The Use of Thin Films as Defect Sealants to Increase the Corrosion Resistance of Thermal Spray Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Production
2.2. Microstructural Characterisation
2.3. Electrochemical Characterisation
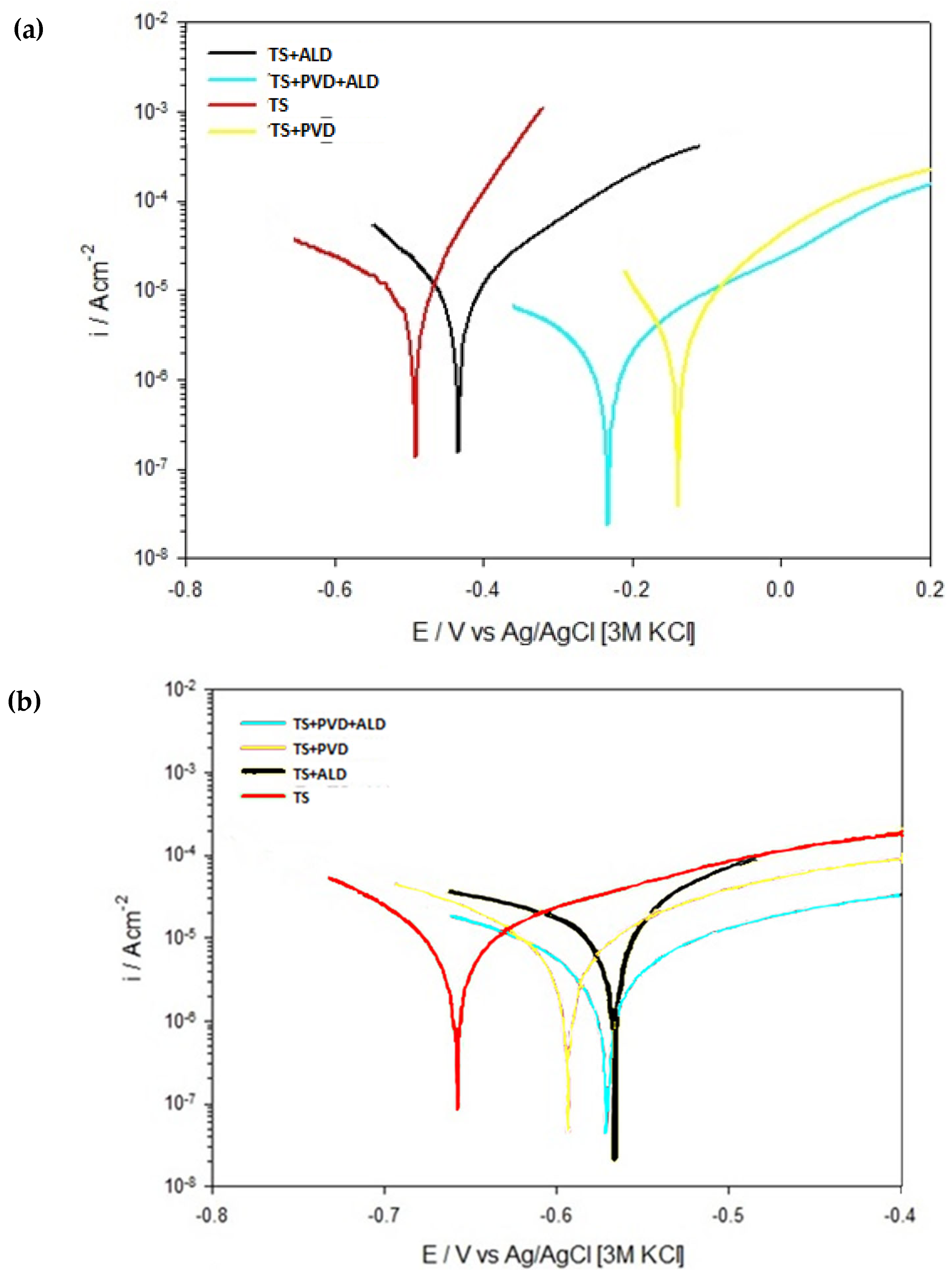
3. Results and Discussion
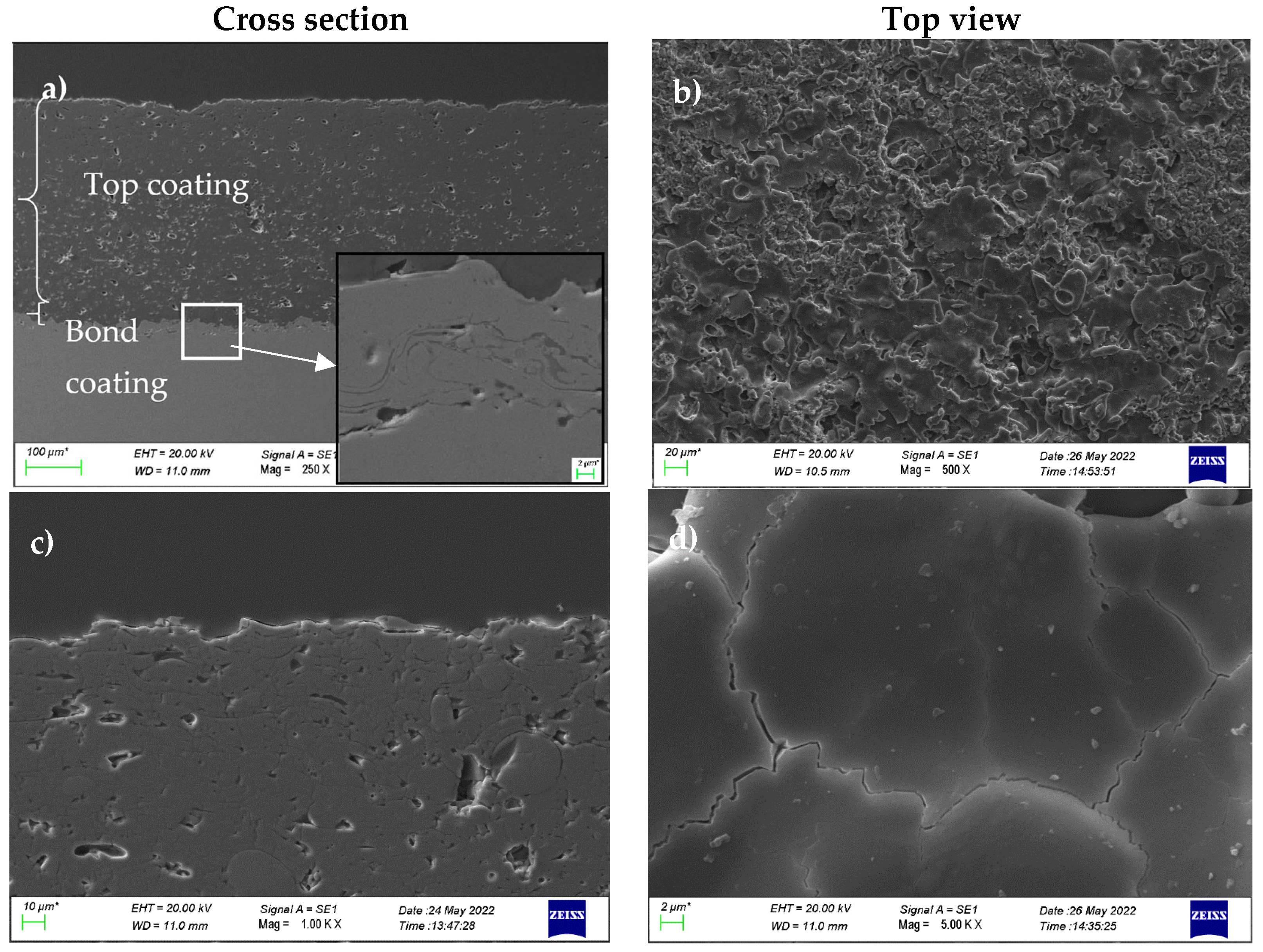
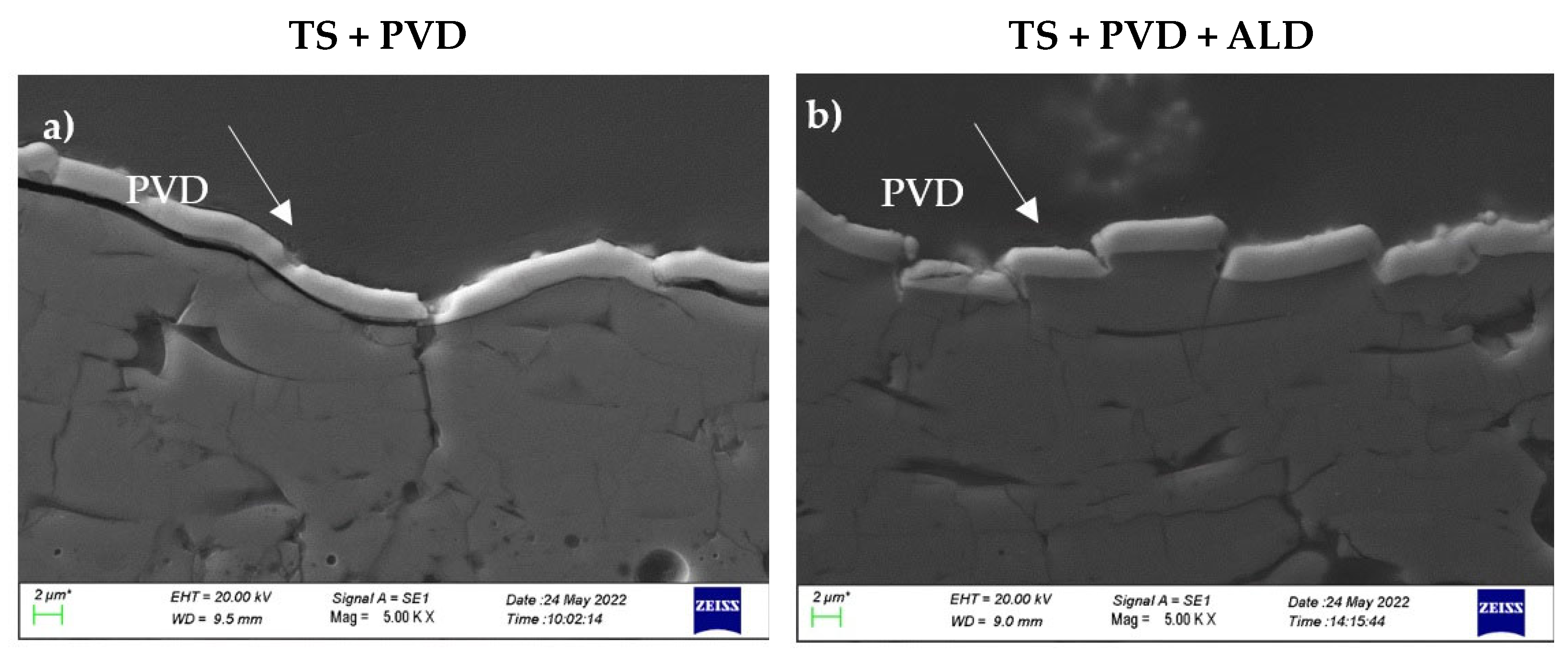
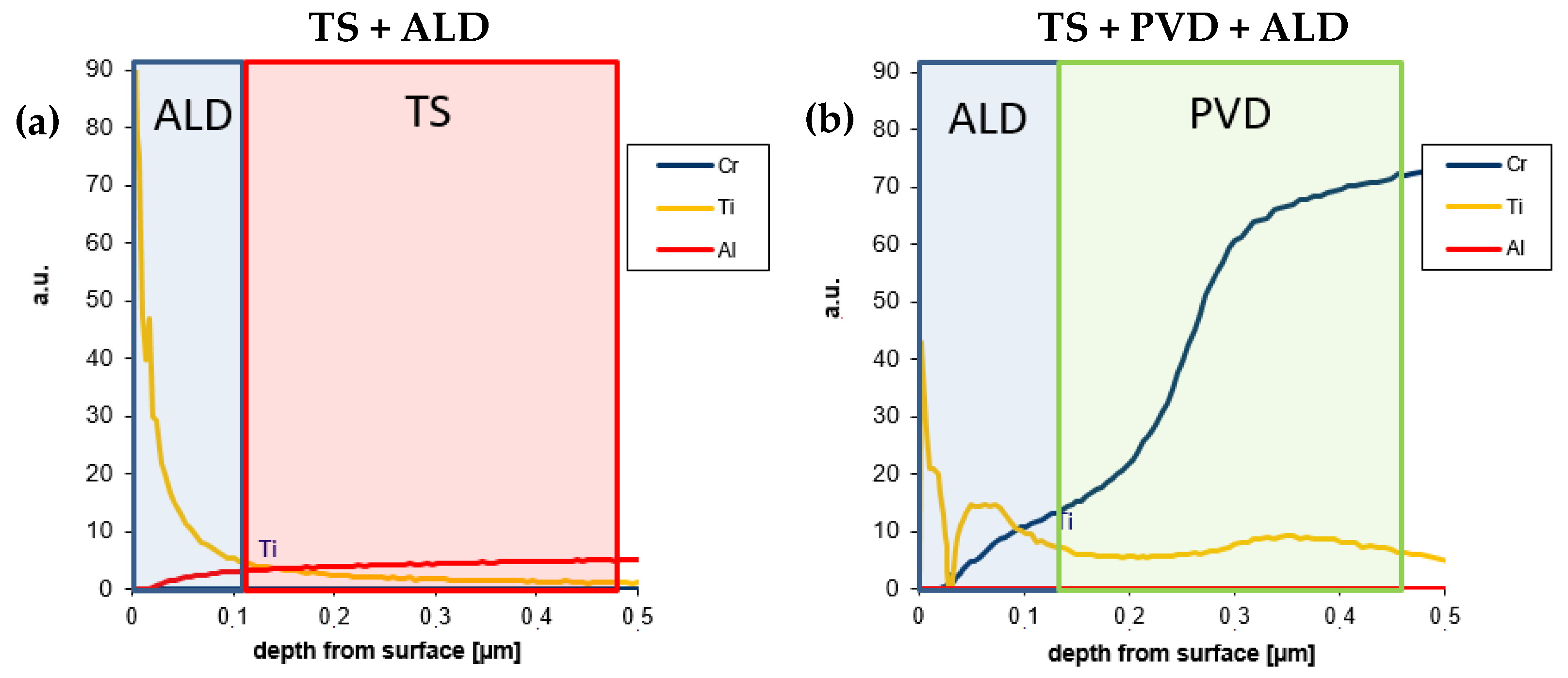
3.1. Microstructural Characterisation
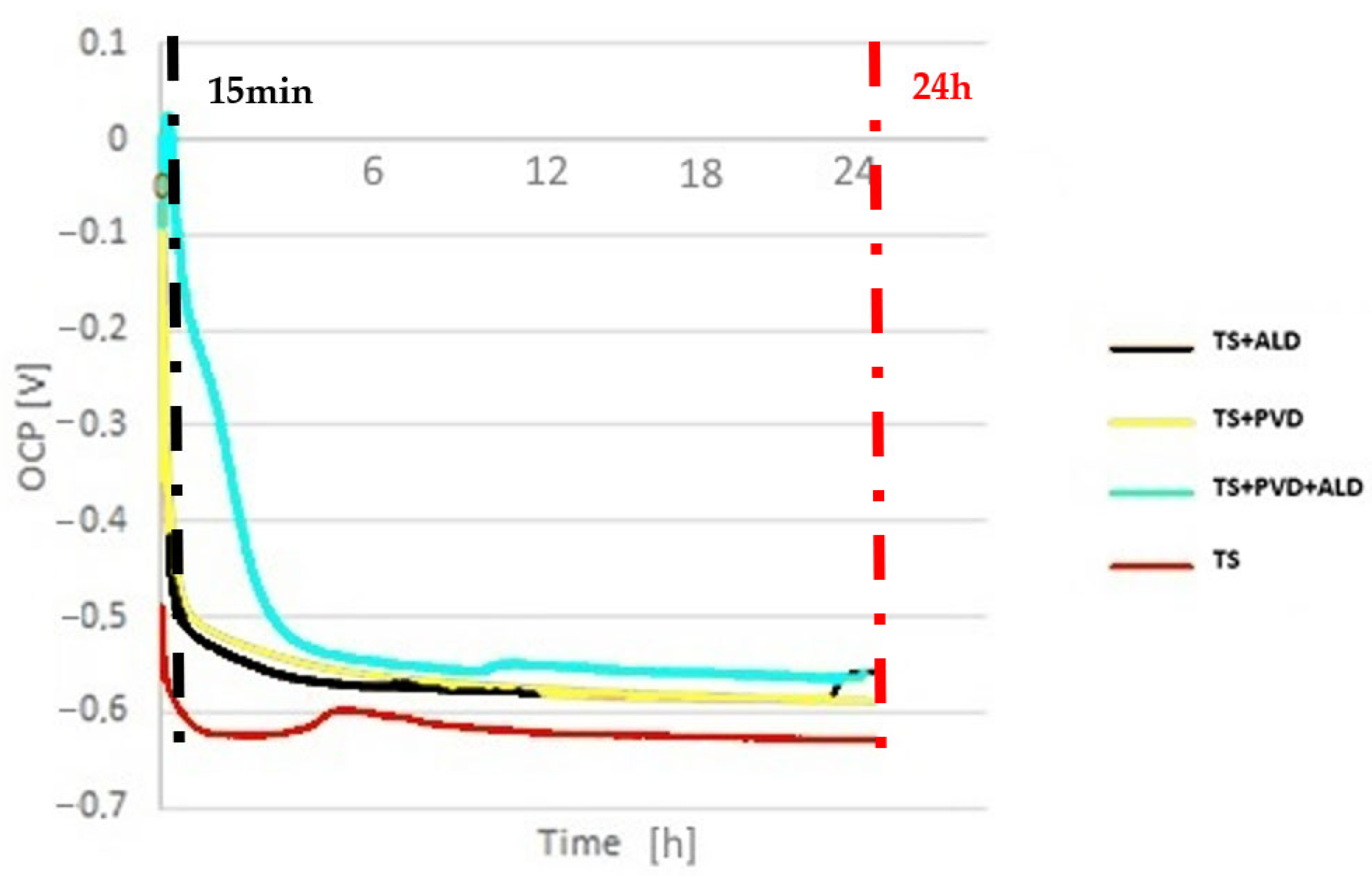
3.2. Electrochemical Characterisation
4. Conclusions
- The thermal spray coating had many intrinsic defects as it deposited, such as pores and shrinkage cracks.
- The PVD coating did not penetrate into the TS coating but covered the outer surface. Some cracks due to the differential thermal expansion between the PVD coating and the TS coating occurred during the cooling of the sample after deposition.
- The ALD coating was detectable with RF-GDOES and appeared to permeate slightly into some surface defects of the thermal spray coating.
- The thin films hindered the permeation of corrosive media, probably by partially sealing the thermal spray coating. The combination of PVD + ALD coatings on the thermal spray was the most efficient sealant. In the latter case, the corrosive media took 3 h to reach the substrate.
