Analysis on Ancient Bloomery Ironmaking Technology: The Earliest Ironmaking Evidence in the Central Plains of China Was Taken as the Research Object
Abstract
:1. Introduction
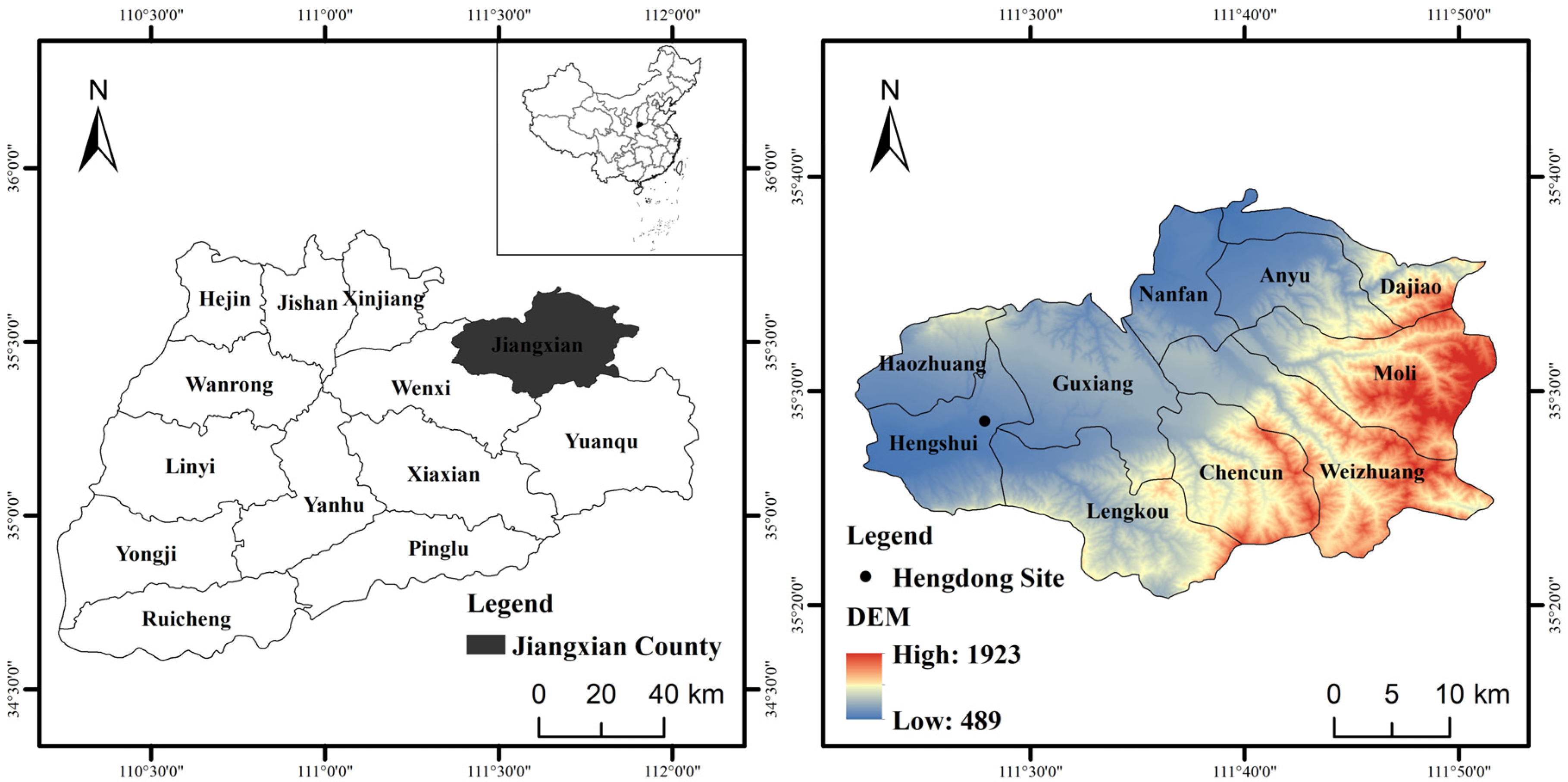
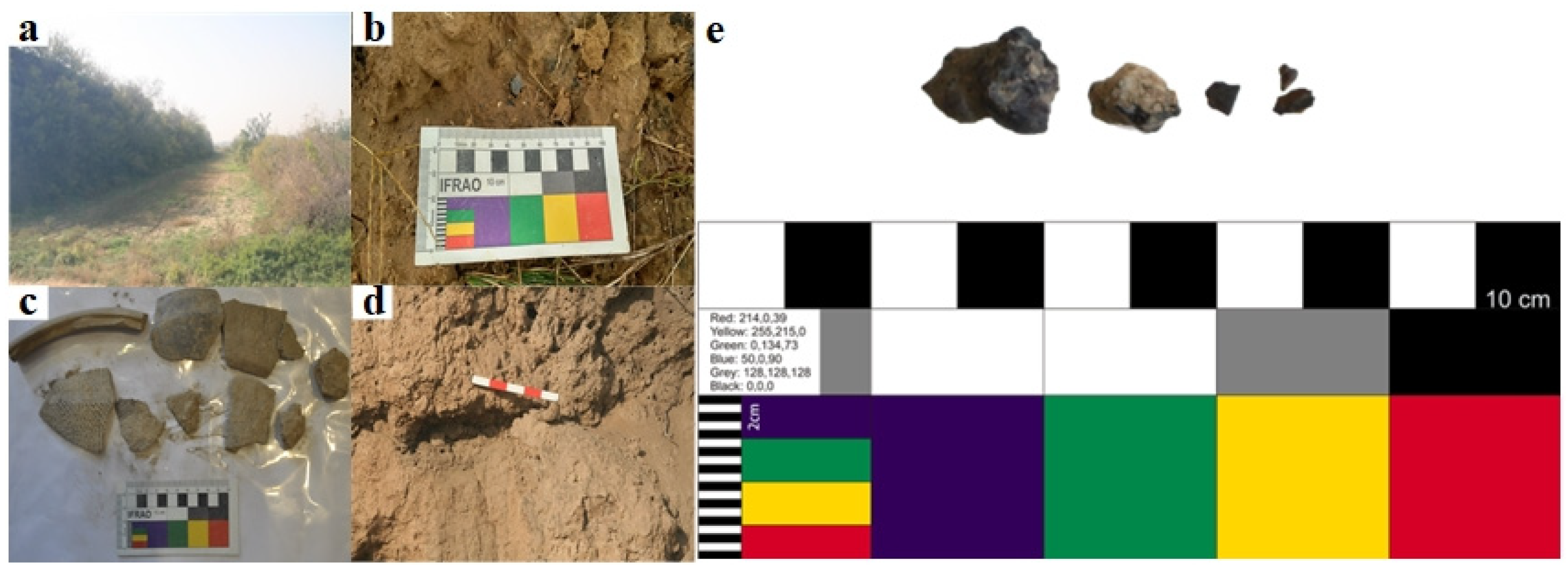
2. General Situation of the Site
3. Quantitative and Characterization Methods
3.1. Quantitative Method
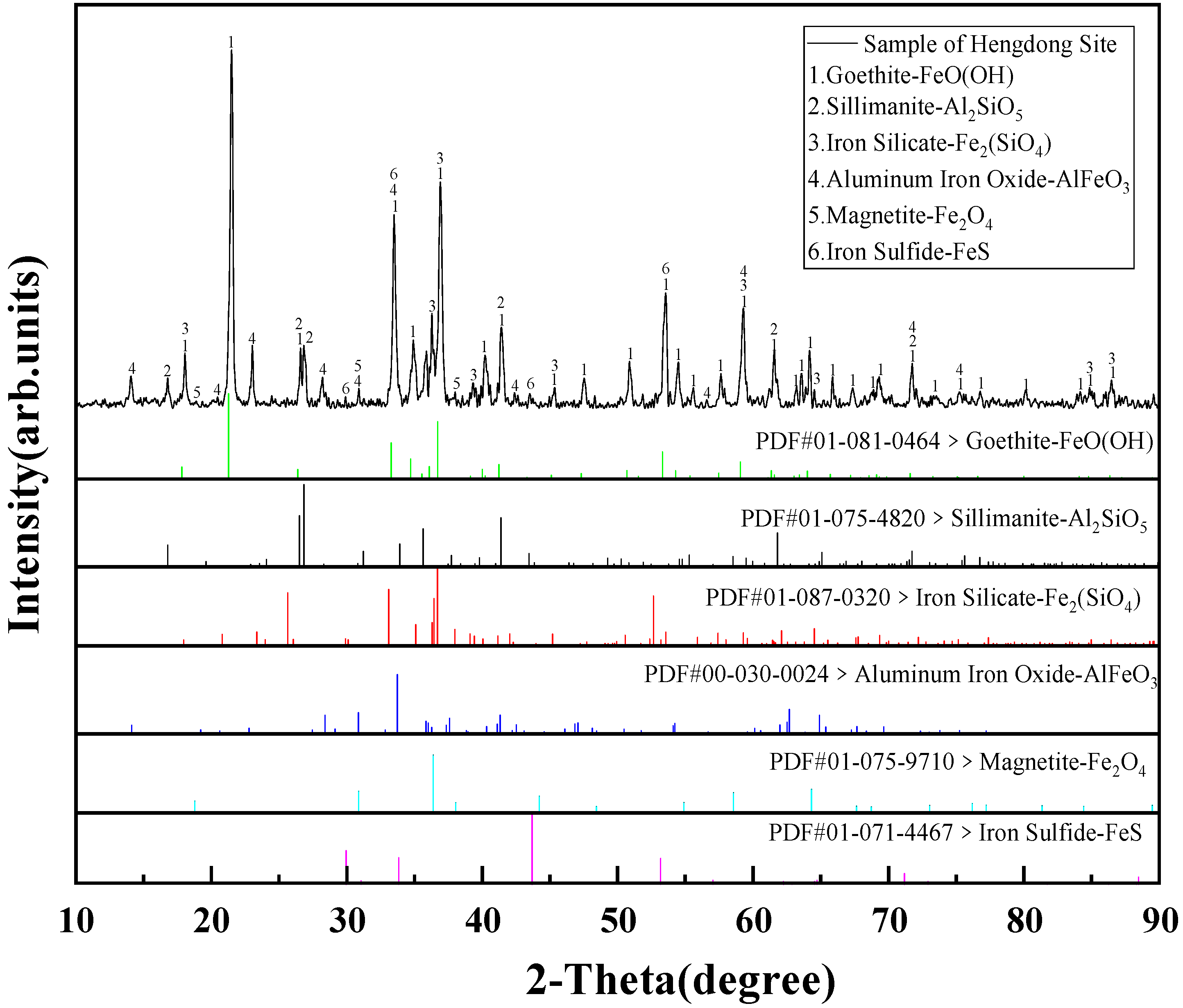
