Abstract
This work examines corrosion behavior of EQ70 high-strength steel under anaerobic conditions in artificial seawater containing sulfate-reducing bacteria (SRB). Polarization and electrochemical impedance spectra (EIS) tests were conducted. The results reveal that corrosion rate initially decreased at the beginning of immersion. However, as the immersion time progressed, the corrosion rate of the high-strength steel increased, attributed to SRB reproduction. The scanning electron microscopy (SEM) results demonstrate that the corrosion was more severe in artificial seawater containing SRB compared to that of seawater without SRB. The morphologies of confocal laser scanning microscopy (CLSM) demonstrate that, after 15 days of immersion, the average depth of the corrosion pits on the sample in the artificial seawater containing SRB was nearly double that of the sample in the SRB-free seawater.
1. Introduction
The behavior in which microbial activities accelerate the corrosion process of materials is called microbiologically influenced corrosion (MIC) [1,2]. A large number of microbes are able to cause MIC [3]. Among them, sulfate-reducing bacteria (SRB) are believed to be among the main culprits leading to MIC. SRB are widely present in underground pipeline systems, oil platforms, and other marine installations in seawater [4,5]. SRB are considered representative anaerobes and can cause the corrosion of iron, zinc, aluminum, and their alloys [6,7,8].
The role of SRB in the context of iron corrosion can be classified into two distinct categories: direct and indirect corrosion. Indirect corrosion is the result of a chemical attack from hydrogen sulfide [9] or other acidic organisms [10]. The process of direct corrosion involves the utilization of cathodic hydrogen and electron transfers derived from iron [11]. In anaerobic conditions or at low oxygen concentrations, SRB have the ability to employ organisms that have been assimilated onto metal surfaces as a means of acquiring carbon sources. The process involves the reduction of sulfate ions (SO42−) present in the surrounding environment to hydrogen sulfide (H2S) or S2−, which interacts with the steel surface, leading to the development of corrosive compounds like iron sulfides. These compounds that adhere to the steel surface have a detrimental effect on the protective oxide layer of steel and can make it more prone to corrosion [12]. Consequently, porous films of corrosion products and extracellular polymeric substances (EPS) form on the metal surface, induced by SRB [13,14,15]. The chemical and electrochemical reactions between SRB and metals accelerate the corrosion process due to the presence of the biocatalyst, which consists of biologically active ferments that are secreted by the SRB in the biofilm [16,17].
Steel facilities in seawater may undergo corrosion damage, resulting in economic loss and safety issues. The corrosion behavior of steel facilities in seawater has attracted significant attention [18,19]. Considerable research efforts have been dedicated to exploring the impact of SRB on corrosion of metals ever since the first documented case in 1934 [20]. To date, several plausible mechanisms have been postulated, including cathodic depolarization by hydrogenase and sulfide, the chelation of extracellular polymeric substances (EPS) with metal ions, direct electron transfer from Fe0, and biocatalytic cathodic sulfate reduction [21,22,23]. However, the authenticity of these corrosion mechanisms heavily relies on the SRB strains, metal material attributes, and the medium characteristics.
EQ70 high-strength steel is a variety of steel renowned for its remarkable strength and durability, as well as its unique properties, which render it highly suitable for deployment in marine environments. Its elevated yield strength and toughness enable it to endure severe conditions, like corrosive seawater, inclement weather, and heavy loads. The exceptional mechanical characteristics of this material make it perfect for constructing marine structures, including oil rigs, ship hulls, and boats, where durability and reliability are crucial. Furthermore, the corrosion resistance of EQ70 steel guarantees a longer lifespan and reduced maintenance costs in marine applications [24]. However, EQ70 high-strength steel, like many other steels, can be susceptible to microbiologically influenced corrosion (MIC) caused by sulfate-reducing bacteria (SRB) strains. The majority of the reports have been centered on the corrosion behavior of other high-strength steel influenced by SRB [13,25] and little is shown in the scientific literature regarding the influence of microorganisms such as SRB on the corrosion behavior of EQ70 high-strength steel.
Electrochemical processes have been widely employed to explore the corrosion behavior of metals affected by microorganisms, and among these, the polarization curve and EIS are commonly utilized methods. This is because the MIC phenomenon is an electrochemical process, and thus these electrochemical techniques are well suited for its investigation. Therefore, this work aimed to study the corrosion behavior of a typical high-strength steel influenced by SRB, EQ70, which is specifically used for marine structures. Electrochemical methods, SEM, and CLSM were employed to investigate the corrosion behavior of EQ70 in artificial seawater with and without SRB strains.
2. Materials and Methods
2.1. Materials
Samples of EQ70 high-strength steel were procured from Henan Runlu Trading Co., Ltd., a supplier in Wugang, China. The chemicals were reagent-grade and used as received, including KH2PO4, (NH4)2Fe(SO4)2 (Tianjin branch Kemiou Chemical Reagent Co., Ltd., Tianjin, China), NH4Cl, CaCl2·6H2O, MgSO4·7H2O, sodium citrate, sodium lactate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), yeast extract (Beijing Aoboxing Biotech Co., Ltd., Beijing, China), and anhydrous ethanol (Tianjin Yongda Chemical Reagent Co., Ltd., Tianjin, China).
2.2. Preparation of High-Strength Steel Samples
The high-strength steel was EQ70, which has the chemical composition shown in Table 1. Two sets of high-strength steel samples were prepared for the tests. One was used for the immersion tests and the other was used for the electrochemical tests. The dimension of the samples used for both the immersion tests and the electrochemical tests was 10 mm × 10 mm × 3 mm. To ensure cleanliness, the samples were first degreased by washing them ultrasonically in acetone. Subsequently, the samples were abraded using emery paper with grit sizes of 240, 400, 800, 1000, and 2000. After polishing with diamond paste, the steel samples were rinsed using deionized water and then dried with ethanol.

Table 1.
Chemical compositions of EQ70 steel (wt. %).
For the electrochemical measurements, a copper wire was welded to the back side of each sample to serve as an electrode. The exposed area of the electrode was 10 mm × 10 mm and the other sides were securely sealed with epoxy resin.
Before the electrochemical tests, the electrodes were abraded using emery paper of a grit size up to 2000, dehydrated using ethanol, and dried in cool air afterward.
2.3. SRB Culturing Procedure
Modified Postgate’s culture medium was obtained by the mixture of KH2PO4 (0.5 g), MgSO4·7H2O (0.06 g), NH4Cl (1 g), CaCl2·6H2O (0.06 g), sodium citrate (0.3 g), yeast extract (1 g), and 70% sodium lactate (6 mL) into 3.5 wt.% artificial seawater (1 L). After assembling the components, the modified culture medium was sealed in sterilized glass bottles and aerated with N2 for 20 min to eliminate the residual oxygen. Then, the modified culture medium was put into an autoclave at 121 °C for 20 min and allowed to cool before being sterilized.
The generated SRB strains are specifically Desulfovibrio caledoniensis. These SRB strains, along with (NH4)2Fe(SO4)2 (0.06 g), were introduced into the cooled modified culture medium and developed slowly in sterilized glass bottles. The mixture was aerated with N2 for about 20 min and kept in a temperature incubator at room temperature for 3 to 4 days.
To prepare the corrosive medium with SRB, SRB strains (5 vol.%) were inoculated into 3.5 wt.% artificial seawater (1 L) contained in a glass bottle, which was then aerated using N2. To avoid contact with air, the glass bottle was stoppered with a rubber plug and sealed using a silicone rubber.
2.4. Immersion Tests
For the immersion tests, the corrosive medium was created by combining either modified culture medium (25 mL) or SRB seed bacteria (25 mL) with 3.5 wt.% artificial seawater (500 mL). The artificial seawater was prepared by dissolving 17.5 g of sea crystals in 500 mL deionized water. These tests were conducted at room temperature, and the steel samples were submerged in the corrosive medium for 15 days.
After immersion in both biotic and abiotic solutions, the EQ70 samples underwent a series of cleaning and preparation steps. First, they were washed with phosphate buffer solutions (PBS) made up of 1.42 g/L of Na2HPO4, 27 g/L of KH2PO4, 8 g/L of NaCl, and 0.2 g/L of KCl. Subsequently, the samples were fastened in glutaraldehyde (5 wt.%) for 2 h and then rinsed with PBS. Following this, the samples were submerged in ethanol for 30 min to facilitate the removal of water. The EQ70 samples submerged in seawater without SRB were cleaned using deionized water and dehydrated with ethanol for about 30 min. Finally, all the EQ70 samples were dried in cool air and sputtered with gold in order to observe the surfaces of the samples using SEM.
The tested samples with corrosion products on the surface were ultrasonically cleaned in hydrochloric acid solution. The hydrochloric acid solution was composed of HCl (500 mL), distilled water (500 mL), and 20 g of hexamethylenetetramine. Once the corrosion products had been removed, the steel samples were washed using deionized water and ethanol and then dried in cool air. The steel samples without corrosion products were also sputtered with gold for the observation of the sample surfaces using SEM and CLSM.
