Distribution and Control of Arsenic during Copper Converting and Refining
Abstract
1. Introduction
2. Thermodynamic Predictions
3. Results and Discussion
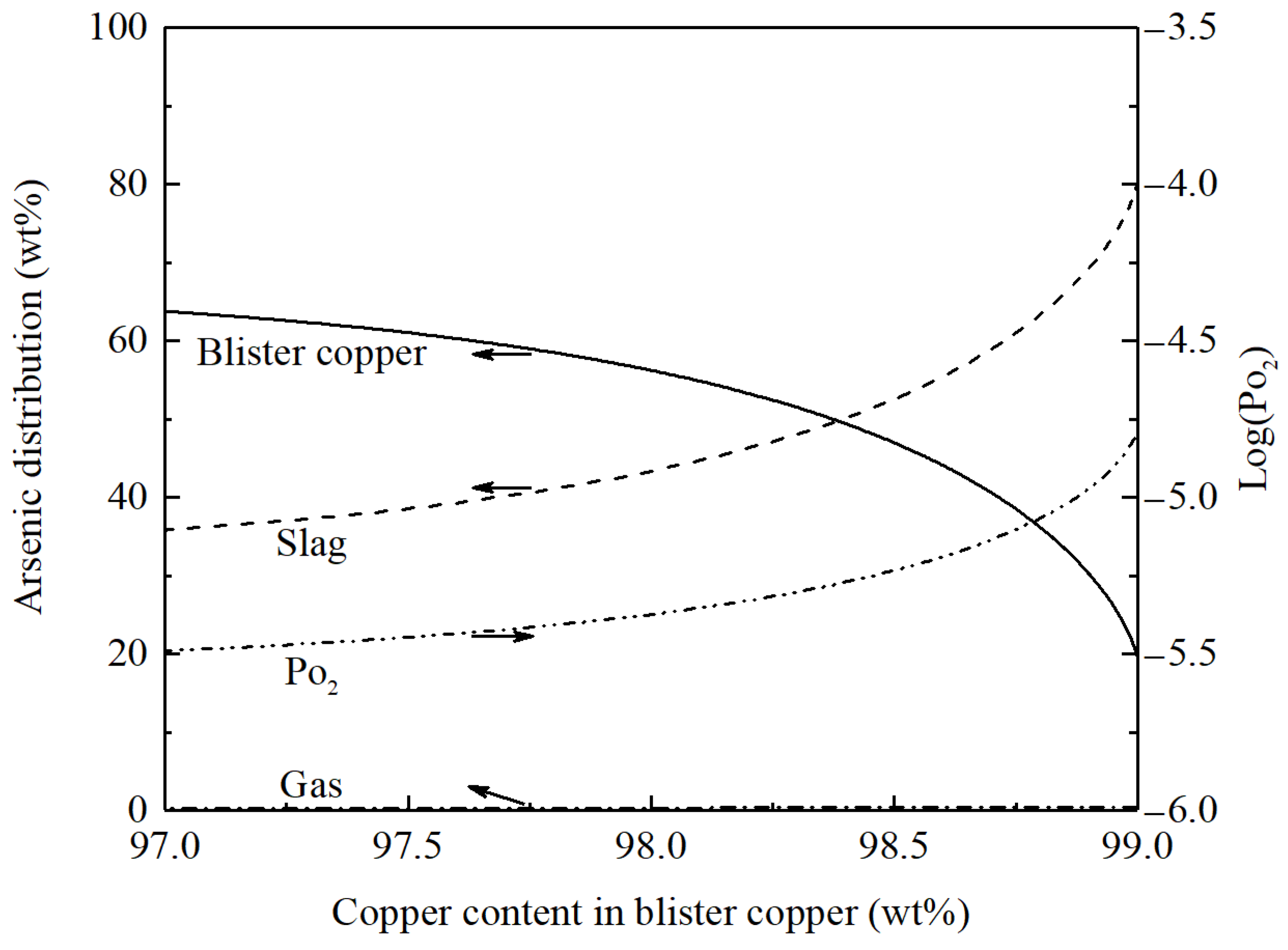
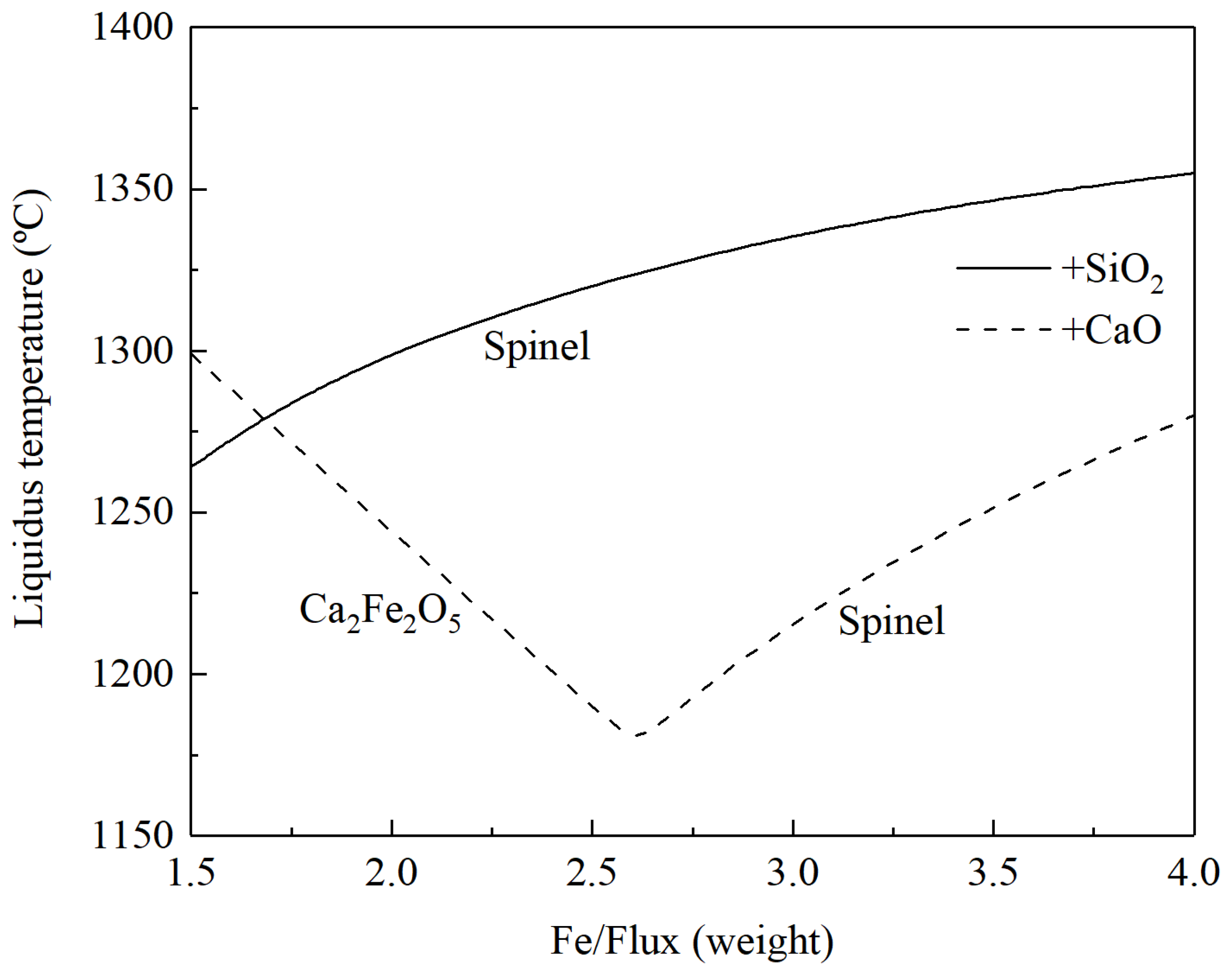
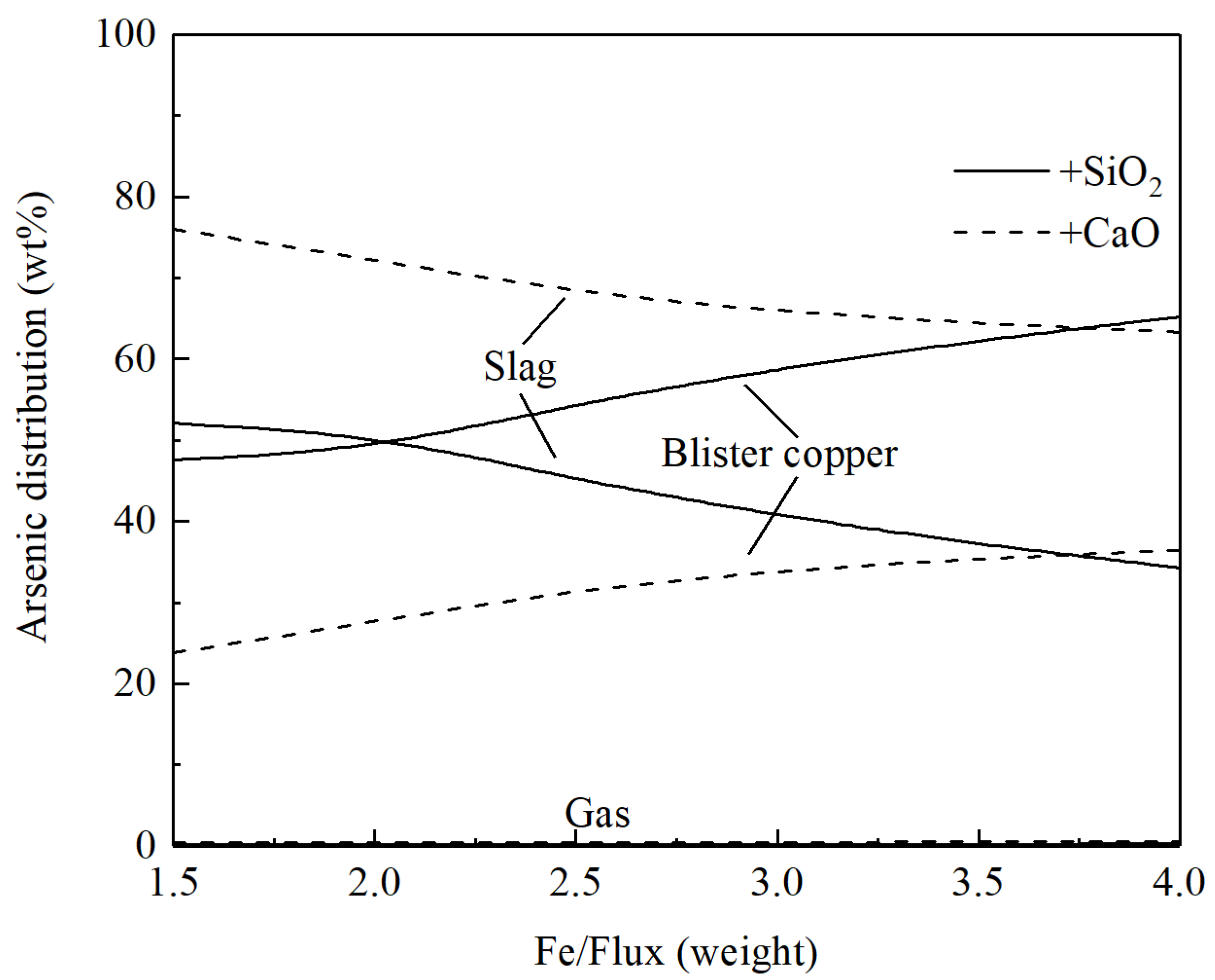
3.1. Converting of Copper Matte
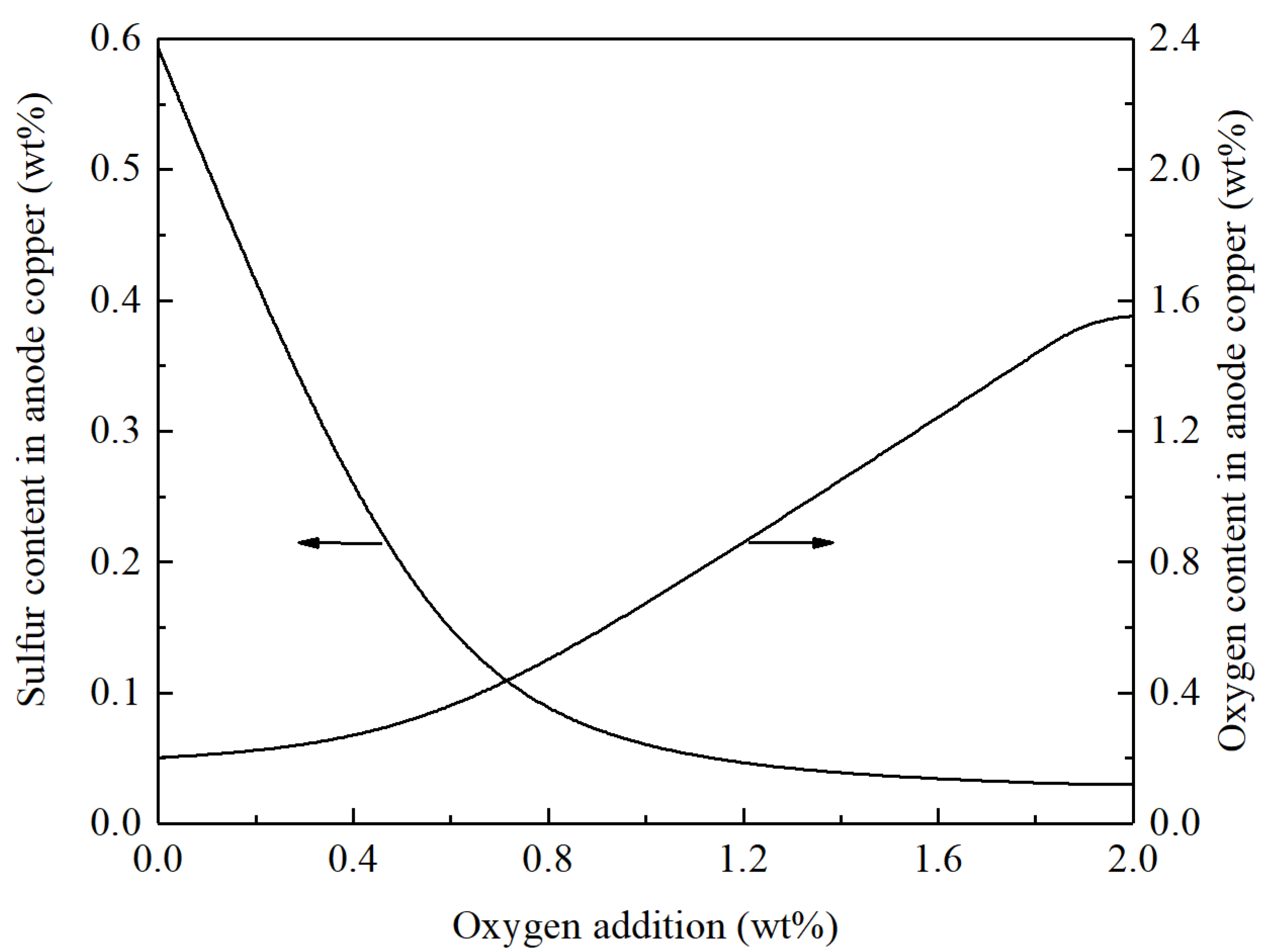
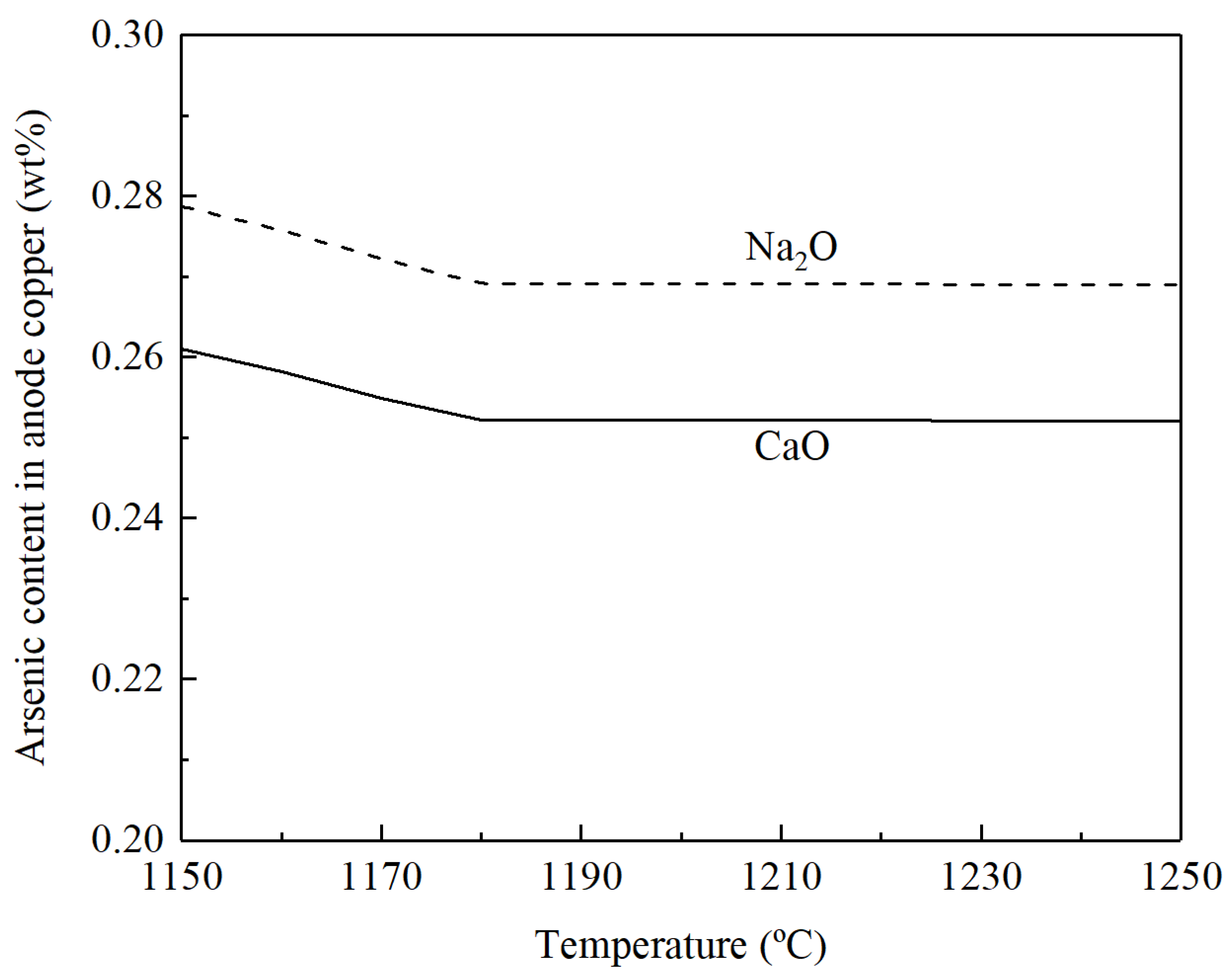
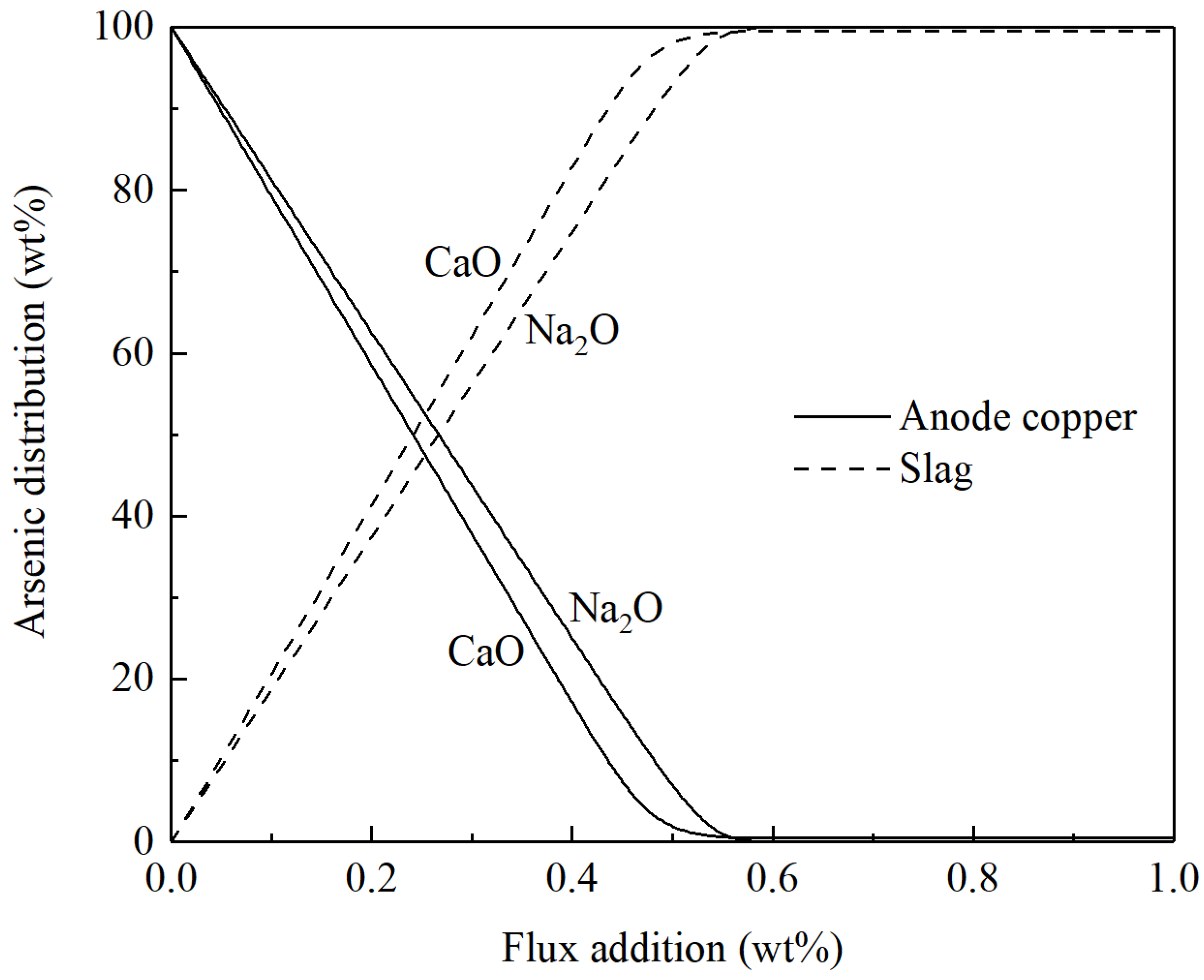
3.2. Fire refining of Blister Copper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlesinger, M.; Sole, K.; Davenport, W.; Alvear, G. Chapter 1—Overview. In Extractive Metallurgy of Copper, 6th ed.; Elsevier: Oxford, UK, 2021; pp. 1–18. ISBN 9780128218754. [Google Scholar]
- Zhang, H.; Wang, Y.; He, Y.; Xu, S.; Hu, B.; Cao, H. Efficient and safe disposition of arsenic by incorporation in smelting slag through copper flash smelting process. Miner. Eng. 2021, 160, 106661. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater. 2021, 401, 123293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liao, J. Development of Bottom-blowing copper smelting technology: A review. Metals 2022, 12, 190. [Google Scholar] [CrossRef]
