An Overview on Atomistic Mechanisms of Heterogeneous Nucleation
Abstract
1. Introduction
2. MD Simulation of Heterogeneous Nucleation
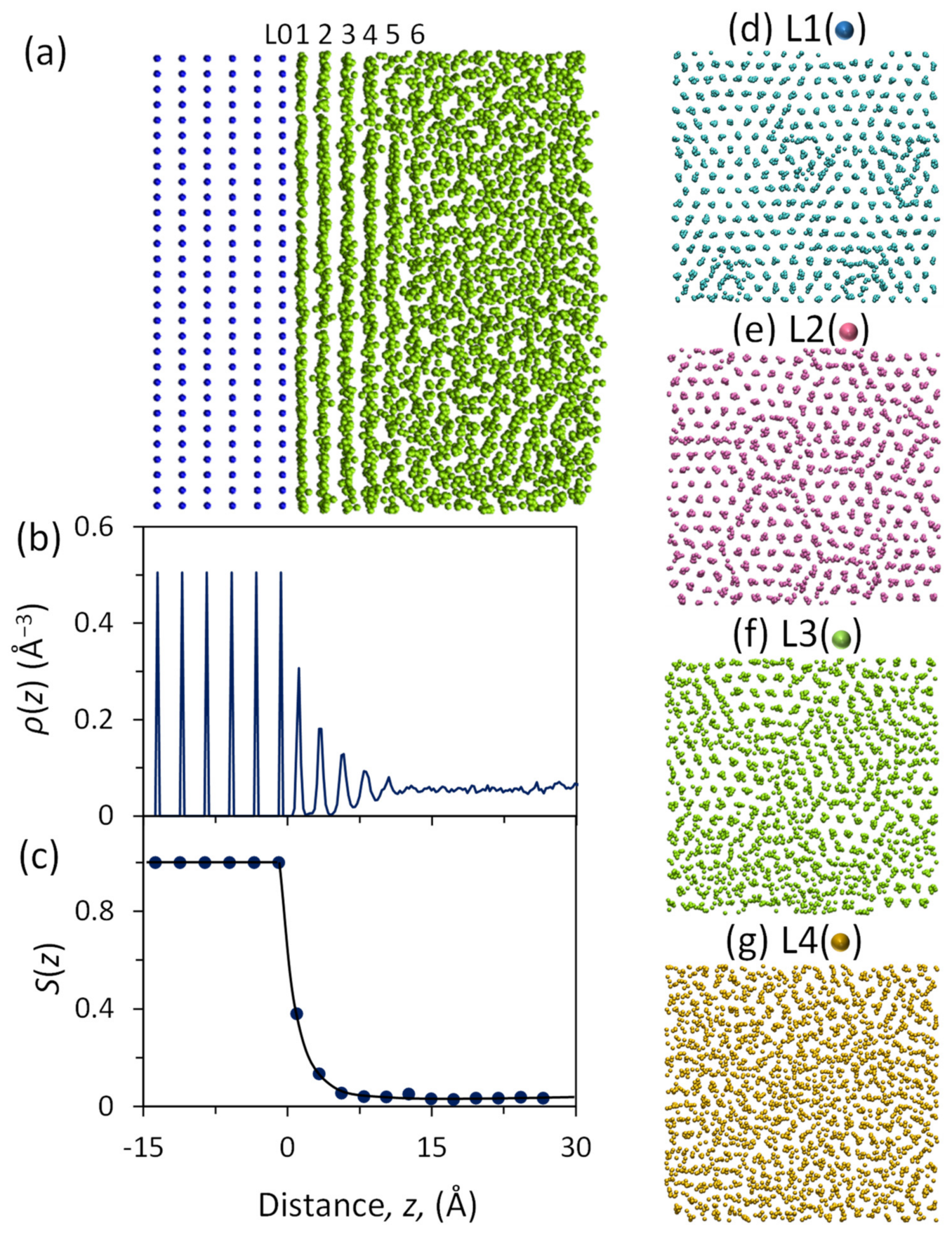
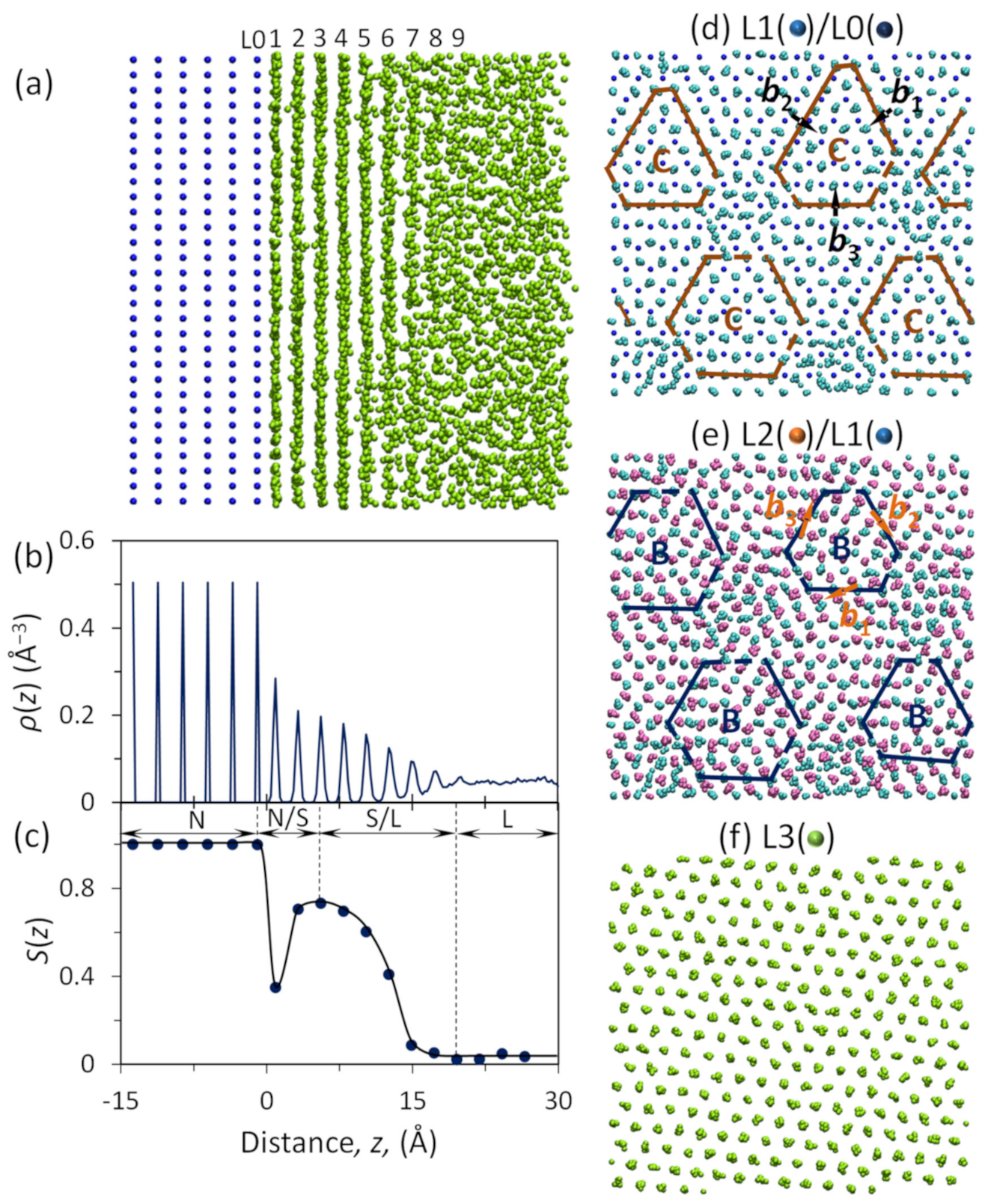
3. Prenucleation and Precursor for Heterogeneous Nucleation
4. Atomistic Mechanisms of Heterogeneous Nucleation
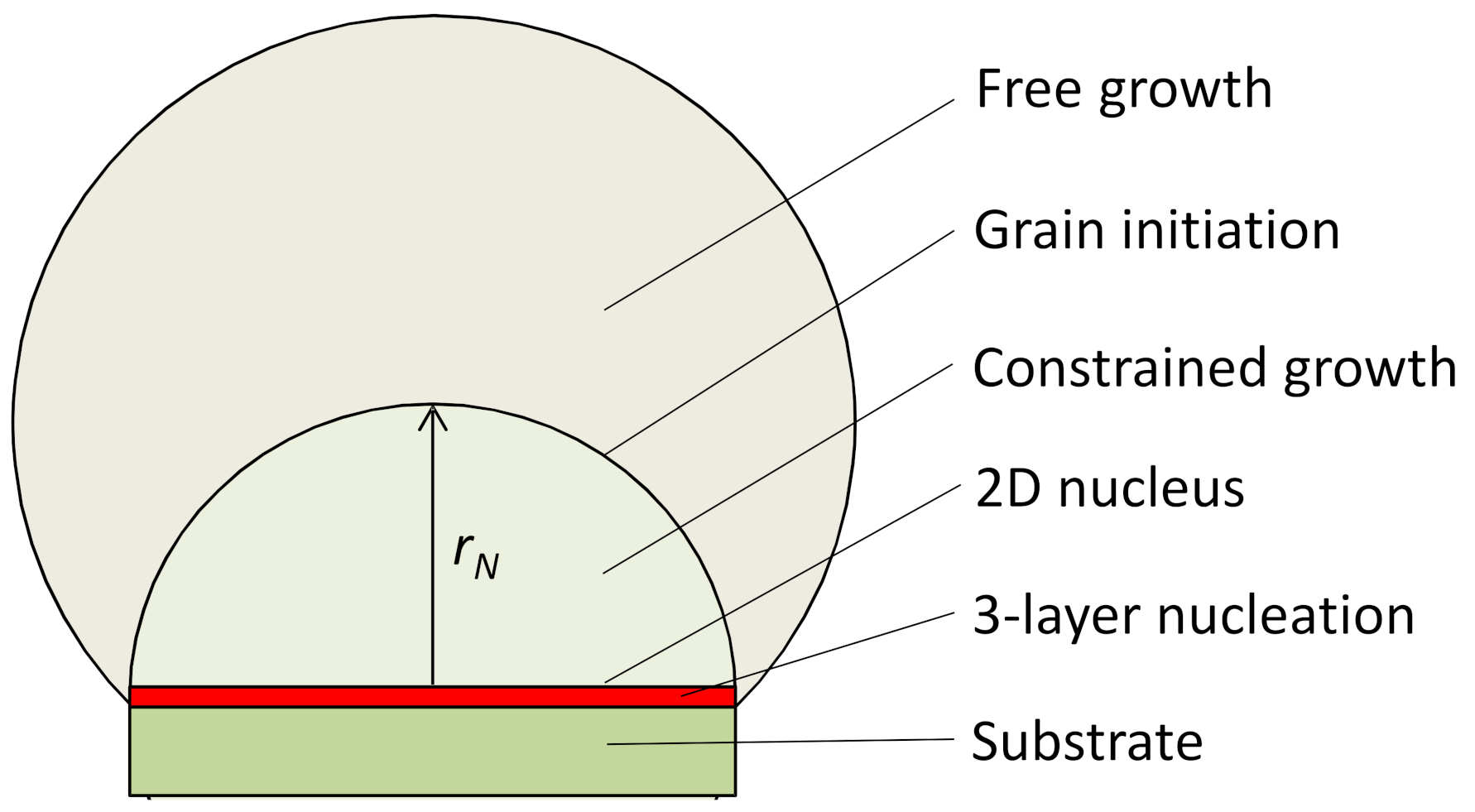
4.1. Definition of Heterogeneous Nucleation
- Prenucleation: to generate a precursor for heterogeneous nucleation.
