Abstract
To determine slag properties and the factors influencing these properties for optimization of operating conditions in the copper flash smelting process, the composition and microstructures of the quenched smelting and converting slags have been analyzed. Thermodynamic software FactSage 8.2 has been used to investigate the effects of matte grade, SO2 partial pressure, and the Fe/SiO2 ratio on the liquidus temperature and the copper content of the smelting slag. The possibility to recover valuable metals from the smelting and converting slags through pyrometallurgical reduction by carbon is also discussed. It was found that the flash smelting slag temperature is usually higher than its liquidus temperature and the copper (1.2% Cu) is mainly present in the slag as dissolved copper. In the copper flash smelting process, the copper content in the slag can be decreased by decreasing the Fe/SiO2 ratio and temperature. In pyrometallurgical slag reduction, most Cu, Mo, and Ni can be recovered as an alloy. The conditions of recovery such as the ratio of smelting slag to converting slag, temperature, and reduction extent have been discussed.
1. Introduction
A total of 80% of copper is extracted from concentrates worldwide by the pyrometallurgical process [1,2]. Flash smelting with the characteristics of copper concentrates directly reacted with oxygen is widely used in the copper matte smelting process as a result of the advantages of high productivity, high automation, and low investment costs [3]. Flash smelting accounts for 43% of all Cu matte smelting [4]. The smelting process is developing toward a high feeding rate, high matte grade, and high oxygen enrichment to increase productivity and SO2 concentration in the off-gas. However, it is confronted with the challenge of the control of copper content in slag and matte grade [5]. Matte and smelting slag are separated normally in the settler of flash smelting furnaces [6]. If the smelting temperature of the slag is lower than its liquidus temperature, spinel is precipitated from the slag, which increases the viscosity of the slag and restrains the separation of matte and slag [7]. In addition, long-term solid precipitation in the settler decreases its effective volume and separation of matte from the slag [8]. The smelting temperature is generally higher than the liquidus temperature of the slag to avoid the formation of spinel. The liquidus temperature of the slag is related to the composition, matte grade, and SO2 partial pressure [9,10]. Copper mainly exists in slag as dissolved copper if the smelting temperature is higher than the liquidus temperature [11]. The composition of slag, temperature, matte grade, O2, and SO2 partial pressures affect the content of dissolved copper in slag [12]. Chemical analysis of smelting and converting slags indicated that they generally contain valuable metals such as copper, molybdenum, zinc, nickel, and tin [13]. Pyrometallurgical technology can be used to recover copper from smelting and converting slags in general [14]. Deep reduction to recover copper and iron from copper slags was also studied where coal, coke, diesel, hydrogen, and natural gas can be used as the reductants [15]. The reduction of the slag using hydrogen can reduce CO2 emission and carbon in the Cu-Fe alloy [16]. Nonetheless, the efficiency of the hydrogen utilization is lower and the cost is higher than the solid reductants. However, other valuable metals such as Mo, Ni, and Zn in slags remain unclear during the recovery process. FactSage thermodynamic software contains powerful databases of optimized solutions, pure substances, and matte, and has been successfully used in the processing of copper concentrates [17], as well as the recovery of valuable metals from solid wastes [18].
This study aims to understand the effects of matte grade, SO2 partial pressure, and Fe/SiO2 on the liquidus temperature and copper content in smelting slag and explore the likelihood of recovering copper, molybdenum, zinc, nickel, and tin from smelting and converting slags.
2. Research Methodology
2.1. Materials
The smelting and converting slags were provided by Jiangxi Copper Smelter where a flash smelting furnace and PS converter are used for smelting and converting, respectively. The slags were collected from slag tap holes using a cooled iron bar, which maintained the microstructures and compositions of the slags at high-temperature conditions. The slag samples were mounted in epoxy resin, polished, and examined using optical and scanning electron microscopy. An MLA 650L scanning electron microscope (SEM-EDS), FEI, Hillsboro, OR, USA, was employed to determine the microstructures of the slags. The bulk compositions of the smelting and converting slags were measured by XRF (Axios max, produced by PANalytical B.V, Almelo, The Netherlands).
2.2. Thermodynamic Predictions
The thermodynamic software, FactSage 8.2, which integrates a substantially optimized oxide, pure substance, and matte database, is widely used in pyrometallurgical processing [19,20]. The databases selected included “FactPs”, “Ftmisc”, and “Ftoxide”. The solution phases selected in the smelting of copper concentrate included “FTmisc-MATT”, “FToxid-SLAGA”, FToxid-SPINC”, FToxid-MeO”, “FToxid-OlivA”, and “FToxid-ZNIT”. The solution phases selected in pyrometallurgical reduction included “FTmisc-FeLQ”, “FTmisc-CuLQ”, “FTmisc-FeCu”, “FTmisc-MATT”, “FToxid-SLAGA”, “FToxid-SPINC”, FToxid-MeO”, “FToxid-cPyrA”, “FToxid-WOLLA”, “FToxid-Bred”, “FToxid-bC2SA”, “FToxid-AC2S”, “FToxid-Mel_A”, “FToxid-OlivA”, “FToxid-Mull”, “FToxid-CORU”, and “FToxid-ZNIT”. Effects of matte grade, SO2 partial pressure, and Fe/SiO2 on liquidus temperature and copper content of slag have been systematically calculated. In addition, the effects of carbon addition on the liquidus temperature of slag and the reduction level of valuable metals in pyrometallurgical reduction have also been calculated.
3. Results and Discussion
3.1. Characterization of Slags
The composition of the smelting and converting slags analyzed by XRF are shown in Table 1. As can be seen from Table 1, Fe/SiO2 values in the smelting and converting slags are 1.3 and 2.0, respectively. The content of copper in the smelting and converting slags is 1.3% and 5.2% Cu2O, respectively. Moreover, the smelting slag contains 0.4% MoO3 and 1.5% ZnO, and the converting slag contains 0.3% NiO, 0.2% SnO, and 2.2% ZnO. Mo, Ni, Sn, and Zn in the slags are valuable elements that should be recovered.

Table 1.
Composition of quenched smelting and converting slags (wt.%).
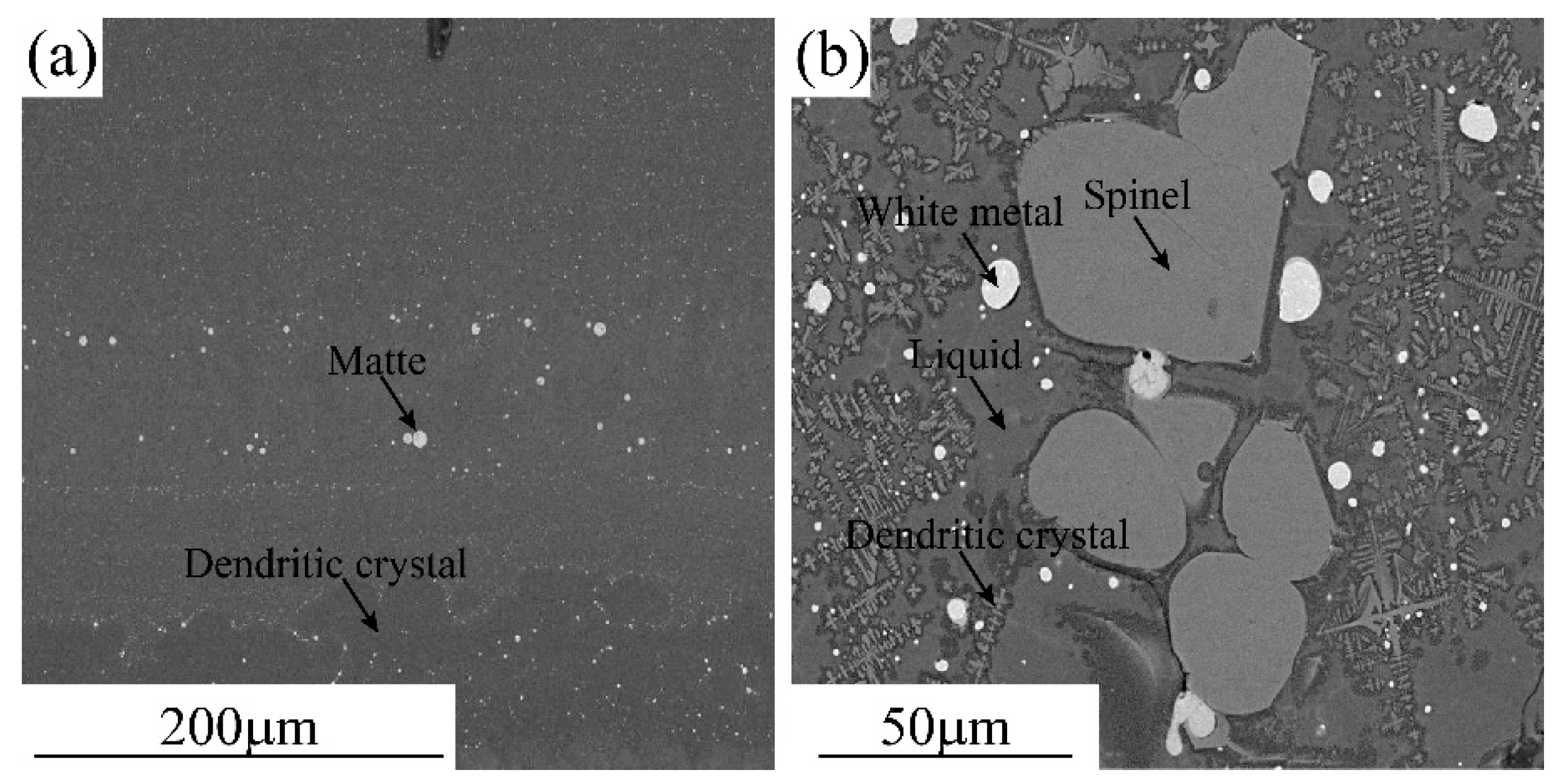
Typical microstructures of the quenched smelting and converting slags are shown in Figure 1. As can be seen from Figure 1a, the slag does contain large solid and matte phases indicating that the smelting temperature was higher than the liquidus temperature of the slag and the separation between the slag and the matte was efficient. The fine matte and dendritic crystals were precipitated from the slag during cooling. The microstructure of the quenched smelting slag confirms that all copper present in the slag was chemically dissolved. The converting slag shown in Figure 1b contains large spinel and matte indicating that the converting temperature (1250 °C) was lower than the liquidus temperature of the slag (1420 °C). The presence of an excessive solid phase in the slag increased the viscosity and affected the separation of the matte droplets from the slag. The copper content in the converting slag (5.2% Cu2O) includes chemically dissolved and entrained matte droplets.