- Gao, D.; Peng, X.; Song, Y.; Zhu, Z.; Dai, Y. Mathematical modeling and numerical optimization of particle heating process in copper flash furnace. Trans. Nonferrous Met. Soc. China 2021, 31, 1506–1517. [Google Scholar] [CrossRef]
- Kucharski, M. Arsenic removal from blister copper by soda injection into melts. Scand. J. Metall. 2002, 31, 246–250. [Google Scholar] [CrossRef]
- Schlesinger, M.; Sole, K.; Davenport, W.; Alvear, G. Chapter 12—Fire refining (S and O removal) and anode casting. In Extractive Metallurgy of Copper, 6th ed.; Elsevier: Oxford, UK, 2021; pp. 313–329. ISBN 9780128218754. [Google Scholar]
- Long, G.; Peng, Y.; Bradshaw, D. A review of copper–arsenic mineral removal from copper concentrates. Miner. Eng. 2012, 36, 179–186. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C.; Mian, M.; Cheema, A. Strategies for arsenic pollution control from copper pyrometallurgy based on the study of arsenic sources, emission pathways and speciation characterization in copper flash smelting systems. Environ. Pollut. 2021, 270, 116203. [Google Scholar] [CrossRef]
- Nagamori, M.; Mackey, P.; Tarassoff, P. Copper solubility in FeO- Fe2O3- SiO2- Al2O3 slag and distribution equilibria of Pb, Bi, Sb and As between slag and metallic copper. Metall. Mater. Trans. B 1975, 6, 295–301. [Google Scholar] [CrossRef]
- Flores, G.; Risopatron, C.; Pease, J. Processing of complex materials in the copper industry: Challenges and opportunities ahead. JOM 2020, 72, 3447–3461. [Google Scholar] [CrossRef]
- Yang, W.; Qian, L.; Jin, B.; Feng, Q.; Li, L.; He, K.; Yang, J. Leaching behaviors of copper and arsenic from high-arsenic copper sulfide concentrates by oxygen-rich sulfuric acid leaching at atmospheric pressure. J. Environ. Chem. Eng. 2022, 10, 107358. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Tian, Q.; Chen, M.; Zhao, B. Reaction mechanism and distribution behavior of arsenic in the bottom blown Copper smelting process. Metals 2017, 7, 302. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, Y.; Ke, L.; Chen, W. Separation and recovery of Cu and As from copper electrolyte through electrowinning and SO2 reduction. Trans. Nonferrous Met. Soc. China 2013, 23, 2166–2173. [Google Scholar] [CrossRef]
