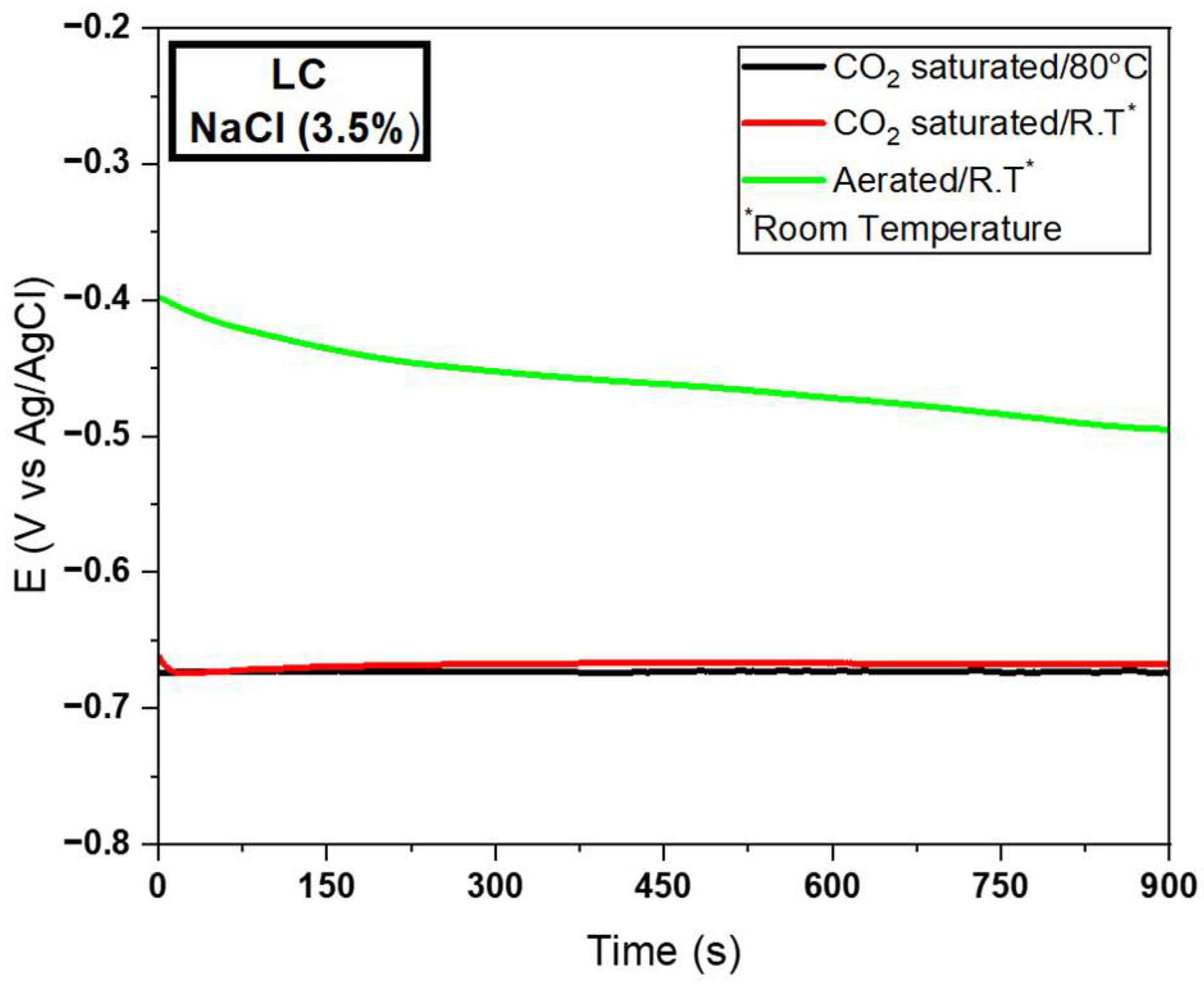
3.2.2. Electrochemical Tests for the LC Wire

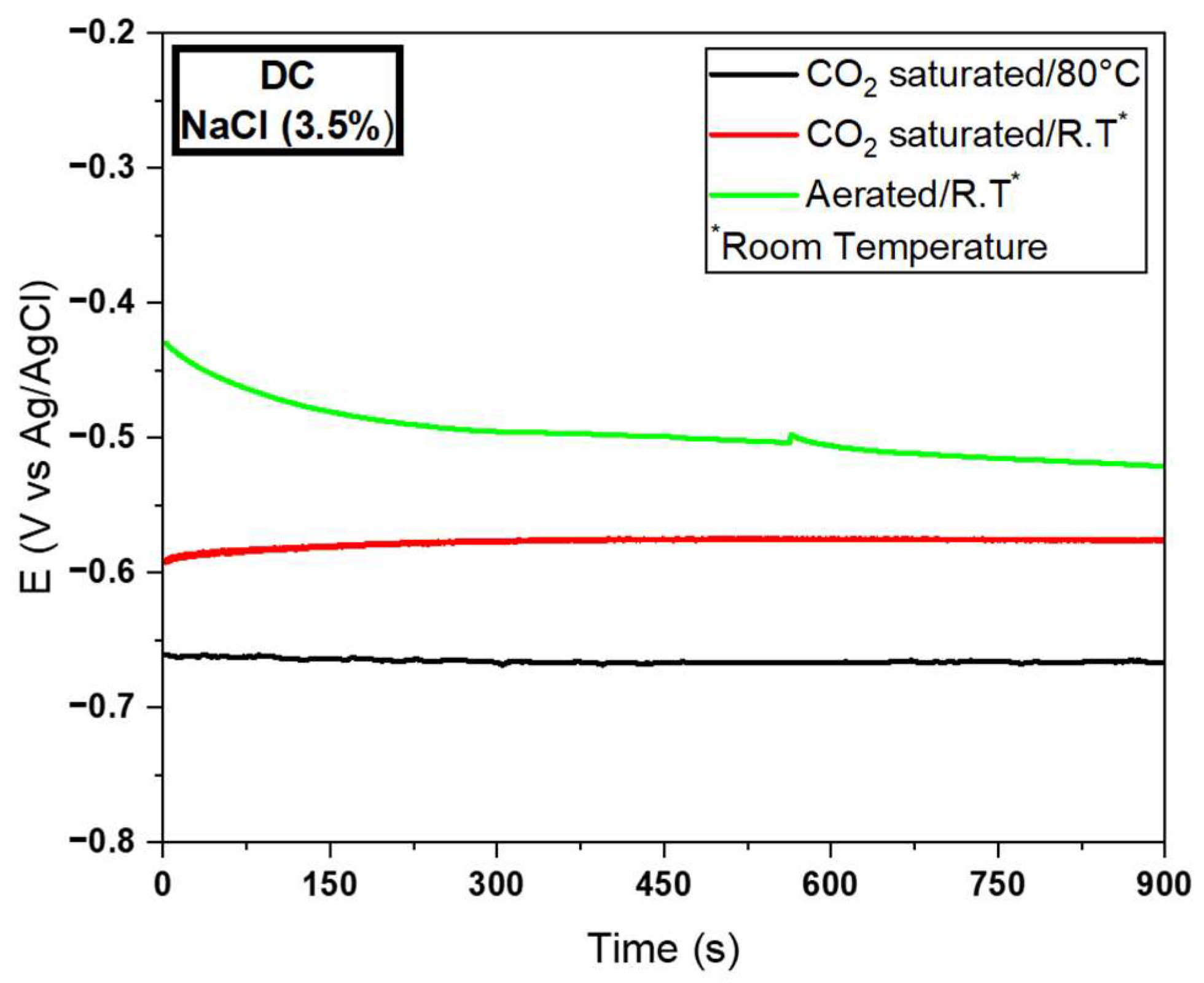
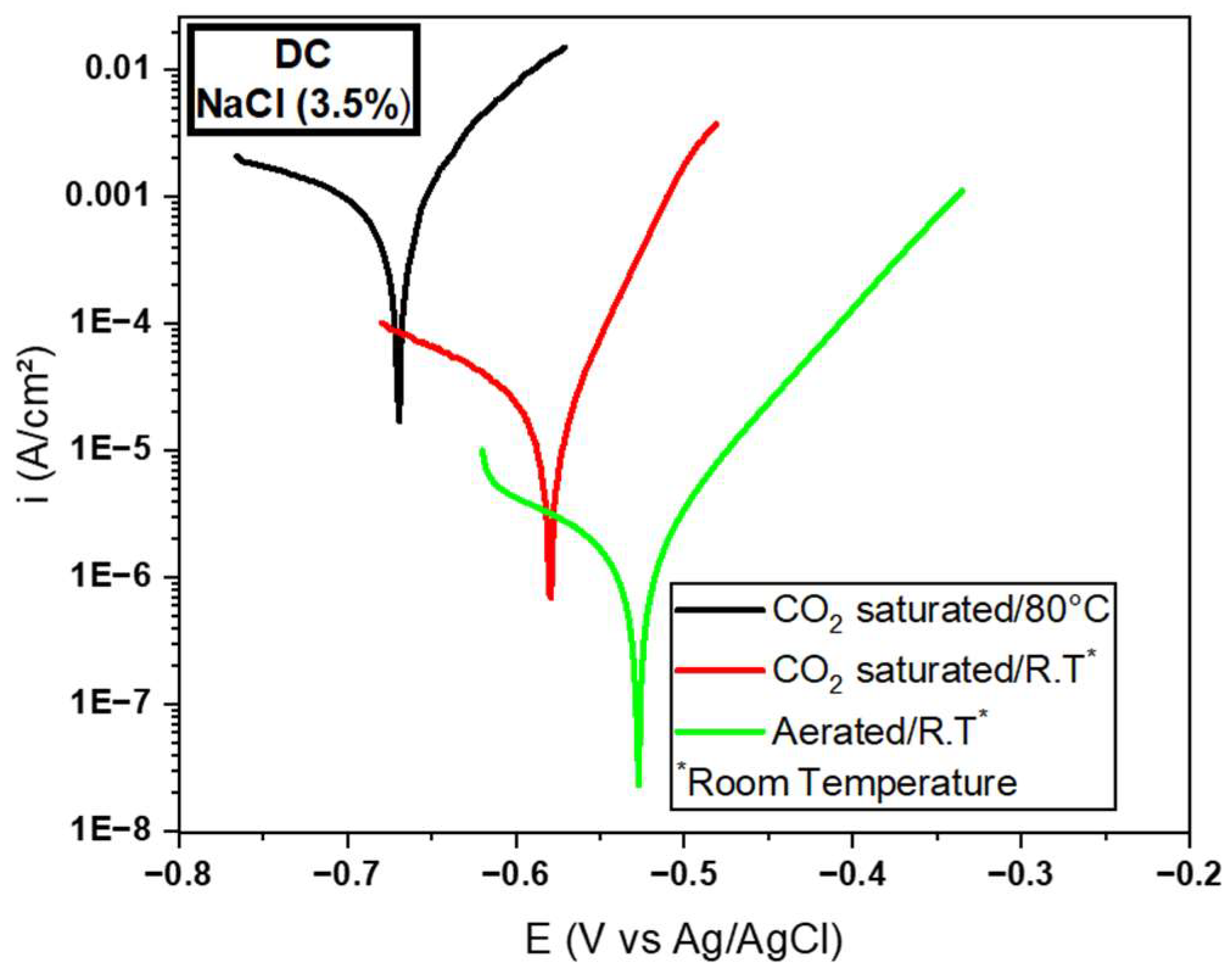
3.2.3. Electrochemical Tests for the DC Wire
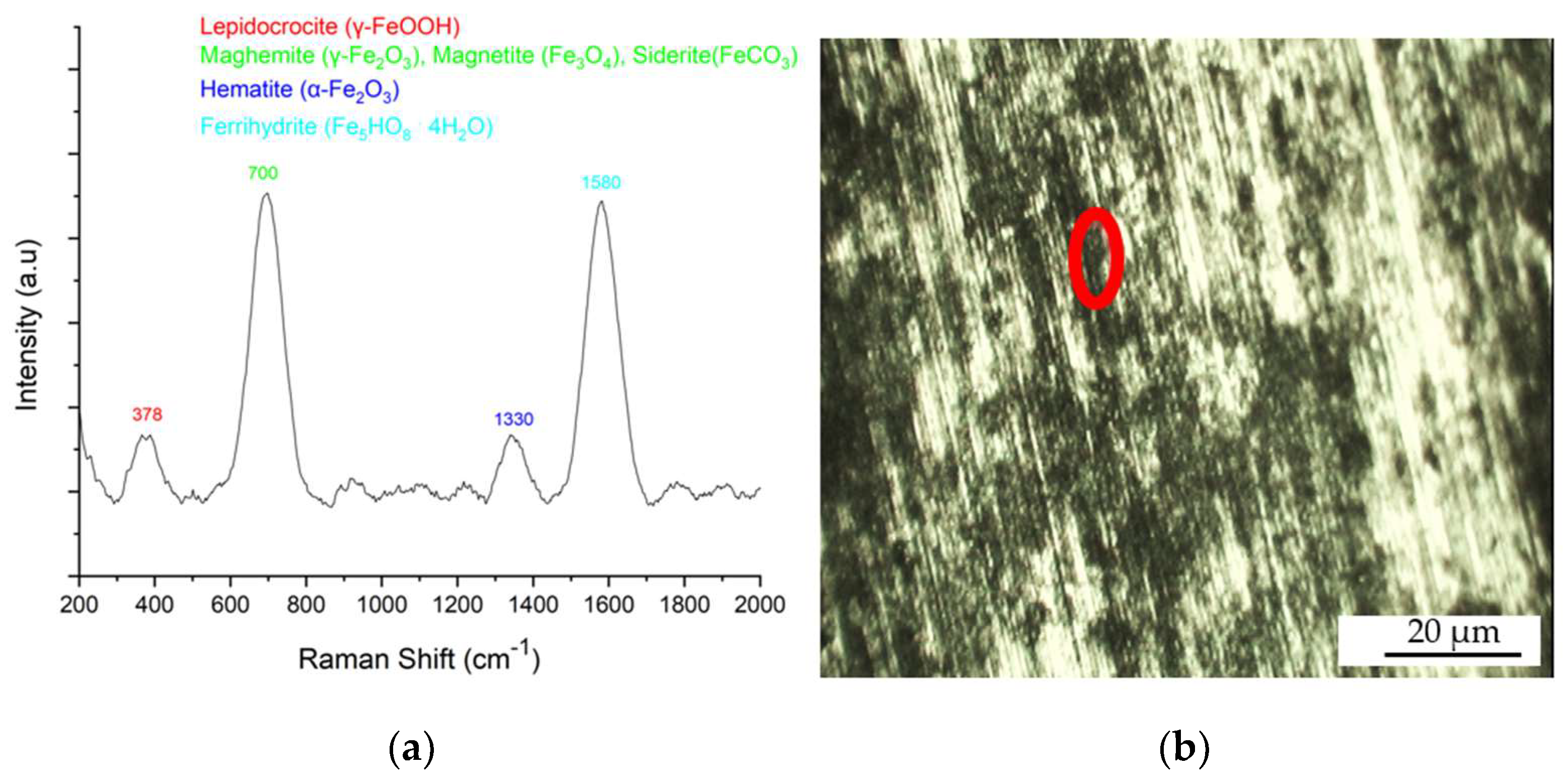
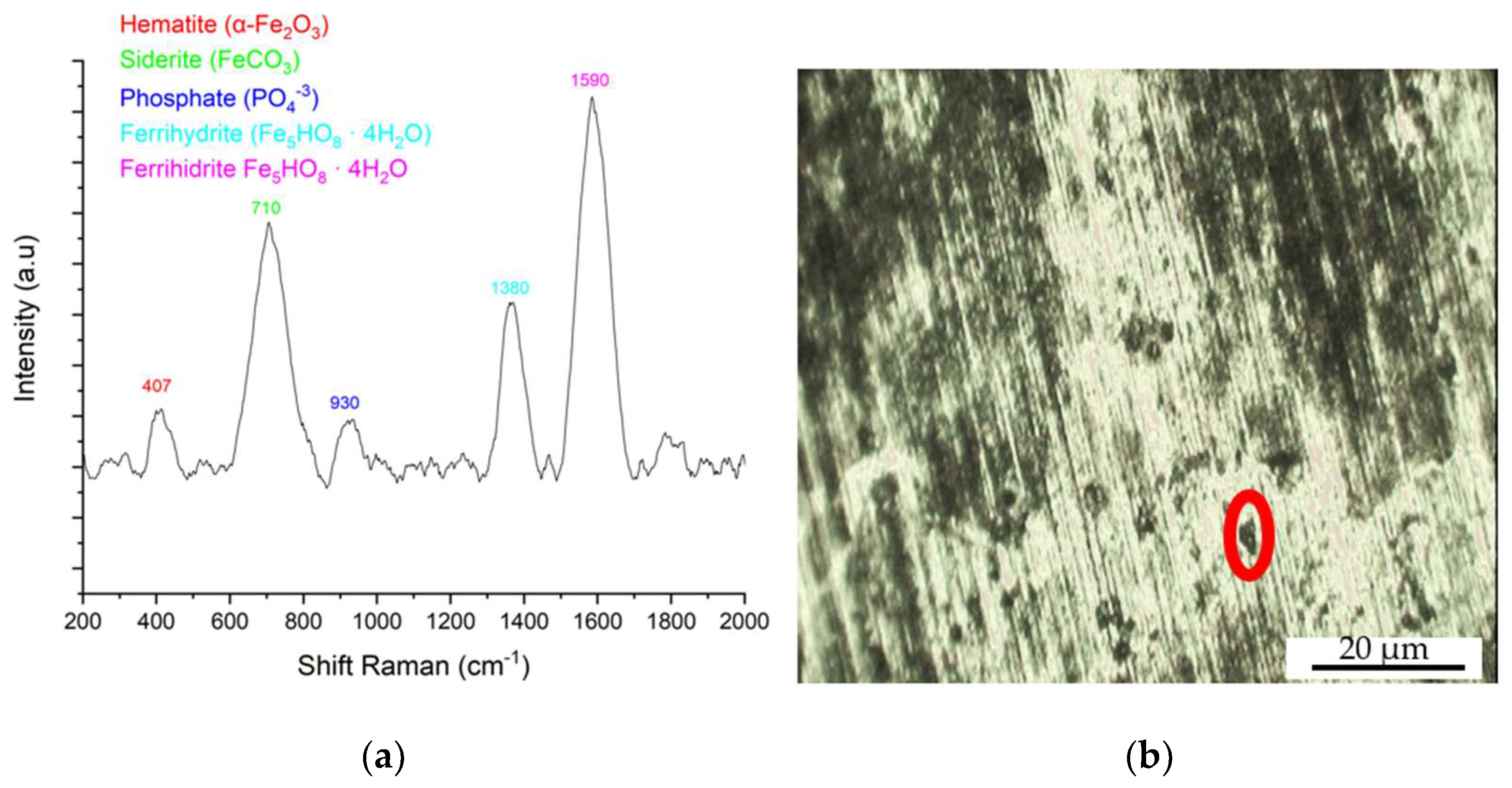
3.3. Characterization of the Corrosion Product by Raman Spectroscopy
SC Sample in Aqueous NaCl Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Jia, R.; Zhang, R.; Yang, S.; Chen, G. A KPCA-BRANN Based Data-Driven Approach to Model Corrosion Degradation of Subsea Oil Pipelines. Reliab. Eng. Syst. Saf. 2022, 219, 108231. [Google Scholar] [CrossRef]
- Cheng, J.; Yan, Q.; Pan, Z.; Wei, W. On-Line Measurement and Characterization of Electrochemical Corrosion of 304L Stainless Steel Pipe Wall in High-Speed Cl-Containing Solution. Metals 2022, 12, 1324. [Google Scholar] [CrossRef]
- Pinheiro, P.H.; Masoumi, M.; Herculano, L.F.G.; Xavier, J.V.B.; de Lima, S.K.B.; Silva, E.S.; Reis, G.S.; Rodrigues, S.F.; de Abreu, H.F.G. Microstructural and Texture Evolution of Pearlite-Drawn Wires for Flexible Marine Pipelines: Investigating the Effect of Heat Treatments on Mechanical Properties. Metals 2023, 13, 805. [Google Scholar] [CrossRef]
- Handoko, W.; Pahlevani, F.; Hossain, R.; Sahajwalla, V. Stress-Induced Phase Transformation and Its Correlation with Corrosion Properties of Dual-Phase High Carbon Steel. J. Manuf. Mater. Process. 2019, 3, 55. [Google Scholar] [CrossRef]
