Abstract
In this work, a series of Gd-based amorphous/crystalline composite fibers (ANCFs) were prepared by regulating the Gd content in Gd-Co-Al alloys using the melt-extracted method. Compared to the amorphous alloy, the ANCFs display excellent magnetic refrigeration capacity (RC). Among them, Gd85Co5Al10 ANCF had the largest RC (841 J kg−1) and the widest working temperature range (245 K). Compared with Gd70Co10Al20, RC and working temperature range increased by 56% and 119%, respectively. This superior property is attributed to the ideal coupling between the amorphous phase and the crystalline. This result opens a new door to optimize the magnetic refrigeration capacity by controlling the amorphous crystalline composite structure.
1. Introduction
Unlike the conventional gas-compression refrigeration, magnetic refrigeration has attracted much attention for being an ideal candidate in refrigeration because of its advantages in environmental friendliness and energy efficiency [1,2,3,4]. Magnetocaloric effect (MCE) is a thermal response caused by the change of magnetic entropy for a varying magnetic field. Magnetocaloric materials with specific magnetocaloric properties are the core issue for their actual applications, e.g., adjustable magnetic transition temperature (TC), large magnetic entropy change (−ΔSM), large RC, and wide working temperature range [5,6]. Previous works show that the rare earth (RE)-based alloys usually perform better in MCE than transition metals such as Fe- or Mn-based alloys [1,2,3,5,7,8,9,10,11,12,13,14,15,16,17,18,19]. For example, RE-based amorphous alloys show reduced Curie temperature (TC), a wider working temperature range, and higher RC [20], which bestows them with superior potential refrigerants for cryogenic refrigeration, such as at liquid nitrogen temperatures or liquid helium temperatures [6,21]. However, the reduced TC limits their refrigeration ability near room temperature [15,20,22,23].
To enhance the temperature range of magnetocaloric materials, the other phases with different TC were induced [6,24,25,26]. For example, the composite of Gd65Mn25Si10 amorphous ribbons and Gd sheets induced by hot-pressing displays a table-like magnetic entropy change in a wide temperature range 73 K [25]. However, without metallurgical grade bonding, composites prepared by physical compounding methods like hot-pressing have weakened strength and thermal conductivity. On the other hand, the amorphous/crystalline composites can also be achieved by the composition design or a part-crystallization annealing for amorphous alloys [6,21,26]. In these processes, the metallurgical grade-binding between different phases can be achieved, which promotes the refrigeration abilities of samples. Besides, the melt extraction method with high heat transfer capacity was used to prepare the fibers, which have relatively high magnetic, thermal, electrical and mechanical properties [6,27,28,29].
In this work, a series of Gd-Co-Al amorphous/nanocrystalline composites fibers (ANCFs) have been prepared by the melt-extraction method. The optimal refrigeration ability was determined by the wide working temperature range from 50 K to 295 K, large −ΔSM (8.7 J kg−1 K−1) and RC (841 J kg−1) when the Gd content is at 85%. Besides, the optimal refrigeration ability of Gd85Co5Al10 originates from the coupling effect between crystalline Gd and an amorphous matrix. This result suggests that the amorphous/crystalline composite formed by composition design has potential to be a new method to fabricate superior magnetocaloric materials.
2. Experimental Details
The master alloys with nominal composition of Gd70Co10Al20, Gd80Co10Al10, Gd85Co5Al10, and Gd80Co5Al15 (at.%) were fabricated by arc melting pure Gd, Co and Al in a Ti-gettered argon atmosphere. In addition, the master alloys were prepared for micron fibers by the melt-extraction method (10 m/s). The phase compositions were examined by X-ray diffraction (XRD, Bruker D8 Advance) with Cu Kα radiation, and the specimen was scanned over a 2θ range from 20° to 90° at 6°/min. The surface and cross-section of the fibers were observed by scanning electron microscopy (SEM, EVO18, ZEISS, Berlin, Germany) at operating voltages of 20 kV. The microstructures were examined by transmission electron microscopy (TEM, Themo Fisher, Talos F200×, Waltham, MA, USA) at operating voltages of 200 kV, bright field image (BF) and selected-area electron diffraction (SAED) of the different electron beam incident axis were used to investigate the microstructure of the ANCF specimen. The magnetic properties were then measured using the Magnetic Property Measurement System (MPMS, Quantum Design Int., San Diego, CA, USA).