2.5. Observation and Analysis
The morphologies of the EQ70 samples before and after immersion in seawater were examined carefully using a camera (FINEPIX HS 35EXR) and a scanning electron microscope (SEM) (XL30 FEG ESEM). Elemental composition analysis of the EQ70 samples was performed using energy-dispersive spectroscopy (EDS). Furthermore, after the removal of the corrosion products, the depth of the corrosion pits and the morphologies of the EQ70 samples were examined using a confocal laser scanning microscope (OLS 400, CLSM).
2.6. Electrochemical Tests
To perform open circuit potential (EOCP), electrochemical impedance spectra (EIS) and polarization measurements, an EG&G 2273 workstation was used connected to a computer. Electrochemical measurements were conducted using a standard three-electrode cell comprised of a saturated calomel electrode (SCE) acting as a reference electrode, a platinum foil electrode acting as a counter electrode and the EQ70 steel sample serving as a working electrode. These measurements were performed in the absence of oxygen using 3.5 wt.% artificial seawater, either containing SRB strains or without SRB strains. EIS data were recorded in a frequency range spanning between 100 kHz and 10 mHz, and the amplitude of the AC signal used was 10 mV. The EIS spectra were fitted and evaluated with the aid of Zsimpwin 1.0 software. The polarization experiments were conducted using a scan rate of 1.0 mV/s and 0.25 V against the open circuit potential (OCP) in the anodic region. To ensure consistency, three parallel samples were tested.
3. Results and Discussion
3.1. Open Circuit Potential (EOCP)
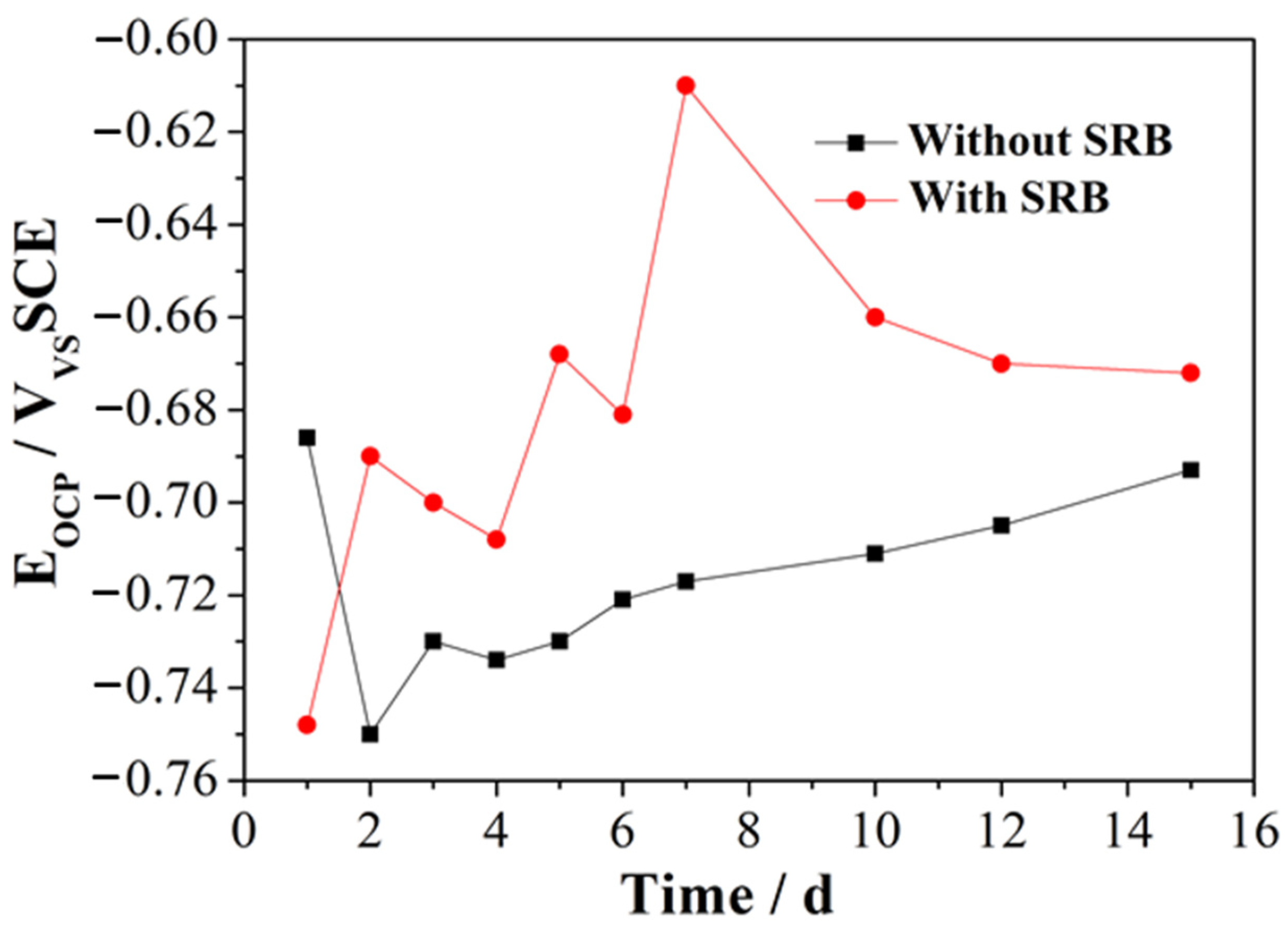
Figure 1 illustrates the change in the EOCP of the samples during immersion in artificial seawater either containing SRB or without SRB strains. In the presence of SRB, the EOCP shows an initial increase during the first 7 days of immersion, along with fluctuation. This rising trend in EOCP suggests the passivation of EQ70 steel and the production of an oxide layer on the EQ70 steel surface [26,27]. The rise in EOCP can also be ascribed to the metabolic activities of SRB and the production of a biofilm on the metal [28,29]. After 7 days of immersion, the EOCP shows a subsequent decrease until the final point of immersion. This decrease in EOCP is most likely caused by the dissolution of the metal oxide layer. In artificial seawater without SRB, the EOCP shifts negatively from the first to the second day of immersion, which can be linked to the disintegration of the passivation film produced on the surface of EQ70 steel [16]. The EOCP constantly increased during the immersion, from 3 to 15 days. This result can be related to the gradual thickening of the corrosion products layer formed on the steel. The variation in EOCP resembles the findings from the study on 316L stainless steel in an environment where metal-oxidizing bacteria were found to be present in a mixture [29].
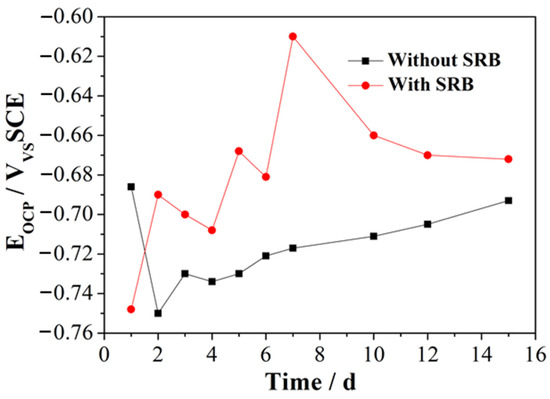
Figure 1.
Change in OCP over time of the two samples immersed in artificial seawater with SRB and without SRB.