- Latif, J.; Khan, Z.A.; Stokes, K. Structural Monitoring System for Proactive Detection of Corrosion and Coating Failure. Sens. Actuators A Phys. 2020, 301, 111693. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Sun, Y.; Wang, G.; Wang, P. Effect of Microstructure on Coalescence-Induced Droplet Jumping Behavior of a Superhydrophobic Surface and Its Application for Marine Atmospheric Corrosion Protection. Metals 2023, 13, 1413. [Google Scholar] [CrossRef]
- Moreira, M.J.; Borges, M.F. Assesment of Electrochemical Machining-Induced Pitting Geometry on Fatigue Performance of Flexible Pipes’ Tensile Armor Wires. Results Eng. 2022, 15, 100485. [Google Scholar] [CrossRef]
- Abubakar, S.A.; Mori, S.; Sumner, J. Effect of Dissolved CO2 on the Interaction of Stress and Corrosion for Pipeline Carbon Steels in Simulated Marine Environments. Metals 2023, 13, 1165. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Chauhan, D.S.; Quraishi, M.A.; Obot, I.B. Influence of Hydrodynamic Condition on 1,3,5-Tris(4-Methoxyphenyl)-1,3,5-Triazinane as a Novel Corrosion Inhibitor Formulation for Oil and Gas Industry. Corros. Eng. Sci. Technol. 2021, 56, 154–161. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Njoku, D.I.; Nwanonenyi, S.C.; Ahanotu, C.C.; Etiowo, K.M. Investigation into the adsorption and inhibition properties of sodium octanoate against CO2 corrosion of C1018 carbon steel under static and hydrodynamic conditions. Sci. Afr. 2023, 20, e01603. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Allahkaram, S.R.; Kermani, M.B. The Effects of Temperature and PH on the Characteristics of Corrosion Product in CO2 Corrosion of Grade X70 Steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Santos, B.A.F.; Souza, R.C.; Serenario, M.E.D.; Gonçalves, M.C.; Mendes Júnior, E.P.; Simões, T.A.; Oliveira, J.R.; Vaz, G.L.; Caldeira, L.; Gomes, J.A.C.P.; et al. The Effect of Different Brines and Temperatures on the Competitive Degradation Mechanisms of CO2 and H2S in API X65 Carbon Steel. J. Nat. Gas. Sci. Eng. 2020, 80, 103405. [Google Scholar] [CrossRef]