3. Results and Discussion
In Figure 1a, the schematic melt-extraction method for preparing Gd-Co-Al fibers is given. The master alloy ingot was melted in a high-frequency-induced current, and the high-speed rotation Mo wheel spun out the melt and formed the fibers finally. The length of prepared fibers was about 15 cm, as shown in Figure 1b. SEM results show that the prepared fibers exhibit narrow distribution of size, and that their diameter is about 70 μm for the Gd85Co5Al10 sample. Besides, the fibers were not cylindrical in a regular manner because of the surface tension during the melt-extraction process, as shown in Figure 1c–e. Notably, fibers have better application performance compared with their bulk or ribbon counterparts because their large surface area enhances the heat-exchange efficiency [27,28,29].
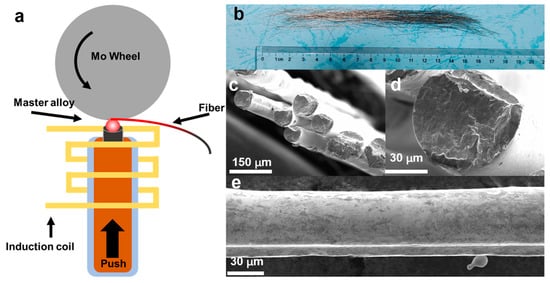
Figure 1.
(a) The diagram of melt-extraction method, (b) the image, and (c–e) the SEM images of Gd85Co5Al10 ANCF.
The XRD patterns of the Gd70Co10Al20, Gd80Co10Al10, Gd80Co5Al15 and Gd85Co5Al10 as-cast fibers is shown in Figure 2. For Gd70Co10Al20, the XRD pattern only shows a broad hump, which indicates its pure amorphous structure. For Gd80Co10Al10, Gd80Co5Al15, and Gd85Co5Al10, crystal peaks appear in their XRD patterns, which were contributed by Gd crystal. It is interesting that the (103 ) peak of Gd appears in the XRD pattern for Gd85Co5Al10, besides the (0002), (100) and (202) peaks. The above results indicate that high Gd content reduces glass-forming abilities and favors the formation of Gd crystal. In other words, the phase compositions of Gd-Co-Al alloys can be adjusted by regulating the Gd content.
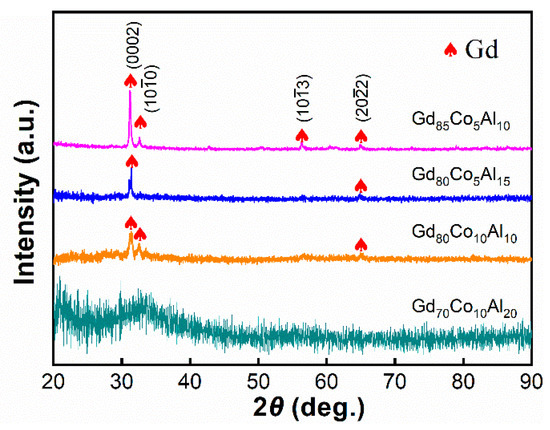
Figure 2.
XRD patterns of Gd-Co-Al metal fibers.
The microstructures of Gd85Co5Al10 were further identified by TEM and EDS (Energy Dispersive Spectroscopy) mapping. The results have shown in Figure 3a–d. As shown in Figure 3a, Gd85Co5Al10 has amorphous/crystalline composite structures, in which the island-like crystal phase is embedded in the river-like amorphous matrix. The size of the crystal phase is 300~500 nm. In Figure 3b, the high-resolution TEM (HRTEM) shows the amorphous maze-like pattern and crystal lattice fringes pattern in regions A and B, respectively. Moreover, Figure 3c shows the selected-area electron diffraction (SAED) for region A, which confirmed its pure amorphous structures. Additionally, in Figure 3d, the SAED for region B is confirmed as the hexagonal Gd (100) zonal axis. The EDS-mapping result further suggests that the nanocrystals were in the Gd phase, as shown in Figure 3e. Based on the above results, we confirmed the nature of ANCF for the Gd85Co5Al10 sample. This conclusion is also applicable to Gd80Co10Al10 and Gd80Co5Al15 samples.
