Abstract
The existence of adsorbed oxygen and oxides on the surface of initial powders has serious effects on the microstructure and mechanical properties of the powder metallurgy alloys. However, the powder surface is inevitably oxidized immediately after the powder preparation. In this work, the oxidation characteristics for the argon atomized powders of a Ni-based superalloy containing Cr, Co, W, Mo, Nb, Ti and Al after exposure at ambient condition for various time were investigated in detail. It is found that various gases can be absorbed on the powder surface, but most of them can be removed by low temperature (<151.5 °C) outgassing procedure. The thermodynamic calculation shows that the oxidation reaction occurs firstly with the alloying elements rather than Ni matrix, whether at room temperature or elevated temperature. The kinetic measurement indicates that the oxygen content on the powder surface approaches a saturation value after 24 h exposure and remains almost stable after 720 h. The oxygen content increases with the decrease of particle size after exposure. X-ray photoelectron spectroscopy characterized that, except the formed oxides, adsorbed oxygen also exists on the powder surface of the as-atomized initial fine powders with particle size <30 μm and the powders with size >18.7 μm after exposure, which may be caused by the internal stress and surface energy of the initial atomized powder. All alloying elements except Ti can form stable oxides directly on the powder surface. For the element of Ti, the metastable TiO forms on the initial powder surface after preparation and it transforms into stable TiO2 or Ti2O3 during exposure. The results provide a deep understanding of absorbed gases and oxide on the surface of powders under treatment and possible desorption approach.
1. Introduction
Powder metallurgy (PM) Ni-based superalloys with working temperature up to 750 °C are ideal materials for turbine disk in the advanced aero-engine [1,2]. Prior particle boundary (PPB) is one of the defects of PM alloys, which has serious effects on the performance of PM parts at high temperatures [3,4,5,6]. Prakash et al. [7] investigated the microstructure and mechanical properties of PM Ni-based superalloy APK-1 and found that the existence of PPB network is detrimental to mechanical properties. Zhou et al. [8] investigated the influences of different grades of PPB in PM FGH96 superalloy during high-cycle fatigue test at 550 °C and found that the presence of PPB reduces the fracture toughness of superalloy. In the fast crack growth zone and the transient zone, the transgranular and long PPB globular surfaces are the main fracture characteristics of the superalloy with serious PPB. These results indicate that PPB is often the source of cracks [7,8] and it has a significant impact on the crack initiation and propagation of the superalloys in service. The origin of PPB has been well investigated, and most researchers [9,10,11] believe that the formation of PPB is mainly due to the existence of adsorbed oxygen or metal oxides on the surface of the powders, resulting in the poor metallurgically bonded interface between the powders during the consolidation process such as hot isostatic pressing (HIP) [3,4].
At present, argon atomization is one of the main approaches for preparing superalloy powders [12,13]. Tan et al. [14] investigated the element distribution of as-prepared Ni-based superalloy powders and found that O, C, and Ti elements are segregated on the powder surface after atomization. The oxygen on the powder surface mainly comes from the oxides formed during atomization process or the oxygen absorbed from external atmosphere. Liu et al. [15] investigated the relationship between surface morphology and oxygen content of argon atomized Ni-based superalloy powders. They found that the surface oxygen content of the powders containing satellite powder is higher than that of smooth powder. In the former a nano oxide layer is formed on the surface, while in the latter, the surface oxygen content mostly comes from the physically adsorbed oxygen. Gao et al. [16] found that the oxygen content of superalloy powder increases with the decrease of particle size and the increase of specific surface area. Xu et al. [17] investigated the influence of temperature on the oxidation behavior of the Ni-based superalloy powders. They found that when the powders were heated at 50~250 °C in muffle furnace at atmospheric gas for 2 h, the oxygen content increases sharply over 150 °C and the powder surface was completely oxidized at 250 °C. Therefore, they suggested that the powder treatment temperature should not be higher than 150 °C if the powder may be exposed to air.