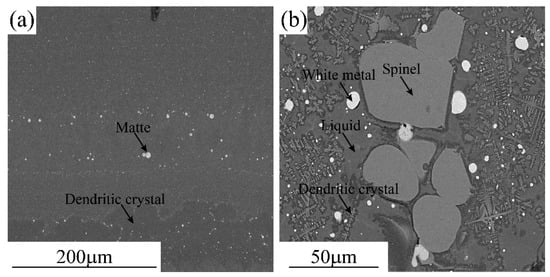
Figure 1.
Typical microstructures of the quenched smelting and converting slags: (a) Smelting slag and (b) Converting slag.
3.2. Control of Liquidus Temperature of Flash Smelting Slag
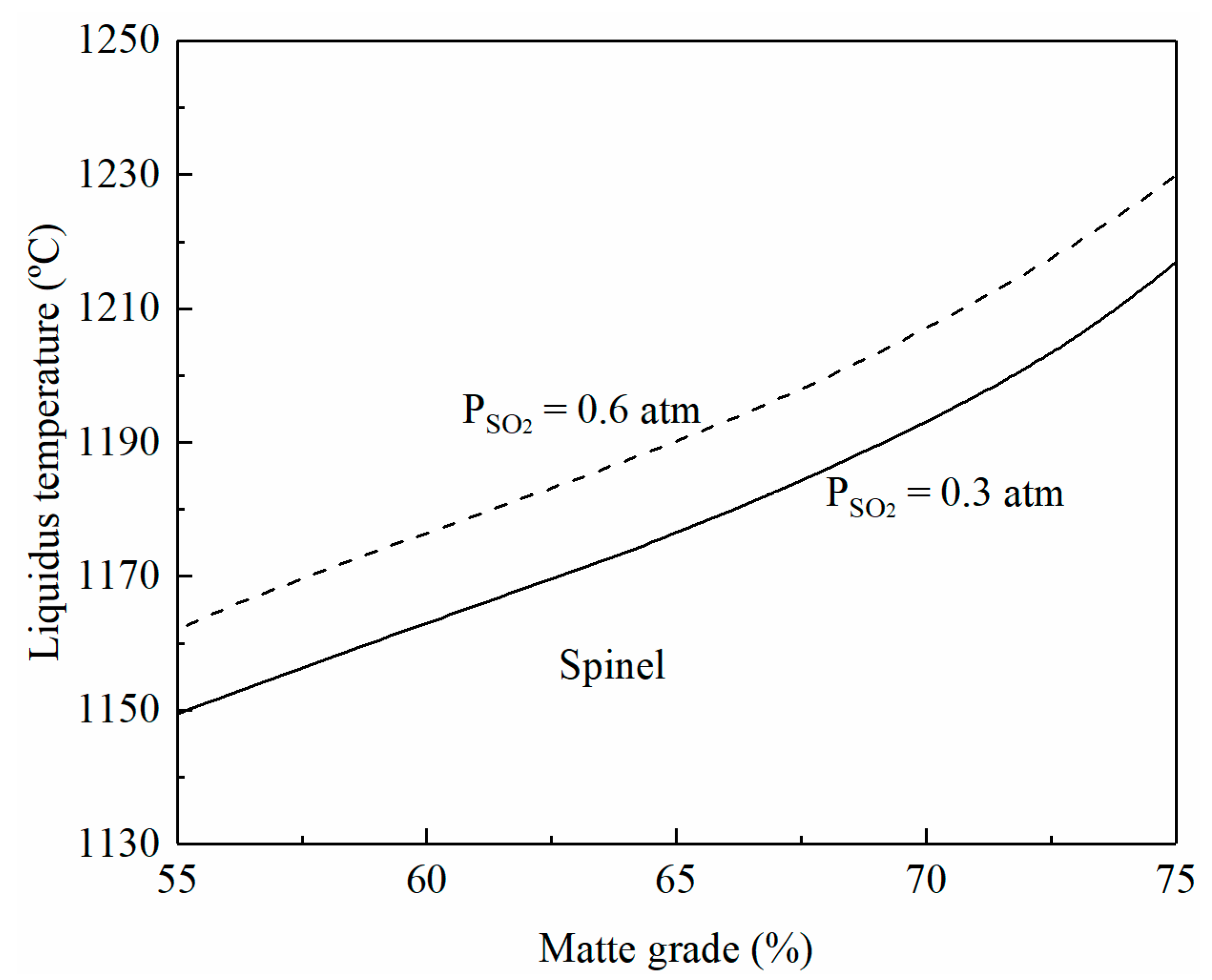
The liquidus temperature of a smelting slag is directly related to its composition and oxygen partial pressure, which determines the FeO/Fe2O3 ratio in the slag [21]. The oxygen partial pressure in the system cannot be measured directly but correlates to the matte grade, SO2 partial pressure, and temperature [22,23]. The matte grade in the smelting process can be controlled by the composition of copper concentrate and the ratio of oxygen to the concentrate. The SO2 partial pressure in the off-gas is determined by the oxygen enrichment in the input gas mixture. Figure 2 shows the effect of matte grade and SO2 partial pressure on the liquidus temperature of the smelting slag whose composition is based on Table 1. FactSage calculations show that the composition of the smelting slag shown in Figure 2 is in the spinel primary phase field. It can be seen that the liquidus temperatures of the smelting slag increase with the increasing matte grade and SO2 partial pressure. At low-oxygen enrichment, PSO2 is approximately 0.3 atm. It can be seen from Figure 2 that the liquidus temperature increases from 1150 to 1215 °C when the matte grade is increased from 55 to 75. On the other hand, the liquidus temperature can be increased by approximately 12 °C when PSO2 is increased from 0.3 to 0.6 atm at a fixed matte grade.
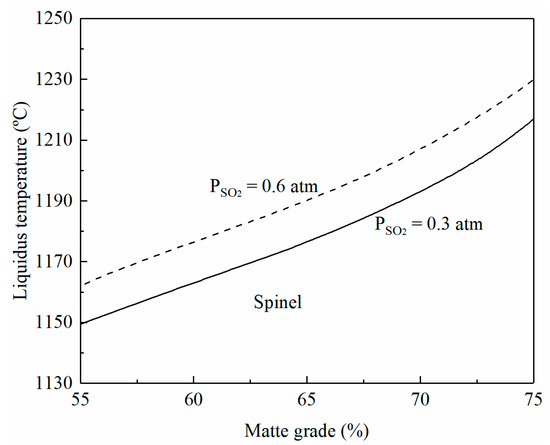
Figure 2.
Effects of matte grade and SO2 partial pressure on liquidus temperature of smelting slag, predicted by FactSage 8.2.