- Mishra, B.; Al-Hassan, S.; Olson, D.L.; Salama, M.M. Development of a Predictive Model for Activation-Controlled Corrosion of Steel in Solutions Containing Carbon Dioxide. Corrosion 1997, 53, 852–859. [Google Scholar] [CrossRef]
- Fu, A.Q.; Tang, X.; Cheng, Y.F. Characterization of corrosion of X70 pipeline steel in thin electrolyte layer under disbonded coating by scanning Kelvin probe. Corros. Sci. 2009, 51, 186–190. [Google Scholar] [CrossRef]
- Panossian, Z.; de Almeida, N.L.; de Sousa, R.M.F.; Pimenta, G.d.S.; Marques, L.B.S. Corrosion of Carbon Steel Pipes and Tanks by Concentrated Sulfuric Acid: A Review. Corros. Sci. 2012, 58, 1–11. [Google Scholar] [CrossRef]
- Gulbrandsen, E.; Kvarekvål, J.; Miland, H. Effect of Oxygen Contamination on Inhibition Studies in Carbon Dioxide Corrosion. Corrosion 2005, 61, 1086–1097. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; Brown, B.; Nesic, S.; Singer, M. Improvement to Water Speciation and FeCO3 Precipitation Kinetics in CO2 Environments: Updates in NaCl Concentrated Solutions. Ind. Eng. Chem. Res. 2021, 60, 17026–17035. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Experimental and Computational Approaches of Sustainable Quaternary Bisammonium Fluorosurfactants for Corrosion Inhibition as Protective Films at Mild Steel/H2SO4 Interface. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126141. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, L.; Zhang, S.; Kaya, S.; Luo, X.; Xiang, B. Corrosion Control of Mild Steel in 0.1 M H2SO4 Solution by Benzimidazole and Its Derivatives: An Experimental and Theoretical Study. RSC Adv. 2017, 7, 23961–23969. [Google Scholar] [CrossRef]
