On Agreement of Experimental Data and Calculated Results in Grain Boundary Segregation
Abstract
1. Introduction
2. Determination of Grain Boundary Composition
3. Calculations of Segregation Energies
4. Fundamentals of the Comparison of Theoretical and Experimental Results
5. Comparison of Experimental and Theoretical Data on Grain Boundary Segregation
5.1. System Fe—5 at.% Si
5.2. System Fe—2.3 at.% V
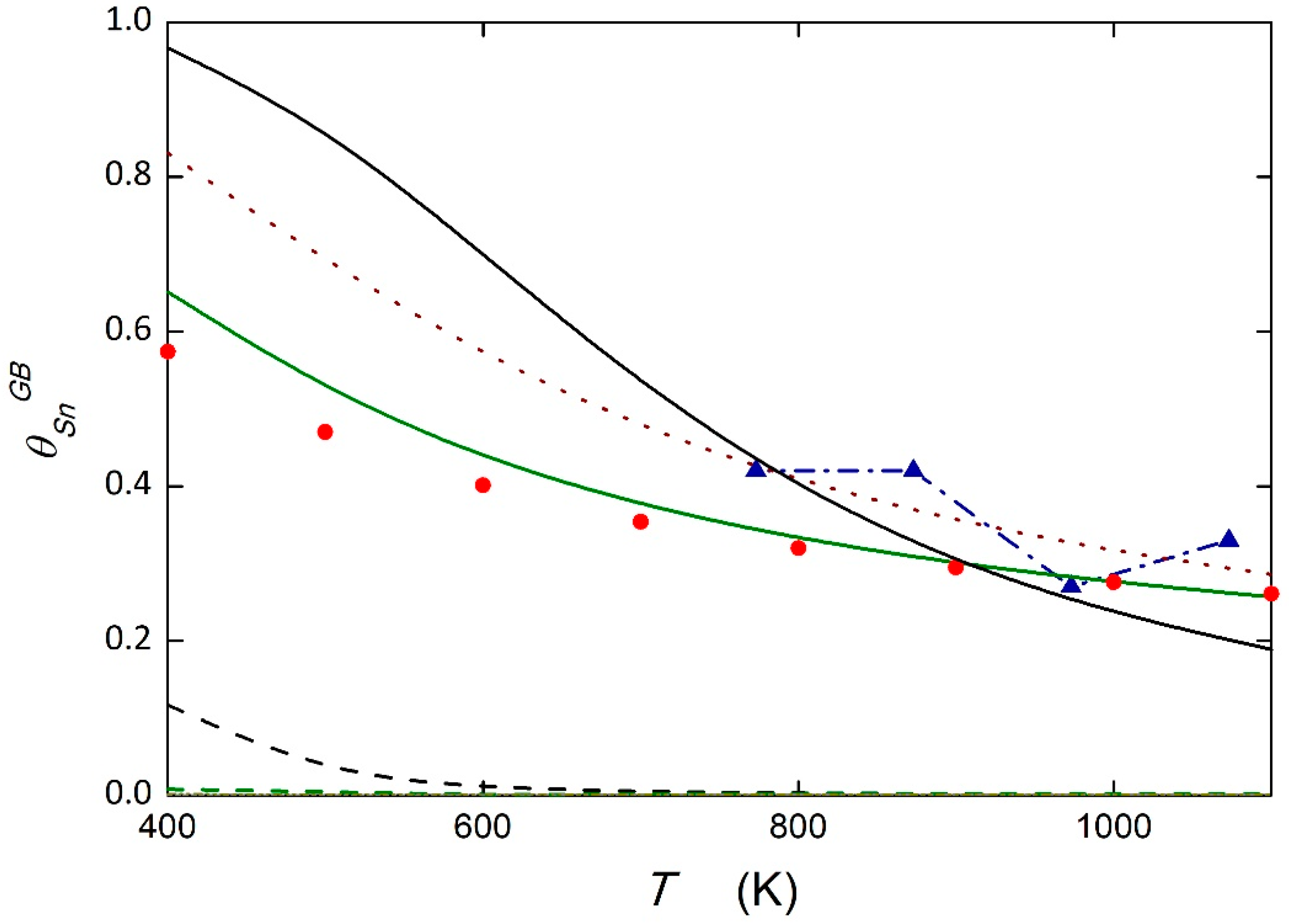
5.3. System Fe—0.065 at.% Sn
6. Discussion
7. Conclusions
- An excellent agreement between experiment and calculation can be obtained when the solid solubility of the solute is high enough, i.e., well above 1 at.%;
- Experimental and calculated data can be compared on basis of averaged values of the segregation energy;
- Averaged values of the segregation energy must be determined using all sites, including the ‘anti-segregation’ ones;
- To predict the value of the segregation entropy, the approach of Scheiber and Romaner [12] distinguishing the upper and bottom branches of the enthalpy–entropy compensation effect seems to be valuable.