The previous works mentioned above are mainly focused on the influences of powder morphology and heating temperature on the oxygen content, but there is less investigated on the oxidation characteristics of different alloy elements in powder. It was reported that the alloying elements Ti and Cr in the Ni-based alloys are easily oxidized to form chromium and titanium oxides even at room temperature, according to thermodynamics and kinetics [18,19]. Once the oxides appeared on the surface of powder, PPB will be easily formed during the hot forming stage, which affect the structure and mechanical properties of PM parts. Although many measures have been taken to avoid the oxidation of the powder, the oxidation seems inevitable during powder preparation and post-treatment such as electrostatic separation, vacuum degassing, and storage, since it is impossible to completely prevent the powder from contacting the air. It is generally accepted that the oxygen in the initial powder will be inherited to the final PM alloy parts, and high oxygen content in the powder will lead to high oxygen content in the hot formed superalloy. Therefore, it is necessary to understand the surface characteristics change after the powder is kept at ambient condition.
However, up to now, there has been little research work on the surface oxidation behavior of the superalloy powders. A deep understanding of the adsorbed gases and the oxidation of the powder surface at ambient condition, especially the states of oxygen and oxide, is still required. In this work, the absorption and desorption of various gases on a Ni-based superalloy powder are analyzed. The oxidation characteristics of the powder surface after exposure at ambient condition were investigated by thermodynamic and kinetic approaches together with surface characterizations. This work provides a reference for taking effective measures to reduce the gas content in the superalloy powder and improve the quality of PM parts.
2. Experimental
The nickel-based superalloy powders with normal composition of 16.14Cr, 12.06Co, 4.35W, 3.96Mo, 3.72Ti, 2.23Al, 0.75Nb, and rest Ni (by wt.%) were prepared by argon atomization. The powders were mechanically sieved under argon atmosphere to obtain various particle sizes i.e., <15 μm, 18.7–30 μm, 37.5–55.5 μm, 60–75 μm, and 100–150 μm. The powders with different size distributions were exposed at ambient condition for 24, 120, 240, 480, 720, 1080, and 1440 h, and their oxygen contents were tested by ONH836 oxygen, nitrogen, and hydrogen analyzer. Thermogravimetric-mass spectrometry (TG-MS) [20] with 5 K/min temperature rise rate was used to characterize the type and content of absorbed gas on the powder surface. The surface morphology and chemical composition of the powders were characterized by scanning electron microscope (SEM, Quanta 200, FEI Company, Eindhoven, Netherlands) with energy dispersive spectrometer (EDS, X-Max N, Oxford Instruments plc, Oxfordshire, UK). The chemical state and composition of the powder surface were analyzed by X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD, Kratos Ltd., Manchester, UK) at 15 kV and 5 mA.
3. Results and Discussion
3.1. Analysis of Adsorbed Gas on the Initial Powder Surface
The initial powders were received after argon atomization. The vacuum storage and vacuum packing are employed to avoid contact with air as much as possible. Figure 1a shows the thermogravimetric analysis (TGA) curve for the as-prepared powder. Figure 1b–g gives the desorption curves for various gases measured by the mass spectrograph (MS) linked with thermal gravimetric analyzer. The relative pressure in Figure 1b–g indicates the amount of gas escaping from the surface of the powder in MS. Figure 1 shows that the powder surface adsorbs not only O2, but also N2, H2O, and a small amount of CH4, NH3, and CO2 at ambient temperature. This is consistent with the research results obtained by Guo et al. [21] and Zhao et al. [22].
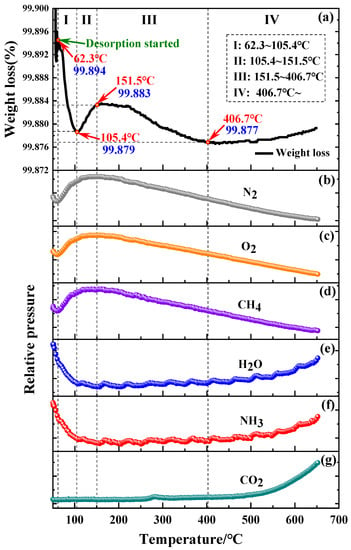
Figure 1.
Thermogravimetric analysis curve of as-prepared powder (a) and desorption curves of adsorbed gases on the powder surface (b–g).