3.2. X-ray Diffraction
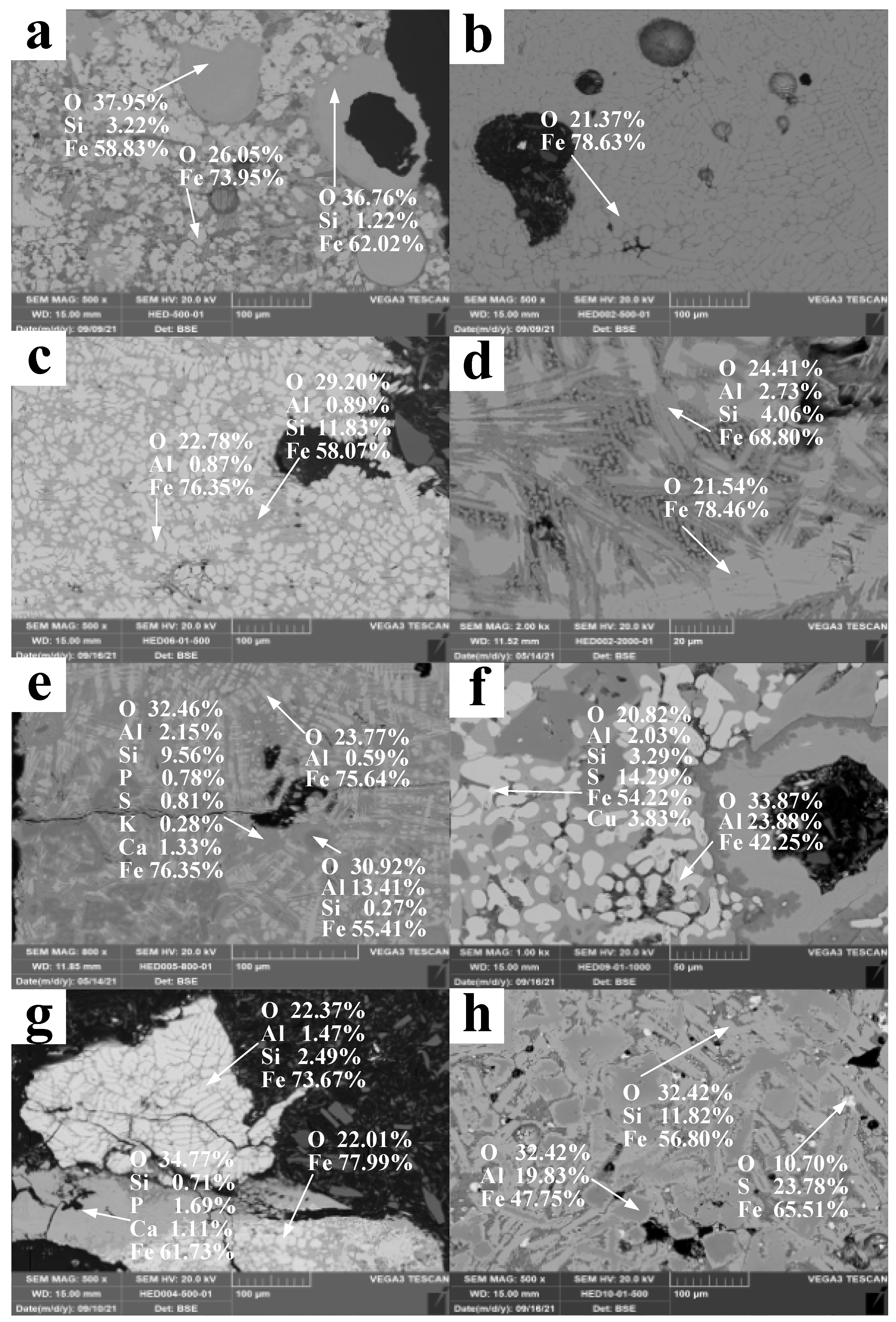
3.3. SEM-EDS
3.4. Tescan Integrated Mineral Analysis (TIMA)
4. Results and Discussion
4.1. Results of Chemical Composition Analysis
4.2. Mineral Phase Composition
4.3. Morphology of Mineral Phase
4.4. Quantitative Mineral Analysis
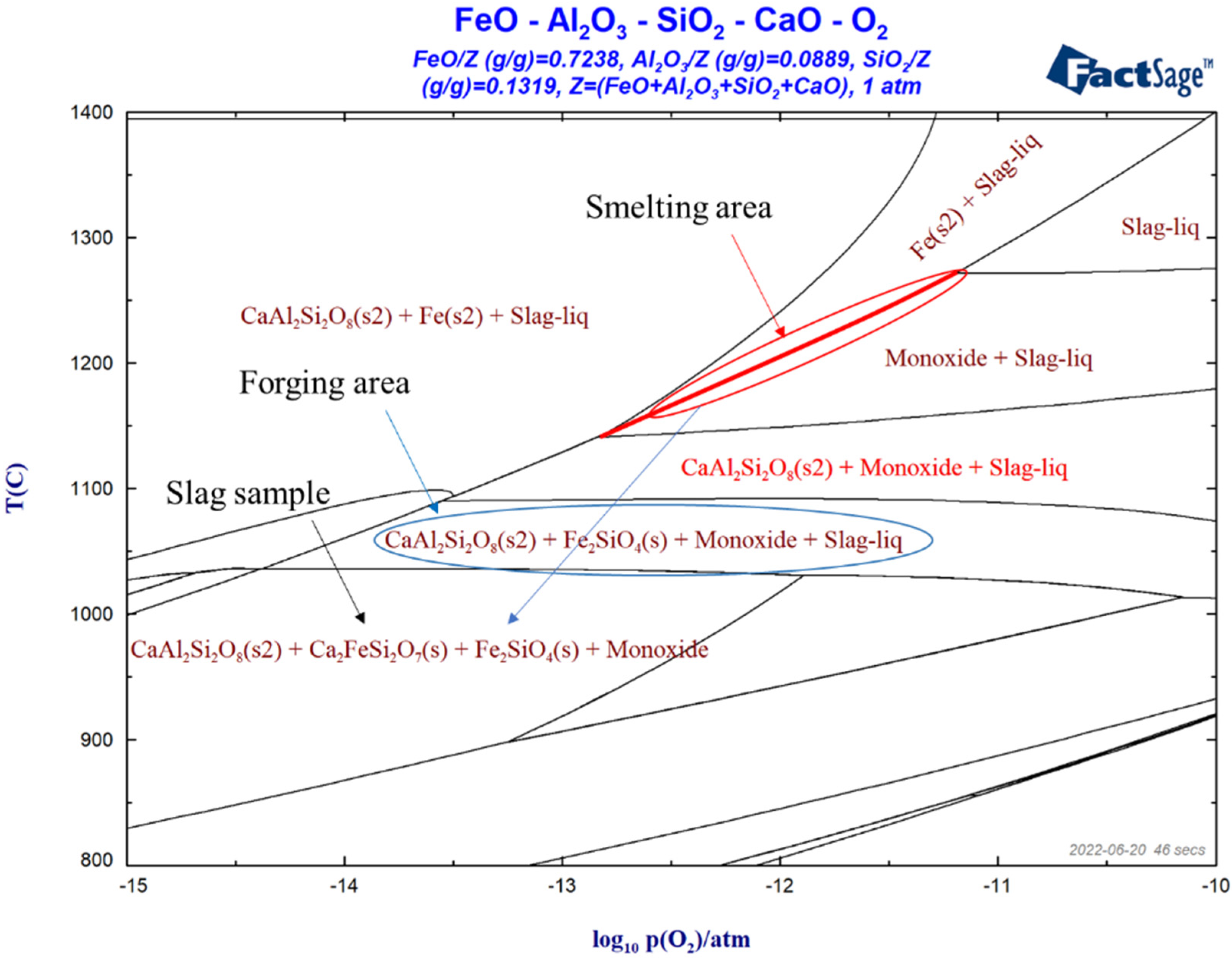
4.5. Thermodynamic Analysis in Smelting Process
5. Conclusions and Significance of the Study
- (1)
- The development of ironmaking technology is a gradual process, which was the result of the long-term development of high temperature technology system and redox control system. Bloomery ironmaking technology appeared earlier and was widely used in most parts of the East Asia, and there may also have been a stage of using block ironmaking in early China. The age difference of the iron wares unearthed in the tombs of Guo State [24], Liangdai village [25] and Tianma-Qucun in the middle Spring and Autumn period [26] should not have exceed 200 years. The test results of this batch of samples showed that the bloomery ironmaking technology was mastered in the core area of the Central Plains of China in the middle and late Western Zhou Dynasty.
- (2)
- Under smelting conditions, FeO existed in slag in the form of solid solution, and slag was a mixed liquid of wustite and molten slag. Since a high smelting temperature cannot be achieved through fuels such as charcoal, and sufficient CO gas cannot be provided for the reduction of iron oxides, the reduction of oxygen potential of slag will have required a long smelting time.