- Swinbourne, D.; Kho, T. Computational thermodynamics modeling of minor element distributions during copper flash converting. Metall. Mater. Trans. B 2012, 43, 823–829. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Zhang, W.; Li, H.; Tong, C. Thermodynamics analysis of distribution behavior of impurity elements during copper flash converting. Trans. Nonferrous Met. Soc. 2017, 27, 1952–1958. (In Chinese) [Google Scholar]
- Li, M.; Zhou, J.; Zhang, W.; Li, H.; Tong, C. Multiphase equilibrium thermodynamics analysis of copper flash converting process. Trans. Nonferrous Met. Soc. 2017, 27, 1494–1503. (In Chinese) [Google Scholar]
- Yuan, Y.; Liu, S. Distribution and removal of arsenic in the process of bottom-blowing continuous copper smelting. China Nonferrous Metall. 2020, 2, 37–40. (In Chinese) [Google Scholar]
- Liu, S. Operation practice of copper matte bottom blowing continuous converting. Nonferrous Met. Extr. Metall. 2016, 12, 17–19. (In Chinese) [Google Scholar]
- Guo, X.; Chen, Y.; Wang, Q.; Wang, S.; Tian, Q. Copper and arsenic substance flow analysis of pyrometallurgical process for copper production. Trans. Nonferrous Met. Soc. China 2022, 32, 364–376. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Li, R.; Wu, F. Distribution of impurity elements in copper pyrometallurgical process and its influence on the recovery. China Nonferrous Metall 2019, 4, 9–16. (In Chinese) [Google Scholar]
- Hidayat, T.; Chen, J.; Hayes, P.C.; Jak, E. Distributions of As, Pb, Sn and Zn as minor elements between iron silicate slag and copper in equilibrium with tridymite in the Cu-Fe-O-Si system. Int. J. Mater. Res. 2021, 112, 178–188. [Google Scholar] [CrossRef]
- Kaur, R.; Swinbourne, D.; Nexhip, C. Nickel, lead and antimony distributions between ferrous calcium silicate slag and copper at 1300 °C. Miner. Process. Extr. Metall. Rev. 2009, 118, 65–72. [Google Scholar] [CrossRef]
- Nagamori, M.; Mackey, P. Distribution equilibria of Sn, Se and Te between FeO-Fe2O3-SiO2-Al2O3-CuO0.5 slag and metallic copper. Metall Mater Trans. B. 1977, 8, 39–46. [Google Scholar] [CrossRef]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Recoveries of rare elements Ga, Ge, In and Sn from waste electric and electronic equipment through secondary copper smelting. Waste Manag. 2018, 71, 400–410. [Google Scholar] [CrossRef]