- Sheikh, A.R. Effect of Microalloying with Ti on the Corrosion Behaviour of Low Carbon Steel in a 3.5 Wt.% NaCl Solution Saturated with CO2. Arch. Foundry Eng. 2023. [Google Scholar] [CrossRef]
- Gupta, R.; Sood, A.K.; Metcalf, P.; Honig, J.M. Raman Study of Stoichiometric and Zn-Doped Fe3O4. Phys. Rev. B 2002, 65, 104430. [Google Scholar] [CrossRef]
- Bahrami, M.J.; Hosseini, S.M.A.; Pilvar, P. Experimental and Theoretical Investigation of Organic Compounds as Inhibitors for Mild Steel Corrosion in Sulfuric Acid Medium. Corros. Sci. 2010, 52, 2793–2803. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.; Jin, S.; Lee, S.; Noda, I.; Jung, Y. Investigation of the Phase Transition Mechanism in LiFePO4 Cathode Using In Situ Raman Spectroscopy and 2D Correlation Spectroscopy during Initial Cycle. Molecules 2019, 24, 291. [Google Scholar] [CrossRef] [PubMed]
- Pyshmintsev, I.Y.; Vavilova, O.v.; Mansurova, E.R.; Korober, S.A.; Maltseva, A.N. Electrochemical Investigation of Corrosion Resistance of Steel For Oil and Gas Pipelines. Metallurg 2023, 2, 27–33. [Google Scholar] [CrossRef]
- de Souza, C.A.C.; Meira, M.; de Assis, L.O.; Barbosa, R.S.; Luna, S. Effect of Natural Substances as Antioxidants and as Corrosion Inhibitors of Carbon Steel on Soybean Biodiesel. Mater. Res. 2021, 24. [Google Scholar] [CrossRef]
- Li, Q.; Liu, B. Erosion-Corrosion of Gathering Pipeline Steel in Oil-Water-Sand Multiphase Flow. Metals 2022, 13, 80. [Google Scholar] [CrossRef]
- Wang, Z.M.; Zhang, J. Corrosion of Multiphase Flow Pipelines: The Impact of Crude Oil. Corros. Rev. 2016, 34, 17–40. [Google Scholar] [CrossRef]
