Investigation of the Heat Treatment Process and Formation Mechanism of Grain Boundary Serration for GH4795 Superalloy
Abstract
:1. Introduction
2. Materials and Experimental
3. Results and Discussion
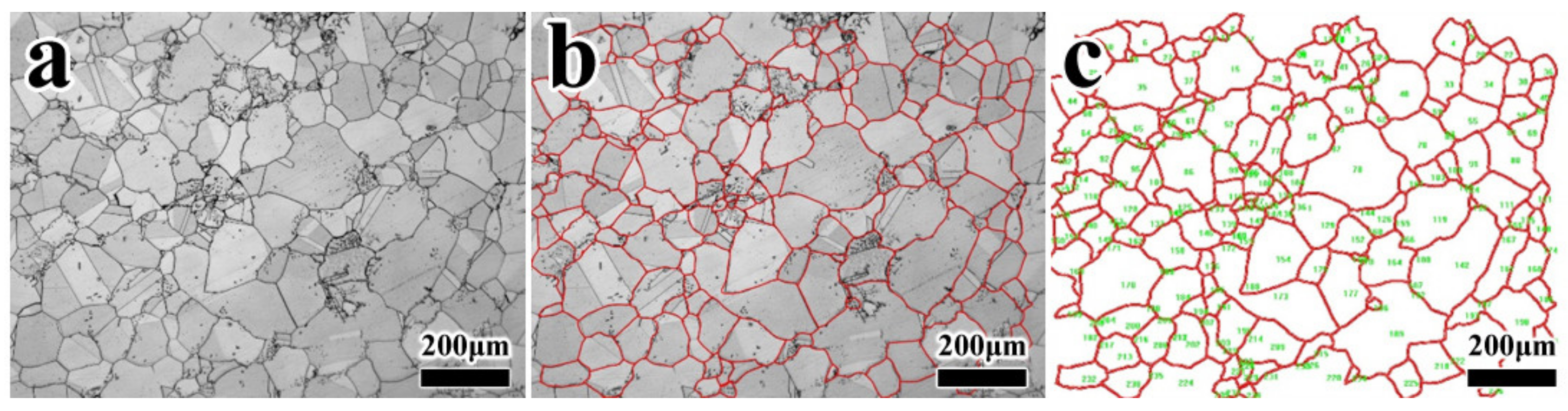
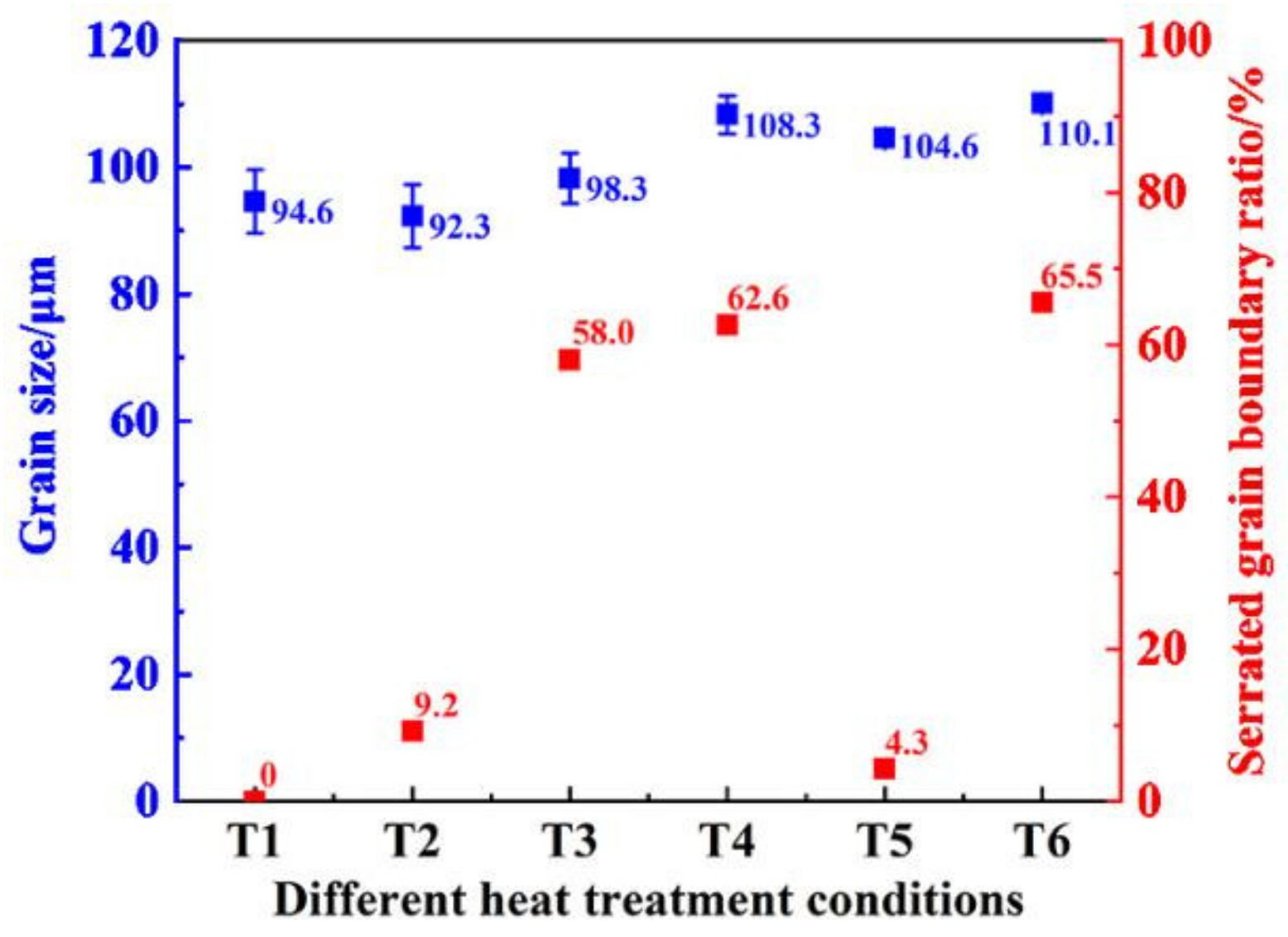
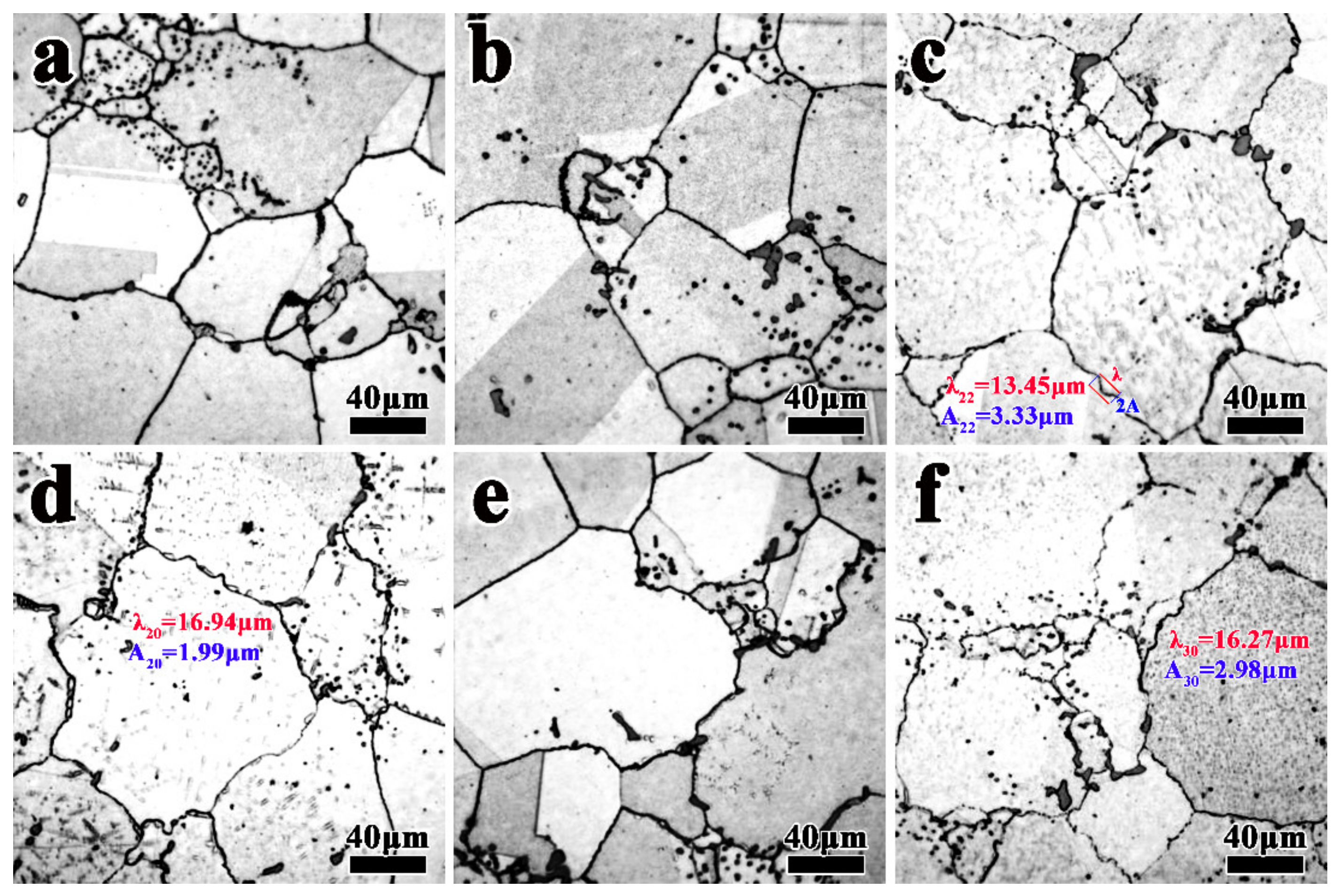
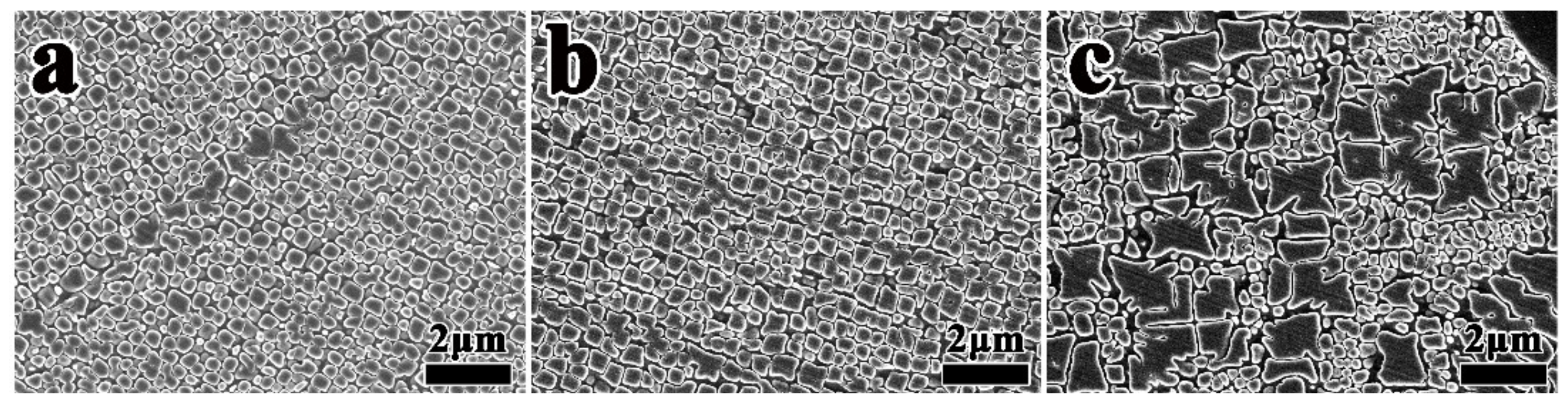
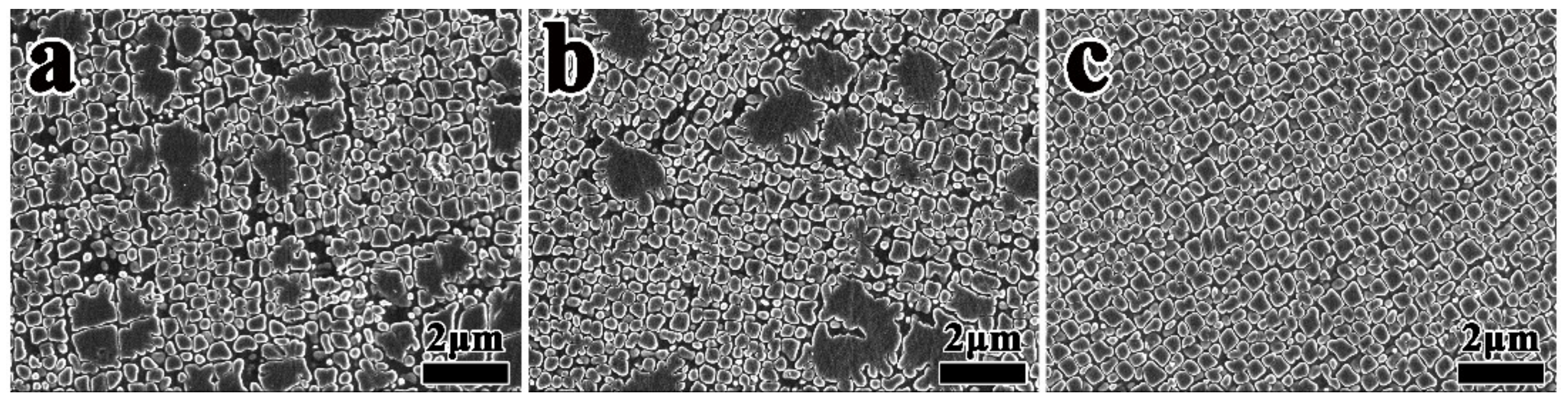
3.1. Effect of Heat Treatment on Grain Boundary Morphology
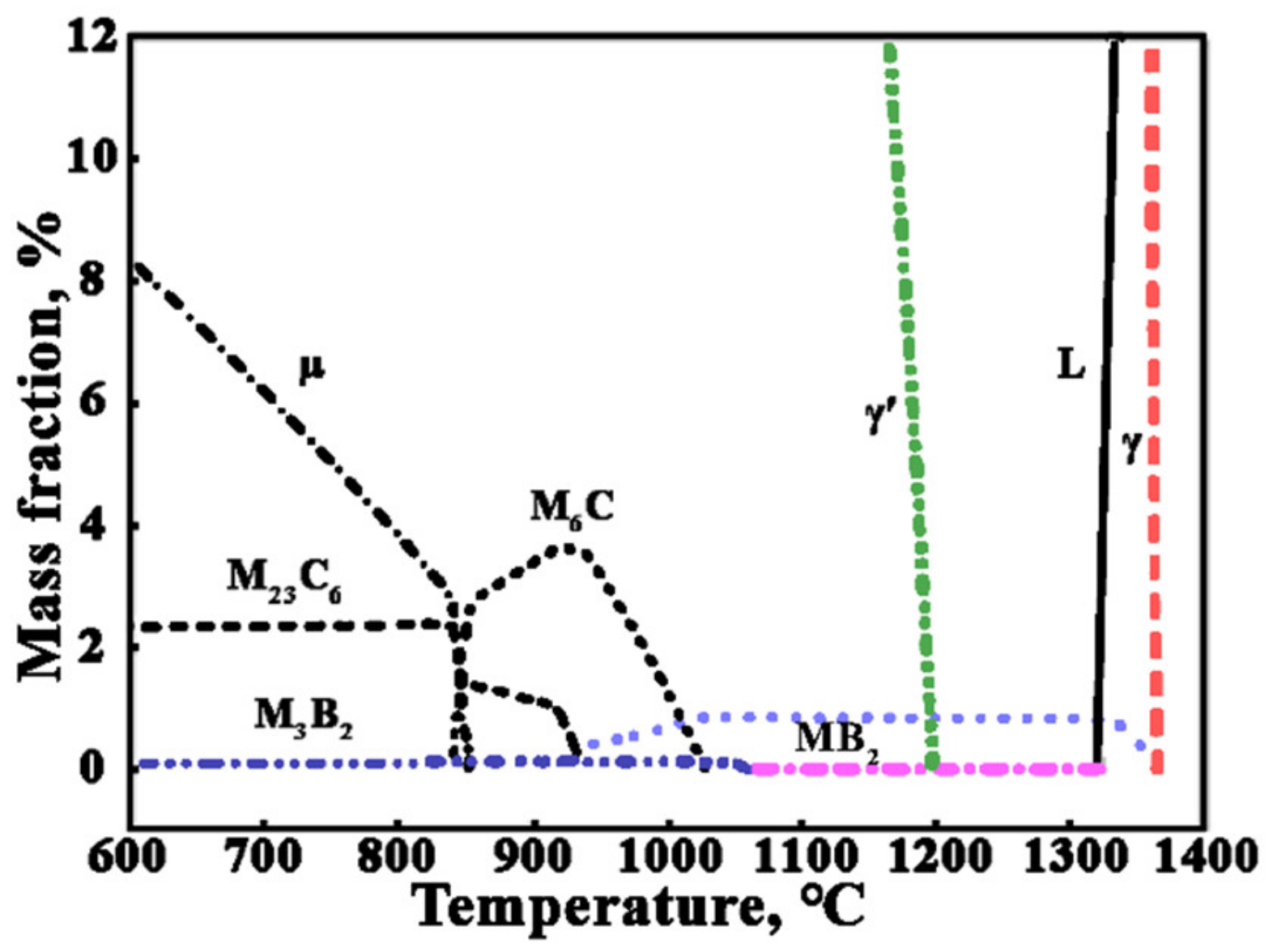
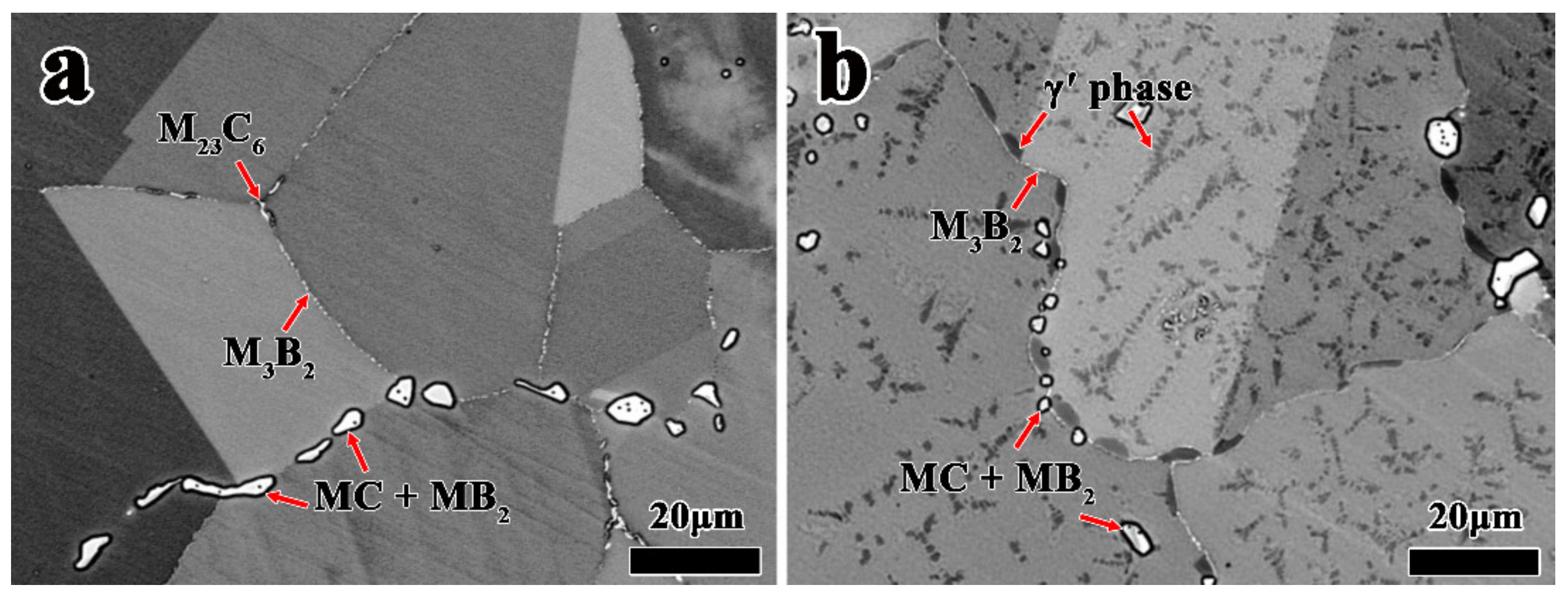
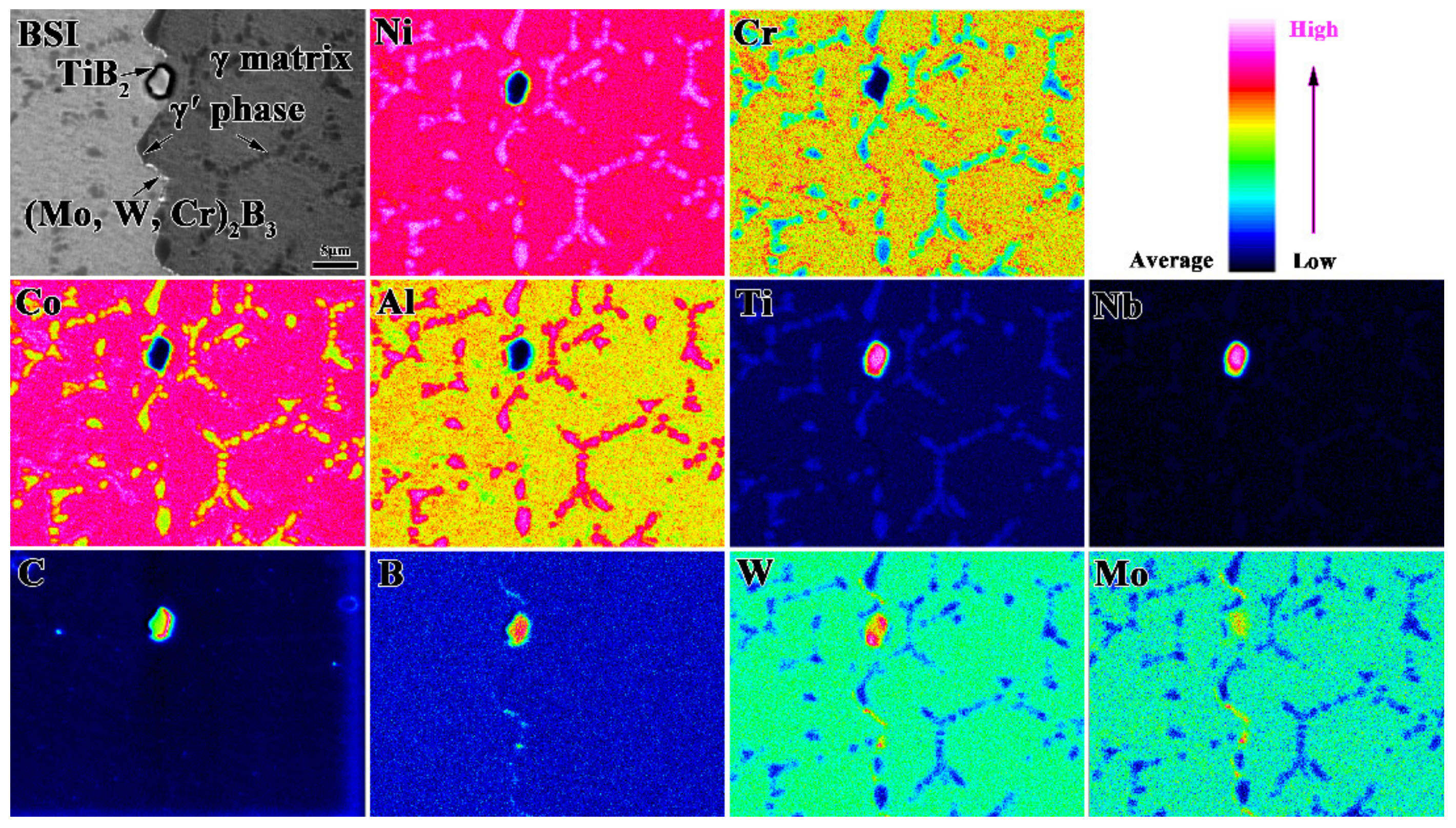
3.2. Effect of Heat Treatment on Precipitated Phases
3.3. The Formation Mechanism of Serrated Grain Boundary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolaÿ, A.; Fiorucci, G.; Franchet, J.M.; Cormier, J.; Bozzolo, N. Influence of strain rate on subsolvus dynamic and post-dynamic recrystallization kinetics of Inconel 718. Acta Mater. 2019, 174, 406–417. [Google Scholar] [CrossRef]
- Ning, Y.Q.; Yao, Z.K.; Lei, Y.Y.; Guo, H.Z.; Fu, M.W. Hot deformation behavior of the post-cogging FGH4096 superalloy with fine equiaxed microstructure. Mater. Charact. 2011, 62, 887–893. [Google Scholar] [CrossRef]
