Abstract
Copper alloys, combining optimized strength with high electrical and thermal conductivity, are analyzed in-depth, in order to meet the increasing requirements of today’s and tomorrow’s applications in the electrical and automotive industries. The conducted research analyzes alloys with up to 0.3 wt.% scandium, as an alloying element with limited solubility in copper. For the simultaneous enhancement of mechanical strength and conductivity, precipitation hardening is the conducted process method, accompanied by experimental and simulation-based investigations. Therefore, the influence of aging temperatures, in the range of 350 °C to 500 °C, is analyzed in combination with 25%, 50%, and 75% prior cold deformation. CuSc starts precipitating at 375 °C, without prior cold working, whereas mechanical deformation refines the growing intermetallic precipitates. Higher temperatures improve the formation of precipitates but carry the risk of overaging. The first key achievement is to use a thoroughly examined thermomechanical treatment, investigating the growth of precipitates to reach significantly higher hardness than the benchmark alloy, CuZr0.15. Furthermore, the analyzed CuSc alloys show advantages in the investigated recrystallization behavior, making them, especially, applicable for higher operating temperatures. Future research will assess ternary alloying combinations, to further scoop the latent potential of CuSc alloys.
1. Introduction
The two most desirable properties of high-performance copper alloys are high strength and high conductivity, including electrical and thermal conductivities. A variety of current and future applications in the automotive and electronic industry, such as relays, switches, welding electrodes, electric motors, generators, casting molds, die-casting plungers, and busbars in commutators, are conceivable for such property profiles.
Different approaches are possible to meet increasing requirements regarding the mechanical properties of alloys. These include dislocation hardening, grain boundary hardening, solid solution hardening, or precipitation hardening [1]. Whereas dislocation and grain boundary hardening show weaknesses at higher operating temperatures [2], solid solution hardening significantly decreases the alloy’s conductivity [1]. Typically, all alloying elements that show limited and, with lower temperature, decreasing solubility in copper indicate the possibility of precipitation hardening [3]. To address the material’s properties, the precipitating volume, number of precipitates, their shape, and their distribution are deciding parameters [4].
Concerning the electrical or thermal conductivity, the solute amount of alloying element within the copper matrix is a crucial factor, due to the scattering of conduction electrons [2,5,6]. Therefore, low-alloyed copper alloys with, alloying elements in the range of 1 wt.%, are in the focus of this research. In addition to a comparison with industrially utilized alloys, new approaches using scandium as an alternative alloying element are highlighted.
A commonly used high-conductivity alloy, with fine grains, improved hardness, and recrystallization behavior, is CuZr0.15 [7,8]. Zirconium has a, comparatively, low maximum solubility in copper, of 0.17 wt.% at 972 °C [9]. The small, and with temperature decreasing, solubility results in precipitation potential [3], generating high conductivity (reaches 95% IACS) and enhanced hardness values [1,10]. Watanabe et al. refer to early coherent fcc precipitates as a GP zone or an intermediate phase [11]. Peng et al. report the presence of rod-shaped Cu5Zr precipitates, which form from a supersaturated state of solid solution to clusters to semi-coherent structures [12]. Nakashima et al. supplement the presence of disk-shaped Cu5Zr phases, with fairly stable ordered fcc precipitates in a wide temperature range [13] which promote the mechanical properties and electrical conductivity up to 700 °C [14]. The cutting and by-passing mechanism interaction influence the hardness of peak-aged and overaged CuZr alloys [12], as the Orowan mechanism is dominant, in case of incoherent precipitates [11].
The light metal scandium is the subject of many research topics, and a commonly used alloying element for lightweight design, in case of high material requirements [15]. Due to multi-modification effects, scandium is an attractive alloying element for aluminum alloys. Combining a precipitation-hardenable alloying system [16,17] with grain refinement [18], smaller secondary dendrite arm spacings [18,19], modification of eutectic AlSi structures [20] and intermetallic AlFeSi phases [21], scandium has multiple benefits. In summary, scandium contributes to different properties of aluminum alloys, such as grain structure, recrystallization, welding or creep behavior, and high-temperature strength, in an attractive way [18]. For several rare earth elements, such as yttrium [22,23] or scandium, a grain-refining influence in copper materials is reported [23], which potentially helped the performance of low-alloyed copper alloys at higher operating temperatures [24].
The phase diagram of copper and scandium shows an excellent potential for precipitation hardening. The maximum solubility of 0.35 wt.% scandium [25,26,27] is less than chromium (0.7 wt.% [28]) and more than zirconium in copper, which are both elements for precipitation hardening in binary copper alloys [29,30]. In addition, the solubility decreases, rapidly, with temperature starting at the maximum solubility at 1138 K [25,26,27]. The Cu-Sc phase diagram shows three intermetallic compounds, including Cu4Sc (15 wt.% Sc), Cu2Sc (26 wt.% Sc), and CuSc (42 wt.% Sc) [26]. A quenched solution of scandium in copper can be used in aging treatments, in order to increase hardness and electrical conductivity [31,32,33,34]. Hao et al. describe the precipitating process of copper containing 0.4 wt.% scandium after 90% cold deformation (at room temperature) and cryogenic rolling before an ensuing aging process [35]: Starting in supersaturated solid solution, regularly distributed scandium-enriched atomic clusters develop in the first step. These nuclei grow to precipitates, including two and more atom layers. Strengthening effects are related to a huge distortion of the surrounding matrix and, therefore, a coherent strengthening mechanism. The huge mismatch between the coherent metastable phase and the surrounding matrix influences the further growth of these precipitates. The number and length of evolving lamellar precipitates increase with further aging time Due to scandium’s larger atom size, and, therefore, an enhanced mismatch, the transition from fully coherent to semi-coherent precipitates is suggested in this stage of development. Afterwards, more atomic layers attach, and the tetragonal orientated lamellar Cu4Sc-precipitates, with a habit plane parallel to the copper’s {111} plane and orientation of and , expand. In conclusion, the highly strengthening effect in these alloys is described as a combination and coexistence of coherent strengthening, with a significant matrix distortion and Orowan by-passing mechanism, at precipitates of a larger size.
Whereas Franczak et al. analyze as-cast structures of CuSc0.15 and CuSc0.3 and the behavior of precipitation without prior cold deformation [33], in direct comparison to the already named benchmark alloy CuZr0.15, Hao et al. focus on the behavior of strongly cold-worked specimens of CuSc0.4 [35], which provides a gap for moderate degrees of cold deformation to be further investigated.
Many industrial production processes address moderate degrees of cold deformation, but those have, rarely, been discussed in detail so far. Evaluating the presented results closes this gap, step by step. This work differs from the known literature and emphasizes the behavior at lower aging temperatures, which shows advantages at optimized peak hardness and fast occurring aging results. The corresponding time-dependent properties analyzed in this work generate a thorough understanding of the alloy’s reactions to heat treatments. To sum up, CuSc0.3 can be optimized with precipitation hardening to reach 200 HV0.1 and 48 MS/m (75% cold working). CuZr0.15 shows hardness peaks only up to 170 HV0.1, at an equal degree of deformation. Otherwise, the electrical conductivity reaches 56 MS/m. Optional cold working before the heat treatment results in significantly smaller precipitates, which have a huge influence on the mechanical properties. Examining recrystallization behavior, alloys containing scandium started at 500 °C, whereas CuZr0.15 started at least 50 K earlier. Therefore, CuSc alloys are suitable for higher operating temperatures.
Giving an overview of the used methods, this publication discusses the correlation of electrical and mechanical properties with metallographic investigations. Besides the identical processing and parallel investigation of CuSc0.3 and CuSc0.15, the comparison with the corresponding benchmark alloy CuZr0.15, which is currently used in various industrial applications, concludes a finalizing picture. Parts of this paper were published in the conference contribution to the German Kupfer-Symposium 2021 [36]. Main statements are taken up for deeper discussion, in the context of this paper, and are reinforced with further investigations.
2. Materials and Methods
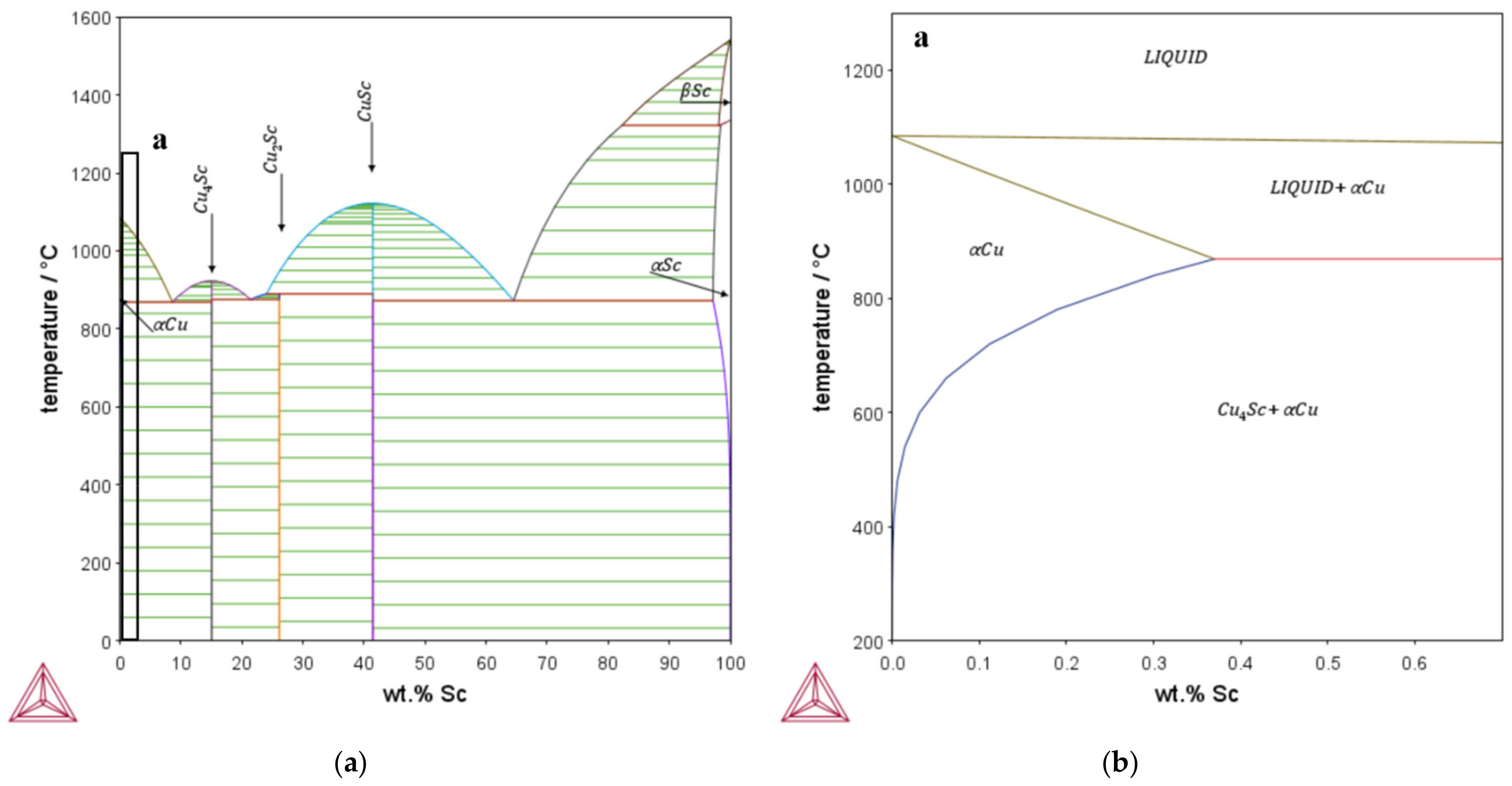
Within the conducted research, copper alloys containing up to 0.3 wt.% Sc were analyzed and compared with identically processed CuZr0.15. Choosing the alloys includes a closer look at the available literature [26,27,34] and previously calculated phase diagrams with the copper database of Thermocalc. To achieve precipitation hardening, the maximum solubility of the alloying element, as shown in Figure 1, was considered. CuSc0.3, CuSc0.15, and CuZr0.15 were chosen, in order to generate an overview of each material’s behavior and provide a reliable comparison between the alloying systems.
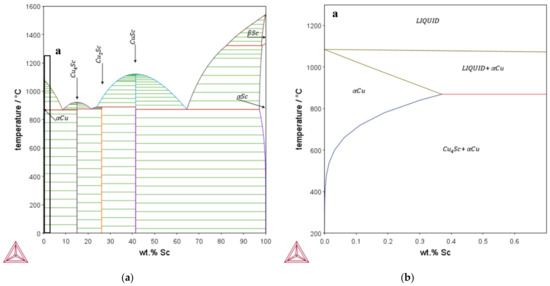
Figure 1.
Phase diagram of the binary system Cu-Sc, calculated with the Thermocalc copper database: (a) overall; (b) magnification of the copper-rich area (detail a).
Within the conducted research analysis, the raw materials Cu-OFE and the corresponding master alloys (CuSc23 and CuZr50) were melted in a graphite crucible, under vacuum conditions. The conducted casting process, using an VC400 casting machine (Indutherm Blue Power Casting Systems, Walzbachtal, Germany), was performed with 500 g casting weight. The graphite crucible was, previously, coated with boron nitride, to avoid reactions between melt and crucible. At 1300 °C melting temperature, casting into a rectangular graphite mold was, also, conducted under vacuum conditions. Afterwards, a specially calibrated optical emission spark spectrometer (Spectrotest, SPECTRO Analytical Instruments GmbH, Kleve, Germany) proved the specimens to have a maximum deviation of 0.05 wt.%, in the target alloy composition.
