Numerical Study on Combustion Behavior of Semi-Coke in Blast Furnace Blowpipe-Tuyere-Combustion Zone
Abstract
:1. Introduction
2. Model Description
2.1. Governing Equations
2.2. Chemical Reaction Model
2.2.1. Releasing of Volatile Matter
2.2.2. Combustion Reaction of Gas Phase
2.2.3. Oxidation and Gasification Models of Residual Carbon
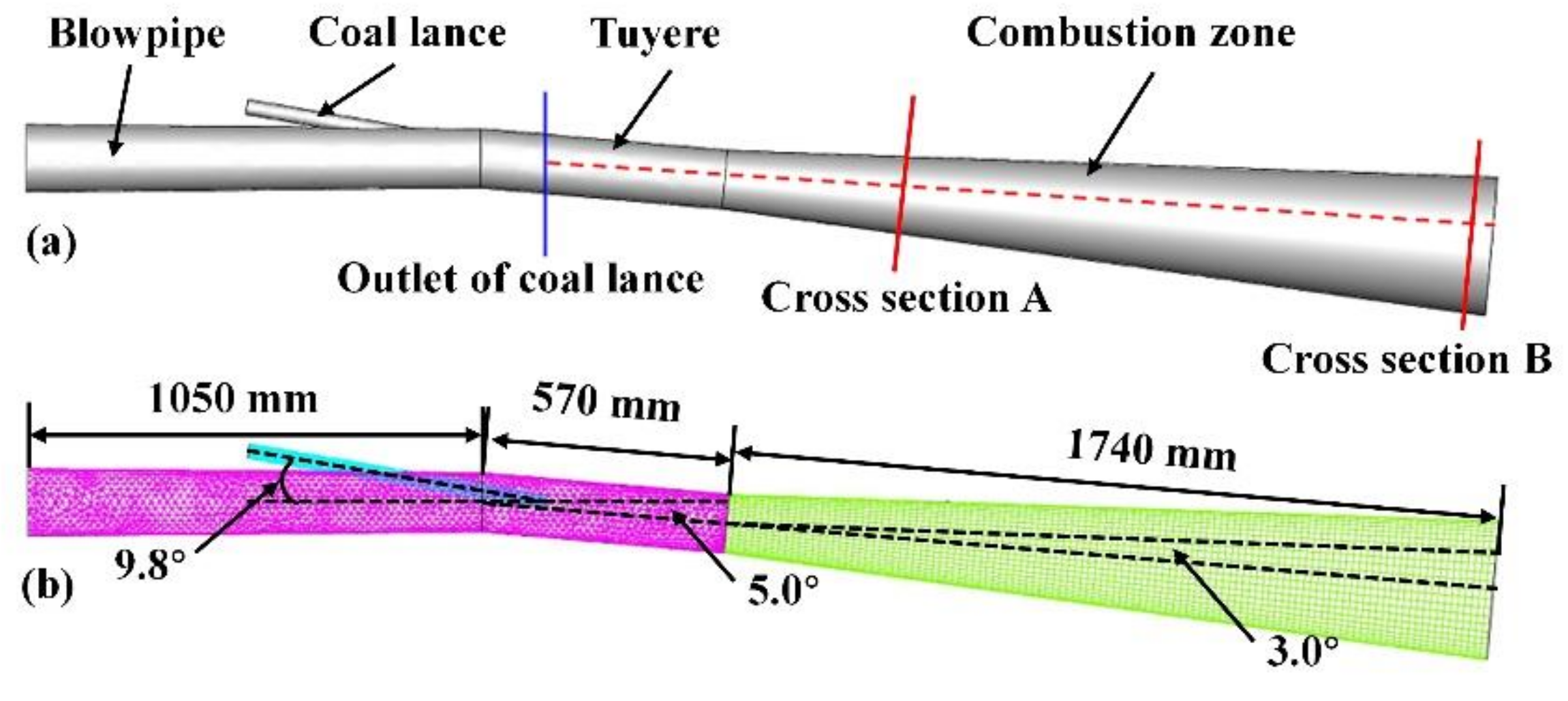
2.3. Geometric Model
2.4. Simulation Conditions
3. Results and Discussion
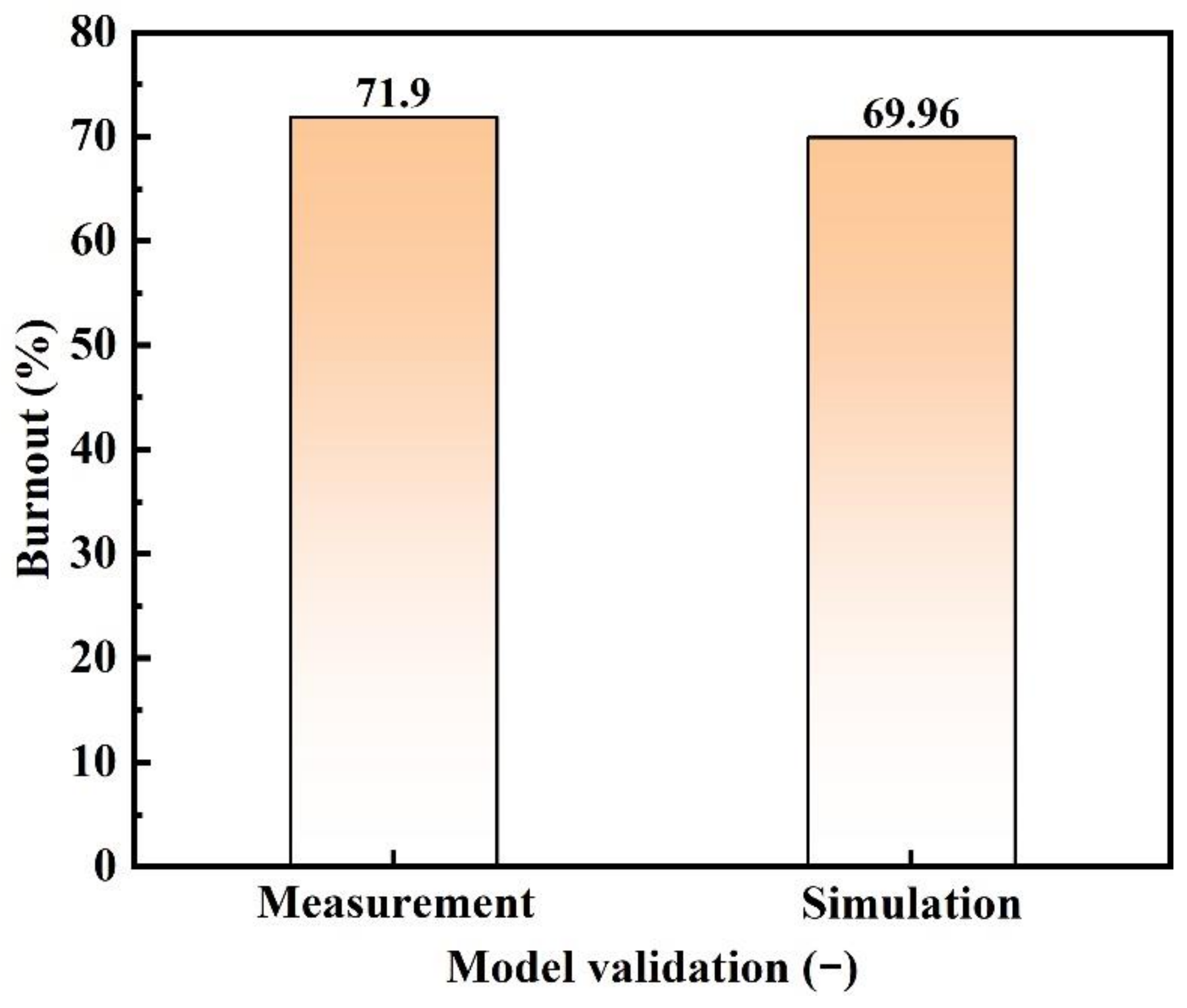
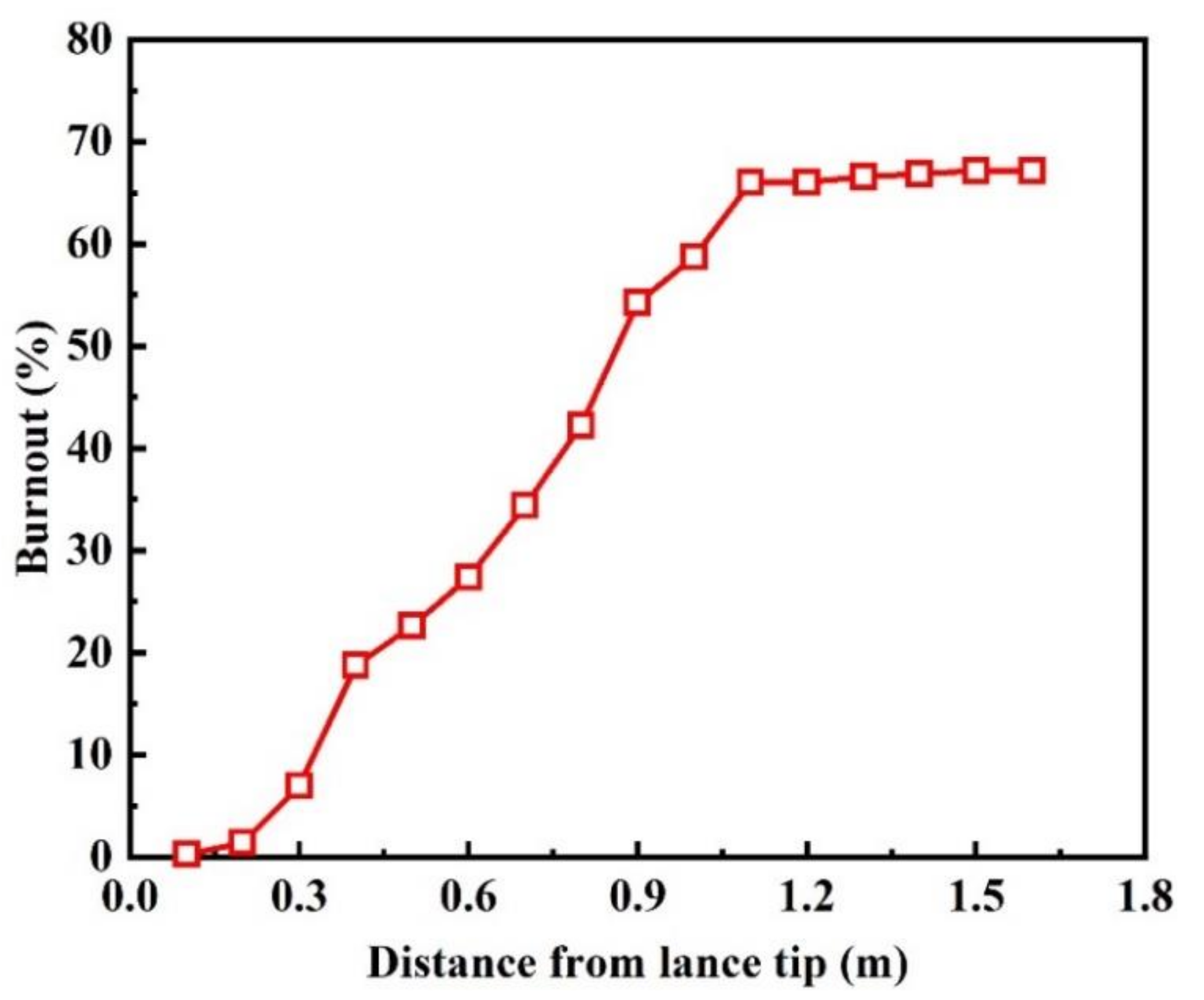
3.1. Model Validation
3.2. Transmission Phenomenon in the Blowpipe-Tuyere-Combustion Zone