In Figure 1b–d, the adsorbed N2, O2 and CH4 start to desorb at 62.3 °C and continue to desorb as the heating temperature increases. However, the TGA curve in Figure 1a does not monotonically increase or decrease, and it falls and rises repeatedly. This should be the combined effect of degassing and oxidation on the powder surface. According to the change of weight gain, the whole degassing process can be divided into 4 stages, as indicated in Figure 1a. At stage I (62.3~105.4 °C), the powder shows a significant weight loss (about 0.015%), which is mainly caused by the desorption of N2, O2 and CH4. These desorptions reach their maximum values at about 105.4 °C. At stage II (105.4~151.5 °C), the powder shows a slight weight gain (about 0.004%) due to the slight oxidation and the formation of NH3·H2O adsorbed on powder surface. At stage III (151.5~406.7 °C), the powder shows a slight weight loss (about 0.006%) due to the desorption of H2O, NH3, and CO2. At stage IV (above 406.7 °C), CO2 starts to desorb significantly, but the oxidation of the metallic elements occurs on the surface of the powder, resulting in continuous weight gain.
Figure 1 also shows that the weight gain occurs in stage II and the continuous weight loss occurs in stage III. As we know, ammonia is easily combined with water and water vapor on the powder surface to form NH3·H2O, which makes the adsorption and desorption of NH3 and H2O occur at the same time. Therefore, the forming and adsorption of NH3·H2O on the powder surface leads to the weight gain of powder at stage II. As temperature increases, Hu et al. [23] believe that water vapor begins to desorb at 140~150 °C, and the maximum desorption temperature is 400 °C, which is basically consistent with the powder weight loss at stage III in TGA curve. In addition, when the temperature exceeds 151.5 °C, the decomposition of NH3·H2O is intensified. As shown in Figure 1e,f, NH3 and H2O are desorbed simultaneously, which causes the weight loss at stage III.
Figure 1 also shows that the amount of CO2 desorption increases at stage III and stage IV, mainly because the CO2 adsorbed on the powder surface begins to desorb from 200 °C. The desorption amount increased with the increase of temperature. It is also possible that the carbon adsorbed on the powder surface reacts with oxygen in the chamber to form C=O, which will further form CO2 and desorb from the powder surface [22].
According to Figure 1b–g, there are 6 kinds of adsorbed gases on the initial powder surface. However, for O2, N2, and H2O, the relative contents of other adsorbed gases are quite low, which has a limited effect on the performance of the alloy and the PM parts. Additionally, based on the TGA curve and desorption curves, it can be found that most of the adsorbed gases on the initial powder surface can be removed by vacuum desorption near 151.5 °C, especially N2, O2, and H2O. This result gives us a suggestion that we can reduce the adsorbed gases on the powder surface by low temperature treatment with various approaches.
Just as the carbon can be oxidized to form CO2 and desorbed from the powder surface at over 200 °C, even under the protection of argon, it was also reported that the alloying elements of Al, Ti, and Cr can be oxidized near the melting temperature of alloy (around 1300 °C), as even the partial pressure of O2 in the gas phase is only 10−10 Pa [24]. Therefore, we must pay attention to the effect of oxygen on alloy powders, since the partial pressure of O2 in the air reaches 2.13 × 104 Pa and it can react with many alloying elements even at the ambient temperature. The oxidation behavior of the powder, thus, should be understood.
3.2. Thermodynamics Calculation of Oxidation Processes
Figure 2 shows the Gibbs free energy curves for various oxidation reactions, which may occur in Ni-based superalloy. The possible reactions and the Gibbs free energy–temperature relationships for various alloying elements are shown below as Equations (1)–(11) [25].
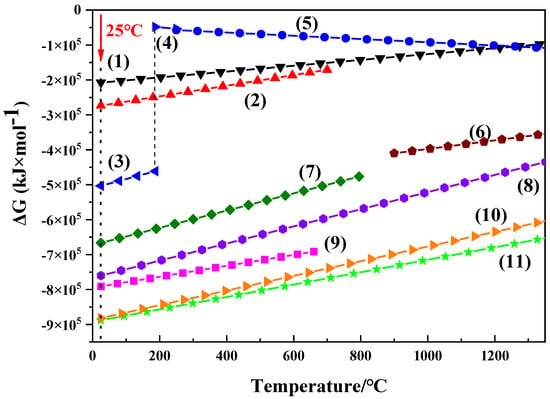
Figure 2.