- Lawitzki, R.; Hassan, S.; Karge, L.; Wagner, J.; Wang, D.; von Kobylinski, J.; Krempaszky, C.; Hofmann, M.; Gilles, R.; Schmitz, G. Differentiation of γ′- and γ″- precipitates in Inconel 718 by a complementary study with small-angle neutron scattering and analytical microscopy. Acta Mater. 2019, 163, 28–39. [Google Scholar] [CrossRef]
- Xie, B.C.; Yu, H.; Sheng, T.; Xiong, Y.H.; Ning, Y.Q.; Fu, M.W. DDRX and CDRX of an as-cast nickel-based superalloy during hot compression at γ′ sub-/super-solvus temperatures. J. Alloys Compd. 2019, 803, 16–29. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Wang, Z.T.; Yu, H.; Ning, Y.Q. Microstructural origin and control mechanism of the mixed grain structure in Ni-based superalloys. J. Alloys Compd. 2022, 900, 163515. [Google Scholar] [CrossRef]
- Tanaka, M.; Iizuka, H.; Ashihara, F. Effects of serrated grain boundaries on the crack growth in austenitic heat-resisting steels during high-temperature creep. J. Mater. Sci. 1988, 23, 3827–3832. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyagawa, O.; Sakaki, T.; Iizuka, H.; Ashihara, F.; Fujishiro, D. Creep rupture strength and grain-boundary sliding in austenitic 21 Cr-4Ni-9Mn steels with serrated grain boundaries. J. Mater. Sci. 1988, 23, 621–628. [Google Scholar] [CrossRef]