- The potentiodynamic curves show a reduction in the corrosion rates of the sealed coatings with respect to the unsealed ones in the samples tested after an immersion time of 15 min. The potentiodynamic measurements after 24 h of immersion were influenced by the corrosion products.
- The analysis of the corrosion morphologies and products after the potentiodynamic tests showed a lower amount of corrosion deposits in the sealed samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Houdková, Š.; Zahálka, F.; Kašparová, M.; Berger, L.M. Comparative Study of Thermally Sprayed Coatings under Different Types of Wear Conditions for Hard Chromium Replacement. Tribol. Lett. 2011, 43, 139–154. [Google Scholar] [CrossRef]
- Baiamonte, L.; Marra, F.; Gazzola, S.; Giovanetto, P.; Bartuli, C.; Valente, T.; Pulci, G. Thermal Sprayed Coatings for Hot Corrosion Protection of Exhaust Valves in Naval Diesel Engines. Surf. Coat. Technol. 2016, 295, 78–87. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, B.S.; Puri, D.; Prakash, S. Use of Plasma Spray Technology for Deposition of High Temperature Oxidation/Corrosion Resistant Coatings-A Review. Mater. Corros. 2007, 58, 92–102. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, R.; Prakash, C.; Singh, S. HA-Based Coating by Plasma Spray Techniques on Titanium Alloy for Orthopedic Applications. Mater. Today Proc. 2021, 50, 612–628. [Google Scholar] [CrossRef]