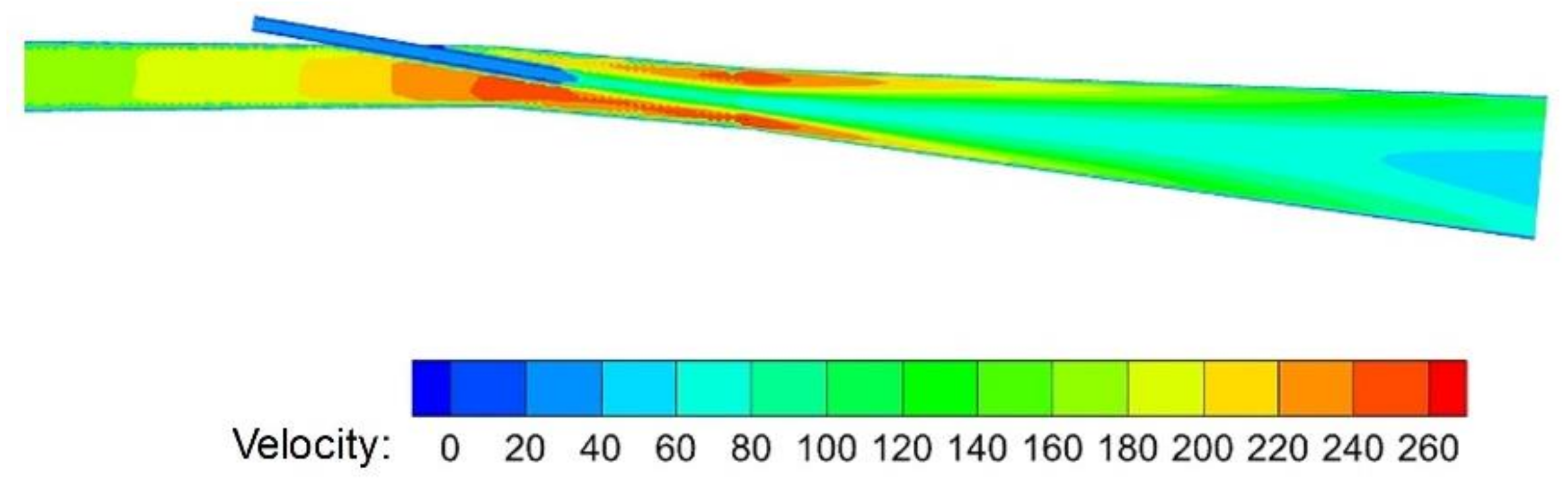
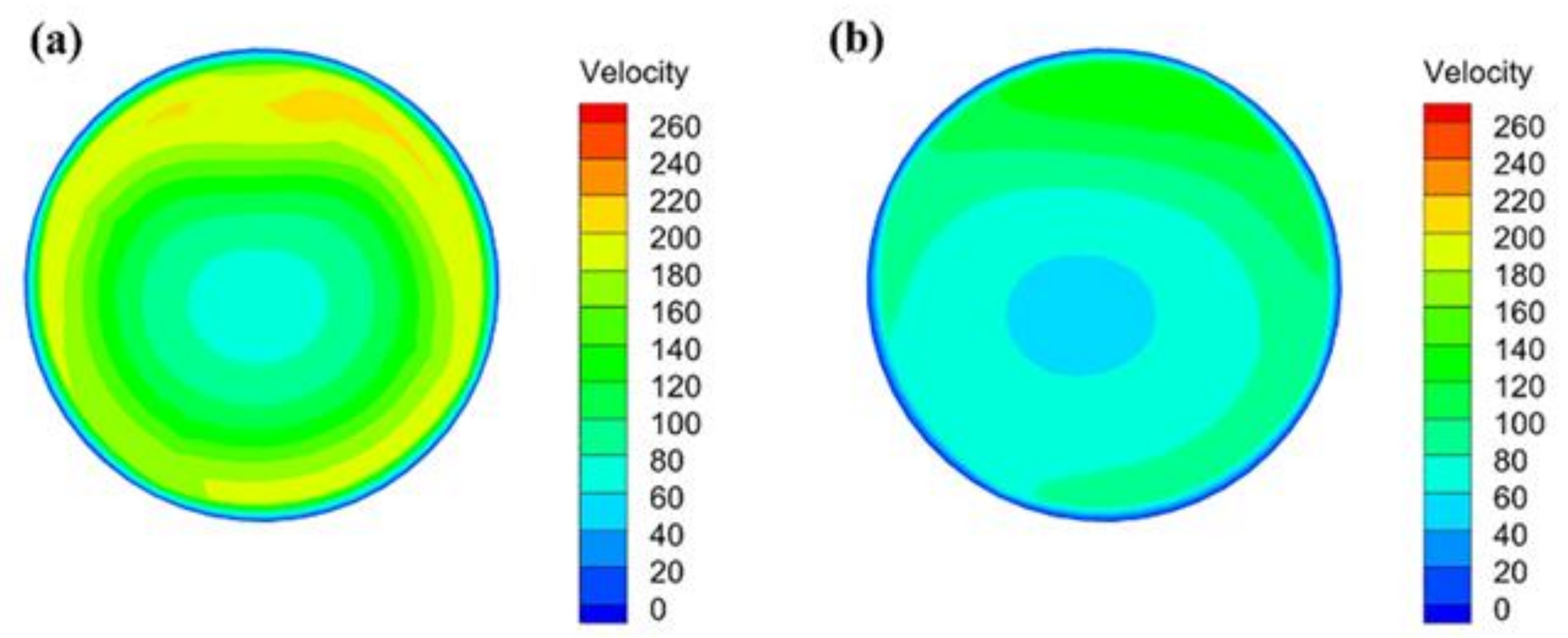
3.2.1. Velocity Distribution
3.2.2. Temperature Distribution
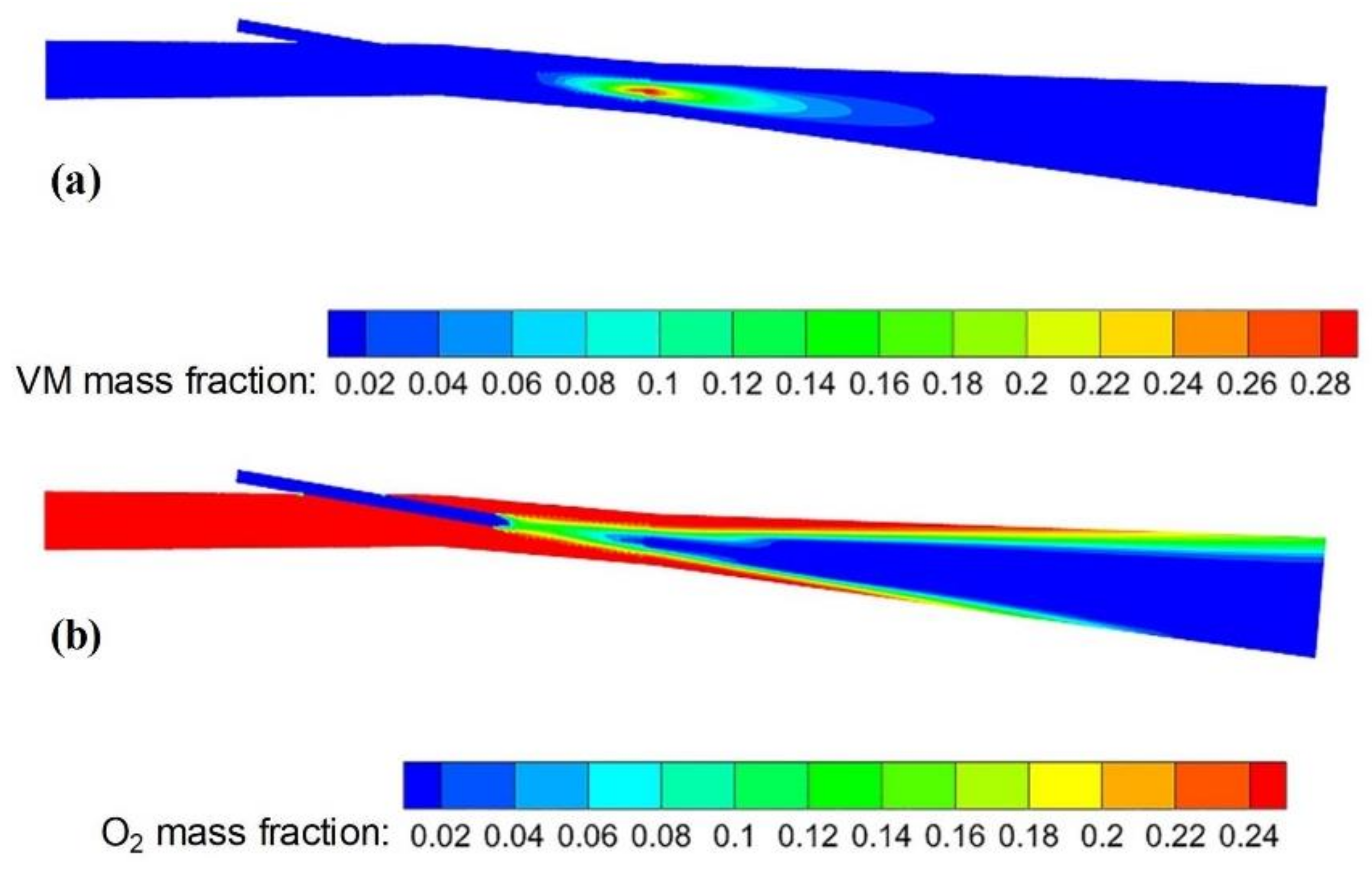
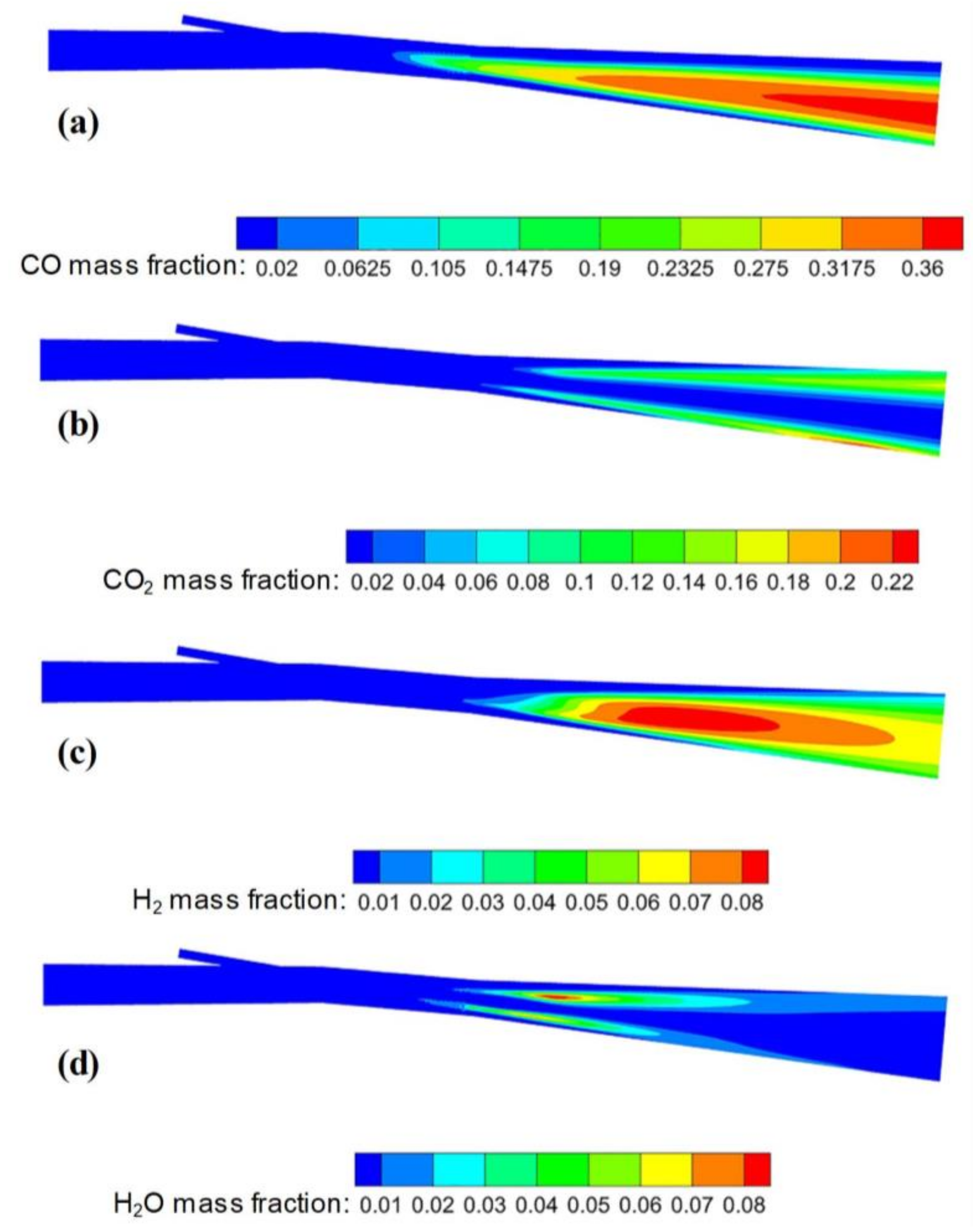
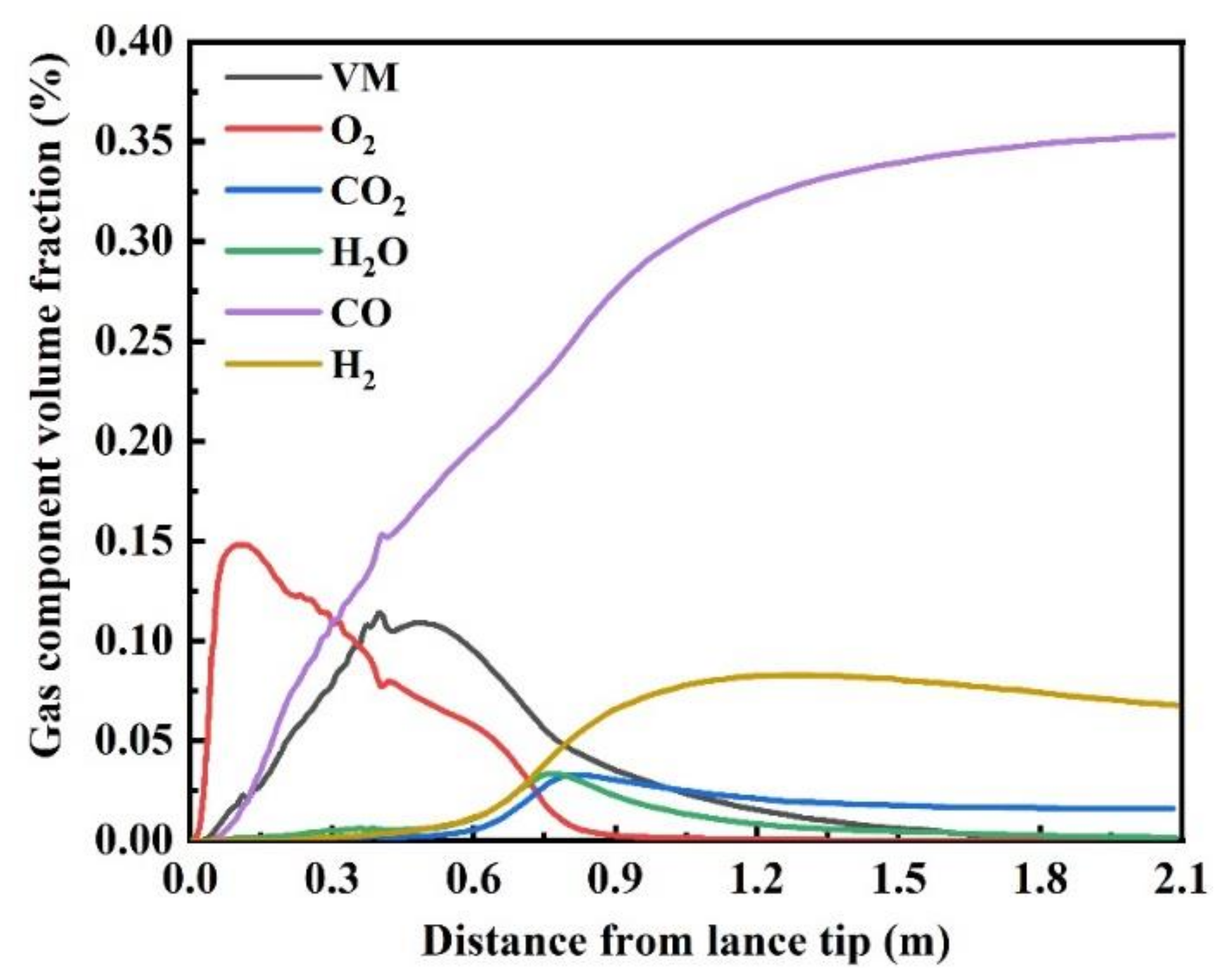
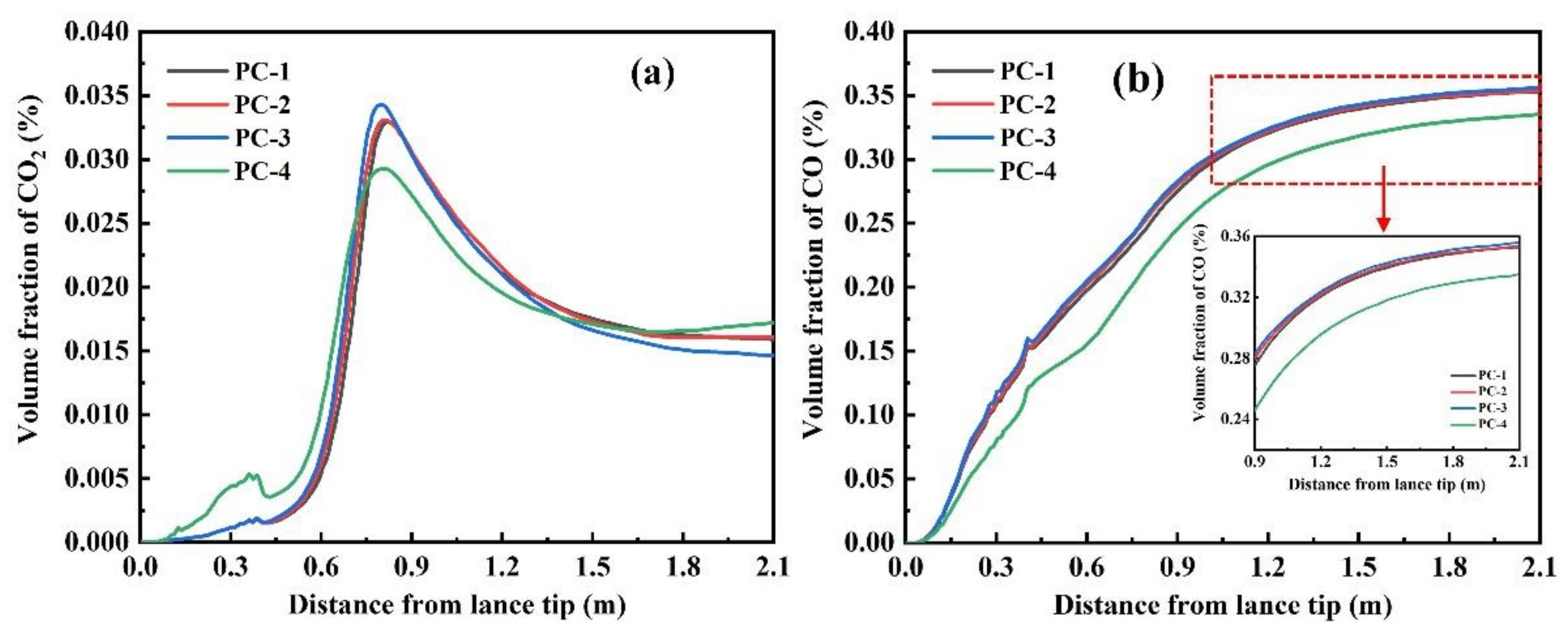