- Hong, H.U.; Nam, S.W. The occurrence of grain boundary serration and its effect on the M23C6 carbide characteristics in an AISI 316 stainless steel. Mater. Sci. Eng. A 2002, 332, 255–261. [Google Scholar] [CrossRef]
- Bhuyan, P.; Reddy, K.V.; Pradhan, S.K.; Pal, S.; Mitra, R.; Mandal, S. A potential insight into the serration behaviour of Σ3n (n ≤ 3) boundaries in Alloy 617. Mater. Chem. Phys. 2020, 248, 122919. [Google Scholar] [CrossRef]
- Tang, Y.T.; Karamched, P.; Liu, J.; Haley, J.C.; Reed, R.C.; Wilkinson, A.J. Grain boundary serration in nickel alloy inconel 600: Quantification and mechanisms. Acta Mater. 2019, 181, 352–366. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.Y.; Kim, K.J.; Hong, H.U.; Nam, S.W. Effects of aging temperature and grain size on the formation of serrated grain boundaries in an AISI 316 stainless steel. Mater. Chem. Phys. 2013, 139, 27–33. [Google Scholar] [CrossRef]
- Kim, K.J.; Hong, H.U.; Nam, S.W. A study on the mechanism of serrated grain boundary formation in an austenitic stainless steel. Mater. Chem. Phys. 2011, 126, 480–483. [Google Scholar] [CrossRef]
- Hong, H.U.; Kim, I.S.; Choi, B.G.; Kim, M.Y.; Jo, C.Y. The effect of grain boundary serration on creep resistance in a wrought nickel-based superalloy. Mater. Sci. Eng. A 2009, 517, 125–131. [Google Scholar] [CrossRef]