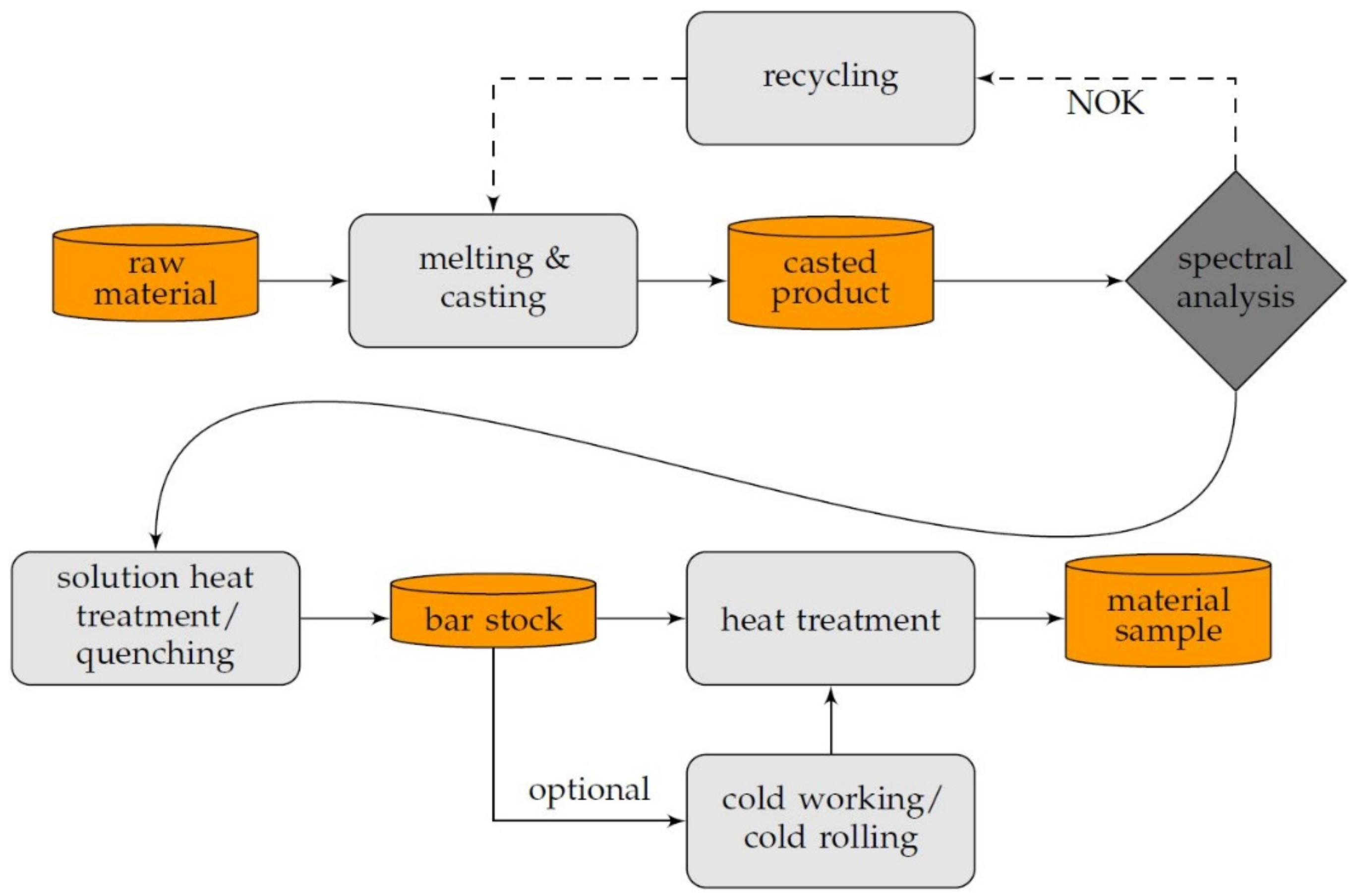
Solution annealing in preheated furnaces (ME65/13, Helmut ROHDE GmbH, Prutting, Germany) for 120 min was conducted at 870 °C for scandium-containing alloys and 960 °C for CuZr0.15, which is the temperature showing the maximum solubility in copper. Followed by water quenching in a recirculated water reservoir, the specimens showed an overall successful solution annealing. The entire temperature monitored heat treatment was conducted at 350 °C, 375 °C, 400 °C, 425 °C, 450 °C, and 500 °C, for up to 48 h, followed by a corresponding cooling to room temperature. Due to the investigated start of precipitation, the lower limit of analyzed temperatures was defined. Exceeding disadvantages regarding the mechanical properties, such as recrystallization and significant dislocation annihilation, determined the upper temperature range of the aging process. In addition, the influence of cold deformation before aging, with 25%, 50%, and 75% cold rolling on a duo roll stand (Bühler, Pforzheim, Germany), was analyzed. Five equidistant cold-rolling steps were appropriated, between the mentioned degrees of deformation. Therefore, the stepwise feed rate was 0.25 mm, starting at an initial thickness of 5 mm, for the copper alloy bars. The duo roll stand was equipped with 150 mm wide rolls, having a diameter of 110 mm, which were driven in opposite directions at a speed of 27 min−1, to allow longitudinal rolling of the specimens. Visualizing the steps of specimen manufacturing, Figure 2 shows the explained steps in an overview.
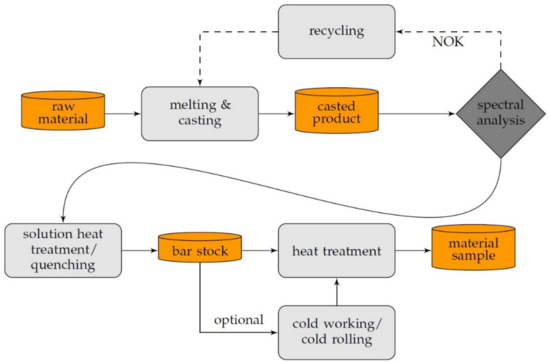
Figure 2.
Schematic of the process of specimen preparation.
In an attempt to identify the material behavior in this work, the electrical conductivity was measured with an eddy current test (Sigmascope SMP10, with TF100A for temperature compensation, Helmut Fischer GmbH, Sindelfingen, Germany). Hardness tests were conducted, continuously, within different processing states, to characterize mechanical properties, using a microhardness tester (NEXUS 412A equipped for DIN EN ISO 6507-1:2018 Vickers hardness test, Innovatest GmbH, Selfkant-Heilder, Germany). The specimen thickness, in the range of 5 mm without cold deformation and 1.25 mm with 75% cold deformation, and the resulting cross sections defined the choice of the hardness test method to be microhardness measurements. The test setup was identical for all of the conducted measurements, to grant comparability, which resulted in the chosen force load of 0.980 N (HV0.1).
All characterizations were accompanied by metallographic analysis, using light microscope (DM2700DM, Leica Microsystems, Wetzlar, Germany) and SEM (Gemini Sigma VP with the used NTS BSD (Carl Zeiss Microscopy Deutschland GmbH, Oberkochen, Germany) and XFlash 6|30 detectors (Bruker Nano GmbH, Berlin, Germany). For better contrasting in the light microscope, the specimens were etched corresponding to Klemm III [37], with a short polishing after etching. Grain size measurements were conducted, using the linear intercept method, in five different transverse sections. Precipitation measurements with SEM analysis were manually performed, due to only a slight visual contrast at identical magnifications, using 30 representative precipitates in two images.
Generally, a precise validation of the alloy composition and entire control of the temperatures optimized the quality of the received results, shown within the following chapters.
3. Results
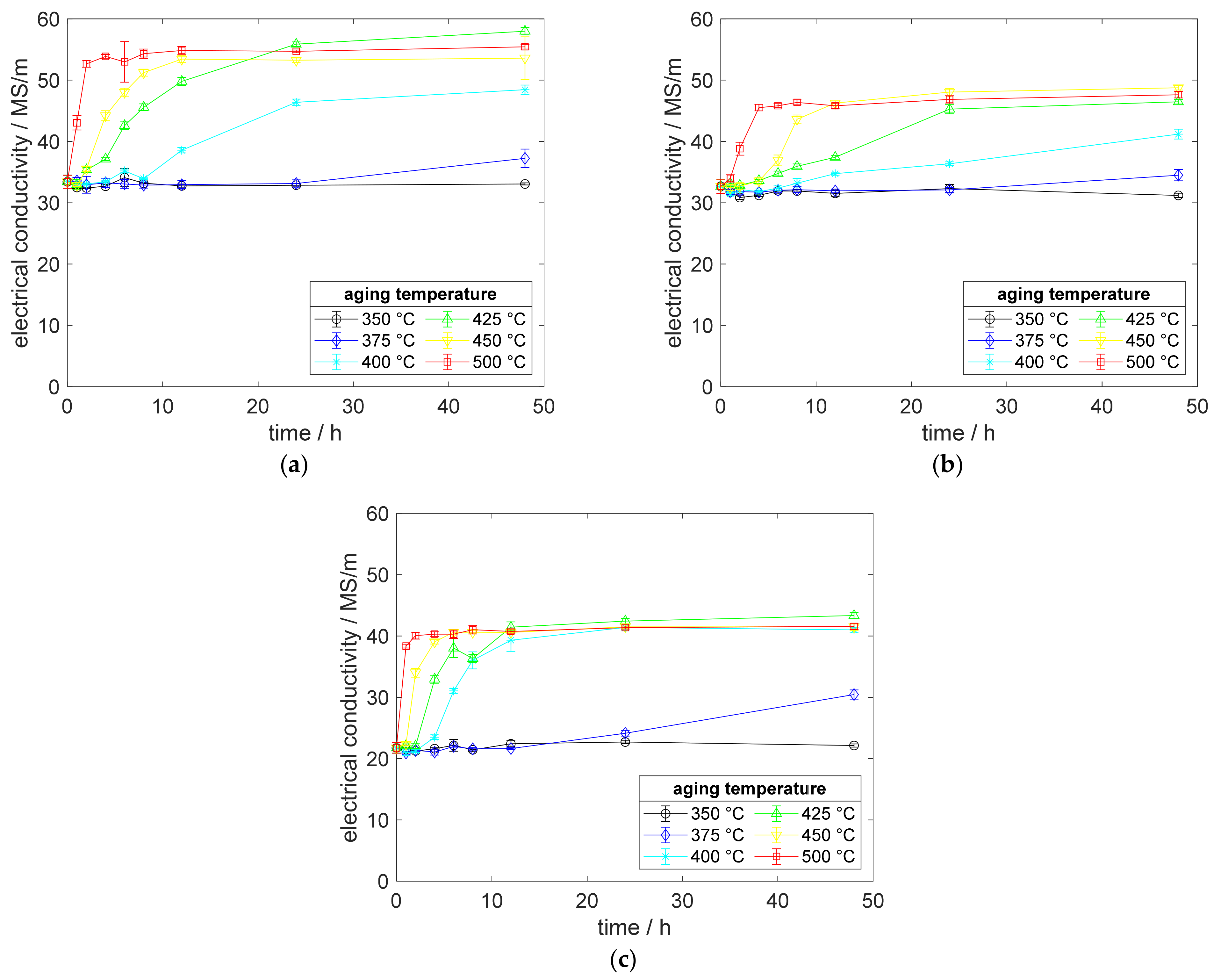
Analyzing the precipitation behavior of scandium-containing copper alloys and the corresponding CuZr0.15 benchmark alloy, Figure 3 shows the reaction on heat treatments after annealing and water quenching, without prior cold deformation. The measurement of electrical conductivity reacted sensibly and, firstly, showed the impacts of aging reactions. CuSc0.15, CuSc0.3, and CuZr0.15 started precipitating at 375 °C, whereas higher aging temperatures accelerated the increase in conductivity for all alloying systems. CuZr0.15 showed its strengths, reaching high electrical conductivities. For both alloying systems, the region of maximum solubility in copper provided the best potential for precipitation. Therefore, the aging curves at 400 °C and 425 °C in Figure 3 show a remarkable and early increase in conductivity. Whereas CuSc0.15 and CuZr0.15 needed a remarkably longer aging time at 400 °C to reach their individual and final level of electrical conductivity, CuSc0.3 showed advantages with less aging time.
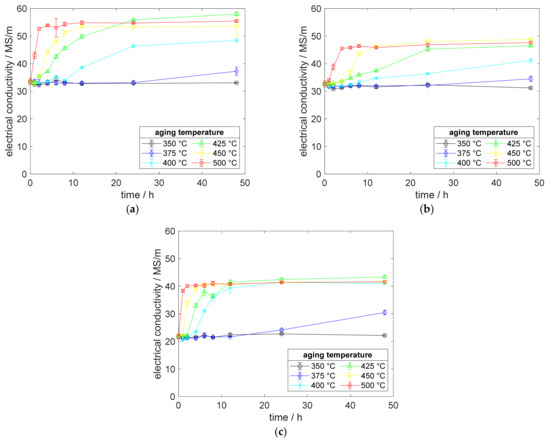
Figure 3.
Electrical conductivity development during aging, without prior cold deformation (solution annealing, quenching, aging) of (a) CuZr0.15; (b) CuSc0.15; (c) CuSc0.3.
Firstly, the behavior of CuSc0.3 is analyzed and, afterwards, compared in the context of the other alloys, CuSc0.15 and CuZr0.15.