- Silva, C.A.; Filho, D.R.; Nunes, G.M.; Bassani, G.S.; Almeida, N.L.; Panossian, Z. Corrosion in Multiphase Slug Flow Loop in Deep-Water Oil and Gas Exploitation. In Proceedings of the Offshore Technology Conference Brasil, Rio de Janeiro, Brazil, 28–31 October 2019. [Google Scholar]
- Wang, Z.M.; Song, G.-L.; Zhang, J. Corrosion Control in CO2 Enhanced Oil Recovery From a Perspective of Multiphase Fluids. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Banaś, J.; Lelek-Borkowska, U.; Mazurkiewicz, B.; Solarski, W. Effect of CO2 and H2S on the Composition and Stability of Passive Film on Iron Alloys in Geothermal Water. Electrochim. Acta 2007, 52, 5704–5714. [Google Scholar] [CrossRef]
- Simpson, L.J.; Melendres, C.A. Surface-Enhanced Raman Spectroelectrochemical Studies of Corrosion Films on Iron in Aqueous Carbonate Solution. J. Electrochem. Soc. 1996, 143, 2146–2152. [Google Scholar] [CrossRef]
- Cen, H.; Chen, Z.; Guo, X. N, S Co-Doped Carbon Dots as Effective Corrosion Inhibitor for Carbon Steel in CO2-Saturated 3.5% NaCl Solution. J. Taiwan Inst. Chem. Eng. 2019, 99, 224–238. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS Characterization of FeCO3 Films from CO2 Corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Islam, M.A.; Farhat, Z.N. Characterization of the Corrosion Layer on Pipeline Steel in Sweet Environment. J. Mater. Eng. Perform. 2015, 24, 3142–3158. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. A Micro-Raman Spectroscopic Study of Marine Atmospheric Corrosion of Carbon Steel: The Effect of Akaganeite. J. Electrochem. Soc. 2015, 162, C495–C502. [Google Scholar] [CrossRef]