- Bale, C.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.; Kang, Y.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Liao, C.; Xie, S.; Zhao, B.; Cai, B.; Wang, L. Thermodynamic and experimental analyses of the carbothermic reduction of tungsten slag. JOM 2021, 73, 1853–1860. [Google Scholar] [CrossRef]
- Yu, W.; Tang, Q.; Chen, J.; Sun, T. Thermodynamic analysis of the carbothermic reduction of a high-phosphorus oolitic iron ore by FactSage. J. Miner. Met. Mater. 2016, 23, 1126–1132. [Google Scholar] [CrossRef]
- Nezhad, M.; Zabett, A. Thermodynamic analysis of the carbothermic reduction of electric arc furnace dust in the presence of ferrosilicon. Calphad 2016, 52, 143–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Huang, L.; Xu, Z. Trend and recovery of arsenic, antimony and bismuth in copper smelting. Nonferrous Met. Sci. Eng. 2019, 10, 13–19. (In Chinese) [Google Scholar]
- Wang, S. Arsenic Distribution Surveying in Copper Smelting Process of Jinlong Copper Co., Ltd. Nonferrous Met. Eng. 2016, 6, 83–86. (In Chinese) [Google Scholar]
- Wang, Q.; Zhong, S.; Guo, X.; Chen, J. Arsenic challenge and control in zijin copper smelter. In Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th International Copper Conference 2019, Vancouver, BC, Canada, 18–21 August 2019; p. 16. [Google Scholar]
- Schlesinger, M.; Sole, K.; Davenport, W.; Alvear, G. Chapter 8—Converting of copper matt. In Extractive Metallurgy of Copper, 6th ed.; Elsevier: Oxford, UK, 2021; pp. 187–229. ISBN 9780128218754. [Google Scholar]
- Yang, X.; Han, Z.; Guo, Y.; Xu, M. Study on lead impurity movement and removal in copper flash converting. China Nonferrous Metall. 2018, 6, 9–11. (In Chinese) [Google Scholar]




| Authors | Technology | Distribution (wt%) | ||
|---|---|---|---|---|
| Blister Copper | Slag | Gas | ||
| Li [16] | Flash | 52 | 42 | 6 |
| Swinbourne [15] | Flash | 60 | 37 | 3 |
| Liu [19] | Bottom blowing | 65.7 | 31.4 | 2.9 |
| Guo [20] | Peirce-smith | 12.9 | 9.4 | 77.7 |
| Zhou [21] | Peirce-smith | 39.0 | 17.0 | 44.0 |
| Zhang [30] | Peirce-smith | 28.0 | 13.0 | 58.0 |
| Wang [31] | Peirce-smith | 66.4 | 5.4 | 28.2 |
| Wang [32] | Peirce-smith | 59.9 | 5.1 | 34.9 |
| Vogt J [33] | Peirce-smith | 50.0 | 32.0 | 18.0 |
