Current Status and Outlook of Temporary Implants (Magnesium/Zinc) in Cardiovascular Applications
Abstract
:1. Introduction
2. Driving Force for Temporary Implants
- Avoiding revision surgery for the patient.
- Minimizing medical costs for the patient.
- Minimizing patient trauma inflicted during the second surgery.
- Saving the doctor’s time.
- Avoid long-term toxicity effects if a permanent implant material is used instead.
- Titanium-based materials.
- Steels.
- Co-Cr based alloys.
- Tantalum.
- Nitinol [31].
- Biocompatible with acceptable or zero cytotoxicity.
- No chronic deleterious effect.
- To maintain mechanical integrity during the healing time.
- Minimal stress-shielding effect.
- Acceptable degradation time, synchronized closely with healing time.
3. Magnesium and Zinc in the Human Body
| Application | Zinc | Magnesium |
|---|---|---|
| Function in Human Body |
|
|
4. Material Requirements for Fully-Bioresorbable Vascular Stents
5. Cardiovascular Applications of Biodegradable Magnesium and Zinc Based Materials
5.1. Criteria for Biodegradable Vascular Stents
5.1.1. Traditional Stents
5.1.2. Required Mechanical Properties
5.2. Magnesium Stents
5.2.1. Mechanical Properties
5.2.2. Biocompatibility and Degradation
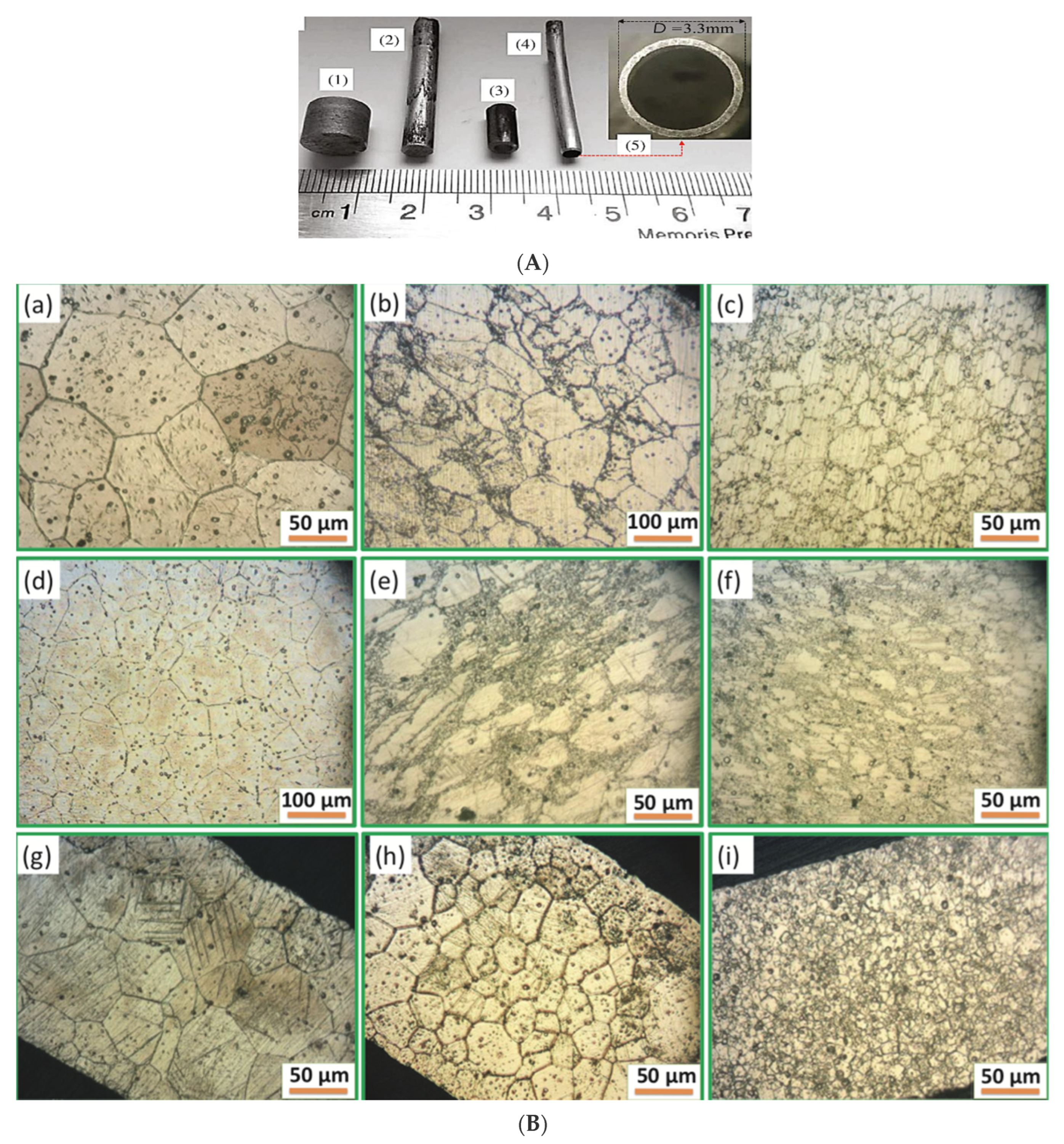
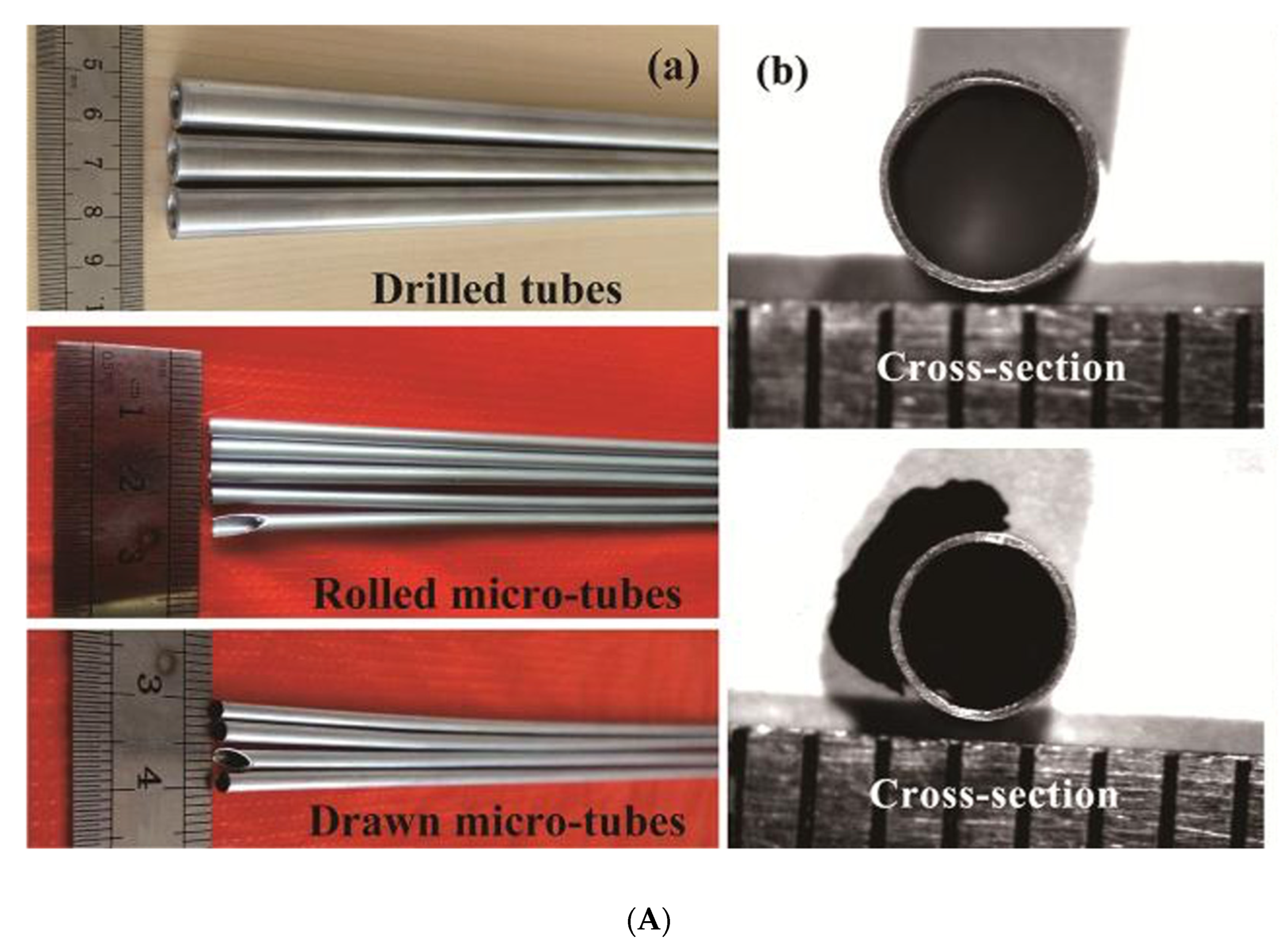
5.3. Zinc Stents
5.3.1. Degradation
5.3.2. Mechanical Properties

5.3.3. Biocompatibility
| References | Alloys | Processes | Dimensions | Mechanical Properties | ||
|---|---|---|---|---|---|---|
| YS (MPa) | UTS (MPa) | EL (%) | ||||
| - | Zn alloys | |||||
| [87] | Zn–0.15Mg | DE + TE | Tube: outer diameter of 4 mm; inner diameter of 1.5 mm | 114 | 250 | 22 |
| Zn–0.5Mg | 159 | 297 | 13 | |||
| Zn–1Mg | 180 | 340 | 6 | |||
| Zn–3Mg | 291 | 399 | 1 | |||
| [28] | Zn–1Mg–1Ca | HR | - | 210 | 260 | 5.5 |
| Zn–1Mg–1Sr | HE | - | 200 | 250 | 7.5 | |
| Zn–1Mg–1Sr | 215 | 265 | 6.8 | |||
| [99] | Zn–1Ca–0.1Sr | HR | Sheet metal: thickness of 2.1 | 196 | 300 | 22.49 |
| [100] | Zn–1Mg–0.1Mn | - | Sheet metal: thickness of 2.1 | 195 | 299 | 26.07 |
| [89] | Zn–1Ca | HE | Rod: diameter of 10 mm | 195 | 240 | 7.5 |
| Zn–1Sr | 220 | 260 | 10.8 | |||
| Zn–1Mg | 205 | 265 | 8.5 | |||
| [87] | Zn–0.5Al | DE + TE | Tube: outer diamter of 4 mm; inner diameter of 1.5 mm | 119 | 203 | 33 |
| Zn–1Al | 134 | 223 | 24 | |||
| [101] | Zn–1Cu | HE | Rod: diameter of 20 mm | 149 | 186 | 21 |
| Zn–2Cu | 199 | 240 | 46.8 | |||
| Zn–3Cu | 213 | 257 | 47.2 | |||
| - | Zn–4Cu | 227 | 270 | 50.6 | ||
| [102] | Zn–3Cu–0.1Mg | HE | Rod: diameter of 20 mm | 340 | 375 | 5 |
| Zn–3Cu–0.5Mg | 400 | 425 | 2 | |||
| Zn–3Cu–1Mg | 425 | 450 | 1 | |||
| [88] | Zn–2.5Ag | HE | Rod: extrusion ratio 14:1 | 155 | 205 | 35 |
| Zn–5.0Ag | 210 | 260 | 38 | |||
| Zn–7.0Ag | 240 | 290 | 32 | |||
| Mg alloys | ||||||
| [103] | AZ31 | DE + CD + CD | Outer diameter of 3 mm, Thickness of 0.18 mm | 172 | - | 16 |
| [104] | Dieless drawing | Outer diameter of 3.35 mm, Thickness of 0.69 mm | - | - | - | |
| [103] | JDBM | DE + CD + CD | Outer diameter of 3 mm, Thickness of 0.18 mm | 123 | - | 26 |
| [74] | - | Double Extrusion | Outer diameter of 3.5 mm, Thickness of 0.18 mm | 220 | 267 | 48.8 |
| [103] | WE43 | DE + CD + CD | Outer diameter of 3 mm, Thickness of 0.18 mm | 113 | - | 10 |
| [80] | CEE + DE + MTE | Outer diameter of 3.3 mm, Thickness of 0.22 mm | - | 410 | 18.5 | |
| [105] | CEE | - | - | 440 at highest | 21 at highest | |
