Exploring the Formation Mechanism, Evolution Law, and Precise Composition Control of Interstitial Oxygen in Body-Centered Cubic Mo
Abstract
:1. Introduction
2. Experimental
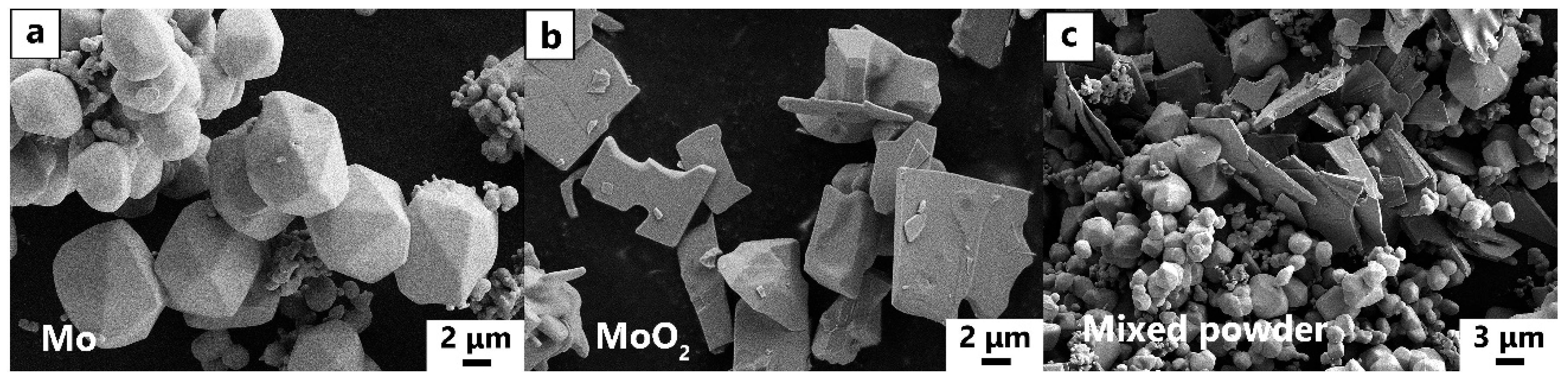
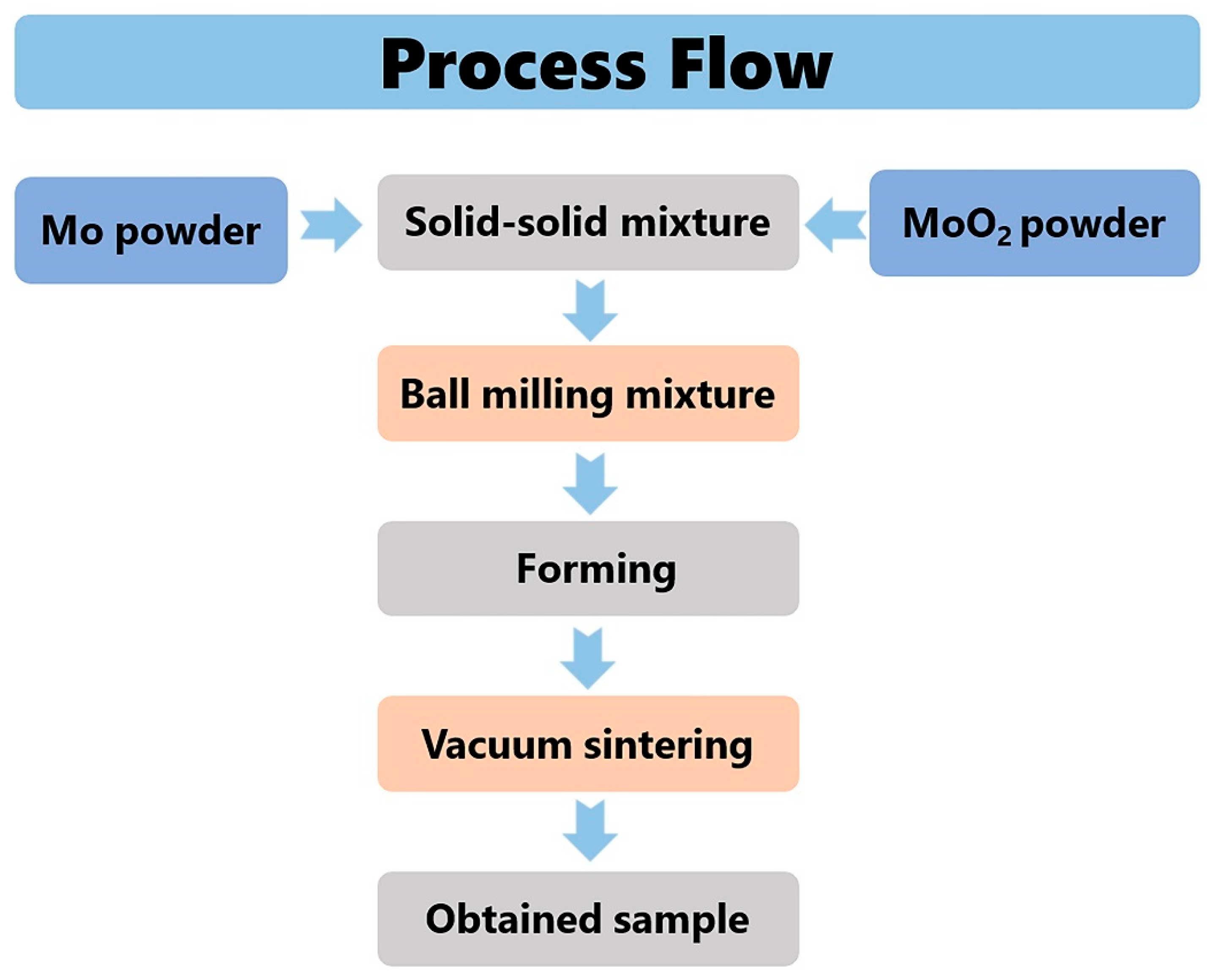
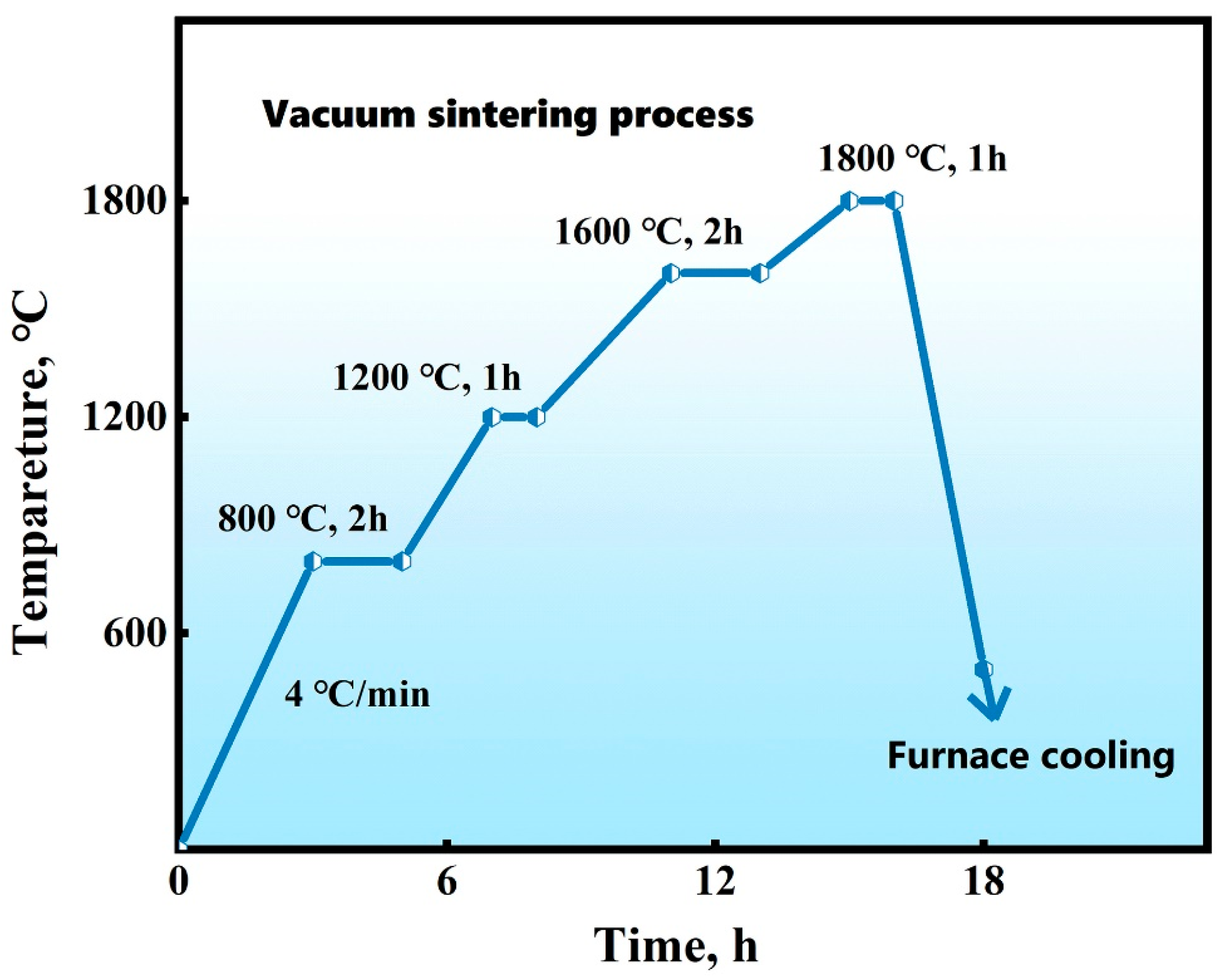
2.1. Materials and Processing
2.2. Characterization
3. Results and Discussion
