Microstructure, Mechanical Properties and Fire Resistance of High Strength Mg-Gd-Y-Zr Alloys
Abstract
:1. Introduction
2. Experiment and Method
2.1. Experimental Materials
2.2. Alloy Preparation
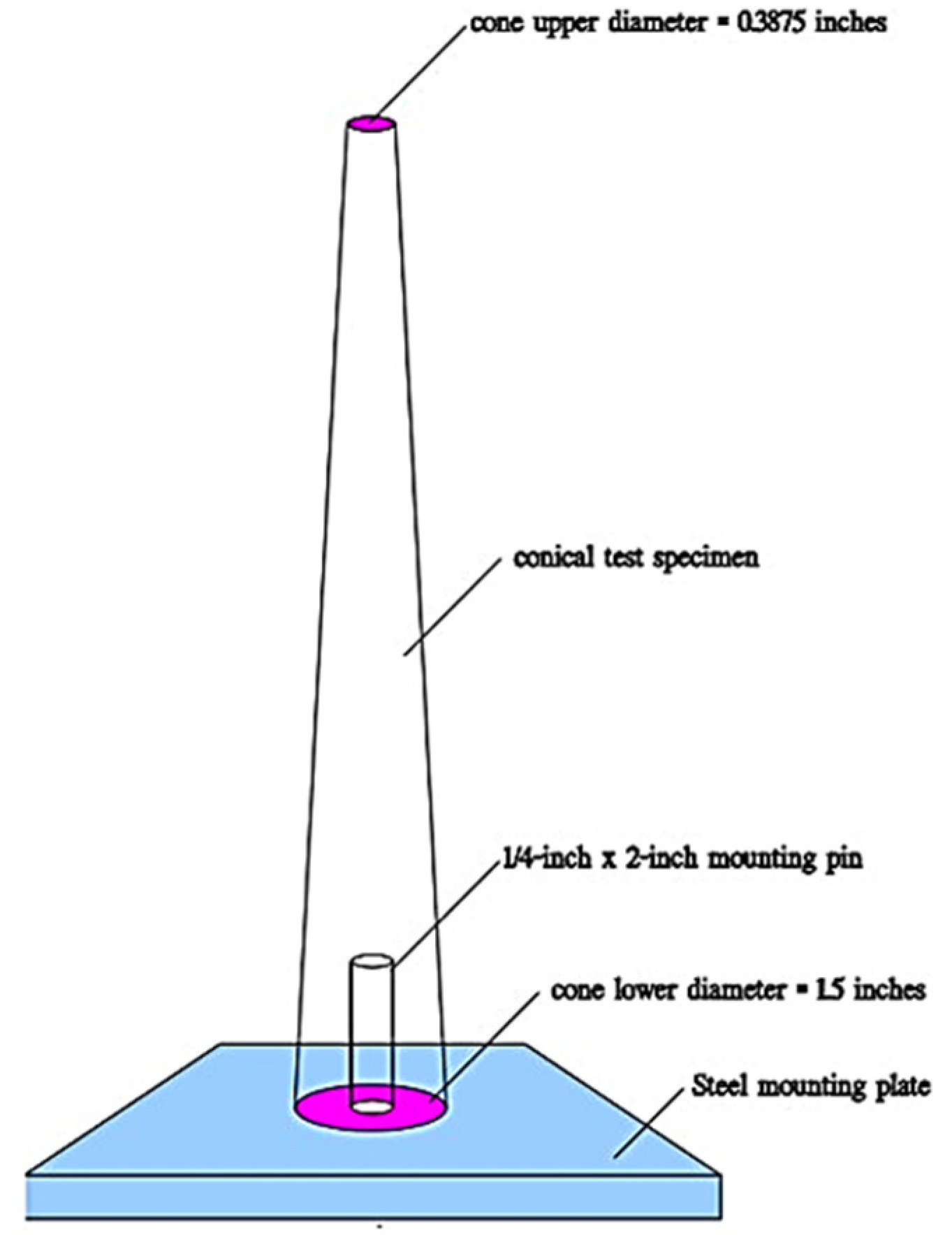
2.3. Tissue Characterization and Performance Testing
3. Results and Discussion
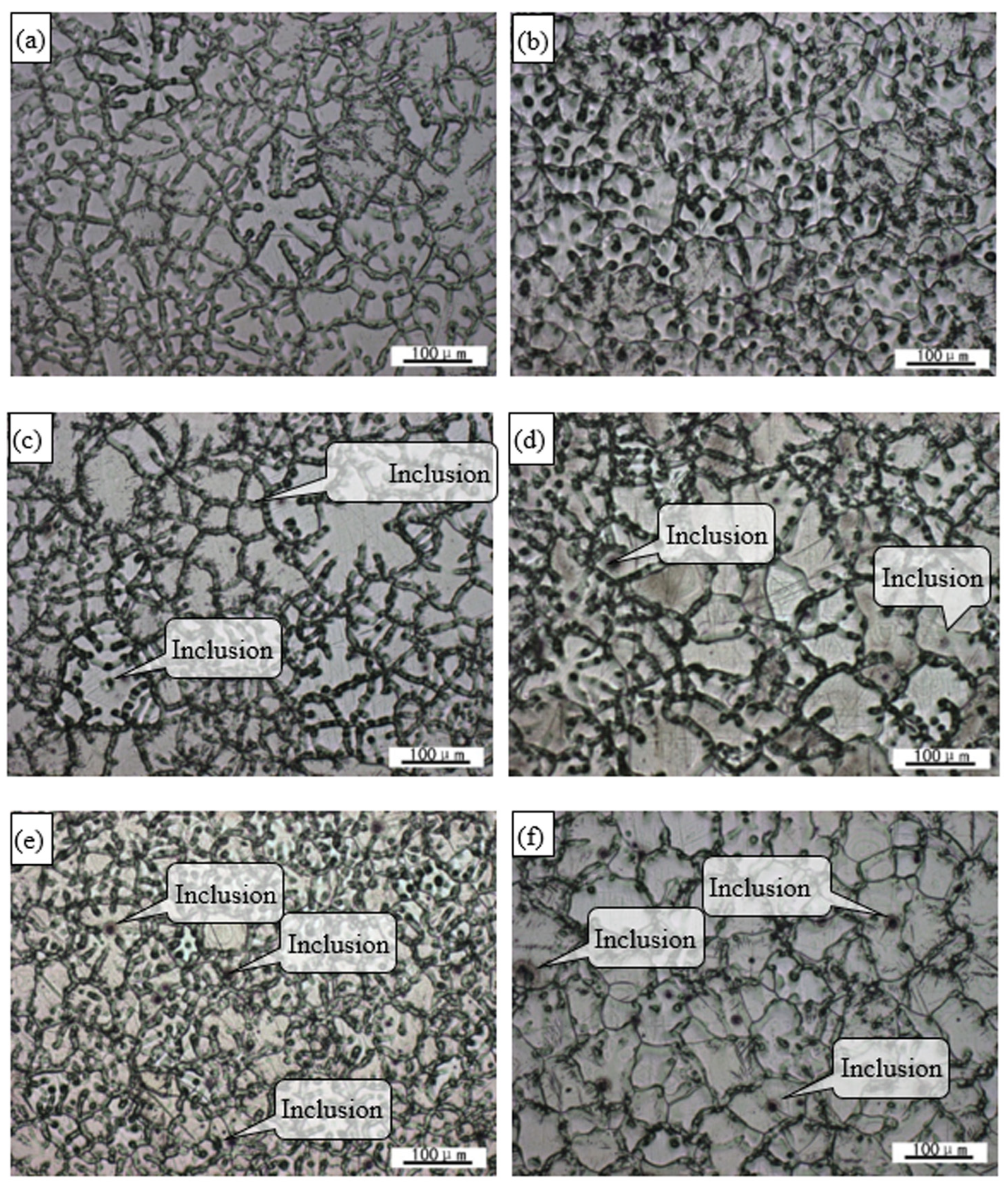
3.1. Microstructure of as-cast Alloy
