Effect of TiN on Sulfide Morphology of Non-Quenched and Tempered Steel
Abstract
:1. Introduction
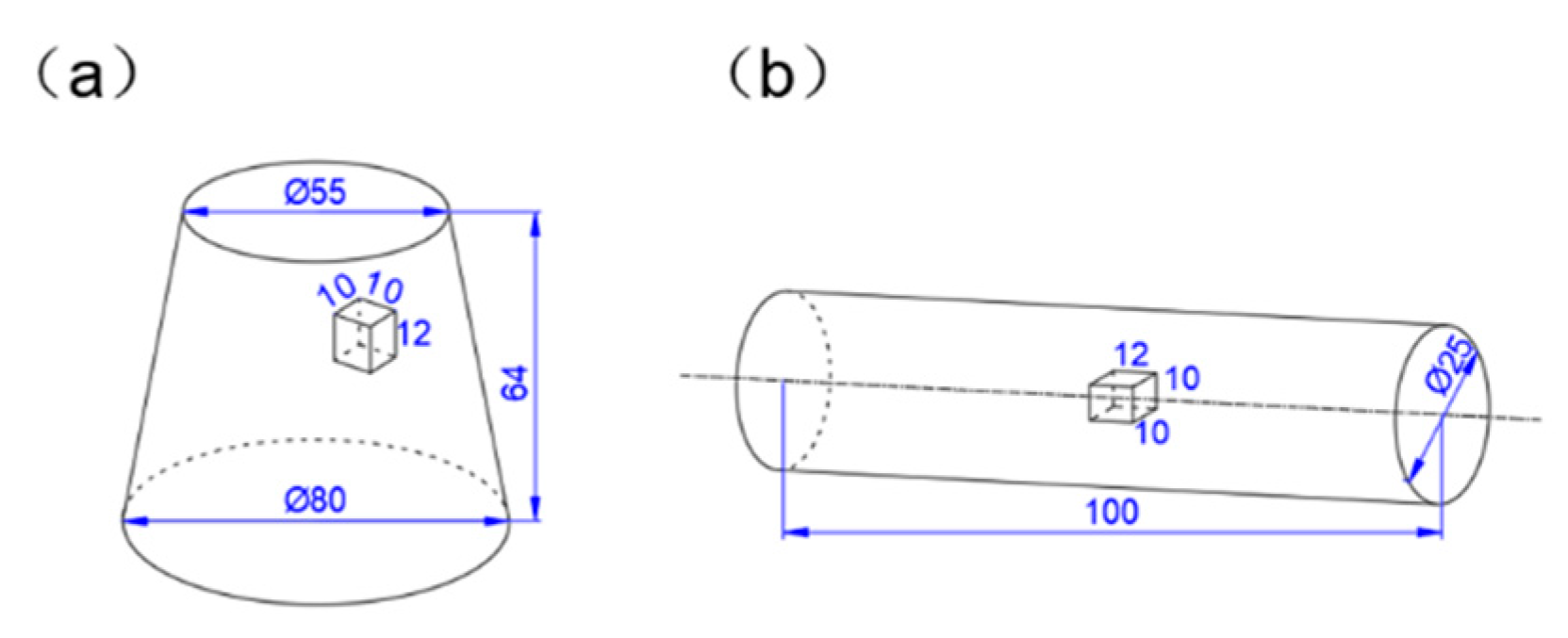
2. Materials and Methods
3. Results
3.1. Effect of Titanium Treatment on Sulfide Morphology in Steel under As-Cast Condition
3.2. Effect of Titanium Treatment on Sulfide Morphology in Steel under Rolling Condition
4. Discussion
5. Conclusions
- (1)
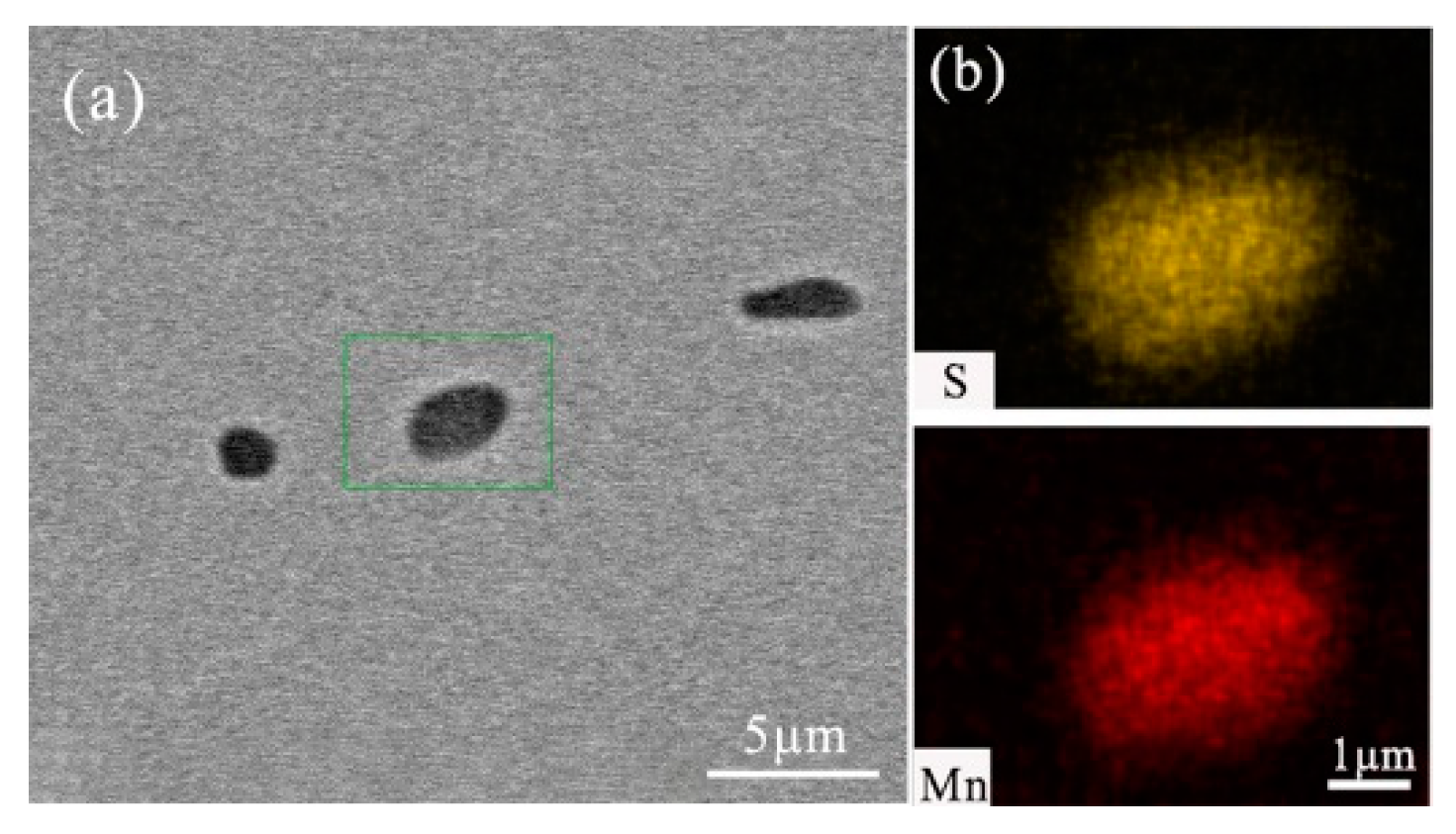
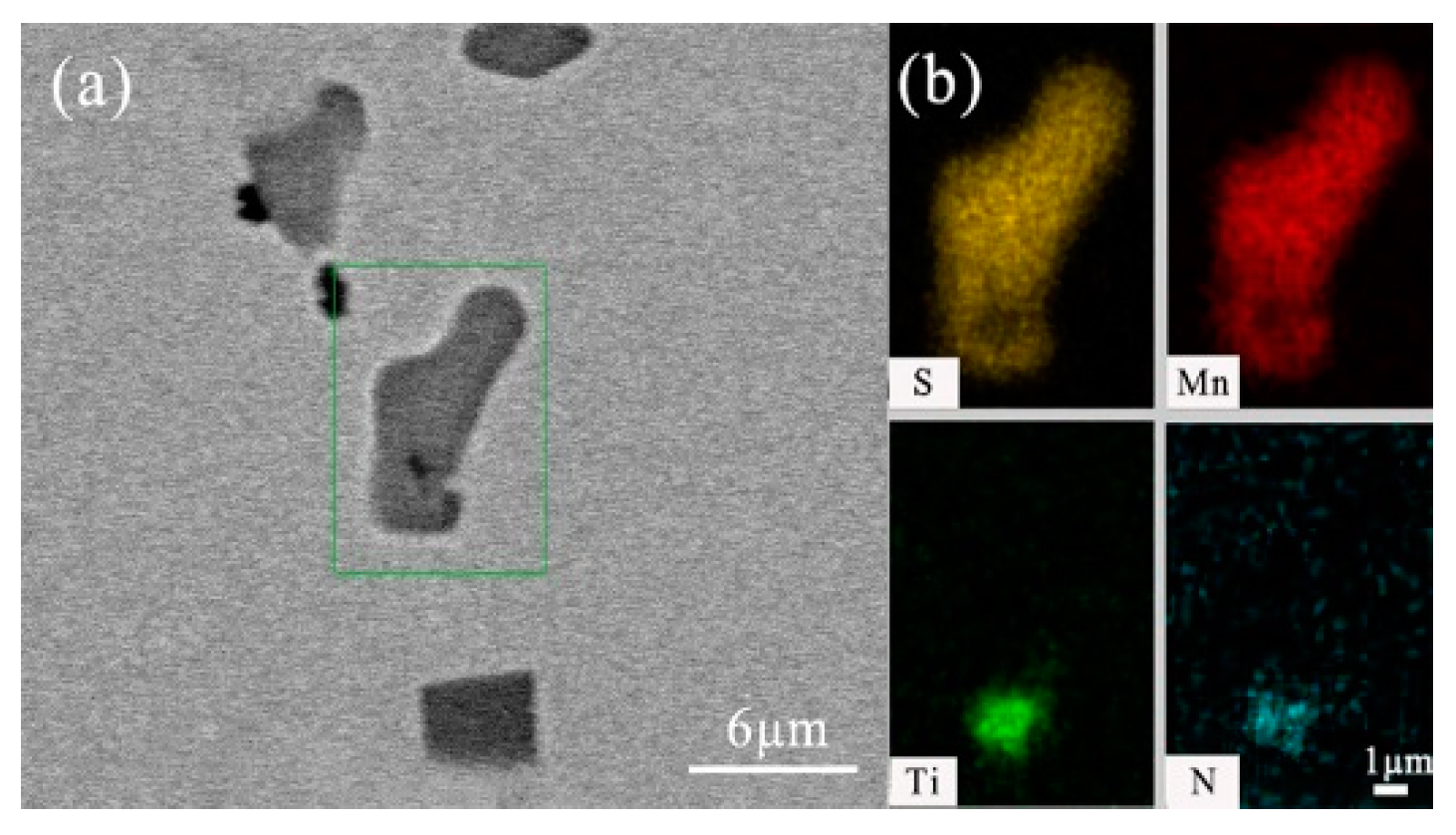
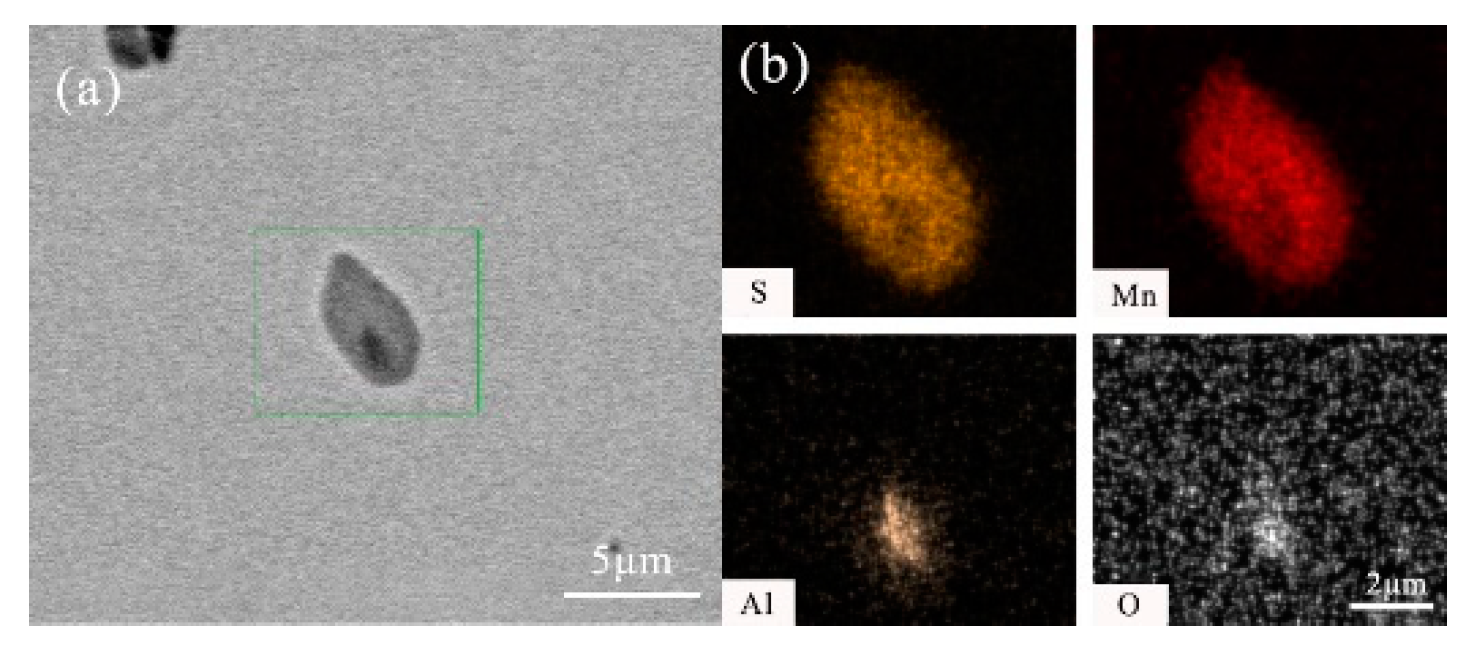