3.1. Results for Copper–Scandium Alloy CuSc0.3
3.1.1. Development of Material Properties
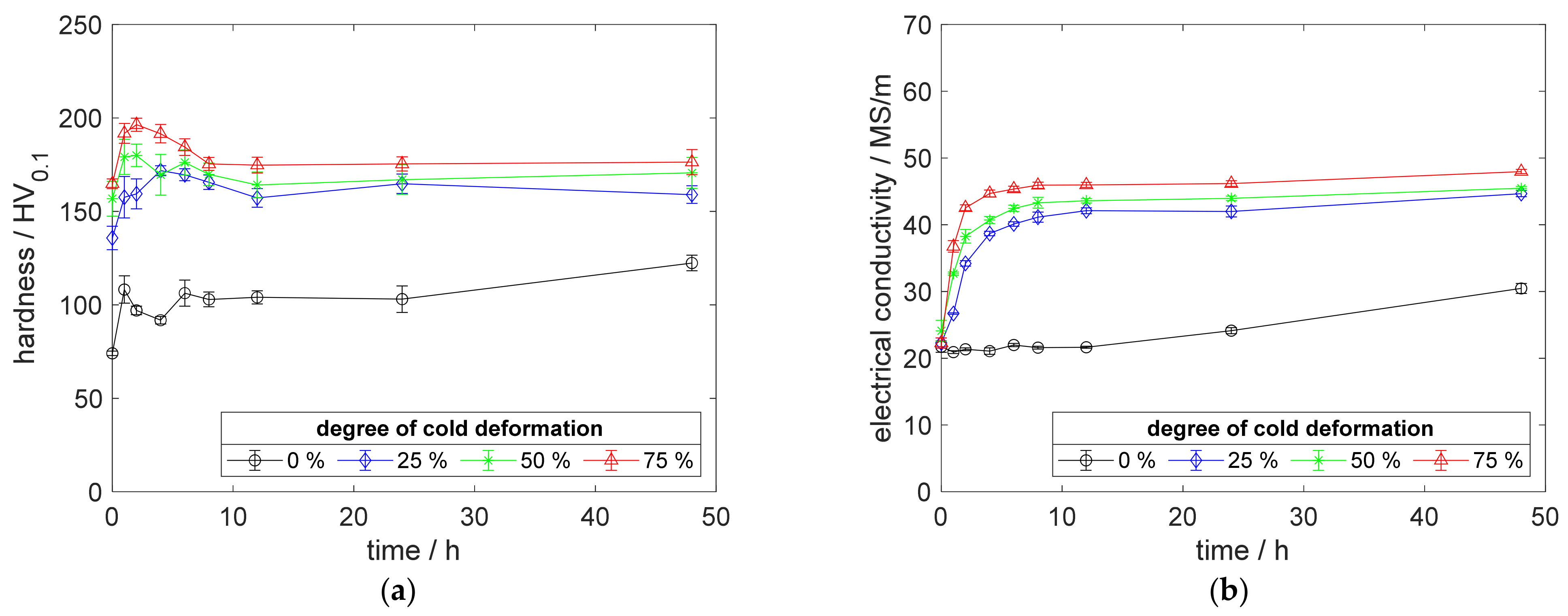
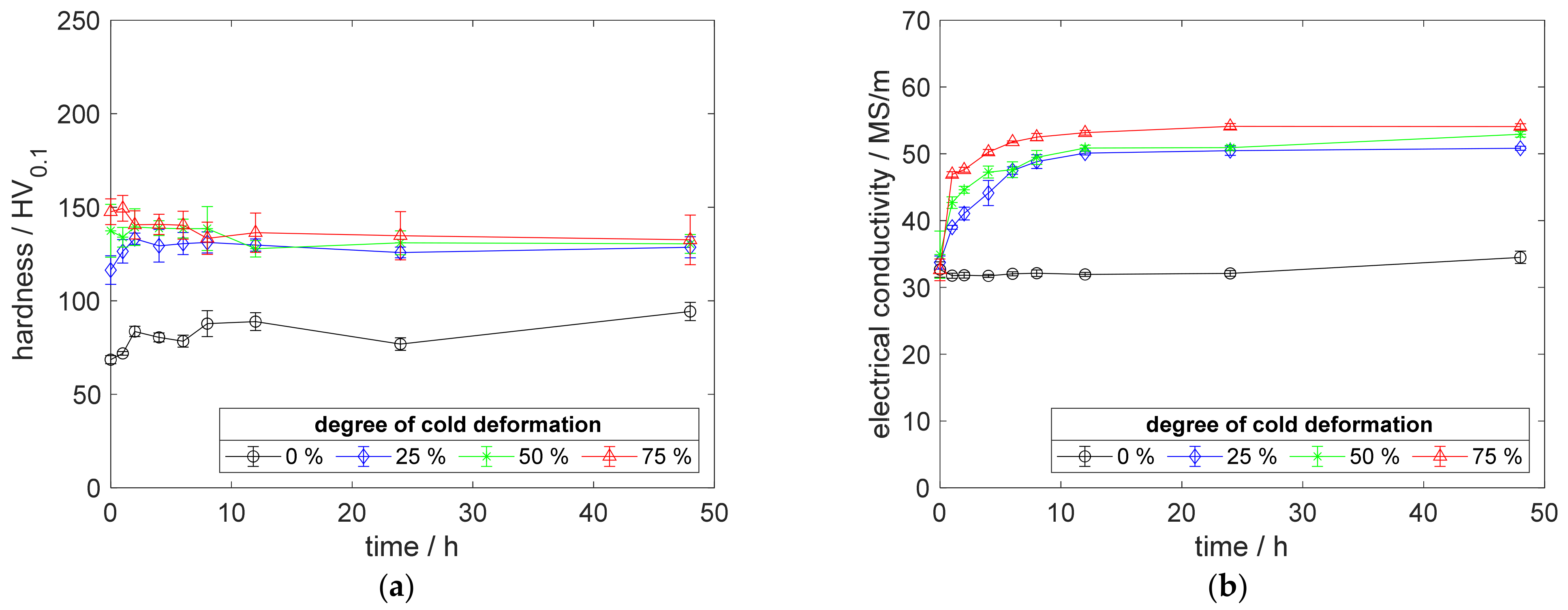
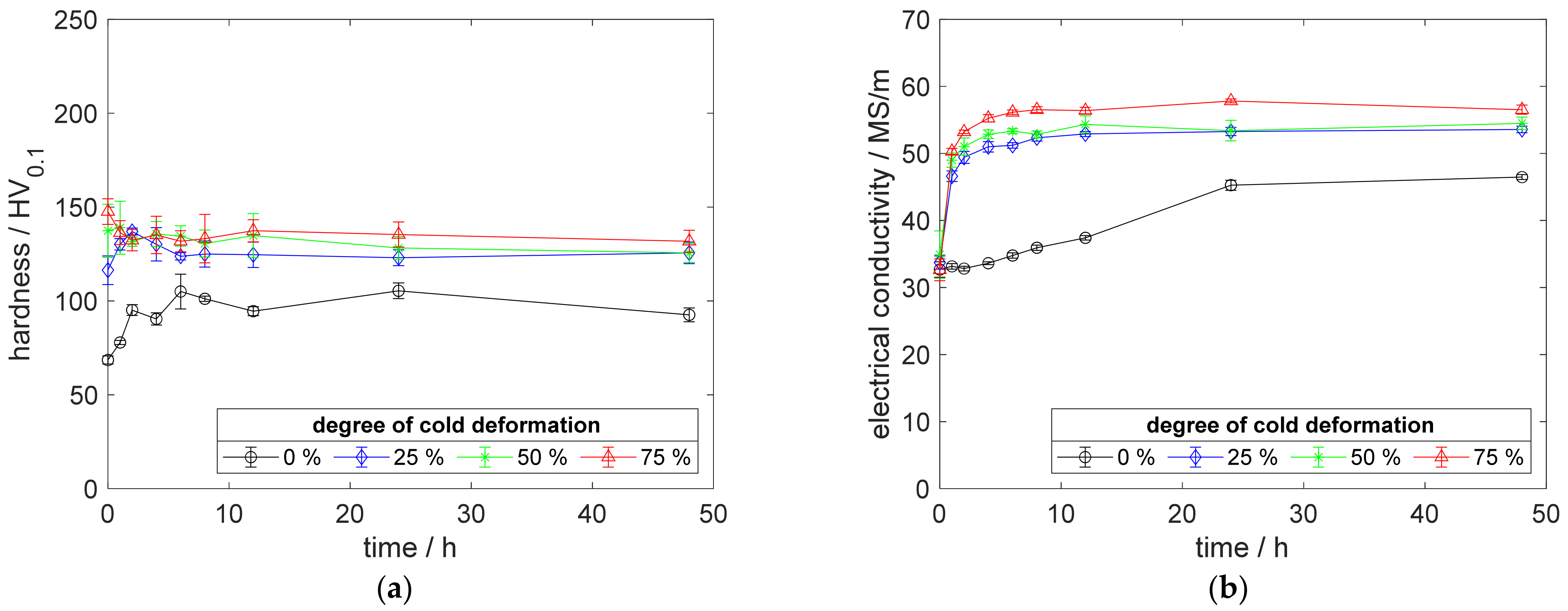
Especially, the influence of cold deformation after annealing as well as quenching on the precipitation process and resulting properties provides a deeper insight into the material’s behavior. The development of hardness (Figure 4a) and electrical conductivity (Figure 4b) of CuSc0.3 at 375 °C aging temperature, clearly, shows the impact of even lower degrees of cold deformation. The introduction of 25%, 50%, or 75% cold deformation accelerated the aging process, especially at lower temperatures. An increased degree of cold deformation resulted in a consistent tendency, which culminated for the specimens with 75% cold deformation providing their peak hardness above 196 HV0.1, after 2 h of aging (Figure 4a). After reaching the peak-aged state, the hardness decreased about 20 HV0.1 (Figure 4a). Furthermore, this specimen reached a maximum electrical conductivity of 48 MS/m (Figure 4b).
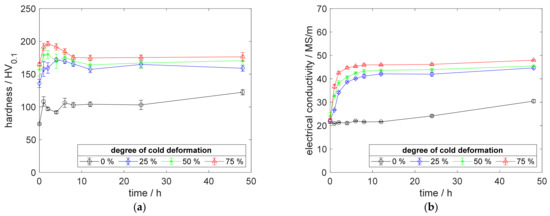
Figure 4.
Property development during aging of CuSc0.3 at 375 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Using only a slight degree of cold deformation (25%) significantly influenced the properties of the material (Figure 4a,b), compared to the specimens without deformation. A higher degree of cold deformation resulted in faster and slightly further increased properties (Figure 4a,b). Whereas the hardness reached a maximum state during the aging process (Figure 4a), the corresponding electrical conductivity increased, continuously (Figure 4b).
A reduced aging temperature of 350 °C did not affect the specimen of CuSc0.3, without prior cold deformation (Figure 5a,b). The lowered temperature resulted in a slower precipitation process, for all degrees of cold deformation. Especially for highly cold-deformed specimens, this slower process resulted in a steadier hardness peak and a moderate hardness decrease during overaging (Figure 5a). The 75% cold-worked specimen reached a hardness peak of 198 HV0.1 after 6 h, which decreased by only 14 HV0.1, due to overaging (Figure 5a). Figure 5b shows the alloy’s maximum electrical conductivity with 45.7 MS/m.
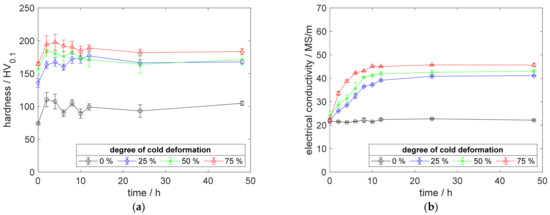
Figure 5.
Property development during aging of CuSc0.3 at 350 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Moreover, a higher aging temperature intensified the sharply demarcated hardness peak. All specimens, treated with cold deformation prior to aging, showed early peak-aged states, followed by a remarkable decrease in hardness (Figure 6a). For example, the hardness of the 75% cold-worked specimen decreased from peak 192 HV0.1 to 164 HV0.1 (Figure 6a), within 1 h.
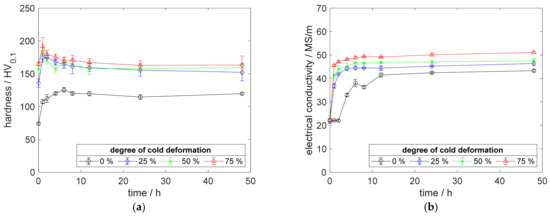
Figure 6.
Property development during aging of CuSc0.3 at 425 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
In terms of electrical conductivity, the specimen without plastic deformation prior to the aging treatment, nearly reached the level of the other variants, if the chosen temperature was 425 °C or higher (Figure 6b).
Generally, the reachable maximum electrical conductivity increased over the aging temperature (Figure 4b, Figure 5b and Figure 6b). At 425 °C, the 75% cold-deformed specimen improved its conductivity by about 27 MS/m, to a maximum of 51 MS/m (Figure 6b).
Further increased aging temperatures (Appendix A.3, Figure A10 and Figure A11) proved the trend and reproducibility. Still, they did not, mainly, contribute to further expanding the Cu-Sc system’s understanding. Furthermore, CuSc0.3 did not recrystallize at any regarded processing state, except for a started grain reformation at 500 °C (48 h), in the case of the 75% cold-worked specimen. The average grain size of CuSc0.3 specimens was 327 µm, which was 232 µm smaller than Cu-OFE and 19 µm larger compared to the benchmark alloy, CuZr0.15 (Appendix A.5, Figure A13). This assured a grain-refining influence of scandium in copper.