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briant, C.L. (Ed.) Impurities in Engineering Materials, Impact, Reliability and Control; Marcel Dekker, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Flewitt, P.E.J.; Wild, R.K. Grain Boundaries, Their Microstructure and Chemistry; John Wiley and Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Lejček, P. Grain Boundary Segregation in Metals; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- McLean, D. Grain Boundaries in Metals; Clarendon Press: Oxford, UK, 1957. [Google Scholar]
- Hondros, E.D. Grain Boundary Segregation Assessment of Investigative Techniques. In Grain Boundary Structure and Properties; Chadwick, G.A., Smith, D.A., Eds.; Academic Press: London, UK, 1976; pp. 265–298. [Google Scholar]
- Hofmann, S. Auger and X-ray Photoelectron Spectroscopy in Materials Science. In A User-Oriented Guide; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain Boundary Segregation Engineering and Austenite Reversion Turn Embrittlement into Toughness: Example of a 9 wt.% Medium Mn Steel. Acta Mater. 2015, 86, 182–192. [Google Scholar] [CrossRef]
- Amram, D.; Schuh, C.A. Interplay Between Thermodynamic and Kinetic Stabilization Mechanisms in Nanocrystalline Fe-Mg alloys. Acta Mater. 2018, 144, 447–458. [Google Scholar] [CrossRef]
- Lejček, P.; Šob, M.; Paidar, V. Interfacial Segregation and Grain Boundary Embrittlement: An Overview and Critical Assessment of Experimental Data and Calculated Results. Prog. Mater. Sci. 2017, 87, 83–139. [Google Scholar] [CrossRef]