- Müller, J.; Speziale, S.; Efthimiopoulos, I.; Jahn, S.; Koch-Müller, M. Raman Spectroscopy of Siderite at High Pressure: Evidence for a Sharp Spin Transition. Am. Mineral. 2016, 101, 2638–2644. [Google Scholar] [CrossRef]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman Mapping of Corrosion Products Formed onto Spring Steels during Salt Spray Experiments. A Correlation between the Scale Composition and the Corrosion Resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Demoulin, A.; Trigance, C.; Neff, D.; Foy, E.; Dillmann, P.; L’Hostis, V. The Evolution of the Corrosion of Iron in Hydraulic Binders Analysed from 46- and 260-Year-Old Buildings. Corros. Sci. 2010, 52, 3168–3179. [Google Scholar] [CrossRef]
- Neff, D.; Bellot-Gurlet, L.; Dillmann, P.; Reguer, S.; Legrand, L. Raman Imaging of Ancient Rust Scales on Archaeological Iron Artefacts for Long-Term Atmospheric Corrosion Mechanisms Study. J. Raman Spectrosc. 2006, 37, 1228–1237. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Taneda, K. In-Situ Raman Spectroscopy for the Passive Oxide Film on Iron in Neutral Borate Solution. ECS Trans. 2009, 16, 125–131. [Google Scholar] [CrossRef]
- Ohtsuka, T. Raman Spectra of Passive Films of Iron in Neutral Borate Solution. Mater. Trans. JIM 1996, 37, 67–69. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Neff, D.; Reguer, S.; Bellot-Gurlet, L.; Dillmann, P.; Bertholon, R. Structural Characterization of Corrosion Products on Archaeological Iron: An Integrated Analytical Approach to Establish Corrosion Forms. J. Raman Spectrosc. 2004, 35, 739–745. [Google Scholar] [CrossRef]