- (3)
- The sample was bloomery ironmaking slag, and the actual operating temperature was between 1050 °C and 1100 °C. It may be that the primary ore contained a certain amount of CaO, which led to the actual smelting temperature slightly lower than that of the bloomery ironmaking technology in other areas. When the sample temperature was lower than 1050 °C, the slag solidified completely.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchwald, V.F.; Wivel, H. Slag Analysis as a Method for the Characterization and Provenancing of Ancient Iron Objects. Mater. Charact. 1998, 40, 73–96. [Google Scholar] [CrossRef]
- Tylecote, R.F. A history of metallurgy. Br. Corros. J. 1977, 12, 137–140. [Google Scholar] [CrossRef]
- Siddique, R.; Cachim, P. Waste and Supplementary Cementitious Materials in Concrete: Characterisation, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Stefanescu, D.M. ASM Handbook Vol. 1A Cast Iron Science and Technology; ASM International: Geauga, OH, USA, 2017; pp. 3–11. [Google Scholar]
- Blakelock, E.; Martinón-Torres, M.; Veldhuijzen, H.A.; Young, T. Slag inclusions in iron objects and the quest for provenance: An experiment and a case study. J. Archaeol. Sci. 2009, 36, 1745–1757. [Google Scholar] [CrossRef]
- Eliyahu-Behar, A.; Yahalom-Mack, N.; Gadot, Y.; Finkelsteina, I. Iron smelting and smithing in major urban centers in Israel during the Iron Age. J. Archaeol. Sci. 2013, 40, 4319–4330. [Google Scholar] [CrossRef]
- Workman, V.; Maeir, A.M.; Eliyahu-Behar, A. In search of the invisible hearth: An experimental perspective on early Levantine iron production. J. Archaeol. Sci. Rep. 2021, 36, 102803. [Google Scholar] [CrossRef]
- Chuenpee, T.; Won-In, K.; Natapintu, S.; Takashima, I.; Dararutana, P. Archaeometallurgical Studies of Ancient Iron Smelting Slags from Ban Khao Din Tai Archaeological Site, Northeastern Thailand. J. Appl. Sci. 2014, 14, 938–943. [Google Scholar] [CrossRef] [Green Version]
- Larreina-Garcia, D.; Li, Y.; Liu, Y.; Martinón-Torresd, M. Bloomery iron smelting in the Daye County (Hubei): Technological traditions in Qing China. Archaeol. Res. Asia 2018, 16, 148–165. [Google Scholar] [CrossRef]