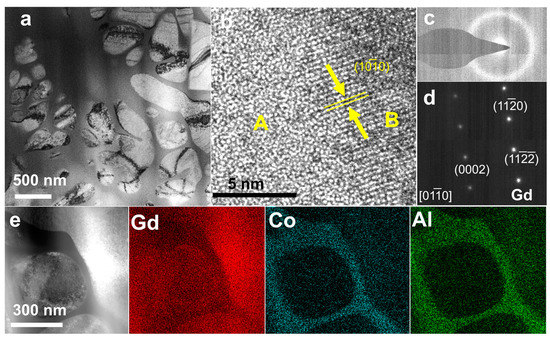
Figure 3.
(a) The morphology of Gd85Co5Al10, (b) HRTEM of Gd85Co5Al10, (c) SAED of the amorphous part of Gd85Co5Al10; (d) SAED of the crystalline part of Gd85Co5Al10; (e) the Gd, Co, Al are the EDS-mapping results.
For these samples, the temperature dependences of magnetic behavior were measured under a 200 Oe magnetic field. As shown in Figure 4a–d, the zero-field cooling (ZFC) curves and field-cooling (FC) curves separate at about 50 K, which means that the spin freezing temperatures for all samples is about 50 K [30]. For Gd70Co10Al20, there is only one TC at 101 K, as shown in the insert of Figure 4a. For ANCFs there were two magnetic transition temperatures belonging to the amorphous and crystalline phases separately, as shown in Figure 4c,d. The TC1 of the amorphous phase were 116, 98, 98 K, and the TC2 of the crystalline phases were 271, 264, 264 K for Gd80Co5Al15, Gd80Co10Al10 and Gd85Co5Al10 ANCFs, respectively. These results are also listed in Table 1.
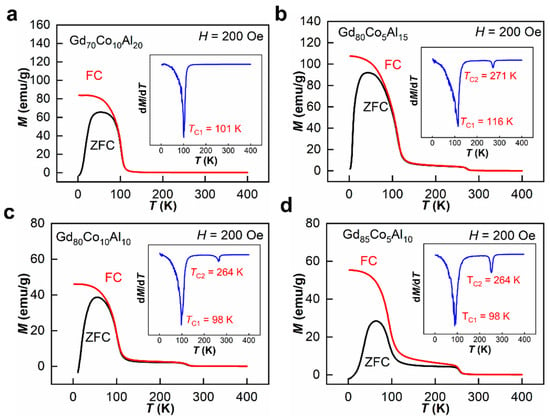
Figure 4.
Temperature dependences of zero field cooling (ZFC) and field cooling (FC) magnetization under a magnetic field of 200 Oe, (a) Gd70Co10Al20, (b) Gd80Co5Al15, (c) Gd80Co10Al10, (d) Gd85Co5Al10. The insets are dM/dT curves of FC, respectively.

Table 1.
The magnetic transition temperature (Ttran), peak value of magnetic entropy change (), full width at half-maximum of −ΔSM peak (ΔTFWHM), refrigeration capacity (RC) under a maximum applied field of 5 T for some selected Gd-based magnetic refrigeration material.
The magnetocaloric properties were investigated by measuring the isothermal magnetization curves for these fibers. In Figure 5a,b, the M-H curves and Arrot plot for Gd85Co5Al15 ANCF are given. In Figure 5a, magnetization continually changes following the changes of an applied magnetic field; thus there were no features of field-induced phase transition, which is the characteristic of first-order magnetic transition [28,34]. In Figure 5b, the positive slopes further confirmed the second-order magnetic phase transition of both the amorphous and crystalline phases for ANCFs [35]. The second-order magnetic transition had almost zero magnetic and thermal hysteresis, benefiting from a wide working temperature range [34,36]. These features ensure the magnetocaloric performances for ANCFs.
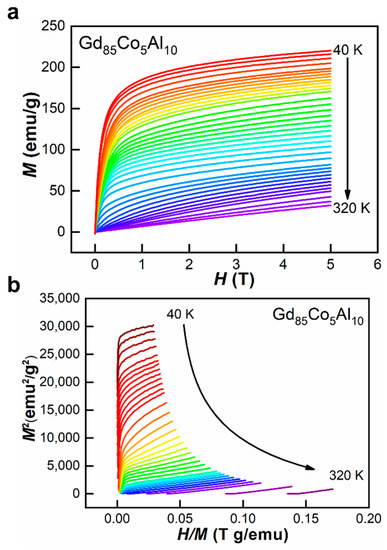
Figure 5.