- Lejček, P.; Šob, M.; Paidar, V.; Vitek, V. Why Calculated Energies of Grain Boundary Segregation Are Unreliable When Segregant Solubility Is Low. Scripta Mater. 2013, 68, 547–550. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S. Modeling Grain Boundary Segregation by Prediction of All the Necessary Parameters. Acta Mater. 2019, 170, 253–267. [Google Scholar] [CrossRef]
- Scheiber, D.; Romaner, L. Impact of the Segregation Energy Spectrum on the Enthalpy and Entropy of Segregation. Acta Mater. 2021, 221, 117393. [Google Scholar] [CrossRef]
- Lejček, P. Characterization of Grain Boundary Segregation in Fe-Si Alloy. Anal. Chim. Acta 1994, 297, 165–178. [Google Scholar] [CrossRef]
- Hondros, E.D.; Seah, M.P. Segregation to Interfaces. Int. Met. Rev. 1977, 22, 262–299. [Google Scholar] [CrossRef]
- Lejček, P.; Zheng, L.; Hofmann, S.; Šob, M. Applied Thermodynamics: Grain Boundary Segregation. Entropy 2014, 16, 1462–1483. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S. Thermodynamics of Grain Boundary Segregation and Applications to Anisotropy, Compensation Effect and Prediction. Crit. Rev. Sol. State Mater. Sci. 2008, 33, 133–163. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Open-Shell Transition Metals. Phys. Rev. B 1993, 48, 13115. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furhmüller, J. Efficient Iterative Schemes for Ab Intio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Coghlan, W.A. The Spectrum of Binding Energies Approach to Grain Boundary Segregation. Metall. Trans. A 1977, 8, 1403–1412. [Google Scholar] [CrossRef]
- Nowicki, T.; Joud, J.C.; Biscondi, M. A Thermodynamic Model of Grain Boundary Segregation for Atomistic Calculations. J. Phys. Colloq. 1990, 51, C1-293–C1-298. [Google Scholar] [CrossRef][Green Version]
- Lejček, P.; Hofmann, S. Interstitial and Substitutional Solute Segregation at Individual Grain Boundaries of α-Iron: Data Revisited. J. Phys. Condens. Matter. 2016, 28, 064001. [Google Scholar] [CrossRef]
- Jin, H.; Elfimov, I.; Militzer, M. Study of the interaction Of Solutes With Σ5 (013) Tilt Grain Boundaries in Iron Using Density-Functional Theory. J. Appl. Phys. 2014, 115, 093506. [Google Scholar] [CrossRef]
- Lejček, P.; Pokluda, J.; Šandera, P.; Horníková, J.; Jenko, M. Solute Segregation At 46.8 (111) Twist Grain Boundary of a Phosphorus Doped Fe–2.3%V Alloy. Surface Sci. 2012, 606, 258–262. [Google Scholar] [CrossRef]