3.2.3. Composition Distribution
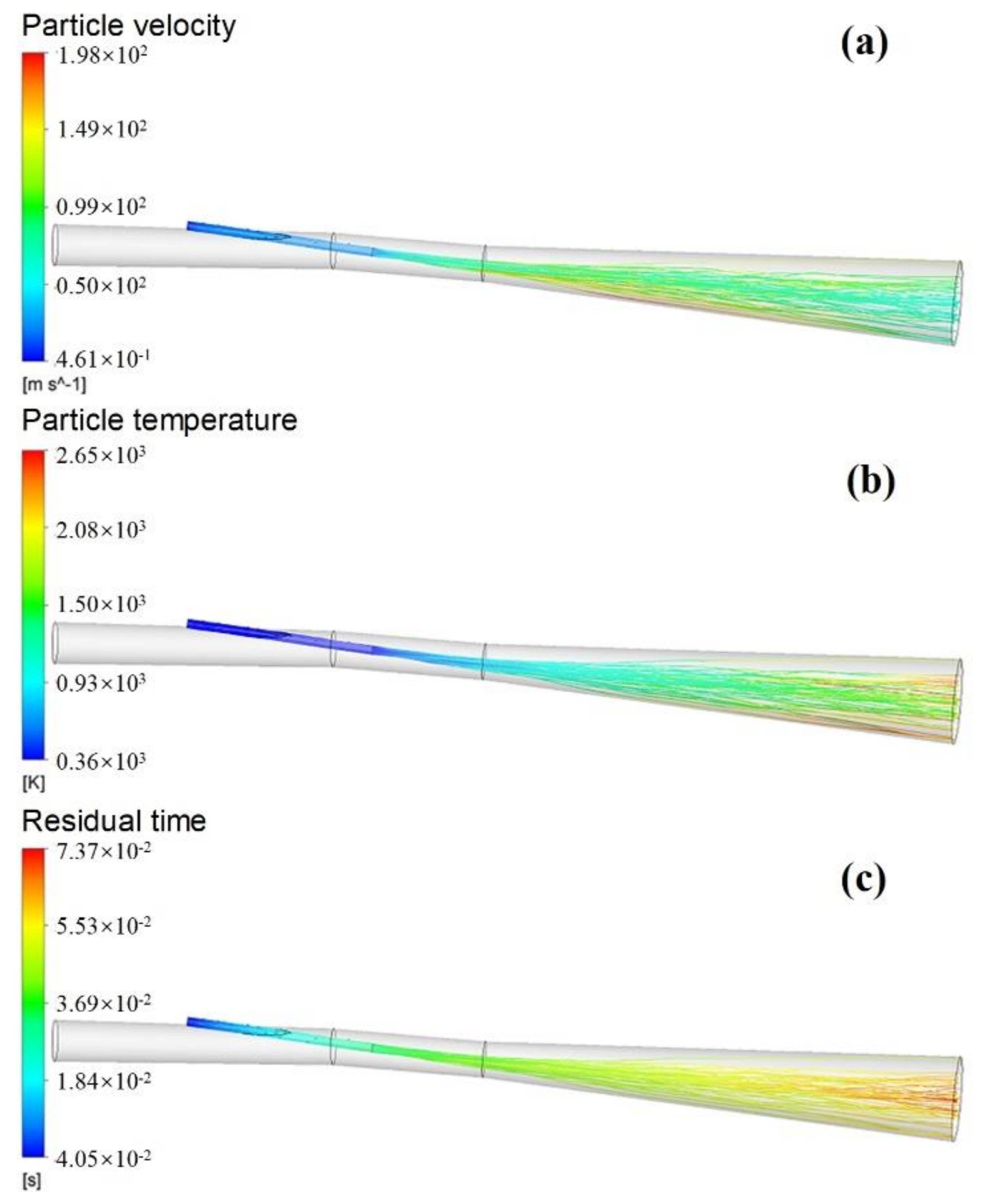
3.2.4. Motion Behavior of Pulverized Coal Particles
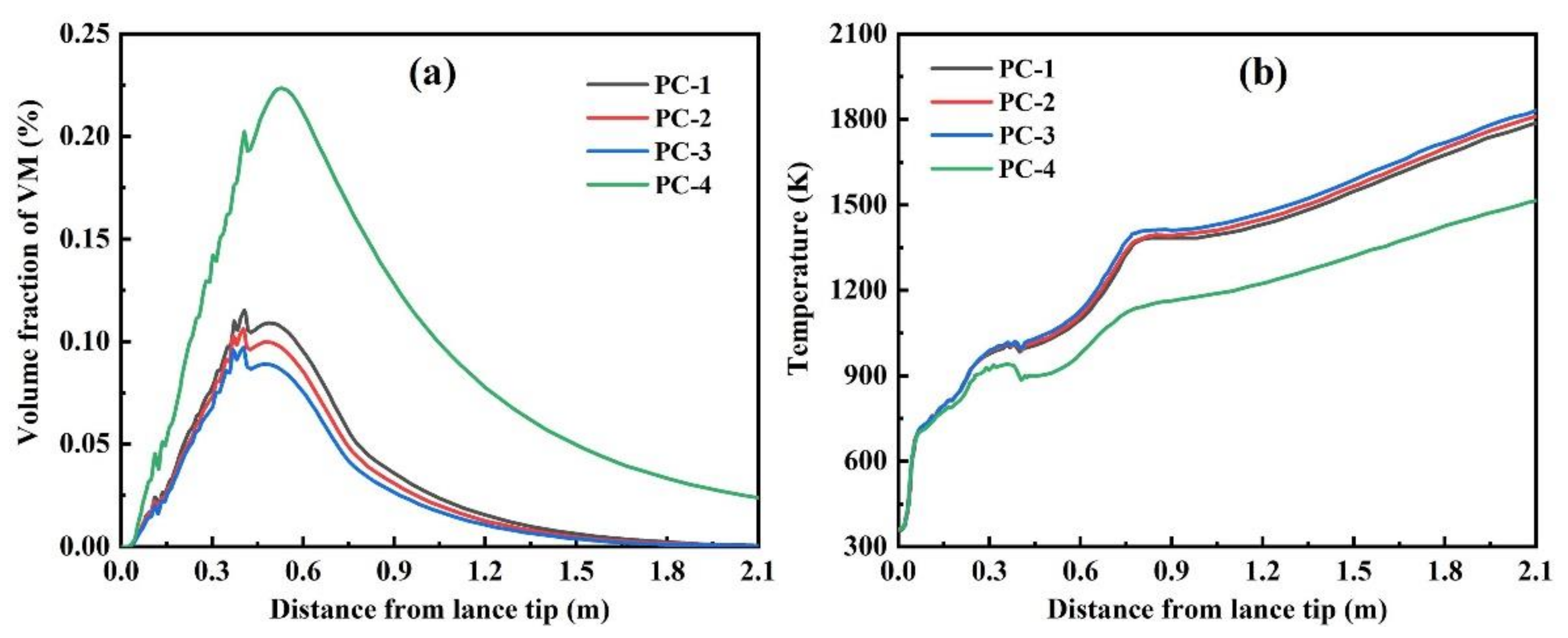
3.3. Effect of Semi-Coke Ratio
4. Conclusions
- (1)
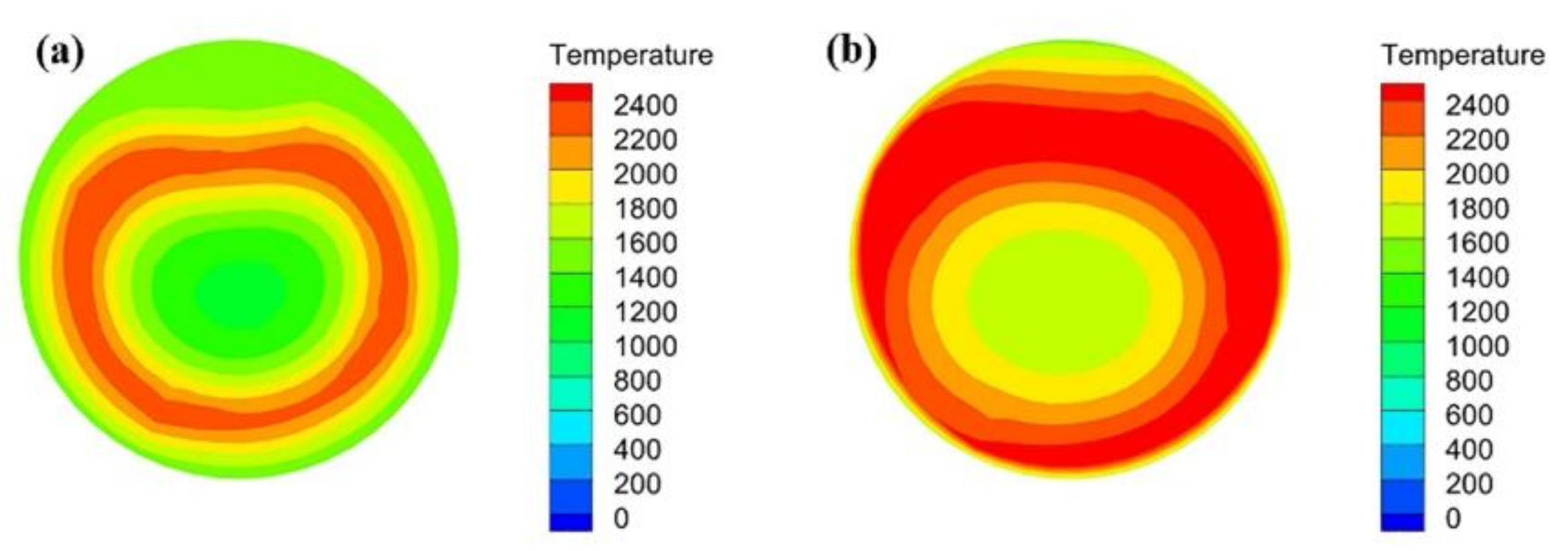
- During the blast furnace injection of semi-coke, the velocity in the central region of the combustion zone is lower than that in the edge region, but with the development of the combustion zone, the velocity distribution of the flow field tends to be uniform. The high temperature zone of the combustion zone is annularly distributed, and its radial distribution continues to expand with the extension of the depth of the combustion zone.
- (2)
- The highest concentrations of CO and H2 in the combustion zone reach 36% and 8%, respectively. The longest residence time of semi-coke particles in the combustion zone is 70 ms under the current simulation conditions.