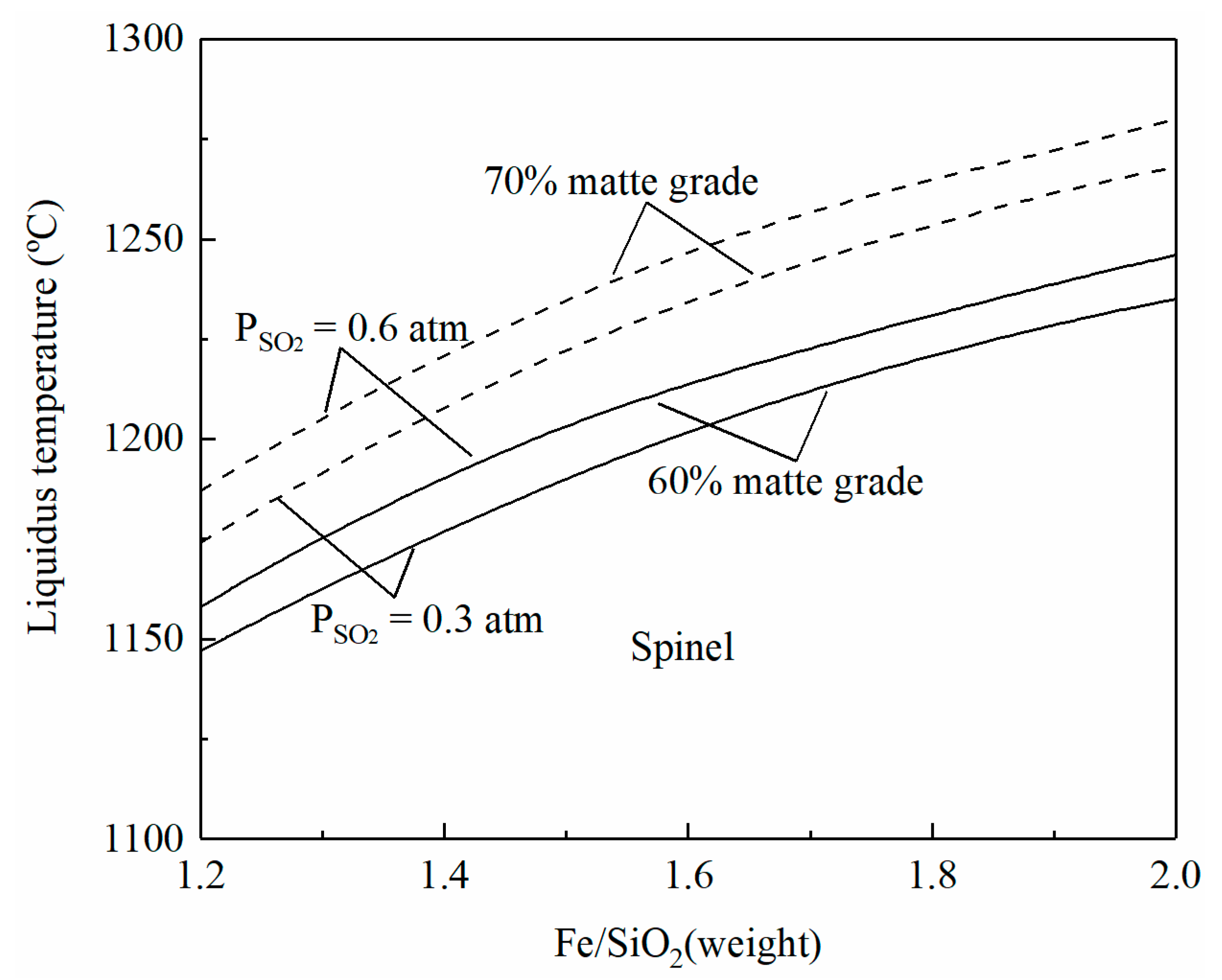
SiO2 is usually added as a flux in the copper smelting process, and the Fe/SiO2 ratio can represent the composition of a copper smelting slag. It can be seen from Figure 3 that at fixed matte grade and PSO2, the liquidus temperatures of a copper smelting slag increase with an increasing Fe/SiO2 ratio, i.e., with a decreasing SiO2 addition. At a fixed operating temperature and production of high-grade matte or high-SO2 off-gas, more SiO2 flux needs to be added to keep the liquidus temperature below the operating temperature. The slope of the liquidus temperature is decreased with the increase in Fe/SiO2. For example, the temperature increase is 30 °C when the Fe/SiO2 ratio increases from 1.3 to 1.5. When the Fe/SiO2 ratio is increased from 1.5 to 1.7, the increment of the liquidus temperature is only 20 °C at SO2 partial pressure 0.6 atm and matte grade 70%.
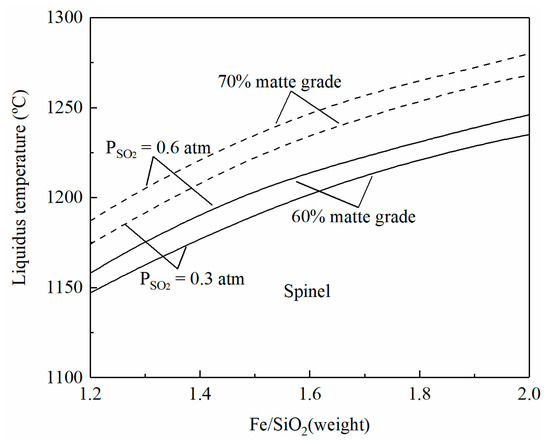
Figure 3.
Effects of the Fe/SiO2 ratio on liquidus temperature of smelting slag, predicted by FactSage 8.2.
In summary, low matte grade, low SO2 partial pressure, and high SiO2 addition can decrease the liquidus temperature of the copper smelting slag. The modern smelting process tends to increase productivity by producing high-grade matte with high-oxygen enrichment. The intensified smelting can treat more copper concentrate and reduce the volume of the off-gas capacity for easy production of sulfuric acid. Consequently, increased SiO2 addition seems to be the efficient direction to control the liquidus temperature of the smelting slag.
3.3. Control of Copper Content in Copper Smelting Slag
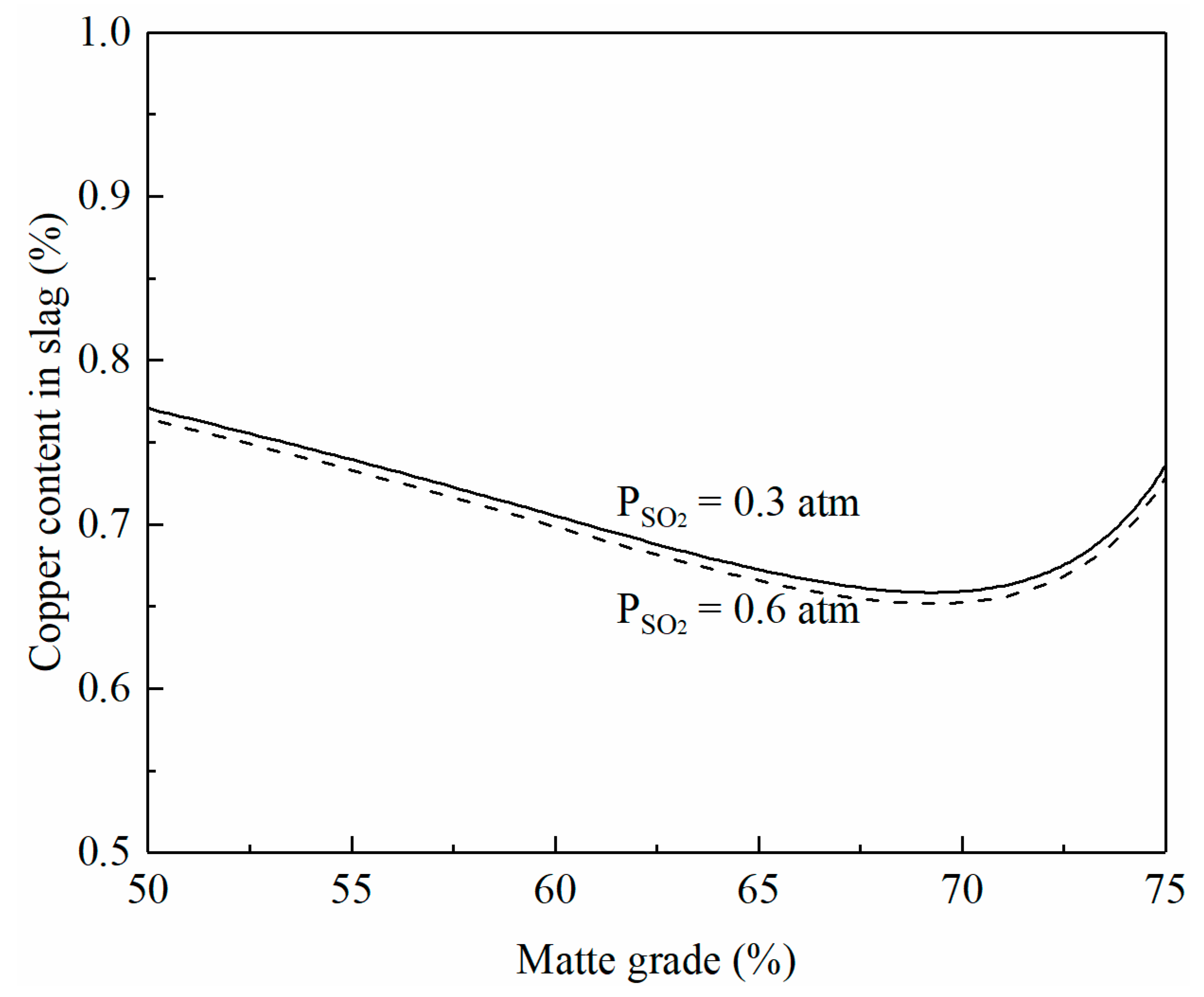
The microstructure of the quenched flash smelting slag shown in Figure 1a indicates that the copper in the smelting slag is present as dissolved copper. The effects of matte grade on copper content in the smelting slag are predicted by FactSage 8.2 and shown in Figure 4. The copper content in smelting slag decreases with the increasing matte grade up to 70% Cu. With a matte grade greater than 70%, the copper content in the slag increases with the increasing matte grade. This trend is confirmed by the experimental studies of Fallah-Mehrjardi et al. [24,25] but is different from the findings reported by Shimpo et al. [26]. There is a general understanding [27] that the sulfidic dissolution of copper in copper smelting decreases with the increasing matte grade. However, the oxidic dissolution of copper in the slag increases with the increasing matte grade. As a result, the copper content in the smelting slag is not a simple function of the matte grade. A total of 70% Cu in the matte seems to be the optimum grade to reduce the copper loss in the slag to 0.65%. It can be seen from the figure that SO2 partial pressure has little effect on the copper content in the slag. This is a good indication that increased oxygen enrichment does not increase the copper content in the smelting slag.