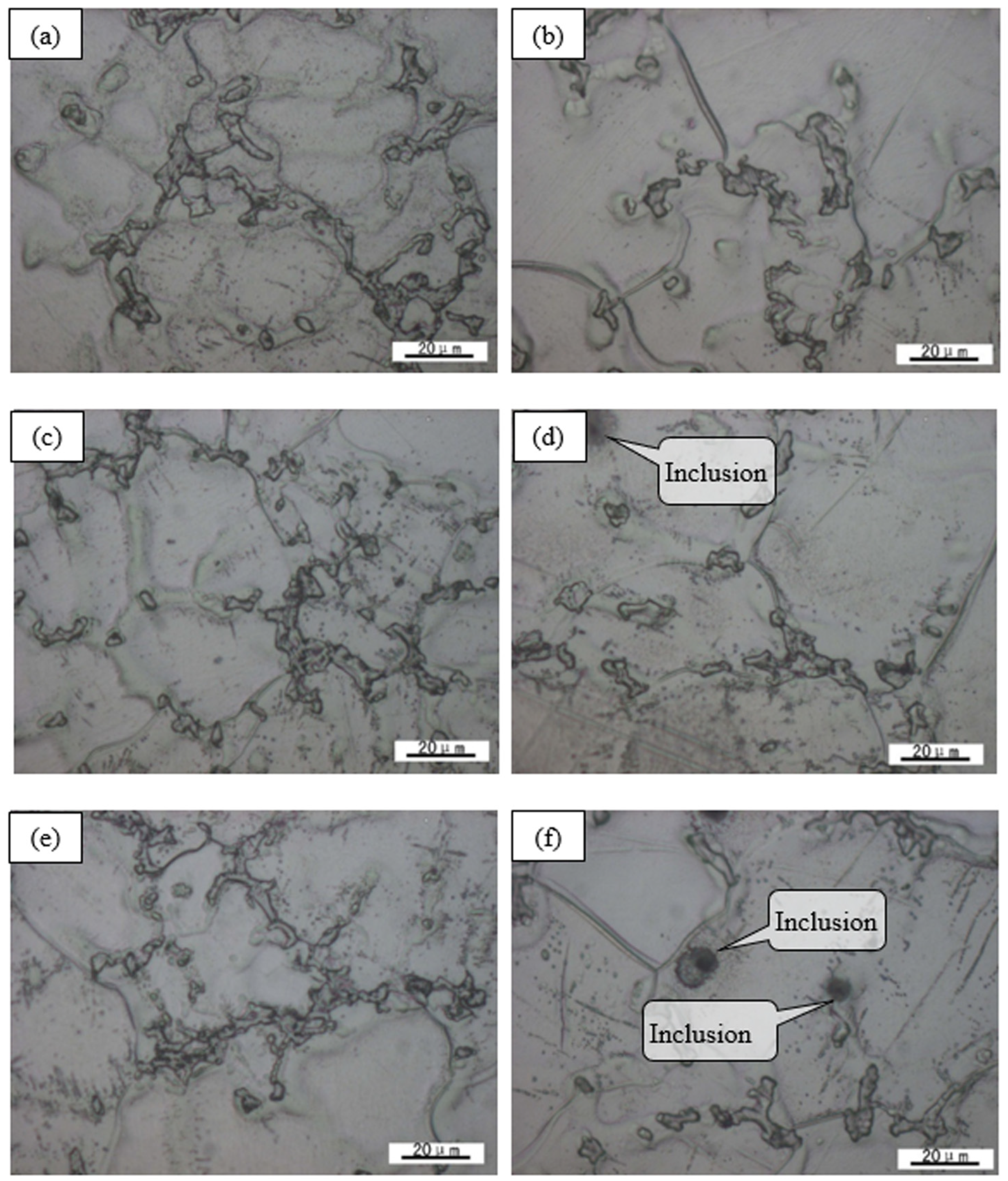
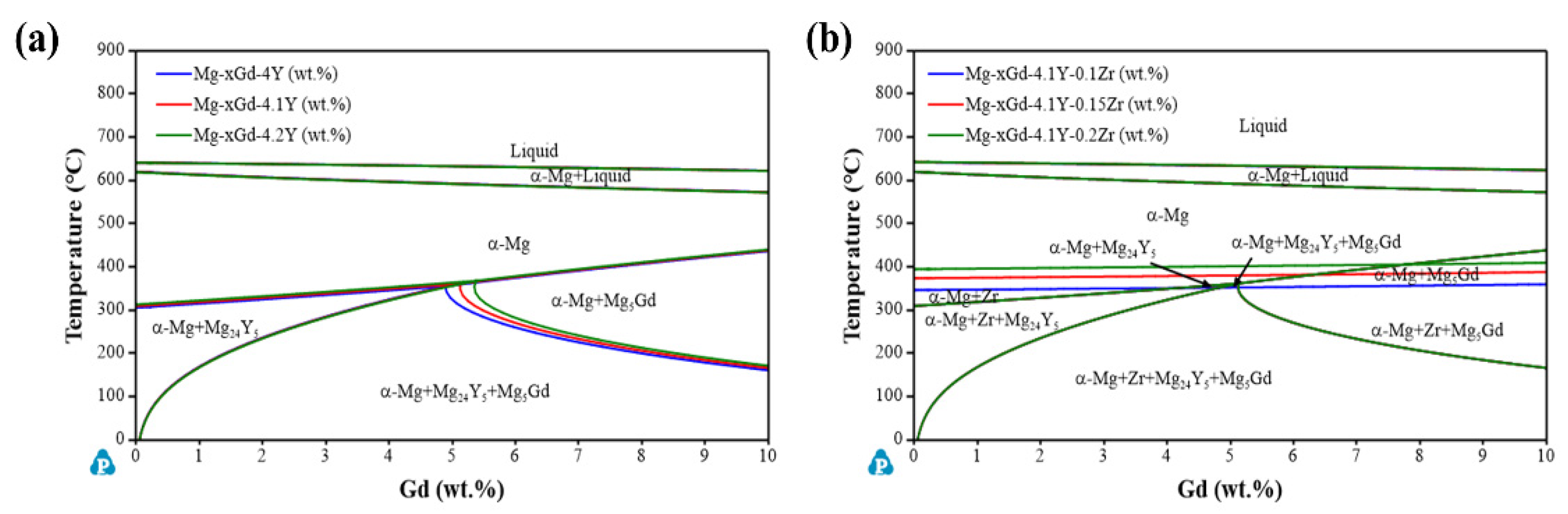
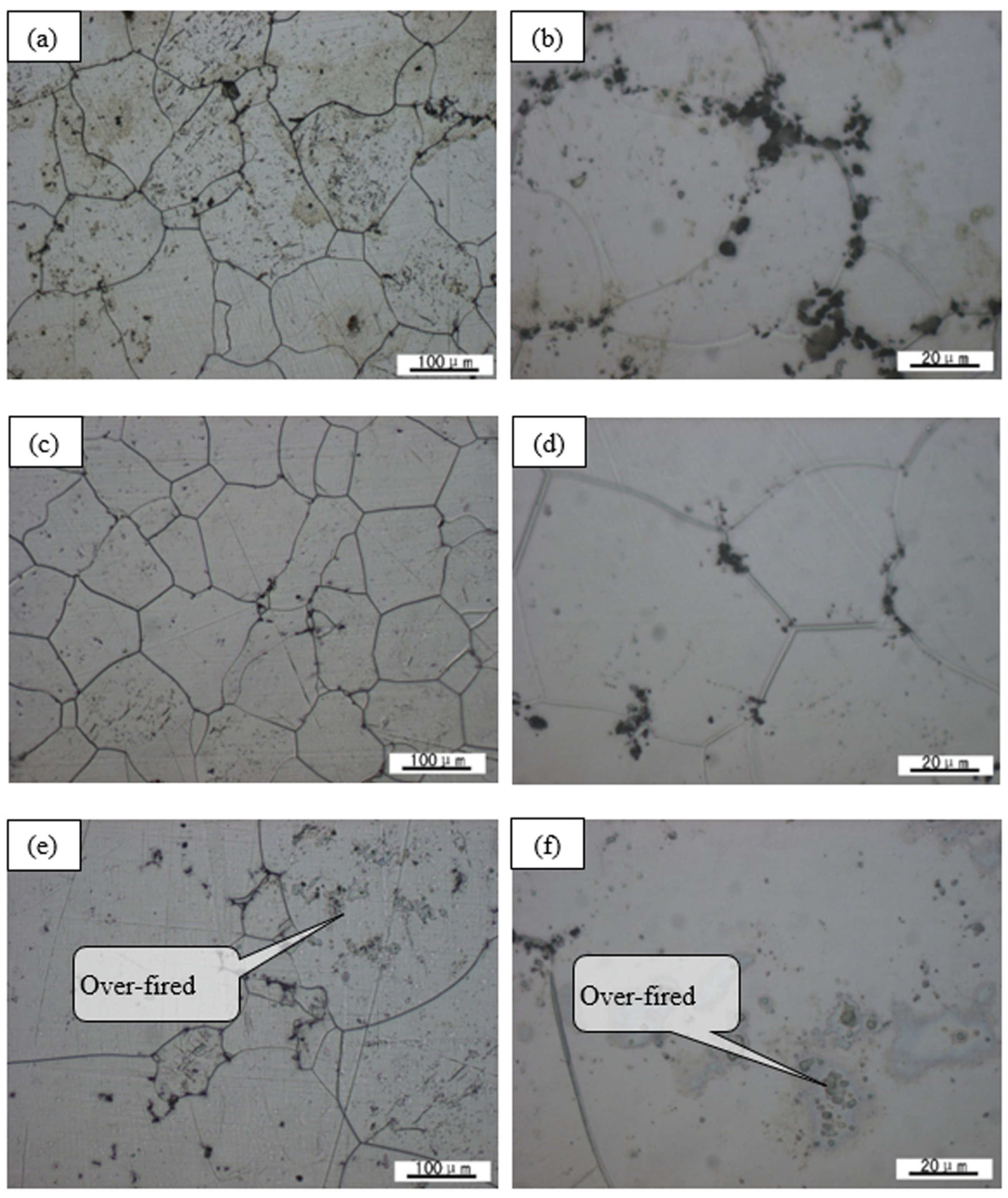
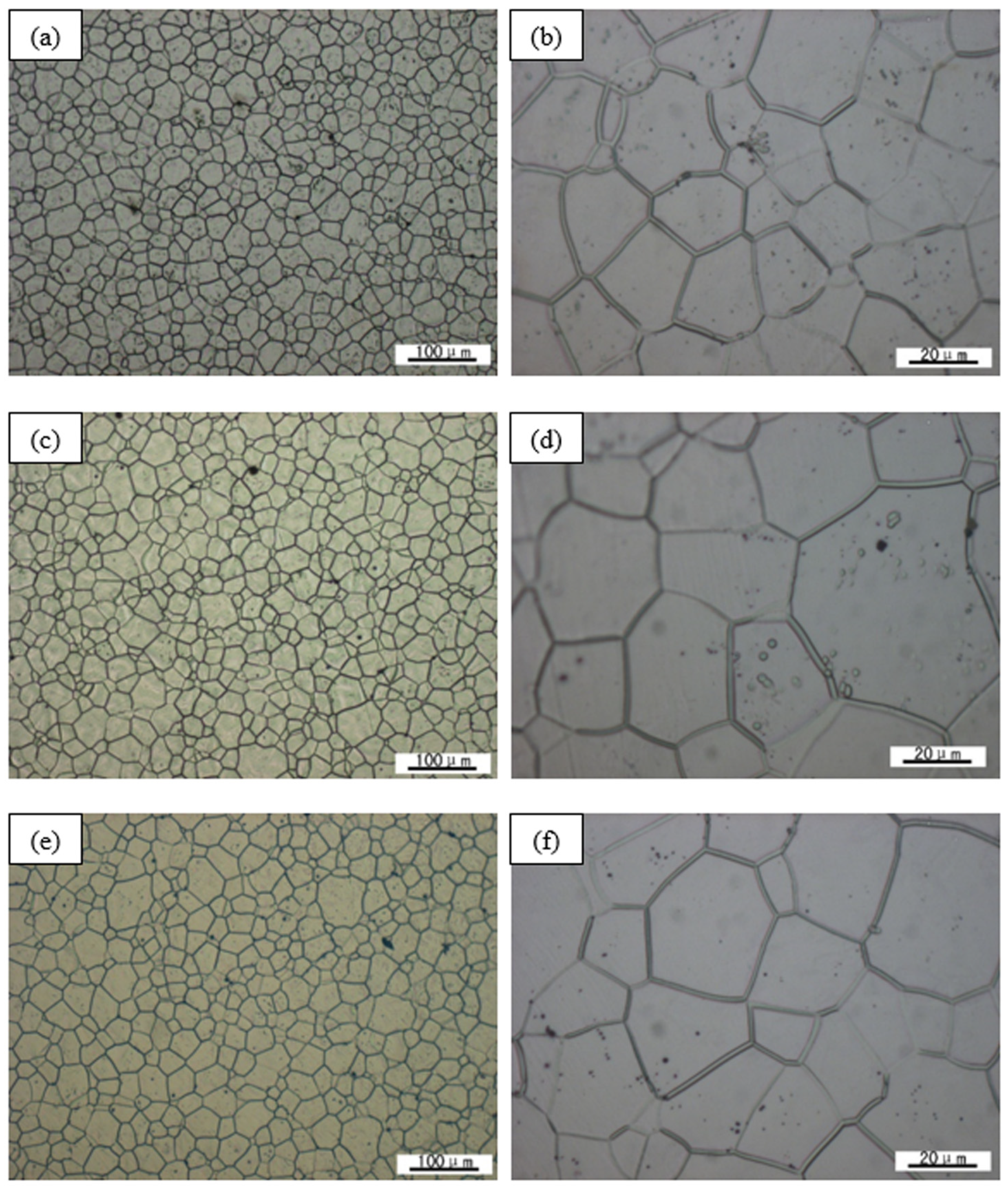
3.2. Alloy Precipitation Behavior and Homogenized Microstructure
3.3. Microstructure of Extruded Alloy
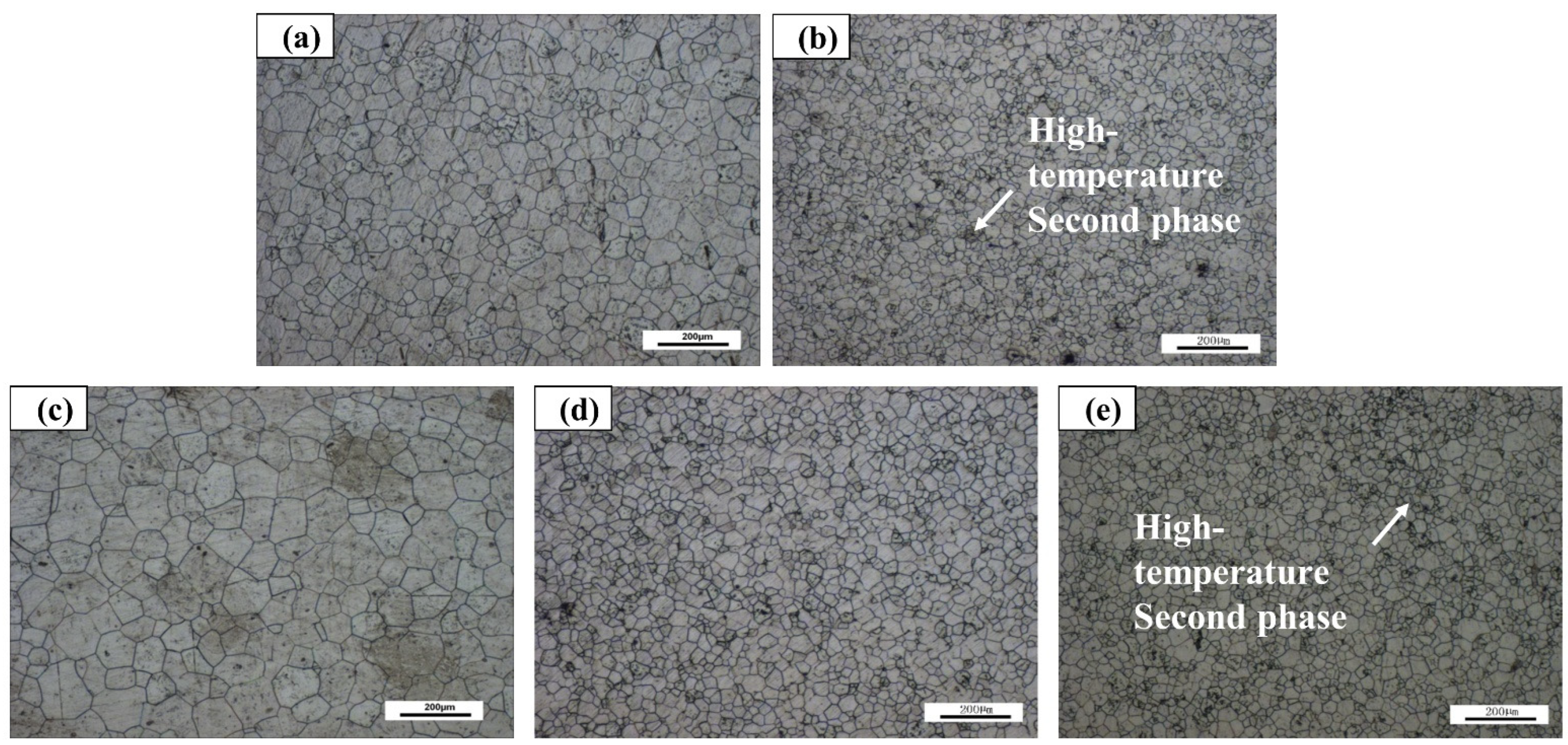
3.4. Microstructure of Aging Alloy
3.5. Normal Temperature Mechanical and Combustion Performance Test of Alloy