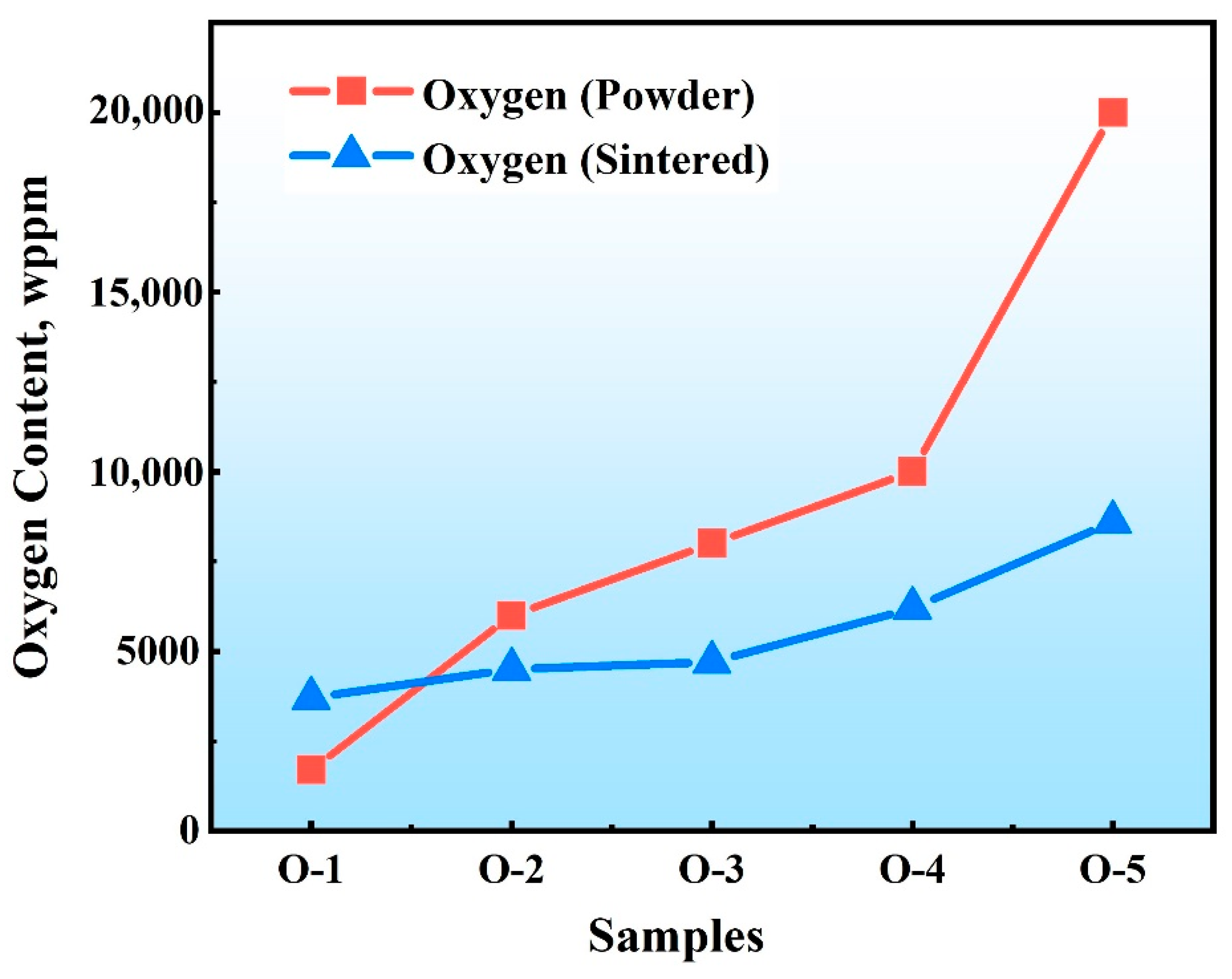
3.1. Oxygen Content and Phase Composition of Mo
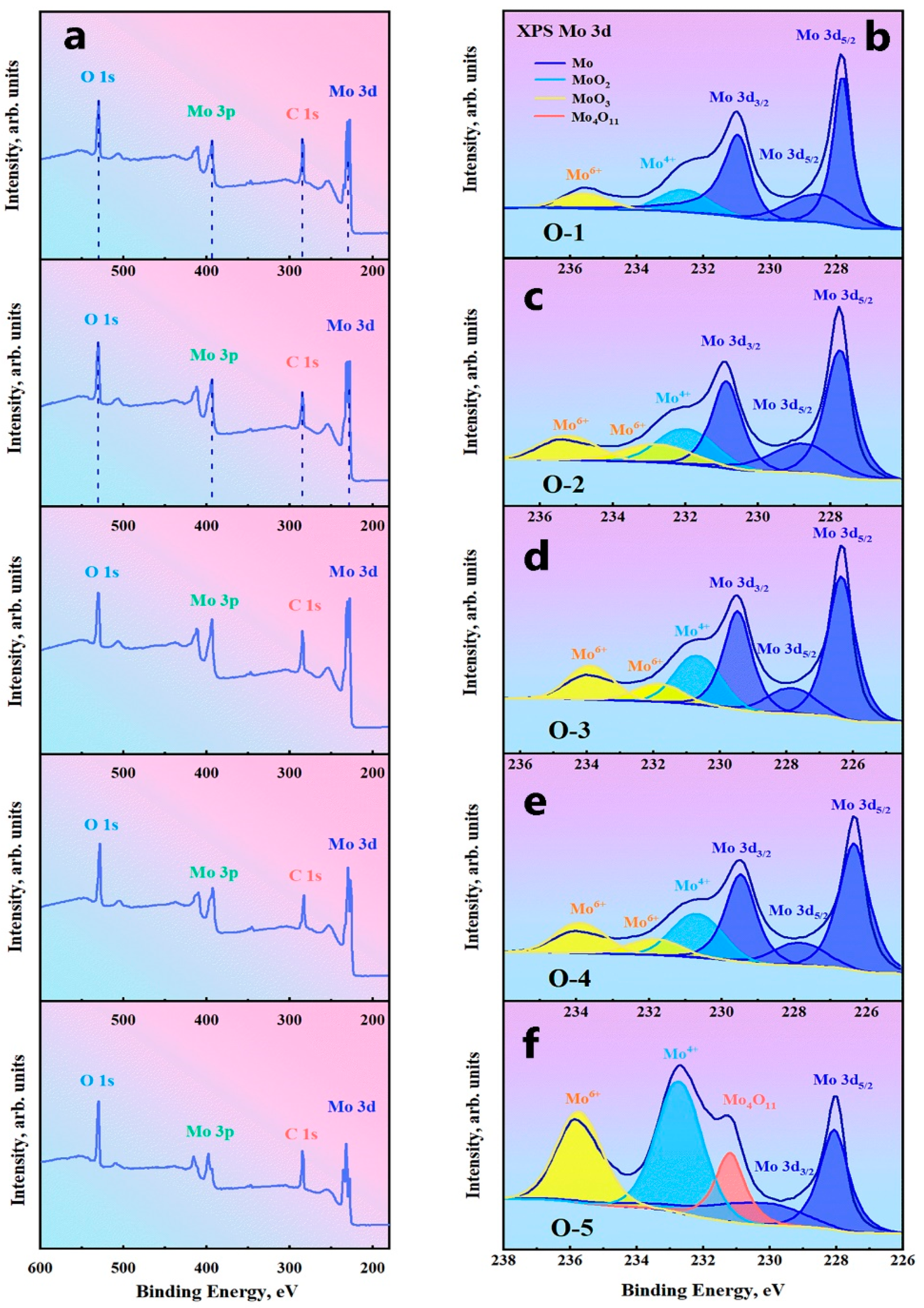
3.2. Oxygen Effects on Mo Microstructure and Composition
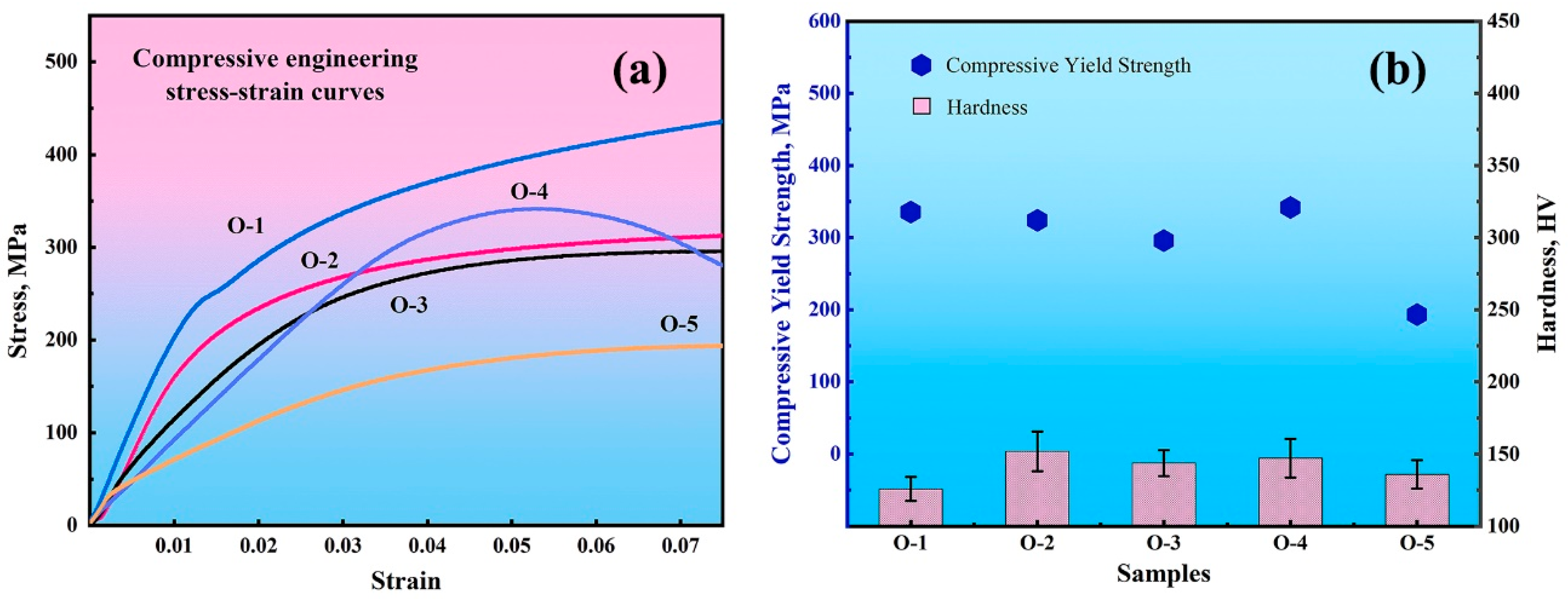
3.3. Oxygen Effects on Mo Mechanical Performance
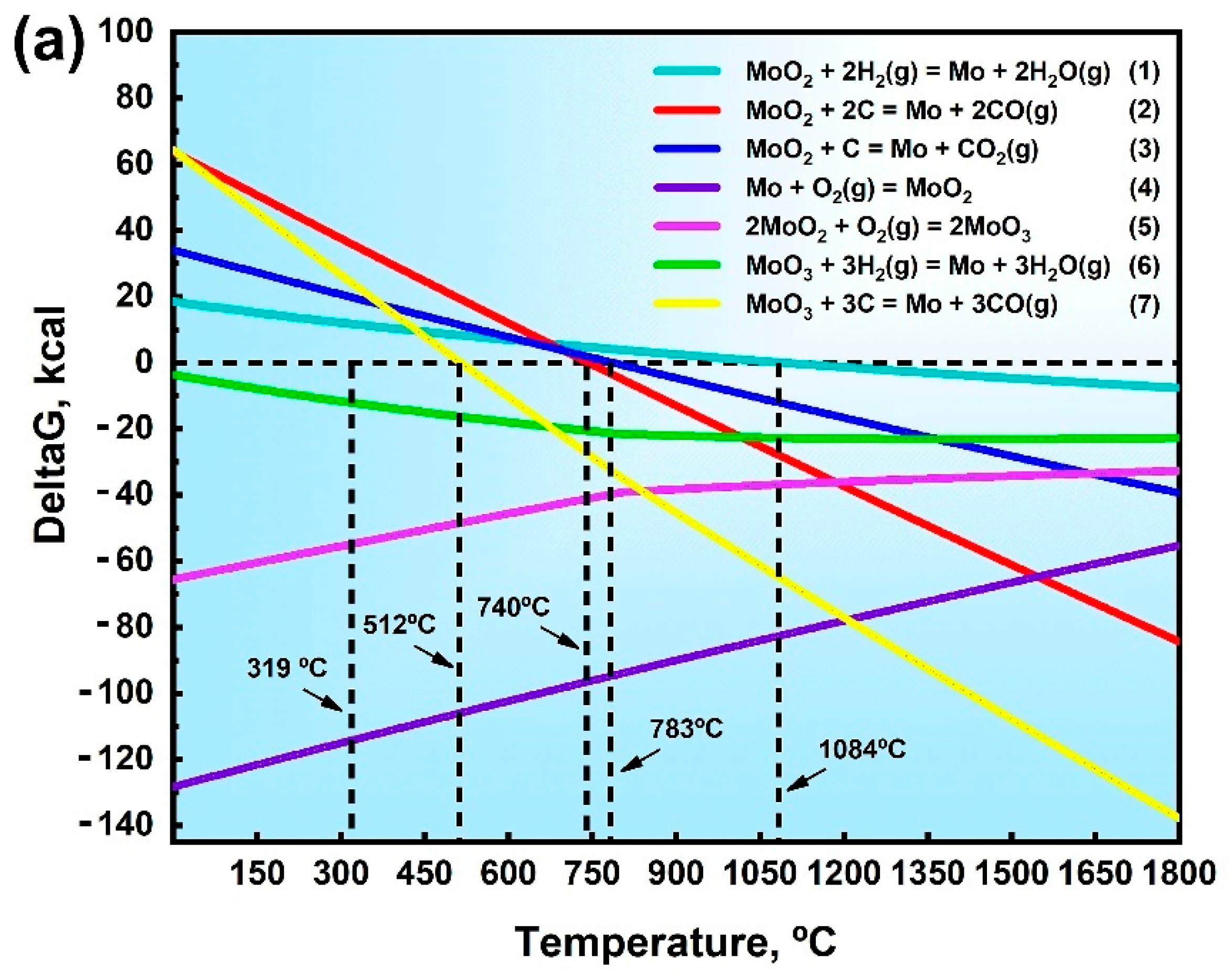
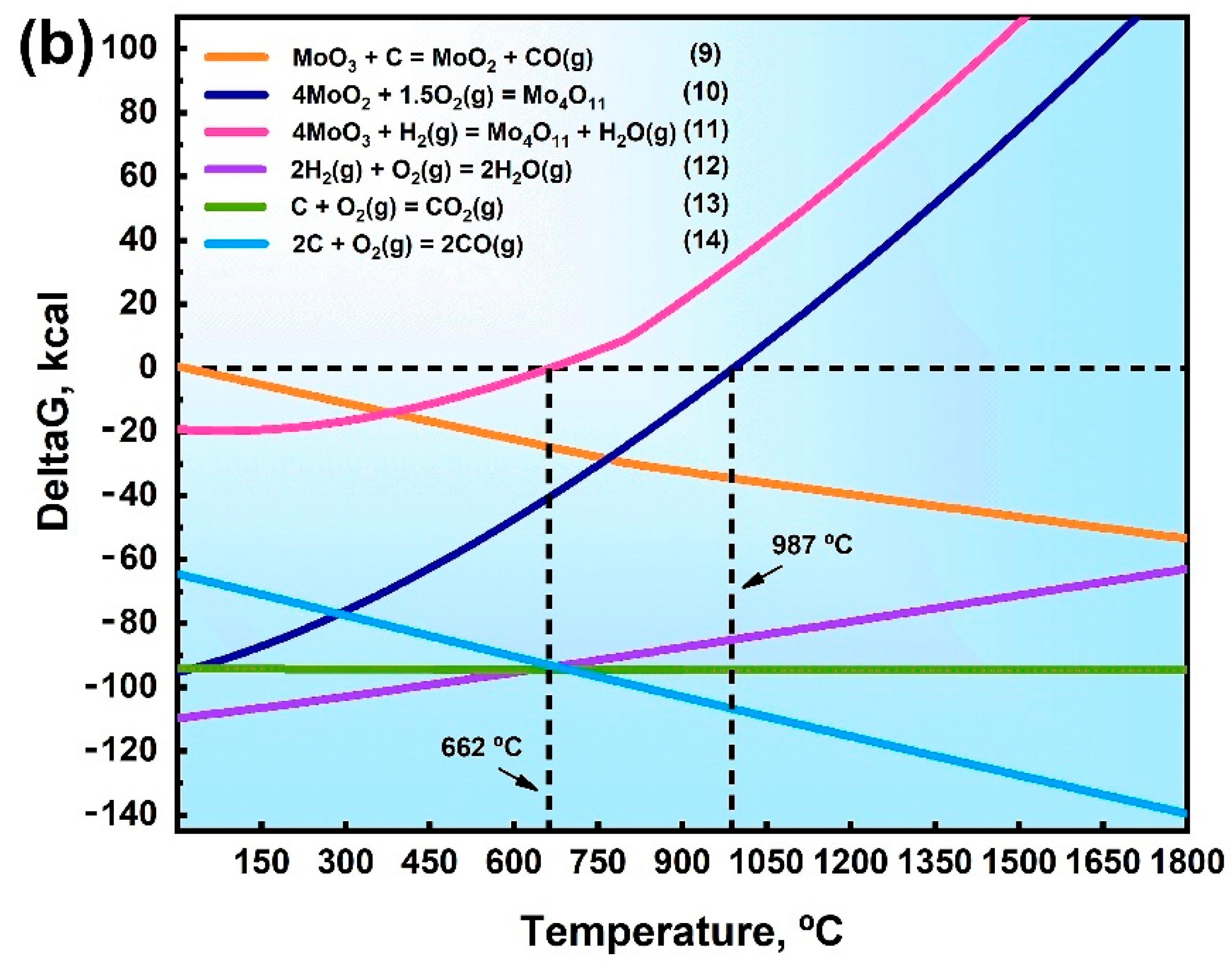