3.2. EIS Analysis
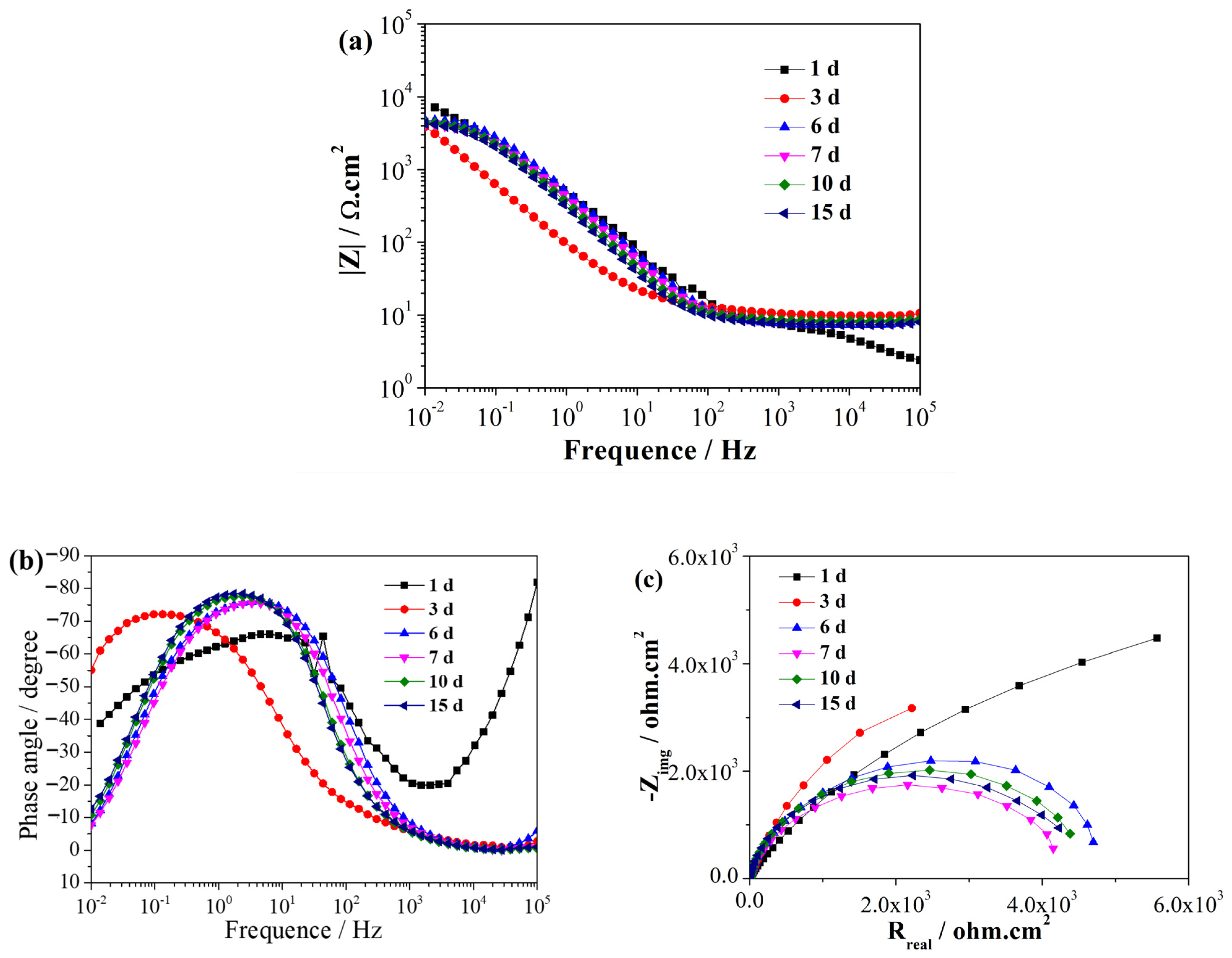
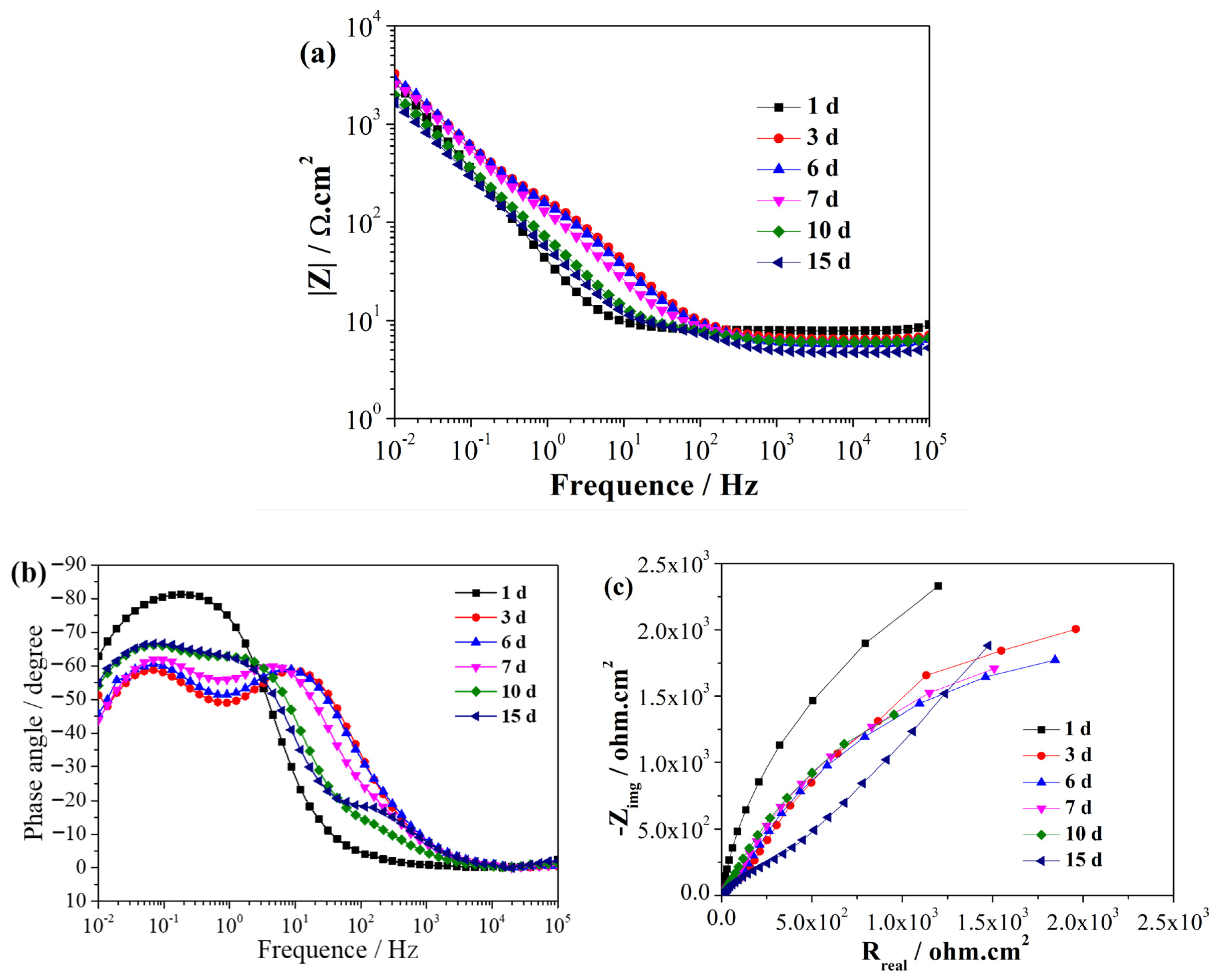
EIS is a non-destructive electrochemical technique used to characterize reactions between metals and biofilms [30]. EIS can be used to analyze the process of SRB adhesion, biofilm formation, and the related corrosion processes [31]. Figure 2 and Figure 3 illustrate the EIS spectra of the EQ70 samples after different exposure times in the artificial seawater without SRB and with SRB, respectively. Immediately upon immersion, the EQ70 sample in artificial seawater without SRB exhibited relatively high impedance values obtained in the low-frequency range (Figure 2a), attributed to the production of a layer of corrosion products that shielded the metal from the corrosive ions in the solution. After 3 days of immersion, the impedance at 0.01 Hz decreased due to the reduced protective ability of the layer. After 6 days, the impedance values at 0.01 Hz stabilized. The above change in the impedance value is similar to the findings from a prior study on 316L stainless steel in sterile artificial seawater [29,32]. During the immersion period from 1 to 7 days, the EQ70 sample in artificial seawater with SRB exhibited an increment in impedance at 0.01 Hz (Figure 3a). This increase is likely due to the passivation effect of the biofilm [33]. However, after 10 and 15 days, the impedance at 0.01 Hz decreased. This decrease may be due to the inhomogeneous biofilm, which can be damaged by the corrosive Cl− and thus the subsequent increase in the corrosion process on the metal surface [34,35,36]. Upon comparing Figure 2b with Figure 3b, a change in the time constant of the EIS plots can be observed, transitioning from a two-time constant in artificial seawater without SRB to a three-time constant in artificial seawater containing SRB. The time constant observed in the high-frequency range is likely linked to the presence of the biofilm formed on the steel surface, while the time constant observed in the low-frequency range can be ascribed to the layer of corrosion products on the steel. Figure 2c and Figure 3c show the Nyquist plots of the EQ70 samples in artificial seawater without SRB and with SRB, respectively. The reduction in the capacitance loop suggests that SRB can accelerate the corrosion of EQ70 steel. Under anaerobic conditions, SRB caused the production of a biofilm on the top of the steel surface [26,37]. A biofilm consumes iron to provide the life energy and the electron for itself [38,39]. Moreover, SRB can transform SO42− into H2S [12], which can further react with metal ions to form metal sulfides. The change of sulfur ions to metal sulfides is an important factor in accelerating metal corrosion [16,40].
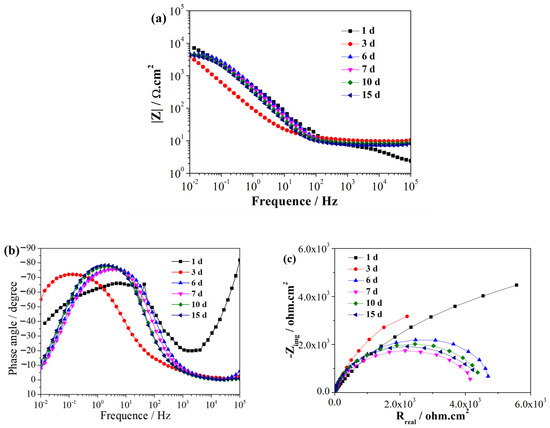
Figure 2.
EIS of the EQ70 sample at various exposure times in artificial seawater without SRB, shown in Bode plots (a, b) and a Nyquist plot (c).
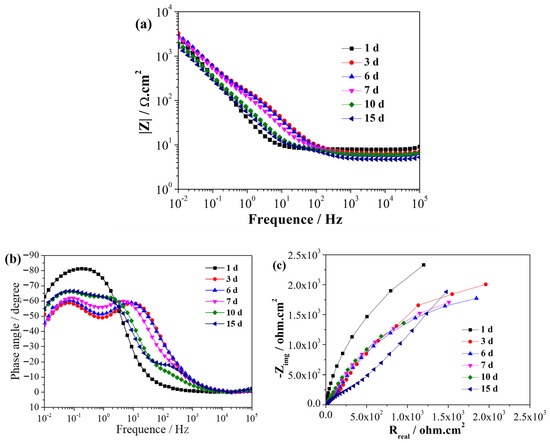
Figure 3.