- After titanium treatment, there are four main kinds of Ti-containing composite sulfides in non-quenched and tempered steel: MnS with TiN as nucleation core, MnS with Al2O3 and TiN as nucleation core, MnS precipitated with TiN and MnS pinned by TiN. The as-cast pure MnS and composite sulfides are basically point or spindle-like, while the forged pure MnS is strip-like, and the composite sulfides tend to be spindle-like.
- (2)
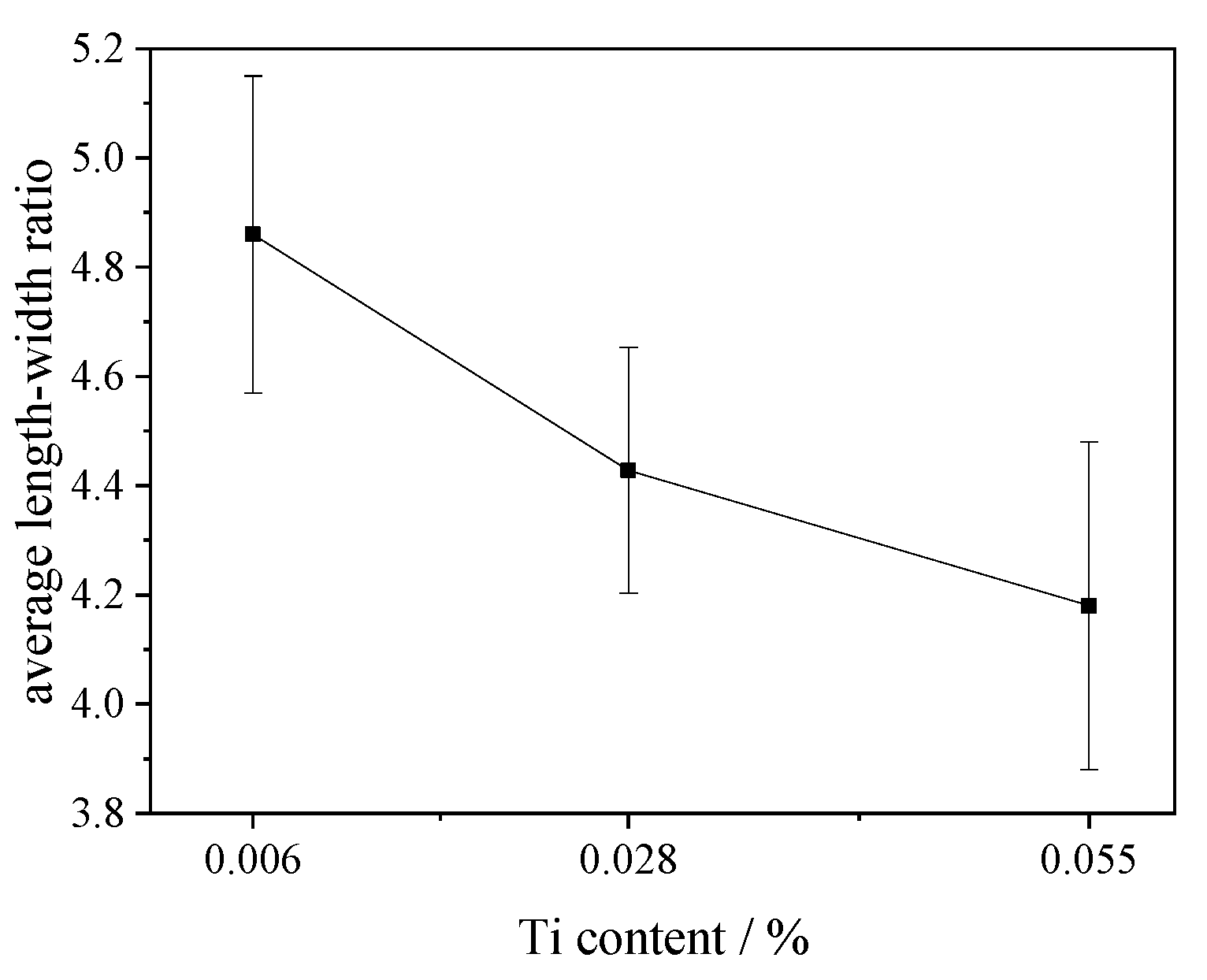
- With the increase in TiN content, the aspect ratio of sulfide decreases from 4.86 to 4.18, and the shape of inclusions tends to be cylindrical, spindle or spherical.
- (3)
- With different Ti contents, the types of sulfide precipitation interval and proportion are different. The increase of Ti and N content will increase the corresponding composite sulfide content. The number of MnS with TiN as the nucleation core increases most, indicating that increasing the content of TiN in steel can increase the proportion of composite sulfide in steel.