Relationship between Gibbs free energy-temperature for possible oxidation reactions in superalloy.
The thermodynamic results indicate that the stable oxides, such as Nb2O5, Al2O3, TiO2, WO3, MoO3, Co3O4, and NiO, can be formed on the surface of superalloy powder at room temperature. In particular, Cr is oxidized to form CrO3 firstly at ambient temperature. CrO3 can be decomposed to CrO2 at 190–197 °C and converts to Cr2O3 completely at 250 °C. Cr can also be oxidized into Cr2O3 directly above 900 °C. The trend of oxidation reactions occurred at ambient temperature is (11) > (10) > (9) > (8) > (7) > (3) > (2) > (1) in Figure 2, and therefore, the oxidation tendency for alloying elements is Ti > Nb > Al > W > Mo > Cr > Co > Ni. The results clearly indicate that the oxidation occurs firstly with the alloying elements rather than Ni matrix, whether it is at room temperature or elevated temperatures. However, it has to be noted that the above results are calculated under equilibrium condition. Some metastable phases may be formed during oxidation through non-equilibrium reactions, such as TiO [25].
3.3. Oxidation Kinetics of Powder at Ambient Temperature
To further study the oxidation kinetics of the superalloy powders, the relationships between the oxygen content and exposure time at ambient temperature for the powders with different particle sizes are investigated. The oxygen content of alloy powders was tracked and measured by an oxygen, nitrogen, and hydrogen analyzer, and the results are shown in Figure 3. The data of 0 h exposure indicates that the oxygen content is measured on the initial powder without exposure. It was tested immediately after the powder was taken out from the vacuum container. With the increase of exposure time, two different types of oxidation characteristics for different particle sizes are observed. The oxygen content of powders with a particle size of 100–150 μm are kept stable below 50 ppm, but the oxygen content in the powders with a particle size below 75 μm increases rapidly in the first 24 h exposure and then increases slowly. In particular, for the powders with a particle size below 15 μm, the oxygen content increases from 220 ppm to 360 ppm after 24 h exposure with 63.6% increment, and it reaches 426.7 ppm after 720 h exposure with ~94% increase. In addition, for all powders, the oxygen content remains relatively stable after 720 h exposure.
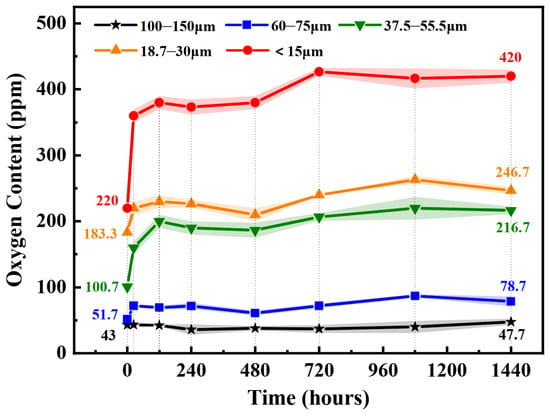
Figure 3.
Oxygen contents of the studied superalloy powder after exposure for different time in air.
Figure 4 shows the powder surface morphologies of the powders with particle size about 50 μm after 0 h, 720 h, and 1440 h exposure. There is no significant difference for various exposure time. The composition analysis indicated that the elements distributions on the powder surface are uniform, and the different areas of the same powder have only slight composition fluctuation. The surface composition of the powders with different particle sizes for different exposure time in air has been characterized by EDS, but no oxygen elements can be detected for all powders due to the detection limitation of EDS, which indicates the oxidation layer is extremely thin. The result also suggests that the superalloy powder has relatively good antioxidant properties at ambient temperature.
Figure 4.
Surface morphology of superalloy powder for different exposure time in air: (a) 0 h; (b) 720 h; (c) 1440 h.