EIS of the EQ70 sample at various exposure times in artificial seawater with SRB strains, shown in Bode plots (a, b) and a Nyquist plot (c).
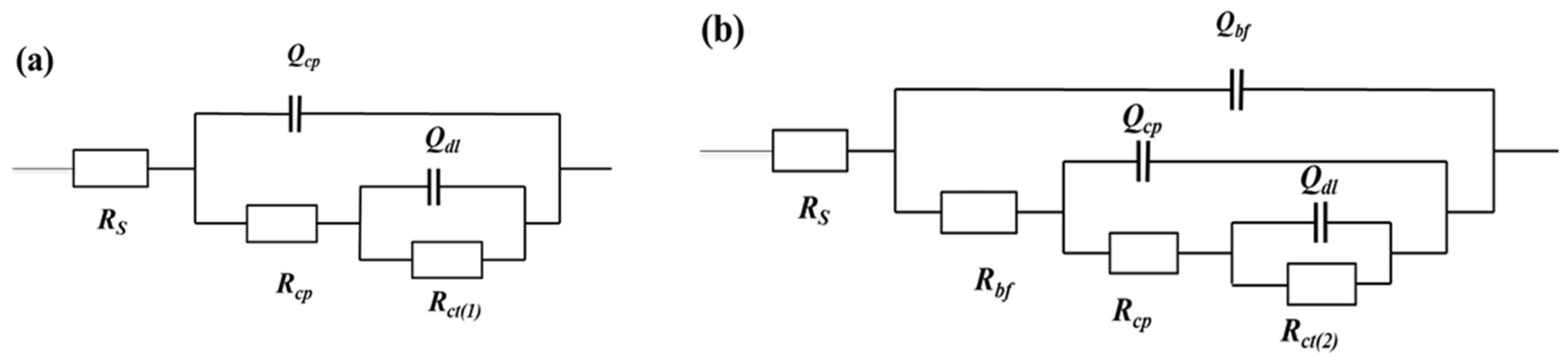
Figure 4 shows the electrical equivalent circuits (EEC) employed in fitting the EIS spectra for the EQ70 samples in artificial seawater without SRB (Figure 4a) and artificial seawater with SRB (Figure 4b). To fit the impedance data for the EQ70 samples in artificial seawater without SRB, an EEC consisting of two-time constants is used, denoted as R(Q(R(QR))). For the impedance data for the EQ70 samples in artificial seawater with SRB, an EEC consisting of three-time constants is used, denoted as R(Q(R(Q(R(QR))))) [2,41]. The constant phase element (CPE or Q) is employed in place of capacitance (C) to address the deviation from pure capacitance for the capacitance of the corrosion product layer, biofilm, and double layer. The CPE is denoted as follows [42]:
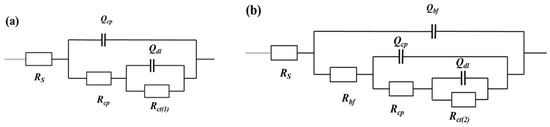
Figure 4.
Electrical equivalent circuits (EECs) employed to fit the EIS data of the EQ70 samples in artificial seawater without SRB (a) and with SRB strains (b). Rs: solution resistance; Qbf: biofilm capacitance; Rbf: biofilm resistance; Qcp: corrosion product layer capacitance; Rcp: corrosion product layer resistance; Qdl: double layer capacitance; Rct(2): charge transfer resistance with SRB; Rct(1): charge transfer resistance without SRB.
In the equation, Y0 represents the magnitude of the CPE, which can be converted into Qdl. ω denotes the angular frequency. The dispersion coefficient, n, reflects the intensity of the dispersion.
The parameters obtained by fitting the EIS spectra can be found in Table 2 and Table 3. For the EQ70 sample in artificial seawater without SRB, the resistance of the corrosion product layer (Rcp) initially increased during the early stage of immersion but decreased in the later stage (see Table 2). The initial increase in the resistance of the corrosion product layer (Rcp) can be attributed to the emergence of a protective layer on the metal surface. This layer, commonly known as the corrosion product layer, acted as a barrier separating the metal from harmful elements in the environment. The formation of the corrosion product layer was accompanied by an increase in thickness and compactness, thereby augmenting its resistance. This enhanced resistance served to impede the corrosion process by restricting the diffusion of corrosive agents, including oxygen and chloride ions, toward the metal surface. However, with the passage of time and prolonged immersion, the corrosion product layer might have undergone alterations, leading to a decrease in compactness and an increase in porosity. This makes it easier for corrosive agents to penetrate the layer, causing a faster corrosion rate and weakening the protective properties of the layer [16,29]. The value of 1/Rct (Rct, charge transfer resistance) indicates the corrosion rate [35,43]. This value increased in the first 7 days of immersion and showed a slight decrease afterward. In particular, 1/Rct reached the maximum value on the 7th day, indicating the highest corrosion rate in the middle of the immersion. For the EQ70 sample in artificial seawater with SRB, the resistance of the biofilm (Rbf) was found to be very low. Additionally, the resistance of the layer of corrosion products was lower than that in artificial seawater without SRB. This result can be attributed to the increase in conductivity introduced by the SRB [9,44]. During the initial 7 days, the 1/Rct gradually increased, indicating that the SRB accelerated the corrosion of EQ70 (see Table 3). After 7 days, the stable 1/Rct value showed that the number of living SRB decreased. Consequently, SRB had a noticeable influence on the corrosion rate of EQ70. From the above results, it can be inferred that SRB strains can accelerate the corrosion of EQ70, particularly in the early stage of the immersion period.

Table 2.
Fitted parameters of the EIS of the EQ70 sample after different exposure times in artificial seawater without SRB.

Table 3.
Fitted parameters of the EIS of the EQ70 sample after different exposure times in artificial seawater with SRB strains.
3.3. Polarization Curves
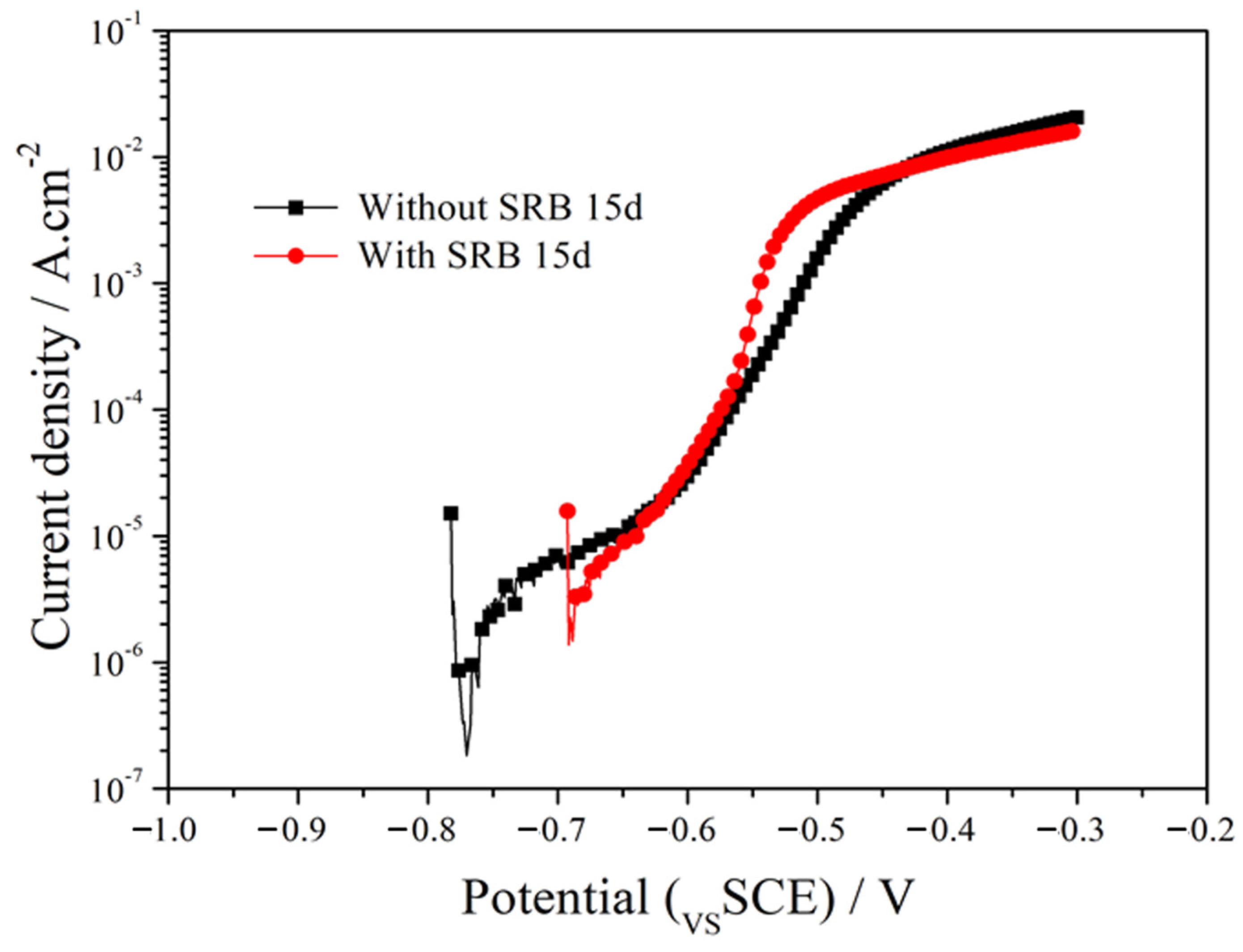
Figure 5 shows the anodic polarization curves of the EQ70 samples after 15 days of exposure to artificial seawater without SRB and with SRB strains. The polarization curves were fitted using Rp extrapolation near the open circuit potential (±15 mV). The corrosion current density (Icorr) was measured as 1.11 × 10−4 A/cm2 for the EQ70 sample in artificial seawater without SRB. Icorr is 1.95 × 10−3 A/cm2 for the EQ70 sample in artificial seawater with SRB strains. The increase in Icorr indicates that SRB can accelerate the corrosion rate of EQ70 steel.