- Li, H.Y.; Sun, J.F.; Hardy, M.C.; Evans, H.E.; Williams, S.J.; Doel, T.J.A.; Bowen, P. Effects of microstructure on high temperature dwell fatigue crack growth in a coarse grain PM nickel based superalloy. Acta Mater. 2015, 90, 355–369. [Google Scholar] [CrossRef]
- Tang, Y.T.; Wilkinson, A.J.; Reed, R.C. Grain boundary serration in nickel-based superalloy Inconel 600: Generation and effects on mechanical behavior. Metall. Mater. Trans. A 2018, 49, 4324–4342. [Google Scholar] [CrossRef]
- Koul, A.K.; Gessinger, G.H. On the mechanism of serrated grain boundary formation in Ni-based superalloys. Acta Mater. 1983, 31, 1061–1069. [Google Scholar] [CrossRef]
- Atrazhev, V.V.; Burlatsky, S.F.; Dmitriev, D.V.; Furrer, D.; Kuzminyh, N.Y.; Lomaev, I.L.; Novikov, D.L.; Stolz, S.; Reynolds, P. The mechanism of grain boundary serration and fan-type structure formation in Ni-based superalloys. Metall. Mater. Trans. A 2020, 51, 3648–3657. [Google Scholar]
- Lu, X.D.; Deng, Q.; Du, J.H.; Qu, J.L.; Zhuang, J.Y.; Zhong, Z.Y. Effect of slow cooling treatment on microstructure of difficult deformation GH4742 superalloy. J. Alloys Compd. 2009, 477, 100–103. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Li, H.Y.; Huang, Z.W. On the formation of serrated grain boundaries and fan type structures in an advanced polycrystalline nickel-base superalloy. J. Mater. Process. Technol. 2009, 209, 1011–1017. [Google Scholar] [CrossRef]
- Lim, Y.S.; Kim, D.J.; Hwang, S.S.; Kim, H.P.; Kim, S.W. M23C6 precipitation behavior and grain boundary serration in Ni-based Alloy 690. Mater. Charact. 2014, 96, 28–39. [Google Scholar] [CrossRef]
- Lu, X.D.; Du, J.H.; Deng, Q.; Zhong, Z.Y. Effect of slow cooling treatment on hot deformation behavior of GH4742 superalloy. J. Alloys Compd. 2009, 486, 195–198. [Google Scholar] [CrossRef]
- Yang, K.; An, T.; Qu, J.L.; Du, J.H.; Qin, H.L.; Zheng, S.Q.; Bi, Z.N. Effects of solution cooling rate on the grain boundary and mechanical properties of GH4710 alloy. Mater. Sci. Eng. A 2022, 832, 142459. [Google Scholar] [CrossRef]
- Hong, H.U.; Jeong, H.W.; Kim, I.S.; Choi, B.G.; Yoo, Y.S.; Jo, C.Y. A study on the formation of serrated grain boundaries and its applications in Nimonic 263. Mater. Sci. Forum. 2010, 638–642, 2245–2250. [Google Scholar] [CrossRef]