- Zou, G.; Meng, Z.; Li, Y.; Huang, Q.; Cui, C. From bowl furnaces to small shaft furnaces: New evidence from ancient bloomery iron smelting site at Liuzhuoling in Guangxi, Southern China, ca. 400 to 700 AD. Archaeol. Anthropol. Sci. 2022, 14, 54. [Google Scholar] [CrossRef]
- Cavallini, M. Thermodynamics applied to iron smelting techniques. Appl. Phys. A 2013, 113, 1049–1053. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; Petersen, S.; et al. FactSage thermochemical software and databases—recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Lianghui, Q. A preliminary study of the characteristics of metallurgical technology in ancient China. In Chinese Studies in the History and Philosophy of Science and Technology; Springer: Dordrecht, The Netherlands, 1996; pp. 219–241. [Google Scholar]
- Iwama, T.; Du, C.M.; Koizumi, S.; Gao, X.; Ueda, S.; Kitamura, S.Y. Extraction of phosphorus and recovery of phosphate from steelmaking slag by selective leaching. Isij Int. 2019. ISIJINT-2019-298. [Google Scholar] [CrossRef] [Green Version]
- Epp, J. X-ray diffraction (XRD) techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Woodhead Publishing: Cambridge, UK, 2016; pp. 81–124. [Google Scholar]
- Wang, A. Types and characteristics of main iron deposits in Zhongtiao Mountain. Huabei Nat. Resour. 2011, 26–27. (In Chinese) [Google Scholar]
- Thomas, G. A Chemical and Mineralogical Investigation of Bloomery Iron-Making in the Bristol Channel Orefield, UK; Cardiff University: Cardiff, UK, 2000. [Google Scholar]
- Mancini, A.; Lothenbach, B.; Geng, G.; Grolimund, D.; Sanchez, D.F.; Fakra, S.C.; Dähn, R.; Wieland, E. Iron speciation in blast furnace slag cements. Cem. Concr. Res. 2021, 140, 106287. [Google Scholar] [CrossRef]
- Charlton, M.F.; Crew, P.; Rehren, T.; Shennan, S.J. Explaining the evolution of ironmaking recipes–An example from northwest Wales. J. Anthropol. Archaeol. 2010, 29, 352–367. [Google Scholar] [CrossRef]
- Zou, G.; Meng, Z.; Li, Y.; Huang, Q.; Cui, C. Preliminary Study on Metallurgical Relics Unearthed from Liuzhuoling Iron Semlting Site in Pingnan, Guangxi. Nonferrous Met. 2022, 14, 54. (In Chinese) [Google Scholar]
- Cui, C.; Li, Y.; Chen, S.; Xi, Q. A Primary Study on the Smelting Relics in Ezhou, Hubei Province. Jianghan Archaeol. 2016, 3, 102–111. (In Chinese) [Google Scholar]