3.4. Characterization of the State of Oxygen
Although SEM and EDS analysis failed to detect the oxides and oxygen on the powder surface, TG-MS analysis, thermodynamic calculation, and kinetic experiments all showed that the oxygen or oxides must exist at ambient temperature. In order to accurately analyze the oxide states of different alloy elements on the superficial layer of the powder, XPS was used for further characterization. The XPS spectra of the initial powder and the powder exposed for 720 h are shown in Figure 5. The results show that oxides already exist on the surface of both initial powder and exposed powder. The valence states that all elements (Al, Co, Cr, Mo, Ni, W, and Nb) on the powders with different particle sizes and exposure time have no significant difference. It indicates that these alloying elements can form similar oxides before and after exposure. However, there are two special cases—one is oxygen and the other is titanium.
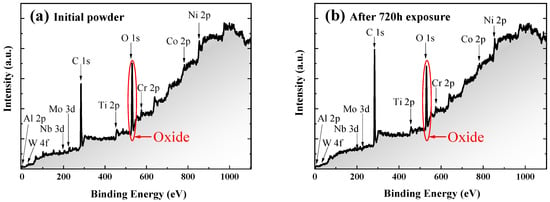
Figure 5.
The overall XPS spectra of (a) the initial powder and (b) the powder after 720 h exposure.
Figure 6 shows the local magnifications of oxygen XPS spectra for initial powder and the powders after 720 h exposure. Figure 6a shows the oxygen XPS spectrum of the initial powder with particle size larger than 37.5 μm. The peaks located at near 529.5 eV and 531.3 eV are those of negative divalent oxygen (O2−) [26]. The appearance of these peaks indicates that the oxygen on the powder surface exists in oxidation state. All possible oxides in this study are shown in the figure, including NiO, Cr2O3, Co3O4, TiO2, Ti2O3, MoO2, MoO3, WO2, WO3, Nb2O5, and Al2O3. However, in Figure 6b, the oxygen XPS for the initial powder with a particle size below 30 μm has not only the peaks near 529.8 eV and 532.0 eV, but also a characteristic peak of adsorbed oxygen near 533.7 eV. It is revealed that oxygen on the surface of small particle powders exists in two states, including oxidation and adsorption.
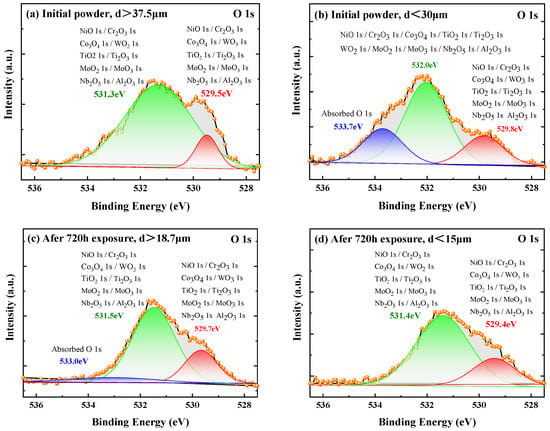
Figure 6.
Surface oxidation states of initial powders with particle sizes of (a) above 37.5 μm; (b) below 30 μm, and of the powders after 720 h exposure with particle size of (c) above 18.7 μm; (d) below 15 μm.
Figure 6c,d shows that the peaks of oxygen on the surface of powder after 720 h exposure. These peaks are different from those of initial powder. There is adsorbed oxygen peak at about 533.0 eV observed for the sample of large particle powder (>18.7 μm), while the absorbed oxygen peak disappeared for the small powder (<15 μm). The changed oxidation state can be explained from the powder preparation process, and the thermodynamic and kinetic properties of powder during exposure. The superalloy powder was prepared by argon atomization in this work. The cooling rate of small particles, such as less than 15 μm, is one order of magnitude higher than that of large particles [27]. The surface of small particle powder suffers greater internal stress, and the alloying elements on the surface are in a higher energy state [14]. Hence, the oxidation easily occurs on the surface of small powders. On the other hand, small particle powders generally have a larger specific surface than large particle powders [16]. The larger specific surface and the existence of oxygen during preparation and storage make it possible for physical adsorption of oxygen on the fine powder surface [15].