3.1.2. Analysis of Precipitates
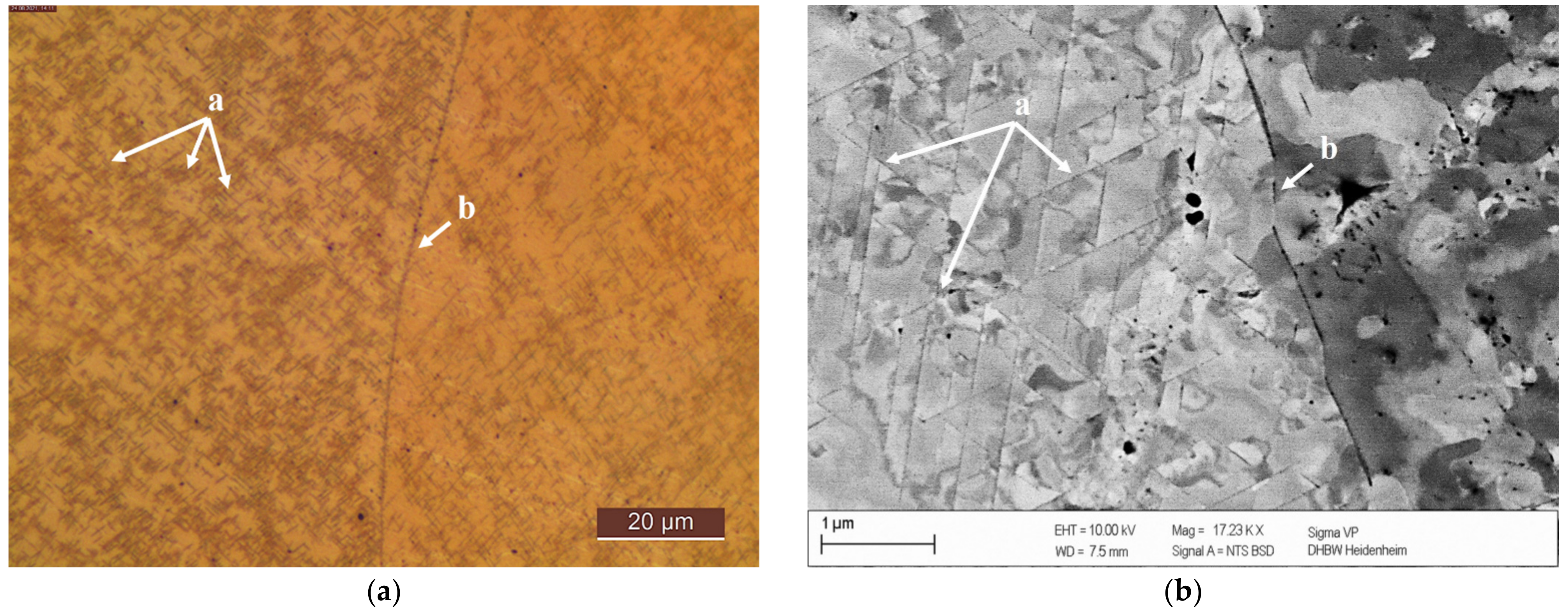
Coarsening of precipitates in CuSc0.3 specimens, without cold deformation prior to the aging process, showed their changing structural orientations at grain boundaries in the light microscope in Figure 7a. Lamellar structures of the homogenously and regularly distributed growing precipitates (details (a) in Figure 7 and Figure 8) were visible in the electron microscope backscatter detector (Figure 7b). Next to grain boundaries, the alloying element tended to migrate into the intergranular space, which resulted in fewer precipitation structures next to the grain boundary (area (b) in Figure 7a,b). This behavior was consistent and reproducible at any analyzed aging temperature (350 °C, 375 °C, 400 °C, 425 °C, 450 °C, 500 °C).
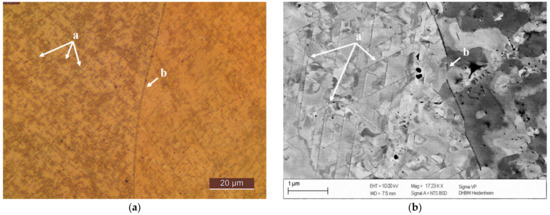
Figure 7.
Precipitates in CuSc0.3 after 48 h at 375 °C (0% cold deformation): (a): light microscope polished with 0.25 µm and etched (Klemm III); (b): REM polished with 0.25 µm)—for (a) and (b): homogenously distributed lamellar precipitates within a grain (a), fewer and smaller precipitates in the surroundings of grain boundary with scandium migration in the intergranular space (b).
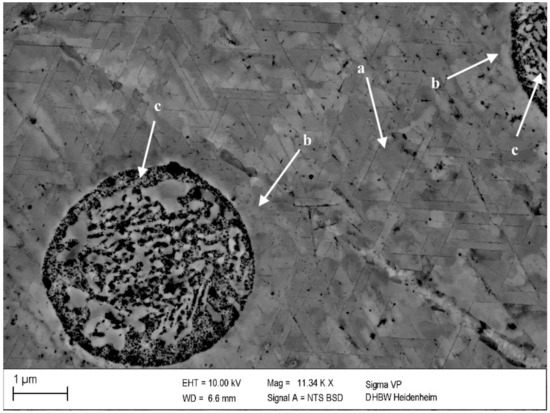
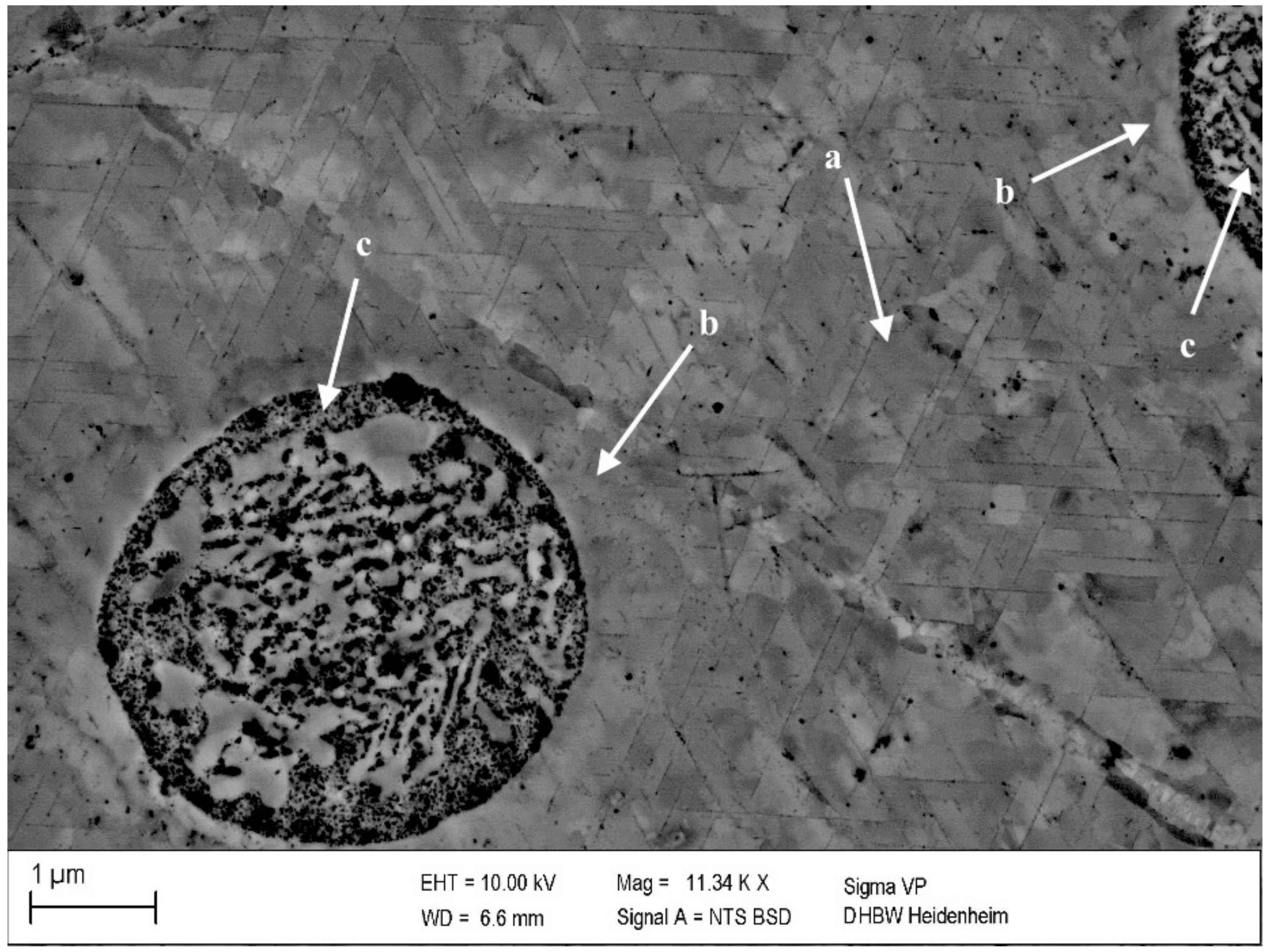
Figure 8.
Precipitates in CuSc0.3 after 48 h at 375 °C (0% cold deformation) (polished with 0.25 µm): homogenously distributed lamellar precipitates within a grain (a), fewer and smaller precipitates in the surroundings of an intermetallic CuSc-phase (b) and intermetallic CuSc-phase (c).
A corresponding behavior of homogenously growing precipitates within the grains is visualized in Figure 8a. Next to undissolved scandium containing intermetallic phases (c), the formation of lamellar precipitates was reduced (b). To form precipitates, the quenched solution of scandium atoms in the copper matrix is necessary. In the following, this condition for precipitation was not sufficient in the area (b) close to the intermetallic phase (c). Therefore, this local change confirmed the detectability of the precipitates by the selected metallographic methods.
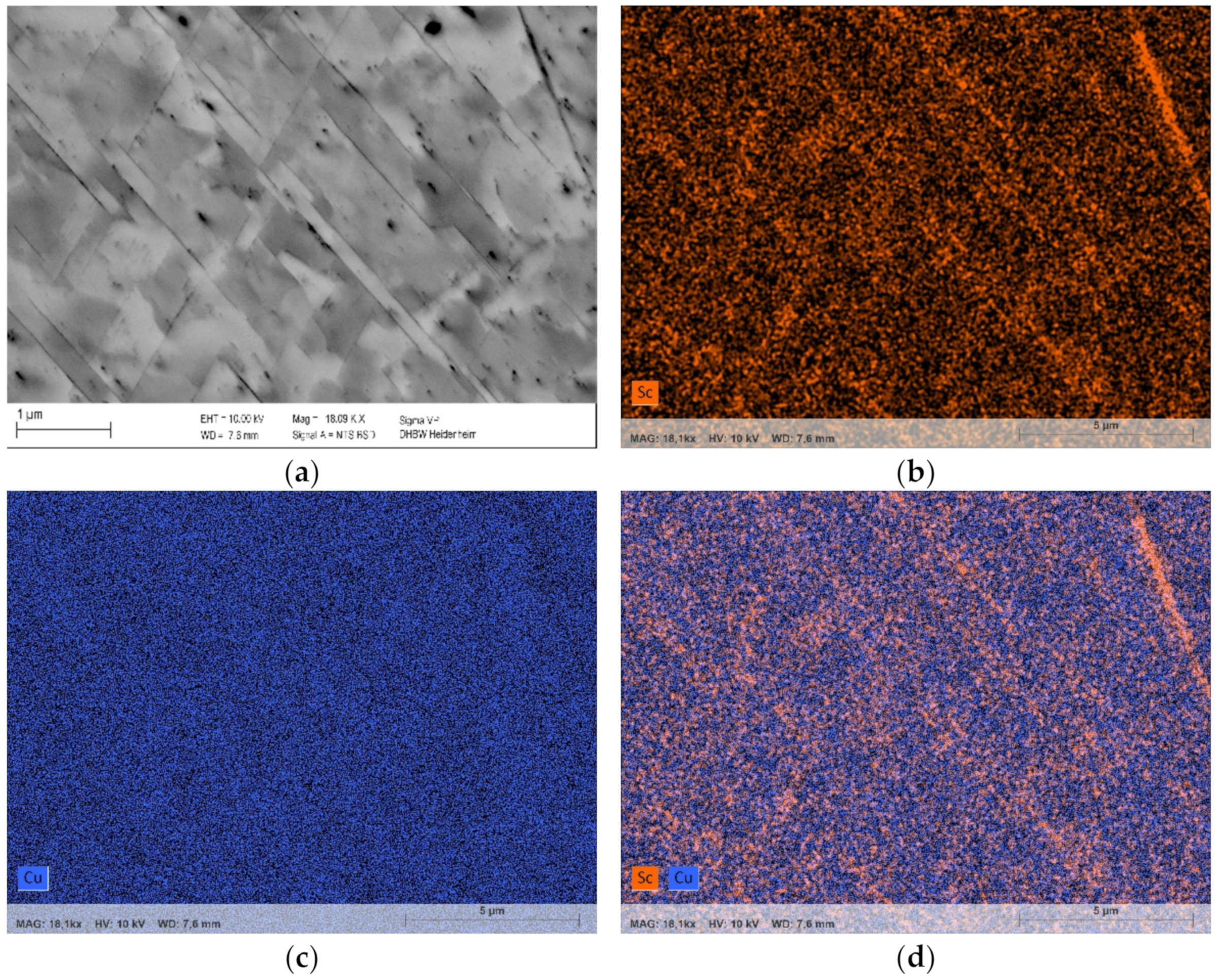
Further investigations using EDS analysis validated the formation of scandium-containing intermetallic phases (Figure 9). Due to the highly copper-containing and layered structure (corresponding to [35]) of Cu4Sc-phases, the resulting material contrast of scandium distribution was only slightly detectable (Figure 9b–d). Nevertheless, the corresponding areas, clearly, outlined the connection between higher scandium concentrations and the lamellar structures that can be identified using the backscatter detector (Figure 9a,b,d).
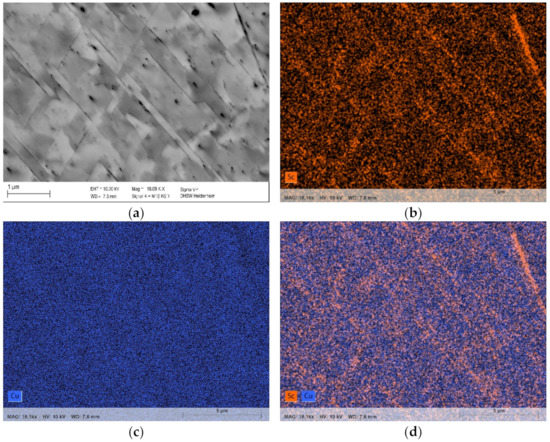
Figure 9.