- Kholtobina, A.S.; Ecker, W.; Pipan, R.; Razumovskiy, V.I. Effect of Alloying Elements on Hydrogen Enhanced Decohesion in Bcc Iron. Comput. Mater. Sci. 2021, 188, 110215. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S.; Všianská, M.; Šob, M. Entropy Matters in Grain Boundary Segregation. Acta Mater. 2021, 206, 116597. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S. Entropy-dominated grain boundary segregation. J. Mater. Sci. 2021, 56, 7464–7473. [Google Scholar] [CrossRef]
- Lejček, P.; Šandera, P.; Horníková, J.; Pokluda, J.; Godec, M. On the Segregation Behavior of Tin and Antimony at Grain Boundaries of Polycrystalline Iron. Appl. Surf. Sci. 2016, 363, 140–144. [Google Scholar] [CrossRef]
- Lejček, P.; Jäger, A.; Gärtnerová, V. Reversed Anisotropy of Grain Boundary Properties and Its Effect on Grain Boundary Engineering. Acta Mater. 2010, 58, 1930–1937. [Google Scholar] [CrossRef]
- Seah, M.P.; Lea, C. Surface Segregation and Its Relation to Grain Boundary Segregation. Philos. Mag. 1975, 31, 627–645. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S. Entropy-Driven Grain Boundary Segregation: Prediction of the Phenomenon. Metals 2021, 11, 1331. [Google Scholar] [CrossRef]
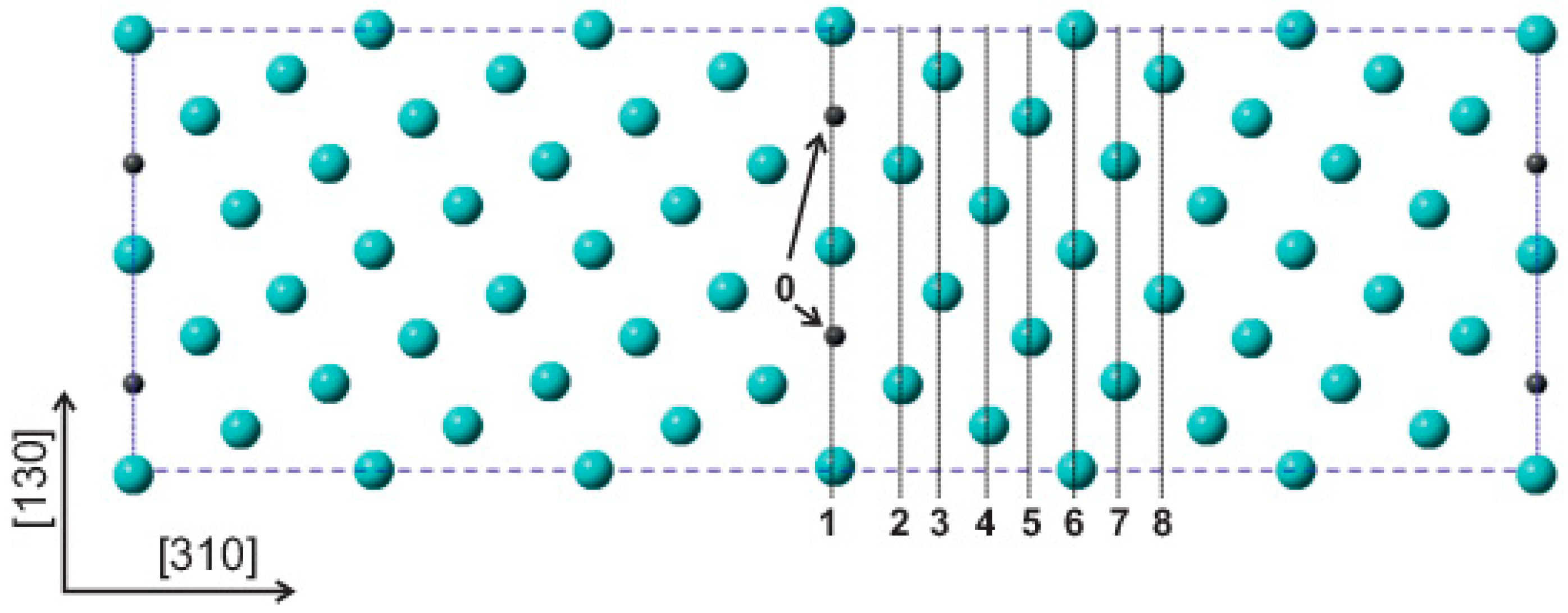
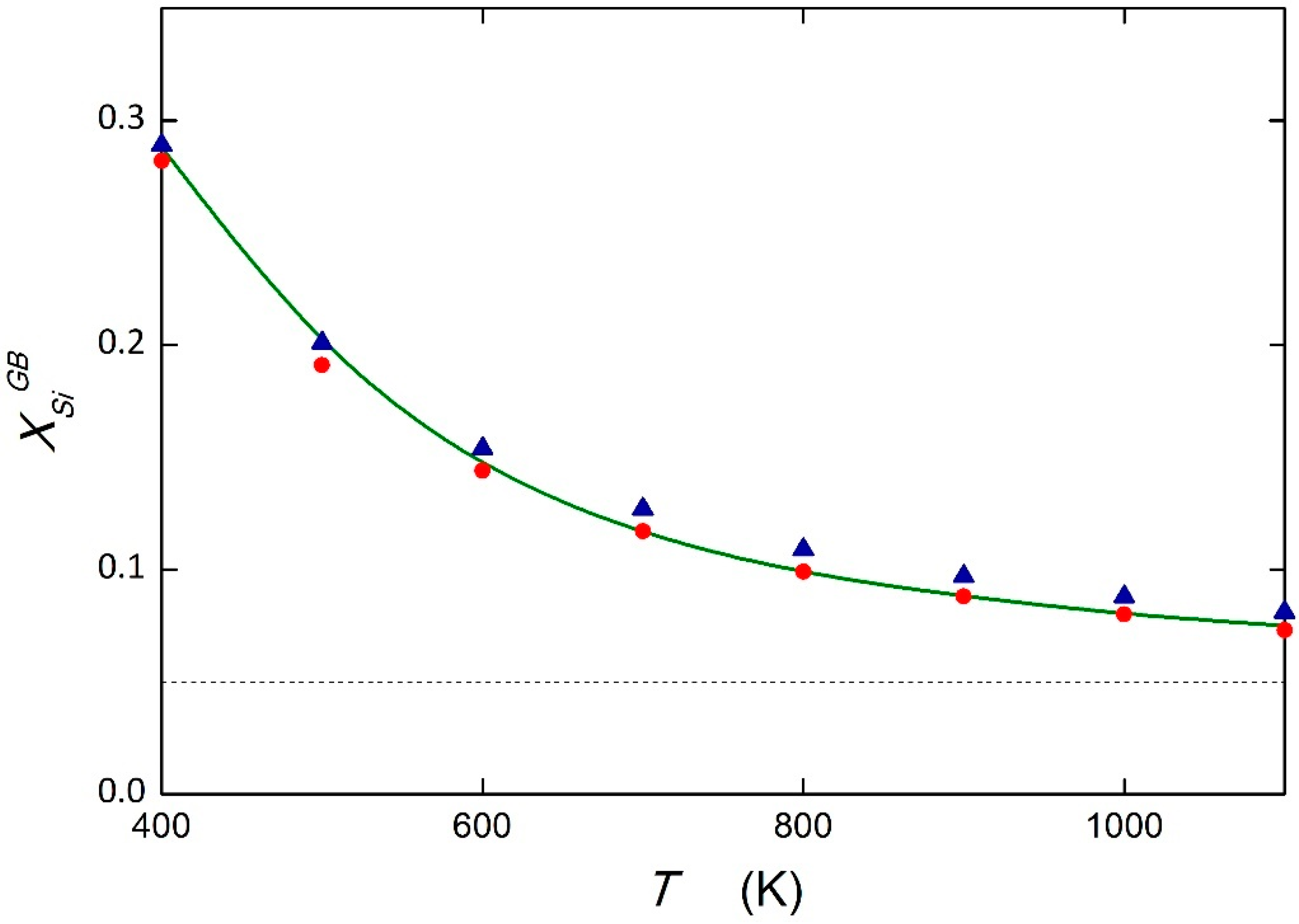