- Kim, K.J.; Hong, H.U.; Nam, S.W. Investigation on the formation of serrated grain boundaries with grain boundary characteristics in an AISI 316 stainless steel. J. Nucl. Mater. 2009, 393, 249–253. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, R.; Kou, H.C.; Li, J.S.; Bai, G.H.; Fu, H.Z. The effect of M23C6 carbides on the formation of grain boundary serrations in a wrought Ni-based superalloy. Mater. Sci. Eng. A 2012, 536, 37–44. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Hao, X.C.; Zha, X.D.; Gao, M.; Ma, Y.C.; Liu, K. Research progress in grain boundary serration in iron/nickel based austenitic polycrystalline alloys. Chin. J. Eng. 2021, 43, 1323–1338. [Google Scholar]
- Haack, M.; Kuczyk, M.; Seidel, A.; López, E.; Brückner, F.; Leyens, C. Investigation on the formation of grain boundary serrations in additively manufactured superalloy Haynes 230. J. Laser Appl. 2020, 32, 032014. [Google Scholar] [CrossRef]
- Son, H.W.; Lee, J.W.; Hyun, S.K. Mechanism of grain boundary serration during hot deformation of AZ31 alloy: Role of grain boundary dislocations and grain boundary sliding. Int. J. Plasticity 2020, 125, 118–132. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, Y.J.; Yang, L.; Li, J.G. Evolution of microstructures at a wide range of solidification cooling rate in a Ni-based superalloy. J. Alloys Compd. 2013, 570, 70–75. [Google Scholar] [CrossRef]