As mentioned, the alloying elements on small particle powder surface are in a high energy state due to rapid cooling. With the extension of exposure or storage time, the alloying elements can react with the adsorbed oxygen rapidly to form oxides. Therefore, the adsorbed oxygen peak disappeared in Figure 6d. However, the energy state of alloying elements on the coarse powder surface is lower and oxidation rate is lower [27]. There are not only oxides formed on the coarse powder, but also a small amount of adsorbed oxygen appeared. This is why the adsorbed oxygen peak appears in Figure 6c. In the present results, the change of surface oxygen state before and after exposure for the powders with different particle sizes indicates that the reaction between oxygen and alloy elements are related to the particle size. Hence, by appropriately reducing the proportion of small particle powder, the adsorbed oxygen on the initial powder surface can be controlled to prevent oxidation during storage. Alternatively, the low temperature desorption treatment mentioned above can be used to remove the adsorbed oxygen on initial powder surface, which can effectively prevent the oxidation problem.
3.5. Characterization of the Oxidation state of Titanium
Titanium is an active metal with various chemical valence states. It can form metastable or stable oxides at ambient temperature, which depends on the oxygen potential at the gas/solid interface and along the inter-diffusion zone [28]. Figure 7a,b shows the titanium XPS spectra for the initial powder and the powder after 720 h exposure, respectively. The results indicate that the titanium exists in pure Ti state and three forms of oxides: TiO, Ti2O3, and TiO2. There is a clear Ti/TiO 2p1/2 peak in the initial powder at 460.3 eV, which disappears after 720 h exposure. After exposure, the relative peak intensity of the Ti2O3/TiO2 2p3/2 peak increases, indicating that some Ti or TiO has been transformed into Ti2O3 or TiO2.
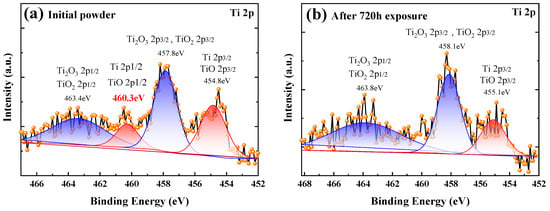
Figure 7.
The oxidation state of titanium on the surface of (a) initial powder, and (b) the powder after 720 h exposure.
Figure 8 showed the Gibbs free energy–temperature curve of the oxidation of Ti based on chemical reactions and Gibbs free energy equations listed as Equations (12)–(16) [22,26,29]. Ti can be oxidized to TiO2, Ti2O3, and TiO in turn at aerobic environment. The metastable TiO can be further transformed into stable TiO2 and Ti2O3. This is consistent with the XPS results, indicating that the TiO formed on the initial powder surface has transformed into stable TiO2 and Ti2O3 after further exposure.
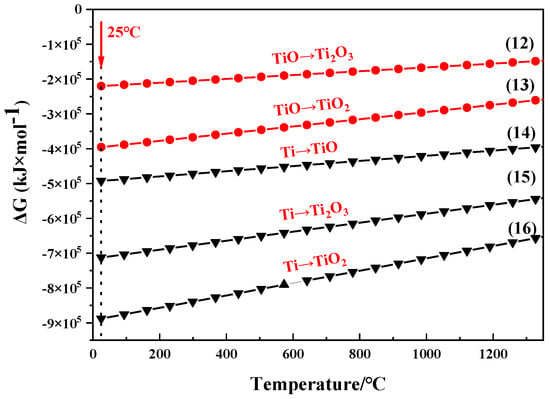
Figure 8.
Relationship between Gibbs free energy-temperature of oxidation reaction of Ti.
4. Conclusions
The oxidation of the Ni-based superalloy powders before and after exposure at ambient temperature in air are investigated in this work. The results indicate that O2, N2, and other gases are easily absorbed on the surface of powder, but most gases can be desorbed by vacuum at low temperature (<151.5 °C). The oxygen adsorption process on the powder surface is almost complete after a short time (<24 h) of exposure in air. There is adsorbed oxygen on the surface of the fine powder before exposure and the coarse powder after exposure. Adsorbed oxygen on fine powder surface can react with the alloying elements to form oxides after exposure at ambient temperature. Most of the alloy elements are oxidized directly and formed stable oxides on the powder surface, but titanium firstly formed the metastable TiO, and then transformed to TiO2 or Ti2O3 after exposure. This work clarified the oxygen absorption and element oxidation behavior of the Ni-based superalloy powder. It suggests that the adsorbed gases on the powder surface can be removed by low temperature vacuum desorption, which can improve the performance of PM parts.