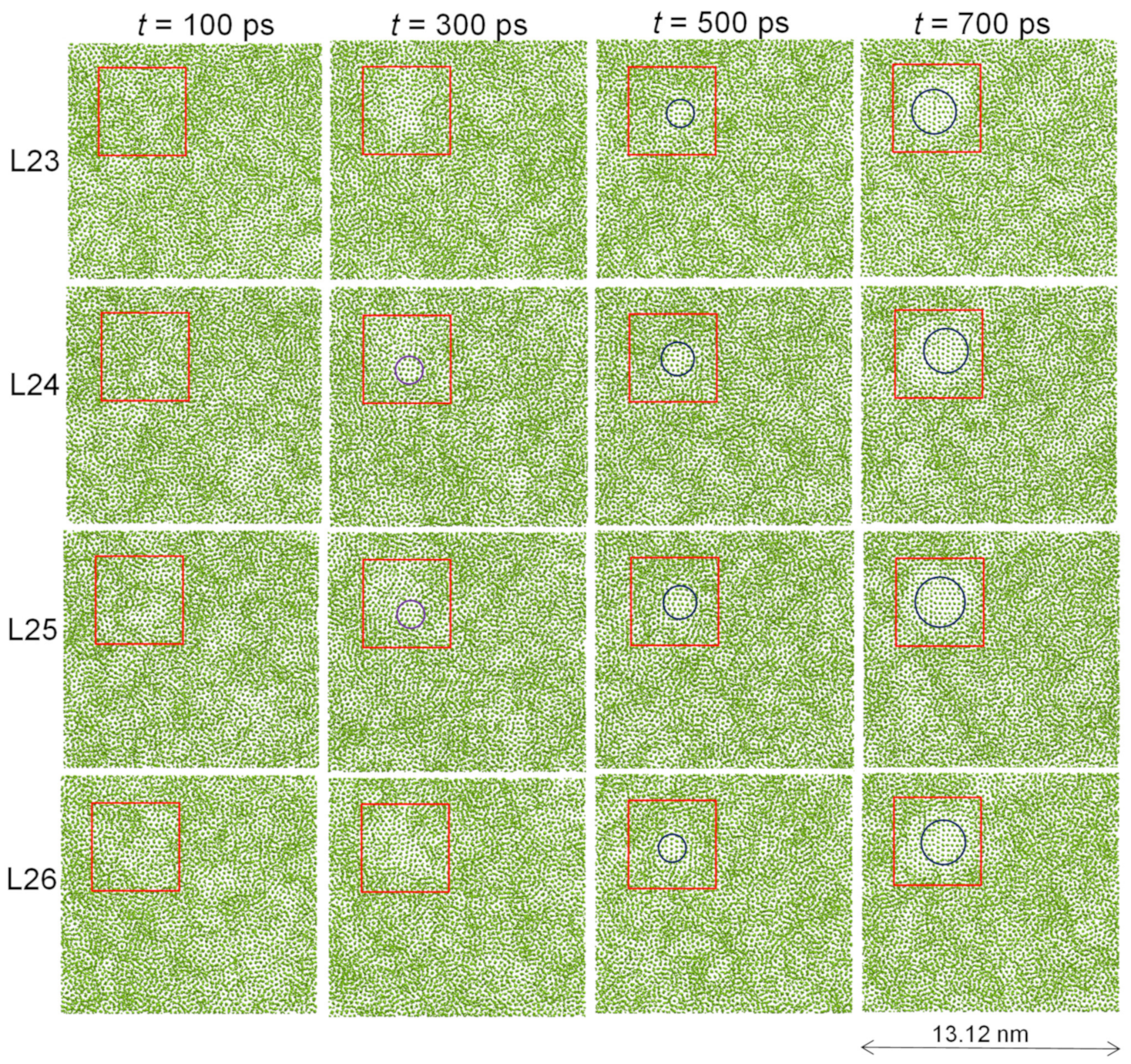
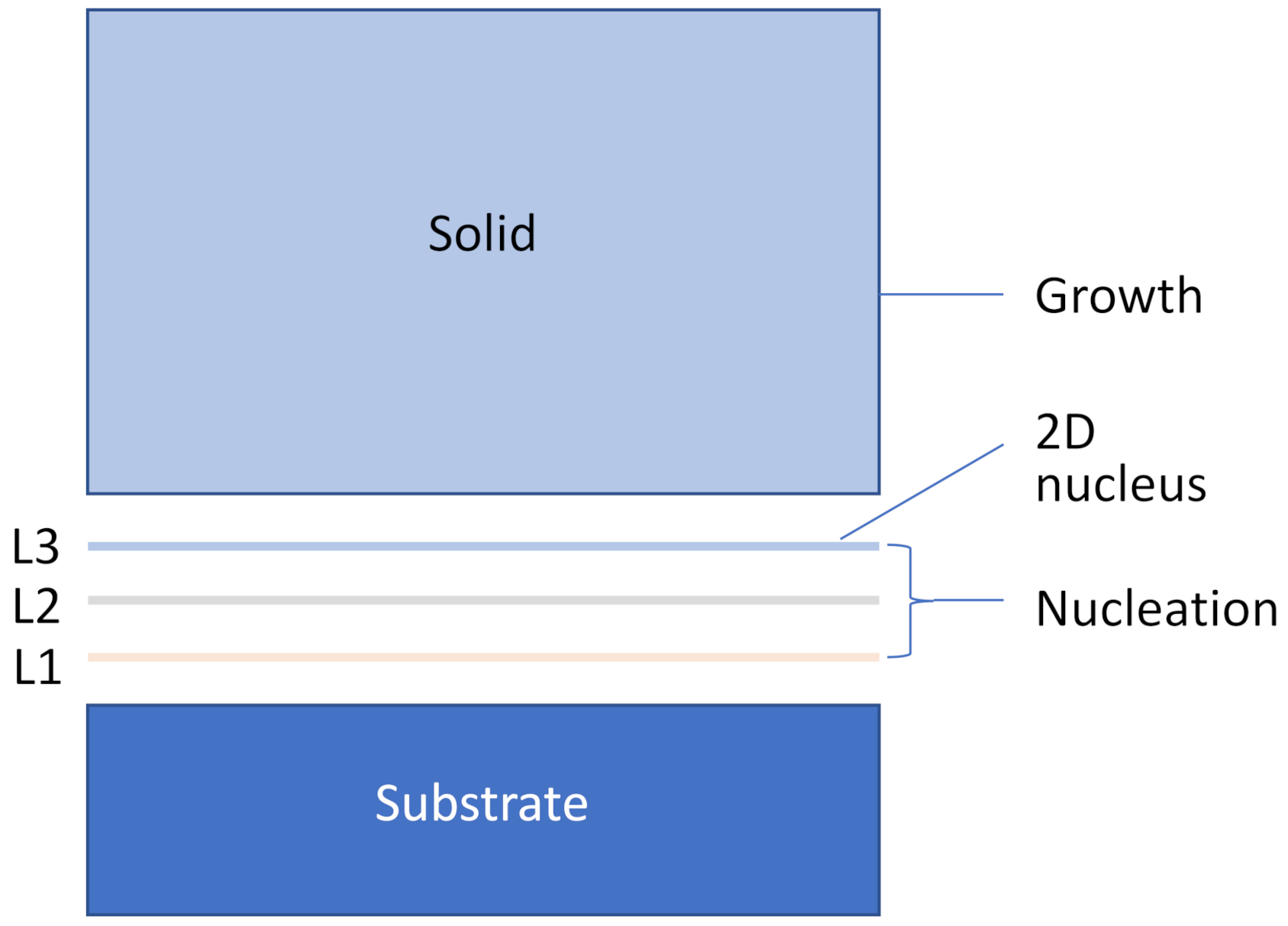
- Three-layer heterogeneous nucleation: to generate a 2D nucleus, which is a crystal plane of the solid.
- Constrained cap formation: spherical cap formation by lowering the temperature to overcome the energy barrier due to curvature constrain. The outcome of constrained cap formation is a hemisphere of the solid.
- Grain initiation: a grain is initiated if the spherical cap can grow beyond the hemisphere.