- Fan, X.Q.; Guo, Z.P.; Wang, X.F.; Yang, J.; Zou, J.W. Morphology evolution of gamma’ precipitates in a powder metallurgy Ni-base superalloy. Mater. Charact. 2018, 139, 382–389. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.T.; Zhang, B.Y.; Ning, Y.Q.; Fu, M.W. Re-precipitation mechanisms of the γ′ phase with sphere, near-sphere, cubic, octets and finally-dendrite in as-cast Ni-based superalloys. J. Alloys Compd. 2021, 876, 160104. [Google Scholar] [CrossRef]
- Mao, W.M.; Yang, P. Migration behaviors of boundaries in polycrystalline metals during grain growth process. Sci. Sin. Technol. 2014, 44, 911–916. [Google Scholar]
- Li, H.; Xia, S.; Zhou, B.X.; Peng, J.C. Study of carbide precipitation at grain bundary in nickel base alloy 690. Acta Metall. Sin. 2011, 47, 853–858. [Google Scholar]




| Ni | Co | Cr | Mo | W | Al | Ti | Nb | C | B | Zr |
|---|---|---|---|---|---|---|---|---|---|---|
| Base | 15.5 | 8.5 | 1.2 | 10.0 | 4.8 | 2.4 | 1.5 | 0.10 | 0.015 | 0.04 |
| No | Solution Treatment | Isothermal Treatment (Temperature, °C) | Isothermal Treatment (Soaking Time, h) |
|---|---|---|---|
| T1 | 1210 °C × 6 h, AC | ||
| T2 | 1210 °C × 6 h, FC | ||