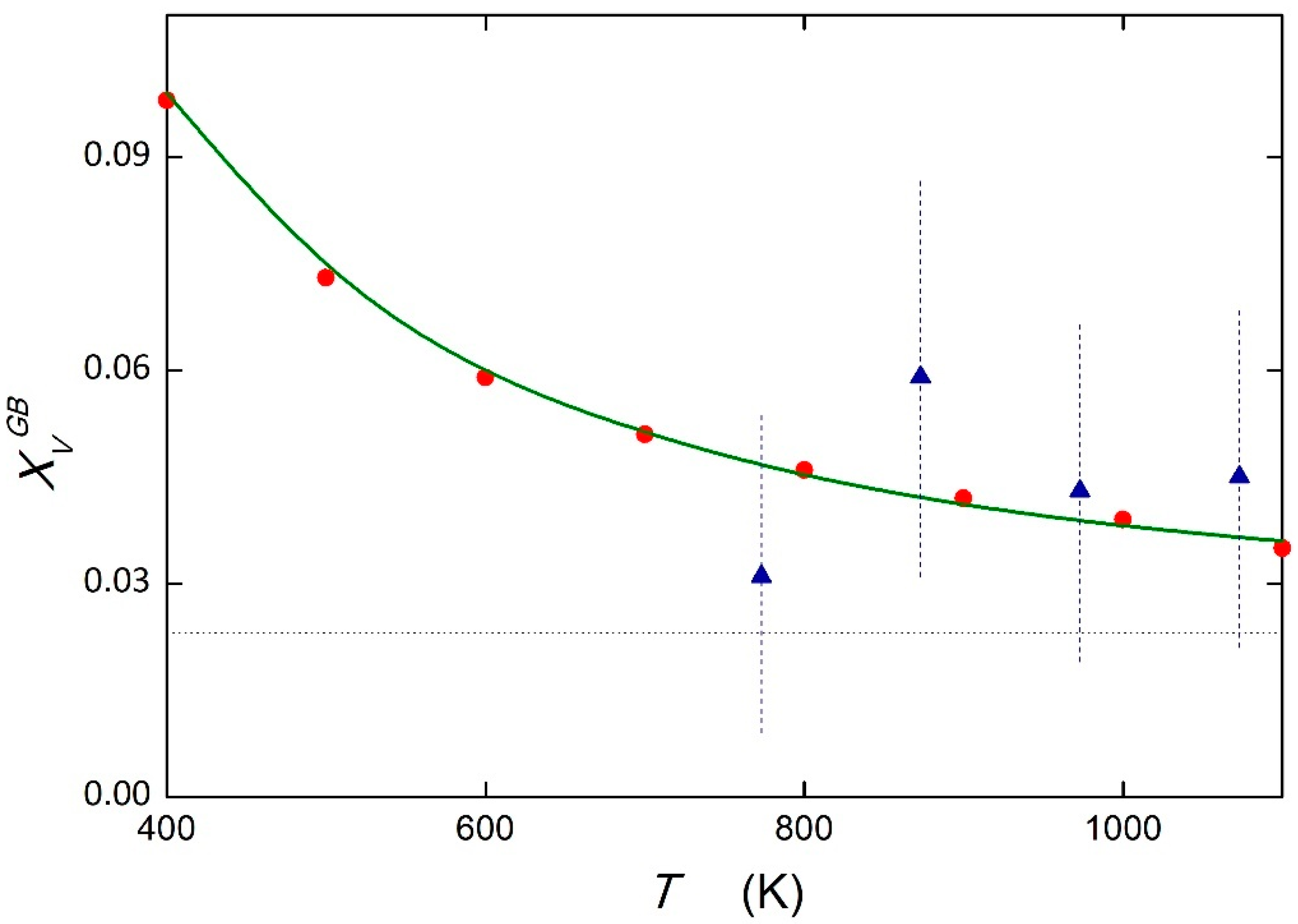
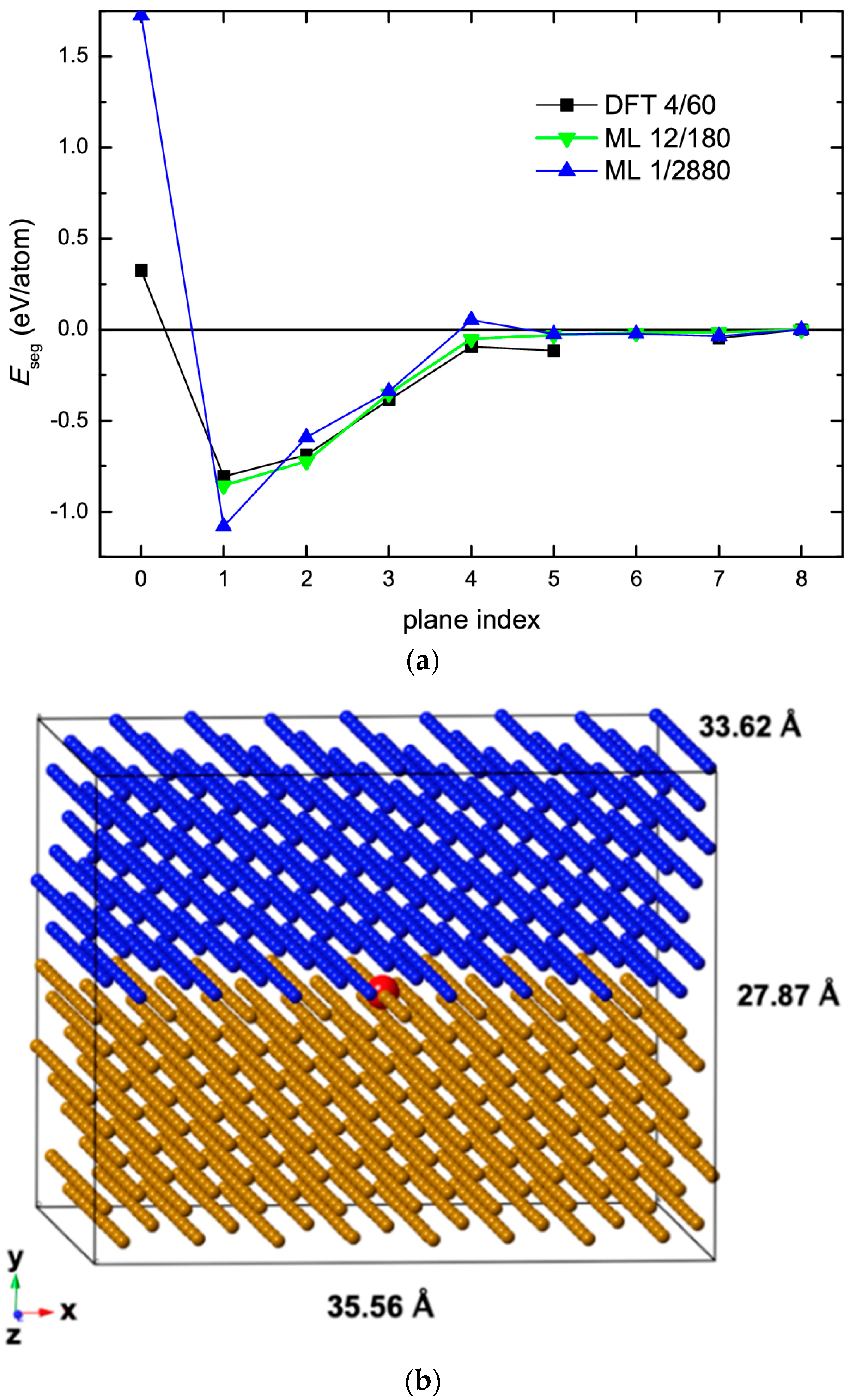
| GB Site | DFT ΔESi [24] (kJ mol−1) | PRED (DFT) ΔSSi [11] (J mol−1 K−1) | AES ΔHSi [23] (kJ mol−1) | AES ΔSSi [23] (J mol−1 K−1) | PRED [11] (kJ mol−1) | PRED [11] (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| 0 | −6.8 | −2.5 | - | - | - | - |
| +1, −1 | −17.4 | −14.3 | - | - | - | - |
| +2, −2 | −3.9 | +0.7 | - | - | - | - |
| +3, −3 | −1 | +3.9 | - | - | - | - |
| AVE | −7.3 | −3.1 | −8 | −3 | −8.4 | −4.4 |
| GB Site | DFT ΔEV [26] (kJ mol−1) | PRED (DFT) ΔSV [11] (J mol−1 K−1) | PRED [11] (kJ mol−1) | PRED [11] (J mol−1 K−1) |
|---|---|---|---|---|
| 0 | −16.4 | −13.2 | - | - |
| +1, −1 | +6.8 | +12.5 | - | - |
| +2, −2 | −12.5 | −8.9 | - | - |
| AVE {111} | −5.6 | −1.2 | −5.5 | −1 |
| GB Site | ML ΔESn (kJ mol−1) | PRED (ML) ΔSSn [11] (J mol−1 K−1) | PRED (ML) ΔSSn [12] (J mol−1 K−1) | DFT ΔESn (kJ mol−1) | PRED (DFT) ΔSSn [11] (J mol−1 K−1) | PRED (DFT) ΔSSn [12] (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| 0i | +166.6 | +239.1 | +239.1 | +31.3 | +88.8 | +88.8 |
| 1 | −104.4 | −111.0 | −62.0 | −78.0 | −81.7 | −32.7 |
| +2, −2 | −57.1 | −58.5 | −9.5 | −66.5 | −68.9 | −19.9 |
| +3, −3 | −32.6 | −31.2 | +17.8 | −37.2 | −36.3 | +12.7 |