| [105] | Mg–Zn–Y–Nd | ECAE + DE + MTE | Outer diameter of 3.3 mm, Thickness of 0.22 mm | - | 340 | 20 |
| [60] | DE + CD annealing | Outer diameter of 2 mm, Thickness of 0.15 mm | 196 | 298 | 20 | |
| [106] | ZM21 | DE + drilling + HIDE + CD | Outer diameter of 2.9 mm, Thickness of 0.2 mm | - | - | - |
| [107] | DE + drilling + IDE + CD | Outer diameter of 2.9 mm, Thickness of 0.217 mm | - | - | - | |
| [108] | Mg–4Zn–1Y | DE + ECAE | Outer diameter of 2.4 mm, Thickness of 0.4 mm | 340 | 353 | 11.5 |
| HDE + annealing | - | 240 | 330 | 20.4 | ||
6. Translational Research on Cardiovascular Applications
Clinical Trials of Stents
| References | Device | No. of patients | Total Duration (Months) | Outcomes | Identifier |
|---|---|---|---|---|---|
| [129,130,131] | AMS–PROGRESS | 63 | 28 months | Stent totally absorbed after four months. 47% of patients suffered restenosis. No myocardial infarction or late thrombosis occurred. | - |
| [129,130,131] | AMS–INSIGHT | 117 | 12 months | Three patients with one-month complications. Lower patency rate for AMS (31.8%) versus PTA (58.0%). | NCT00572494 |
| [132,133] | DREAMS–BIOSOLVE-I | 46 | 36 months | One myocardial infarction occurred (not stentrelated). No late thrombosis. Late lumen loss was higher than inert drug-eluting stents. LLL lower at 36 months | NCT01168830 |
| [134,135] | DREAMS-2G (Magmaris©)–BIOSOLVEII | 121 | 36 months | No thrombosis after 24 months. Late lumen loss reduced vs. DREAMS. The device was absorbed at 95% after 12 months. | NCT01960504 |
| [135] | DREAMS-2G (Magmaris©)–BIOSOLVEIII | 61 | 36 months | No thrombosis detected. One post-intervention death (not procedure-related) | NCT02716220 |
| [136] | MAGNEZIX© | 26 | 6 months | Outstanding results for ROM, MTPJ, and AOFAS. Equivalent performance to titanium screws for the treatment of mild hallux valgus deformities. | - |
| [137] | RESOMET© (K-MET) | 53 | 12 months | The normal range of grip power. No change in ROM and DASH. At 12 months, screws were completely replaced by new bones. | NCT02456415 |
| [138] | Pure Magnesium | 48 | 12 months | Increased bone flap stability. Serum levels were normal. Two patients suffered collapse of the femoral head. At 12 months, there was a higher Harris hip score than control. | ChiCTR-TRC-13003238 |
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mozafari, M. Handbook of Biomaterials Biocompatibility; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Albrektsson, T.; Berglundh, T.; Lindhe, J. Clinical Periodontology and Implant Dentistry; Blackwell Munksgaard, A Blackwell Publishing Company: Oxford, UK, 2003. [Google Scholar]
- Legeros, R.Z.; Craig, R.G. Strategies to affect bone remodeling: Osteointegration. J. Bone Miner. Res. 1993, 8, S583–S596. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Ratheesh, V.; Manakari, V.; Parande, G.; Gupta, M.; Wong, R. The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Puleo, D.; Nanci, A. Understanding and controlling the bone–implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Gasser, B.; Eschbach, L.; Biehl, V. Metallische Implantatwerkstoffe: Reintitan und Titanlegierungen. OP-J. RMS 2000, 16, 7–11. [Google Scholar]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef]
- Marti, A. Cobalt-base alloys used in bone surgery. Injury 1999, 31, D18–D21. [Google Scholar] [CrossRef]
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105. [Google Scholar] [CrossRef]