Precipitation in CuSc0.3 without prior cold deformation and aging for 48 h at 500 °C (polished with 0.25 µm). Lamellar distribution of enhanced Sc-contents, analyzed with EDS: (a) backscatter; (b) EDS distribution of Sc; (c) EDS distribution of Cu; (d) EDS distribution of Sc and Cu.
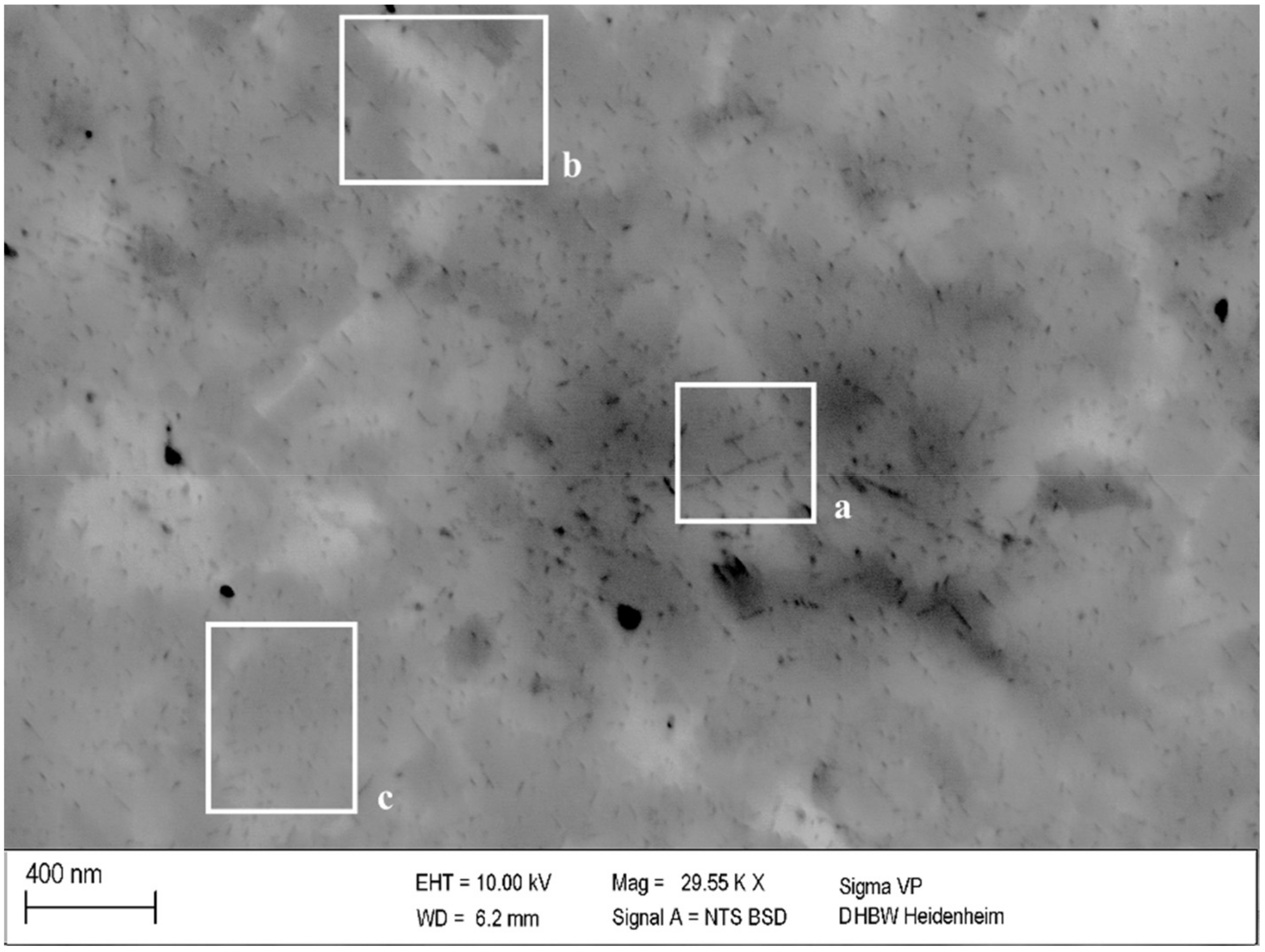
Previous degrees of cold deformation affected measured values of hardness and electrical conductivity (Figure 4, Figure 5 and Figure 6), by influencing the formation and growth of precipitates. This impact can be seen in the correlation with SEM investigations in Figure 10. Generally, the precipitates of a cold-worked specimen in Figure 10 were significantly smaller than the corresponding ones in Figure 8 without prior cold deformation. Nevertheless, local influences resulted, directly, in the size of growing precipitates (Figure 10a–c).
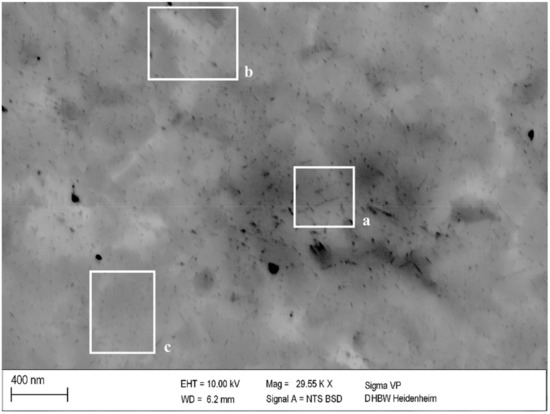
Figure 10.
Precipitation in 25% cold worked and aged specimen of CuSc0.3 at 375 °C for 48 h (polished with 0.25 µm): larger (a), medium (b), and smaller (c) precipitation structures.
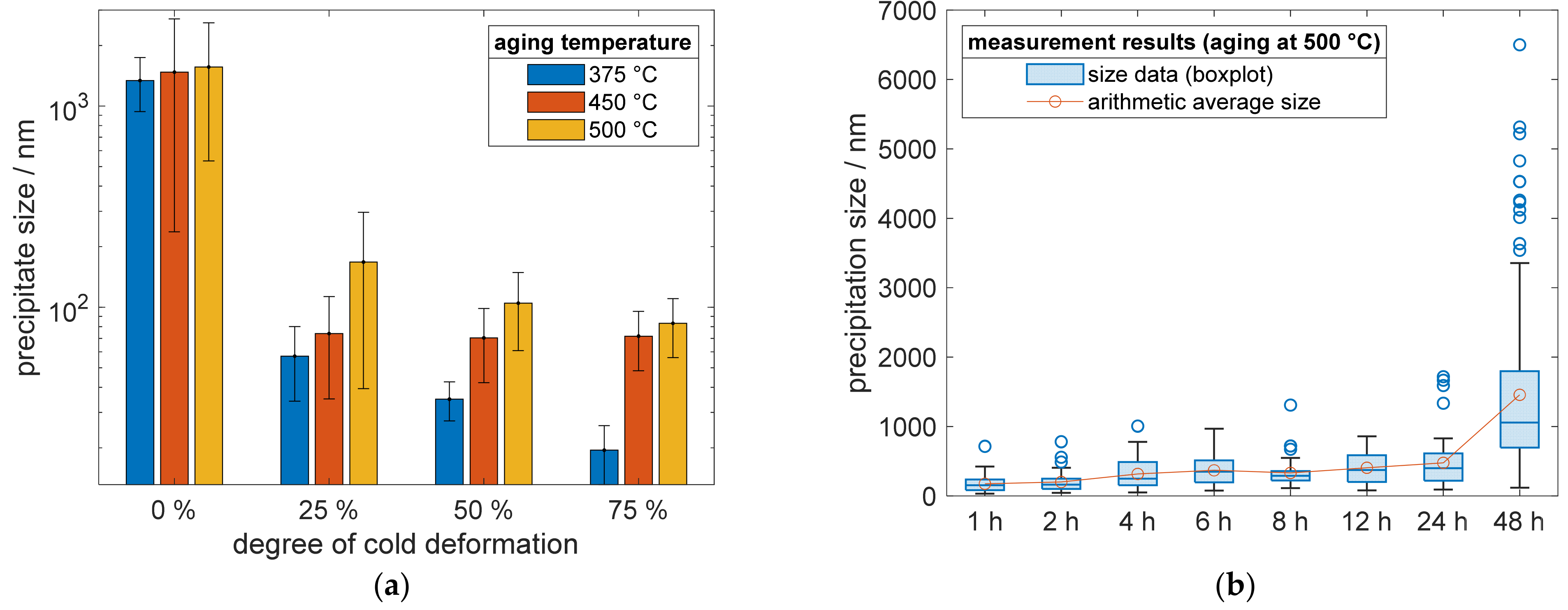
In addition, the quantification of precipitate sizes proved the discussed impacts. The sizes were reproducible and consistent (Figure 11a) for all analyzed temperatures, 375 °C, 450 °C, and 500 °C, whereby increased aging temperatures promoted larger phases of Cu4Sc. The results of a prior cold deformation for all temperatures, in the bar chart of Figure 11a, showed a clear trend of decreasing precipitation sizes, while increasing the degree of cold deformation. The bars visualizing the material condition in Figure 11a, after 48 h aging treatment for direct comparison. The continuing time-dependent evolutions of Cu4Sc phases for specimens without prior cold deformation, are shown in Figure 11b for an aging treatment at 500 °C. With increasing aging time, the average precipitation size increased, showing their growing evolution. Parallel to the further growth of older precipitates, new ones continued to nucleate and grew in their early stages. Therefore, the spread of quartiles and whiskers, as well as the occurrence of outliers, increased.
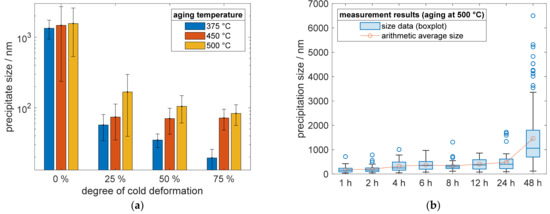
Figure 11.
Precipitate growth in CuSc0.3 (measured length of lamellar precipitation structures): (a) influence of optional cold working, before the following aging treatment at different temperatures, on resulting precipitate sizes; (b) precipitate growth of specimens without cold deformation, prior to the aging process at 500 °C.
3.2. Results for Copper–Scandium Alloy CuSc0.15
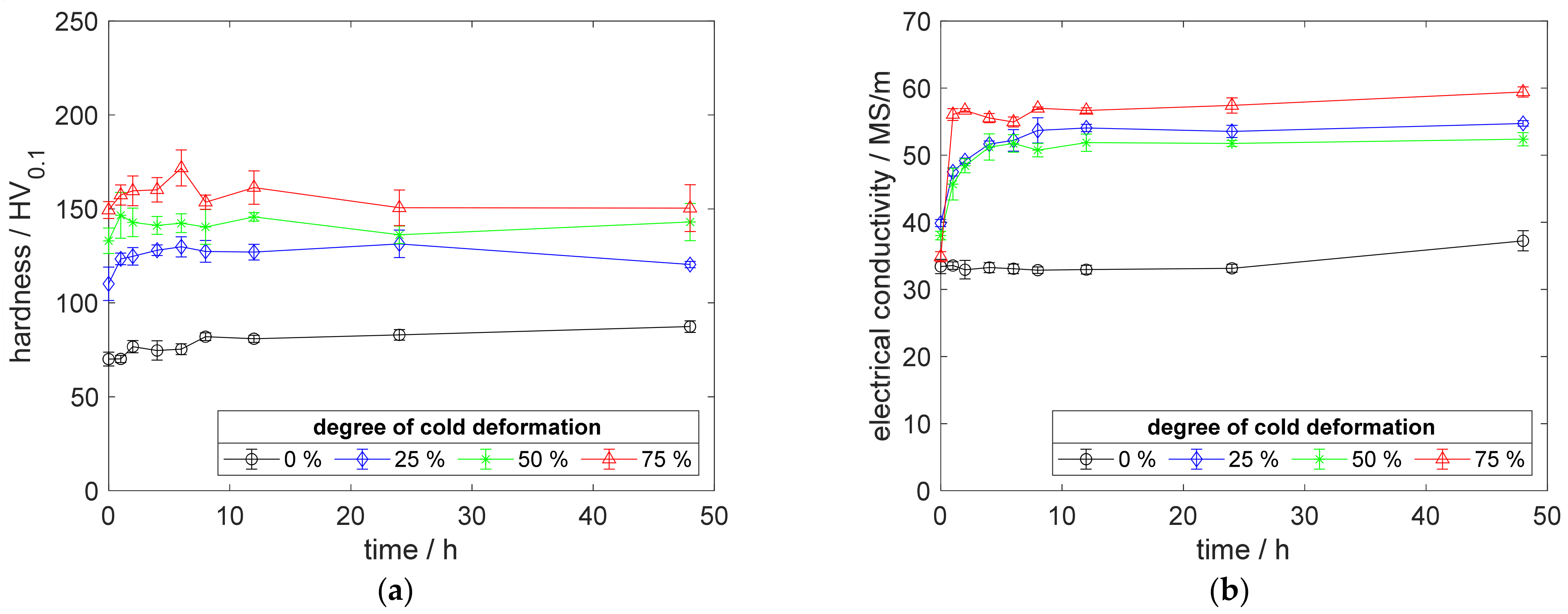
To compare the results on CuSc with the CuZr0.15 benchmark alloy, a reduced content of 0.15 wt.% scandium was the main point of this investigation. Starting to precipitate, with an increasing electrical conductivity for specimens without prior cold deformation, CuSc0.15 (Figure 12b) showed qualitative behavior similar to CuSc0.3 (Figure 4b). Cold deformation promoted precipitation and, therefore, a significant increase in conductivity (Figure 12b). At 375 °C aging temperature, CuSc0.15 reached an electric conductivity of 54.11 MS/m, which is a 6.1 MS/m increase in comparison to CuSc0.3 (Figure 4b).