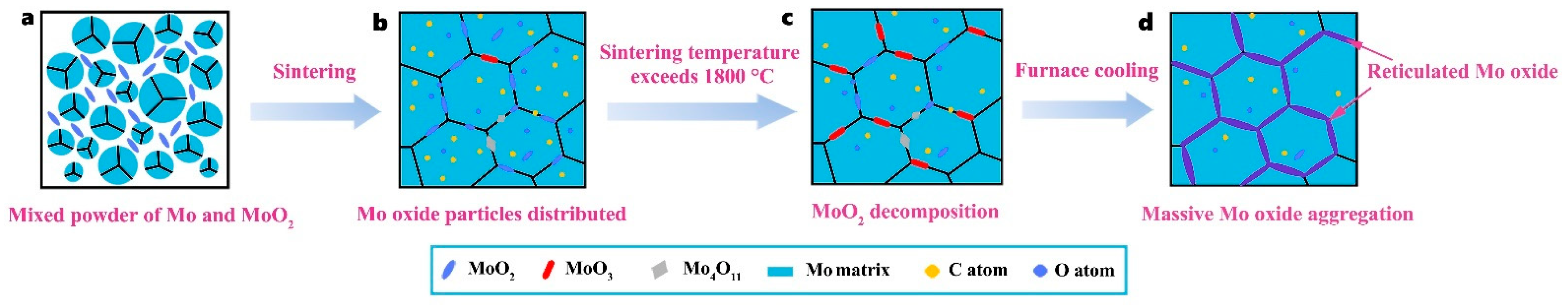
3.4. Oxygen Formation Mechanism at Grain Boundaries
4. Conclusions
- (1)
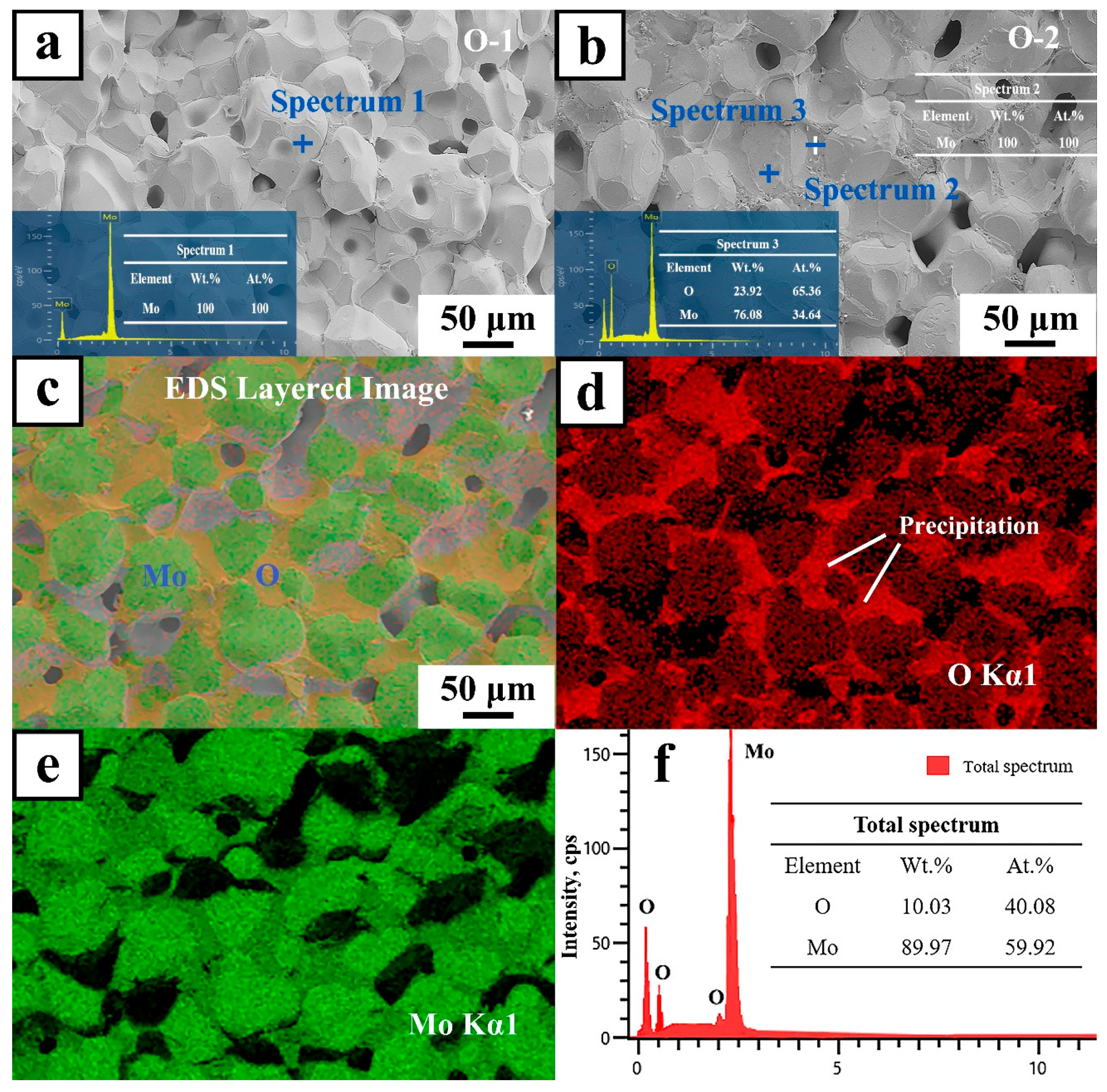
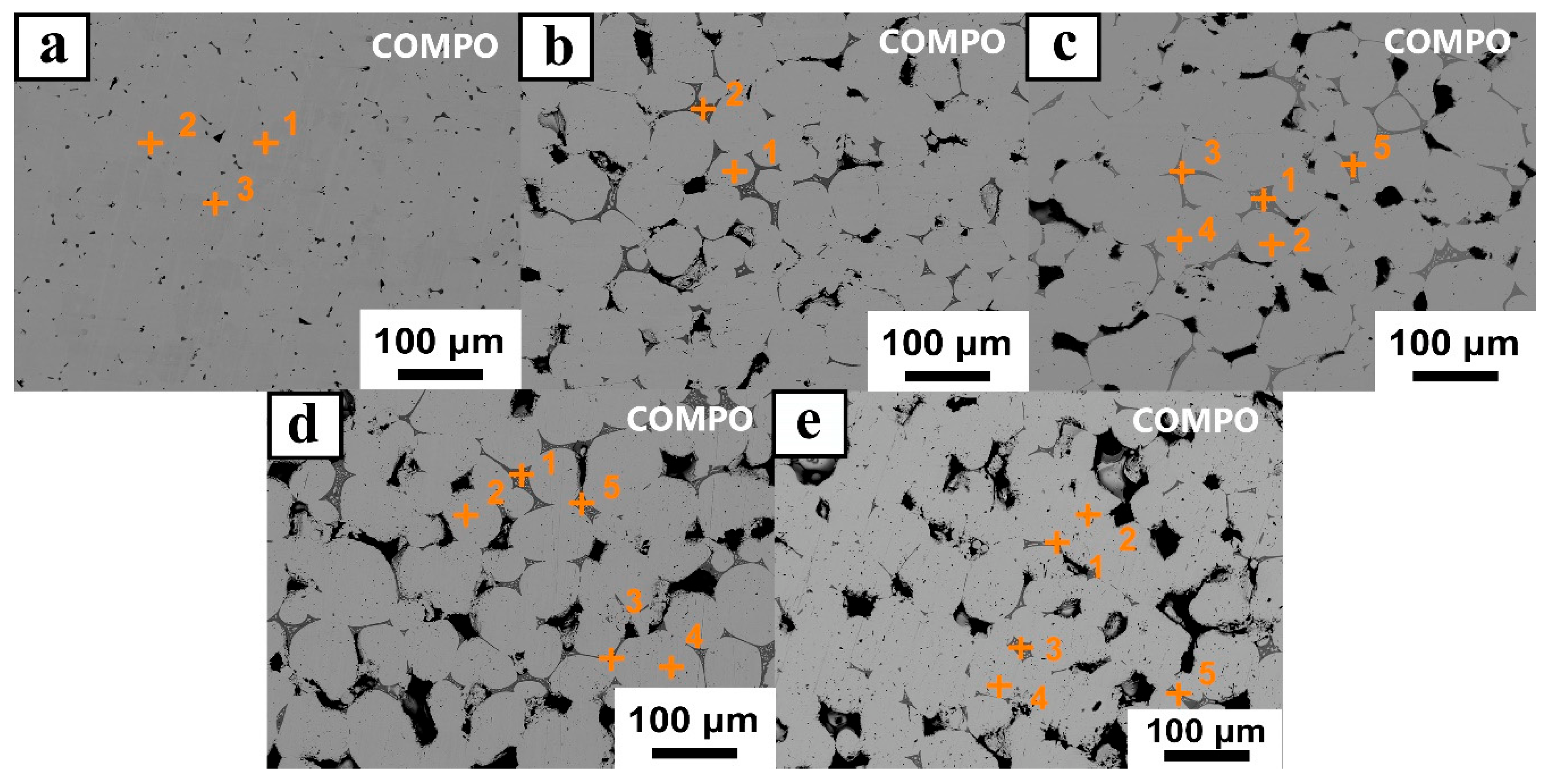
- Mo samples with different O contents (3700–8600 wppm) can be prepared by adding different amounts of MoO2. Under vacuum sintering conditions, the O content in the sintered samples can be controlled by changing the chemical composition design method of MoO2 powder. The O element in the powder and sintering can be quantitatively detected by the oxygen–nitrogen analyzer. SEM analysis of O doping showed that the fracture mode was intergranular fracture, and the fracture morphology did not change with an increase in O content.