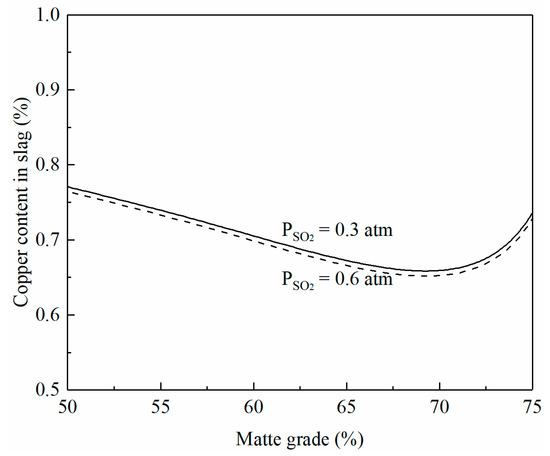
Figure 4.
Effects of matte grade and SO2 partial pressure on copper content in smelting slag at 1250 °C and Fe/SiO2 1.3, predicted by FactSage 8.2.
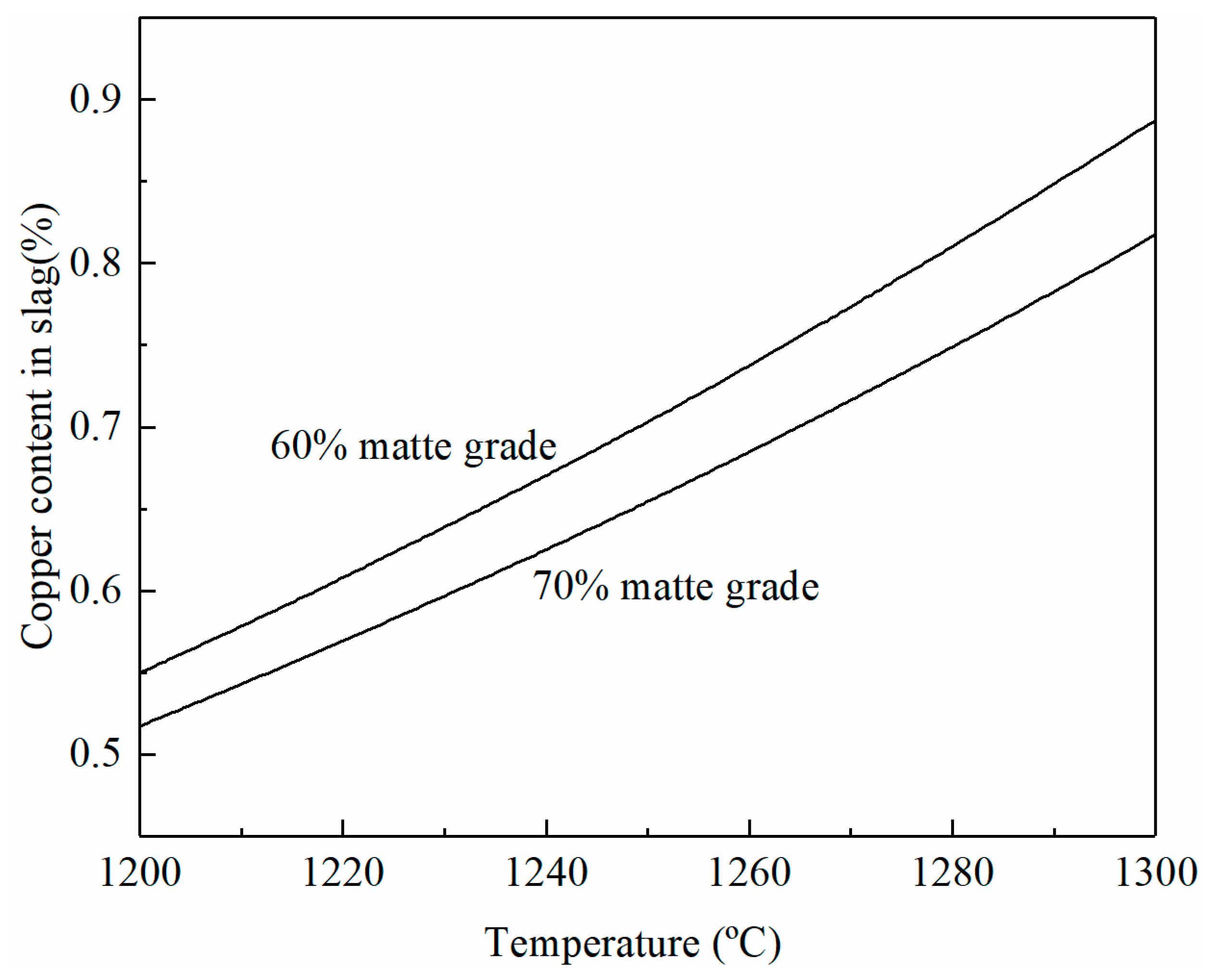
The effects of temperature and matte grade on copper content in the smelting slag are shown in Figure 5 at a fixed SO2 partial pressure of 0.3 atm and a Fe/SiO2 ratio of 1.3. It can be seen that the copper content in the smelting slag increases rapidly with the increasing temperature. Nearly 0.3% Cu is increased in the slag if the temperature is increased from 1200 to 1300 °C. This is a significant copper loss as the volume of the smelting slag is very high. An increase in the matte grade from 60 to 70% can decrease the copper solubility in the slag.
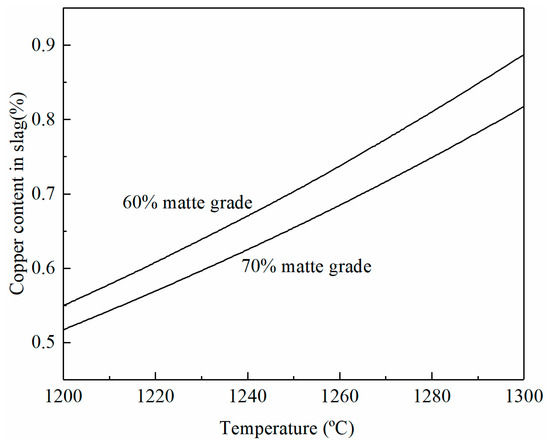
Figure 5.
Effects of temperature and matte grade on copper content in smelting slag at a fixed SO2 partial pressure of 0.3 atm and a Fe/SiO2 ratio of 1.3, predicted by FactSage 8.2.