- Prasadh, S.; Manakari, V.; Parande, G.; Srivatsan, T.; Wong, R.; Gupta, M. Bioresorbable Nano-Hydroxyapatite Reinforced Magnesium Alloplastic Bone Substitute for Biomedical Applications: A Study, Nanocomposites VI: Nanoscience and Nanotechnology in Advanced Composites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 71–82. [Google Scholar]
- Park, J.B.; Bronzino, J.D. Biomaterials: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kujur, M.S.; Manakari, V.; Parande, G.; Prasadh, S.; Wong, R.; Mallick, A.; Gupta, M. Effect of samarium oxide nanoparticles on degradation and invitro biocompatibility of magnesium. Mater. Today Commun. 2021, 26, 102171. [Google Scholar] [CrossRef]
- Kujur, M.S.; Manakari, V.; Parande, G.; Prasadh, S.; Wong, R.; Mallick, A.; Gupta, M. Development of rare-earth oxide reinforced magnesium nanocomposites for orthopaedic applications: A mechanical/immersion/biocompatibility perspective. J. Mech. Behav. Biomed. Mater. 2021, 114, 104162. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Tang, X.; Gong, T.; Ye, W.; Pan, C.; Ding, H.; Luo, X.; Li, X.; Wang, Q.M. Surface modification with ECM-inspired SDF-1α/laminin-loaded nanocoating for vascular wound healing. ACS Appl. Mater. Interfaces 2017, 9, 30373–30386. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Prasadh, S.; Manakari, V.; Parande, G.; Wong, R.C.W.; Gupta, M. Hollow silica reinforced magnesium nanocomposites with enhanced mechanical and biological properties with computational modeling analysis for mandibular reconstruction. Int. J. Oral Sci. 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Suresh, S.; Ratheesh, V.; Wong, R.; Gupta, M. Biocompatibility of Metal Matrix Composites Used for Biomedical Applications. Encycl. Mater. Compos. 2021, 1, 474–501. [Google Scholar]
- Hou, R.; Victoria-Hernandez, J.; Jiang, P.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.; Yi, S.; Letzig, D.; Feyerabend, F. In vitro evaluation of the ZX11 magnesium alloy as potential bone plate: Degradability and mechanical integrity. Acta Biomater. 2019, 97, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Velazguez, J.; Jimenez, A.; Chomon, B.; Villa, T. Magnesium supplementation and bone turnover. Nutr. Rev. 1999, 57, 227. [Google Scholar]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Wan, Y.; Cui, T.; Li, W.; Li, C.; Xiao, J.; Zhu, Y.; Ji, D.; Xiong, G.; Luo, H. Mechanical and biological properties of bioglass/magnesium composites prepared via microwave sintering route. Mater. Des. 2016, 99, 521–527. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, M.; Gao, J.; Hu, J.; Zhang, Y. Corrosion process of pure magnesium in simulated body fluid. Mater. Lett. 2008, 62, 2181–2184. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Persaud-Sharma, D.; McGoron, A. Biodegradable magnesium alloys: A review of material development and applications, Journal of Biomimetics. Biomater. Tissue Eng. 2011, 12, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F. Zinc deficiency: A special challenge. J. Nutr. 2007, 137, 1101–1105. [Google Scholar] [CrossRef] [Green Version]