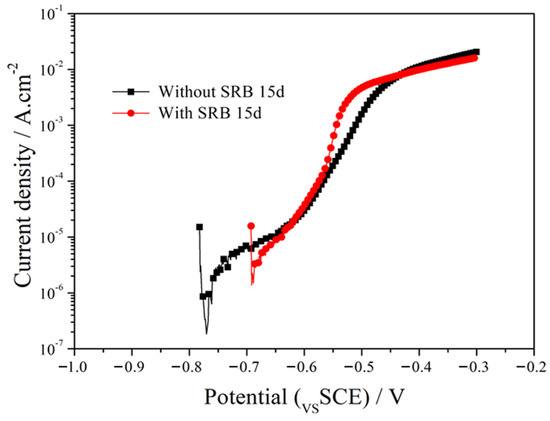
Figure 5.
Anodic polarization curves of EQ70 samples after 15 days of exposure in artificial seawater, in the presence or absence of SRB.
3.4. Surface Morphologies
3.4.1. Macroscopical Observation
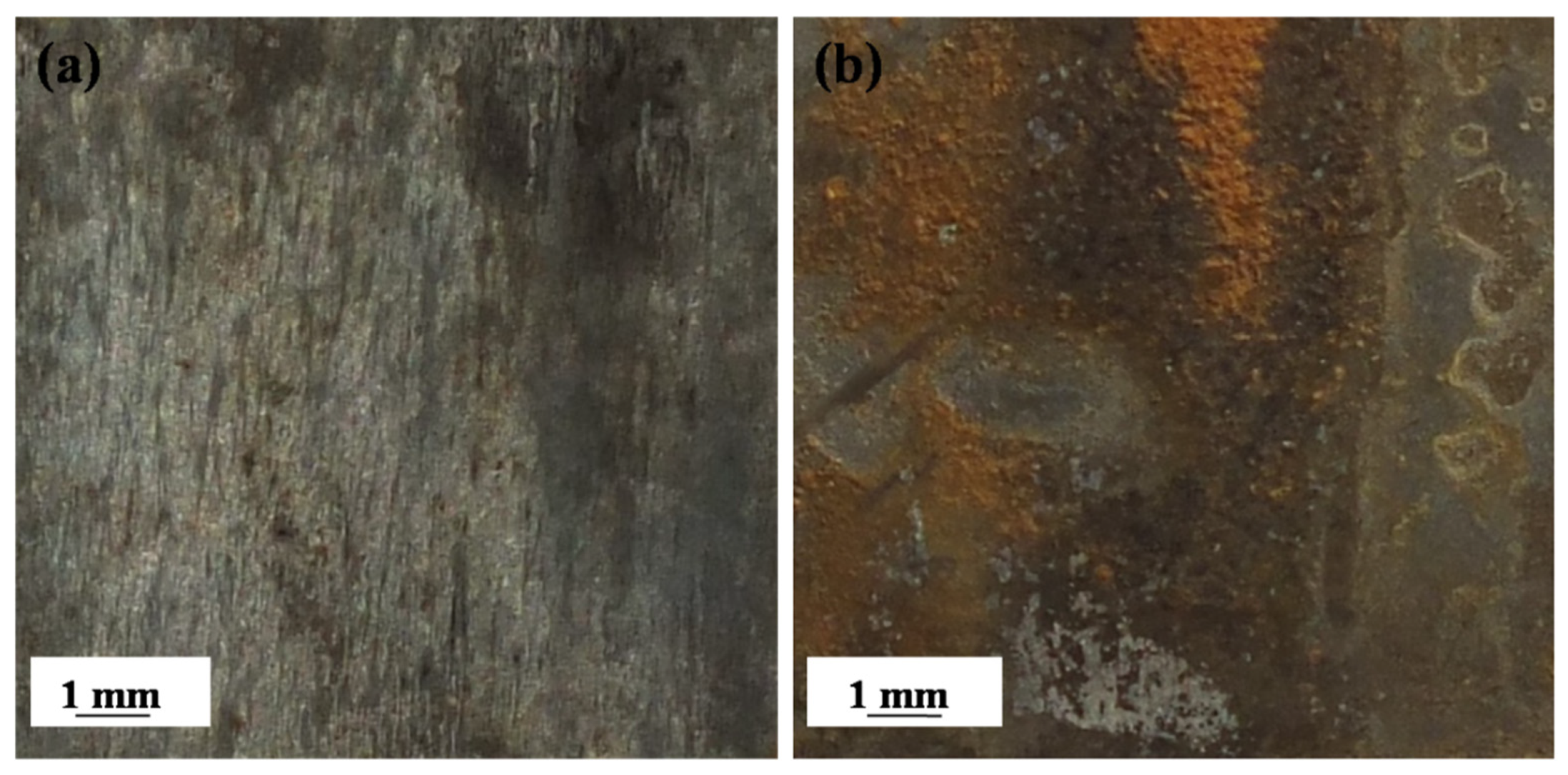
Figure 6 displays photographs of the EQ70 samples after 15 days of exposure to artificial seawater without SRB and with SRB strains. Upon exposure to artificial seawater without SRB for 15 days, a thin layer of rust was observed on the surface of the EQ70 sample (Figure 6a). However, after 15 days of exposure to artificial seawater with the SRB strains, much more red rust could be seen on the EQ70 sample (Figure 6b), indicating extensive corrosion activity on the EQ70 sample affected by SRB.
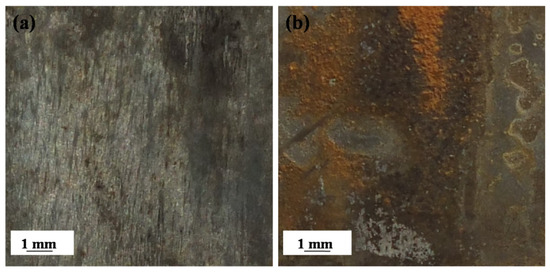
Figure 6.
Photographs of EQ70 samples after 15 days of exposure to artificial seawater without SRB (a) and with SRB strains (b).