- (2)
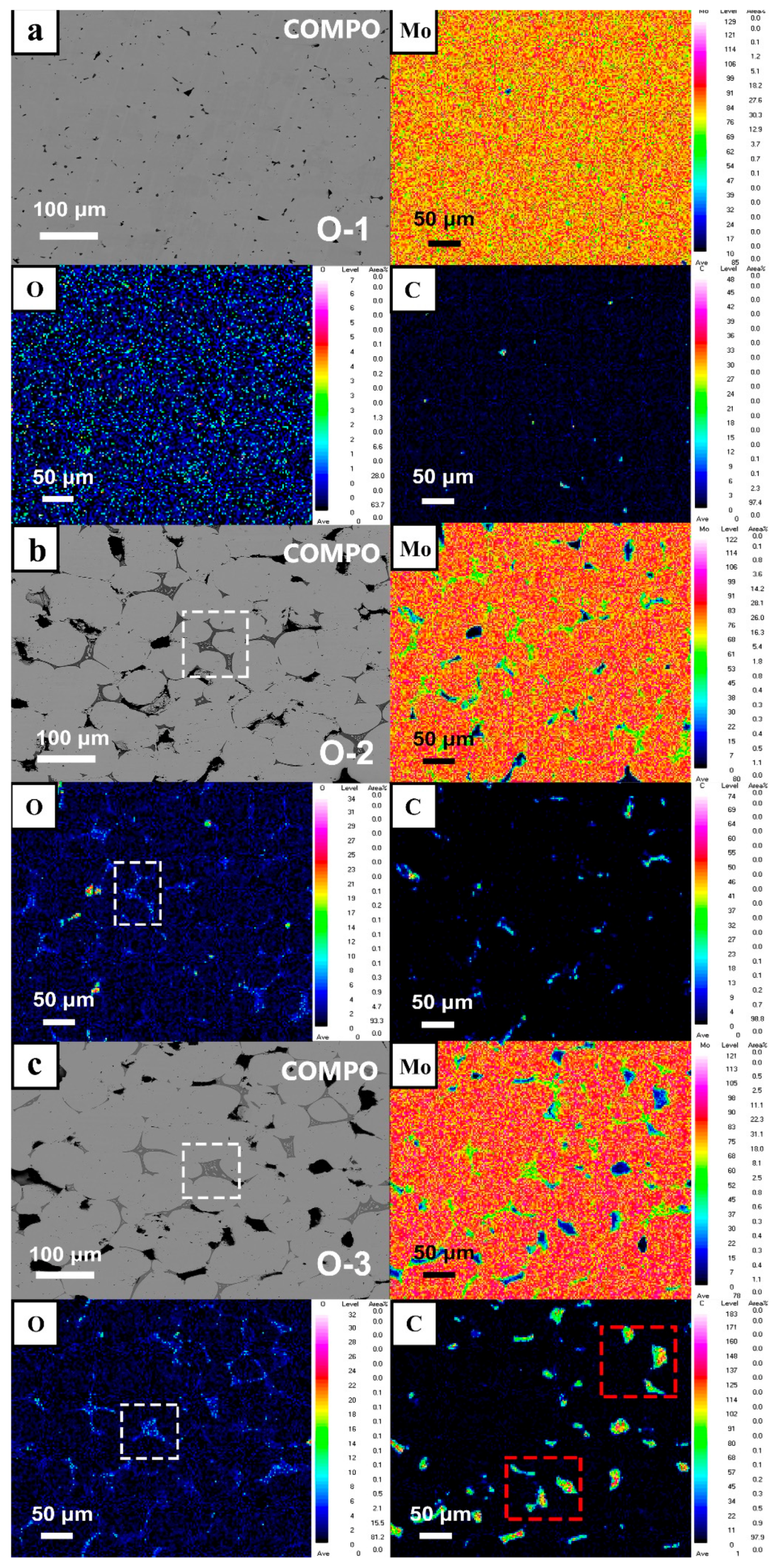
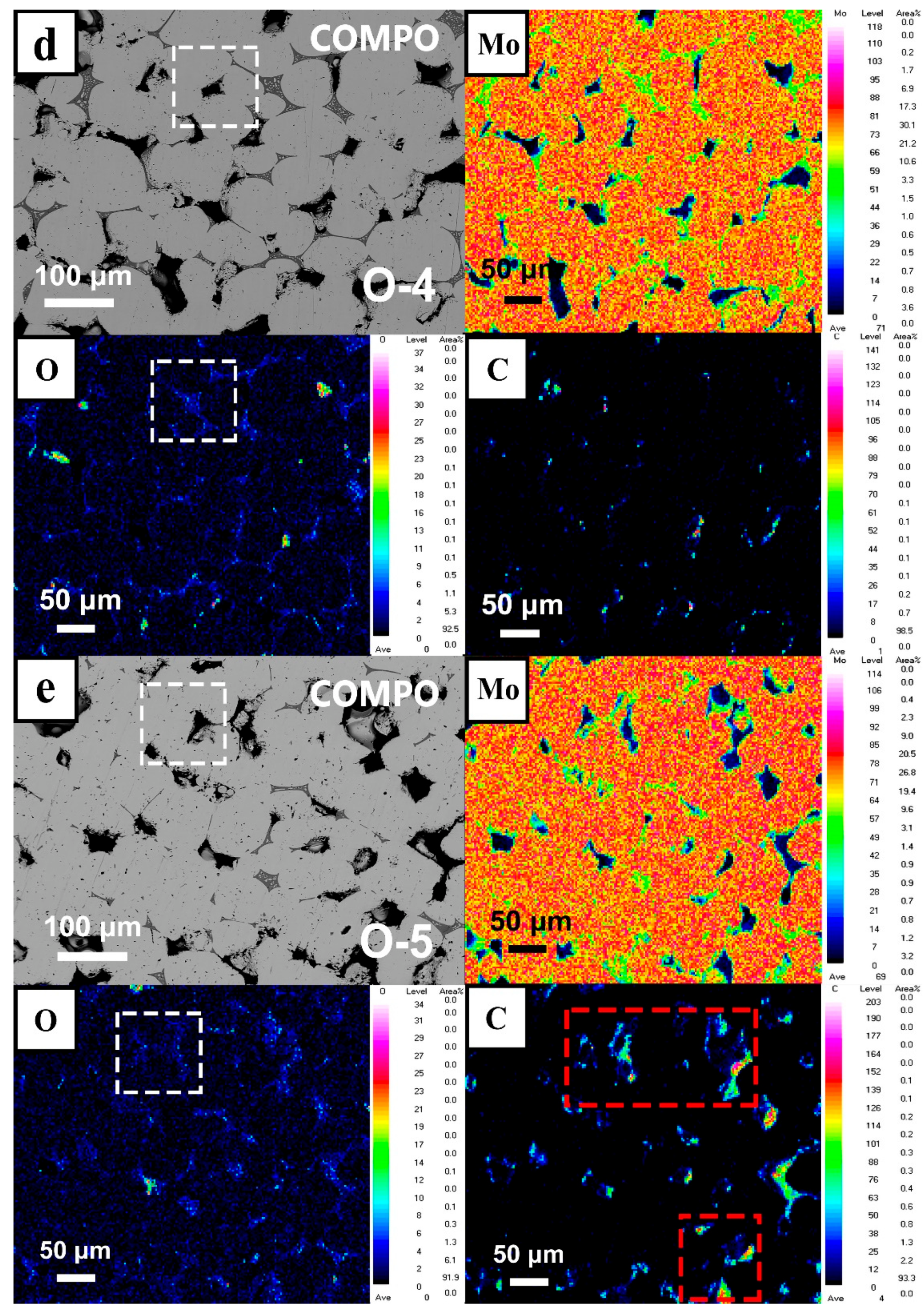
- O preferred to segregate at GBs with an increase in O content, and Mo oxides appeared in the grain boundary area of the fracture by SEM, EDS, and EPMA technology. It indicated that the solid doping process with addition of MoO2 powder achieved enrichment of O at GBs and solid solution of O with Mo matrix, resulting in a maximum O element content of 8600 wppm.