(a) Isothermal magnetization curves and (b) Arrott plots of Gd85Co5Al10 ANCF.
The ΔSM in the different applied magnetic fields can be calculated using the following equation [37]:
Figure 6a–d show the temperature dependences of −ΔSM in the different magnetic fields. In Figure 6a, for Gd70Co10Al20, there is only one −ΔSM peak around 100 K due to its pure amorphous structures, and the peak value is 6.36 J kg−1 K−1 (H = 5 T). For ANCFs, two −ΔSM peaks belong to the amorphous and crystalline phases, respectively. With the increase of Gd content, the peak values of −ΔSM near 100 K decreases gradually. Meanwhile, the peak values of −ΔSM around 270 K were strengthened with the increase of the crystalline Gd phase. At H = 5 T, the first −ΔSM peak values were 4.31 J kg−1 K−1, 4.06 J kg−1 K−1 and 4.24 J kg−1 K−1, and at the second −ΔSM peak values were 3.29 J kg−1 K−1, 2.64 J kg−1 K−1 and 5.07 J kg−1 K−1 for Gd80Co10Al10, Gd80Co5Al15 and Gd85Co5Al10 ANCFs, respectively. These values are listed in Table 1. The above results show that the amount of Gd crystalline phases of ANCFs can enhance the RC near room temperature. Especially, Gd85Co5Al10 ANCF not only had the highest −ΔSM peak value but also the crystalline phase had a similar −ΔSM peak value to that of the amorphous phase, thus the ratio of amorphous and crystalline phases is optimal in Gd85Co5Al10 ANCF. Because of this optimal ratio, Gd85Co5Al10 ANCF keeps a large magnetic entropy change in 50~300 K, thus Gd85Co5Al10 ANCF is promising for a large application temperature span. Although the −ΔSM peak values decreased due to the composite structure for ANCF, it is worth noting that such values were still larger than those of transition metals [38,39,40,41,42].
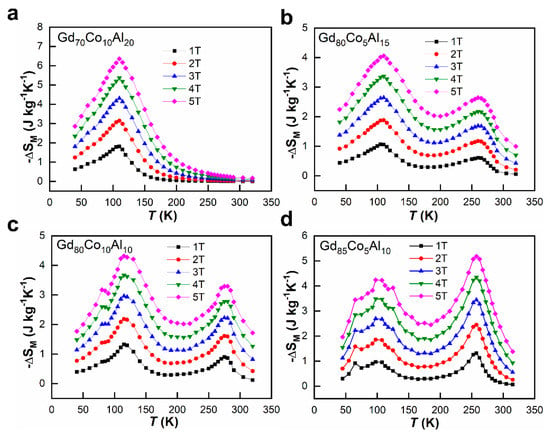
Figure 6.
−ΔSM as a function of temperature under the magnetic fields of 1, 2, 3, 4 and 5 T for (a) Gd70Co10Al20; (b) Gd80Co5Al15; (c) Gd80Co10Al10; (d) Gd85Co5Al10.
RC is an important parameter indicating the efficiency of magnetic refrigerant. Here RC is obtained by using the following equation:
where T1 and T2 are identified by the temperature values at half height of magnetic entropy change peaks. For amorphous Gd70Co10Al20, the RC is 538 J kg−1. For Gd80Co10Al10, Gd80Co5Al15 and Gd85Co5Al10 ANCF, the RCs were 622 J kg−1, 625 J kg−1, and 841 J kg−1, respectively. Compared to Gd70Co10Al20, the RC of ANCF is significantly improved due to the formation of crystalline Gd. Gd85Co5Al10 ANCF shows the best performance, which is an increase of about 56% compared to pure amorphous Gd70Co10Al20. Just as important, the range of working temperature range for Gd85Co5Al10 can be as wide as 245 K from room temperature to liquid nitrogen temperature, which was also the best performance and was about 119% higher than Gd70Co10Al20. Such a performance also proved the optimal ratio of amorphous and crystalline phases in Gd85Co5Al10 ANCF.
To further distinguish the contributions from both phases in ANCF, the −ΔSM-T curves were fitted by the Gaussian peak function. The reliability of the fitting results was confirmed by the coefficient of determination R2 (≥0.917). In Figure 7a, there is only one fitted peak belonging to the amorphous phase of Gd70Co10Al20. For ANCF, the contribution from the amorphous phase decreased with increasing Gd content, while the contribution from the crystalline phase increased, as shown in Figure 7b–d. In the Gd85Co5Al10 ANCF, amorphous and crystalline phases comparably contributed to magnetocaloric performance.