- (3)
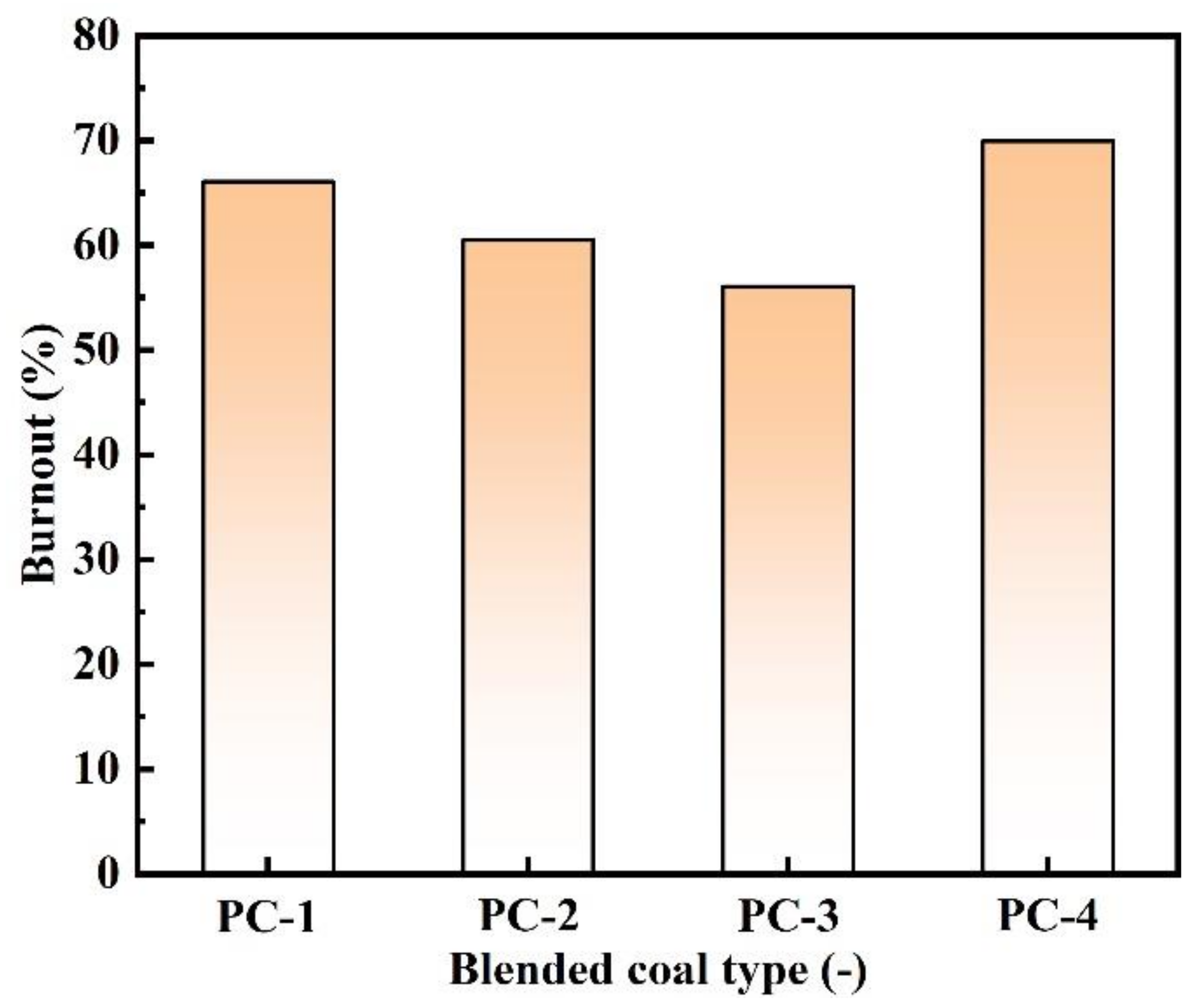
- With the decrease of the semi-coke ratio in the blended coal, the fixed carbon content and the calorific value of the blended coal increase, but the burnout of the blended coal reduces. The burnout of bituminous coal is about 70%, which is significantly higher than that of blended coal. Therefore, an appropriate amount of bituminous coal can be added to improve the combustion performance of pulverized coal in actual production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Shen, Y. Modelling and optimisation of biomass injection in ironmaking blast furnaces. Prog. Energy Combust. Sci. 2021, 87, 100952. [Google Scholar] [CrossRef]
- Nielson, S.; Okosun, T.; Damstedt, B.; Jampani, M.; Zhou, C.Q. Tuyere-Level Syngas Injection in the Blast Furnace: A Computational Fluid Dynamics Investigation. Processes 2021, 9, 1447. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, J.; Bi, C.; Ning, X.; Tan, P.; Wang, G. Effect of coke powder and semi-coke on combustion characteristics of blast furnace blended coal. Iron Steel 2019, 54, 10–18. [Google Scholar]
- Chen, S.; Cheng, M.; Xu, J.; Liu, X.; Yu, D.; Xu, M. Numerical analysis on reduction of ultrafine particulate matter by a kaolin additive during pulverized coal combustion. Energy Fuels 2021, 35, 9538–9549. [Google Scholar] [CrossRef]
- He, J.; Zou, C.; Zhao, J.; Liang, D.; Xi, J.; Ma, C. Comparison of semi-coke with traditional pulverized coal injection and iron ore sintering fuels based on chemical structure and combustion behavior. J. Iron Steel Res. Int. 2022, 29, 725–740. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.; Zhou, H.; Ning, X.; Zhang, C. Numerical Simulation Study on the Effects of Co-Injection of Pulverized Coal and Hydrochar into the Blast Furnace. Sustainability 2022, 14, 4407. [Google Scholar] [CrossRef]
- Tian, J.; Ni, H.; Han, Y.; Shen, Z.; Wang, Q.; Long, X.; Zhang, Y.; Cao, J. Primary PM2.5 and trace gas emissions from residential coal combustion: Assessing semi-coke briquette for emission reduction in the Beijing-Tianjin-Hebei region, China. Atmos. Environ. 2018, 191, 378–386. [Google Scholar] [CrossRef]
- Zou, C.; Ma, C.; Zhao, J.; Wen, L.; Bai, C. Research status and suggestion of mid-low temperature pyrolysis semi-coke as the PCI fuel in blast furnace. Clean Coal Technol. 2017, 23, 57–64. [Google Scholar]
- Li, J.; Li, X.J.; Li, B.P.; Song, C.Y.; Zhang, X.P.; Qin, X.W. Experimental analysis on use of semi-coke as fuel for blast furnace injection. China Metall. 2019, 29, 14–18. [Google Scholar]
- Bi, C.G.; Huang, C.C.; Ning, X.J.; Zhang, J.L.; Wang, G.W.; Peng, Z.F. Best ratio of semi-coke for blast furnace injection. Iron Steel 2020, 55, 25–32. [Google Scholar]
- Gong, L.W. Semi-coke coal injection practice in Taiyuan Steel’s BF. Ironmaking 2018, 37, 16–19. [Google Scholar]
- Du, G.; Yang, S. An investigation into coal blending and the addition of semi-coke. Iron Steel Vanadium Titan. 2013, 34, 64–68. [Google Scholar]
- Steer, J.; Greenslade, M.; Marsh, R. A Comparison of Laboratory Coal Testing with the Blast Furnace Process and Coal Injection. Metals 2021, 11, 1476. [Google Scholar] [CrossRef]
- Liu, L.; Kuang, S.; Jiao, L.; Guo, B.; Yu, A. Optimization of pulverized coal injection (PCI) rate in an ironmaking blast furnace by an integrated process model. Fuel 2022, 315, 122832. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, C.; Zhao, J.; Wang, F. Combustion Characteristics of Coal for Pulverized Coal Injection (PCI) Blending with Steel Plant Flying Dust and Waste Oil Sludge. ACS Omega 2021, 6, 28548–28560. [Google Scholar] [CrossRef]
- Belošević, S.; Tomanović, I.; Crnomarković, N.; Milićević, A. Full-scale CFD investigation of gas-particle flow, interactions and combustion in tangentially fired pulverized coal furnace. Energy 2019, 179, 1036–1053. [Google Scholar] [CrossRef]
- Zhang, J.; Ito, T.; Ishii, H.; Ishihara, S.; Fujimori, T. Numerical investigation on ammonia co-firing in a pulverized coal combustion facility: Effect of ammonia co-firing ratio. Fuel 2020, 267, 117166. [Google Scholar] [CrossRef]
- Goto, K.; Murai, R.; Murao, A.; Sato, M.; Asanuma, M.; Ariyama, T. Massive combustion technology of solid fuel injected into blast furnace. In Proceedings of the International Blast Furnace Lower Zone Symposium, Wollongong, Australia, 25 November 2002. [Google Scholar]
- Du, S.-W.; Chen, W.-H. Numerical prediction and practical improvement of pulverized coal combustion in blast furnace. Int. Commun. Heat Mass Transf. 2006, 33, 327–334. [Google Scholar] [CrossRef]