- (4)
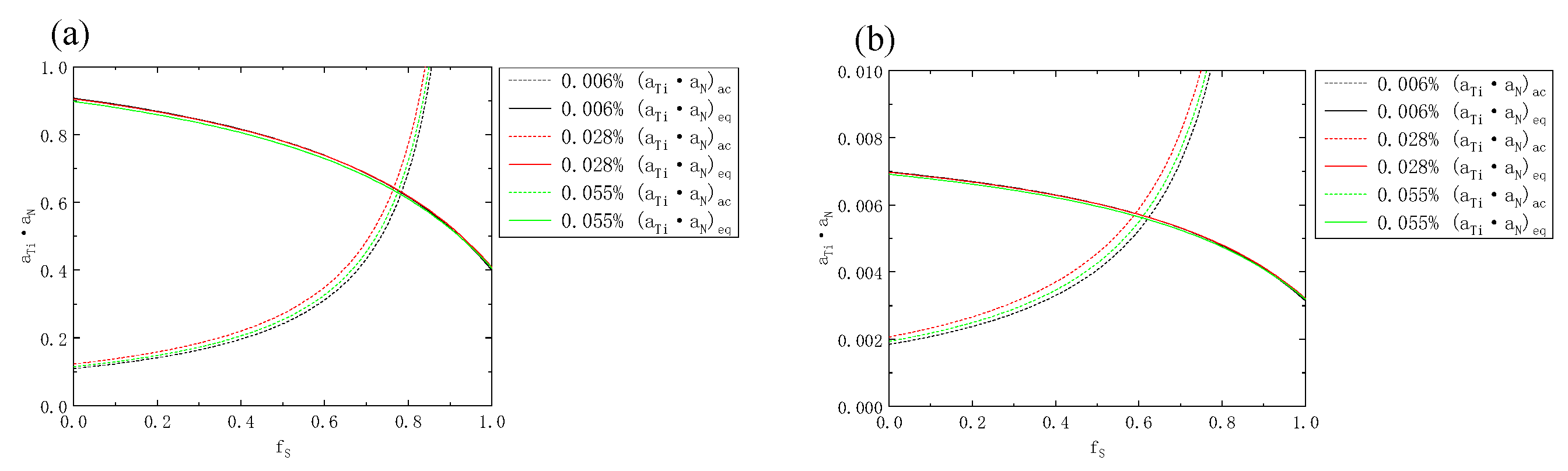
- The Ti content of the three samples increased from 0.006% to 0.055%. In both the liquid phase and the solid phase of the solid–liquid two-phase region, the solid phase rate of the initial precipitation of TiN decreased from 0.79 to 0, and the initial precipitation temperature increased from 1447 °C to higher than the liquidus temperature. The initial precipitation solid fraction of MnS was about 0.77 and 0.60, respectively, and the initial precipitation temperature was about 1450 °C and 1470 °C, respectively.
- (5)
- Titanium treatment provides a new idea for improving the morphology of sulfides. The formation of composite sulfides by using TiN precipitated at high temperatures is conducive to improving the morphology control and distribution uniformity of sulfides in steel. The determination of reasonable Ti and N contents according to the composition ranges of Mn and S, and the guarantee that the precipitation temperature is higher than the precipitation temperature of MnS are crucial to the morphology control of sulfides in steel. At present, it is not enough to judge whether the effect of the current treatment is sufficent, but the experimental results can clearly provide us with a direction of regulation. At the same time, the possible negative effects of titanium addition should be considered, such as leading to TiN coarsening, increased TiO2 formation and nozzle clogging. In the future, more experiments and industrial experiments should be combined to determine the reasonable control effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, K.D.; Wang, P.; Liu, W.P. Development status and application prospect of non-quenched tempered steel. Heat Treat. Metals 2011, 36, 80–85. [Google Scholar]
- Pi, J.H.; Bai, Y.Q.; Zhen, R. A Study on the Effect of Aging on Mechanical Properties of Cold-Formed Non-quenched Steel via Nanoindentation. J. Mater. Eng. Perform. 2017, 26, 28–33. [Google Scholar] [CrossRef]