- Zou, G.; Li, Y.; Yang, Z.; Gao, C.; Huang, Q. Preliminary Study on Ancient Iron Smelting Site in Datang Village, Huaihua City, Hunan Province. Nonferrous Met. 2020, 92–98. (In Chinese) [Google Scholar]
- Huang, Q.; Li, Y. A preliminary study on tieshitang smelting site in Pingnan County, Guangxi. Sichuan Cult. Relics 2012, 92–96. (In Chinese) [Google Scholar]
- Wang, Y.; Liu, Y.; Jiang, T.; Chen, K. Scientific Research of Bimetallic Objects Unearthed from M2009 in the Guo State Cemetery at Sanmenxia. Spectrosc. Spectr. Anal. 2019, 39, 3154–3158. (In Chinese) [Google Scholar]
- Chen, J.; Yang, J.; Sun, B.; Pan, Y. Fabrication technology of copper iron complex unearthed from M27 of Liangdai village Site. Sci. Sin. 2009, 39, 1574–1581. (In Chinese) [Google Scholar]
- Han, R. A metallographic study on early iron objects of China. Wen Wu 1998, 1998, 87. (In Chinese) [Google Scholar]
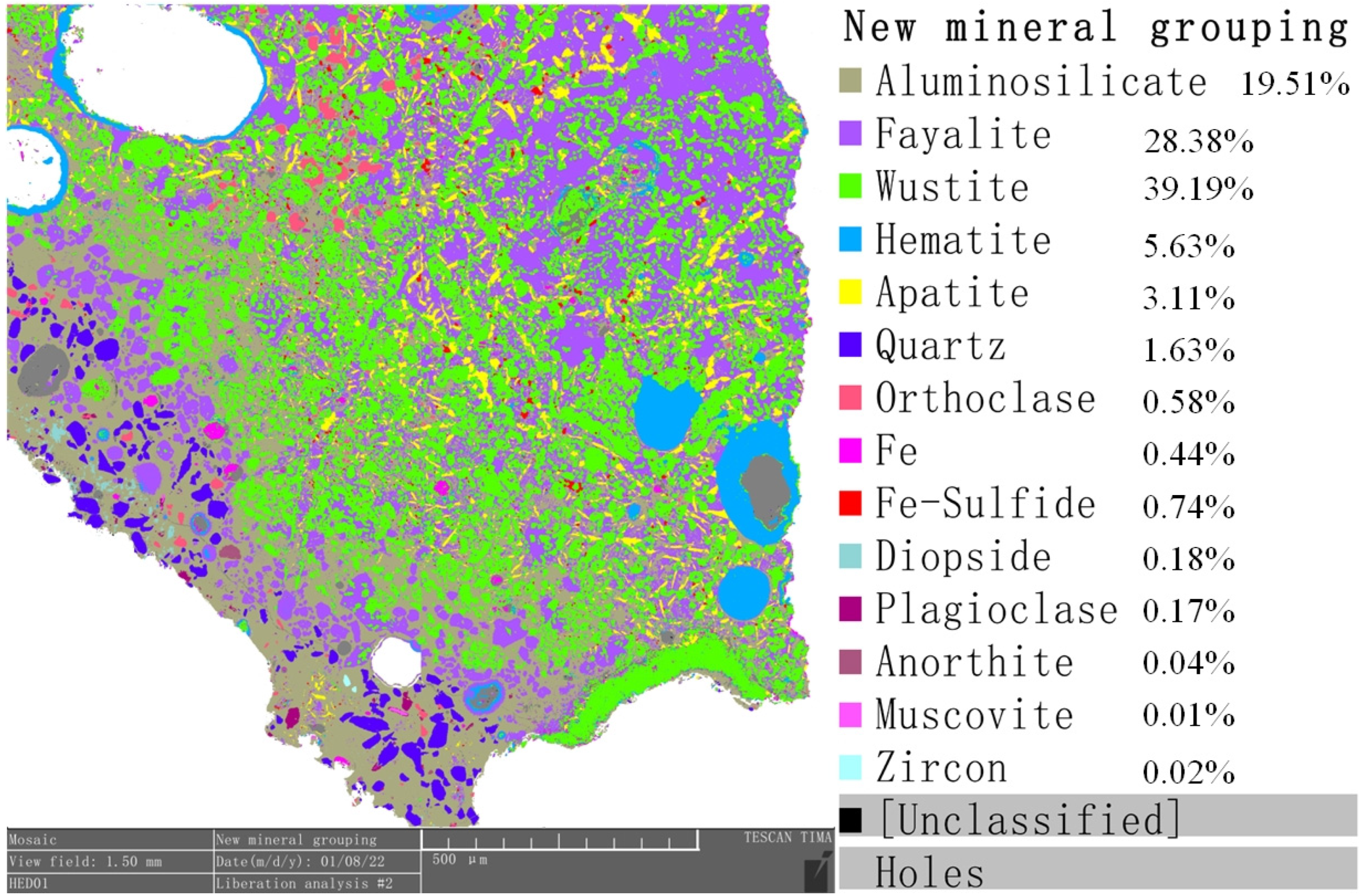
| T Fe | Fe2+ | Fe3+ | M Fe | Cu | Na | Mg | Al | Si | P | S | K | Ca |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 64.18 | 43.53 | 20.28 | 0.37 | 0.037 | 0.34 | 1.05 | 3.64 | 4.76 | 0.15 | 0.16 | 0.19 | 3.07 |
| FeO | Al2O3 | SiO2 | CaO |
|---|---|---|---|
| 72.36 | 8.89 | 13.19 | 5.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, Y.; Zhu, R.; Wang, H. Analysis on Ancient Bloomery Ironmaking Technology: The Earliest Ironmaking Evidence in the Central Plains of China Was Taken as the Research Object. Metals 2022, 12, 1307. https://doi.org/10.3390/met12081307
Li S, Li Y, Zhu R, Wang H. Analysis on Ancient Bloomery Ironmaking Technology: The Earliest Ironmaking Evidence in the Central Plains of China Was Taken as the Research Object. Metals. 2022; 12(8):1307. https://doi.org/10.3390/met12081307
Chicago/Turabian StyleLi, Shuoyang, Yanxiang Li, Rong Zhu, and Hongyang Wang. 2022. "Analysis on Ancient Bloomery Ironmaking Technology: The Earliest Ironmaking Evidence in the Central Plains of China Was Taken as the Research Object" Metals 12, no. 8: 1307. https://doi.org/10.3390/met12081307
APA StyleLi, S., Li, Y., Zhu, R., & Wang, H. (2022). Analysis on Ancient Bloomery Ironmaking Technology: The Earliest Ironmaking Evidence in the Central Plains of China Was Taken as the Research Object. Metals, 12(8), 1307. https://doi.org/10.3390/met12081307