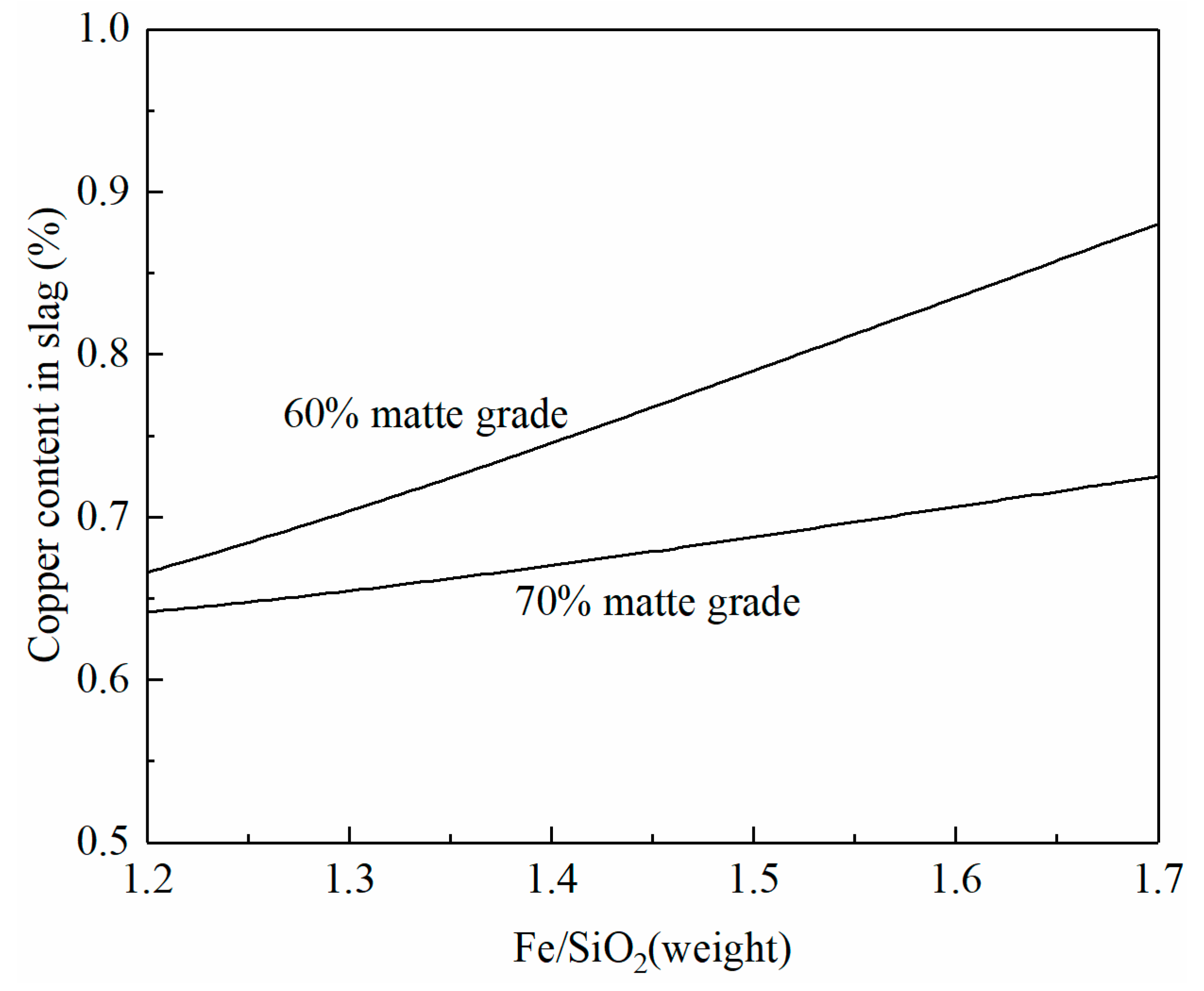
Figure 6 shows the copper content in the smelting slag as a function of the Fe/SiO2 ratio in the slag. It can be seen that copper content in the smelting slag increases with the increasing Fe/SiO2 ratio in the slag. However, the increment is different at different matte grades. When 70% matte is produced, the copper content in the slag increases slowly with the increasing Fe/SiO2 ratio in the slag. An increase in the Fe/SiO2 ratio from 1.2 to 1.7 only increases the copper content in slag by 0.05%. In contrast, an increase in the Fe/SiO2 ratio from 1.2 to 1.7 can increase the copper content in slag by 0.2% with 60% matte production. The increase in Fe/SiO2 means a decrease in SiO2 addition and slag volume. Copper loss in the slag needs to consider both Cu% in the slag and slag volume. The selection of the Fe/SiO2 ratio in the copper smelting slag needs to balance liquidus temperature, copper content in slag, and slag volume.
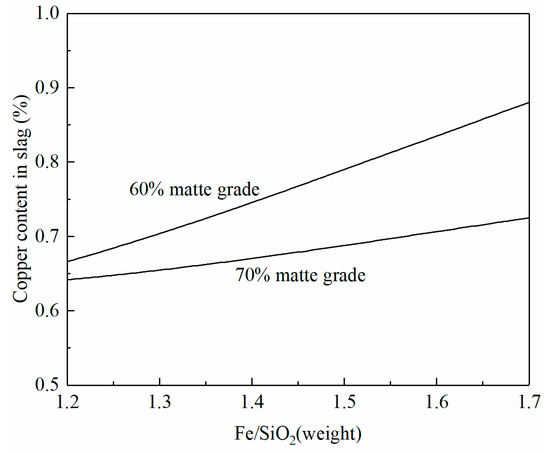
Figure 6.
Effect of the Fe/SiO2 ratio on copper content in smelting slag at 1250 °C and a fixed SO2 partial pressure of 0.3 atm, calculated by FactSage 8.2.
Copper in the smelting slag can be recovered by a pyrometallurgical process or flotation process. The flotation process can only recover matte and metal particles. Fine matte and copper metal may precipitate from the smelting slag on slow cooling. However, the slag needs to be ground very finely to liberate the copper matte and metal from hard oxides, which increases the cost of copper recovery. Therefore, the selection of an optimum condition including an optimized matte grade, low temperature, and optimized Fe/SiO2 ratio can reduce the copper loss in the flash smelting slag.
3.4. Recovery of Valuable Metals from Smelting and Converting Slags
It can be seen from Table 1 that the smelting and converting slags provided by the smelter contain significant amounts of valuable elements such as copper, molybdenum, zinc, nickel, and tin. Copper can be effectively recovered by the flotation process. However, it is unclear whether other elements can be recovered in the flotation process. The possibility of recovering all valuable elements in the pyrometallurgical process is evaluated by the thermodynamic calculations of FactSage 8.2. Smelting and converting slags can be treated individually or together depending on the economic evaluation. The composition values given in Table 1 are not sufficient for the calculation of reduction reactions. According to the smelting temperature and matte grade provided by the smelter, the oxygen partial pressure and Fe2+/Fe3+ ratio in the smelting slag were calculated by FactSage 8.2 and are shown in Table 2. Similarly, the oxygen partial pressure and Fe2+/Fe3+ ratio in the converting slag were also calculated by assuming the slag was in equilibrium with Cu2S. Using the information given in Table 1 and Table 2, the reduction of the smelting and converting slags by carbon were predicted by FactSage 8.2 and discussed below.

Table 2.
Fe2+/Fe3+ ratio in the smelting and converting slags calculated by FactSage 8.2.