- Levy, G.K.; Leon, A.; Kafri, A.; Ventura, Y.; Drelich, J.W.; Goldman, J.; Vago, R.; Aghion, E. Evaluation of biodegradable Zn-1% Mg and Zn-1% Mg-0.5% Ca alloys for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 174. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Gupta, M.; Ling, S.N.M. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Parande, G.; Manakari, V.; Prasadh, S.; Chauhan, D.; Rahate, S.; Wong, R.; Gupta, M. Strength retention, corrosion control and biocompatibility of Mg–Zn–Si/HA nanocomposites. J. Mech. Behav. Biomed. Mater. 2020, 103, 103584. [Google Scholar] [CrossRef]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, A.; Beckmann, E.; Kaufeld, T.; Umminger, J.; Fleissner, F.; Koigeldiyev, N.; Krueger, H.; Puntigam, J.; Haverich, A.; Shrestha, M. Total aortic arch repair: Risk factor analysis and follow-up in 199 patients. Eur. J. Cardio-Thorac. Surg. 2016, 50, 940–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.P.; Huff, C.M.; Roubin, G.S. Vascular disease in the older adult. J. Geriatr. Cardiol. 2016, 13, 727. [Google Scholar] [PubMed]
- Selvaraj, D.; Raja, J.; Prasath, S. Interdisciplinary approach for bilateral maxillary canine: First premolar transposition with complex problems in an adult patient. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. S2), S190. [Google Scholar] [CrossRef]
- Theron, J.G.; Payelle, G.G.; Coskun, O.; Huet, H.F.; Guimaraens, L. Carotid artery stenosis: Treatment with protected balloon angioplasty and stent placement. Radiology 1996, 201, 627–636. [Google Scholar] [CrossRef]
- Bonati, L.H.; Lyrer, P.; Ederle, J.; Featherstone, R.; Brown, M.M. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst. Rev. 2012, 9, CD000515. [Google Scholar] [CrossRef]
- Hiremath, G.; Qureshi, A.M.; Prieto, L.R.; Nagaraju, L.; Moore, P.; Bergersen, L.; Taggart, N.W.; Meadows, J. Balloon Angioplasty and Stenting for Unilateral Branch Pulmonary Artery Stenosis Improve Exertional Performance. JACC Cardiovasc. Interv. 2019, 12, 289–297. [Google Scholar] [CrossRef]
- Rittersma, S.Z.; de Winter, R.J.; Koch, K.T.; Bax, M.; Schotborgh, C.E.; Mulder, K.J.; Tijssen, J.G.; Piek, J.J. Impact of strut thickness on late luminal loss after coronary artery stent placement. Am. J. Cardiol. 2004, 93, 477–480. [Google Scholar] [CrossRef]
- Levy, J.A.; Podeszwa, D.A.; Lebus, G.; Ho, C.A.; Wimberly, R.L. Acute complications associated with removal of flexible intramedullary femoral rods placed for pediatric femoral shaft fractures. J. Pediatric Orthop. 2013, 33, 43–47. [Google Scholar] [CrossRef]
- Onche, I.; Osagie, O.; Nuhu, S.I. Removal of orthopaedic implants: Indications, outcome and economic implications. J. West Afr. Coll. Surg. 2011, 1, 101. [Google Scholar]
- Artang, R.; Dieter, R.S. Analysis of 36 reported cases of late thrombosis in drug-eluting stents placed in coronary arteries. Am. J. Cardiol. 2007, 99, 1039–1043. [Google Scholar] [CrossRef]
- Buchanan, K.; Steinvil, A.; Waksman, R. Does the new generation of drug-eluting stents render bare metal stents obsolete? Cardiovasc. Revascularization Med. 2017, 18, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Filion, K.B.; Roy, A.M.; Baboushkin, T.; Rinfret, S.; Eisenberg, M.J. Cost-effectiveness of drug-eluting stents including the economic impact of late stent thrombosis. Am. J. Cardiol. 2009, 103, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Hsieh, W.-h.; Massaro, J.M.; Ho, K.K.; D’Agostino, R.; Cutlip, D.E. Stent thrombosis in randomized clinical trials of drug-eluting stents. New Engl. J. Med. 2007, 356, 1020–1029. [Google Scholar] [CrossRef]
- Pleva, L.; Kukla, P.; Hlinomaz, O. Treatment of coronary in-stent restenosis: A systematic review. J. Geriatr. Cardiol. 2018, 15, 173. [Google Scholar] [PubMed]
- Waksman, R. A new generation of drug-eluting stents: Indications and outcomes of bioresorbable vascular scaffolds. Clevel. Clin. J. Med. 2017, 84, e20–e24. [Google Scholar] [CrossRef] [PubMed]