- Chellaganesh, D.; Khan, M.A.; Jappes, J.T.W. Thermal Barrier Coatings for High Temperature Applications-A Short Review. Mater. Today Proc. 2021, 45, 1529–1534. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal Spray High-Entropy Alloy Coatings: A Review; Springer: Berlin, Germany, 2020; Volume 29, ISBN 1166602001. [Google Scholar]
- Baiamonte, L.; Pulci, G.; Hlede, E.; Marra, F.; Bartuli, C. Thermal Spray Coatings for Corrosion and Wear Protection of Naval Diesel Engines Components. Metall. Ital. 2014, 106, 9–13. [Google Scholar]
- Sahith, M.S.; Giridhara, G.; Kumar, R.S. Development and Analysis of Thermal Barrier Coatings on Gas Turbine Blades-A Review. Mater. Today Proc. 2018, 5, 2746–2751. [Google Scholar] [CrossRef]
- Bolelli, G.; Giovanardi, R.; Lusvarghi, L.; Manfredini, T. Corrosion Resistance of HVOF-Sprayed Coatings for Hard Chrome Replacement. Corros. Sci. 2006, 48, 3375–3397. [Google Scholar] [CrossRef]
- Galedari, S.A.; Mahdavi, A.; Azarmi, F.; Huang, Y.; McDonald, A. A Comprehensive Review of Corrosion Resistance of Thermally-Sprayed and Thermally-Diffused Protective Coatings on Steel Structures. J. Therm. Spray Technol. 2019, 28, 645–677. [Google Scholar] [CrossRef]
- Li, H.; Ke, Z.; Li, J.; Xue, L.; Yan, Y. An Effective Low-Temperature Strategy for Sealing Plasma Sprayed Al2O3-Based Coatings. J. Eur. Ceram. Soc. 2018, 38, 1871–1877. [Google Scholar] [CrossRef]
- Liscano, S.; Gil, L.; Staia, M.H. Effect of Sealing Treatment on the Corrosion Resistance of Thermal-Sprayed Ceramic Coatings. Surf. Coat. Technol. 2004, 188–189, 135–139. [Google Scholar] [CrossRef]