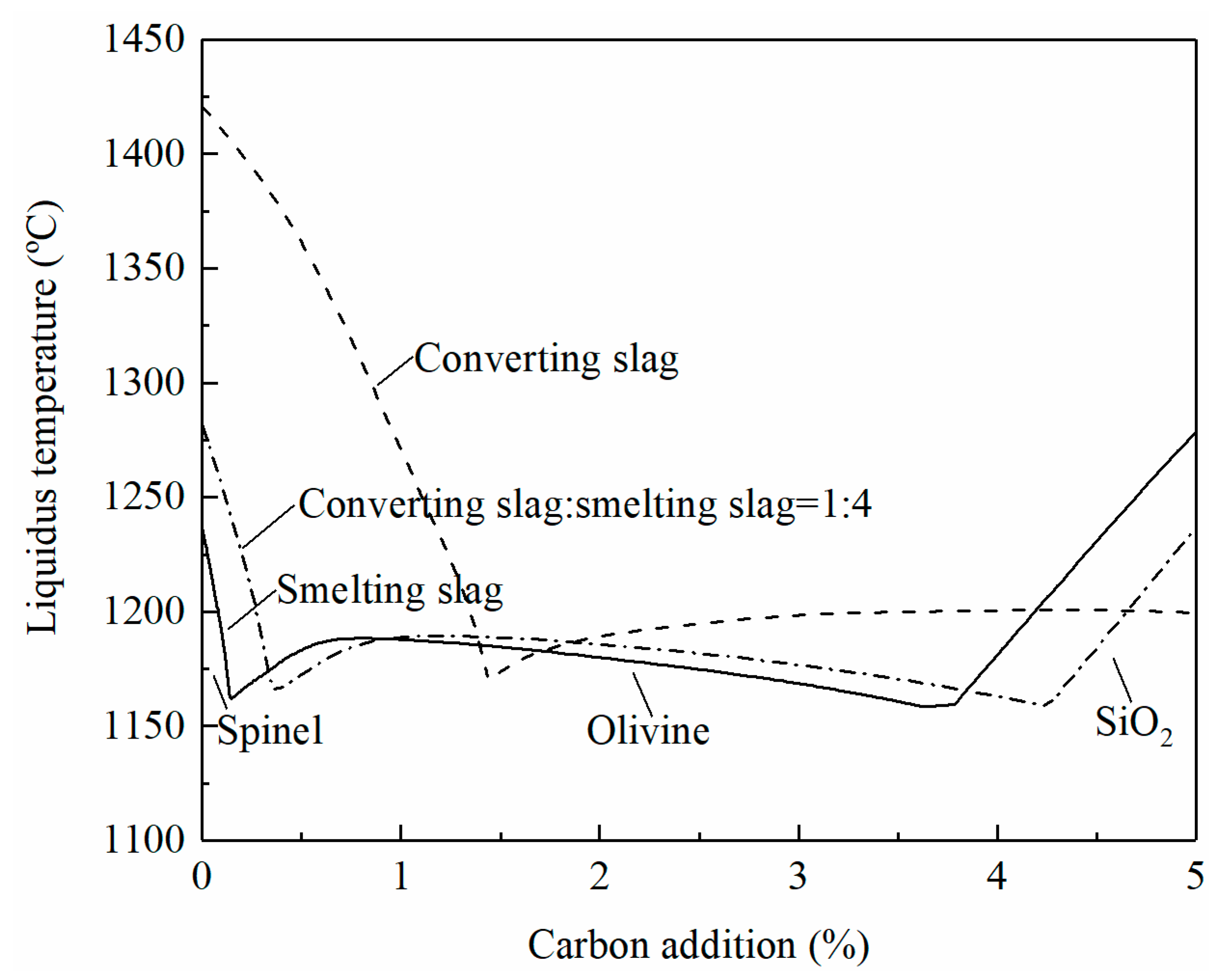
At high temperature reduction, the oxides of copper, molybdenum, zinc, nickel, iron, and tin in the smelting and converting slags can be reduced with carbon addition. The liquidus temperatures of the slag will change accordingly with the reduction. The effect of carbon addition on liquidus temperatures when treating individual slag or mixed slags (80% smelting slag and 20% converting slag) is shown in Figure 7. It can be seen that spinel is the primary phase at low carbon addition and the liquidus temperatures decrease with increasing the carbon percentage. More carbon addition brings the slags to the olivine primary phase field where the liquidus temperatures are lower than 1200 °C. The smelting slag enters into the SiO2 primary phase field with further addition of carbon. The liquidus temperatures rapidly increase with the increasing carbon in the SiO2 primary phase field. In a copper smelter, the ratio of the smelting slag to converting slag is approximately 4:1. It can be seen from Figure 7 that treatment of the mixed smelting and converting slags is possible. The liquidus temperatures can be controlled below 1200 °C without flux addition.
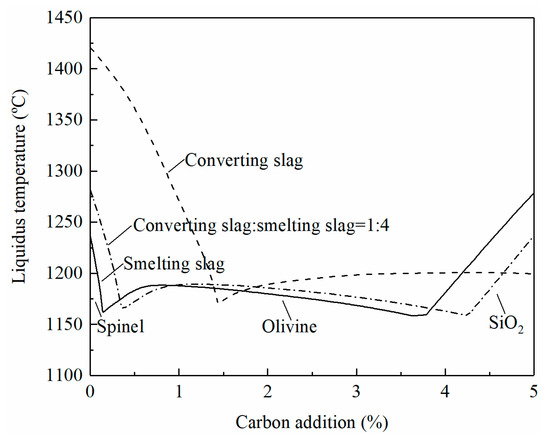
Figure 7.
Effect of carbon addition on the liquidus temperature of slag, calculated by FactSage 8.2.
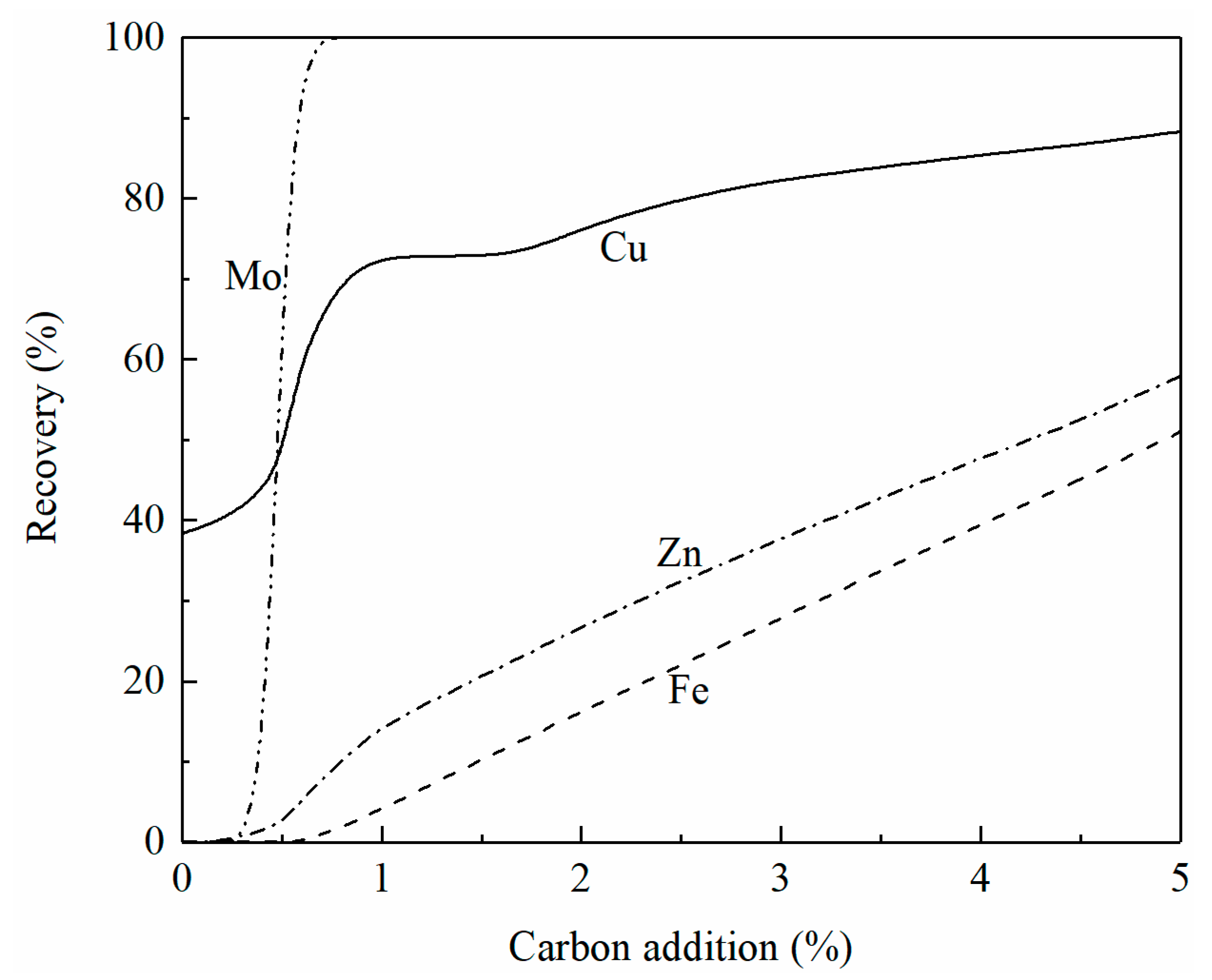
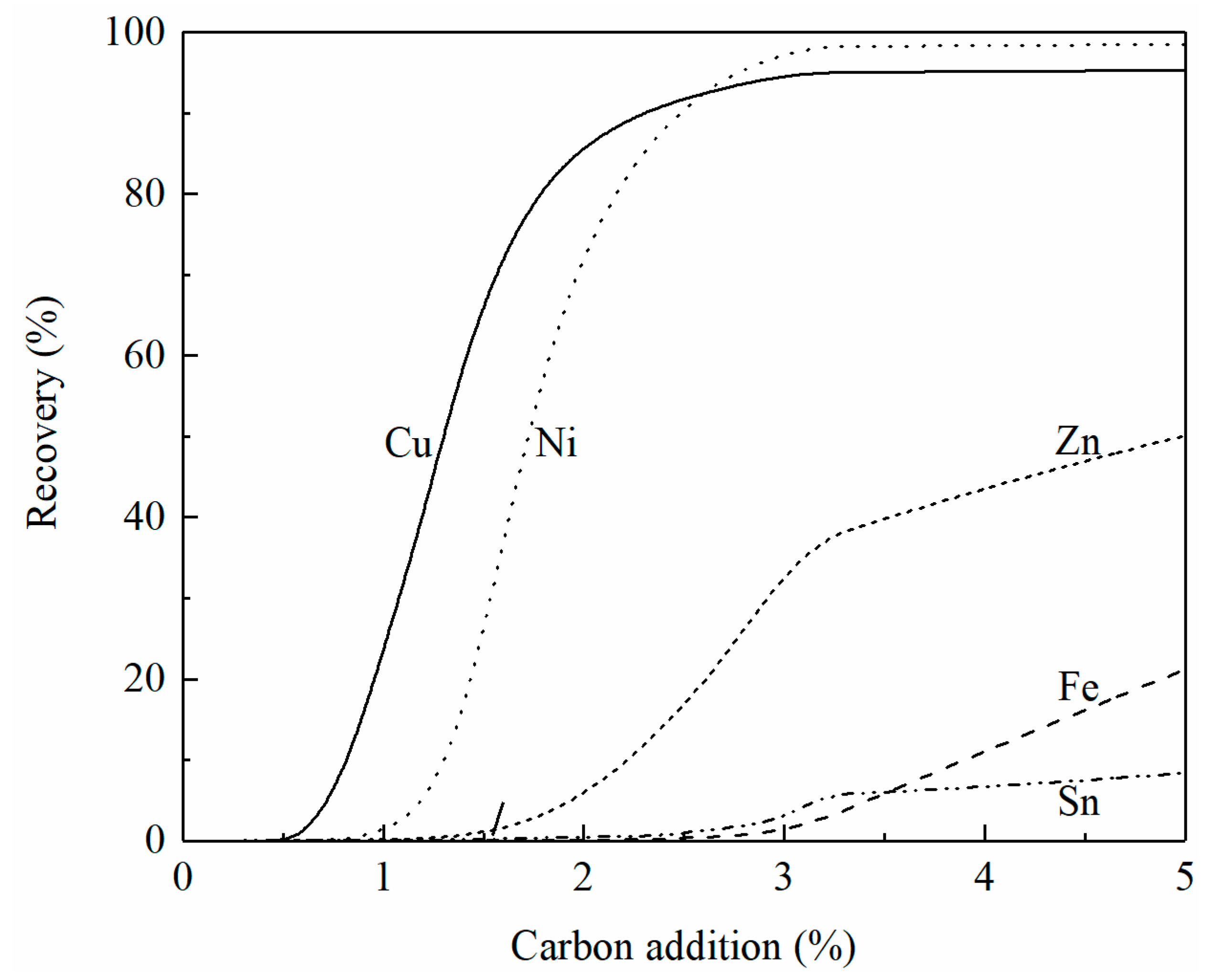