- Erne, P.; Schier, M.; Resink, T.J. The road to bioabsorbable stents: Reaching clinical reality? Cardiovasc. Interv. Radiol. 2006, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Waksman, R.; Pakala, R. Biodegradable and bioabsorbable stents. Curr. Pharm. Des. 2010, 16, 4041–4051. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Xi, Y.L.; Chai, D.L. In vitro degradation and cytotoxicity of Mg/Ca composites produced by powder metallurgy. Acta Biomater. 2010, 6, 1783–1791. [Google Scholar] [CrossRef]
- Babaji, P.; Singh, A.; Lau, H.; Lamba, G.; Somasundaram, P. Deletion of short arm of chromosome 18, Del (18p) syndrome. J. Indian Soc. Pedod. Prev. Dent. 2014, 32, 68. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable metals for cardiovascular stents: From clinical concerns to recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poncin, P.; Proft, J. Stent Tubing: Understanding the desired attributes. In Medical Device Materials: Proceedings of the Materials & Processes for Medical Devices Conference; ASM International: Materials Park, OH, USA, 2004; pp. 253–259. [Google Scholar]
- Wang, J.; Zhou, Y.; Yang, Z.; Zhu, S.; Wang, L.; Guan, S. Processing and properties of magnesium alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C 2018, 90, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary stents: Historical development, current status and future directions. Br. Med. Bull. 2013, 106, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Köster, R.; Vieluf, D.; Kiehn, M.; Sommerauer, M.; Kähler, J.; Baldus, S.; Meinertz, T.; Hamm, C.W. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 2000, 356, 1895–1897. [Google Scholar] [CrossRef]
- Iijima, R.; Ikari, Y.; Amiya, E.; Tanimoto, S.; Nakazawa, G.; Kyono, H.; Hatori, M.; Miyazawa, A.; Nakayama, T.; Aoki, J. The impact of metallic allergy on stent implantation: Metal allergy and recurrence of in-stent restenosis. Int. J. Cardiol. 2005, 104, 319–325. [Google Scholar] [CrossRef]
- Camenzind, E.; Steg, P.G.; Wijns, W. Response to Camenzind et al. Circulation 2007, 115, 1440–1455. [Google Scholar] [CrossRef] [Green Version]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Circ. Cardiovasc. Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Hermawan, H.; Dubé, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, B.; Cai, Z. Recent Progress on Mg-and Zn-Based Alloys for Biodegradable Vascular Stent Applications. J. Nanomater. 2019, 2019, 1310792. [Google Scholar] [CrossRef]
- Hu, T.; Yang, C.; Lin, S.; Yu, Q.; Wang, G. Biodegradable stents for coronary artery disease treatment: Recent advances and future perspectives. Mater. Sci. Eng. C 2018, 91, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Luffy, S. Magnesium Alloys for Use as an Intraluminal Tracheal Stent; University of Pittsburgh: Pittsburgh, PA, USA, 2013. [Google Scholar]
- Luffy, S.A.; Wu, J.; Kumta, P.N.; Gilbert, T.W. Evaluation of magnesium alloys for use as an intraluminal tracheal for pediatric applications in a rat tracheal bypass model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1844–1853. [Google Scholar] [CrossRef]
- Lee, J.; Han, P.; Song, E.; Kim, D.; Lee, H.; Labowsky, M.; Taavitsainen, J.; Ylä-Herttuala, S.; Hytönen, J.; Gülcher, M. Magnetically coated bioabsorbable stents for renormalization of arterial vessel walls after stent implantation. Nano Lett. 2018, 18, 272–281. [Google Scholar] [CrossRef]