| Cu | Fe | S | As | O | Ca | Al2O3 | CaO | MgO | |
|---|---|---|---|---|---|---|---|---|---|
| Concentrate | 29.50 | 32.60 | 31.80 | 0.30 | 0.00 | 0.00 | 2.60 | 2.30 | 0.90 |
| Matte | 50.01 | 23.70 | 24.45 | 0.37 | 1.18 | 0.28 | 0.00 | 0.00 | 0.00 |
| Blister copper | 98.50 | 0.00 | 0.73 | 0.43 | 0.34 | 0.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Weng, T.; Tan, K.; Liao, J.; Zhao, B.; Xie, S. Distribution and Control of Arsenic during Copper Converting and Refining. Metals 2023, 13, 85. https://doi.org/10.3390/met13010085
Xu F, Weng T, Tan K, Liao J, Zhao B, Xie S. Distribution and Control of Arsenic during Copper Converting and Refining. Metals. 2023; 13(1):85. https://doi.org/10.3390/met13010085
Chicago/Turabian StyleXu, Feiyan, Tao Weng, Keqin Tan, Jinfa Liao, Baojun Zhao, and Sui Xie. 2023. "Distribution and Control of Arsenic during Copper Converting and Refining" Metals 13, no. 1: 85. https://doi.org/10.3390/met13010085
APA StyleXu, F., Weng, T., Tan, K., Liao, J., Zhao, B., & Xie, S. (2023). Distribution and Control of Arsenic during Copper Converting and Refining. Metals, 13(1), 85. https://doi.org/10.3390/met13010085