4. Conclusions
- (1)
- The alloy has good casting characteristics, uniform distribution of ingot elements, and stable yield of main alloy elements. The outer microstructure of the alloy is finer than the core microstructure, and the second phase is finely dispersed. The size and distribution of the alloy phases of different compositions are similar, which verifies the reliability of the semi-continuous casting production quality.
- (2)
- The optimal homogenization treatment specification of the alloy is 500 °C/6 h. If the temperature is too low, the atomic diffusion is insufficient, and the eutectic compound and the refractory phase are distributed in the grain boundary and within the grain, respectively. If the temperature is too high, the grain will grow abnormally and burnt out.
- (3)
- After the alloy was subjected to an extrusion temperature of 430 °C, an extrusion speed of 3–5 mm/s, an extrusion ratio of 25, and an artificial aging treatment of 200 °C/64 h, the tensile strength was 405 MPa, and the yield strength was 275 MPa. the elongation was 8.12%.
- (4)
- The flame retardant properties of the alloys are closely related to the elements and their addition amounts. The as-cast alloys have little difference in the flame retardant properties; while the extruded alloys have flame retardant properties due to uneven precipitation behavior under high temperature gradient and high energy density heating. Poor; aging alloys exhibit excellent flame retardant properties due to pre-precipitation of high temperature refractory phases.