- (3)
- Thermodynamic calculations can be used as a criterion for oxide reactions in molybdenum, and the experimental results can be verified using XPS techniques. Due to addition of a large number of O elements, it was identified by EPMA and XPS that O elements in GBs exist as MoO2, MoO3, and intermediate oxides of Mo4O11 phases, and Mo oxides were distributed in GBs in a reticulated manner.
- (4)
- The sintered O-1 had the best compressive strength of 1384.85 MPa. With an increase in O content, the compressive strength decreased gradually. Good compressive strength is demonstrated because of the solid solution strengthening effect. When the content of O increased, element O existed in the form of oxide, which led to a reduction in compressive strength, and the minimum compressive strength was 7.84 MPa. The O-4 sample with an O content of 6200 wppm had the highest yield strength (341.74 MPa). With the increase in O contents, the hardness increased slightly from 125.86 HV to 151.92 HV and the grain size increased from 17.35 μm to 24.7 μm.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.Y.; Zhang, L.; Wang, G.H.; Qin, M.L.; Qu, X.H.; Kang, P.S. Influence of impurities on hot-rolled molybdenum for high temperature applications. Int. J. Refract. Met. Hard Mater. 2020, 92, 105294. [Google Scholar] [CrossRef]
- Dekhtyar, A.I.; Karasevska, O.P.; Bondarchuk, V.I. Effect of plastic bending on high temperature creep resistance of molybdenum single crystals. Int. J. Refract. Met. Hard Mater. 2021, 95, 105461. [Google Scholar] [CrossRef]
- Cho, G.S.; Ahn, G.B.; Choe, K.H. Creep microstructures and creep behaviors of pure molybdenum sheet at 0.7 Tm. Int. J. Refract. Met. Hard Mater. 2016, 60, 52–57. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G.J.; Jiang, F.; Ding, X.D.; Sun, Y.J.; Sun, J.; Ma, E. Nanostructured high-strength molybdenum alloys with unprecedented tensile ductility. Nat. Mater. 2013, 12, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Sarkar, A.; Suwas, S. Investigation of stress-strain response, microstructure and texture of hot deformed pure molybdenum. Int. J. Refract. Met. Hard Mater. 2018, 73, 168–182. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.J.; Shan, Z.W.; Li, J.; Sun, J.; Ma, E. A new regime for mechanical annealing and strong sample-size strengthening in body centred cubic molybdenum. Nat. Commun. 2011, 2, 547. [Google Scholar] [CrossRef] [Green Version]
- Jing, K.; Liu, R.; Xie, Z.M.; Ke, J.G.; Wang, X.P.; Fang, Q.F.; Liu, C.S.; Wang, H.; Li, G.; Wu, X.B. Excellent high-temperature strength and ductility of the ZrC nanoparticles dispersed molybdenum. Acta Mater. 2022, 227, 117725. [Google Scholar] [CrossRef]
- Cairang, W.; Li, T.S.; Xue, D.Z.; Yang, H.J.; Cheng, P.M.; Chen, C.; Sun, Y.J.; Zeng, Y.; Ding, X.D.; Sun, J. Enhancement of the corrosion resistance of Molybdenum by La2O3 dispersion. Corros. Sci. 2021, 186, 109469. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, P.; Wang, K.S.; Li, S.L.; Xing, H.R.; Chang, T.; Feng, P.F.; Li, L.P. Microstructure and texture evolution of pure molybdenum during hot deformation. Mater. Charact. 2020, 159, 110010. [Google Scholar] [CrossRef]
- Xie, M.X.; Li, Y.X.; Shang, X.T.; Wang, X.W.; Pei, J.Y. Microstructure and mechanical properties of a fiber welded socket-joint made of powder metallurgy molybdenum alloy. Metals 2019, 9, 640. [Google Scholar] [CrossRef]
- Minkin, A.M.; Kozlov, V.M. Preferred growth of molybdenum thin films during magnetron sputtering. J. Solid State Chem. 2021, 304, 122543. [Google Scholar] [CrossRef]
- Sargent, G.A.; Shaw, B.J. Stress relaxation and the ductile-brittle transition temperature of molybdenum. Acta Metall. 1966, 14, 909–912. [Google Scholar] [CrossRef]
- Novoselov, I.I.; Yanilkin, A.V. Impact of segregated interstitials on structures and energies of tilt grain boundaries in Mo. Comput. Mater. Sci. 2016, 112, 276–281. [Google Scholar] [CrossRef]
- Miller, M.K.; Kenik, E.A.; Mousa, M.S.; Russell, K.F.; Bryhan, A.J. Improvement in the ductility of molybdenum alloys due to grain boundary segregation. Scr. Mater. 2002, 46, 299–303. [Google Scholar] [CrossRef]
- Oku, M.; Suzuki, S.; Kurishita, H.; Yoshinaga, H. Chemical states of oxygen segregated intergranular fracture surfaces of molybdenum. Appl. Surf. Sci. 1986, 26, 42–50. [Google Scholar] [CrossRef]
- Li, S.L.; Hu, P.; Han, J.Y.; Ge, S.W.; Hua, X.J.; Xing, H.R.; Deng, J.; Hu, B.L.; Yang, F.; Wang, K.S. The formation mechanism of micro-nano secondary phase in solid-liquid doped TZM alloy. Mater. Charact. 2022, 186, 111800. [Google Scholar] [CrossRef]
- Hu, B.L.; Wang, K.S.; Hu, P.; Li, S.L.; Deng, J.; Zuo, Y.G.; Xing, H.R.; Feng, P.F.; Paley, V. Fracture behavior of the La-doped molybdenum-titanium-zirconium alloy. Mater. Sci. Eng. A 2019, 759, 167–171. [Google Scholar] [CrossRef]
- Hu, B.L.; Wang, K.S.; Hu, P.; Su, Y.T.; Li, S.L.; Xing, H.R.; Han, J.Y.; Ge, S.W.; Hua, X.J.; Fu, J.B.; et al. Effect of secondary phases on the strength and elongation of a novel Mo-TiC-ZrC-C alloy. Int. J. Refract. Met. Hard Mater. 2020, 92, 105336. [Google Scholar] [CrossRef]
- Hu, B.L.; Wang, K.S.; Hu, P.; Zhou, Y.H.; Deng, J.; Chen, W.J.; Feng, P.F.; Zhang, J.P.; Yu, H.L. Secondary phases formation in lanthanum-doped titanium-zirconium-molybdenum alloy. J. Alloy. Compd. 2018, 757, 340–347. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Hu, P.; Xing, H.R.; Yang, F.; Wang, X.Y.; Ge, S.W.; Hua, X.J.; He, C.J.; Zhang, X.Y.; Wang, K.S. Preparation of nanoporous flake molybdenum powder by sol-gel reduction method. Mater. Charact. 2022, 187, 111879. [Google Scholar] [CrossRef]
- Wang, K.S.; Tan, J.F.; Hu, P.; Yu, Z.T.; Yang, F.; Hu, B.L.; Song, R.; He, H.C.; Volinsky, A.A. La2O3 effects on TZM alloy recovery, recrystallization and mechanical properties. Mater. Sci. Eng. A 2015, 636, 415–420. [Google Scholar] [CrossRef]
- Primig, S.; Leitner, H.; Clemens, H.; Lorich, A.; Knabl, W.; Stickler, R. On the recrystallization behavior of technically pure molybdenum. Int. J. Refract. Met. Hard Mater. 2010, 28, 703–708. [Google Scholar] [CrossRef]
- Brosse, J.B.; Fillit, R.; Biscondi, M. Intrinsic intergranular brittleness of molybdenum. Scr. Metall. 1981, 15, 619–623. [Google Scholar] [CrossRef]
- Lin, M.; Yu, H.; Ding, Y.; Wang, G.; Olden, V.; Alvaro, A.; He, J.; Zhang, Z. A predictive model unifying hydrogen enhanced plasticity and decohesion. Scr. Mater. 2022, 215, 114707. [Google Scholar] [CrossRef]