Figure 12.
Property development during aging of CuSc0.15 at 375 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Whereas CuSc0.3 showed hardening effects during aging (Figure 4a, Figure 5a and Figure 6a), for all specimens and degrees of cold deformation, at 375 °C only the 25% cold-worked specimen of CuSc0.15 showed a significant increase in hardness (171.8 HV0.1 peak hardness). After the peak aged state, the hardness decreased by about 12.8 HV0.1 (Figure 12b). A higher degree of cold deformation did not bring further hardness increases but ended with a reduction in hardness, within the first hour of temperature exposure (Figure 12a).
As already observed for CuSc0.3, an increased aging temperature showed a similar impact on the material’s electrical conductivity. The higher aging temperature resulted in increased electrical conductivities for CuSc0.15 (Figure 12b and Figure 13b) and CuSc0.3 (Figure 4b, Figure 5b and Figure 6b). Comparing the 75% cold-worked specimens at aging temperatures of 375 °C and 425 °C (Figure 12b and Figure 13b), the higher temperature gained 3.72 MS/m, regarding the highest reachable electrical conductivity.
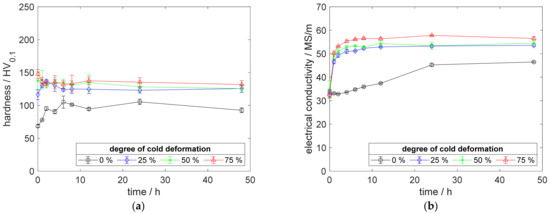
Figure 13.
Property development during aging of CuSc0.15 at 425 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Furthermore, the hardness development at 375 °C (Figure 12a) occurred even more intensively at 425 °C (Figure 13a). The measured hardness decreased faster and more intensively for specimens with 50% or 75% cold deformation, prior to the aging treatment (Figure 13a). As for the aging treatment at 375 °C, the 25% cold-deformed specimen remained unaffected and reached its peak hardness at 175.6 HV0.1 (Figure 13a). After the peak aged state, its hardness decreased by about 23.4 HV0.1. Therefore, at 425 °C, the peak-aged state reached a slightly higher hardness but was followed by a sharper overaging. The presented aging temperatures match perfectly the trend of the other aging temperatures completing the investigated range of 350 °C to 500 °C (Appendix A.2, Figure A5, Figure A6, Figure A7 and Figure A8).
CuSc0.15 started to recrystallize within the experiments after 48 h of heat treatment at 500 °C (specimens with 50% and 75% prior cold deformation). The average grain size of 368 µm was marginally larger for CuSc0.3, but it still emphasized the grain-refining influence of scandium in copper (Appendix A.5, Figure A13).
3.3. Results for Copper–Zirconium Alloy CuZr0.15
The analysis of CuZr0.15 was conducted to obtain a direct comparison with a commonly used reference alloy with identical processing.
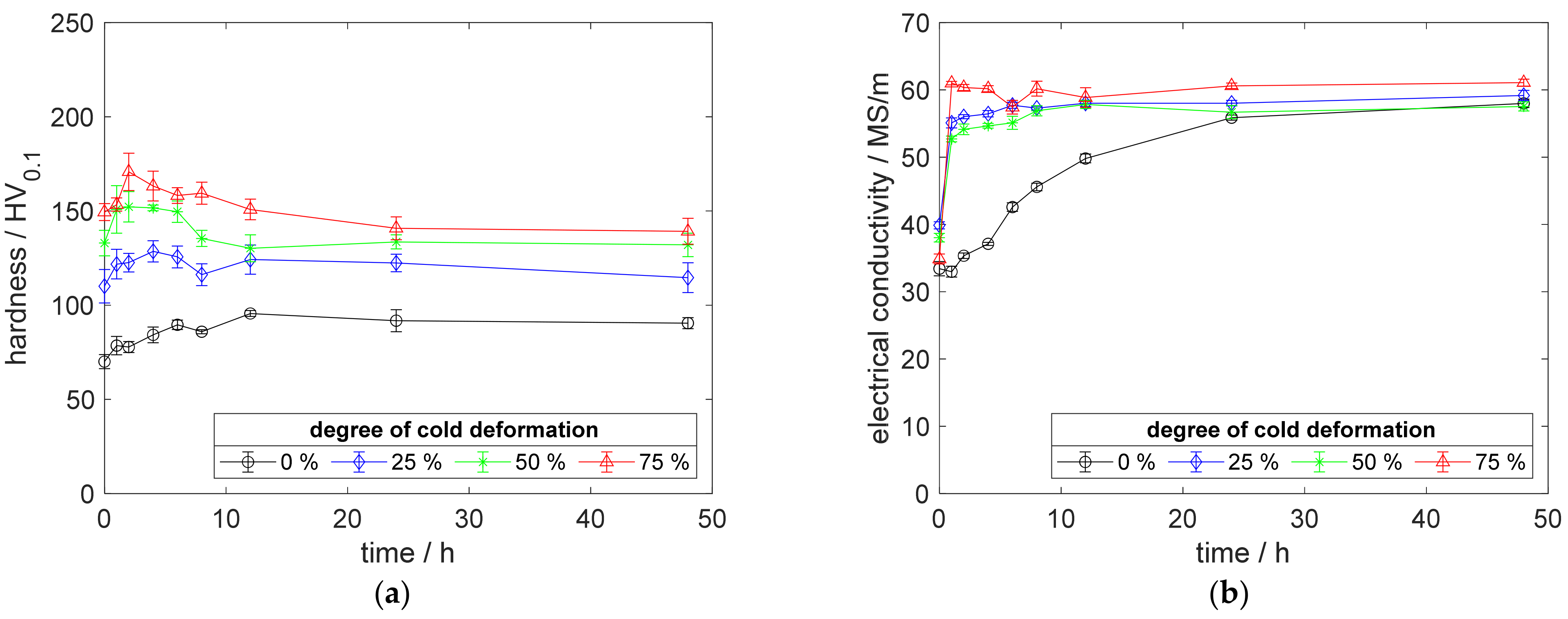
The aging process at 375 °C showed an immediate increase in the electrical conductivity for all cold-worked specimens (Figure 14b). The utilized temperature was not sufficient, to promote the reaction for the specimen without prior cold deformation, neither for hardness nor for sensible conductivity.
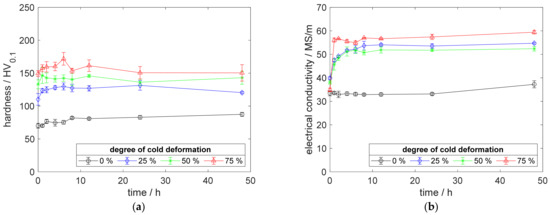
Figure 14.
Property development during aging of CuZr0.15 at 375 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Furthermore, Figure 14b shows an excellent conductivity of specimens made from CuZr0.15, with a maximum of 59 MS/m for 75% and 54.7 MS/m for 25% cold-worked specimens. This alloy, nearly, reached the conductivity of the raw Cu-OFE material. In direct comparison to scandium-containing alloys at this temperature (Figure 4b and Figure 12b), CuZr0.15 highlights its advantages in conductivity, especially in contrast to the equal alloying content of CuSc0.15 specimens.
In addition, CuZr0.15 showed its potential for precipitation hardening in Figure 14a. At 375 °C aging temperature, the peak hardness of 171.8 HV0.1 was followed by a hardness decrease, due to overaging of about 21.4 HV0.1. Regarding the peak hardness, the CuSc0.3 alloy reached more than 24.6 HV0.1 higher values with less hardness decreases during overaging up to 48 h (Figure 4a).
Increasing the aging temperature followed the trend of reduced peak hardness (Figure 15a) and increased reachable electrical conductivity (Figure 15b). Aging the alloy at 425 °C or higher (Appendix A.1) realized comparable maximum conductivities, for specimens with and without cold deformation before aging (Figure 15b). A direct comparison of CuSc0.15 and CuZr0.15, which use the same alloy content of 0.15 wt.%, is, particularly, interesting because they showed significant differences regarding the hardness development (Figure 12a, Figure 13a, Figure 14a and Figure 15a). The presented aging temperatures match perfectly the trend of the other aging temperatures completing the investigated range of 350 °C to 500 °C (Appendix A.1, Figure A1, Figure A2, Figure A3 and Figure A4).
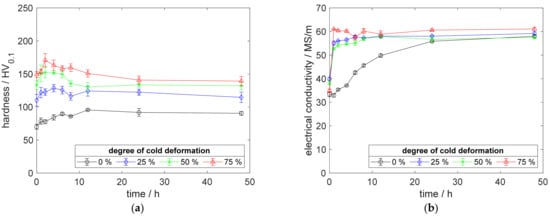
Figure 15.
Property development during aging of CuZr0.15 at 425 °C (solution annealing, quenching, cold working, aging): (a) Hardness; (b) Electrical conductivity.
Specimens of CuZr0.15, being cold worked prior to the aging process (50% and 75%), started to recrystallize comparably early after 48 h, at about 400 °C to 425 °C. Appendix A.4, Figure A12 provides further impressions on the recrystallization behavior. The average grain size of 308 µm was only slightly smaller than for CuSc0.3 (Appendix A.5, Figure A13).
4. Discussion
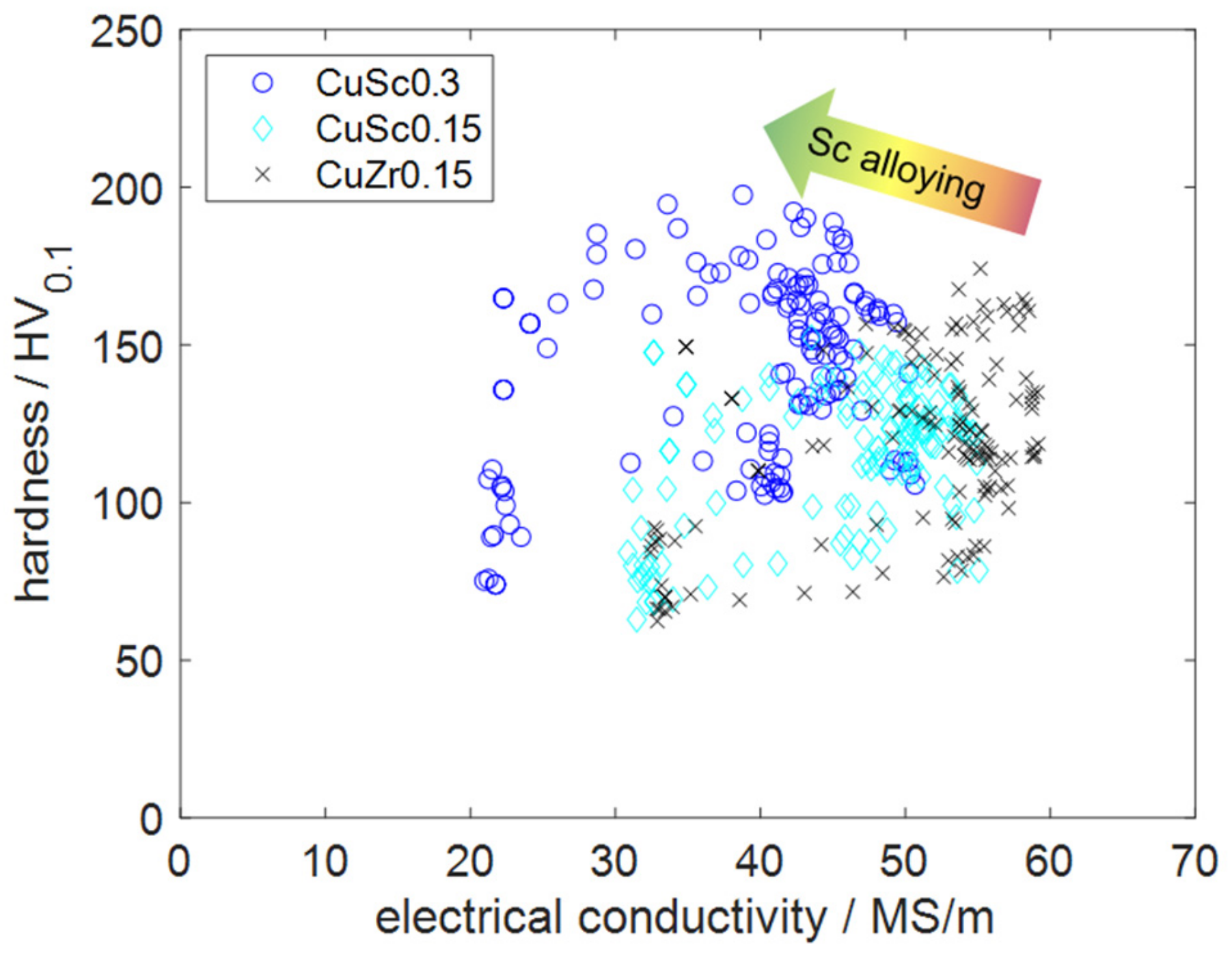
The main goal of this experimental study was to analyze the precipitation behavior of low-alloyed CuSc alloys and compare their properties with the commonly used benchmark alloy CuZr0.15, visualized in Figure 16.
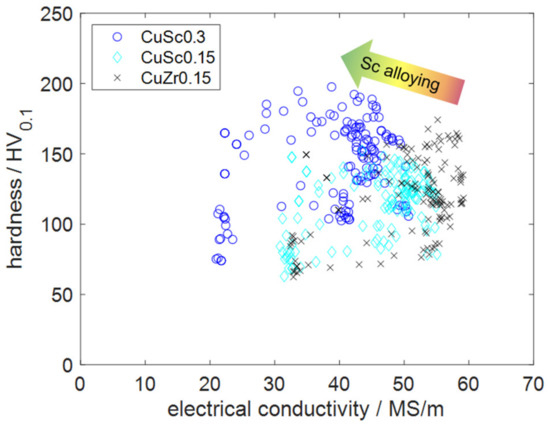
Figure 16.