3.4.2. SEM and EDS
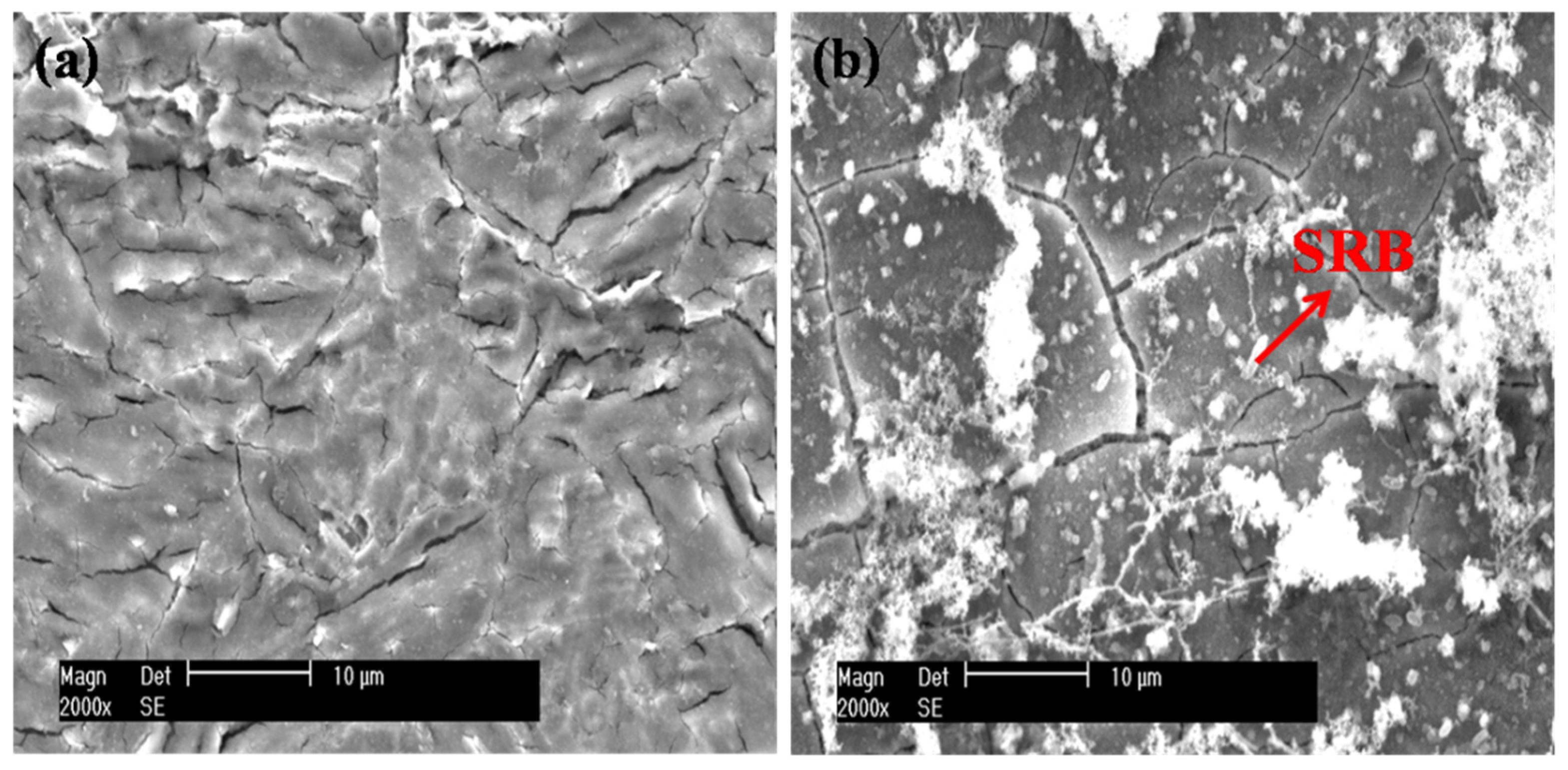
Figure 7 presents the SEM images of the EQ70 samples after 15 days of exposure to artificial seawater without SRB and with SRB strains. In Figure 7a, light corrosion is observed for the EQ70 sample in artificial seawater without SRB. In Figure 7b, corrosion products and SRB strains can be seen on the EQ70 sample in artificial seawater with SRB strains. The EDS analysis reveals that the content of O (28.2 wt.%) on the EQ70 surface in artificial seawater with SRB strains is much higher than that (8.3 wt.%) on the EQ70 surface in artificial seawater without SRB. This indicates the generation of carbohydrates by SRB through their metabolic activity on the steel surface [17,31,36], suggesting the appearance of SRB and EPS on the steel. Furthermore, the content of Fe (38.5 wt.%) on the EQ70 surface in artificial seawater with SRB strains is less than that (69.3 wt.%) on the EQ70 surface in artificial seawater without SRB, implying that a portion of Fe on the steel surface has transformed to FeS or Fe2O3, as induced by SRB.
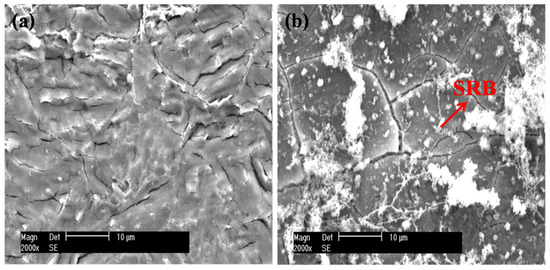
Figure 7.
SEM micrographs of EQ70 samples after 15 days of exposure to artificial seawater without SRB (a) and with SRB strains (b).
3.4.3. CLSM Analysis
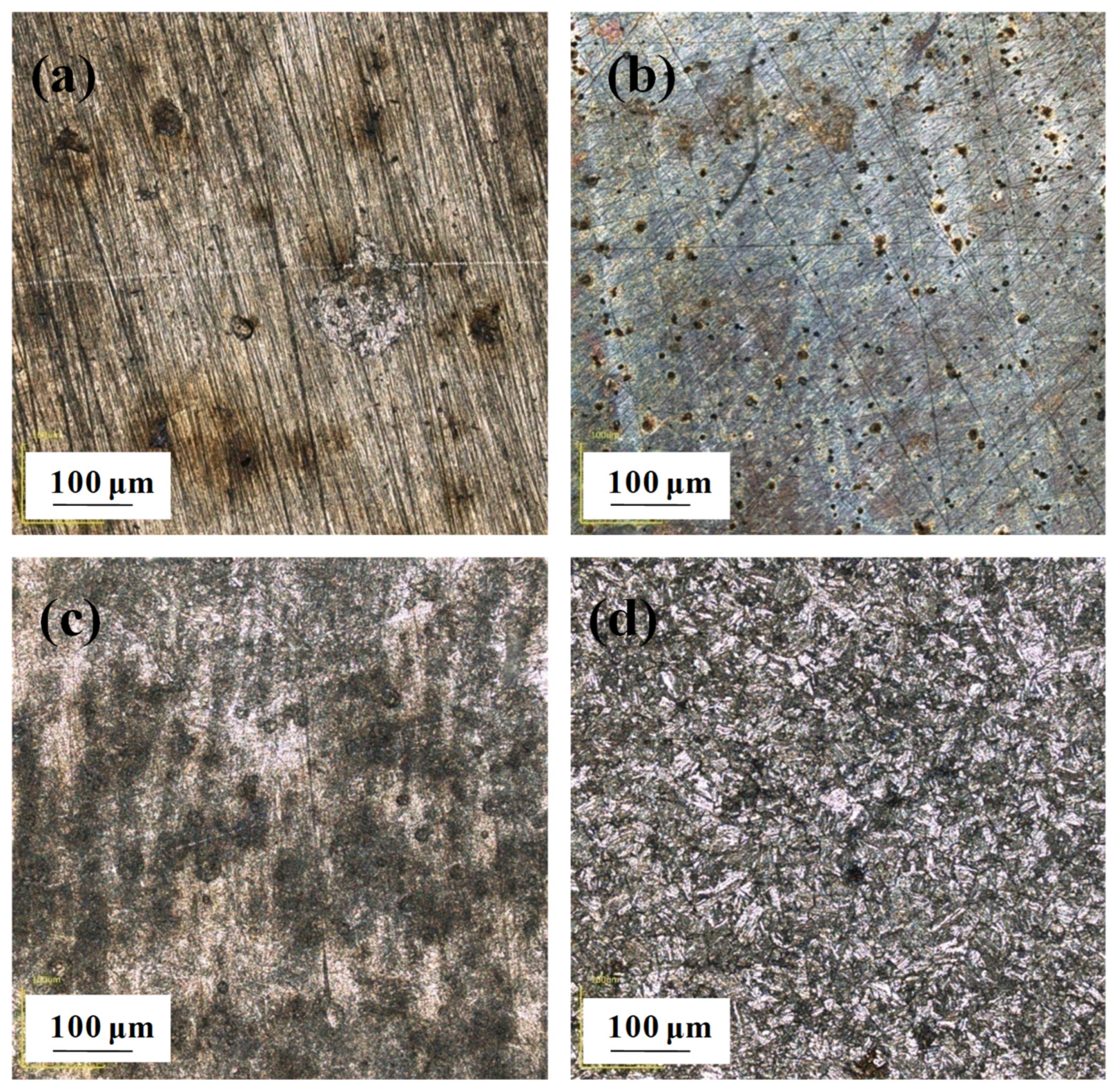
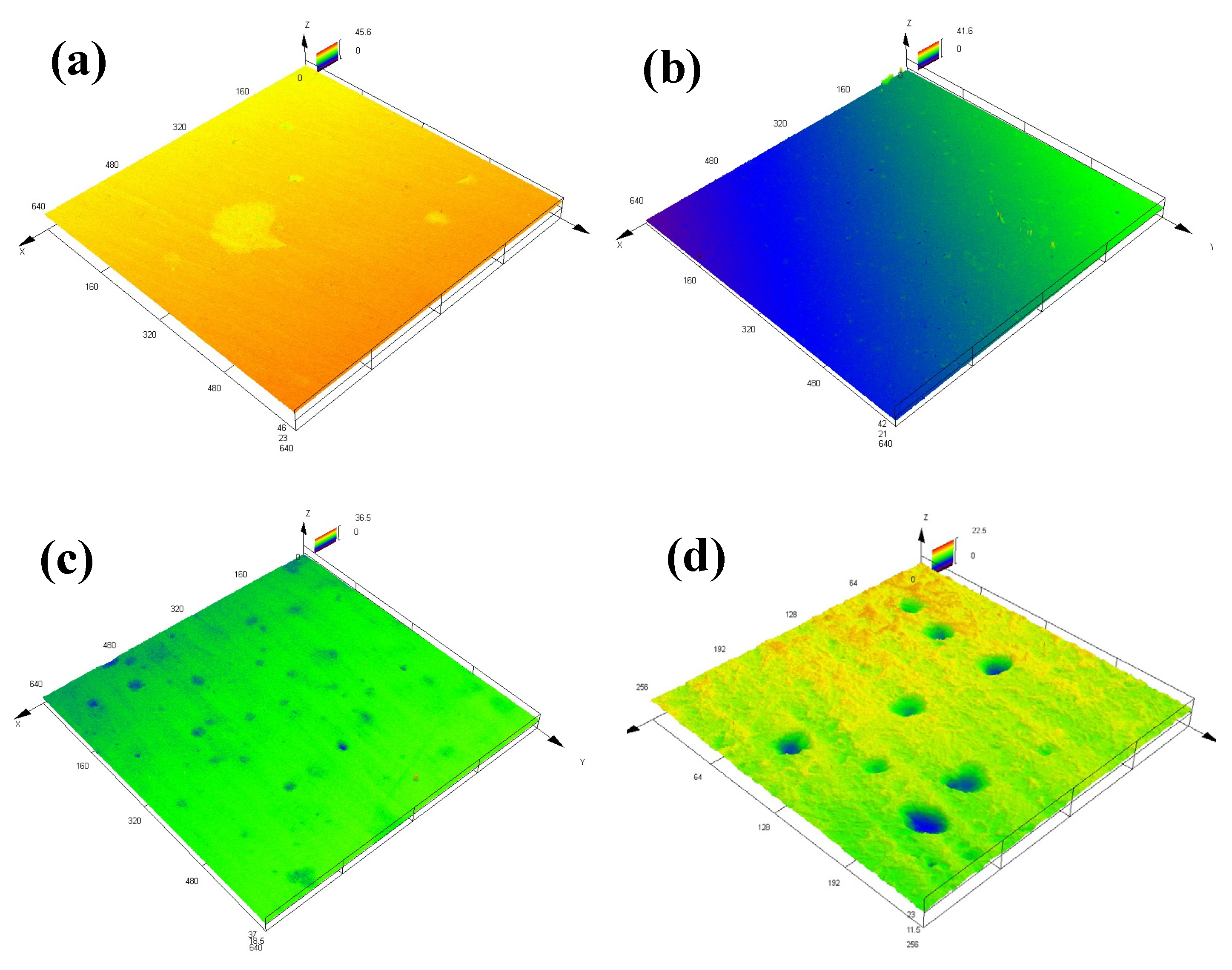
According to the CLSM micrographs (Figure 8) and the typical 3D CLSM images (Figure 9), it is obvious that corrosion of the EQ70 sample after 15 days of exposure was more serious than that after 4 days of exposure. From Table 4, it can be seen that the pit depth increases from 4 days to 15 days. The presence of SRB leads to a deeper pit depth on the steel compared to that when no SRB is present in the artificial seawater. In particular, after 15 days of exposure, the average pit depth on the steel surface in artificial seawater with SRB (16.5 µm) was approximately twice as deep as the average pit depth on the steel surface in artificial seawater without SRB (8.1 µm).