- Leitner, K.; Scheiber, D.; Jakob, S.; Primig, S.; Clemens, H.; Povoden Karadeniz, E.; Romaner, L. How grain boundary chemistry controls the fracture mode of molybdenum. Mater. Des. 2018, 142, 36–43. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Li, Y.H.; Hong, L.; Zhou, H.B. Influence of carbon and oxygen on the core structure and peierls stress of screw dislocation in molybdenum. Metals 2022, 12, 507. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, L.J.; Ning, J.; Sun, Y.J.; Na, S.J. Strengthening mechanisms of combined alloying with carbon and titanium on laser beam welded joints of molybdenum alloy. J. Manuf. Process. 2021, 68, 1637–1649. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.Y.; Jiang, S.Y.; Li, X.P.; Zhang, B.G.; Feng, J.C. Stress relief and purification mechanisms for grain boundaries of electron beam welded TZM alloy joint with zirconium addition. J. Mater. Process. Technol. 2018, 251, 168–174. [Google Scholar] [CrossRef]
- Miller, M.K.; Bryhan, A.J. Effect of Zr, B and C additions on the ductility of molybdenum. Mater. Sci. Eng. A 2002, 327, 80–83. [Google Scholar] [CrossRef]
- Lei, Z.F.; Liu, X.J.; Wu, Y.; Wang, H.; Jiang, S.H.; Wang, S.D.; Hui, X.D.; Wu, Y.D.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef]
- Yang, P.J.; Li, Q.J.; Tsuru, T.; Ogata, S.; Zhang, J.W.; Sheng, H.W.; Shan, Z.W.; Sha, G.; Han, W.Z.; Li, J.; et al. Mechanism of hardening and damage initiation in oxygen embrittlement of body-centred-cubic niobium. Acta Mater. 2019, 168, 331–342. [Google Scholar] [CrossRef]
- Yang, P.J.; Li, Q.J.; Han, W.Z.; Li, J.; Ma, E. Designing solid solution hardening to retain uniform ductility while quadrupling yield strength. Acta Mater. 2019, 179, 107–118. [Google Scholar] [CrossRef]
- Moser, M.; Lorand, S.; Bussiere, F.; Demoisson, F.; Couque, H.; Bernard, F. Influence of carbon diffusion and the presence of oxygen on the microstructure of molybdenum powders densified by SPS. Metals 2020, 10, 948. [Google Scholar] [CrossRef]
- Zhang, J.; Han, W.Z. Oxygen solutes induced anomalous hardening, toughening and embrittlement in body-centered cubic vanadium. Acta Mater. 2020, 196, 122–132. [Google Scholar] [CrossRef]
- Xing, H.R.; Hu, P.; Han, J.Y.; Li, S.L.; Ge, S.W.; Hua, X.J.; Hu, B.L.; Yang, F.; Wang, K.S.; Feng, P.F. Effects of oxygen on microstructure and evolution mechanism of body-centred-cubic molybdenum. Int. J. Refract. Met. Hard Mater. 2022, 103, 105747. [Google Scholar] [CrossRef]
- Veverka, J.; Vilémová, M.; Chlup, Z.; Hadraba, H.; Lukáč, F.; Csáki, Š.; Matějíček, J.; Vontorová, J.; Chráska, T. Evolution of carbon and oxygen concentration in tungsten prepared by field assisted sintering and its effect on ductility. Int. J. Refract. Met. Hard Mater 2021, 97, 105499. [Google Scholar] [CrossRef]
- Lang, D.; Pöhl, C.; Holec, D.; Schatte, J.; Povoden Karadeniz, E.; Knabl, W.; Clemens, H.; Primig, S. On the chemistry of the carbides in a molybdenum base Mo-Hf-C alloy produced by powder metallurgy. J. Alloy. Compd. 2016, 654, 445–454. [Google Scholar] [CrossRef]
- Waugh, A.R.; Southon, M.J. Surface studies with an imaging atom-probe. Surf. Sci. 1977, 68, 79–85. [Google Scholar] [CrossRef]
- Waugh, A.R.; Southon, M.J. Surface analysis and grain-boundary segregation measurements using atom-probe techniques. Surf. Sci. 1979, 89, 718–724. [Google Scholar] [CrossRef]
- Miller, M.K.; Kurishita, H. APFIM Characterization of Grain Boundary Segregation in Titanium Carbide-Doped Molybdenum. Le J. De Phys. IV 1996, 6, C5-265–C5-270. [Google Scholar] [CrossRef]
- Scheiber, D.; Pippan, R.; Puschnig, P.; Ruban, A.; Romaner, L. Ab-initio search for cohesion-enhancing solute elements at grain boundaries in molybdenum and tungsten. Int. J. Refract. Met. Hard Mater. 2016, 60, 75–81. [Google Scholar] [CrossRef]
- Ma, H.B.; Ding, X.K.; Zhang, L.B.; Sun, Y.J.; Liu, T.; Ren, Q.; Liao, Y.H. Segregation of interstitial light elements at grain boundaries in molybdenum. Mater. Today Commun. 2020, 25, 101388. [Google Scholar] [CrossRef]
- Tahir, A.M.; Janisch, R.; Hartmaier, A. Ab initio calculation of traction separation laws for a grain boundary in molybdenum with segregated C impurites. Model. Simul. Mater. Sci. Eng. 2013, 21, 075005. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Li, Y.H.; Gong, H.F.; Ren, Q.Y.; Ma, F.F.; Liu, T.; Lu, G.H.; Zhou, H.B. Suppressing effect of carbon on oxygen-induced embrittlement in molybdenum grain boundary. Comput. Mater. Sci. 2021, 198, 110676. [Google Scholar] [CrossRef]
- Leitner Née Babinsky, K.; Lutz, D.; Knabl, W.; Eidenberger Schober, M.; Huber, K.; Lorich, A.; Clemens, H.; Maier Kiener, V. Grain boundary segregation engineering in as-sintered molybdenum for improved ductility. Scr. Mater. 2018, 156, 60–63. [Google Scholar] [CrossRef]
- Babinsky, K.; Weidow, J.; Knabl, W.; Lorich, A.; Leitner, H.; Primig, S. Atom probe study of grain boundary segregation in technically pure molybdenum. Mater. Charact. 2014, 87, 95–103. [Google Scholar] [CrossRef]
- Leitner, K.; Felfer, P.J.; Holec, D.; Cairney, J.; Knabl, W.; Lorich, A.; Clemens, H.; Primig, S. On grain boundary segregation in molybdenum materials. Mater. Des. 2017, 135, 204–212. [Google Scholar] [CrossRef]
- Babinsky, K.; Knabl, W.; Lorich, A.; De Kloe, R.; Clemens, H.; Primig, S. Grain boundary study of technically pure molybdenum by combining APT and TKD. Ultramicroscopy 2015, 159, 445–451. [Google Scholar] [CrossRef]
- Bera, S.; Shivaprasad, S.M.; Sharma, J.K.N. Observation of electron transfer in the silicidation and oxidation of molybdenum by AES and EELS. Appl. Surf. Sci. 1994, 74, 13–17. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bezerra, M.A.; Santos, A.S.; dos Santos, W.N.L.; Novaes, C.G.; de Oliveira, O.M.C.; Oliveira, M.L.; Garcia, R.L. Atomic absorption spectrometry-A multi element technique. Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Padmasubashini, V.; Ganguly, M.K.; Satyanarayana, K.; Malhotra, R.K. Determination of tungsten in niobium-tantalum, vanadium and molybdenum bearing geological samples using derivative spectrophotometry and ICP-AES. Talanta 1999, 50, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.I. Determination of trace elements in high purity tungsten by solid phase extraction/ICP-MS. Mater. Trans. 2008, 49, 2054–2057. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, T.; Okochi, H.; Hasegawa, R. Determination of ultra low contents of oxygen in high purity iron. Mater. Trans. JIM 1993, 34, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.R.; Hu, P.; Li, S.L.; Zuo, Y.G.; Han, J.Y.; Hua, X.J.; Wang, K.S.; Yang, F.; Feng, P.F.; Chang, T. Adsorption and diffusion of oxygen on metal surfaces studied by first-principle study: A review. J. Mater. Sci. Technol. 2021, 62, 180–194. [Google Scholar] [CrossRef]