The effect of carbon addition on the recovery of copper, iron, zinc, and molybdenum from the smelting slag at 1250 °C is shown in Figure 8. It can be seen that copper is first reduced followed by molybdenum. With less than 1% carbon addition, 100% molybdenum oxide has been reduced. When 5% carbon is added, the recovery of copper, zinc, and iron is 88%, 58%, and 51%, respectively. It can be seen from Figure 7 that the liquidus temperature is above 1250 °C when 5% carbon is added to the smelting slag. To add more carbon to increase the recovery of copper and zinc, flux such as CaO needs to be added to lower the liquidus temperature of the slag.
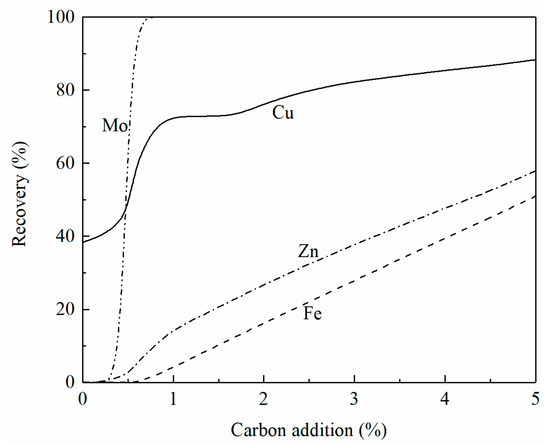
Figure 8.
Effect of carbon addition on the recovery of copper, iron, zinc, and molybdenum from smelting slag at 1250 °C, calculated by FactSage 8.2.
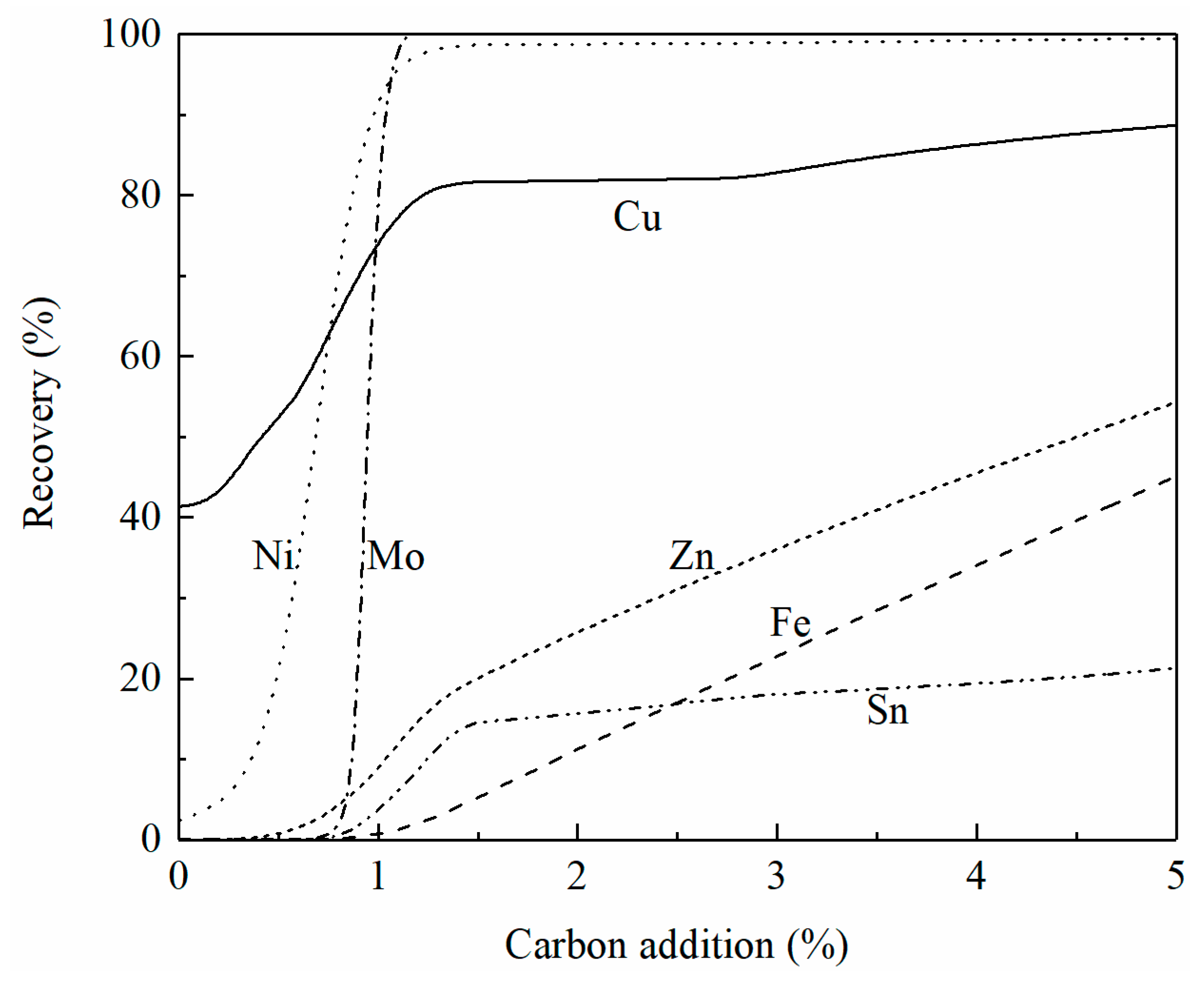
Figure 9 shows the effect of carbon addition on the recovery of copper, iron, zinc, nickel, and tin from converting slag at 1250 °C. Note that the converting slag contains less sulfur than the smelting slag which causes different reduction behavior. With a 5% carbon addition, over 95% of copper and nickel have been recovered from the slag. Less than 50% of the zinc and 10% of the tin are reduced from the slag. Deep reduction is required to fully reduce the oxides of zinc and tin.
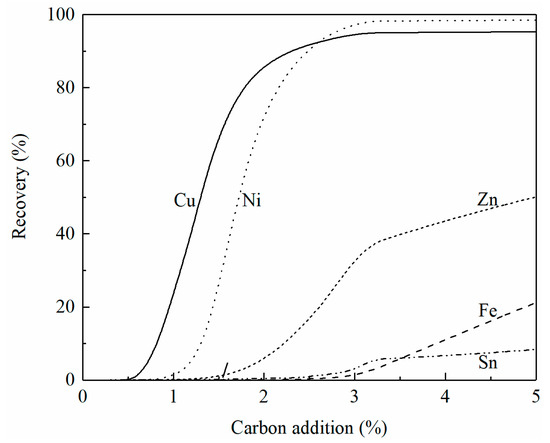
Figure 9.