- Lu, J.L.; Wang, Y.P.; Wang, Q.M.; Cheng, H.J.; Cheng, G.G. Effect of MnS Inclusions Distribution on Intragranular Ferrite Formation in Medium Carbon Non-Quenched and Tempered Steel for Large-Sized Crankshaft. ISIJ Int. 2019, 59, 524–530. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Gekonde, H.O.; Zhang, X. Design of steels for high speed machining. Ironmak. Steelmak. 1999, 26, 333–338. [Google Scholar] [CrossRef]
- Ghosh, A.; Sahoo, S.; Ghosh, M.; Ghosh, R.N.; Chakrabarti, D. Effect of microstructural parameters, microtexture and matrix strain on the Charpy impact properties of low carbon HSLA steel containing MnS inclusions. Mater. Sci. Eng. A 2014, 613, 37–47. [Google Scholar] [CrossRef]
- Ito, Y.; Yonezawa, N.; Matsubara, K. The Effect of Carbon on the Composition of the Eutectic Conjugation in the Fe-Mn-S System and the Equilibrium of Sulfides in Solid Steels. Tetsu-Hagane 1979, 65, 1149–1158. [Google Scholar] [CrossRef]
- Ito, Y.; Masumitsu, N.; Matsubara, K. Formation of Manganese Sulfide in Steel. Iron Steel Inst. Jpn. 1981, 21, 477–484. [Google Scholar] [CrossRef]
- Ito, Y.; Yonezawa, N.; Matsubara, K. The Composition of Eutectic Conjugation in Fe-Mn-S System. Trans. Iron Steel Inst. Jpn. 2019, 20, 19–25. [Google Scholar] [CrossRef]
- Bigelow, L.K.; Flemings, M.C. Sulfide inclusions in steel. Metall. Mater. Trans. B 1975, 6, 275–283. [Google Scholar] [CrossRef]
- Ray, A.; Paul, S.K.; Jha, S. Effect of Inclusions and Microstructural Characteristics on the Mechanical Properties and Fracture Behavior of a High- Strength Low-Alloy Steel. J. Mater. Eng. Perform. 1995, 4, 679–688. [Google Scholar] [CrossRef]
- Xiao, G.H.; Dong, H.; Wang, M.Q.; Hui, W.J. Effect of Mg/Ca-Treatment on Morphology of Sulfide in Non-Quenched and Tempered Steel. Iron Steel 2011, 46, 65–69. [Google Scholar]