- Knuuttila, J.; Sorsa, P.; Mäntylä, T. Sealing of Thermal Spray Coatings by Impregnation. J. Therm. Spray Technol. 1999, 8, 249–257. [Google Scholar] [CrossRef]
- Park, I.C.; Kim, S.J. Corrosion Behavior in Seawater of Arc Thermal Sprayed Inconel 625 Coatings with Sealing Treatment. Surf. Coat. Technol. 2017, 325, 729–737. [Google Scholar] [CrossRef]
- Tang, P.; He, D.; Li, W.; Shang, L.; Zhai, H.; Wang, L.; Zhang, G. Achieving Superior Hot Corrosion Resistance by PVD/HVOF Duplex Design. Corros. Sci. 2020, 175, 108845. [Google Scholar] [CrossRef]
- Marcano, D.; Mauer, G.; Vaßen, R.; Weber, A. Manufacturing of High Performance Solid Oxide Fuel Cells (SOFCs) with Atmospheric Plasma Spraying (APS) and Plasma Spray-Physical Vapor Deposition (PS-PVD). Surf. Coat. Technol. 2017, 318, 170–177. [Google Scholar] [CrossRef]
- Zhai, H.; Ning, W.; Li, W.; Li, C.; Jia, J.; Xiao, R. Effect of Sealing Treatment on the Corrosion Resistance of Detonation-Sprayed Fe-Based Amorphous Coating. J. Mater. Eng. Perform. 2023, 32, 8419–8429. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2022, 10, 447. [Google Scholar] [CrossRef]
- Pougoum, F.; Qian, J.; Martinu, L.; Klemberg-Sapieha, J.; Zhou, Z.; Li, K.Y.; Savoie, S.; Lacasse, R.; Potvin, E.; Schulz, R. Study of Corrosion and Tribocorrosion of Fe3Al-Based Duplex PVD/HVOF Coatings against Alumina in NaCl Solution. Surf. Coat. Technol. 2019, 357, 774–783. [Google Scholar] [CrossRef]