Effect of carbon addition on the recovery of copper, iron, zinc, nickel, and tin in converting slag at 1250 °C, calculated by FactSage 8.2.
It is shown in Figure 7 that the liquidus temperatures of the reduced slag mixture can be controlled below 1250 °C. Figure 10 shows the effect of carbon addition on the recovery of copper, iron, zinc, molybdenum, nickel, and tin from the mixed slag (smelting slag:converting slag = 4:1) at 1250 °C. It can be seen that almost all nickel and molybdenum can be recovered from the mixed slag with a 5% carbon addition. In addition, 85% of the copper can also be recovered. However, only part of the zinc oxide and tin oxide is reduced at the same condition. Considering the liquidus temperature of the reduced slag and recovery of multi-elements, it is more beneficial to treat the smelting slag and converting slag together.
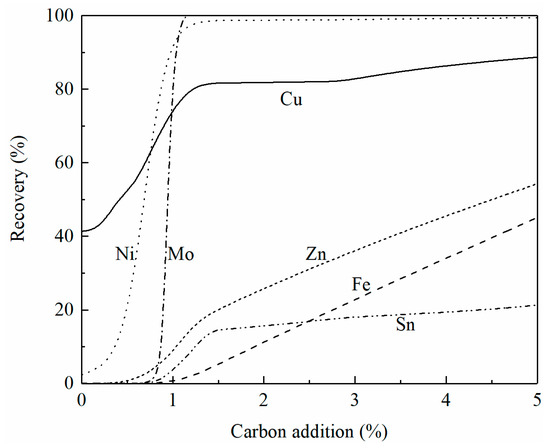
Figure 10.
Effect of carbon addition on the recovery of copper, zinc, molybdenum, nickel, iron, and tin from the mixed slag (smelting slag:converting slag = 4:1) at 1250 °C, calculated by FactSage 8.2.
4. Conclusions
Quenched flash smelting slag and converting slag have been analyzed as a base for thermodynamic predictions. The control of liquidus temperatures and copper content in the smelting slag and the recovery of valuable elements from the slags have been calculated by FactSage 8.2. It was found that the smelting temperature in the flash furnace was higher than the liquidus temperature of the slag and all copper was present in the flash smelting slag as dissolved copper. High matte grade, SO2 partial pressure, and Fe/SiO2 can cause high liquidus temperature of the smelting slag. Optimum matte grade, low temperature, and low Fe/SiO2 are beneficial to decrease the copper content in the smelting slag. Copper, nickel, and molybdenum can be recovered in alloy by reduction of the mixed smelting and converting slags. Recovery of zinc and tin from the slags requires deep reduction and flux addition.
Author Contributions
Methodology, B.Z.; software, B.Z.; resources, X.Y.; writing – original draft, S.X.; writing – review & editing, X.Y., F.L. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Guixi Smelter of Jiangxi Copper for providing the smelting and converting slags.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Potysz, A.; Hullebusch, E.; Kierczak, J.; Grybos, M.; Lens, P.; Guibaud, G. Copper metallurgical slags–current knowledge and fate: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Moskalyk, R.; Alfantazi, A. Review of copper pyrometallurgical practice: Today and tomorrow. Miner. Eng. 2003, 16, 893–919. [Google Scholar] [CrossRef]
- Zhao, B.; Liao, J. Development of Bottom-blowing copper smelting technology: A review. Metals 2022, 12, 190. [Google Scholar] [CrossRef]
- Wan, X.; Shen, L.; Jokilaakso, A.; Eriç, H.; Taskinen, P. Experimental Approach to Matte-Slag Reactions in the Flash Smelting Process. Miner. Process. Extr. Metall. Rev. 2021, 42, 231–241. [Google Scholar] [CrossRef]
- Kojo, I.; Jokilaakso, A.; Hanniala, P. Flash smelting and converting furnaces: A 50 year retrospect. JOM 2000, 52, 57–61. [Google Scholar] [CrossRef]
- Fagerlund, K.; Jalkanen, H. Microscale simulation of settler processes in copper matte smelting. Metall. Mater. Trans. B 2000, 31, 439–451. [Google Scholar] [CrossRef]
- Liao, J.; Liao, C.; Zhao, B. Comparison of Copper Smelting Slags Between Flash Smelting Furnace and Bottom-Blowing Furnace. In Proceedings of the 12th International Symposium on High-Temperature Metallurgical Processing, Orlando, FL, USA, 15–18 March 2021. [Google Scholar]
- Zhou, J.; Chen, Z.; Zhou, P.; Yu, J.; Liu, A. Numerical simulation of flow characteristics in settler of flash furnace. Trans. Nonferrous Met. Soc. China 2012, 22, 1517–1525. [Google Scholar] [CrossRef]
- Zhao, B.; Xie, S.; Zhou, L. Study on slag-matte-gas equilibrium in copper flash smelting process. Nonferrous Met. (Extr. Metall.) 2022, 3, 38–43. (In Chinese) [Google Scholar]
- Sridhar, R.; Toguri, J.; Simeonov, S. Copper losses and thermodynamic considerations in copper smelting. Metall. Mater. Trans. B 1997, 28, 191–200. [Google Scholar] [CrossRef]
- Bacedoni, M.; Moreno-Ventas, I.; Ríos, G. Copper flash smelting process balance modelling. Metals 2020, 10, 1229. [Google Scholar] [CrossRef]
- Cornejo, K.; Chen, M.; Zhao, B. Control of Copper Loss in Flash Smelting Slag. In Materials Engineering—From Ideas to Practice: An EPD Symposium in Honor of Jiann-Yang Hwang; Springer: Orlando, FL, USA, 2021. [Google Scholar]
- Zhang, H.; Wang, Y.; He, Y.; Xu, S.; Hu, B.; Cao, H.; Zhou, J.; Zheng, G. Efficient and safe disposition of arsenic by incorporation in smelting slag through copper flash smelting process. Miner. Eng. 2021, 160, 106661. [Google Scholar] [CrossRef]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour. Conserv. Recy. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Qu, G.; Wei, Y.; Li, B.; Wang, H.; Yang, Y.; McLean, A. Distribution of copper and iron components with hydrogen reduction of copper slag. J. Alloy Compd. 2020, 824, 153910. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Zheng, C. Reduction Kinetics of Copper Slag by H2. Minerals 2022, 12, 548. [Google Scholar] [CrossRef]
- Xu, F.; Weng, T.; Tan, K.; Liao, J.; Zhao, B.; Xie, S. Distribution and Control of Arsenic during Copper Converting and Refining. Metals 2023, 13, 85. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Hu, J. Co-treatment of electroplating sludge, copper slag, and spent cathode carbon for recovering and solidifying heavy metals. J. Hazard. Mater. 2021, 417, 126020. [Google Scholar]
- Bale, C.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.; Kang, Y.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Bale, C.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Ben Mahfoud, R.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Zhao, B.; Hayes, P.; Jak, E. Effects of CaO, Al2O3 and MgO on liquidus temperatures of copper smelting and converting slags under controlled oxygen partial pressures. J. Min. Metall. B 2013, 49, 153. [Google Scholar] [CrossRef]
- Jak, E. Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field. In Proceedings of the IX International Conference on Molten Slags, Fluxes and Salts, Beijing, China, 27–30 May 2012. [Google Scholar]
- Yazawa, A. Thermodynamic evaluations of extractive metallurgical processes. Metall. Mater. Trans. B 1979, 10, 307–321. [Google Scholar] [CrossRef]
- Fallah-Mehrjardi, A.; Hidayat, T.; Hayes, P.; Jak, E. Experimental investigation of gas/slag/matte/tridymite equilibria in the Cu-Fe-O-S-Si system in controlled gas atmospheres: Experimental results at 1473 K (1200 °C) and P(SO2) = 0.25 atm. Metall. Mater. Trans. B 2017, 48, 3017–3026. [Google Scholar] [CrossRef]
- Fallah-Mehrjardi, A.; Hayes, P.; Jak, E. The effect of CaO on gas/slag/matte/tridymite equilibria in fayalite-based copper smelting slags at 1473 K (1200 °C) and P(SO2) =0.25 atm. Metall. Mater. Trans. B 2018, 49, 602–609. [Google Scholar] [CrossRef]
- Shimpo, R.; Goto, S.; Ogawa, O.; Asakura, L. A study on the equilibrium between copper matte and slag. Can. Metall. Quart. 1986, 25, 113–121. [Google Scholar] [CrossRef]
- Furuta, S.; Tanaka, S.; Hamamoto, M.; Inada, H. Analysis of copper loss in slag in Tamano type flash smelting furnace. In Sohn International Symposium: International Symposium of Sulfide Smelting; Wiley-TMS: San Diego, CA, USA.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).