- Li, G.; Wang, N.; Li, X.; Ma, N.; Liu, T.; Sun, Y.; Liu, P.; Miao, Z.; Zhang, Y. Balloon-mounted versus self-expanding stent outcomes in symptomatic middle cerebral artery stenosis combined with poor collaterals in China: A multicenter registry study. World Neurosurg. 2019, 124, e675–e681. [Google Scholar] [CrossRef]
- Lu, W.; Yue, R.; Miao, H.; Pei, J.; Huang, H.; Yuan, G. Enhanced plasticity of magnesium alloy micro-tubes for vascular stents by double extrusion with large plastic deformation. Mater. Lett. 2019, 245, 155–157. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, A.; Drelich, J.; Goldman, J. Rates of in vivo (arterial) and in vitro biocorrosion for pure magnesium. J. Biomed. Mater. Res. Part A 2015, 103, 341–349. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility. Bioact. Mater. 2020, 5, 611–623. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Xu, M.; Hu, Y.-D.; Gao, F.; Gong, T.; Liu, T.; Li, X.; Pan, C.-J. Biofunctionization of biodegradable magnesium alloy to improve the in vitro corrosion resistance and biocompatibility. Appl. Surf. Sci. 2018, 451, 20–31. [Google Scholar] [CrossRef]
- Pan, C.-J.; Hou, Y.; Wang, Y.-N.; Gao, F.; Liu, T.; Hou, Y.-H.; Zhu, Y.-F.; Ye, W.; Wang, L.-R. Effects of self-assembly of 3-phosphonopropionic acid, 3-aminopropyltrimethoxysilane and dopamine on the corrosion behaviors and biocompatibility of a magnesium alloy. Mater. Sci. Eng. C 2016, 67, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Amani, S.; Faraji, G.; Mehrabadi, H.K.; Abrinia, K.; Ghanbari, H. A combined method for producing high strength and ductility magnesium microtubes for biodegradable vascular stents application. J. Alloys Compd. 2017, 723, 467–476. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Drelich, A.J.; Zhao, S.; Guillory, R.J., II; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Buxbaum, R.E.; Rajachar, R.M.; Goldman, J. New approaches in evaluating metallic candidates for bioabsorbable stents. Emerg. Mater. Res. 2012, 1, 237–255. [Google Scholar] [CrossRef]
- Bowen, P.K.; Seitz, J.M.; Guillory, R.J.; Braykovich, J.P.; Zhao, S.; Goldman, J.; Drelich, J.W. Evaluation of wrought Zn–Al alloys (1, 3, and 5 wt% Al) through mechanical and in vivo testing for stent applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 245–258. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed ZnAg alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, X.; Zheng, Y.; Cong, Y.; Zhou, F.; Qiu, K.; Wang, X.; Chen, S.; Huang, L.; Tian, L. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ren, Y.; Wang, L.; Yang, B.; Li, H.; Qin, G. Abnormal effect of Mn addition on the mechanical properties of as-extruded Zn alloys. Mater. Sci. Eng. A 2017, 701, 129–133. [Google Scholar] [CrossRef]
- Edwards, G.R.; Shyne, J.C.; Sherby, O.D. Strain softening in powder metallurgy zinc. Metall. Trans. 1971, 2, 2955–2958. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Z.; Cui, Y.; Zhang, Y.; Yu, S.; Qu, G.; Gong, H. Processing of a novel Zn alloy micro-tube for biodegradable vascular stent application. J. Mater. Sci. Technol. 2016, 32, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Amani, S.; Faraji, G. Processing and properties of biodegradable magnesium microtubes for using as vascular stents: A brief review. Met. Mater. Int. 2019, 25, 1341–1359. [Google Scholar] [CrossRef]