- Goti, E.; Mura, A.; di Confiengo, G.M.G.; Casalegno, V. The tribological performance of super-hard Ta: C DLC coatings obtained by low-temperature PVD. Ceram. Int. 2023, in press. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Andreatta, F.; Lekka, M.; Guzman, L. Atomic Layer Deposition: State-of-the-Art and Research/Industrial Perspectives. Corros. Rev. 2011, 29, 5–6. [Google Scholar] [CrossRef]
- Leppäniemi, J.; Sippola, P.; Peltonen, A.; Aromaa, J.J.; Lipsanen, H.; Koskinen, J. Effect of Surface Wear on Corrosion Protection of Steel by CrN Coatings Sealed with Atomic Layer Deposition. ACS Omega 2018, 3, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Guzman, L.; Lanzutti, A.; Fedrizzi, L.; Saikkonen, M. Chemical and Electrochemical Characterization of Hybrid PVD + ALD Hard Coatings on Tool Steel. Electrochem. Commun. 2009, 11, 2060–2063. [Google Scholar] [CrossRef]
- Härkönen, E.; Kolev, I.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Fenker, M.; Toth, L.; Radnoczi, G.; et al. Sealing of Hard CrN and DLC Coatings with Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2014, 6, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Santinacci, L. Atomic Layer Deposition: An Efficient Tool for Corrosion Protection. Curr. Opin. Colloid Interface Sci. 2023, 63, 101674. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Cao, K.; Chen, R. Advances in Atomic Layer Deposition. Nanomanufacturing Metrol. 2022, 5, 191–208. [Google Scholar] [CrossRef]