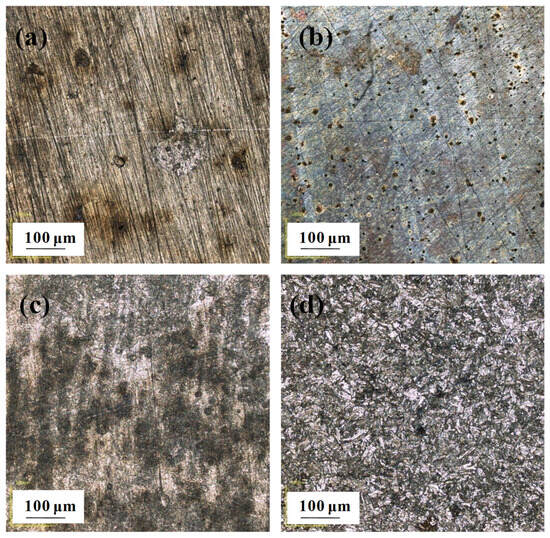
Figure 8.
Macro optical images obtained through confocal laser scanning microscopy of EQ70 samples after 4 and 15 days of exposure in artificial seawater with SRB strains (a,c) and without SRB (b,d).
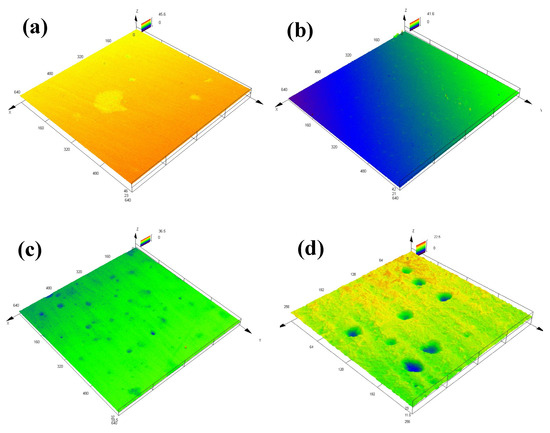
Figure 9.
Three-dimensional confocal laser scanning microscopy images showing typical pits on EQ70 samples after 4 and 15 days of exposure in artificial seawater with SRB strains (a,c) and without SRB (b,d).

Table 4.
Depth of pits of the EQ70 samples after exposure for 4 and 15 days in artificial seawater without and with SRB strains.
4. Conclusions
The corrosion behavior of EQ70 steel was investigated using artificial seawater under anaerobic conditions in the presence of SRB. Macroscopic morphology and SEM analysis revealed the existence of severe corrosion on the EQ70 steel surface after immersion in artificial seawater with SRB strains. Our calculation based on CLSM data indicates that the average pit depth on the steel surface in artificial seawater with SRB strains (16.5 µm) is almost twice that (8.1 µm) on the steel surface in artificial seawater without SRB after 15 days of immersion. The results of this study demonstrate that the presence of SRB strains influenced corrosion of EQ70 high-strength steel in seawater environments by increasing the corrosion rate.
Author Contributions
Y.S. performed the experiments, analyzed the data and wrote the original draft; I.K.N., B.W., S.G. and H.C. performed the data analysis, wrote the partial draft and revised the paper; H.S. conceived, designed the experiments and revised the paper; J.W. conceived and designed the experiments and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 52171089 and 51571202.
Data Availability Statement
The data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the Effect of Carbon Steel Exposure in Sulfide Containing Solutions to Microbially Induced Corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Puentes-Cala, E.; Tapia-Perdomo, V.; Espinosa-Valbuena, D.; Reyes-Reyes, M.; Quintero-Santander, D.; Vasquez-Dallos, S.; Salazar, H.; Santamaría-Galvis, P.; Silva-Rodríguez, R.; Castillo-Villamizar, G. Microbiologically Influenced Corrosion: The Gap in the Field. Front. Environ. Sci. 2022, 10, 924842. [Google Scholar] [CrossRef]
- Dou, W.; Xu, D.; Gu, T. Biocorrosion Caused by Microbial Biofilms Is Ubiquitous around Us. Microb. Biotechnol. 2021, 14, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, S.R.G.; Macrae, A.; Peixoto, R.S.; da Costa Rachid, C.T.C.; Mansoldo, F.R.P.; Alviano, D.S.; Alviano, C.S.; Ferreira, D.F.; de Queiroz Venâncio, F.; Ferreira, D.F.; et al. Sulphate-Reducing Bacterial Community Structure from Produced Water of the Periquito and Galo de Campina Onshore Oilfields in Brazil. Sci. Rep. 2021, 11, 20311. [Google Scholar] [CrossRef]
- Kamarisima; Hidaka, K.; Miyanaga, K.; Tanji, Y. The Presence of Nitrate- and Sulfate-Reducing Bacteria Contributes to Ineffectiveness Souring Control by Nitrate Injection. Int. Biodeterior. Biodegrad. 2018, 129, 81–88. [Google Scholar] [CrossRef]
- Liduino, V.; Galvão, M.; Brasil, S.; Sérvulo, E. SRB-Mediated Corrosion of Marine Submerged AISI 1020 Steel under Impressed Current Cathodic Protection. Colloids Surf. B Biointerfaces 2021, 202, 111701. [Google Scholar] [CrossRef]
- Chen, L.; Wei, B.; Xu, X. Effect of Sulfate-Reducing Bacteria (Srb) on the Corrosion of Buried Pipe Steel in Acidic Soil Solution. Coatings 2021, 11, 625. [Google Scholar] [CrossRef]
- Tambe, S.P.; Jagtap, S.D.; Chaurasiya, A.K.; Joshi, K.K. Evaluation of Microbial Corrosion of Epoxy Coating by Using Sulphate Reducing Bacteria. Prog. Org. Coat. 2016, 94, 49–55. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, B.; Zhao, Y.; Pehkonen, S.O. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros. Sci. 2013, 74, 353–366. [Google Scholar] [CrossRef]
- Qi, B.; Dun, Z.; Dandan, L.; Peng, W. Effects of two main metabolites of sulphate-reducing bacteria on the corrosion of Q235 steels in 3.5 wt.% NaCl media. Corros. Sci. 2012, 65, 405–413. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, D.; Liu, H.; Li, Y.; Hou, B. Influence of sulphate-reducing bacteria on environmental parameters and marine corrosion behavior of Q235 steel in aerobic conditions. Electrochim. Acta 2010, 55, 1528–1534. [Google Scholar] [CrossRef]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, M.; Hou, B. Influence of Sulfate-Reducing Bacteria on the Corrosion Behavior of High Strength Steel Eq70 under Cathodic Polarization. PLoS ONE 2016, 11, e0162315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Sun, H.; Ge, Y.; Wang, C.; Li, L.; Kang, J.; Qian, H.; Gao, Q. Corrosion Behavior on 20# Pipeline Steel by Sulfate-Reducing Bacteria in Simulated NaCl Alkali/Surfactant/Polymer Produced Solution. ACS Omega 2023, 8, 13955–13966. [Google Scholar] [CrossRef]
- Kokilaramani, S.; AlSalhi, M.S.; Devanesan, S.; Narenkumar, J.; Rajasekar, A.; Govarthanan, M. Bacillus Megaterium-Induced Biocorrosion on Mild Steel and the Effect of Artemisia Pallens Methanolic Extract as a Natural Corrosion Inhibitor. Arch. Microbiol. 2020, 202, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, Y.; Yu, D.; Lan, G.; Wang, Z.; Mou, X. Studies on the Impact of Fluid Flow on the Microbial Corrosion Behavior of Product Oil Pipelines. J. Pet. Sci. Eng. 2016, 146, 803–812. [Google Scholar] [CrossRef]
- Stipaničev, M.; Turcu, F.; Esnault, L.; Schweitzer, E.W.; Kilian, R.; Basseguy, R. Corrosion Behavior of Carbon Steel in Presence of Sulfate-Reducing Bacteria in Seawater Environment. Electrochim. Acta 2013, 113, 390–406. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T. Carbon Source Starvation Triggered More Aggressive Corrosion against Carbon Steel by the Desulfovibrio Vulgaris Biofilm. Int. Biodeterior. Biodegrad. 2014, 91, 74–81. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Bai, Q.; Zhang, L.L.; Peng, X.; Kong, X.F. Potential Distribution Characteristics of Mild Steel in Seawater. Corros. Sci. 2012, 57, 202–208. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-Term Immersion Corrosion of Steels in Seawaters with Elevated Nutrient Concentration. Corros. Sci. 2014, 81, 110–116. [Google Scholar] [CrossRef]