Author Contributions
Funding
Conflicts of Interest
References
- Pan, F.; Zhang, J.; Wang, J.; Yang, M.; Han, E.; Chen, R. Key R&D activities for development of new types of wrought magnesium alloys in China. Trans. Nonferrous Met. Soc. China 2010, 20, 1249–1258. [Google Scholar]
- Jiang, B.; Zeng, X.; Song, J. Development Strategies for China’s Advanced Magnesium Alloy Industry Toward 2035. Chin. J. Eng. Sci. 2020, 22, 76–83. [Google Scholar]
- National Natural Science Foundation of China, Chinese Academy of Sciences. Materials Science of China’s Discipline Development Strategy in the Next 10 Years; Science Press: Beijing, China, 2012. [Google Scholar]
- Zhang, D. Thoughts on the Development Strategy of my country’s Magnesium Alloy Industry. World Nonferrous Met. 2004, 7, 4–20. [Google Scholar]
- Ding, W.; Zeng, X. Development and application of Mg materials in China. Chin. J. Met. 2010, 46, 1450–1457. [Google Scholar]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research-A review. J. Magnes. Alloy. 2021, 10, 1–61. [Google Scholar] [CrossRef]
- Gradinger, R.; Gneiger, S.; Betz, A. Investigations on Fire-Resistant Magnesium Alloys for Aerospace Applications. Mater. Sci. Forum 2015, 828, 492–498. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- U.S. Department of Transportation, Federal Aviation Administration. Evaluating the Flammability of Various Magnesium Alloys During Laboratory–and Full-Scale Aircraft Fire Tests; Federal Aviation Administration: Atlantic City, NJ, USA, 2013. [Google Scholar]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Tan, Q.; Yin, Y.; Mo, N.; Zhang, M. Recent understanding of the oxidation and burning of magnesium alloys. Surf. Innov. 2019, 7, 71–92. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Wang, Q.; Zhang, M.; Wang, A.; Li, M. Flame resistance and oxidation behavior of Mg-Y-Ce-Zr alloys. Mater. Res. Express 2018, 6, 016536. [Google Scholar] [CrossRef]
- Qin, L.; Ding, J.; Fang, Z.; Zhao, W. Effect of Ce Addition on the Ignition-Proof Properties and Surface Tension of AZ91D-2.5Ca (Wt.%) Magnesium Alloy. Mater. Sci. Forum. 2014, 788, 82–87. [Google Scholar] [CrossRef]
- Tan, Q.; Mo, N.; Jiang, B.; Pan, F. Combined influence of Be and Ca on improving the high-temperature oxidation resistance of the magnesium alloy Mg-9Al-1Zn. Corros. Sci. 2017, 122, 1–11. [Google Scholar] [CrossRef]
- Inoue, S.-I.; Yamasaki, M.; Kawamura, Y. Oxidation behavior and incombustibility of molten Mg-Zn-Y alloys with Ca and Be addition. Corros. Sci. 2019, 149, 133–143. [Google Scholar] [CrossRef]
- Bian, M.; Huang, X.; Chino, Y. Improving flame resistance and mechanical properties of magnesium–silver–calcium sheet alloys by optimization of calcium content. J. Alloy. Compd. 2020, 837, 155551. [Google Scholar] [CrossRef]
- Li, R.G.; Li, H.R.; Pan, H.C.; Xie, D.S.; Zhang, J.H.; Fang, D.Q.; Dai, Y.Q.; Zhao, D.Y.; Zhang, H. Achieving exceptionally high strength in binary Mg-13Gd alloy by strong texture and substantial precipitates. Scr. Mater. 2021, 193, 142–146. [Google Scholar] [CrossRef]
- Wan, Y.; Tang, B.; Gao, Y.; Tang, L.; Sha, G.; Zhang, B.; Liang, N.; Liu, C.; Jiang, S.; Chen, Z.; et al. Bulk nanocrystalline high-strength magnesium alloys prepared via rotary swaging. Acta Mater. 2020, 200, 274–286. [Google Scholar] [CrossRef]
- Cao, W.; Chen, S.L.; Zhang, F.; Wu, K. Pandat software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. CAPHAD Comput. Coupling Phase Diagr. Thermochem. 2009, 31, 328–342. [Google Scholar] [CrossRef]