Comparison of the analyzed alloys, regarding the two target properties.
The industrial application of CuZr is commonly used, if the requirements include high electrical or thermal conductivity paired with enhanced mechanical properties and better temperature resistance than pure copper. CuCr, CuMg, or the CuBe family with CuNiSi alloys might be options regarding further increased mechanical properties. Regarding electrical conductivity, these materials would be accompanied by several disadvantages [1,7,10,38]. CuSc alloys fit into the surrounding of several benchmark alloys, which were defined by CuZr0.15 in this study. The scatter plot of Figure 16, clearly, illustrates how the investigated copper alloys containing scandium behaved in comparison with the chosen benchmark, in the case of similar production processes. CuSc0.3 alloys appeared to be higher-performing alloys, due to their enhanced mechanical properties. The highest reachable absolute hardness of CuSc0.3 was 198 HV0.1, which exceeded CuZr0.15 by 24 HV0.1.
Regarding reachable electrical conductivity, CuZr0.15 defined the most beneficial alloy behavior, which is accentuated by common industrial applications for this alloy. With the conducted aging treatment, the electrical conductivities of CuZr0.15 and the scandium-containing alloys were significantly increased. Comparing the recrystallization behavior within the conducted experiments, the scandium-containing alloys CuSc0.3 and CuSc0.15 appeared to provide more advantages than the benchmark. Especially during enhanced product lifetimes, these properties could be deciding, in cases of higher operating temperatures and enhanced mechanical requirements.
4.1. Regarding the Electrical Conductivity
The measured electrical conductivity was a sensitive indicator for developing demixing processes. Generally, all alloys’ development of electrical conductivity, temperatures, and process variations showed continuously increased material properties, proving a precipitation reaction in all regarded alloys. The purity of the copper matrix rises, subsequently, with precipitation formation and growth.
Starting at the temperature’s impact on the precipitation of CuSc0.3, CuSc0.15 and CuZr0.15, in Figure 3, have shown how higher aging temperatures accelerated the increase in conductivity.
Due to its low solubility in copper [9] and the precipitation of intermetallic zirconium phases [12,13], this alloy, nearly, reached the conductivity level of the used raw material Cu-OFE. It defined the high standards for comparing the other specimens (Figure 3). Especially in early aging stages, CuSc0.15 and CuSc0.3 showed significant increases in electrical conductivity as well (Figure 3). CuSc0.15 used less than half the element’s maximum solubility concentration in copper. Utilizing the conducted aging process, the resulting microstructure of CuSc0.15 influenced the properties of the material less than CuSc0.3 and ended up in higher reachable electrical conductivities (Figure 3).
4.2. Utilizing Cold Deformation
The introduction of 25%, 50%, or 75% cold deformation increased the density of dislocations, which contributed to the materials’ mechanical properties in the first step, due to dislocation hardening [39]. Several influences should be considered, regarding the aging process: first, the defect structure, vacancies, and local strains’ dislocations can contribute as catalytic influences on nucleation [40]. Second, dislocations support the diffusion as high diffusivity paths, especially at lower temperatures [3,41]. Furthermore, the vacancy concentration increases by cold deformation [35] and facilitates diffusion [3]. For CuSc0.3 alloys, the trend of reaching peak-aged hardness by enhanced prior cold deformation accelerated. Regarding the precipitation starting temperature of CuSc0.3, as shown in Figure 3c, a peak-aging treatment at 375 °C took 2 h for a 75% and 50% cold-deformed specimen, 4 h for a 25% cold deformed specimen, and 48 h for a specimen without a cold deformation. The enhanced density of defects accelerated the precipitation remarkably, especially at lower aging temperatures.
This effect was visualized, regarding the electrical conductivity development of CuSc0.3, in Figure 4b and Figure 5b, in direct comparison to Figure 6b. Temperatures below 400 °C showed a substantial difference of peak conductivities, between CuSc0.3 specimens without a prior cold deformation and the cold-worked options. Regarding the named targeting goals of the optimum simultaneous combination of enhanced mechanical properties and conductivity, the lower aging temperatures were, especially, favorable.
For CuSc0.15, this impact appeared consistently and further intensified regarding the high degrees of cold deformation. For a reduced scandium content of 0.15 wt.%, the aging temperature of the specimen without prior cold deformation needed to reach 450 °C, to provide comparable conductivities to the cold-worked specimen.
The corresponding benchmark alloy CuZr0.15 showed a less sensitive reaction. Specimen of CuZr0.15, without cold working before the aging process, reached the others’ level at 425 °C aging temperature or higher. This direct comparison shows the importance of reproducible and comparable analyses to define the operational ranges and advantages of different alloys.
4.3. Analyzing the Hardening Mechanism
Due to the coarsening precipitates and the transition between the highly strengthening interaction of the coherent strengthening with the coexisting Orowan mechanism [35], to a singular Orowan mechanism, the hardness decreased after the peak-aged condition was reached. These observations were in good agreement with the published results of Hao et al. [35]. The nucleation and growth of precipitates during the whole aging process is a continuing process, which resulted in an enlarged variation of precipitation sizes in Figure 11b, with the aging time. Next to the growth of existing precipitates, new structures were further built.
A lower aging temperature reduced the risk of rapidly growing precipitates, Ostwald-ripening, and rapid overaging (Figure 5a). In combination with an enhanced degree of cold deformation, many fine precipitates were promoted (Figure 10) and resulted in the most beneficial results for CuSc0.3 (Figure 4a, Figure 5a and Figure 6a). Therefore, a lower aging temperature and lower diffusion in the material, combined with many potential nucleation sites for precipitates, appeared to provide the most advantageous absolute combination.
For the specimen with cold deformation before the aging treatment, the annihilation of dislocations needs to be considered during the whole production process. Especially, the high aging temperatures promoted, with easier diffusion, the decrease in the dislocation-hardening contribution. Therefore, the total difference, between the solution annealed and the optionally cold-worked initial state and the peak-aged condition, was inclined to increase for samples without a prior cold deformation. For an aging treatment at 375 °C, the most significant hardness increase of the 0% cold-deformed specimen was 48 HV0.1. In contrast, the 75% specimen showed an absolute difference of 32 HV0.1.
Nevertheless, CuSc0.3 showed outstanding absolute peak hardness values, reached with a sufficient influence of cold working prior to the aging process (Figure 4a, Figure 5a and Figure 6a). Whereas the electrical conductivity of as-cast solution annealed, quenched and aged specimens of CuSc0.3 were able to reach the level of cold-worked specimens (Figure 4b, Figure 5b and Figure 6b), so the hardness was more affected by the production process (Figure 4a, Figure 5a and Figure 6a). CuSc0.15 showed an even more meaningful interaction of hardening mechanisms during the aging treatment. The concurring effects of dislocation annihilation, material regeneration, and precipitation hardening need to be considered, in cases of decreasing hardness of specimens that were cold worked and aged at 375 °C to 425 °C (Figure 12a and Figure 13a). Recrystallization did not affect the results at this temperature range. Compared to an equal alloying content in CuZr0.15, the force to build a huge amount of strengthening precipitates must be sufficient to compensate concurring effects.
Considering the background of classical nucleation and growth theories, the driving force to nucleation and precipitation growth relies on a supersaturated solid solution [40,42]. Since nucleation and precipitation growth are strongly impacted by supersaturation [43,44], the potential of precipitation hardening for quenched alloys, supersaturated near their maximum solubility, is the largest. The discussed mechanisms underline how CuSc0.15 was influenced by a lowered degree of supersaturated solid solution. CuSc0.3 and CuZr0.15 were close to their maximum solubility in copper. To sum up, the in-depth analysis of the material’s behavior appears to be essential, for further alloy optimization and a beneficial production process.
4.4. Regarding Recrystallization Behavior and As-Cast Microstructure
Alloying elements reduce the mobility of grain boundaries, and fine precipitates contribute, through pinning effects, to the recrystallization and grain growth [3]. One strength of low-alloyed copper alloys is the improved softening behavior compared to pure copper [7]. The described recrystallization resistance of CuSc0.15, in comparison to conventionally used CuZr0.15 containing the same alloying content, emphasized the improvement potential of using CuSc alloys.
Additionally, the impacts on the as-cast states microstructure of copper, when alloying with zirconium or scandium, were considered to be comparable.
4.5. Summary
All experimental data on the hardness and electrical conductivity of specimens, without cold working performed prior to aging, were consistent and correlated with the published results of Franczak et al. [33]. Furthermore, the investigated visual precipitates appeared in lamellar structures, corresponding to the published investigation of strengthening effects [35] and their expected structure [2].
Hao et al. demonstrated the precipitation mechanisms, formation, and growth of Cu4Sc phases from the supersaturated solid solution, with detailed TEM investigations [35]. Focusing on moderate degrees of cold deformation before the aging process, the conducted research closed the gap between specimens aged directly after solution annealing and quenching, by Franczac et al. [33], and specimens with enhanced degrees of deformation prior to the aging process, by Hao et al. [35]. Referring to Hao et al., XRD measurements were conducted to verify the same crystallographic structure of the precipitates. The Cu4Sc-phase could not be proved yet in the specimen of this work because of limited resolution during the measurement and hardly definable peaks. Due to the strong correlation between aging times and aging temperatures, it can be assumed that the published Cu4Sc phase and its precipitation process were occurring in the conducted research activities. The corresponding shape and size of precipitates, as visualized in Figure 7a, Figure 8a, Figure 9 and Figure 10, confirmed and proved this. Furthermore, the trend of small precipitates, due to cold deformation and enhanced defect density (Figure 11), combined with the directly manipulated materials’ properties (Figure 4, Figure 5 and Figure 6), contributes, as further reinforcement.
For CuSc alloys, a suitable adjustment of the thermomechanical treatment was essential, for best material performance. Before aging treatment, a cold deformation of only 25% significantly promoted the precipitation process. Further-increased cold deformations affected the properties of the material (Figure 4, Figure 5 and Figure 6), on a less noticeable scale. This impact on materials’ properties correlated directly with investigations of resulting precipitation sizes, in Figure 11a. All results contributed to a consistent understanding of CuSc alloys and optimized a targeted manipulation of the precipitating intermetallic Cu4Sc phases.
5. Conclusions
Thermomechanical treatment had an important influence on precipitation hardening and, therefore, significantly impacted the material properties of CuSc alloys. This research analyzed alloys with up to 0.3 wt.% Sc, in direct comparison to CuZr0.15. The results agreed with the corresponding literature [2,33,35] and refined the thermomechanical treatment. The following conclusions represent the experimental results of this study:
- All alloys, CuZr0.15, CuSc0.15, and CuSc0.3, showed significant increases in the electrical conductivity, due to the aging treatment. Using a lower content of alloying elements resulted in a higher reachable electrical conductivity.
- The aging process was observed up to 48 h at 350 °C to 500 °C. Temperatures below 450 °C showed the greatest potential for precipitation hardening. After the peak-aged state of material hardness was reached, overaging followed and resulted in hardness decreases. Favorable temperature ranges were comparable for CuZr and CuSc alloys.
- Alloys with alloy content in the range of maximum solubility in copper (CuSc0.3 and CuZr0.15) showed the most significant potential for hardness increase, due to precipitation hardening. Regarding hardness, CuSc0.3 appeared to be outstanding, compared to the benchmark alloy CuZr0.15.
- For CuSc0.3, cold working highly supported the precipitation process and tremendously increased the peak hardness. A lower aging temperature and a higher degree of cold deformation resulted in excellent mechanical properties.
- Scandium-containing copper alloys appeared to be well-equipped, regarding their recrystallization behavior. Moreover, the discussed advantages directly compared to CuZr0.15 might suggest applications at higher operating temperatures.
The thorough discussion of this research work has contributed to an in-depth view of how copper-based alloys can be designed for various application profiles. Especially, moderate degrees of deformation have been emphasized during this research work, which had not been regarded in the literature so far.
Whereas CuZr0.15 showed its strength for very high conductivity requirements and moderate mechanical requirements, CuSc0.3 showed its advantages for enhanced strength and high conductivity requirements, paired with an improved recrystallization behavior. Therefore, CuSc0.3 showed its potential for high-performance copper alloys at enhanced operating conditions.
Further studies are needed to determine the deciding material properties and analyze whether these findings at room temperature can contribute to advanced material behavior at higher operating temperatures. To further scoop the potential of CuSc alloys, ternary combinations will be assessed.
Author Contributions
J.D. is the principal author of this article, who carried out most of this study for their doctoral research. R.H. conducted Thermocalc simulations and assisted in REM analysis and grain size measurements. U.P. and A.Z. supervised the research project. R.H., U.P., A.Z. and G.N. helped in scripting and finalizing the article. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Baden-Wuerttemberg Ministry of Science, Research and the Arts and the Baden-Wuerttemberg Cooperative State University, Stuttgart, via the funding program Open Access Publishing.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
A.1. Results for Copper–Zirconium Alloy CuZr0.15
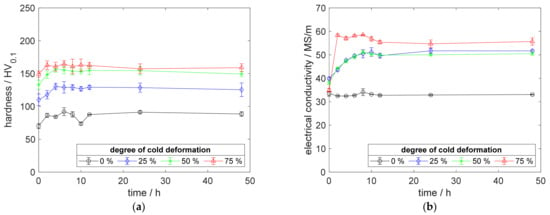
Figure A1.
Property development during aging of CuZr0.15 at 350 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A1.
