Wear Resistance of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Eli Alloy Processed by Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Samples
2.2. Obtaining Coatings
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- NBRISO/ASTM52900; Additive Manufacturing—General Principles—Terminology. ISO: Rio de Janeiro, Brazil, 2015.
- Atzeni, E.; Salmi, A. Study on Unsupported Overhangs of AlSi10Mg Parts Processed by Direct Metal Laser Sintering (DMLS). J. Manuf. Processes 2015, 20, 500–506. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Yao, J.; Ding, R.; Li, K.; Du, B.; Zhao, L.; Yuan, Y. Study on the impact behavior of arch micro-strut (ARCH) lattice structure by selective laser melting (SLM). Rapid Prototyp. J. 2022; in press. [Google Scholar] [CrossRef]
- Linares, J.-M.; Chaves-Jacob, J.; Lopez, Q.; Sprauel, J.-M. Fatigue life optimization for 17-4Ph steel produced by selective laser melting. Rapid Prototyp. J. 2022, 28, 1182–1192. [Google Scholar] [CrossRef]
- Giganto, S.; Martínez-Pellitero, S.; Cuesta, E.; Zapico, P.; Barreiro, J. Proposal of design rules for improving the accuracy of selective laser melting (SLM) manufacturing using benchmarks parts. Rapid Prototyp. J. 2022, 28, 1129–1143. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2022, 28, 87–100. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Characteristic and in Vitro Bioactivity of a Microarc-Oxidized TiO2-Based Coating after Chemical Treatment. Acta Biomater. 2007, 3, 817–827. [Google Scholar] [CrossRef]
- Becerikli, M.; Kopp, A.; Kröger, N.; Bodrova, M.; Wallner, C.; Wagner, J.M.; Dadras, M.; Jettkant, B.; Pöhl, F.; Lehnhardt, M.; et al. A Novel Titanium Implant Surface Modification by Plasma Electrolytic Oxidation (PEO) Preventing Tendon Adhesion. Mater. Sci. Eng. C 2021, 123, 112030. [Google Scholar] [CrossRef]
- Ciocca, L.; Fantini, M.; De Crescenzio, F.; Corinaldesi, G.; Scotti, R. Direct Metal Laser Sintering (DMLS) of a Customized Titanium Mesh for Prosthetically Guided Bone Regeneration of Atrophic Maxillary Arches. Med. Biol. Eng. Comput. 2011, 49, 1347–1352. [Google Scholar] [CrossRef]
- Ganesh, B.K.C.; Ramanaiah, N.; Rao, P.V.C. Effect of Surface Treatment on Tribological Behavior of Ti-6Al-4V Implant Alloy. J. Miner. Mater. Charact. Eng. 2012, 11, 735. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Friction and Surface Behavior of Selected Titanium Alloys during Reciprocating-Sliding Motion. Wear 2001, 249, 157–167. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Silva, F.S.; Carvalho, O.; Miranda, G. Wear Behavior of Ti6Al4V Biomedical Alloys Processed by Selective Laser Melting, Hot Pressing and Conventional Casting. Trans. Nonferrous Met. Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, K.K.; Islam, A.; Keshri, A.K. Corrosion Behaviour of Plasma Sprayed Graphene Nanoplatelets Reinforced Hydroxyapatite Composite Coatings in Simulated Body Fluid. Ceram. Int. 2020, 46, 13539–13548. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, N.; Kumar, D.; Hegab, H. Design, Development and Tribological Characterization of Ti–6Al–4V/Hydroxyapatite Composite for Bio-Implant Applications. Mater. Chem. Phys. 2020, 243, 122662. [Google Scholar] [CrossRef]
- Costa, A.I.; Sousa, L.; Alves, A.C.; Toptan, F. Tribocorrosion Behaviour of Bio-Functionalized Porous Ti Surfaces Obtained by Two-Step Anodic Treatment. Corros. Sci. 2020, 166, 108467. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Kovacı, H.; Yetim, T.; Yetim, A.F.; Çelik, A. Tribological and Electrochemical Properties of TiO2 Films Produced on Cp-Ti by Sol-Gel and SILAR in Bio-Simulated Environment. Surf. Coat. Technol. 2018, 352, 513–521. [Google Scholar] [CrossRef]
- Bertuccioli; Garzoni; Martini; Morri; Rondelli Plasma Electrolytic Oxidation (PEO) Layers from Silicate/Phosphate Baths on Ti-6Al-4V for Biomedical Components: Influence of Deposition Conditions and Surface Finishing on Dry Sliding Behaviour. Coatings 2019, 9, 614. [CrossRef] [Green Version]
- Simchen, F.; Sieber, M.; Lampke, T. Electrolyte Influence on Ignition of Plasma Electrolytic Oxidation Processes on Light Metals. Surf. Coat. Technol. 2017, 315, 205–213. [Google Scholar] [CrossRef]
- Durdu, S.; Deniz, Ö.F.; Kutbay, I.; Usta, M. Characterization and Formation of Hydroxyapatite on Ti6Al4V Coated by Plasma Electrolytic Oxidation. J. Alloy. Compd. 2013, 551, 422–429. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Parfenova, L.V.; Dyakonov, G.S.; Danilko, K.V.; Mukaeva, V.R.; Farrakhov, R.G.; Lukina, E.S.; Valiev, R.Z. Surface Functionalization via PEO Coating and RGD Peptide for Nanostructured Titanium Implants and Their in Vitro Assessment. Surf. Coat. Technol. 2019, 357, 669–683. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mohedano, M.; Martínez-Corriá, R.; Ramos, V.; Arrabal, R.; Matykina, E. In Vitro and in Vivo Evaluation of PEO-Modified Titanium for Bone Implant Applications. Surf. Coat. Technol. 2018, 347, 358–368. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G.E. Plasma Electrolytic Oxidation of Titanium in a Phosphate/Silicate Electrolyte and Tribological Performance of the Coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Alves, S.A.; Bayón, R.; de Viteri, V.S.; Garcia, M.P.; Igartua, A.; Fernandes, M.H.; Rocha, L.A. Tribocorrosion Behavior of Calcium- and Phosphorous-Enriched Titanium Oxide Films and Study of Osteoblast Interactions for Dental Implants. J. Bio Tribo Corros. 2015, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Dong, Q.; Yu, H.; Wang, X.; Wang, D. Microstructure of Porous TiO2 Coating on Pure Ti by Micro-Arc Oxidation. Adv. Eng. Mater. 2006, 8, 754–759. [Google Scholar] [CrossRef]
- Santos, P.B.; Baldin, E.K.; Krieger, D.A.; de Castro, V.V.; Aguzzoli, C.; Fonseca, J.C.; Rodrigues, M.; Lopes, M.A.; de Fraga Malfatti, C. Wear Performance and Osteogenic Differentiation Behavior of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Alloys: Potential Application for Bone Tissue Repairs. Surf. Coat. Technol. 2021, 417, 127179. [Google Scholar] [CrossRef]
- Yu, J.-M.; Choe, H.-C. Morphology Changes and Bone Formation on PEO-Treated Ti-6Al-4V Alloy in Electrolyte Containing Ca, P., Sr, and Si Ions. Appl. Surf. Sci. 2019, 477, 121–130. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Shamanian, M.; Minneboo, M.; Modaresifar, K.; van Hengel, I.A.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Osteogenic and Antibacterial Surfaces on Additively Manufactured Porous Ti-6Al-4V Implants: Combining Silver Nanoparticles with Hydrothermally Synthesized HA Nanocrystals. Mater. Sci. Eng. C 2021, 120, 111745. [Google Scholar] [CrossRef]
- Yan, X.; Chen, C.; Bolot, R.; Ma, W.; Deng, C.; Wang, J.; Ren, Z.; Liao, H.; Liu, M. Improvement of Tribological Performance by Micro-Arc Oxidation Treatment on Selective Laser Melting Ti6Al4V Alloy. Mater. Res. Express 2019, 6, 096509. [Google Scholar] [CrossRef]
- Exley, C. The Toxicity of Aluminium in Humans. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Yellamma, K.; Saraswathamma, S.; Kumari, B.N. Cholinergic System under Aluminium Toxicity in Rat Brain. Toxicol. Int. 2010, 17, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An Investigation of Ceramic Coating Growth Mechanisms in Plasma Electrolytic Oxidation (PEO) Processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic Study of Electrolytic Plasma and Discharging Behaviour during the Plasma Electrolytic Oxidation (PEO) Process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Han, I.; Choi, J.H.; Zhao, B.H.; Baik, H.K.; Lee, I.-S. Changes in Anodized Titanium Surface Morphology by Virtue of Different Unipolar DC Pulse Waveform. Surf. Coat. Technol. 2007, 201, 5533–5536. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Sun, M.; Zhang, Q.; Yao, J. Influence of Microstructure of TC4 Substrate on the MAO Coating. Surf. Eng. 2020, 36, 827–836. [Google Scholar] [CrossRef]