- Shan, C.X.; Hou, X.; Choy, K.L. Corrosion Resistance of TiO2 Films Grown on Stainless Steel by Atomic Layer Deposition. Surf. Coatings Technol. 2008, 202, 2399–2402. [Google Scholar] [CrossRef]
- Staszuk, M.; Reimann, Ł.; Pakuła, D.; Pawlyta, M.; Musztyfaga-Staszuk, M.; Czaja, P.; Beneš, P. Investigations of TiO2/NanoTiO2 Bimodal Coatings Obtained by a Hybrid PVD/ALD Method on Al-Si-Cu Alloy Substrate. Coatings 2022, 12, 338. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, W.H.; Wang, J.Y. A Model for Quantification of GDOES Depth Profiles. Vacuum 2015, 113, 5–10. [Google Scholar] [CrossRef]
- Yung, T.Y.; Chen, T.C.; Tsai, K.C.; Lu, W.F.; Huang, J.Y.; Liu, T.Y. Thermal Spray Coatings of Al, ZnAl and Inconel 625 Alloys on SS304L for Anti-Saline Corrosion. Coatings 2019, 9, 32. [Google Scholar] [CrossRef]



| Thermal Spray | PVD | ALD | |
|---|---|---|---|
| TS | X | ||
| TS + PVD | X | X | |
| TS + ALD | X | X | |
| TS + PVD + ALD | X | X | X |
| Sample | Ecorr [V] | Icorr [10−6 A/cm2] | |
|---|---|---|---|
| 15 min | TS | −0.51 ± 0.11 | 9.0 ± 1.11 |
| TS + PVD | −0.17 ± 0.07 | 3.1 ± 0.31 | |
| TS + ALD | −0.42 ± 0.08 | 6.2 ± 0.57 | |
| TS + PVD + ALD | −0.22 ± 0.08 | 1.1 ± 0.22 | |
| 24 h | TS | −0.67 ± 0.012 | 9.8 ± 2.12 |
| TS + PVD | −0.58 ± 0.13 | 4.1 ± 1.11 | |