- Du, S.-W.; Chen, W.-H.; Lucas, J. Performances of pulverized coal injection in blowpipe and tuyere at various operational conditions. Energy Convers. Manag. 2007, 48, 2069–2076. [Google Scholar] [CrossRef]
- Gu, M.; Chen, G.; Zhang, M.; Huang, D.; Chaubal, P.; Zhou, C.Q. Three-dimensional simulation of the pulverized coal combustion inside blast furnace tuyere. Appl. Math. Model. 2010, 34, 3536–3546. [Google Scholar] [CrossRef]
- Shen, Y.S.; Yu, A.B.; Austin, P.R.; Zulli, P. CFD study of in-furnace phenomena of pulverised coal injection in blast furnace: Effects of operating conditions. Powder Technol. 2012, 223, 27–38. [Google Scholar] [CrossRef]
- Shen, Y.S.; Yu, A.B. Modelling of injecting a ternary coal blend into a model ironmaking blast furnace. Miner. Eng. 2016, 90, 89–95. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Shen, Y. CFD Study of Hydrogen Injection in Blast Furnaces: Tuyere Co-injection of Hydrogen and Coal. Metall. Mater. Trans. B 2021, 52, 2971–2991. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hu, Z.; Shen, Y. CFD study of hydrogen injection through tuyeres into ironmaking blast furnaces. Fuel 2021, 302, 120804. [Google Scholar] [CrossRef]
- Hu, Z.J.; Liu, Y.R.; Xu, H.; Zhu, J.M.; Wu, S.L.; Shen, Y.S. Co-combustion of semicoke and coal in an industry ironmaking blast furnace: Lab experiments, model study and plant tests. Fuel Process. Technol. 2019, 196, 106165. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Ning, X.; Zhang, J.; Wang, C. Numerical simulation of combustion behaviors of hydrochar derived from low-rank coal in the raceway of blast furnace. Fuel 2020, 278, 118267. [Google Scholar] [CrossRef]
- Muto, M.; Yuasa, K.; Kurose, R. Numerical simulation of soot formation in pulverized coal combustion with detailed chemical reaction mechanism. Adv. Powder Technol. 2018, 29, 1119–1127. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, B.; Yu, A.; Maldonado, D.; Austin, P.; Zulli, P. Three-dimensional modelling of coal combustion in blast furnace. ISIJ Int. 2008, 48, 777–786. [Google Scholar] [CrossRef] [Green Version]

| Mass conservation | |
| Momentum conservation | |
| Energy conservation | |
| Turbulent kinetic energy | |
| Turbulent dissipation rate | |
| Gas mass fraction | |
| Mass | |
| Momentum | |
| Energy |
| Heterogeneous Chemical Reactions | Er/(J/kmol) | ||
|---|---|---|---|
| C(s) + 0.5O2 → CO | 1.36 × 106 | 0.68 | 1.30 × 108 |
| C(s) +CO2 → 2CO | 6.78 × 104 | 0.73 | 1.63 × 108 |
| C(s) + H2O → CO + H2 | 8.55 × 104 | 0.84 | 1.40 × 108 |
| Parameters | Semi-Coke | Coke Powder |
|---|---|---|
| Fixed carbon | 75.06 | 84.11 |
| Ash | 11.65 | 14.62 |
| Volatile | 13.29 | 1.27 |
| C | 85.83 | 97.83 |
| H | 2.95 | 0.25 |
| O | 10.44 | 0.33 |
| N | 0.78 | 1.59 |
| Operational Parameters | Value |
|---|---|
| Blast flow rate (Nm3/h) | 7110.67 |
| Blast temperature (K) | 1463 |
| Coal injection rate (kg/h) | 0.5707 |
| Pulverized coal temperature (K) | 360 |
| Conveying gas (N2) volume flux (Nm3/h) | 2750 |
| Conveying gas (N2) temperature (K) | 326 |
| Oxygen enrichment ratio (%) | 3.96 |
| Iron production per day (t) | 10,436.7 |
| Blend Coal | Industrial Analysis | Elemental Analysis | |||||
|---|---|---|---|---|---|---|---|
| Fixed Carbon | Ash | VM | C | H | O | N | |
| PC-1 | 75.97 | 11.95 | 12.09 | 87.03 | 2.68 | 9.43 | 0.86 |
| PC-2 | 76.87 | 12.24 | 10.89 | 88.23 | 2.41 | 8.42 | 0.94 |
| PC-3 | 77.78 | 12.54 | 9.68 | 89.43 | 2.14 | 7.41 | 1.02 |
| PC-4 | 63.01 | 5.66 | 31.33 | 81.31 | 4.05 | 13.35 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Zheng, Z.; Wang, R.; Hu, Q.; Li, Y.; You, Z. Numerical Study on Combustion Behavior of Semi-Coke in Blast Furnace Blowpipe-Tuyere-Combustion Zone. Metals 2022, 12, 1272. https://doi.org/10.3390/met12081272
You Y, Zheng Z, Wang R, Hu Q, Li Y, You Z. Numerical Study on Combustion Behavior of Semi-Coke in Blast Furnace Blowpipe-Tuyere-Combustion Zone. Metals. 2022; 12(8):1272. https://doi.org/10.3390/met12081272
Chicago/Turabian StyleYou, Yang, Zhuang Zheng, Rui Wang, Qingqing Hu, Yanhui Li, and Zhixiong You. 2022. "Numerical Study on Combustion Behavior of Semi-Coke in Blast Furnace Blowpipe-Tuyere-Combustion Zone" Metals 12, no. 8: 1272. https://doi.org/10.3390/met12081272
APA StyleYou, Y., Zheng, Z., Wang, R., Hu, Q., Li, Y., & You, Z. (2022). Numerical Study on Combustion Behavior of Semi-Coke in Blast Furnace Blowpipe-Tuyere-Combustion Zone. Metals, 12(8), 1272. https://doi.org/10.3390/met12081272