- Free growth: beyond the hemisphere the solid can grow isothermally without any energy barrier.
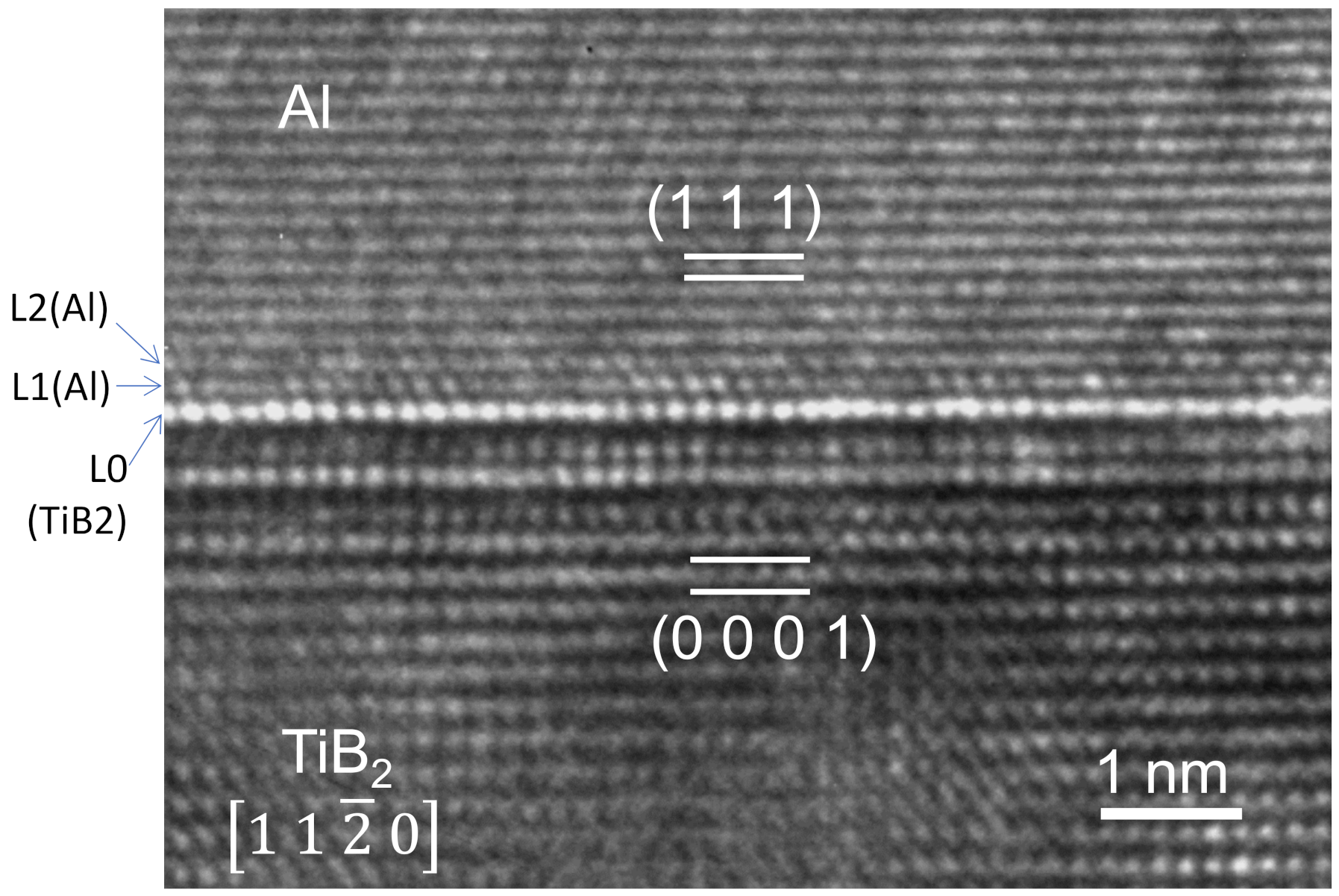
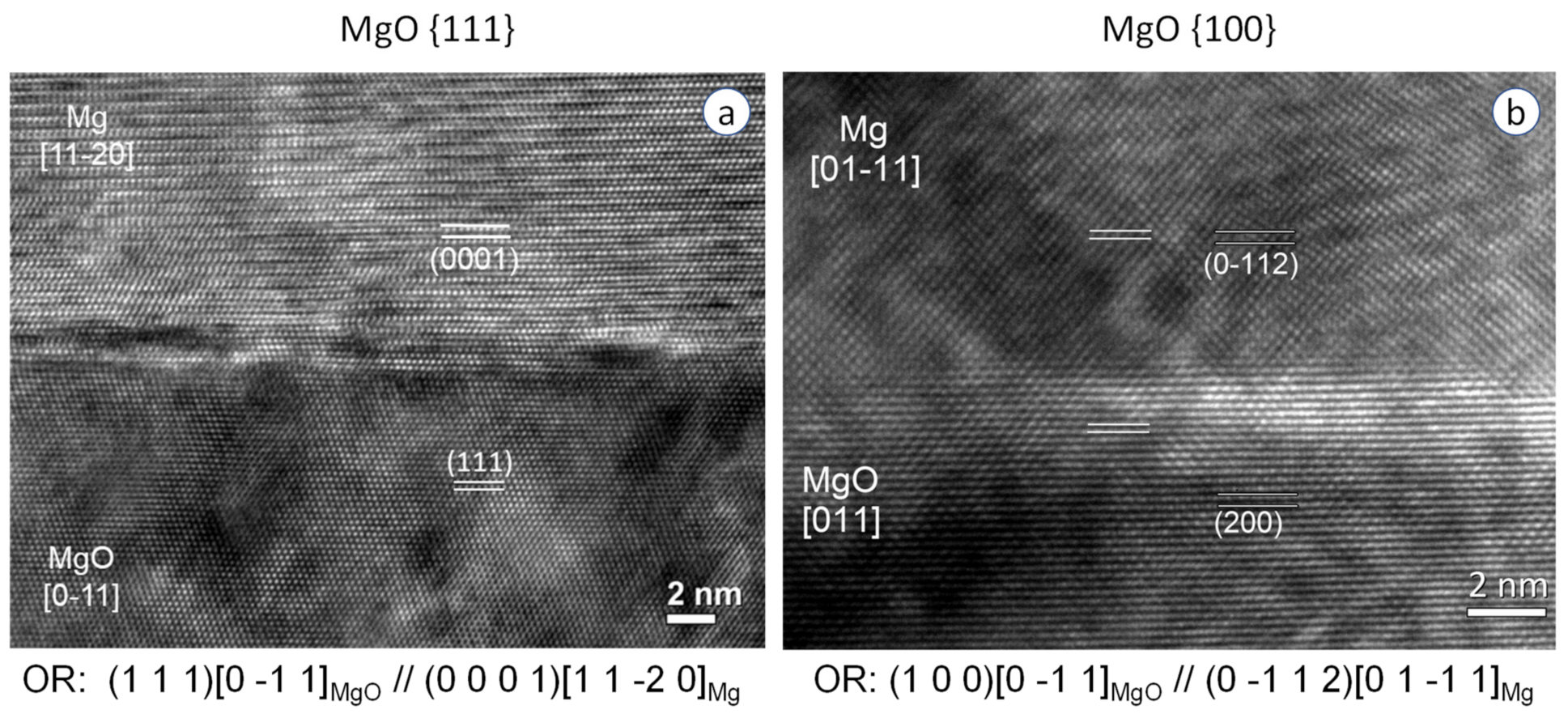
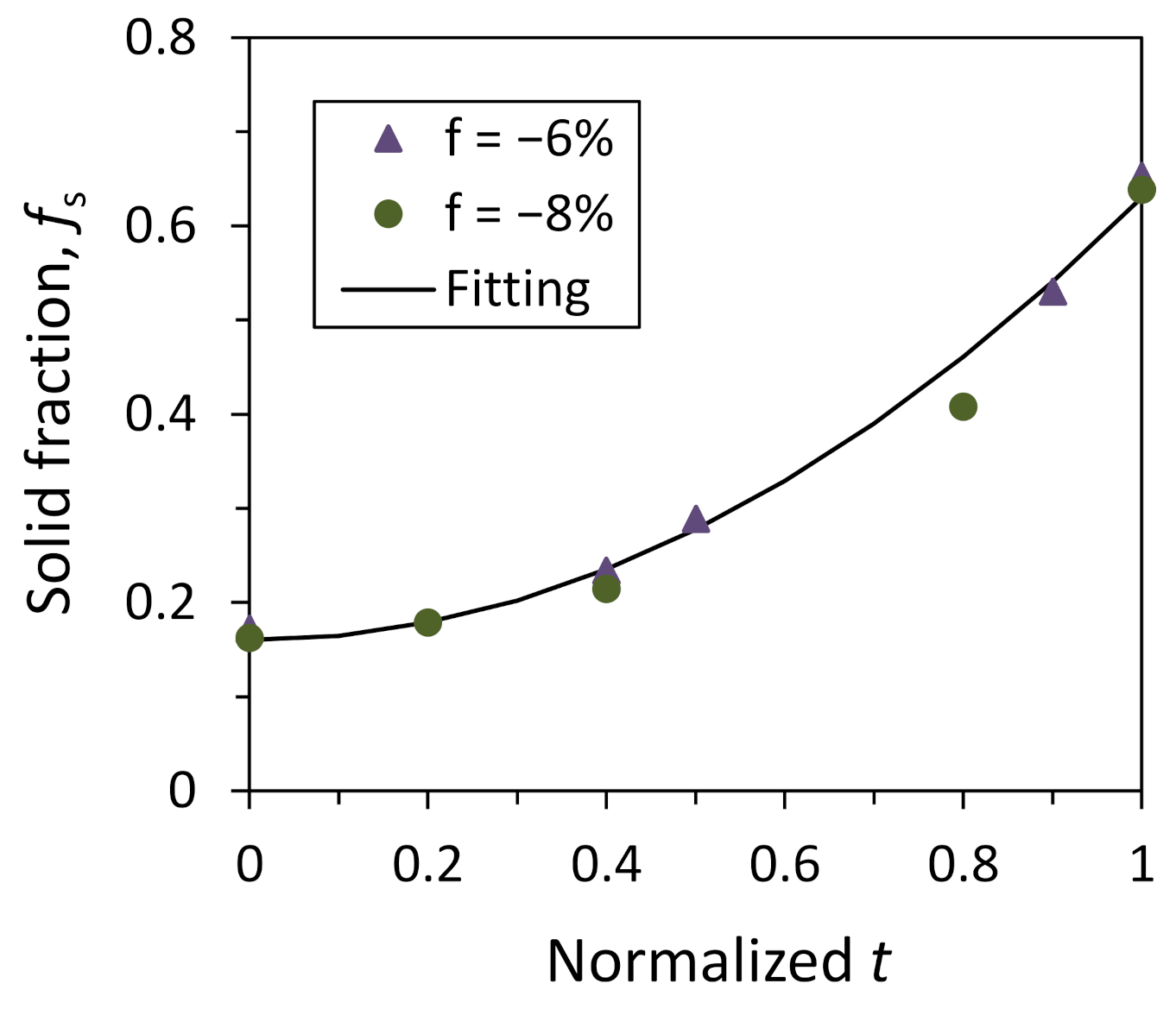
4.2. Heterogeneous Nucleation on a Substrate with Small Negative Misfit
4.3. Heterogeneous Nucleation on a Substrate with Small Positive Misfit
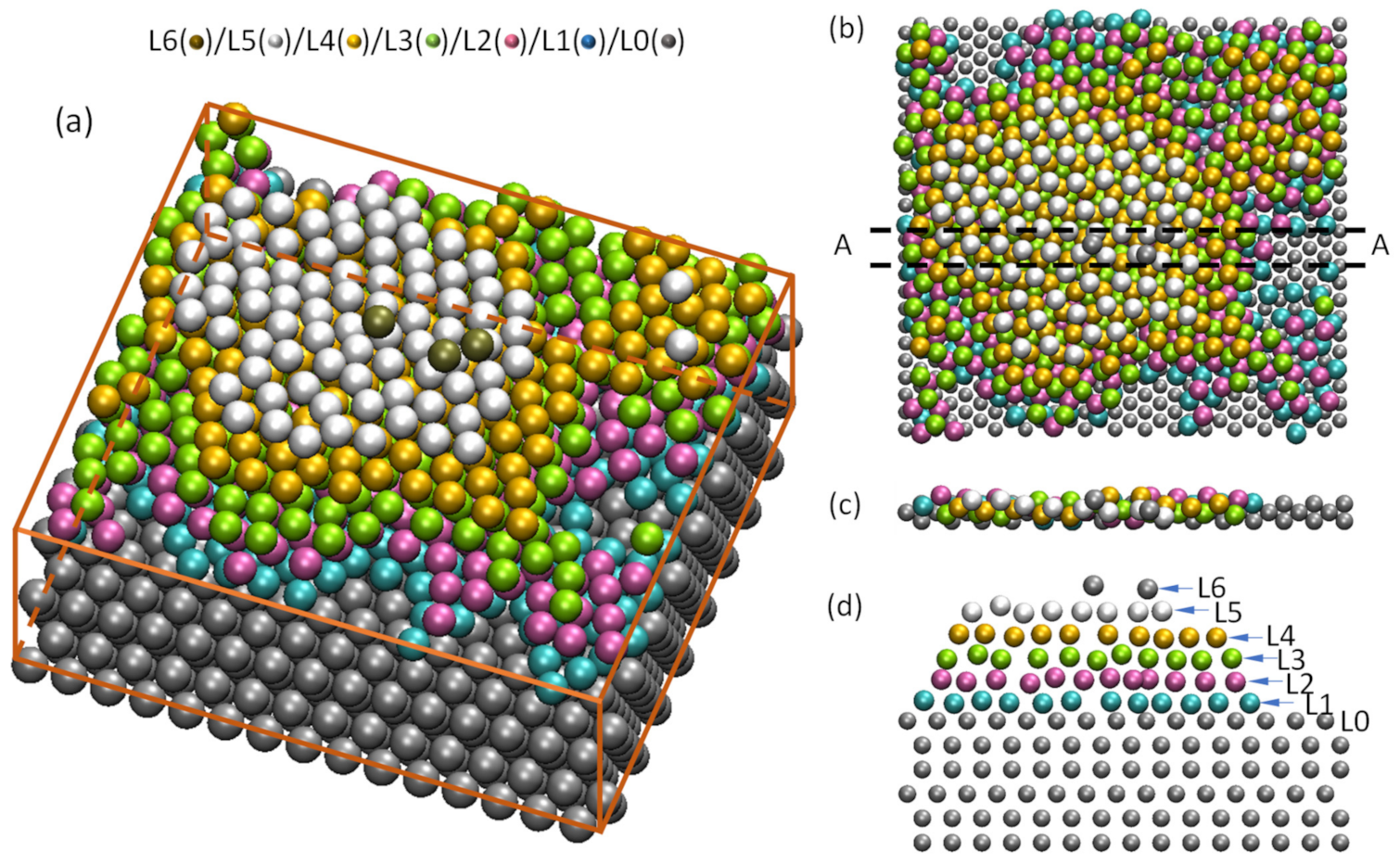
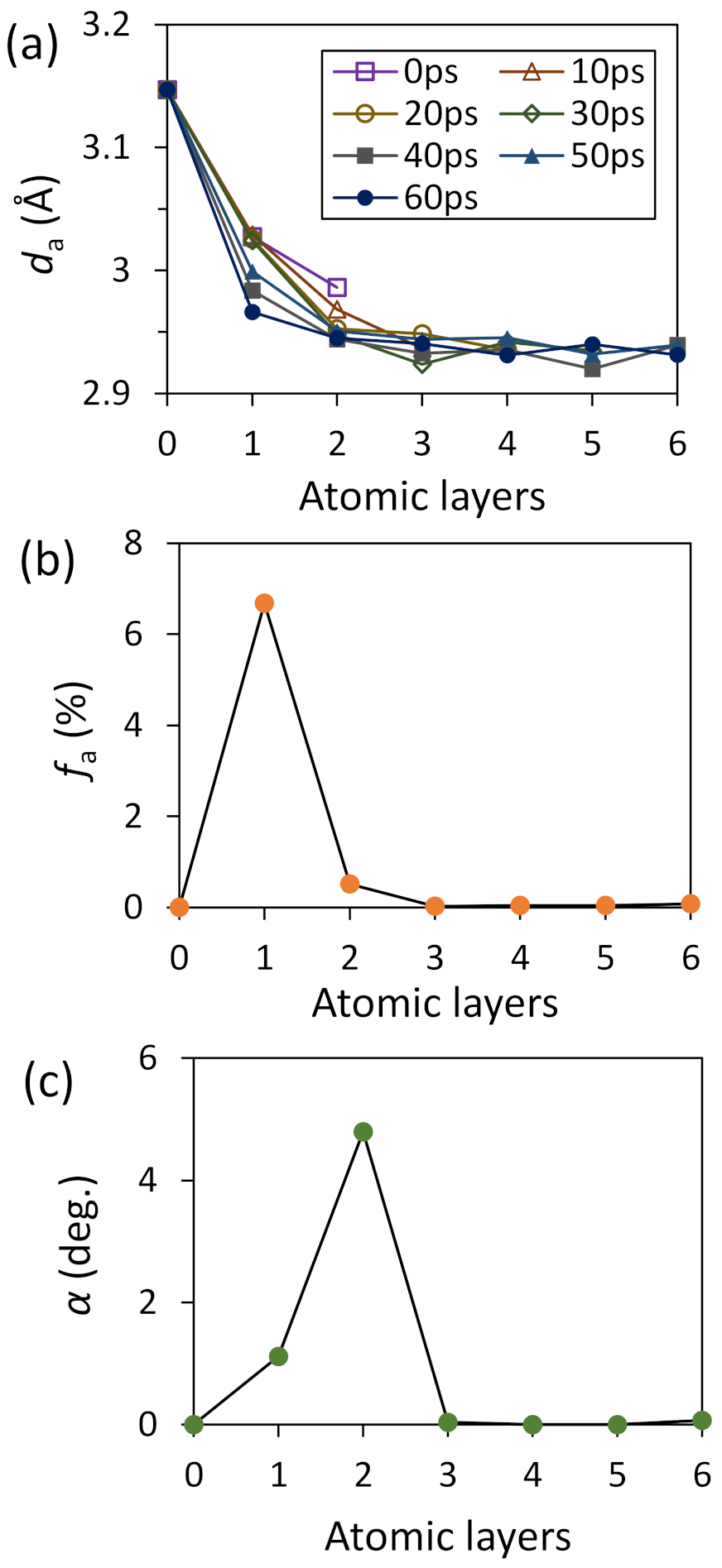