- Von Wolzogen Kiinr, C.A.H.; Van der Vlugf, L.R. Degrafiteering Van Gietijzer Als Electrobiochemisch Proces in Anaerobe Gronden. Water 1934, 18, 147–165. (In Dutch) [Google Scholar]
- Loto, C.A. Microbiological corrosion: Mechanism, control and impact—A review. Int. J. Adv. Manuf. Technol. 2017, 92, 4241–4252. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Mussmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron Corrosion by Novel Anaerobic Microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef]
- Li, H.; Xu, D.; Li, Y.; Feng, H.; Liu, Z.; Li, X.; Gu, T.; Yang, K. Extracellular Electron Transfer is a Bottleneck in the Microbiologically Influenced Corrosion of C1018 Carbon Steel by the Biofilm of SulfateReducing Bacterium Desulfovibrio vulgaris. PLoS ONE 2015, 10, 0136183. [Google Scholar] [CrossRef]
- Tao, S.-F.; Xia, Y.-J.; Wang, F.-M.; Li, J.; Fan, D.-D. Effect of Heat Treatment Technique on the Low Temperature Impact Toughness of Steel EQ70 for Offshore Structure. High Temp. Mater. Proc. 2017, 36, 825–830. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Wang, P.; Zhang, D.; Chen, S.; Tan, F. Corrosion of 907 Steel Influenced by Sulfate-Reducing Bacteria. J. Mater. 2019, 28, 1469–1479. [Google Scholar] [CrossRef]
- Li, F.; An, M.; Liu, G.; Duan, D. Effects of Sulfidation of Passive Film in the Presence of SRB on the Pitting Corrosion Behaviors of Stainless Steels. Mater. Chem. Phys. 2009, 113, 971–976. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O. Microbiologically Influenced Corrosion of 304 Stainless Steel by Aerobic Pseudomonas NCIMB 2021 Bacteria: AFM and XPS Study. Colloids Surf. B Biointerfaces 2007, 59, 87–99. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Sun, C.; Yu, Z.; Hou, B. The Corrosion of Two Aluminium Sacrificial Anode Alloys in SRB-Containing Sea Mud. Corros. Sci. 2014, 83, 375–381. [Google Scholar] [CrossRef]
- Moradi, M.; Duan, J.; Ashassi-Sorkhabi, H.; Luan, X. De-Alloying of 316 Stainless Steel in the Presence of a Mixture of Metal-Oxidizing Bacteria. Corros. Sci. 2011, 53, 4282–4290. [Google Scholar] [CrossRef]
- Nwokolo, I.K.; Shi, H.; Ikeuba, A.I.; Gao, N.; Li, J.; Ahmed, S.; Liu, F. Synthesis, Characterization and Investigation of Anticorrosion Properties of an Innovative Metal–Organic Framework, ZnMOF-BTA, on Carbon Steel in HCl Solution. Coatings 2022, 12, 1288. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Rasheed, P.A.; Al-Qahtani, N.; Abdullah, A.M.; Hamdy, F.; Sharaf, M.A.; Hassan, M.K.; Mahmoud, K.A.; Mohamed, A.M.; Jarjoura, G.; et al. Microbiologically-Influenced Corrosion of the Electroless-Deposited NiP-TiNi—Coating. Arab. J. Chem. 2021, 14, 103445. [Google Scholar] [CrossRef]
- Udoh, I.I.; Shi, H.; Daniel, E.F.; Li, J.; Gu, S.; Liu, F.; Han, E.H. Active Anticorrosion and Self-Healing Coatings: A Review with Focus on Multi-Action Smart Coating Strategies. J. Mater. Sci. Technol. 2022, 116, 224–237. [Google Scholar] [CrossRef]
- Dong, X.; Zhai, X.; Yang, J.; Guan, F.; Zhang, Y.; Duan, J.; Hou, B. Two Metabolic Stages of SRB Strain Desulfovibrio Bizertensis Affecting Corrosion Mechanism of Carbon Steel Q235. Corros. Commun. 2023, 10, 56–68. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. A Study of Bacteria Adhesion and Microbial Corrosion on Different Stainless Steels in Environment Containing Desulfovibrio Vulgaris. R. Soc. Open Sci. 2021, 8, 201577. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Z. EIS Investigation and Structural Characterization of Different Hot-Dipped Zinc-Based Coatings in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2013, 8, 7753–7767. [Google Scholar] [CrossRef]
- Li, H.; Zhou, E.; Zhang, D.; Xu, D.; Xia, J.; Yang, C.; Feng, H.; Jiang, Z.; Li, X.; Gu, T.; et al. Microbiologically Influenced Corrosion of 2707 Hyper-Duplex Stainless Steel by Marine Pseudomonas Aeruginosa Biofilm. Sci. Rep. 2016, 6, 20190. [Google Scholar] [CrossRef]
- Lv, M.; Du, M.; Li, X.; Yue, Y.; Chen, X. Mechanism of Microbiologically Influenced Corrosion of X65 Steel in Seawater Containing Sulfate-Reducing Bacteria and Iron-Oxidizing Bacteria. J. Mater. Res. Technol. 2019, 8, 4066–4078. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Thakur, P.; Saxena, P.; Rauniyar, S.; Gopalakrishnan, V.; Singh, R.N.; Gadhamshetty, V.; Gnimpieba, E.Z.; Jasthi, B.K.; Sani, R.K. Gene Sets and Mechanisms of Sulfate-Reducing Bacteria Biofilm Formation and Quorum Sensing with Impact on Corrosion. Front. Microbiol. 2021, 12, 754140. [Google Scholar] [CrossRef]
- Clark, M.E.; He, Z.; Redding, A.M.; Joachimiak, M.P.; Keasling, J.D.; Zhou, J.Z.; Arkin, A.P.; Mukhopadhyay, A.; Fields, M.W. Transcriptomic and Proteomic Analyses of Desulfovibrio Vulgaris Biofilms: Carbon and Energy Flow Contribute to the Distinct Biofilm Growth State. BMC Genom. 2012, 13, 138. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Yan, M.; Sun, C.; Yu, C.; Ke, W. Synergistic Effect of Sulfate-Reducing Bacteria and Elastic Stress on Corrosion of X80 Steel in Soil Solution. Corros. Sci. 2014, 83, 38–47. [Google Scholar] [CrossRef]
- Qin, J.; Shi, X.; Li, H.; Zhao, R.; Li, G.; Zhang, S.; Ding, L.; Cui, X.; Zhao, Y.; Zhang, R. Performance and Failure Process of Green Recycling Solutions for Preparing High Degradation Resistance Coating on Biomedical Magnesium Alloys. Green. Chem. 2022, 24, 8113–8130. [Google Scholar] [CrossRef]
- Liu, H.; Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion Behavior of Carbon Steel in the Presence of Sulfate Reducing Bacteria and Iron Oxidizing Bacteria Cultured in Oilfield Produced Water. Corros. Sci. 2015, 100, 484–495. [Google Scholar] [CrossRef]
- Wang, J.; Hou, B.; Xiang, J.; Chen, X.; Gu, T.; Liu, H. The Performance and Mechanism of Bifunctional Biocide Sodium Pyrithione against Sulfate Reducing Bacteria in X80 Carbon Steel Corrosion. Corros. Sci. 2019, 150, 296–308. [Google Scholar] [CrossRef]
- Tuck, B.; Watkin, E.; Somers, A.; Machuca, L.L. A Critical Review of Marine Biofilms on Metallic Materials. NPJ Mater. Degrad. 2022, 6, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).