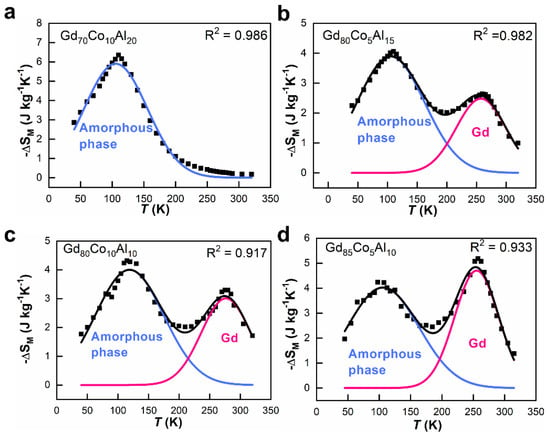
Figure 7.
The fitting results of the contribution of both the amorphous phase (blue curve) and the crystalline phase (red curve) to the total −ΔSM-T curve (solid square) of fibers under a field change of 5 T. (a) Gd70Co10Al20; (b) Gd80Co5Al15; (c) Gd80Co10Al10; (d) Gd85Co5Al10.
The fitted peak value of −ΔSM and RC for both phases are shown in Figure 8a,b. The −ΔSM and RC of Gd70Co10Al20 were completely in the amorphous phase. It was obvious that with the increase of Gd content, the − and RC of crystal Gd gradually increased, which further indicated the positive correlation between the Gd content in alloy and the crystal Gd content in ANCFs. Once the Gd crystalline phase appeared, the magnetic entropy changed peaks and RC was enhanced obviously, which reflected the coupling effect between the amorphous and Gd phase improved the refrigeration ability. For the best magnetocaloric performance of Gd85Co5Al10 ANCF, the magnetic entropy change peaks were 4.0 J kg− K− and 4.7 J kg−a K−a, and the RC values were 477 J kg−J and 364 J kg−J for amorphous and crystalline phases, respectively. In other words, the optimal ratio of amorphous and crystalline phases was established in Gd85Co5Al10 ANCF.
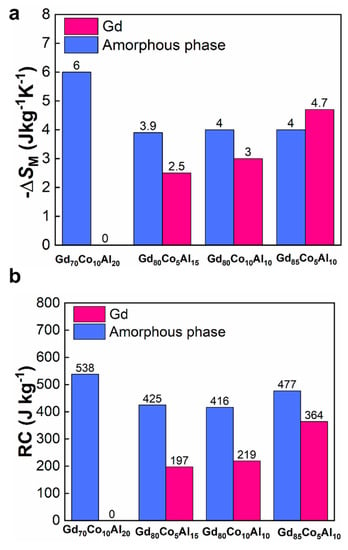
Figure 8.
(a) −ΔSM and (b) RC for Gd-Co-Al fibers contributed by amorphous and crystalline phases, respectively.
To analyze the MCE performance characteristics of ANCFs, the RC and TC of typical Gd-based comparisons are summarized in Figure 9, including Gd-based metallic glasses (MG), Gd-based crystals, and Gd85Co5Al10. These results are also listed in Table 1. The Gd85Co5Al10 ANCF possessed the largest RC of 841 J kg−1 and widest working temperature range. Specifically, the wide working temperature window (from 50 K to 295 K) of Gd85Co5Al10 is critical for its application in refrigerators. This makes refrigeration applicable at both room temperature and liquid nitrogen temperature.
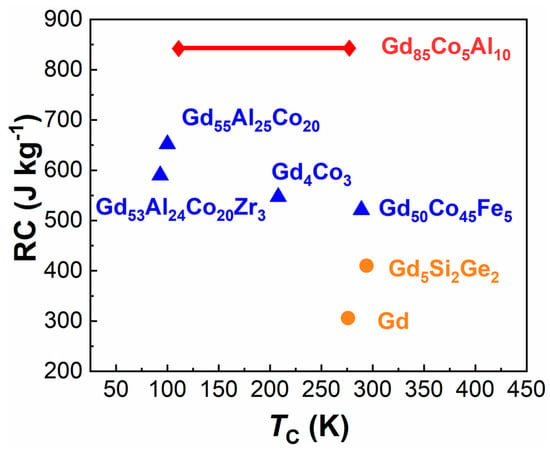
Figure 9.