- Fu, J.X.; Zhu, Y.Y.; Li, X.; Liu, H.; Gong, T.; Li, J.; Hu, D.L.; Xu, J.; Zhang, M.; Wu, Y.X.; et al. A 45MnVS Easy—Cutting Non—Quenched and Tempered Steel Containing Magnesium and Calcium and Its Manufacturing Method. Chinese Patent Application CN105803308A, 19 March 2016. [Google Scholar]
- Liu, W.; Yang, S.F.; Li, J.S.; Li, J.K.; Zhang, S. Modification of sulphide inclusions in 20 CrMnTi steel by calcium-magnesium treatment. Iron Steel 2017, 52, 21–27. [Google Scholar]
- Li, D.Z. Globularizing of sulfide inclusions and its influence on the propesties of steel. J. Iron Steel Res. 1992, 4, 97–101. [Google Scholar]
- Lu, J.L.; Cheng, G.G.; Chen, L.; Xiong, G.J.; Wang, L.S. Distribution and Morphology of MnS Inclusions in Resulfurized Non-Quenched and Tempered Steel with Zr Addition. ISIJ Int. 2018, 58, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.J.; Li, J.; Fan, D.D.; Dong, H.B. The Effect of Aluminum on the Divorced Eutectic Transformation of MnS Inclusions. Metall. Mater. Trans. B 2021, 52, 1118–1131. [Google Scholar] [CrossRef]
- Li, M.L.; Wang, F.M.; Li, C.R.; Yang, Z.B.; Meng, Q.Y.; Tao, S.F. Effects of cooling rate and Al on MnS formation in medium-carbon non-quenched and tempered steels. Int. J. Miner. Metall. Mater. 2015, 22, 589–597. [Google Scholar] [CrossRef]
- Tomita, Y.; Saito, N.; Tsuzuki, T.; Tokunaga, Y.; Okamoto, K. Improvement in HAZ Toughness of Steel by TiN-MnS Addition. ISIJ Int. 1994, 34, 829–835. [Google Scholar] [CrossRef]
- Oikawa, K.; Ishida, K.; Nishizawa, T. Effect of Titanium Addition on the Formation and Distribution of MnS Inclusions in Steel during Solidification. ISIJ Int. 1997, 37, 332–338. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Bai, X.F.; Barati, M.; Mclean, A. Formation of Inclusions in Ti-Stabilized 17Cr Austenitic Stainless Steel. Metall. Mater. Trans. B 2016, 47, 3274–3284. [Google Scholar] [CrossRef]