- Neff, D.; Dillmann, P.; Bellot-Gurlet, L.; Beranger, G. Corrosion of Iron Archaeological Artefacts in Soil: Characterisation of the Corrosion System. Corros. Sci. 2005, 47, 515–535. [Google Scholar] [CrossRef]
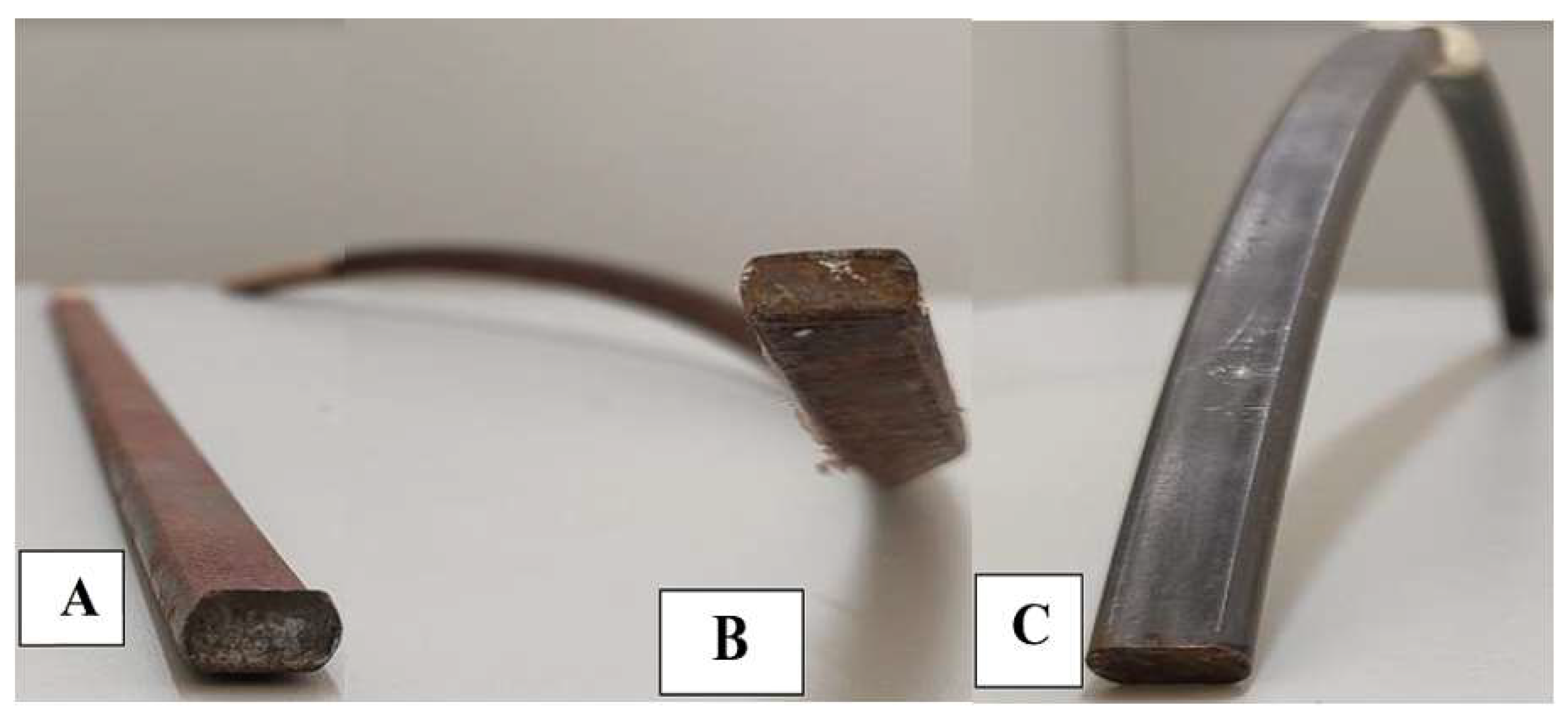

| Wire | C | Mn | Si | S + P | Cr + Ni +V | Al | Mo +Ti | Fe |
|---|---|---|---|---|---|---|---|---|
| A | 0.77 | 0.50 | 0.20 | 0.014 | 0.054 | - | 0.007 | bal |
| B | 0.76 | 0.56 | 0.18 | 0.014 | 0.041 | - | 0.005 | bal |
| C | 0.73 | 0.58 | 0.26 | 0.008 | 0.038 | 0.033 | 0.005 | Bal |
| Cementite Condition | Condition in 3.5% NaCl |
|---|---|
| SC, LC and DC | Saturated with CO2 at 80 °C |
| SC, LC and DC | Saturated with CO2 at room temperature |
| SC, LC and DC | Aerated and at room temperature |
| SAMPLE | STATISTIC | Solution (3.5% of NaCl) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) CO2 Saturated/80 °C | (2) CO2 Saturated/R.T. | (3) Aerated/R.T. | ||||||||
| Electrochemical Parameters | ||||||||||
| OCP (V vs Ag/AgCl) | I (A/cm²) | Rp (Ω.cm²) | OCP (V vs Ag/AgCl) | I (A/cm²) | Rp (Ω.cm²) | OCP (V vs Ag/AgCl) | I (A/cm²) | Rp (Ω. cm²) | ||
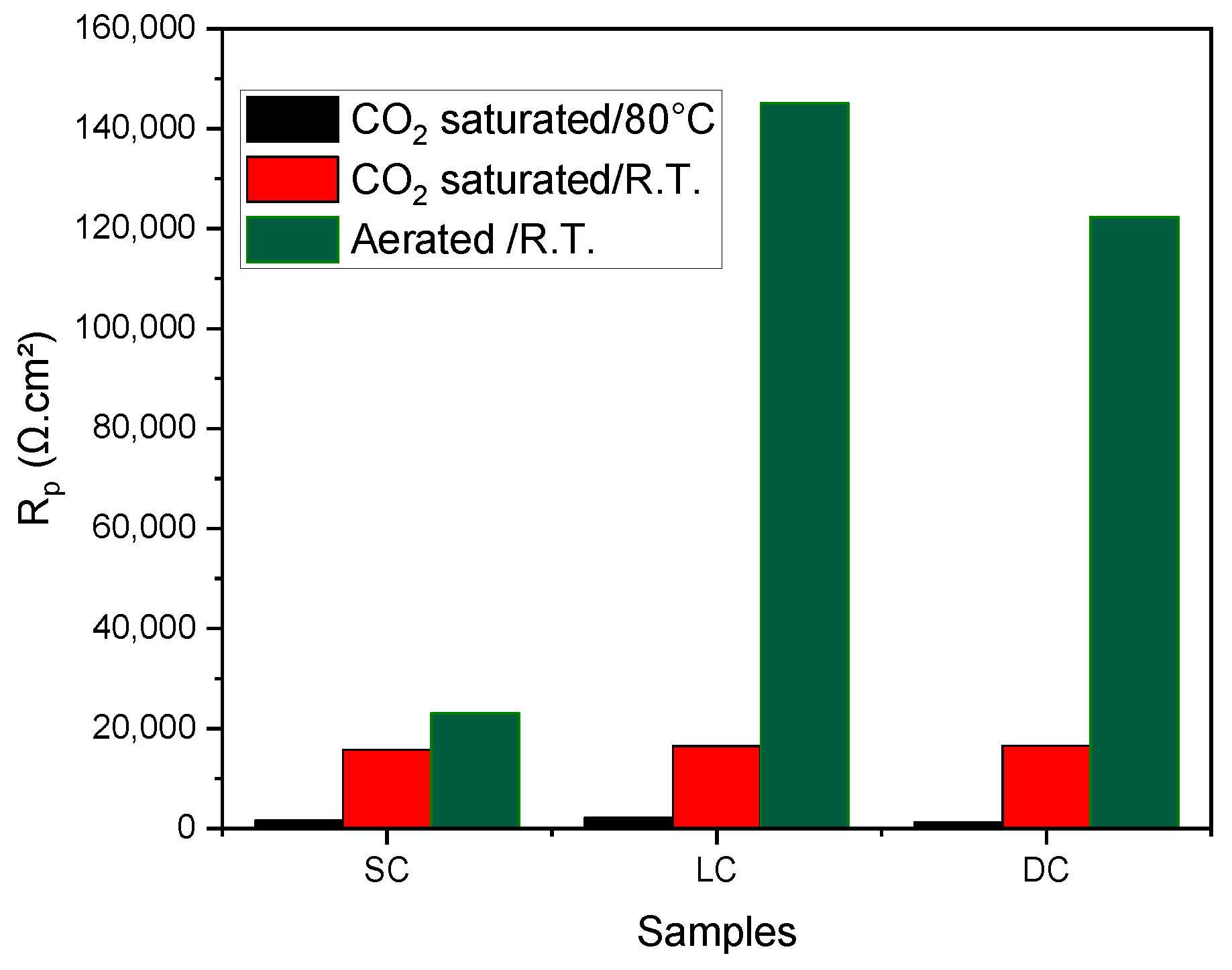
| SC | MD | −0.68 | 4.24E−4 | 1603.77 | −0.64 | 4.07E−5 | 15,724.81 | −0.44 | 1.91E−5 | 23,036.64 |
| SD | 0.01 | 3.24E−4 | 0.01 | 8.55E−6 | 0.02 | 8.15E−6 | ||||
| LC | ||||||||||
| MD | −0.68 | 3.15E−4 | 2158.73 | −0.66 | 4.01E−5 | 16,458.85 | −0.47 | 3.24E−6 | 145,061.72 | |
| SD | 0.01 | 2.10E−4 | 0.01 | 1.01E−5 | 0.02 | 7.00E−8 | ||||
| DC | ||||||||||
| MD | −0.66 | 5.41E−4 | 1219.96 | −0.56 | 3.38E−5 | 16,568.04 | −0.51 | 4.17E−6 | 122,302.15 | |
| SD | 0.01 | 4.31E−4 | 0.02 | 6.25E−6 | 0.01 | 6.01E−8 | ||||
| Corrosion Resistance | Solutions (3.5% de NaCl) | ||
|---|---|---|---|
| 1 CO2 Saturated/80 °C | 2 CO2 Saturated/T.A. | 3 Aerated/T.A. | |