The RC and TC of different Gd-based magnetic refrigeration materials, including metal glass (triangular), crystal (circular), and Gd85Co5Al10 (rhombus) listed in Table 1.
4. Conclusions
By adjusting the Gd content, the transition of Gd-Co-Al magnetic refrigeration material from amorphous fiber to ANCF was realized. We found that the RC of Gd85Co5Al10 reaches 841 J kg−1 and the temperature range extended from 50 K to 295 K, which contributed to excellent MCE. This result suggests that the amorphous/crystalline composite, produced by controlling the Gd content, is a superior method to obtain a promising magnetic refrigerant from room temperature to liquid nitrogen temperature, which is not only for achieving giant RC but also for obtaining a wide temperature range.
Author Contributions
Data curation, F.C. and K.H.; Formal analysis, F.C., K.H., M.G., Y.Z., W.X., J.H., C.Z., L.S. and J.-Q.W.; Funding acquisition, J.H.; Investigation, F.C., K.H., M.G., Y.Z., W.X., J.H., C.Z., L.S. and J.-Q.W.; Methodology, F.C., J.H., C.Z., L.S. and J.-Q.W.; Writing—original draft, F.C., K.H., M.G., Y.Z., J.H., C.Z., L.S. and J.-Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC 51827801), Youth Innovation Promotion Association CAS (No. 2019296), Zhejiang Provincial Natural Science Foundation of China (LR22E010004).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guillou, F.; Pathak, A.K.; Paudyal, D.; Mudryk, Y.; Wilhelm, F.; Rogalev, A.; Pecharsky, V.K. Non-hysteretic first-order phase transition with large latent heat and giant low-field magnetocaloric effect. Nat. Commun. 2018, 9, 2925. [Google Scholar] [CrossRef] [PubMed]
- Kimel, A.V.; Kirilyuk, A.; Tsvetkov, A.; Pisarev, R.V.; Rasing, T. Laser-induced ultrafast spin reorientation in the antiferromagnet TmFeO3. Nature 2004, 429, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, Y.; Tong, X.; Zhang, H.; Wang, H.; Liu, X.J.; Ma, L.; Suo, H.L.; Lu, Z.P. Rare-earth high-entropy alloys with giant magnetocaloric effect. Acta Mater. 2017, 125, 481–489. [Google Scholar] [CrossRef]
- Fukamichi, K.; Fujita, A.; Fujieda, S. Large magnetocaloric effects and thermal transport properties of La(FeSi)(13) and their hydrides. J. Alloys Compd. 2006, 408, 307–312. [Google Scholar] [CrossRef]
- Huo, J.T.; Wang, J.Q.; Wang, W.H. Denary high entropy metallic glass with large magnetocaloric effect. J. Alloys Compd. 2019, 776, 202–206. [Google Scholar] [CrossRef]
- Feng, J.Q.; Liu, Y.H.; Sui, J.H.; He, A.N.; Xia, W.X.; Wang, W.H.; Wang, J.Q.; Huo, J.T. Giant refrigerant capacity in Gd-based amorphous/nanocrsytalline composite fibers. Mater. Today Phys. 2021, 21, 100528. [Google Scholar] [CrossRef]
- Feng, J.Q.; Li, F.M.; Wang, G.; Wang, J.Q.; Huo, J.T. Magnetocaloric effect in ercu-based metallic glass composite. J. Non Cryst. Solids 2020, 536, 120004. [Google Scholar] [CrossRef]
- Franco, V.; Borrego, J.M.; Conde, C.F.; Conde, A.; Stoica, M.; Roth, S. Refrigerant capacity of FeCrMoCuGaPCB amorphous alloys. J. Appl. Phys. 2006, 100, 083903. [Google Scholar] [CrossRef]
- Franco, V.; Conde, A. Scaling laws for the magnetocaloric effect in second order phase transitions: From physics to applications for the characterization of materials. Int. J. Refrig. 2010, 33, 465–473. [Google Scholar] [CrossRef]