Author Contributions
Conceptualization, W.Z. and D.J.; methodology, D.J. and Z.L. (Zhongwu Liu); formal analysis, W.Z. and W.X.; investigation, W.Z. and D.J.; resources, Z.L. (Zhou Li) and G.Z.; data curation, W.Z. and W.X.; writing—original draft preparation, W.Z.; writing—review and editing, Z.L. (Zhongwu Liu); supervision, W.Q. and Z.L. (Zhongwu Liu); project administration, Z.L. (Zhongwu Liu) and G.Z.; funding acquisition, Z.L. (Zhongwu Liu) and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Key R&D Program of China (Grant Nos. 2021YFB3704000 and 2019YFA0705300). A PhD scholarship by South China University of Technology is also gratefully acknowledged.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.R.; Wang, X.C.; Zhong, B.; Wei, D.S.; Jiang, X.H. Estimation of fatigue parameters in total strain life equation for powder metallurgy superalloy FGH96 and other metallic materials. Int. J. Fatigue. 2019, 122, 116–124. [Google Scholar] [CrossRef]
- Umeda, T.; Okane, T.; Kurz, W. Phase selection during solidification of peritectic alloys. Acta Mater. 1996, 44, 4209–4216. [Google Scholar] [CrossRef]
- Yao, C.G.; Meng, S.; Li, X.L.; Yi, D.Q.; Lü, H.J.; Wang, B. Effects of powder oxygen content on mechanical properties and microstructure of FGH4169 alloy. Mater. Sci. Eng. Powder Metall. 2017, 22, 33–40. [Google Scholar]
- Warren, R.; Ingesten, N.G.; Winberg, L.; Ronnhult, T. Particle surfaces and prior particle boundaries in Hf modified PM astroloy. Powder Metall. 1984, 27, 141–146. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Y.W.; Chi, Y.; Liu, J.T.; Jia, J.; Han, S.B. Effect of content of Hf and Zr on equilibrium phase and PPB in FGH96 P/M superalloy. Trans. Mater. Heat Treat. 2013, 34, 60–67. [Google Scholar]
- Zhang, Y.W.; Liu, J.T. Development in powder metallurgy superalloy. Mater. China 2013, 32, 1–11. [Google Scholar]
- Prakash, T.L.; Chari, Y.N.; Bhagiradha Rao, E.S.; Thamburaj, R. Microstructures and mechanical properties of hot isostatically pressed powder metallurgy alloy APK-1. Metall. Trans. A 1983, 14, 733–742. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Liu, C.K.; Zhao, W.X.; Zheng, Z.; Zhong, Y. Prior particle boundary of PM FGH96 superalloy and its in-situ high-cycle fatigue at elevated temperature. J. Aeronaut. Mater. 2017, 37, 83–89. [Google Scholar]
- Qin, S.Y.; Yan, L.G.; Zhang, X.F. Removing prior particle boundaries in a powder superalloy based on the interaction between pulsed electric current and chain-like structure. J. Mater. Sci. Technol. 2021, 87, 95–100. [Google Scholar] [CrossRef]
- Qiu, C.L.; Attallah, M.M.; Wu, X.H.; Andrews, P. Influence of hot isostatic pressing temperature on microstructure and tensile properties of a nickel-based superalloy powder. Mater. Sci. Eng. A 2013, 564, 176–185. [Google Scholar] [CrossRef]
- Appa Rao, G.A.; Srinvas, M.; Sarma, D.S. Effect of oxygen content of powder on microstructure and mechanical properties of hot isostatically pressed superalloy Inconel 718. Mater. Sci. Eng. A 2006, 435, 84–99. [Google Scholar]
- Zeoli, N.; Gu, S. Computational validation of an isentropic plug nozzle design for gas atomisation. Comput. Mater. Sci. 2008, 42, 245–258. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Z.; Xu, W.Y.; Zhang, G.Q. The study of argon atomized superalloy powders. Powder Metall. Ind. 2010, 20, 1–5. [Google Scholar]