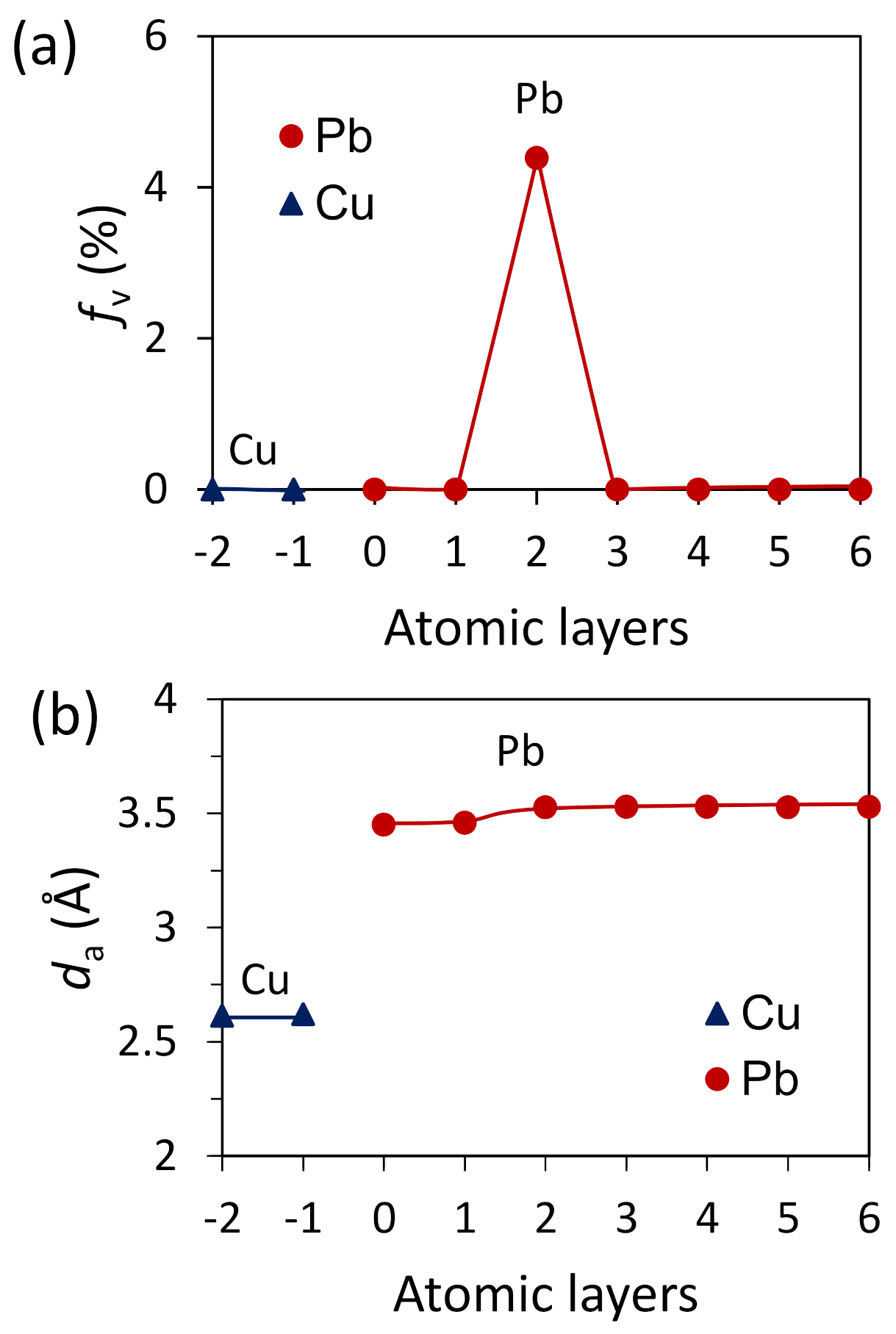
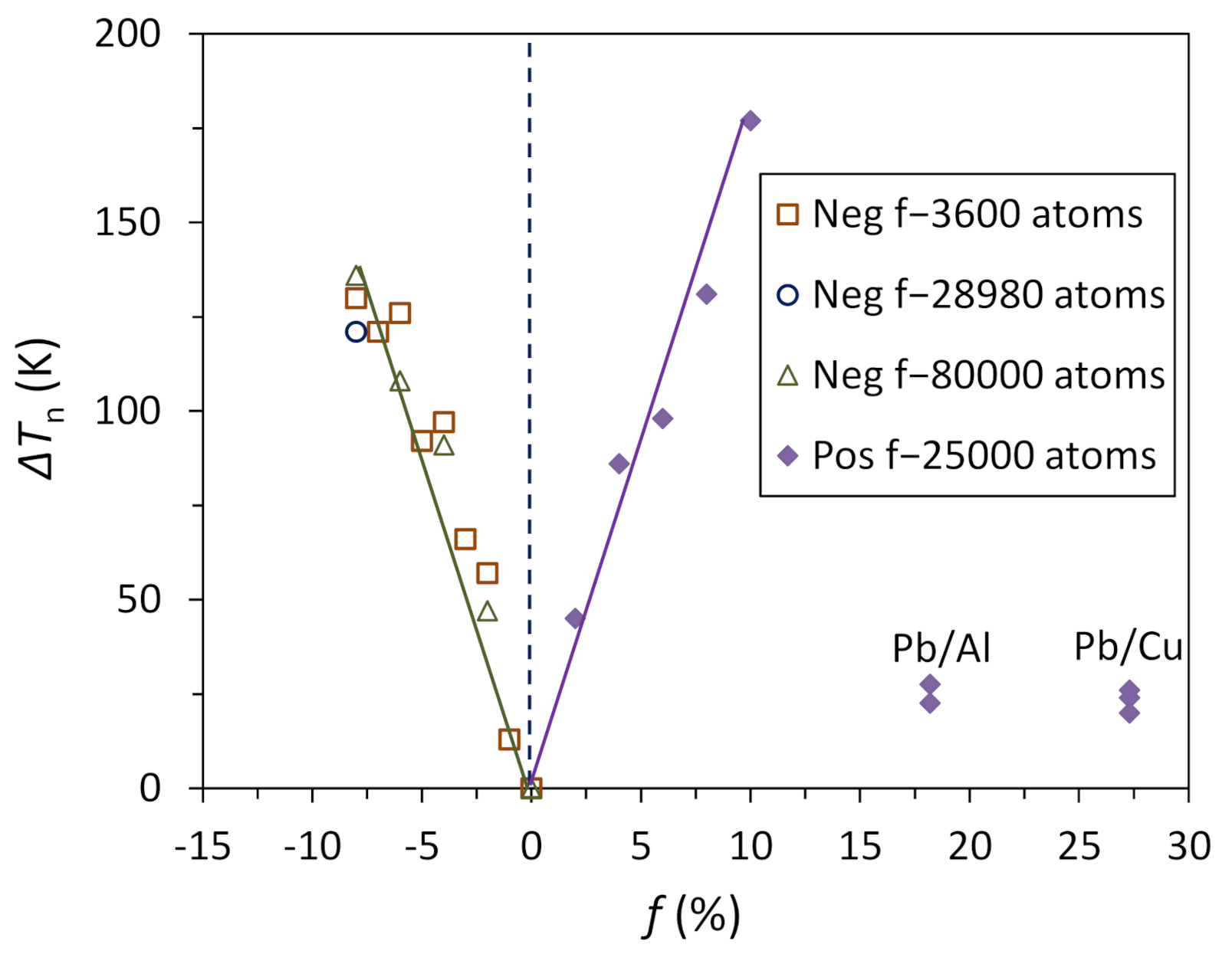
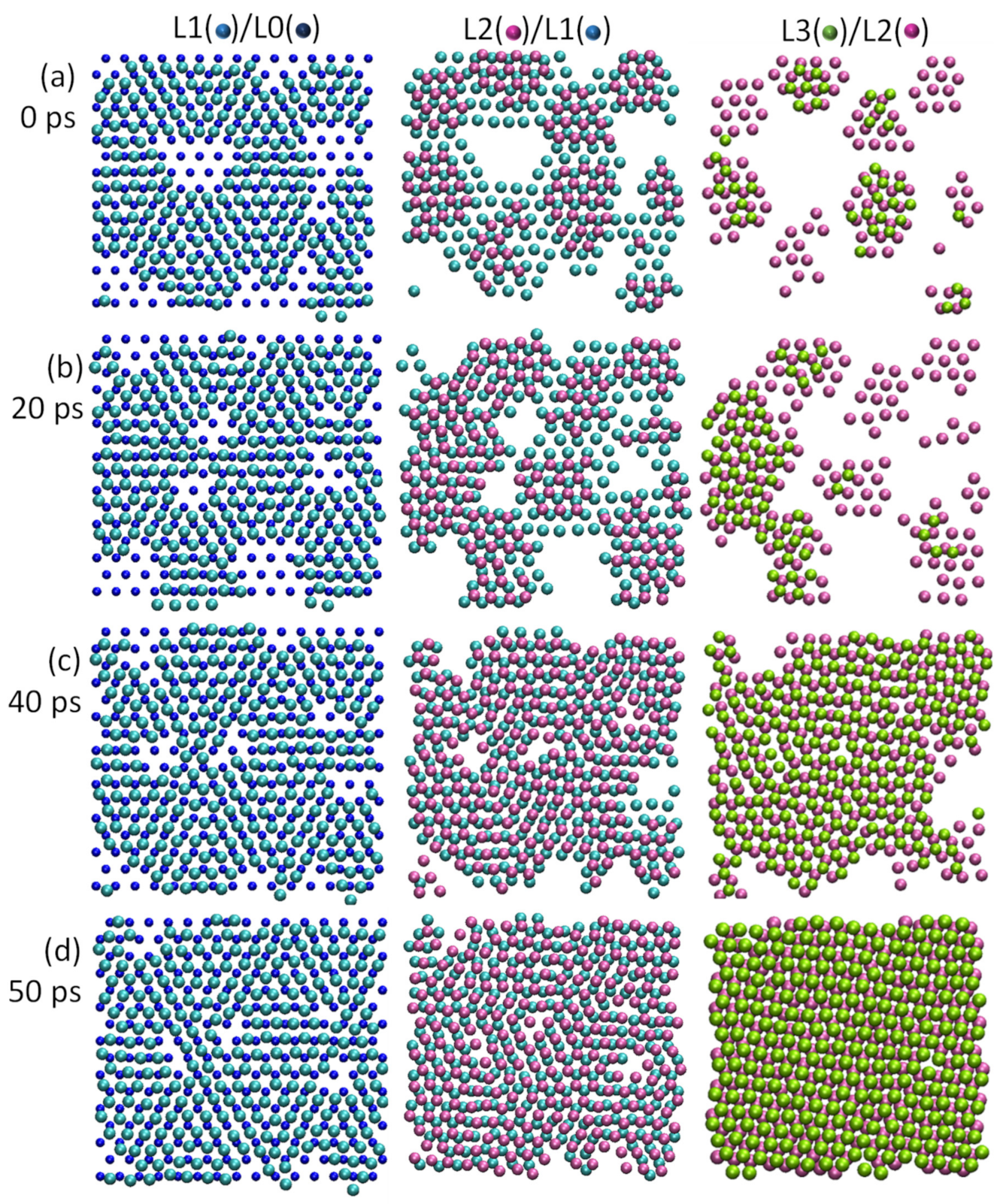
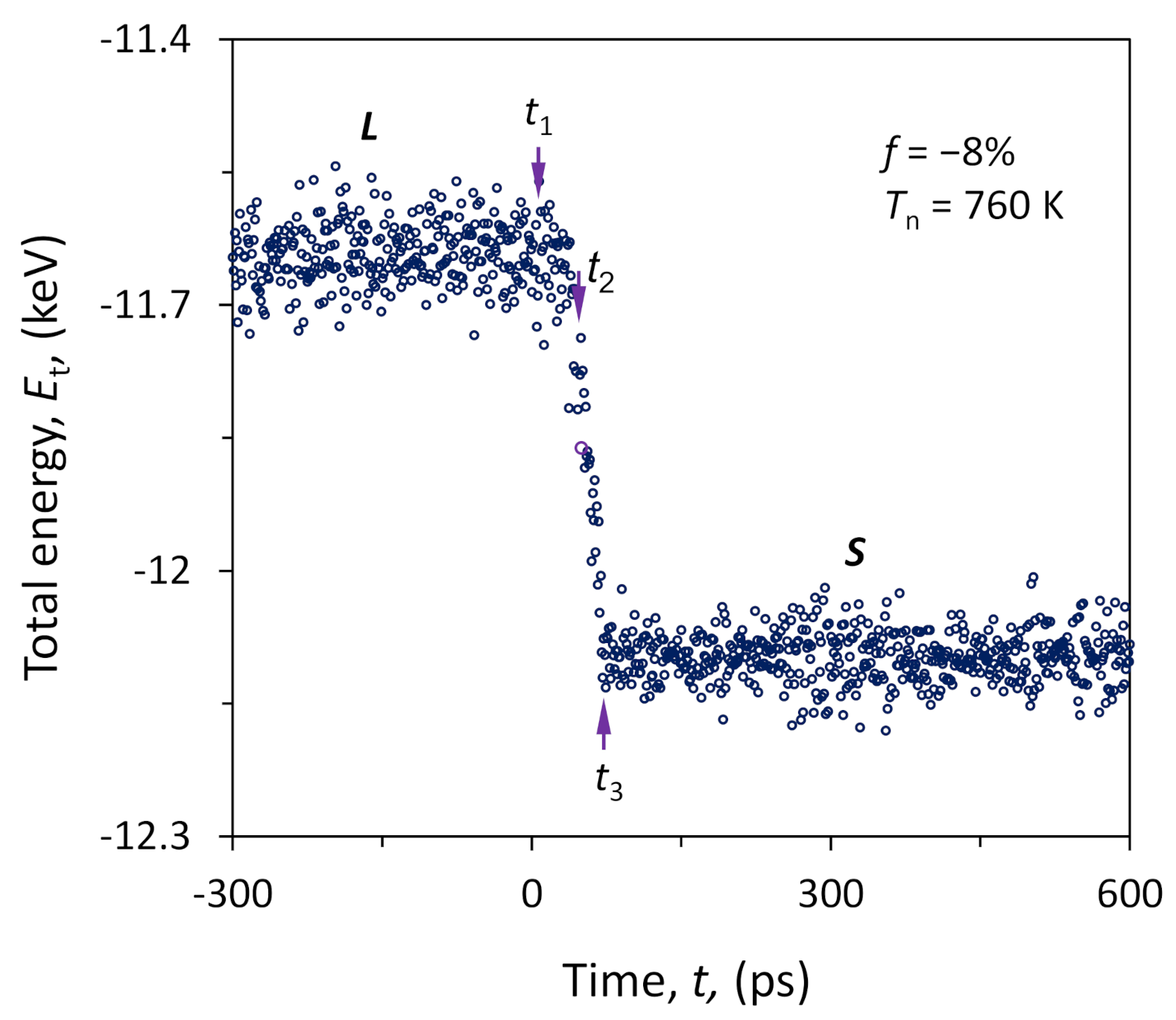
4.4. Heterogeneous Nucleation on a Substrate with Large Misfit
4.5. Heterogeneous Nucleation on Amorphous Substrate
5. General Discussion
5.1. Atomistic Mechanisms of Heterogeneous Nucleation
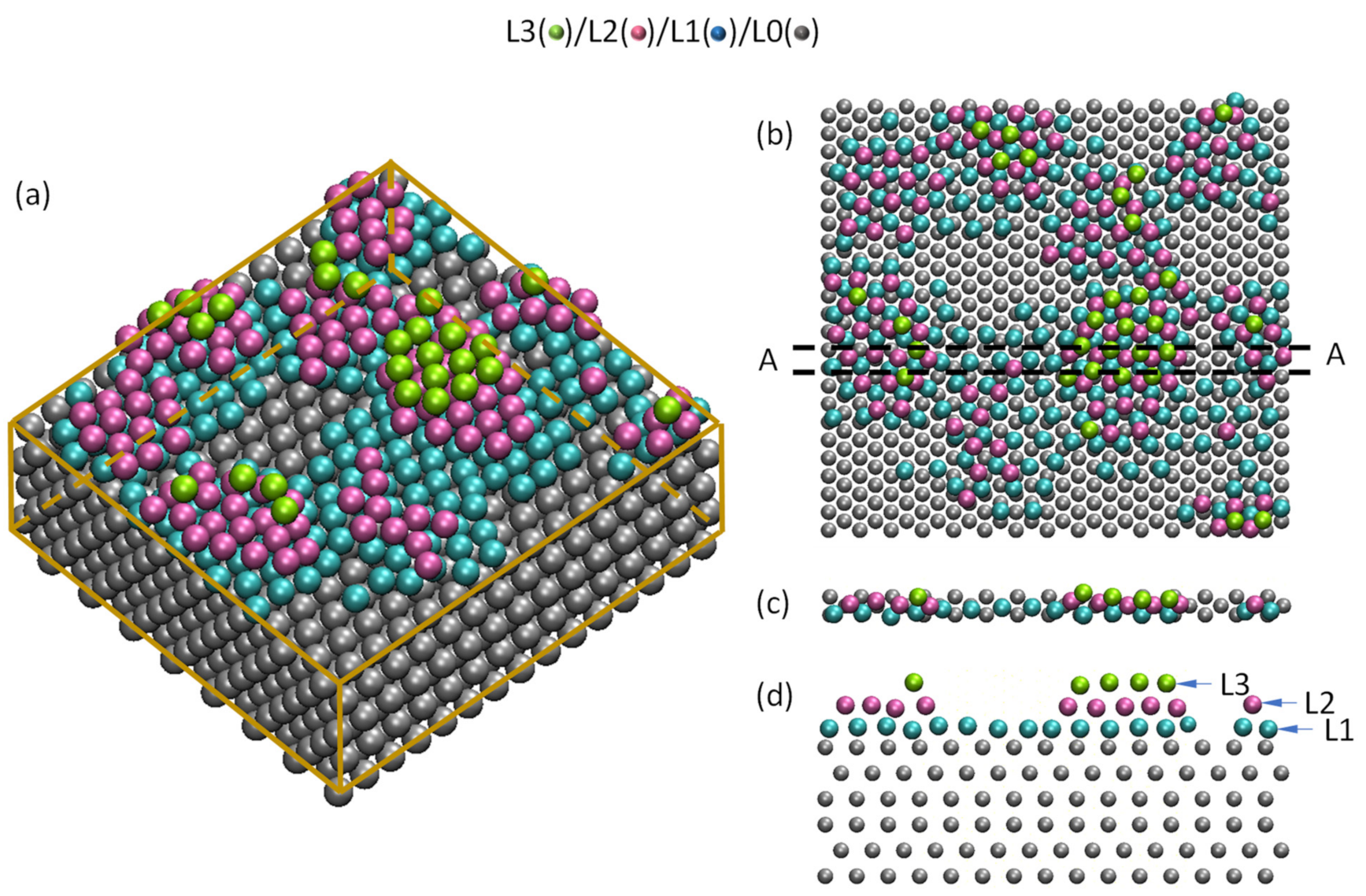
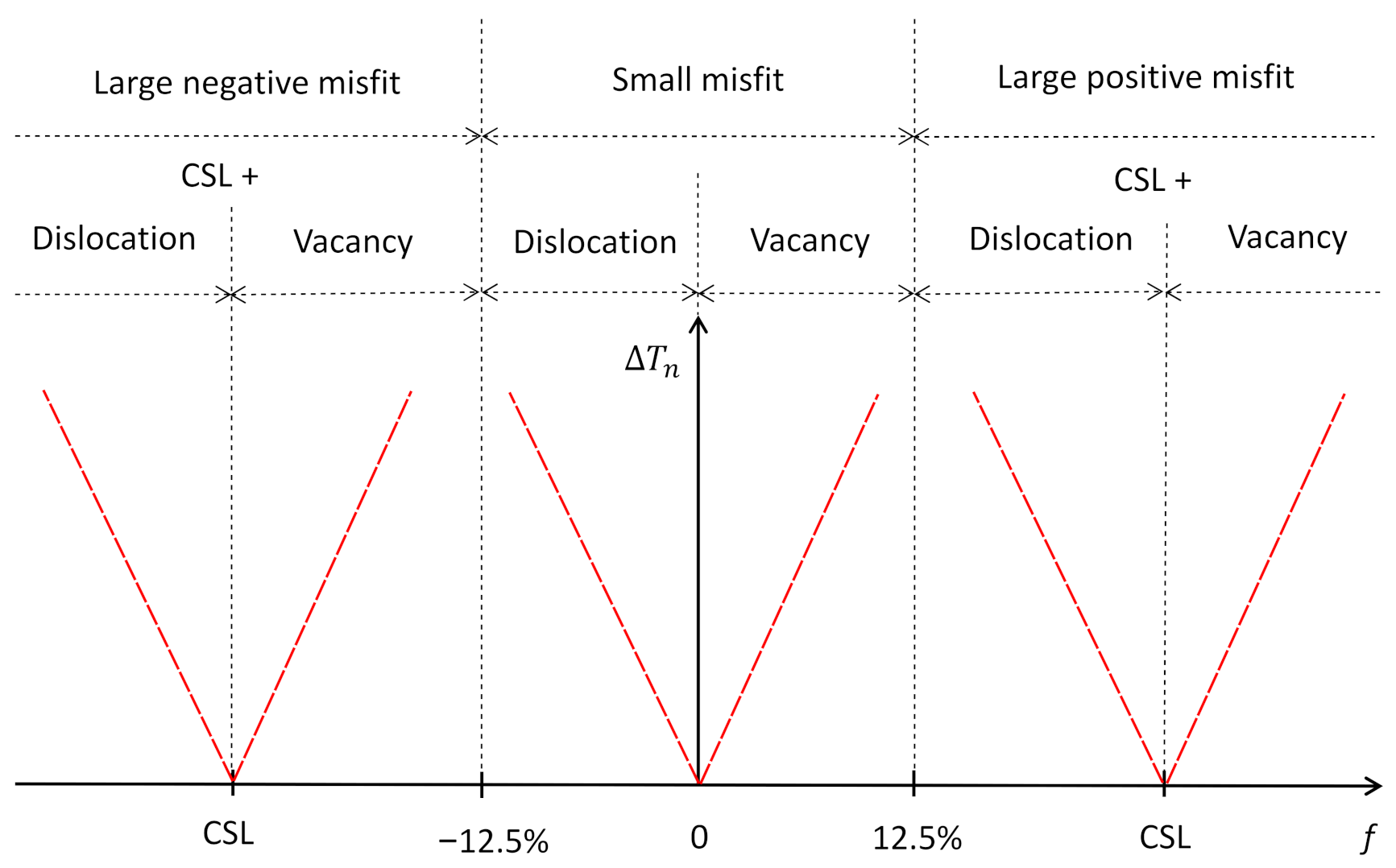