- García-Mintegui, C.; Córdoba, L.C.; Buxadera-Palomero, J.; Marquina, A.; Jiménez-Piqué, E.; Ginebra, M.-P.; Cortina, J.L.; Pegueroles, M. Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef]
- Shearier, E.R.; Bowen, P.K.; He, W.; Drelich, A.; Drelich, J.; Goldman, J.; Zhao, F. In vitro cytotoxicity, adhesion, and proliferation of human vascular cells exposed to zinc. ACS Biomater. Sci. Eng. 2016, 2, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn–Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Bosman, W.; van der Burg, B.B.; Schuttevaer, H.; Thoma, S.; Joosten, P.P.H. Infections of intravascular bare metal stents: A case report and review of literature. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sun, J.; Yang, Y.; Zhou, F.; Pu, Z.; Li, L.; Zheng, Y. Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn–Mg–Sr alloys as biodegradable metals. Mater. Lett. 2016, 162, 242–245. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn–Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Niu, J.; Pei, J.; Zhang, H.; Huang, H.; Yuan, G. The processing of Mg alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C 2015, 48, 400–407. [Google Scholar] [CrossRef]
- Furushima, T.; Manabe, K.-I. Large reduction die-less mandrel drawing of magnesium alloy micro-tubes. CIRP Ann. 2018, 67, 309–312. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G.; Mehrabadi, H.K.; Baghani, M. Manufacturing and mechanical characterization of Mg-4Y-2Nd-0.4 Zr-0.25 La magnesium microtubes by combined severe plastic deformation process for biodegradable vascular stents. Proc. Inst. Mech. Eng. Part B: J. Eng. Manuf. 2019, 233, 1196–1205. [Google Scholar] [CrossRef]
- Wang, L.; Fang, G.; Qian, L.; Leeflang, S.; Duszczyk, J.; Zhou, J. Forming of magnesium alloy microtubes in the fabrication of biodegradable stents. Prog. Nat. Sci. Mater. Int. 2014, 24, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.; Ai, W.-J.; Leeflang, S.; Duszczyk, J.; Zhou, J. Multipass cold drawing of magnesium alloy minitubes for biodegradable vascular stents. Mater. Sci. Eng. C 2013, 33, 3481–3488. [Google Scholar] [CrossRef]
- Ge, Q.; Dellasega, D.; Demir, A.G.; Vedani, M. The processing of ultrafine-grained Mg tubes for biodegradable stents. Acta Biomater. 2013, 9, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Parande, G.; Gupta, M.; Wong, R. Compositional Tailoring of Mg–2Zn–1Ca Alloy Using Manganese to Enhance Compression Response and In-Vitro Degradation. Materials 2022, 15, 810. [Google Scholar] [CrossRef] [PubMed]
- Schildwächter, M. Biotronik Announces CE Mark for Magmaris, the First Clinically-Proven Bioresorbable Magnesium Scaffold. J. Invasive Cardiol. 2016. Available online: https://news.biotronik.com/biotronik-announces-ce-mark-for-magmaris-the-first-clinically-proven-bioresorbable-magnesium-scaffold/ (accessed on 12 April 2022).
- Chen, J.; Tan, L.; Yang, K. Recent advances on the development of biodegradable magnesium alloys: A review. Mater. Technol. 2016, 31, 681–688. [Google Scholar] [CrossRef]
- Kitabata, H.; Waksman, R.; Warnack, B. Bioresorbable metal scaffold for cardiovascular application: Current knowledge and future perspectives. Cardiovasc. Revascularization Med. 2014, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bühler, D. Press release BIOTRONIK Announces Completion of Enrollment in SFA Arm of the BIOFLEX-I Study. Available online: https://news.biotronik.com/press-release--biotronik-announces-completion-of-enrollment-in-sfa-arm-of-the-bioflex-i-study/ (accessed on 12 April 2022).