| highest | LC | DC | LC |
| intermediate | SC | LC | DC |
| lowest | DC | SC | SC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fideles, F.F.d.M.; Florez, M.A.C.; Rodrigues, M.V.G.; Cardoso, J.L.; Aranas, C., Jr.; Rodrigues, S.F.; Lima, M.N.d.S.; Pascoal, C.V.P.; de Moura, T.A.; Reis, G.S.; et al. Influence of the Morphology of Eutectoid Steels on Corrosion Resistance in NaCl Aqueous Medium with and without CO2. Metals 2023, 13, 1782. https://doi.org/10.3390/met13101782
Fideles FFdM, Florez MAC, Rodrigues MVG, Cardoso JL, Aranas C Jr., Rodrigues SF, Lima MNdS, Pascoal CVP, de Moura TA, Reis GS, et al. Influence of the Morphology of Eutectoid Steels on Corrosion Resistance in NaCl Aqueous Medium with and without CO2. Metals. 2023; 13(10):1782. https://doi.org/10.3390/met13101782
Chicago/Turabian StyleFideles, Francisco Felipe de M., Mauro Andres C. Florez, Maria Veronica G. Rodrigues, Jorge Luiz Cardoso, Clodualdo Aranas, Jr., Samuel F. Rodrigues, Marcos Natan da S. Lima, Caio Victor P. Pascoal, Thiago Alves de Moura, Gedeon S. Reis, and et al. 2023. "Influence of the Morphology of Eutectoid Steels on Corrosion Resistance in NaCl Aqueous Medium with and without CO2" Metals 13, no. 10: 1782. https://doi.org/10.3390/met13101782
APA StyleFideles, F. F. d. M., Florez, M. A. C., Rodrigues, M. V. G., Cardoso, J. L., Aranas, C., Jr., Rodrigues, S. F., Lima, M. N. d. S., Pascoal, C. V. P., de Moura, T. A., Reis, G. S., Silva, E. S., & de Abreu, H. F. G. (2023). Influence of the Morphology of Eutectoid Steels on Corrosion Resistance in NaCl Aqueous Medium with and without CO2. Metals, 13(10), 1782. https://doi.org/10.3390/met13101782