- He, K.N.; Song, C.; Hou, J.; Xu, Y.C.; You, Y.W.; Kong, X.S.; Liu, C.S. The influence of transition metal solutes on the dissolution and diffusion of oxygen in tungsten. J. Nucl. Mater. 2020, 537, 152250. [Google Scholar] [CrossRef]
- Zeng, Q.Q.; Liu, Z.X.; Liang, W.F.; Ma, M.F.; Deng, H.Q. A first-principles study on Na and O adsorption behaviors on Mo (110) surface. Metals 2021, 11, 1322. [Google Scholar] [CrossRef]
- Li, S.L.; Hu, P.; Zuo, Y.G.; Xing, H.R.; Han, J.Y.; Ge, S.W.; Hua, X.J.; Hu, B.L.; Cui, C.J.; Wang, K.S. Precise control of oxygen for titanium-zirconium-molybdenum alloy. Int. J. Refract. Met. Hard Mater. 2022, 103, 105768. [Google Scholar] [CrossRef]
- Atkins, E. Elements of X-ray Diffraction; Addison-Wesley Publishing Co., Inc.: Glenview, IL, USA, 1956; Volume 29, p. 572. [Google Scholar] [CrossRef]
- Battu, A.K.; Makeswaran, N.; Ramana, C.V. Fabrication, characterization and optimization of high conductivity and high quality nanocrystalline molybdenum thin films. J. Mater. Sci. Technol. 2019, 35, 2734–2741. [Google Scholar] [CrossRef]
- Browning, P.N.; Fignar, J.; Kulkarni, A.; Singh, J. Sintering behavior and mechanical properties of Mo-TZM alloyed with nanotitanium carbide. Int. J. Refract. Met. Hard Mater. 2017, 62, 78–84. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, L.J.; Ning, J.; Sun, Y.J.; Na, S.J. On the role of pre-nitriding on improving the weldability of molybdenum alloy. Mater. Des. 2021, 198, 109377. [Google Scholar] [CrossRef]
- Xing, H.R.; Hu, P.; Zhou, Y.H.; Li, S.L.; Zuo, Y.G.; Cheng, Q.; Wang, K.S.; Yang, F.; Feng, P.F.; Chang, T. The microstructure and texture evolution of pure molybdenum sheets under various rolling reductions. Mater. Charact. 2020, 165, 110357. [Google Scholar] [CrossRef]
- Atkins, P.; De Paula, J. Physical Chemistry, 8th ed.; W.H. Freeman and Company: New York, NY, USA, 2006; p. 1060. ISBN 0716787598. [Google Scholar]
- Mrotzek, T.; Hoffmann, A.; Martin, U. Hardening mechanisms and recrystallization behaviour of several molybdenum alloys. Int. J. Refract. Met. Hard Mater. 2006, 24, 298–305. [Google Scholar] [CrossRef]
- Chang, L.; Phillips, B. Phase Relations in Refractory Metal-Oxygen Systems. J. Am. Ceram. Soc. 2006, 52, 527–533. [Google Scholar] [CrossRef]
- Lee, S.K.; Yoon, S.H.; Chung, I.; Hartwig, A.; Kim, B.K. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 2011; Volume 49. [Google Scholar]
- Brox, B.; Olefjord, I. ESCA Studies of MoO2 and MoO3. Surf. Interface Anal. 1988, 13, 3–6. [Google Scholar] [CrossRef]
- Shimoda, M.; Hirata, T.; Yagisawa, K.; Okochi, M.; Yoshikawa, A. Deconvolution of Mo 3d X-ray photoemission spectraγ-Mo4O11: Agreement with prediction from bond length-bond strength relationships. J. Mater. Sci. Lett. 1989, 8, 1089–1091. [Google Scholar] [CrossRef]
- Srivastava, S.C.; Seigle, L.L. Solubility and thermodynamic properties of oxygen in solid molybdenum. Metall. Mater. Trans. B 1974, 5, 49. [Google Scholar] [CrossRef]
- Kumar, A.; Eyre, B.L. Grain Boundary Segregation and Intergranular Fracture in Molybdenum. Proc. R. Soc. A 1980, 370, 431–458. [Google Scholar] [CrossRef]
- Brewer, L.; Lamoreaux, R.H. The Mo-O system (Molybdenum-Oxygen). Bull. Alloy. Phase Diagr. 1980, 1, 85–89. [Google Scholar] [CrossRef]



| Number | O | MoO2 | Mo |
|---|---|---|---|
| 1 | - | - | Bal. |
| 2 | 0.6 | 4.92 | Bal. |
| 3 | 0.8 | 6.56 | Bal. |
| 4 | 1 | 8.2 | Bal. |
| 5 | 2 | 16.4 | Bal. |
| Number | Powder Samples | Sintered Samples | |
|---|---|---|---|
| 1 | O-1 | 1700 | 3700 |
| 2 | O-2 | 6000 | 4500 |
| 3 | O-3 | 8000 | 4700 |
| 4 | O-4 | 10,000 | 6200 |
| 5 | O-5 | 20,000 | 8600 |
| Position | Test No. | Element (wt.%) | |||
|---|---|---|---|---|---|
| C | O | Mo | Total | ||
| O-1 | 1 | 0.281 | 0.49 | 101.26 | 102.04 |
| 2 | 0.335 | 0.561 | 101.95 | 102.85 | |
| 3 | 0.497 | 0.155 | 101.91 | 102.56 | |
| Average | 0.371 | 0.4 | 101.53 | 102.3 | |
| O-2 | 1 | 0.747 | 1.728 | 102.11 | 104.59 |
| 2 | 3.175 | 23.99 | 76.718 | 103.89 | |
| Average | 1.961 | 12.86 | 89.415 | 104.24 | |
| O-3 | 1 | 0.269 | 25.65 | 76.314 | 102.23 |
| 2 | 0.739 | 0.643 | 103.17 | 102.68 | |
| 3 | 0.689 | 25.95 | 75.927 | 102.57 | |
| 4 | 0.885 | 0.663 | 101.4 | 102.97 | |
| 5 | 0.682 | 25.28 | 76.013 | 101.97 | |
| Average | 0.653 | 15.64 | 86.565 | 102.48 | |
| O-4 | 1 | 0.207 | 24.40 | 76.146 | 100.75 |
| 2 | 0.302 | 1.115 | 100.93 | 102.35 | |
| 3 | 0.663 | 25.386 | 76.931 | 102.98 | |
| 4 | 0.745 | 1.061 | 103.13 | 104.94 | |
| 5 | 0.688 | 24.57 | 76.343 | 101.6 | |
| Average | 0.521 | 15.31 | 86.697 | 102.52 | |
| O-5 | 1 | 0.629 | 26.43 | 77.11 | 104.17 |
| 2 | 0.979 | 1.241 | 101.48 | 103.7 | |
| 3 | 0.403 | 26.08 | 76.390 | 102.87 | |
| 4 | 0.571 | 1.042 | 99.939 | 101.55 | |
| 5 | 0.23 | 26.52 | 75.8 | 102.55 | |
| Average | 0.562 | 16.26 | 86.144 | 102.97 | |
| Number | O Content wppm | Compressive Strength MPa | Yield Strength MPa | Vickers Hardness HV | Grain Size μm |
|---|---|---|---|---|---|
| O-1 | 3700 | 1384.85 | - | 125.86 ± 8.28 | 17.35 |
| O-2 | 4500 | 98.83 | 323.95 | 151.92 ± 13.79 | 18.36 |
| O-3 | 4700 | 35.97 | 295.8 | 143.81 ± 9.01 | 20.11 |
| O-4 | 6200 | 12.43 | 341.74 | 147.16 ± 13.42 | 21.53 |
| O-5 | 8600 | 7.84 | 193.58 | 136.01 ± 9.88 | 24.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, H.-R.; Hu, P.; He, C.-J.; Zhang, X.-Y.; Yang, F.; Han, J.-Y.; Ge, S.-W.; Hua, X.-J.; Zhang, W.; Wang, K.-S.; et al. Exploring the Formation Mechanism, Evolution Law, and Precise Composition Control of Interstitial Oxygen in Body-Centered Cubic Mo. Metals 2023, 13, 1. https://doi.org/10.3390/met13010001
Xing H-R, Hu P, He C-J, Zhang X-Y, Yang F, Han J-Y, Ge S-W, Hua X-J, Zhang W, Wang K-S, et al. Exploring the Formation Mechanism, Evolution Law, and Precise Composition Control of Interstitial Oxygen in Body-Centered Cubic Mo. Metals. 2023; 13(1):1. https://doi.org/10.3390/met13010001
Chicago/Turabian StyleXing, Hai-Rui, Ping Hu, Chao-Jun He, Xiang-Yang Zhang, Fan Yang, Jia-Yu Han, Song-Wei Ge, Xing-Jiang Hua, Wen Zhang, Kuai-She Wang, and et al. 2023. "Exploring the Formation Mechanism, Evolution Law, and Precise Composition Control of Interstitial Oxygen in Body-Centered Cubic Mo" Metals 13, no. 1: 1. https://doi.org/10.3390/met13010001
APA StyleXing, H.-R., Hu, P., He, C.-J., Zhang, X.-Y., Yang, F., Han, J.-Y., Ge, S.-W., Hua, X.-J., Zhang, W., Wang, K.-S., & Volinsky, A. A. (2023). Exploring the Formation Mechanism, Evolution Law, and Precise Composition Control of Interstitial Oxygen in Body-Centered Cubic Mo. Metals, 13(1), 1. https://doi.org/10.3390/met13010001