- Longhitano, G.A.; Arenas, M.A.; Conde, A.; Larosa, M.A.; Jardini, A.L.; de Carvalho Zavaglia, C.A.; Damboreneac, J.J. Heat Treatments Effects on Functionalization and Corrosion Behavior of Ti-6Al-4V ELI Alloy Made by Additive Manufacturing. J. Alloy. Compd. 2018, 765, 961–968. [Google Scholar] [CrossRef]
- Baldin, E.K.; Santos, P.B.; de Castro, V.V.; Aguzzoli, C.; Maurmann, N.; Girón, J.; Pranke, P.; de Fraga Malfatti, C. Plasma Electrolytic Oxidation (PEO) Coated CP-Ti: Wear Performance on Reciprocating Mode and Chondrogenic–Osteogenic Differentiation. J. Bio Tribo Corros. 2021, 8, 29. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Hu, B.; Yao, Z.; Xia, Q.; Jiang, Z. Preparation of a Novel Yellow Ceramic Coating on Ti Alloys by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2016, 307, 1297–1302. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive Coatings on Ti6Al4V Alloy Formed by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- Yao, Z.Q.; Ivanisenko, Y.; Diemant, T.; Caron, A.; Chuvilin, A.; Jiang, J.Z.; Valiev, R.Z.; Qi, M.; Fecht, H.-J. Synthesis and Properties of Hydroxyapatite-Containing Porous Titania Coating on Ultrafine-Grained Titanium by Micro-Arc Oxidation. Acta Biomater. 2010, 6, 2816–2825. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of Plasma Electrolytic Oxidation of Titanium Substrates: Mechanism, Properties, Applications and Limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M. The Tribological Properties of Bioceramic Coatings Produced on Ti6Al4V Alloy by Plasma Electrolytic Oxidation. Ceram. Int. 2014, 40, 3627–3635. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.; Ntomprougkidis, V.; Migot, S.; Bruyère, S.; Soldera, F.; Belmonte, T.; Henrion, G. Formation of a Metastable Nanostructured Mullite during Plasma Electrolytic Oxidation of Aluminium in “Soft” Regime Condition. Mater. Des. 2019, 180, 107977. [Google Scholar] [CrossRef]
- Hwang, I.-J.; Choe, H.-C. Hydroxyapatite Coatings Containing Zn and Si on Ti-6Al-4Valloy by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2018, 432, 337–346. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Transmission Electron Microscopy of Coatings Formed by Plasma Electrolytic Oxidation of Titanium. Acta Biomater. 2009, 5, 1356–1366. [Google Scholar] [CrossRef]