- For systems with small negative misfit (−12.5% < f < 0), misfit is accommodated by dislocation mechanism; edge dislocation network in L1 and screw dislocation network in L2.
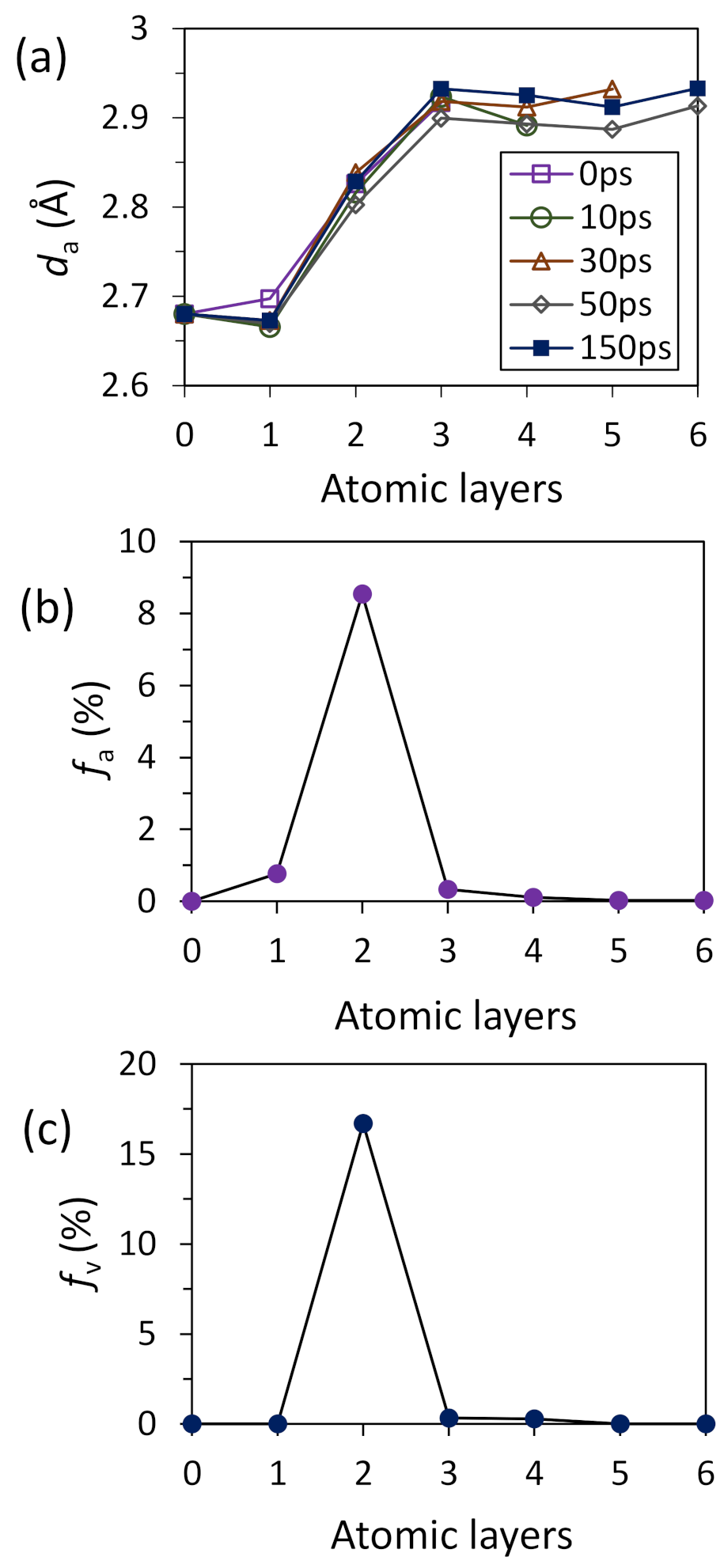
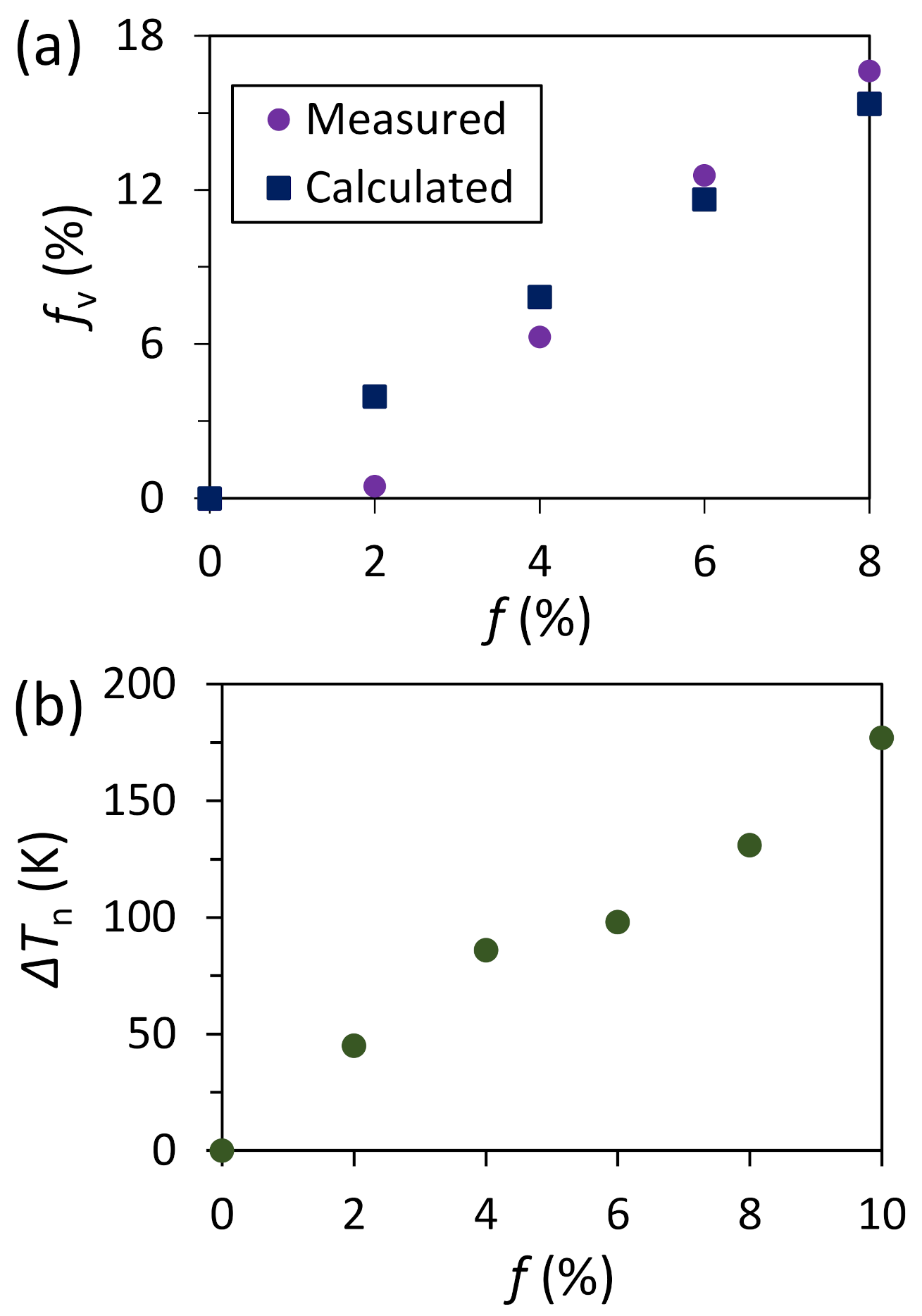
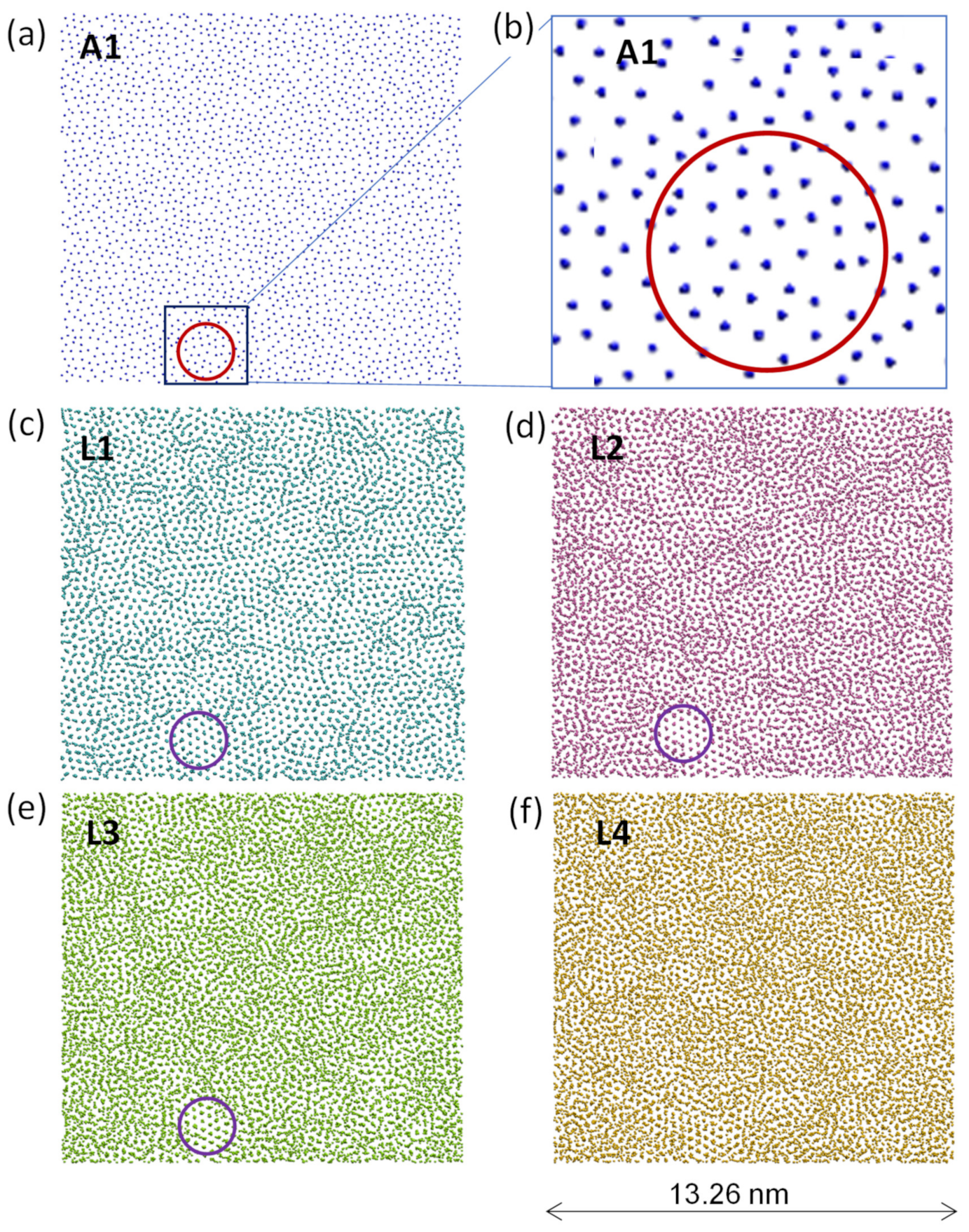
- For systems with small positive misfit (0 < f < 12.5%), misfit is accommodated by vacancy mechanism; L1 is epitaxy to the substrate and L2 contains vacancies to accommodate all the misfit.