- Gerold, B. Implant Made of a Biodegradable Magnesium Alloy. U.S. Patent No. 8,915,953, 23 December 2014. [Google Scholar]
- Savage, D.R.; Nguyen, J.D. Biodegradable Metal Stent and Method of Making. U.S. Patent No. 15/315,846, 6 April 2017. [Google Scholar]
- Prasadh, S.; Krishnan, A.V.; Lim, C.; Gupta, M.; Wong, R. Titanium versus magnesium plates for unilateral mandibular angle fracture fixation: Biomechanical evaluation using 3-dimensional finite element analysis. J. Mater. Res. Technol. 2022, 18, 2064–2076. [Google Scholar] [CrossRef]
- Prasadh, S.; Raguraman, S.; Wong, R.; Gupta, M. Metallic Foams in Bone Tissue Engineering, Nanoscale Engineering of Biomaterials: Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 181–205. [Google Scholar]
- Zartner, P.; Cesnjevar, R.; Singer, H.; Weyand, M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby; Catheter. Cardiovasc. Interv. 2005, 66, 590–594. [Google Scholar]
- Zartner, P.; Buettner, M.; Singer, H.; Sigler, M. First biodegradable metal stent in a child with congenital heart disease: Evaluation of macro and histopathology; Catheter. Cardiovasc. Interv. 2007, 69, 443–446. [Google Scholar] [CrossRef]
- McMahon, C.J.; Oslizlok, P.; Walsh, K.P. Early restenosis following biodegradable stent implantation in an aortopulmonary collateral of a patient with pulmonary atresia and hypoplastic pulmonary arterie. Catheter. Cardiovasc. Interv. 2007, 69, 735–738. [Google Scholar] [CrossRef]
- Schranz, D.; Zartner, P.; Michel-Behnke, I.; Akintürk, H. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheter. Cardiovasc. Interv. 2006, 67, 671–673. [Google Scholar] [CrossRef]
- Maeng, M.; Jensen, L.O.; Falk, E.; Andersen, H.R.; Thuesen, L. Negative vascular remodelling after implantation of bioabsorbable magnesium alloy stents in porcine coronary arteries: A randomised comparison with bare-metal and sirolimus-eluting stents. Heart 2009, 95, 241–246. [Google Scholar] [CrossRef]
- Wittchow, E.; Adden, N.; Riedmüller, J.; Savard, C.; Waksman, R.; Braune, M. Bioresorbable drug-eluting magnesium-alloy scaffold: Design and feasibility in a porcine coronary model. EuroIntervention 2013, 8, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Robinson, K.A.; Crocker, I.R.; Wang, C.; Gravanis, M.B.; Cipolla, G.D.; Hillstead, R.A.; King, S.B., III. Intracoronary low-dose β-irradiation inhibits neointima formation after coronary artery balloon injury in the swine restenosis model. Circulation 1995, 92, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Shen, L.; Niu, J.; Zhang, J.; Ding, W.; Wu, Y.; Fan, R.; Yuan, G. Nanophasic biodegradation enhances the durability and biocompatibility of magnesium alloys for the next-generation vascular stents. Nanoscale 2013, 5, 9517–9522. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhou, H.; Chen, L.; Niu, J.; Zhang, L.; Yuan, G.; Song, C. Enhanced biocompatibility and long-term durability in vivo of Mg-Nd-Zn-Zr alloy for vascular stent application. J. Alloys Compd. 2017, 720, 245–253. [Google Scholar] [CrossRef]
- Mao, L.; Chen, J.; Zhang, X.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.; Song, C. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, B.; Wang, P.; Wang, X.; Zhang, B.; Shi, Q.; Xi, T.; Chen, M.; Guan, S. Enhanced in vitro and in vivo performance of Mg–Zn–Y–Nd alloy achieved with APTES pretreatment for drug-eluting vascular stent application. ACS Appl. Mater. Interfaces 2016, 8, 17842–17858. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar] [CrossRef]
- Waksman, R.; Erbel, R.; Bonnier, H.; Di Mario, C.; Wijns, W.; Weissman, N.J. In Proceedings of the 19th Annual Transcatheter Cardiovascular Therapeutics Symposium, Washington, DC, USA, 20–25 October 2007.
- Waksman, R.; Erbel, R.; di Mario, C.; Bartunek, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C. Early-and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2009, 2, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Vermeersch, P.; Weissman, N.; Prati, F.; Bruining, N.; Waksman, R. Safety and performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. EuroIntervention: J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2016, 12, e160–e166. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Kische, S.; Abizaid, A.; Tölg, R.; Alves, L.P.; van Mieghem, N.; Verheye, S.; von Birgelen, C.; Christiansen, E. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: Pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention 2017, 13, 432–439. [Google Scholar]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasadh, S.; Raguraman, S.; Wong, R.; Gupta, M. Current Status and Outlook of Temporary Implants (Magnesium/Zinc) in Cardiovascular Applications. Metals 2022, 12, 999. https://doi.org/10.3390/met12060999
Prasadh S, Raguraman S, Wong R, Gupta M. Current Status and Outlook of Temporary Implants (Magnesium/Zinc) in Cardiovascular Applications. Metals. 2022; 12(6):999. https://doi.org/10.3390/met12060999
Chicago/Turabian StylePrasadh, Somasundaram, Sreenivas Raguraman, Raymond Wong, and Manoj Gupta. 2022. "Current Status and Outlook of Temporary Implants (Magnesium/Zinc) in Cardiovascular Applications" Metals 12, no. 6: 999. https://doi.org/10.3390/met12060999
APA StylePrasadh, S., Raguraman, S., Wong, R., & Gupta, M. (2022). Current Status and Outlook of Temporary Implants (Magnesium/Zinc) in Cardiovascular Applications. Metals, 12(6), 999. https://doi.org/10.3390/met12060999