- Yu, S.; Yu, Z.; Wang, G.; Han, J.; Ma, X.; Dargusch, M.S. Biocompatibility and Osteoconduction of Active Porous Calcium–Phosphate Films on a Novel Ti–3Zr–2Sn–3Mo–25Nb Biomedical Alloy. Colloids Surf. B Biointerfaces 2011, 85, 103–115. [Google Scholar] [CrossRef]
- dos Santos, A.; Araujo, J.R.; Landi, S.M.; Kuznetsov, A.; Granjeiro, J.M.; de Sena, L.Á.; Achete, C.A. A Study of the Physical, Chemical and Biological Properties of TiO2 Coatings Produced by Micro-Arc Oxidation in a Ca–P-Based Electrolyte. J. Mater. Sci. Mater. Med. 2014, 25, 1769–1780. [Google Scholar] [CrossRef]
- Ríos, J.M.; Quintero, D.; Castaño, J.G.; Echeverría, F.; Gómez, M.A. Comparison among the Lubricated and Unlubricated Tribological Behavior of Coatings Obtained by PEO on the Ti6Al4V Alloy in Alkaline Solutions. Tribol. Int. 2018, 128, 1–8. [Google Scholar] [CrossRef]
- de Viteri, V.S.; Bayón, R.; Igartua, A.; Barandika, G.; Moreno, J.E.; Peremarch, C.P.-J.; Pérez, M.M. Structure, Tribocorrosion and Biocide Characterization of Ca, P and I Containing TiO2 Coatings Developed by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2016, 367, 1–10. [Google Scholar] [CrossRef]
- Qian, J.; Yin, X.; Wang, N.; Liu, L.; Xing, J. Preparation and Tribological Properties of Stearic Acid-Modified Hierarchical Anatase TiO2 Microcrystals. Appl. Surf. Sci. 2012, 258, 2778–2782. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chung, C.-J.; Yang, T.-C.; Tang, C.-H.; He, J.-L. Microscopic Observations of Osteoblast Growth on Micro-Arc Oxidized β Titanium. Appl. Surf. Sci. 2013, 266, 73–80. [Google Scholar] [CrossRef]
- Chung, C.-J.; Su, R.-T.; Chu, H.-J.; Chen, H.-T.; Tsou, H.-K.; He, J.-L. Plasma Electrolytic Oxidation of Titanium and Improvement in Osseointegration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Mashtalyar, D.V.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Filonina, V.S.; Suchkov, S.N.; Gnedenkov, S.V. Composite coatings formed on Ti by PEO and fluoropolymer treatment. Appl. Surf. Sci. 2021, 536, 147976. [Google Scholar] [CrossRef]
- Han, J.; Cheng, Y.; Tu, W.; Zhan, T.-Y.; Cheng, Y. The Black and White Coatings on Ti-6Al-4V Alloy or Pure Titanium by Plasma Electrolytic Oxidation in Concentrated Silicate Electrolyte. Appl. Surf. Sci. 2018, 428, 684–697. [Google Scholar] [CrossRef]