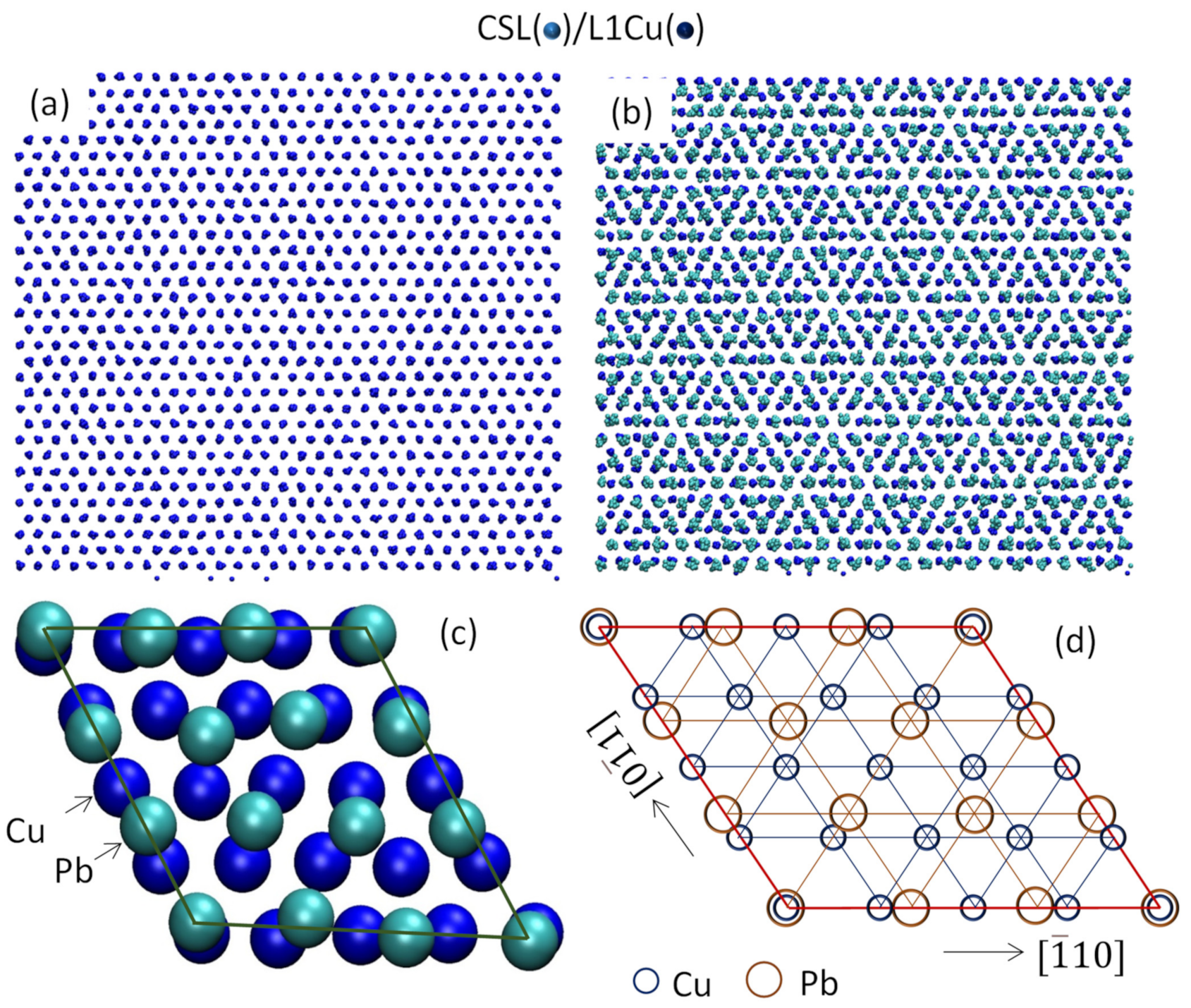
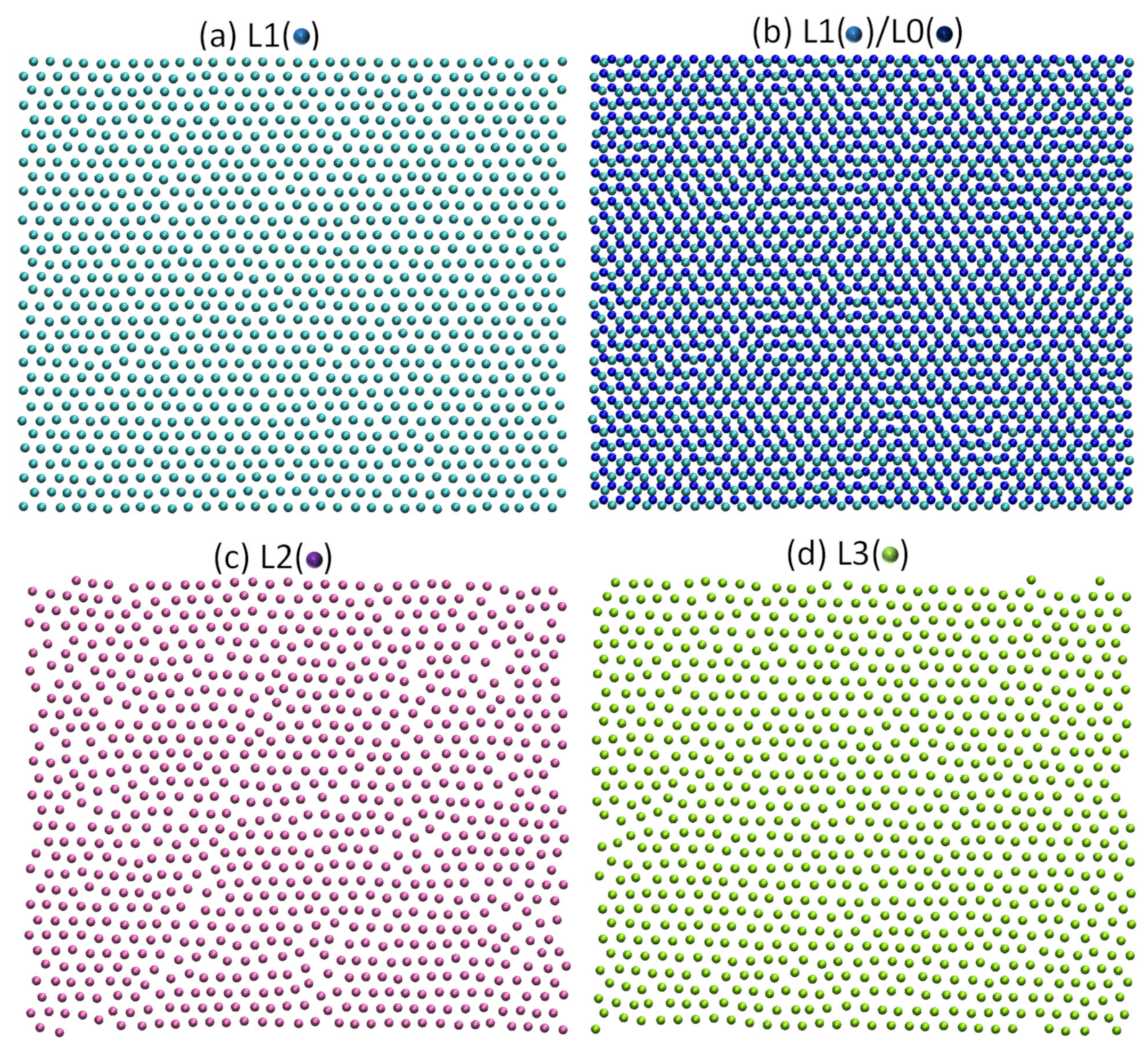
- For systems with large misfit (|f| > 12.5%), misfit is accommodated in two steps: formation of CSL during prenucleation to accommodate the majority of lattice misfit (fCSL), and the residual misfit fr (fr = f – fcsl) is accommodated during heterogeneous nucleation by dislocation mechanism if the residual misfit is less than 0, or by vacancy mechanism if the residual misfit is larger than 0.
5.2. Structural Templating
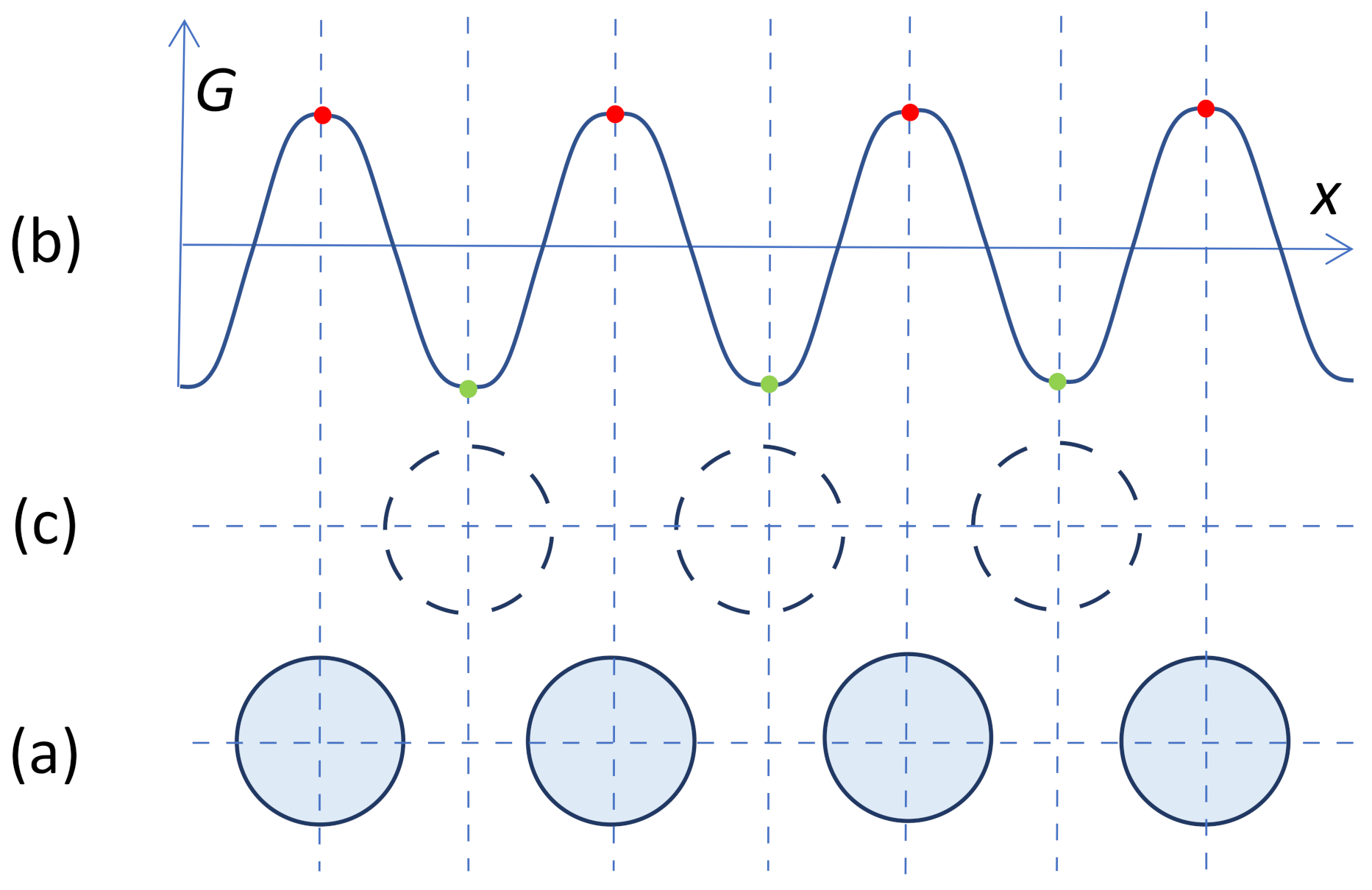
- Only solid atoms in the (i − 1)th layer can template solid atoms in the ith layer. This means no solid atoms can “sit” on top of liquid atoms.