- Huo, J.T.; Zhao, D.Q.; Bai, H.Y.; Axinte, E.; Wang, W.H. Giant magnetocaloric effect in Tm-based bulk metallic glasses. J. Non Cryst. Solids 2013, 359, 1–4. [Google Scholar] [CrossRef]
- Li, F.M.; Feng, J.Q.; Yi, J.; Wang, G.; Wang, J.Q.; Huo, J.T. Magnetocaloric properties of LaFe11.4Si1.6 based amorphous alloys. J. Alloys Compd. 2020, 845, 156191. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhao, D.Q.; Bai, H.Y.; Wang, W.H.; Pan, M.X. Room temperature table-like magnetocaloric effect in amorphous Gd50Co45Fe5 ribbon. J. Phys. D Appl. Phys. 2016, 49, 055004. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Magnetocaloric effect in Gd-based bulk metallic glasses. Appl. Phys. Lett. 2006, 89, 081914. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Zhang, J.X.; Lai, J.K.L.; Shek, C.H. Magnetic entropy in nanocomposite binary gadolinium alloys. J. Appl. Phys. 1996, 80, 76–80. [Google Scholar] [CrossRef]
- Shen, H.X.; Wang, H.; Liu, J.S.; Xing, D.W.; Qin, F.X.; Cao, F.Y.; Chen, D.M.; Liu, Y.F.; Sun, J.F. Enhanced magnetocaloric and mechanical properties of melt-extracted Gd55Al25Co20 micro-fibers. J. Alloys Compd. 2014, 603, 167–171. [Google Scholar] [CrossRef]
- Tang, B.Z.; Guo, D.Q.; Xia, L.; Ding, D.; Chan, K.C. Magnetoelastic and magnetocaloric properties of Tb62.5Co37.5 amorphous alloy. J. Alloys Compd. 2017, 728, 747–751. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, J.Z.; Xia, L. Fe87Zr7B4Co2 amorphous alloy with excellent magneto-caloric effect near room temperature. Intermetallics 2018, 95, 85–88. [Google Scholar] [CrossRef]
- Yuan, F.; Du, J.; Shen, B.L. Controllable spin-glass behavior and large magnetocaloric effect in Gd-Ni-Al bulk metallic glasses. Appl. Phys. Lett. 2012, 101, 032405. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Zhong, X.C.; Liu, Z.W.; Zeng, D.C.; Franco, V.; Zhang, J.L. Magnetocaloric effect and critical behavior of amorphous (Gd4Co3)1−xSix alloys. J. Magn. Magn. Mater. 2013, 343, 184–188. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, W.H. Magnetocaloric effect in rare earth-based bulk metallic glasses. J. Alloys Compd. 2010, 495, 209–216. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, L.; Du, J. Table-like magnetocaloric effect in Gd–Ni–Al amorphous/nanocrystalline composites. J. Phys. D Appl. Phys. 2017, 50, 355601. [Google Scholar] [CrossRef]
- Fu, H.; Zou, M. Magnetic and magnetocaloric properties of ternary Gd–Co–Al bulk metallic glasses. J. Alloys Compd. 2011, 509, 4613–4616. [Google Scholar] [CrossRef]
- Shao, L.; Xue, L.; Luo, Q.; Wang, Q.; Shen, B. The role of Co/Al ratio in glass-forming GdCoAl magnetocaloric metallic glasses. Materialia 2019, 7, 100419. [Google Scholar] [CrossRef]
- Bao, Y.; Shen, H.X.; Huang, Y.J.; Xing, D.W.; Wang, H.; Liu, J.S.; Li, H.C.; Yin, H.B.C.; Jiang, S.D.; Sun, J.F.; et al. Table-like magnetocaloric behavior and enhanced cooling efficiency of a Bi-constituent Gd alloy wire-based composite. J. Alloys Compd. 2018, 764, 789–793. [Google Scholar] [CrossRef]
- Zhong, X.C.; Shen, X.Y.; Mo, H.Y.; Jiao, D.L.; Liu, Z.W.; Qiu, W.Q.; Zhang, H.; Ramanujan, R.V. Table-like magnetocaloric effect and large refrigerant capacity in Gd65Mn25Si10-Gd composite materials for near room temperature refrigeration. Mater. Today Commun. 2018, 14, 22–26. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, L.L.; Du, J. Magnetic entropy change in Gd95Fe2.8Al2.2 amorphous/nanocrystalline ribbons. Scr. Mater. 2017, 130, 170–173. [Google Scholar] [CrossRef]