- Kumari, R.; Blawert, C.; Majumdar, J.D. Microstructures and Properties of Plasma Electrolytic Oxidized Ti Alloy (Ti-6Al-4V) for Bio-Implant Application. Met. Mat. Trans. A 2016, 47, 788–800. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Pardo, A.; Matykina, E. Bioactive Multi-Elemental PEO-Coatings on Titanium for Dental Implant Applications. Mater. Sci. Eng. C 2019, 97, 738–752. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, C.; Liao, Z.; Liu, W. The Improvement of Wettability, Biotribological Behavior and Corrosion Resistance of Titanium Alloy Pretreated by Thermal Oxidation. Tribol. Int. 2014, 79, 174–182. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The Anatase Phase of Nanotopography Titania Plays an Important Role on Osteoblast Cell Morphology and Proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeu, F.; Sampaio, M.; Carvalho, O.; Pinto, E.; Alves, N.; Gomes, J.R.; Silva, F.S.; Miranda, G. Tribological Behavior of Ti6Al4V Cellular Structures Produced by Selective Laser Melting. J. Mech. Behav. Biomed. Mater. 2017, 69, 128–134. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, T.; Nie, X. Wear Failure Behaviour of Titanium-Based Oxide Coatings on a Titanium Alloy under Impact and Sliding Forces. J. Alloy Compd. 2013, 578, 336–344. [Google Scholar] [CrossRef]
- Longhitano, G.A.; Larosa, M.A.; Jardini, A.L.; Zavaglia, C.A.d.C.; Ierardi, M.C.F. Correlation between Microstructures and Mechanical Properties under Tensile and Compression Tests of Heat-Treated Ti-6Al–4V ELI Alloy Produced by Additive Manufacturing for Biomedical Applications. J. Mater. Process. Technol. 2018, 252, 202–210. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ma, W.; Wang, Y.; Yang, J.; Liu, S.; Tang, H. Corrosion and Wear Properties of Micro-Arc Oxidation Treated Ti6Al4V Alloy Prepared by Selective Electron Beam Melting. Trans. Nonferrous Met. Soc. China 2020, 30, 2132–2142. [Google Scholar] [CrossRef]
- Lawn, B.R.; Borrero-Lopez, O.; Huang, H.; Zhang, Y. Micromechanics of Machining and Wear in Hard and Brittle Materials. J. Am. Ceram. Soc. 2021, 104, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Sankara Narayanan, T.S.N.; Kim, J.; Park, H.W. High Performance Corrosion and Wear Resistant Ti-6Al-4V Alloy by the Hybrid Treatment Method. Appl. Surf. Sci. 2020, 504, 144388. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Sabour Rouhaghdam, A.; Shahrabi, T. Abrasive Wear Behaviour of Si3N4/TiO2 Nanocomposite Coatings Fabricated by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2010, 205, S41–S46. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.; Brochard, F.; Briançon, J.-L.; Noël, C.; Belmonte, T.; Czerwiec, T.; Henrion, G. Delay in Micro-Discharges Appearance during PEO of Al: Evidence of a Mechanism of Charge Accumulation at the Electrolyte/Oxide Interface. Appl. Surf. Sci. 2017, 410, 29–41. [Google Scholar] [CrossRef] [Green Version]