- In an ordered patch, the number of solid atoms in the ith layer is always less than that in the (i − 1)th layer. This is the physical origin of curvature development during crystal growth, as will be discussed elsewhere [12].
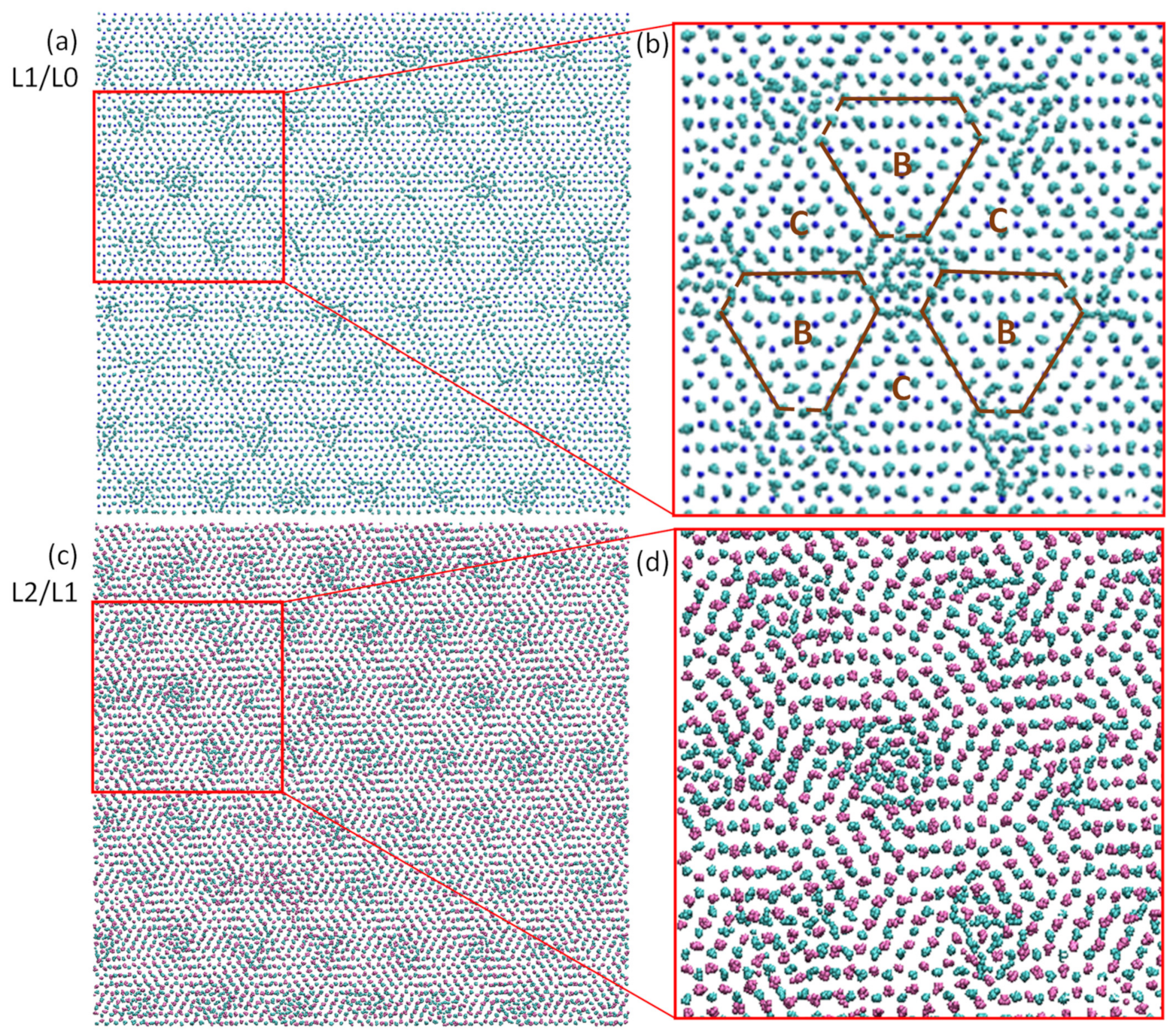
- In the case of fcc Al with an ABCA… stacking sequence, if the position of (i − 1)th layer is assumed to “A”, the positions of the solid atoms in the ith layer can be: (1) located at either “B” or “C” positions in a normal ordered region; (2) located between “B” and “C” positions in the core of a dislocation; and (3) found in both “B” and “C” positions at the nodes of a dislocation network, and the trajectories of such atoms form a circular pattern centred on top of a solid atom in the (i − 1)th layer (see Figure 28).
- Although atoms that hop between “B” and “C” positions have higher mobility than those located at either “B” or “C” positions, they can template solid atoms in the (i + 1)th layer in position “A”.
5.3. Nature of Heterogeneous Nucleation
5.3.1. Heterogeneous Nucleation Transforms a L/N Interface into a S/N Interface plus a L/S Interface
5.3.2. Heterogeneous Nucleation Is Spontaneous thus Barrierless
5.3.3. Heterogeneous Nucleation Is Deterministic rather Than Stochastic
6. Summary
- Only solid atoms in the (i − 1)th layer can template solid atoms in the ith layer. This means no solid atoms can “sit” on top of liquid atoms;
- In an ordered patch, the number of solid atoms in the ith layer is always less than that in the (i − 1)th layer. This is the physical origin of curvature development during crystal growth;
- In the case of fcc Al with an ABCA… stacking sequence, if the position of (i − 1)th layer is assumed to “A”, the positions of the solid atoms in the ith layer can be: (1) located at either “B” or “C” positions in a normal ordered region; (2) located between “B” and “C” positions in the core of a dislocation; and (3) found in both “B” and “C” positions at the nodes of a dislocation network, and the trajectories of such atoms form a circular pattern centred on top of a solid atom in the (i − 1)th layer;
- Although atoms that hop between “B” and “C” positions have higher mobility than those located at either “B” or “C” positions, they can template solid atoms in the (i + 1)th layer in position “A”.
- The atomistic mechanisms for accommodating lattice misfit are described as:
- For systems with small negative misfit (−12.5% < f < 0), misfit is accommodated by dislocation mechanism, i.e., edge dislocation network in L1 and screw dislocation network in L2;
- For systems with small positive misfit (0 < f < 12.5%), misfit is accommodated by vacancy mechanism, where L1 is epitaxy to the substrate and L2 contains vacancies to accommodate all the misfit;
- For systems with large misfit (|f| > 12.5%), misfit is accommodated in two steps: formation of CSL during prenucleation to accommodate the majority of the misfit (fCSL), and the residual misfit fr (fr = f – fcsl) is accommodated during heterogeneous nucleation by dislocation mechanism if the residual misfit is less than 0 or by vacancy mechanism if the residual misfit is larger than 0.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartels-Rausch, T. Chemistry: Ten things we need to know about ice and snow. Nature 2013, 494, 27–29. [Google Scholar] [CrossRef]
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal nucleation in liquids: Open questions and future challenges in molecular dynamics simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.L. Overview: Application of heterogeneous nucleation in grain-refining of metals. J. Chem. Phys. 2016, 145, 211704. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Polymorph selection: The role of nucleation, crystal growth and molecular modelling. Curr. Opin. Drug Discov. Devel. 2007, 10, 746–755. [Google Scholar] [PubMed]
- Michaels, T.C.T.; Šarić, A.; Curk, S.; Bernfur, K.; Arosio, P.; Meisl, G.; Dear, A.J.; Cohen, S.I.A.; Dobson, C.M.; Vendruscolo, M.; et al. Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Aβ42 peptide. Nat. Chem. 2020, 12, 445–451. [Google Scholar] [CrossRef]
- Wolde, P.R.; Frenkel, D. Homogeneous nucleation and the Ostwald step rule. Phys. Chem. Chem. Phys. 1999, 1, 2191–2196. [Google Scholar] [CrossRef]
- Vekilov, P.G. Two-step mechanism for the nucleation of crystals from solution. J. Cryst. Growth 2005, 275, 65–76. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef]
- Smeets, P.J.M.; Finney, A.R.; Habraken, W.J.E.M.; Nudelman, F.; Friedrich, H.; Laven, J.; De Yoreo, J.J.; Rodger, P.M.; Sommerdijk, N.A.J.M. A classical view on nonclassical nucleation. Proc. Natl. Acad. Sci. USA 2017, 114, E7882–E7890. [Google Scholar] [CrossRef]
- Gebauer, D.; Raiteri, P.; Gale, J.D.; Cölfen, H. On classical and non-classical views on nucleation. Am. J. Sci. 2018, 318, 969–988. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H.; Wang, Y.; Que, Z.P. A new atomistic mechanism for heterogeneous nucleation in the systems with negative lattice misfit: Creating a 2D template for crystal growth. Metals 2021, 11, 478. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H. Heterogeneous nucleation and grain initiation on a single substrate. Metals 2022, 12, 1454. [Google Scholar] [CrossRef]
- Easton, M.A.; Qian, M.; Prasad, A.; StJohn, D.H. Recent advances in grain refinement of light metals and alloys. Curr. Opin. Solid State Mater. Sci. 2016, 20, 13–24. [Google Scholar] [CrossRef]
- Kelton, K.F.; Greer, A.L. Nucleation in Condensed Mater: Applications in Materials and Biology; Elsevier Science: Oxford, UK, 2010. [Google Scholar]
- Nose, S.; Yonezawa, F. Isothermal-isobaric computer simulations of melting and crystallization of a Lennard-Jones system. J. Chem. Phys. 1986, 84, 1803–1814. [Google Scholar] [CrossRef]
- Auer, S.; Frenkel, D. Prediction of absolute crystal nucleation rate in hard-sphere colloids. Nature 2001, 409, 1020–1023. [Google Scholar] [CrossRef]
- Shibuta, Y.; Sakane, S.; Takaki, T.; Ohno, M. Submicrometer-scale molecular dynamics simulation of nucleation and solidification from undercooled melt: Linkage between empirical interpretation and atomistic nature. Acta Mater. 2016, 105, 328–337. [Google Scholar] [CrossRef]
- Mahata, A.; Zaeem, M.A.; Baskes, M. Understanding homogeneous nucleation in solidification of aluminum by molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2018, 26, 025007. [Google Scholar] [CrossRef]
- Volmer, M.; Weber, A.Z. Nucleus formation in supersaturated systems. Z. Für Phys. Chem. 1926, 119, 277–301. [Google Scholar] [CrossRef]
- Becker, R.; Döring, W. Kinetic treatment of nucleation in supersaturated vapors. Ann. Phys. 1935, 24, 719–752. [Google Scholar] [CrossRef]
- Zeldovich, J.B. On the theory of new phase formation. Cavitation. Acta Physicochim. USSR 1943, 18, 1–22. [Google Scholar]
- Turnbull, D. Kinetics of heterogeneous nucleation. J. Chem. Phys. 1950, 18, 198. [Google Scholar] [CrossRef]
- Turnbull, D. Kinetics of solidification of supercooled liquid mercury droplets. J. Chem. Phys. 1952, 20, 411–424. [Google Scholar] [CrossRef]
- Moore, K.I.; Zhang, D.L.; Cantor, B. Solidification of Pb particles embedded in Al. Acta Metall. Mater. 1990, 38, 1327–1342. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hoose, C.; Wang, B. Different contact angle distributions for heterogeneous ice nucleation in the community atmospheric aodel version 5. Atmos. Chem. Phys. 2014, 14, 10411–10430. [Google Scholar] [CrossRef]
- Cantor, B. Heterogeneous nucleation and adsorption. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2003, 361, 409–417. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. Solidification behaviour of Pb droplets embedded in a Cu matrix. Acta Metall. Mater. 1992, 40, 3339–3347. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. Heterogeneous nucleation of Al2Cu in Al-Cu eutectic liquid droplets embedded in an Al matrix. Acta Metall. Mater. 1994, 42, 3045–3053. [Google Scholar] [CrossRef]
- Kim, W.T.; Zhang, D.L.; Cantor, B. Nucleation of solidification in liquid droplets. Metall. Trans. A 1991, 22A, 2487–2501. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. Solidification of tin droplets embedded in an aluminium matrix. J. Mater. Sci. 1991, 26, 2868–2878. [Google Scholar] [CrossRef]
- Turnbull, D.; Vonnegut, B. Nucleation catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Fan, Z. An epitaxial model for heterogeneous nucleation on potent substrates. Metall. Mater. Trans. A 2013, 44, 1409–1418. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Qian, M.; Taylor, J.A. Crystallography of grain refinement in Mg–Al based alloys. Acta Mater. 2005, 53, 3261. [Google Scholar] [CrossRef]