| TS + ALD | −0.55 ± 0.09 | 8.2 ± 2.31 | |
| TS + PVD + ALD | −0.54 ± 0.11 | 2.1 ± 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzutti, A.; Sordetti, F.; Marin, E.; Andreatta, F.; Carabillo, A.; Querini, M.; Porro, S.; Rondinella, A.; Magnan, M.; Fedrizzi, L. The Use of Thin Films as Defect Sealants to Increase the Corrosion Resistance of Thermal Spray Coatings. Metals 2023, 13, 1778. https://doi.org/10.3390/met13101778
Lanzutti A, Sordetti F, Marin E, Andreatta F, Carabillo A, Querini M, Porro S, Rondinella A, Magnan M, Fedrizzi L. The Use of Thin Films as Defect Sealants to Increase the Corrosion Resistance of Thermal Spray Coatings. Metals. 2023; 13(10):1778. https://doi.org/10.3390/met13101778
Chicago/Turabian StyleLanzutti, Alex, Francesco Sordetti, Elia Marin, Francesco Andreatta, Antonio Carabillo, Matteo Querini, Samuele Porro, Alfredo Rondinella, Michele Magnan, and Lorenzo Fedrizzi. 2023. "The Use of Thin Films as Defect Sealants to Increase the Corrosion Resistance of Thermal Spray Coatings" Metals 13, no. 10: 1778. https://doi.org/10.3390/met13101778
APA StyleLanzutti, A., Sordetti, F., Marin, E., Andreatta, F., Carabillo, A., Querini, M., Porro, S., Rondinella, A., Magnan, M., & Fedrizzi, L. (2023). The Use of Thin Films as Defect Sealants to Increase the Corrosion Resistance of Thermal Spray Coatings. Metals, 13(10), 1778. https://doi.org/10.3390/met13101778