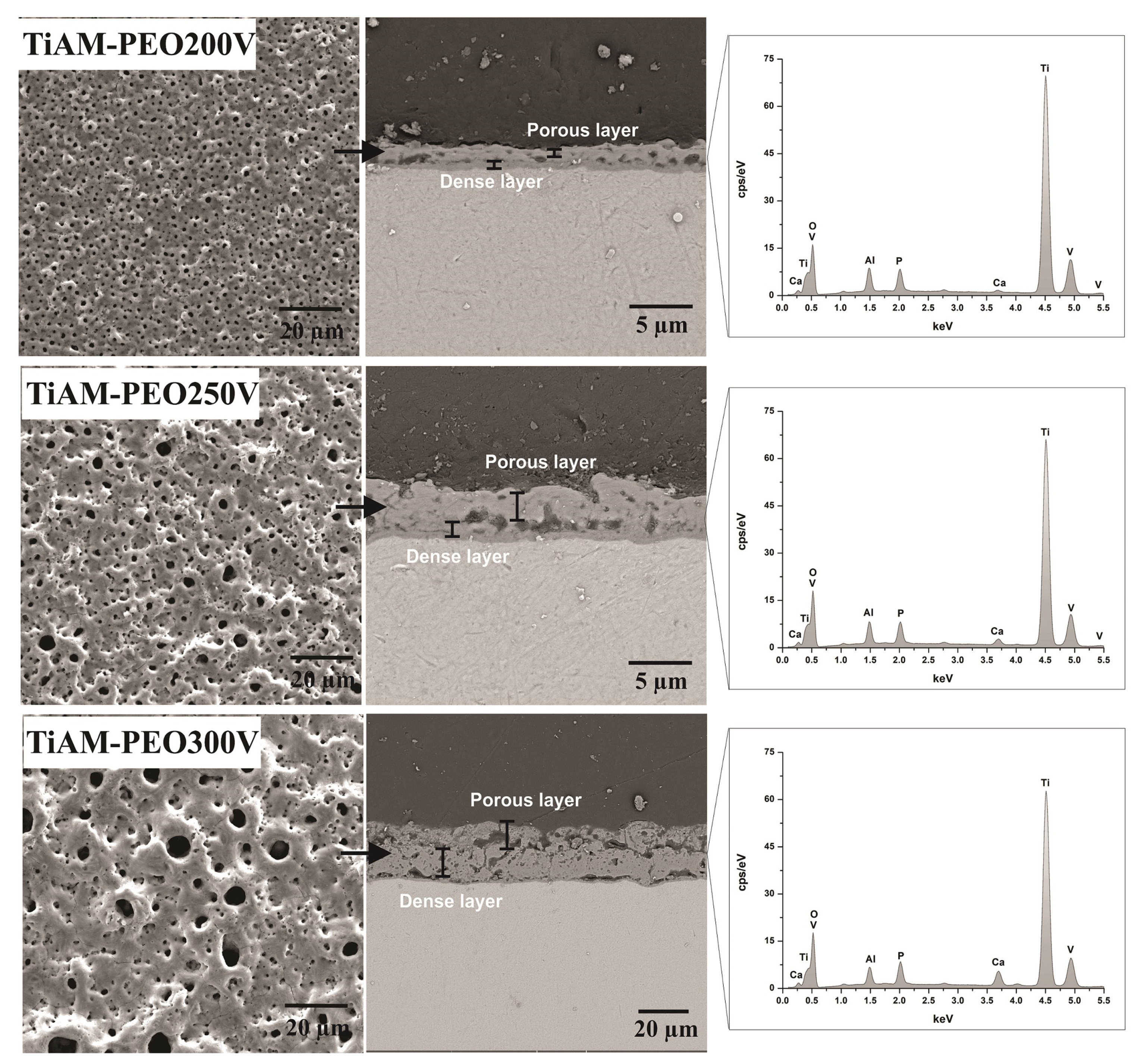



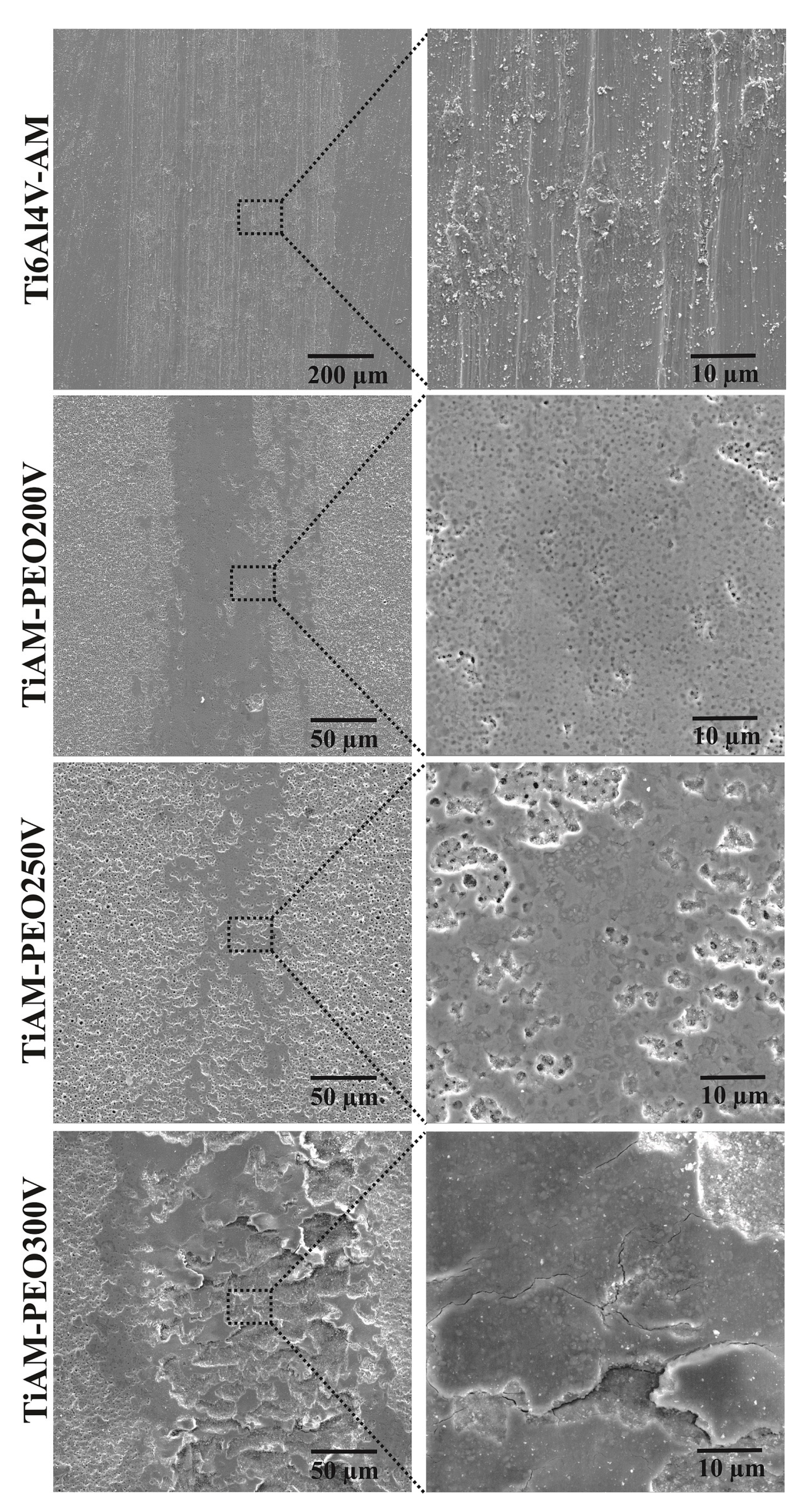

| Sample/Properties | Ti6Al4V-AM | TiAM-PEO200v | TiAM-PEO250v | TiAM-PEO300v |
|---|---|---|---|---|
| Ra (µm) | 0.13 ± 0.02 | 0.17 ± 0.04 | 0.28 ± 0.01 | 0.72 ± 0.05 |
| Rz (µm) | 1.3 ± 0.62 | 1.50 ± 0.36 | 2.33 ± 0.32 | 4.55 ± 0.28 |
| Layer thickness (µm) | - | 2.05 ± 0.13 | 4.50 ± 0.33 | 23.83 ± 1.5 |
| Outer layer (µm) | - | 1.53 ± 0.26 | 3.64 ± 0.44 | 22.53 ± 1.25 |
| Inner layer (µm) | - | 0.41 ± 0.04 | 0.69 ± 0.18 | 1.16 ± 0.22 |
| Pore population density (pores.mm−2) | - | 17.9 × 104 | 10.0 × 104 | 50.4 × 103 |
| Greater pore area (µm2) | - | 1.62 | 5.32 | 18.31 |
| Smaller area pore (µm2) | - | 0.06 | 0.07 | 0.11 |
| Sample | Sliding Distance (m) | Wear Rate (mm3/s) |
|---|---|---|
| Ti6Al4V-AM | 32.4 | 9.79 × 10−5 |
| TiAM-PEO200v | 129.6 | 1.99 × 10−6 |
| TiAM-PEO250v | 129.6 | 2.19 × 10−6 |
| TiAM-PEO300v | 129.6 | 2.60 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.B.; de Castro, V.V.; Baldin, E.K.; Aguzzoli, C.; Longhitano, G.A.; Jardini, A.L.; Lopes, É.S.N.; de Andrade, A.M.H.; de Fraga Malfatti, C. Wear Resistance of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Eli Alloy Processed by Additive Manufacturing. Metals 2022, 12, 1070. https://doi.org/10.3390/met12071070
Santos PB, de Castro VV, Baldin EK, Aguzzoli C, Longhitano GA, Jardini AL, Lopes ÉSN, de Andrade AMH, de Fraga Malfatti C. Wear Resistance of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Eli Alloy Processed by Additive Manufacturing. Metals. 2022; 12(7):1070. https://doi.org/10.3390/met12071070
Chicago/Turabian StyleSantos, Pedro Bell, Victor Velho de Castro, Estela Kerstner Baldin, Cesar Aguzzoli, Guilherme Arthur Longhitano, André Luiz Jardini, Éder Sócrates Najar Lopes, Antonio Marcos Helgueira de Andrade, and Célia de Fraga Malfatti. 2022. "Wear Resistance of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Eli Alloy Processed by Additive Manufacturing" Metals 12, no. 7: 1070. https://doi.org/10.3390/met12071070
APA StyleSantos, P. B., de Castro, V. V., Baldin, E. K., Aguzzoli, C., Longhitano, G. A., Jardini, A. L., Lopes, É. S. N., de Andrade, A. M. H., & de Fraga Malfatti, C. (2022). Wear Resistance of Plasma Electrolytic Oxidation Coatings on Ti-6Al-4V Eli Alloy Processed by Additive Manufacturing. Metals, 12(7), 1070. https://doi.org/10.3390/met12071070