- Cai, K.K. Casting and Solidification; The Metallurgical Industry Press: Beijing, China, 1987; pp. 108–120. [Google Scholar]
- Luo, Y.Z.; Zhang, J.M.; Liu, Z.M.; Xiao, C.; Wu, S.Z. In situ observation and thermodynamic calculation of MnS in 49MnVS3 non-quenched and tempered steel. Acta Metall. Sin. 2011, 24, 326–334. [Google Scholar]
- Chen, J.X. Manual of Chart and Data in Common Use of Steel Making; The Metallurgical Industry Press: Beijing, China, 1998; pp. 221–227. [Google Scholar]
- Fu, J.; Zhu, J.; Di, L.; Tong, F.S.; Liu, D.L.; Wang, L.Y. Study on the precipitation behavior of TiN in the microalloyed steels. Metall. Trans. 2000, 8, 801–804. [Google Scholar]
- Cicutti, C.E.; Madias, J.; Gonzalez, J.C. Control of microinclusions in calcium treated aluminum killed steels. Ironmak. Steelmak. 1997, 24, 155–159. [Google Scholar]
- Hino, M.; Ito, K. Thermodynamic Data for Steelmaking; Senda, Tohoku University Press: Tokyo, Japan, 2010; pp. 259–264. [Google Scholar]
- Huang, X.H. Ferrous Metallurgy Principle, 4th ed.; The Metallurgical Industry Press: Beijing, China, 1997; p. 181. [Google Scholar]

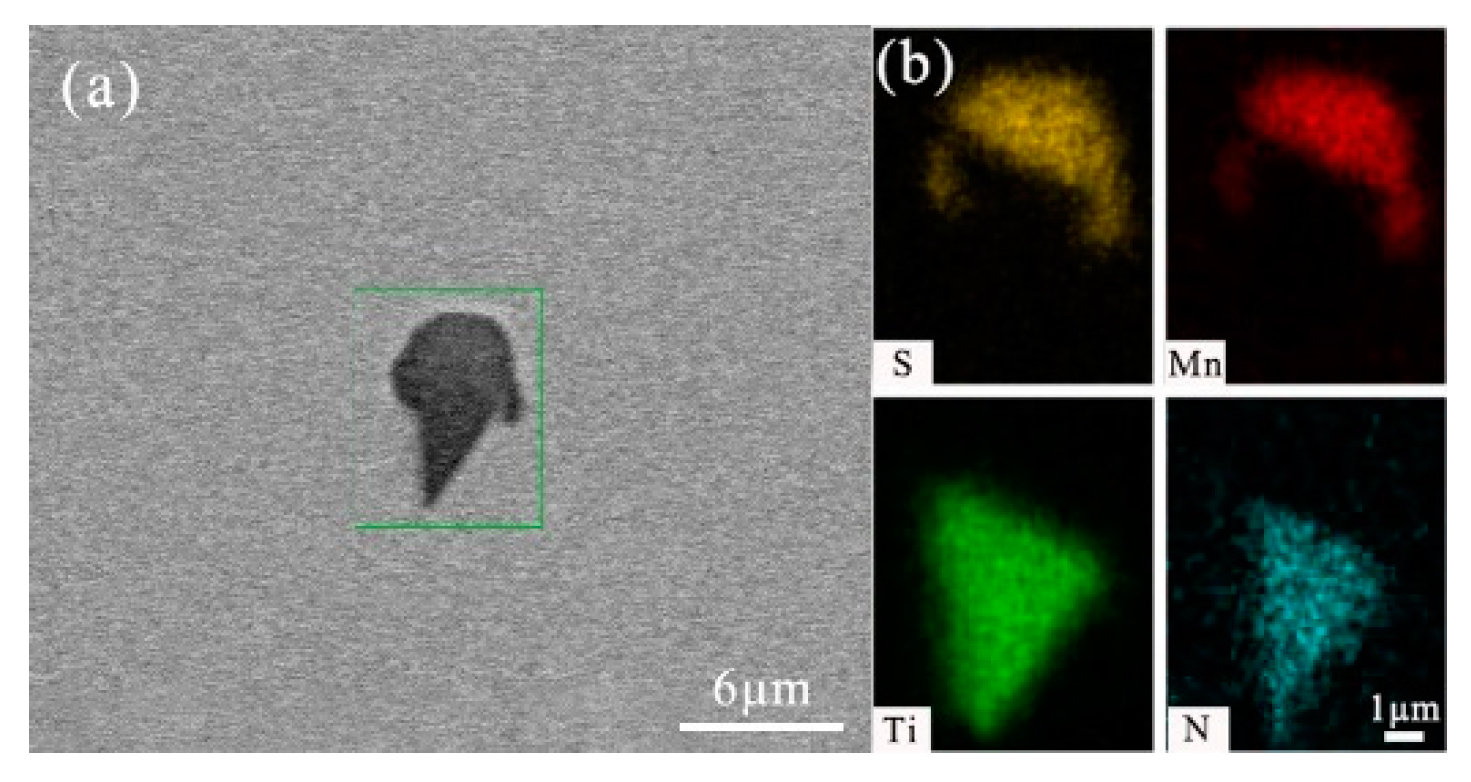



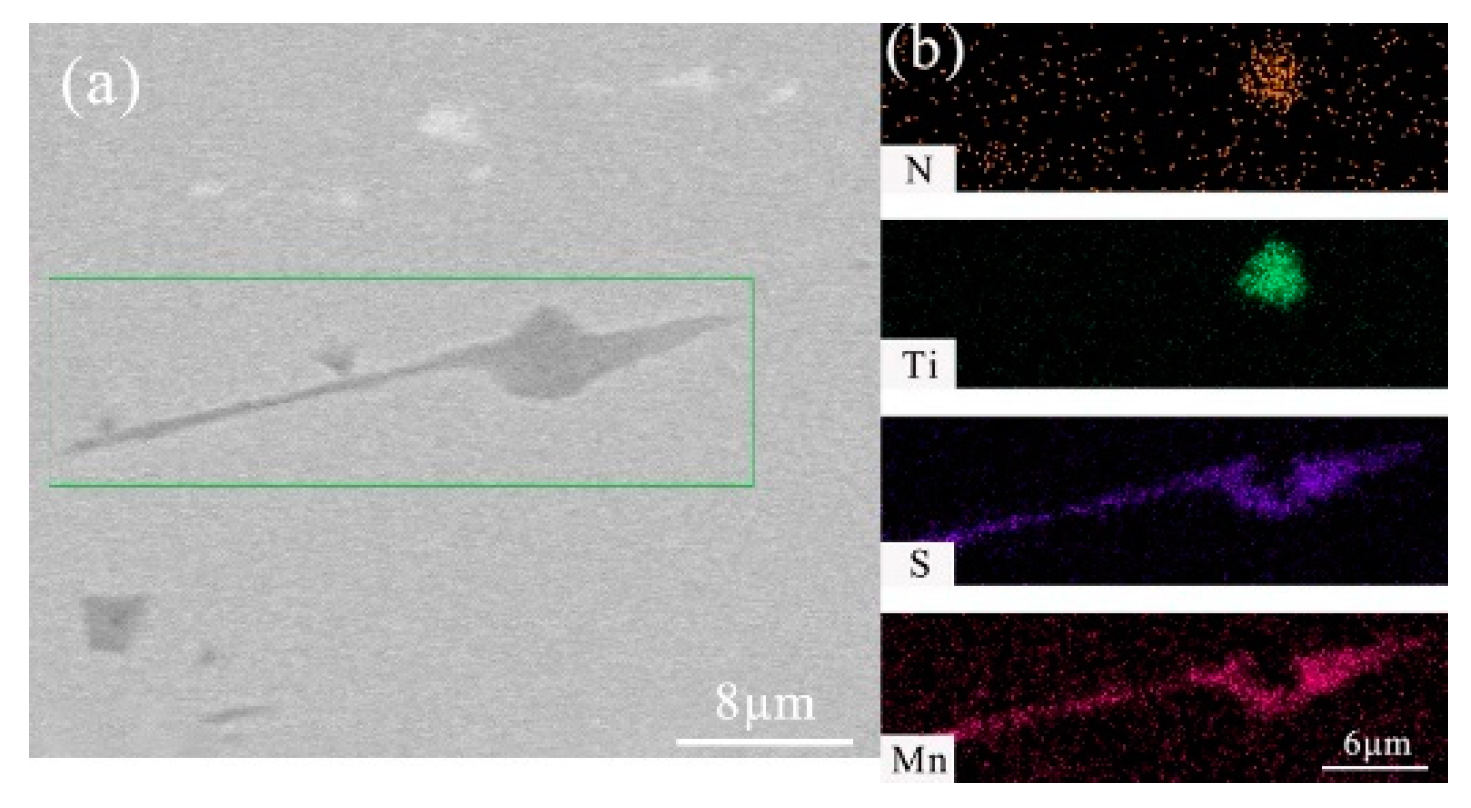
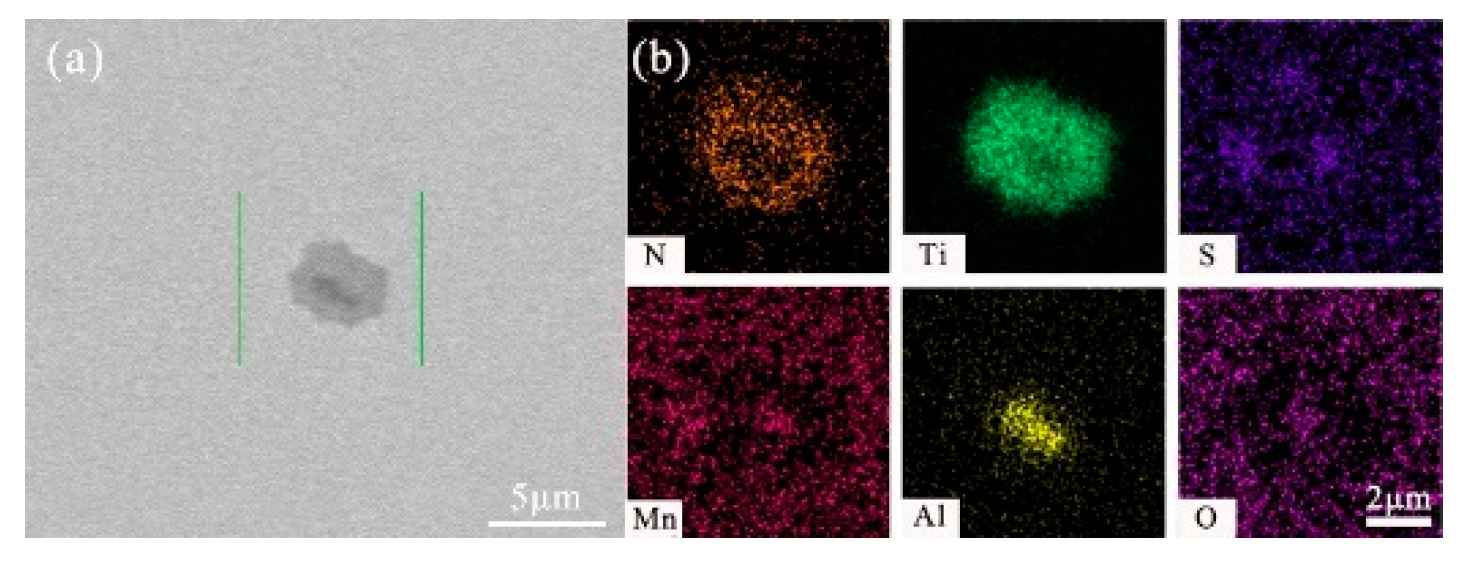
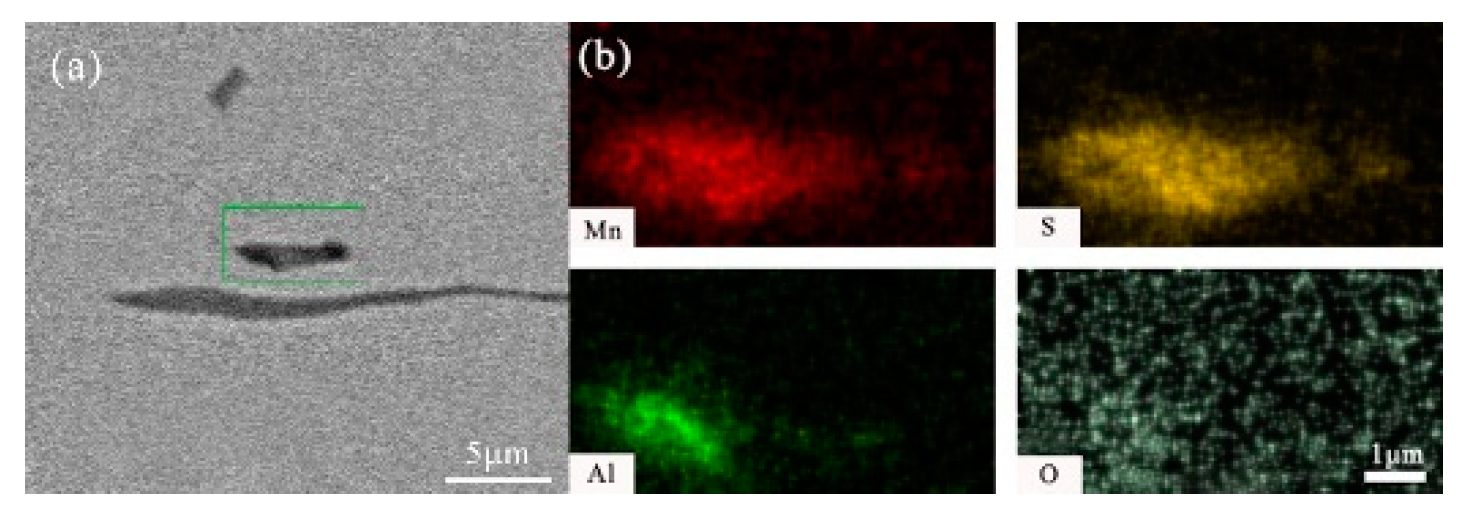
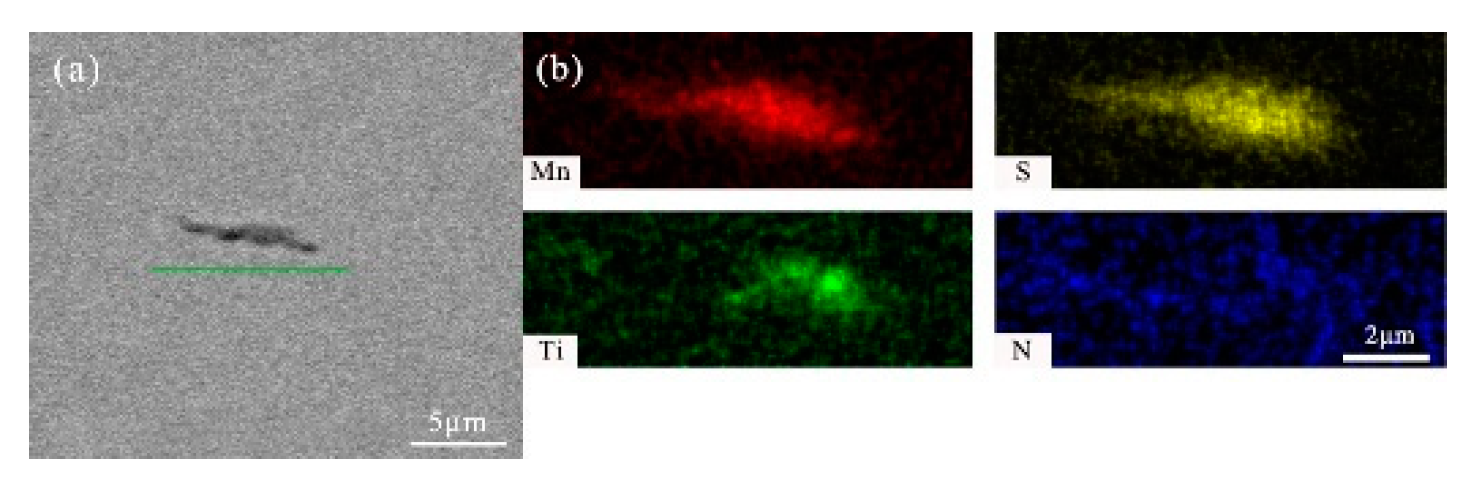



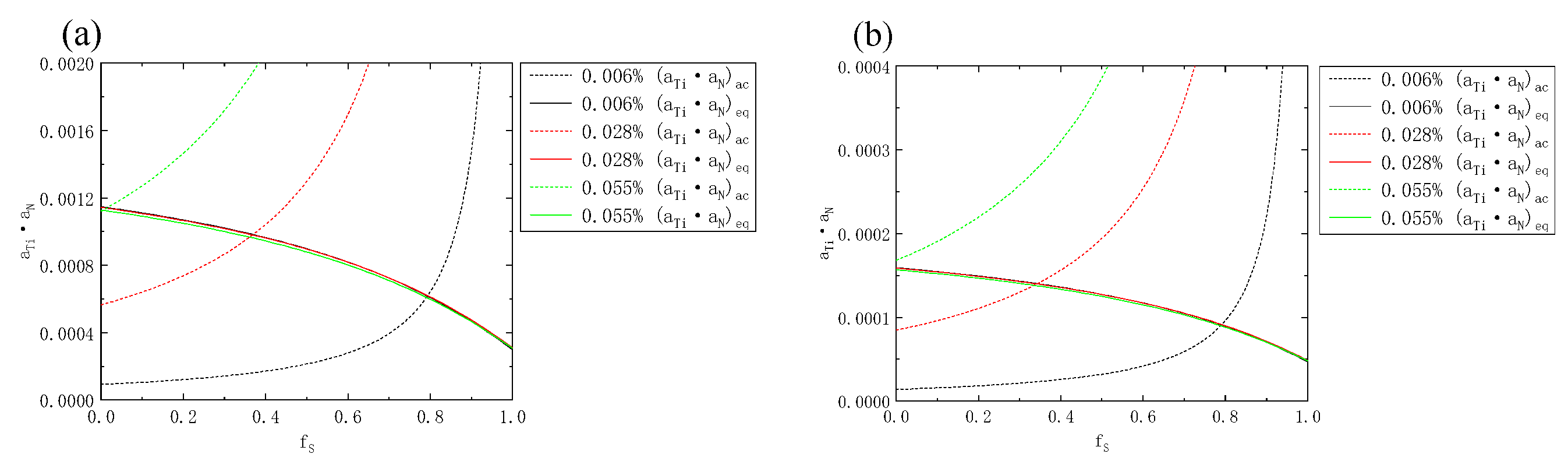
| No. | C | Si | P | Mn | S | Ti | N | Al | V | Nb | O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.333 | 0.388 | 0.009 | 1.812 | 0.044 | 0.006 | 0.018 | 0.005 | 0.026 | 0.024 | 0.0019 |
| 2 | 0.314 | 0.538 | 0.009 | 1.659 | 0.046 | 0.028 | 0.024 | 0.006 | 0.026 | 0.021 | 0.0030 |
| 3 | 0.330 | 0.547 | 0.009 | 1.566 | 0.045 | 0.055 | 0.025 | 0.016 | 0.027 | 0.024 | 0.0025 |
| Ti Content/ωt % | Pure MnS | Ti-Containing Composite Sulfides | Other Composite Sulfides | |||
|---|---|---|---|---|---|---|
| MnS with TiN as the Core | MnS Precipitated with TiN | MnS Pinned by TiN | MnS with Oxide as the Core | MnS Precipitated with Oxide | ||