| +4, −4 | +5.1 | +10.7 | +59.7 | −9.0 | −5.0 | +44.0 |
| +5, −5 | −2.3 | +2.4 | +51.4 | −11.2 | −7.4 | +41.6 |
| +6, −6 | −2.3 | +2.4 | +51.4 | - | - | - |
| +7, −7 | −3.5 | +1.1 | +50.1 | −4.5 | 0 | 49.0 |
| AVE ΔSSi [11] | −8.8 | −1.3 | - | −25.3 | −19.0 | - |
| AVE ΔSSi [12] | −8.8 | - | +44.2 | −25.3 | - | +25.9 |
| PRED [11] | −7 | - | +46 | −7 | - | +46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černý, M.; Šesták, P.; Všianská, M.; Lejček, P. On Agreement of Experimental Data and Calculated Results in Grain Boundary Segregation. Metals 2022, 12, 1389. https://doi.org/10.3390/met12081389
Černý M, Šesták P, Všianská M, Lejček P. On Agreement of Experimental Data and Calculated Results in Grain Boundary Segregation. Metals. 2022; 12(8):1389. https://doi.org/10.3390/met12081389
Chicago/Turabian StyleČerný, Miroslav, Petr Šesták, Monika Všianská, and Pavel Lejček. 2022. "On Agreement of Experimental Data and Calculated Results in Grain Boundary Segregation" Metals 12, no. 8: 1389. https://doi.org/10.3390/met12081389
APA StyleČerný, M., Šesták, P., Všianská, M., & Lejček, P. (2022). On Agreement of Experimental Data and Calculated Results in Grain Boundary Segregation. Metals, 12(8), 1389. https://doi.org/10.3390/met12081389