| T3 | 1210 °C × 6 h, FC | 1160 °C | 1 h |
| T4 | 1210 °C × 6 h, FC | 1160 °C | 2 h |
| T5 | 1210 °C × 6 h, FC | 1175 °C | 2 h |
| T6 | 1210 °C × 6 h, FC | 1150 °C | 2 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zhang, W.; Xie, B.; Yu, H.; Ning, Y.; Zhang, B. Investigation of the Heat Treatment Process and Formation Mechanism of Grain Boundary Serration for GH4795 Superalloy. Metals 2022, 12, 1521. https://doi.org/10.3390/met12091521
Huang S, Zhang W, Xie B, Yu H, Ning Y, Zhang B. Investigation of the Heat Treatment Process and Formation Mechanism of Grain Boundary Serration for GH4795 Superalloy. Metals. 2022; 12(9):1521. https://doi.org/10.3390/met12091521
Chicago/Turabian StyleHuang, Shuo, Wenyun Zhang, Bingchao Xie, Hao Yu, Yongquan Ning, and Beijiang Zhang. 2022. "Investigation of the Heat Treatment Process and Formation Mechanism of Grain Boundary Serration for GH4795 Superalloy" Metals 12, no. 9: 1521. https://doi.org/10.3390/met12091521
APA StyleHuang, S., Zhang, W., Xie, B., Yu, H., Ning, Y., & Zhang, B. (2022). Investigation of the Heat Treatment Process and Formation Mechanism of Grain Boundary Serration for GH4795 Superalloy. Metals, 12(9), 1521. https://doi.org/10.3390/met12091521