| 0.006 | 85.5% | - | 4.8% | 1.6% | 6.5% | 1.6% |
| 0.028 | 74.4% | 5.1% | 20.1% | - | 0.4% | - |
| 0.055 | 61.5% | 19.2% | 9.6% | 7.7% | 2.0% | - |
| Ti Content/ωt % | 0.006 | 0.028 | 0.055 |
|---|---|---|---|
| 1501 °C | 1500 °C | 1499 °C | |
| 1388 °C | 1390 °C | 1390 °C |
| Element j | C | Si | Mn | P | S | Ti | Al | V | Nb | O | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [26] | −0.165 [27] | 0.05 [27] | 0.0043 [27] | −0.06 | −0.27 | 0.042 | 0 | 0 | 0 | −3.4 | −2.041 |
| [26] | 0.13 | 0.048 | −0.02 | 0.059 | 0.007 | −0.59 | 0.01 | −0.123 | −0.068 | −0.12 | 0 |
| [27] | −0.07 | 0.39 | 0 | −0.0035 | −0.048 | 0 | 0 | 0 | 0.0035 | −0.083 | −0.091 |
| [27] | 0.112 | 0.063 | −0.026 | 0.29 | −0.028 | −0.072 | 0.035 | −0.016 | −0.013 | −0.27 | 0.01 |
| Ti Content/ωt % | 0.006 | 0.028 | 0.055 |
|---|---|---|---|
| MnS liquid fs/solid fs | 0.78/0.62 | 0.77/0.59 | 0.77/0.59 |
| TiN liquid fs/solid fs | 0.79/0.79 | 0.37/0.34 | 0/0 |
| MnS T (liquid fs)/T (solid fs) | 1449 °C/1469 °C | 1451 °C/1472 °C | 1449 °C/1470 °C |
| TiN T (liquid fs)/T (solid fs) | 1447 °C/1447 °C | 1487 °C/1488 °C | >1499 °C/>1499 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Li, X.; Wang, Z.; Liu, W. Effect of TiN on Sulfide Morphology of Non-Quenched and Tempered Steel. Metals 2022, 12, 1402. https://doi.org/10.3390/met12091402
Wu H, Li X, Wang Z, Liu W. Effect of TiN on Sulfide Morphology of Non-Quenched and Tempered Steel. Metals. 2022; 12(9):1402. https://doi.org/10.3390/met12091402
Chicago/Turabian StyleWu, Huajie, Xin Li, Zhe Wang, and Wei Liu. 2022. "Effect of TiN on Sulfide Morphology of Non-Quenched and Tempered Steel" Metals 12, no. 9: 1402. https://doi.org/10.3390/met12091402
APA StyleWu, H., Li, X., Wang, Z., & Liu, W. (2022). Effect of TiN on Sulfide Morphology of Non-Quenched and Tempered Steel. Metals, 12(9), 1402. https://doi.org/10.3390/met12091402