- Tan, L.M.; Li, Y.P.; Liu, C.Z.; Yang, C.; Ding, H.H.; Huang, L.; Liu, F.; Qin, Z.J.; Jiang, L. The evolution history of superalloy powders during hot consolidation and plastic deformation. Mater. Charact. 2018, 140, 30–38. [Google Scholar] [CrossRef]
- Liu, N.; Li, Z.; Zhang, G.Q.; Yuan, H.; Xu, W.Y.; Zhang, Y. Oxidation characteristics of nickel-based superalloy powders prepared by argon gas atomization. Chin. J. Rare Met. 2011, 32, 481–485. [Google Scholar]
- Gao, Z.J.; Zhang, G.Q.; Li, Z.; Yuan, H.; Xu, W.Y.; Liu, N. Effect of size distribution and oxygen content of powder on microstructure of HIPed superalloy FGH96. Chin. J. Rare Met. 2012, 36, 665–670. [Google Scholar]
- Xu, W.Y.; Li, Z.; Liu, Y.F.; Zhang, L.; Zhang, G.Q. Influence of temperature on the oxidation behaviors of the nickel-based superalloy powders. Powder Metall. Technol. 2020, 38, 192–196. [Google Scholar]
- Shimizu, K.; Kobayashi, K.; Thompson, G.E.; Wood, G.C. The apparent induction period for γ-Al2O3 development in thermal oxide films on aluminium. Oxid. Met. 1991, 36, 1–13. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J.; Dietrich, J.R.; Senkov, O.N. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, X.Q.; Li, Q.M.; Gao, X.; Liu, W.L. Application of themogravimetric mass spectrometry system with vacuum replacement technology in the thermal decomposition of sodium azide. Anal. Instrum. 2019, 1, 32–35. [Google Scholar]
- Guo, W.M.; Wu, J.T.; Chen, G.S.; Zhou, B.; Zhao, M.H. The influence of vacuum degasing pretreatment on microstructure and properties of superalloy FGH95. J. Aeronaut. Mater. 2003, 23, 21–24. [Google Scholar]
- Kong, L.R.; Zhang, S.Y. Theoretical explanation of the special temperature dependence of rate constant for oxidation of carbon. Univ. Chem. 2016, 31, 84–88. [Google Scholar] [CrossRef]
- Hu, B.F.; Li, H.Y. Microstructure of argon-atomized FGH95 and imported René95 superalloy powders after heat treatment. Chin. J. Eng. 1987, 9, 12–18. [Google Scholar]
- Ma, W.B.; Liu, G.Q.; Hu, B.F.; Zhang, Y.W.; Liu, J.T. Prior particle boundary and its effect on tensile properties of PM FGH96 superalloy. Mater. Sci. Eng. Powder Metall. 2013, 18, 1–7. [Google Scholar]
- Liang, Y.J.; Che, M.C. Handbook of Thermodynamics of Inorganic Materials; Northeast University Press: Liaoning, China, 1993; pp. 449–479. ISBN 7-81006-532-7. [Google Scholar]
- Shen, Y.L.; Guo, M.L.; Xia, X.H.; Shao, G.S. Role of materials chemistry on the electrical/electronic properties of CuO thin films. Acta Mater. 2015, 85, 122–131. [Google Scholar] [CrossRef]
- Fang, P.J.; Xu, Y.; Li, X.G.; Chen, Y. Influence of atomizing gas and cooling rate on solidification characterization of nickel-based superalloy powders. Rare Met. Mater. Eng. 2018, 47, 423–430. [Google Scholar]
- Wang, P.W.; Woo, J.; Avila, M.; Garicia, J.; Bronson, A.; Varma, S.K. In situ surface oxidation of Ti44Al11Nb alloy at room temperature. J. Mater. Sci. 2003, 38, 489–497. [Google Scholar] [CrossRef]
- Merritt, R.R.; Hyde, B.G.; Bursil, L.A.; Philp, D.K. The thermodynamics of titanium+oxygen system: An isothermal gravimetric study of the composition range Ti3O5 to TiO2 at 1304K. Philos. Trans. R. Soc. A 1973, 274, 627–661. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).