- Tóth, G.I.; Tegze, G.; Pusztai, T.; Gránásy, L. Heterogeneous crystal nucleation: The effect of lattice mismatch. Phys. Rev. Lett. 2012, 108, 025502. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Zhang, D.; Xia, M.; Wang, Y.; Li, J.G. The role of lattice misfit on heterogeneous nucleation of pure aluminium. Metall. Mater. Trans. A 2016, 47, 5012–5022. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.Q.; Hu, Q.D.; Xia, M.X.; Wang, Y.; Li, J.G. Interfacial tuning for the nucleation of liquid AlCu alloy. Acta Mater. 2017, 139, 75–85. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H. A molecular dynamics study of heterogeneous nucleation in generic liquid/substrate systems with positive lattice misfit. Mater. Res. Express 2020, 7, 126501. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Heterogeneous nucleation mechanisms in systems with large lattice misfit demonstrated by the Pb(l)/Cu(s) system. Metals 2022. in print. [Google Scholar]
- Wang, J.S.; Horsfield, A.; Schwingenschlögl, U.; Lee, P.D. Heterogeneous nucleation of solid Al from the melt by Al3Ti: Molecular dynamics simulations. Phys. Rev. B 2010, 82, 184203. [Google Scholar] [CrossRef]
- Wearing, D.; Horsfield, A.P.; Xu, W.W.; Lee, P.D. Which wets TiB2 inoculant particles: Al or Al3Ti? J. Alloys Compd. 2016, 664, 460–468. [Google Scholar] [CrossRef]
- Palafox-Hernandez, J.P.; Laird, B.B. Orientation dependence of heterogeneous nucleation at the Cu-Pb solid-liquid interface. J. Chem. Phys. 2016, 145, 211914. [Google Scholar] [CrossRef] [PubMed]
- Sosso, G.C.; Li, T.S.; Donadio, D.; Tribello, G.A.; Michaelides, A. Microscopic mechanism and kinetics of ice formation at complex interfaces: Zooming in on kaolinite. J. Phys. Chem. Lett. 2016, 7, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Cabriolu, R.; Li, T.S. Ice nucleation on carbon surface supports the classical theory for heterogeneous nucleation. Phys. Rev. E 2015, 91, 052402. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Li, T.; Li, H. Molecular dynamics study on the heterogeneous nucleation of liquid Al-Cu alloys on different kinds of copper substrates. Phys. Chem. Chem. Phys. 2018, 20, 29856–29865. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, T.; Shibuta, Y. Molecular dynamics simulation of athermal heterogeneous nucleation of solidification. Comput. Mater. Sci. 2019, 164, 74–81. [Google Scholar] [CrossRef]
- Mithen, J.P.; Sear, R.P. Computer simulation of epitaxial nucleation of a crystal on a crystalline surface. J. Chem. Phys. 2014, 140, 084504. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.L. Liquid metals: Supercool order. Nat. Mater. 2006, 5, 13–14. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Prenucleation induced by crystalline substrates. Metall. Mater. Trans. A 2018, 49, 2766–2777. [Google Scholar] [CrossRef]
- Fang, C.M.; Men, H.; Fan, Z. Effect of substrate chemistry on prenucleation. Metall. Mater. Trans. A 2018, 49, 6231–6242. [Google Scholar] [CrossRef]
- Jiang, B.; Men, H.; Fan, Z. Atomic ordering in the liquid adjacent to an atomic-level rough substrate surface. Comput. Mater. Sci. 2018, 153, 73–81. [Google Scholar] [CrossRef]
- Zope, R.R.; Mishin, Y. Interatomic potentials for atomistic simulations of the Ti-Al system. Phys. Rev. B 2003, 68, 024102. [Google Scholar] [CrossRef]
- Todorov, I.T.; Smith, W.; Trachenko, K.; Dove, M.T. DL_POLY_3: New dimensions in molecular dynamics simulations via massive parallelism. J. Mater. Chem. 2006, 16, 1911–1918. [Google Scholar] [CrossRef]
- Hashibon, A.; Adler, J.; Finnis, M.W.; Kaplan, W.D. Ordering at solid-liquid interfaces between dissimilar materials. Interface Sci. 2001, 9, 175–181. [Google Scholar] [CrossRef]
- Hook, J.R.; Hall, H.E. Solid State Physics, 2nd ed.; Wiley: Chichester, UK, 1991. [Google Scholar]
- Jackson, K.A. The interface kinetics of crystal growth processes. Interface Sci. 2002, 10, 159–169. [Google Scholar] [CrossRef]
- Steinhardt, P.J.; Nelson, D.R.; Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 1983, 28, 784–805. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Coadou, C.L.; Fratzl, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef]
- Kaplan, W.D.; Kauffmann, Y. Structural order in liquids induced by interfaces with crystal. Annu. Rev. Mater. Res. 2006, 36, 1–48. [Google Scholar] [CrossRef]
- Oh, S.H.; Kauffmann, Y.; Scheu, C.; Kaplan, W.D.; Rühle, M. Ordered liquid aluminium at the interface with sapphire. Science 2005, 310, 661–663. [Google Scholar] [CrossRef]
- Kauffmann, Y.; Oh, S.H.; Koch, C.T.; Hashibon, A.; Scheu, C.; Rühle, M.; Kaplan, W.D. Quantitative analysis of layering and in-plane ordering at an alumina-aluminium solid-liquid interface. Acta Mater. 2011, 59, 4378–4386. [Google Scholar] [CrossRef]
- Geysermans, P.; Gorse, D.; Pontikis, V. Molecular dynamics study of the solid-liquid interface. J. Chem. Phys. 2000, 113, 6382–6389. [Google Scholar] [CrossRef]
- Hashibon, A.; Adler, J.; Finnis, M.W.; Kaplan, W.D. Atomistic study of structural correlations at a liquid-solid interface. Comp. Mater. Sci. 2002, 24, 443–452. [Google Scholar] [CrossRef]
- Yang, Y.; Olmsted, D.L.; Asta, M.; Laird, B.B. Atomistic characterization of the chemically heterogeneous Al-Pb solid-liquid interface. Acta Mater. 2012, 60, 4960–4971. [Google Scholar] [CrossRef]
- Palafox-Hernandez, J.P.; Laird, B.B.; Asta, M. Atomistic characterization of the Cu-Pb solid-liquid interface. Acta Mater. 2011, 59, 3137–3144. [Google Scholar] [CrossRef]
- Men, H.; Fang, C.M.; Fan, Z. Prenucleation at the liquid/substrate interface: An overview. Metals 2022. in review. [Google Scholar]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al-Ti-B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Molecular dynamics simulations on effect of surface roughness of amorphous substrate on nucleation in liquid Al. Metals 2022, 12, 1529. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xia, M.X.; Arumuganathar, S. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing. Acta Mater. 2009, 57, 4891–4901. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Fan, Z.Y. Mechanism of zirconium poisoning effect on TiB2 inoculation in aluminium alloys. In Light Metals; Springer: Cham, Switzerland, 2016; pp. 725–729. [Google Scholar]
- O’reilly, K.A.Q.; Cantor, B. Solidification behavior of Al particles embedded in a Zr aluminide matrix. Acta Metall. Mater. 1995, 43, 405–417. [Google Scholar] [CrossRef]
- Zhang, D.L.; Cantor, B. Melting behavior of In and Pb particles embedded in an Al matrix. Acta Metall. Mater. 1991, 39, 1595–1602. [Google Scholar] [CrossRef]
- Goswami, R.; Chattopadhyay, K.; Kim, W.T.; Cantor, B. Heterogeneous nucleation of Pb particles embedded in a Zn matrix. Metall. Trans. A 1992, 23A, 3207. [Google Scholar]
- Zhang, D.L.; Cantor, B. Heterogeneous nucleation of In particles embedded in an AI matrix. Phil. Mag. A 1990, 62, 557–572. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, F.; Song, H.J.; Mendelev, M.I.; Wang, C.-Z.; Ho, K.-M. Temperature dependence of the solid-liquid interface free energy of Ni and Al from molecular dynamics simulation of nucleation. J. Chem. Phys. 2018, 149, 174501. [Google Scholar] [CrossRef] [PubMed]
- Hirth, J.P.; Lothe, J. Theory of Dislocations, 2nd ed.; John Wiley: New York, NY, USA, 1982. [Google Scholar]
- Gillan, M.J. Calculation of the vacancy formation energy in aluminium. J. Phys. Condens. Matter 1989, 1, 689. [Google Scholar] [CrossRef]





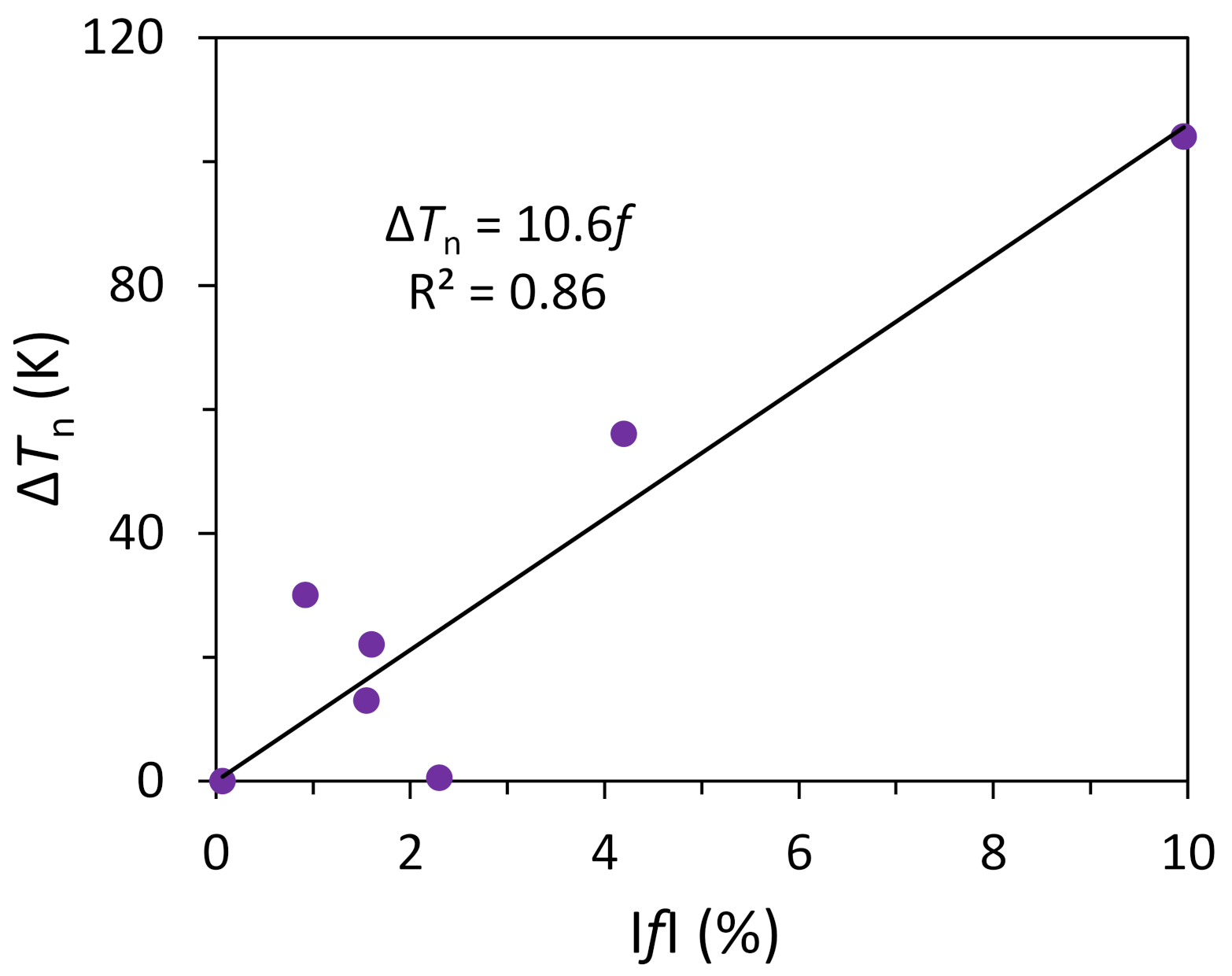
| Droplets | Matrix | OR | f or fr (%) | ΔTn (K) |
|---|---|---|---|---|
| Al | Al3Zr | {001}<100>Al//{00l}<100>Al3Zr | 0.07 | 0 |
| Cd | Al | {0001}<11-20>Cd//{111}<110>A1 | 4.2 | 56 |
| Sn | Al | {1 00}<010>Sn//{1 1 1}<-211>AI; {1 00}<011>Sn//{1 00}<0 11>Al | −9.96 | 104 |
| Pb | Al | {111}<011>Pb//{111}<011>Al | −1.6 | 22 |
| Pb | Zn | (111)<1-10>Pb//(0001)<11-20>Zn | −0.92 | 30 |
| Pb | Cu | {100}<010>Pb//{100}<010>Cu | 2.3 | 0.5 |
| In | Al | {111}<110>In//{111}<110>Al | 1.55 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Men, H. An Overview on Atomistic Mechanisms of Heterogeneous Nucleation. Metals 2022, 12, 1547. https://doi.org/10.3390/met12091547
Fan Z, Men H. An Overview on Atomistic Mechanisms of Heterogeneous Nucleation. Metals. 2022; 12(9):1547. https://doi.org/10.3390/met12091547
Chicago/Turabian StyleFan, Zhongyun, and Hua Men. 2022. "An Overview on Atomistic Mechanisms of Heterogeneous Nucleation" Metals 12, no. 9: 1547. https://doi.org/10.3390/met12091547
APA StyleFan, Z., & Men, H. (2022). An Overview on Atomistic Mechanisms of Heterogeneous Nucleation. Metals, 12(9), 1547. https://doi.org/10.3390/met12091547