- Vostry, P.; Smola, B.; Stulõâkova, I.; von Buch, F.; Mordike, B.L. Microstructure Evolution in Isochronally Heat Treated Mg-Gd Alloys. Phys. Status Solidi 1999, 175, 491–500. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, B. Atomic Scale Investigation on Precipitates and Defects of Mg-RE Alloys: A Review. Adv. Eng. Mater. 2018, 21, 1800734. [Google Scholar] [CrossRef]
- Solomon, E.L.S.; Marquis, E.A. Deformation behavior of β’ and β’’’ precipitates in Mg-RE alloys. Mater. Lett. 2018, 216, 67–69. [Google Scholar] [CrossRef]
- Smola, B.; Stulí Ková, I.; Buch, F.V.; Mordike, B.L. Structural aspects of high performance Mg alloys design. Mater. Sci. Eng. A 2002, 324, 113–117. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Dong, J.; Zeng, X.Q.; Ding, W. Precipitation and its effect on the mechanical properties of a cast Mg-Gd-Nd-Zr alloy. Mater. Sci. Eng. A 2008, 489, 44–54. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Dong, J.; Zeng, X.Q.; Ding, W. Effect of pre-deformation on aging characteristics and mechanical properties of a Mg-Gd-Nd-Zr alloy. Mater. Sci. Eng. A 2008, 491, 103–109. [Google Scholar] [CrossRef]
- Li, D.; Dong, J.; Zeng, X.; Lu, C. Transmission electron microscopic investigation of the β1→β phase transformation in a Mg-Dy-Nd alloy. Mater. Charact. 2010, 61, 818–823. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Shi, R.; Zhou, N.; Xu, Z.; Zhu, Y.M.; Nie, J.F.; Wang, Y. Simulation study of precipitation in an Mg-Y-Nd alloy. Acta Mater. 2012, 60, 4819–4832. [Google Scholar] [CrossRef]
- Li, Y.; Qu, C.; Wang, J.; Xu, R. Exceptional aging hardening behaviour of nanocrystalline Mg–Y-Nd-Gd-Zr alloy prepared by high pressure torsion. J. Alloy. Compd. 2020, 813, 152123. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, B.; Xie, W.; Luo, Q.; Li, Q. Anti-phase boundary energy of β series precipitates in Mg-Y-Nd system. Scr. Mater. 2021, 193, 127–131. [Google Scholar] [CrossRef]





| Samples | Alloys Element (in wt.%) | Designed Density (g/cm3) | Actual Density (g/cm3) | ||
|---|---|---|---|---|---|
| Gd | Y | Zr | |||
| GWK-a | 5.29/5.41/5.20 (5.30) | 4.14/4.20/3.98 (4.11) | 0.13/0.13/0.14 (0.13) | 1.866 | 1.84 |
| GWK-b | 5.51/5.44/5.56 (5.50) | 4.25/4.14/4.27 (4.22) | 0.19/0.19/0.20 (0.19) | 1.890 | 1.86 |
| GWK-c | 5.42/5.58/5.38 (5.46) | 4.06/4.13/3.88 (4.02) | 0.20/0.19/0.21 (0.20) | 1.868 | 1.84 |
| GWK-a Alloy | UTS (Rm/MPa) | YS (RP0.2/MPa) | El. (A%) | Modulus E/G/B (GPa) |
|---|---|---|---|---|
| As-cast | ||||
| As-extruded | ||||
| As-aged 200 °C/12 h | ||||
| As-aged 200 °C/24 h | ||||
| As-aged 200 °C/64 h | ||||
| As-aged 200 °C/72 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Zhao, Y.; Dong, X.; Yu, W.; Feng, J.; Yu, H. Microstructure, Mechanical Properties and Fire Resistance of High Strength Mg-Gd-Y-Zr Alloys. Metals 2022, 12, 1456. https://doi.org/10.3390/met12091456
Qian Y, Zhao Y, Dong X, Yu W, Feng J, Yu H. Microstructure, Mechanical Properties and Fire Resistance of High Strength Mg-Gd-Y-Zr Alloys. Metals. 2022; 12(9):1456. https://doi.org/10.3390/met12091456
Chicago/Turabian StyleQian, Yafeng, Yanhui Zhao, Xiaorui Dong, Wei Yu, Jianhang Feng, and Hui Yu. 2022. "Microstructure, Mechanical Properties and Fire Resistance of High Strength Mg-Gd-Y-Zr Alloys" Metals 12, no. 9: 1456. https://doi.org/10.3390/met12091456
APA StyleQian, Y., Zhao, Y., Dong, X., Yu, W., Feng, J., & Yu, H. (2022). Microstructure, Mechanical Properties and Fire Resistance of High Strength Mg-Gd-Y-Zr Alloys. Metals, 12(9), 1456. https://doi.org/10.3390/met12091456