- Shen, H.X.; Wang, H.; Liu, J.S.; Cao, F.Y.; Qin, F.X.; Xing, D.W.; Chen, D.M.; Liu, Y.F.; Sun, J.F. Enhanced magnetocaloric properties of melt-extracted GdAlCo metallic glass microwires. J. Magn. Magn. Mater. 2014, 372, 23–26. [Google Scholar] [CrossRef]
- Sheng, W.; Wang, J.Q.; Wang, G.; Huo, J.T.; Wang, X.M.; Li, R.W. Amorphous microwires of high entropy alloys with large magnetocaloric effect. Intermetallics 2018, 96, 79–83. [Google Scholar] [CrossRef]
- Jiang, S.D.; Wang, H.; Huang, Y.J.; Shen, H.X.; Xing, D.W.; Ning, Z.L.; Sun, J.F. Effect of strain rate on tensile behavior in amorphous fibers by an in-situ video extensometer system. Mater. Sci. Eng. A 2020, 782, 139252. [Google Scholar] [CrossRef]
- Sherrington, D.; Kirkpatrick, S. Solvable model of a spin-glass. Phys. Rev. Lett. 1975, 35, 1792–1796. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zheng, Z.G.; Cao, W.H.; Shek, C.H. Magnetic behavior of Gd4Co3 metallic glass. J. Magn. Magn. Mater. 2013, 326, 157–161. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Pecharsky, V.K.; Tsokol, A.O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479–1539. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Magnetocaloric effect and magnetic refrigeration. J. Magn. Magn. Mater. 1999, 200, 44–56. [Google Scholar] [CrossRef]
- Yan, J.; Shi, K.W.; Deng, S.H.; Zhao, W.J.; Lu, H.Q.; Sun, Y.; Chen, Y.L.; Wang, C. The influence of combination of the first-order and second-order phase transitions on magnetocaloric effects in Mn3Cu1−xFexN. Solid State Commun. 2018, 282, 33–37. [Google Scholar] [CrossRef]
- Fan, J.; Ling, L.; Hong, B.; Zhang, L.; Pi, L.; Zhang, Y. Critical properties of the perovskite manganiteLa0.1Nd0.6Sr0.3MnO3. Phys. Rev. B 2010, 81, 144426. [Google Scholar] [CrossRef]
- Guo, D.; Moreno-Ramírez, L.M.; Romero-Muñiz, C.; Zhang, Y.; Law, J.-Y.; Franco, V.; Wang, J.; Ren, Z. First- and second-order phase transitions in RE6Co2Ga (RE = Ho, Dy or Gd) cryogenic magnetocaloric materials. Sci. China Mater. 2021, 64, 2846–2857. [Google Scholar] [CrossRef]
- Huo, J.T.; Huo, L.S.; Li, J.W.; Men, H.; Wang, X.M.; Inoue, A.; Chang, C.T.; Wang, J.Q.; Li, R.W. High-entropy bulk metallic glasses as promising magnetic refrigerants. J. Appl. Phys. 2015, 117, 073902. [Google Scholar] [CrossRef]
- Atalay, S.; Gencer, H.; Kolat, V.S. Magnetic entropy change in Fe74−xCrxCu1Nb3Si13B9 (x = 14 and 17) amorphous alloys. J. Non Cryst. Solids 2005, 351, 2373–2377. [Google Scholar] [CrossRef]
- Franco, V.; Blazquez, J.S.; Conde, A. The influence of Co addition on the magnetocaloric effect of Nanoperm-type amorphous alloys. J. Appl. Phys. 2006, 100, 064307. [Google Scholar] [CrossRef]
- Kim, K.S.; Min, S.G.; Yu, S.C.; Oh, S.K.; Kim, Y.C.; Kim, K.Y. The large magnetocaloric effect in amorphous Fe91−xYxZr9 (x = 0, 5, 10) alloys. J. Magn. Magn. Mater. 2006, 304, e642–e644. [Google Scholar] [CrossRef]
- Kolano-Burian, A.; Kowalczyk, M.; Kolano, R.; Szymczak, R.; Szymczak, H.; Polak, M. Magnetocaloric effect in Fe–Cr–Cu–Nb–Si–B amorphous materials. J. Alloys Compd. 2009, 479, 71–73. [Google Scholar] [CrossRef]
- Min, S.G.; Kim, K.S.; Yu, S.C.; Suh, H.S.; Lee, S.W. Analysis of magnetization and magnetocaloric effect in amorphous FeZrMn ribbons. J. Appl. Phys. 2005, 97, 10M310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).