Property development during aging of CuZr0.15 at 350 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
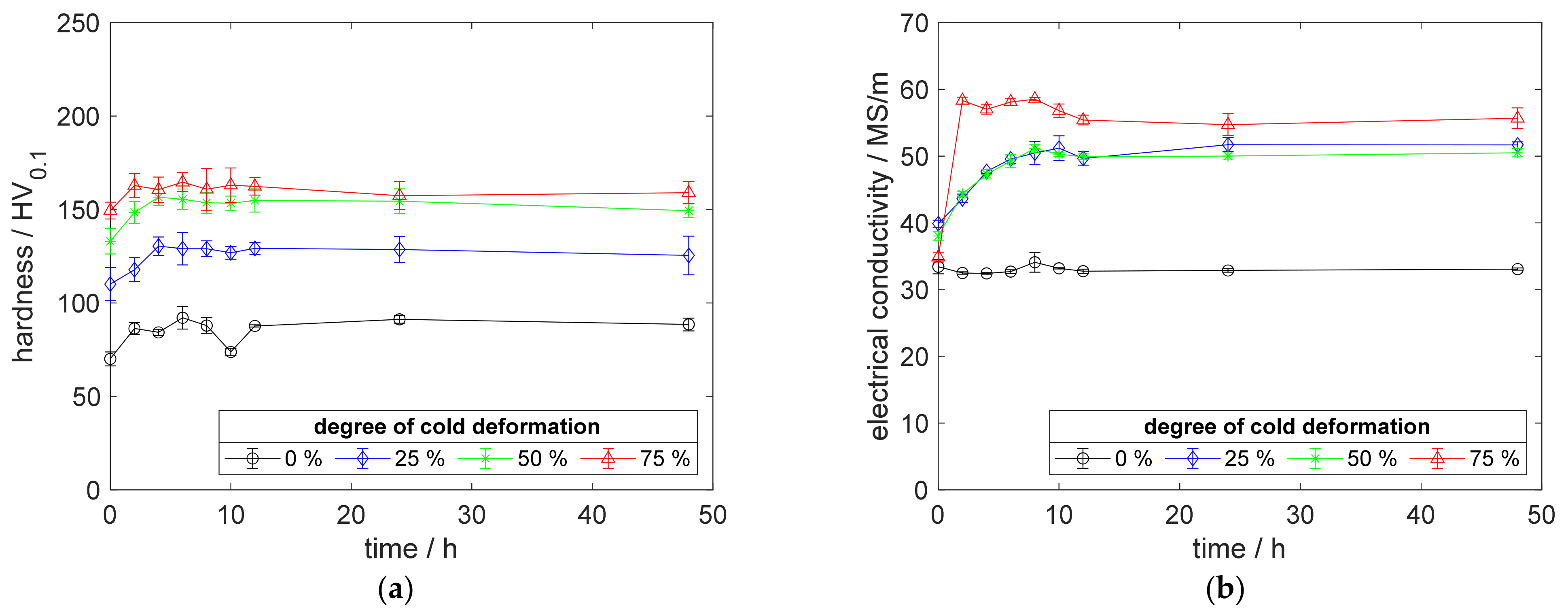
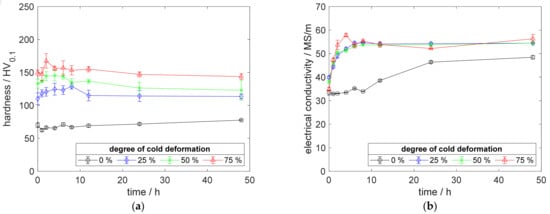
Figure A2.
Property development during aging of CuZr0.15 at 400 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A2.
Property development during aging of CuZr0.15 at 400 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
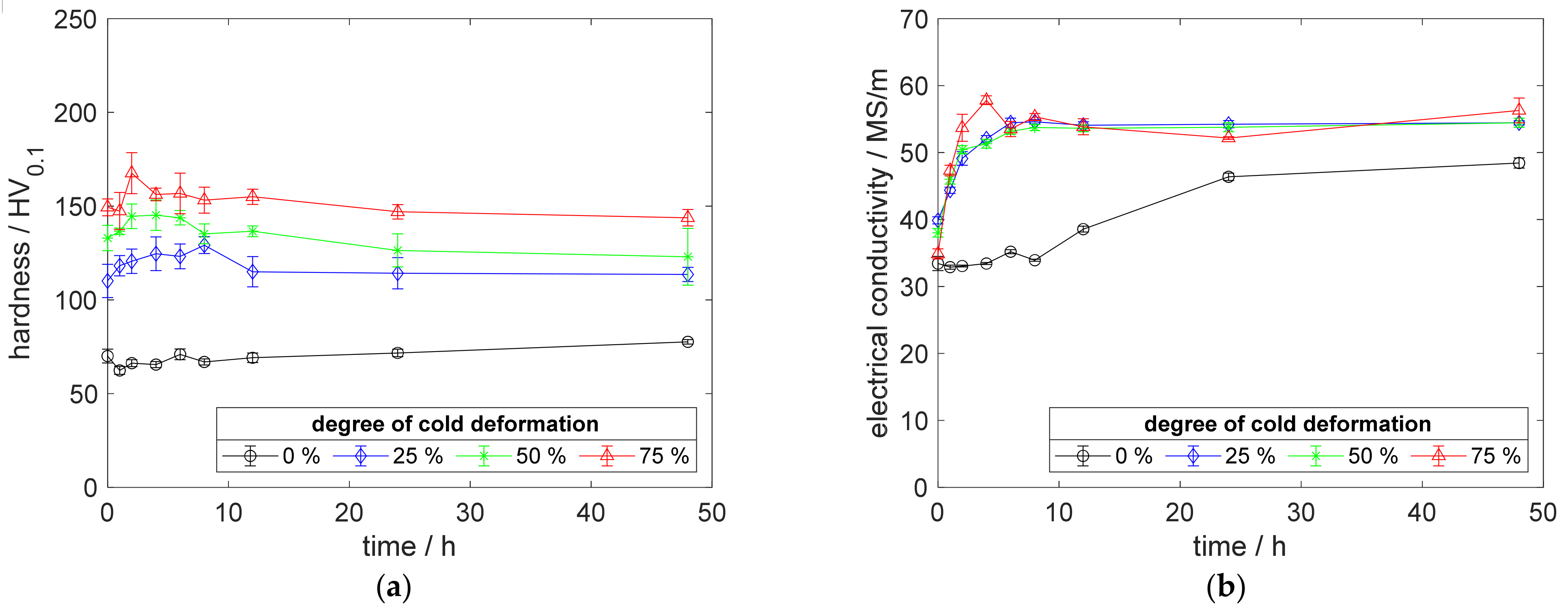
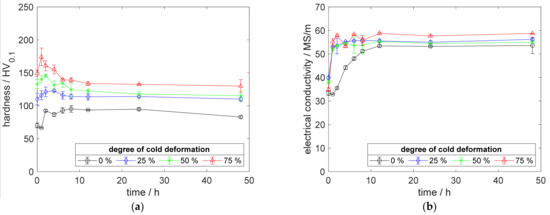
Figure A3.
Property development during aging of CuZr0.15 at 450 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A3.
Property development during aging of CuZr0.15 at 450 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
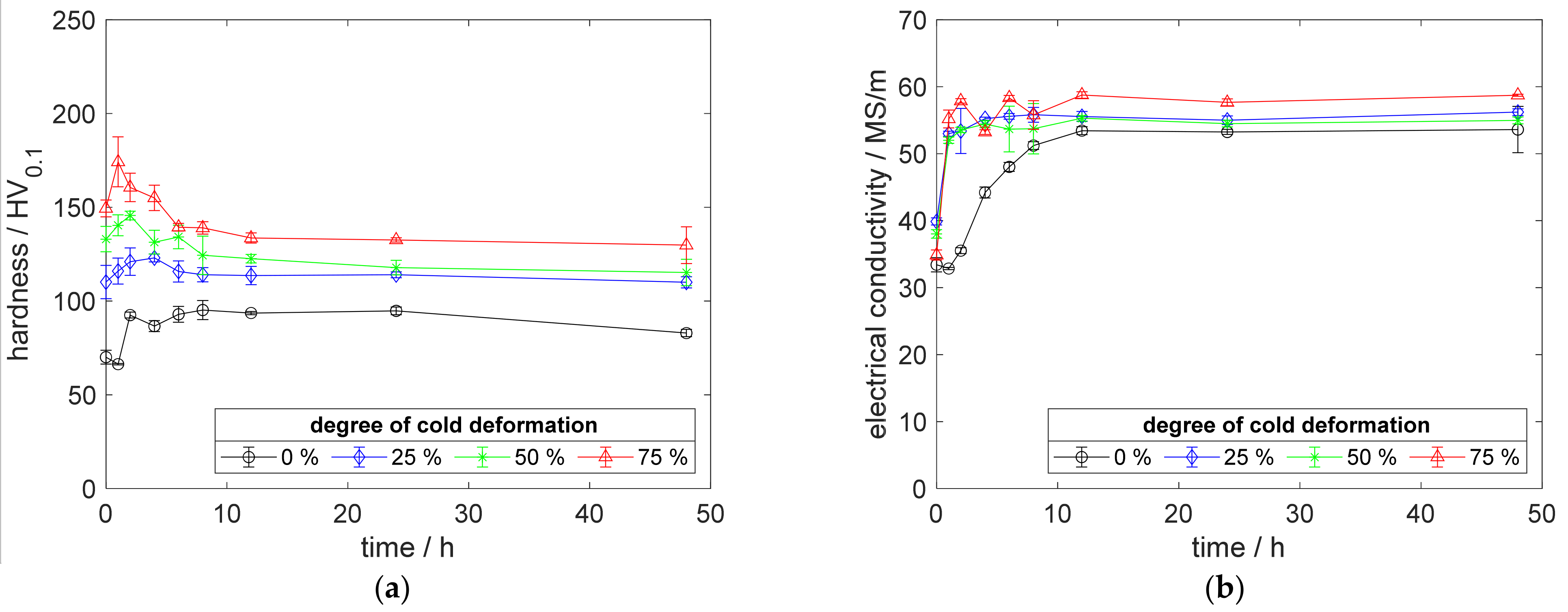
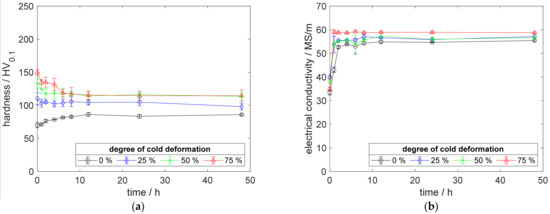
Figure A4.
Property development during aging of CuZr0.15 at 500 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A4.
Property development during aging of CuZr0.15 at 500 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
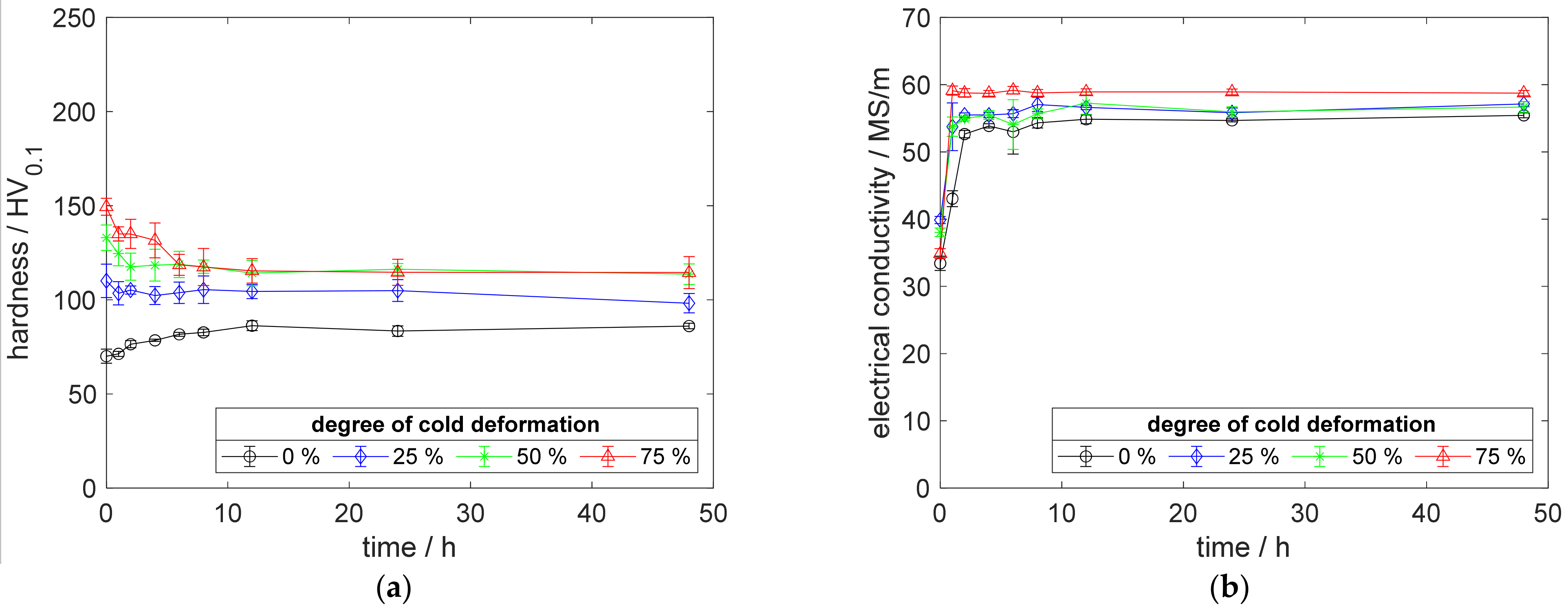
A.2. Results for Copper–Scandium Alloy CuSc0.15
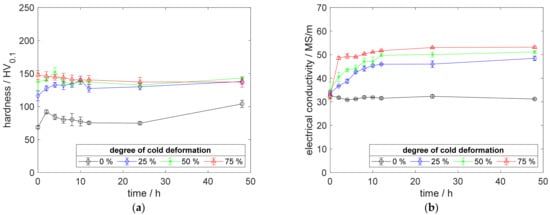
Figure A5.
Property development during aging of CuSc0.15 at 350 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A5.
Property development during aging of CuSc0.15 at 350 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
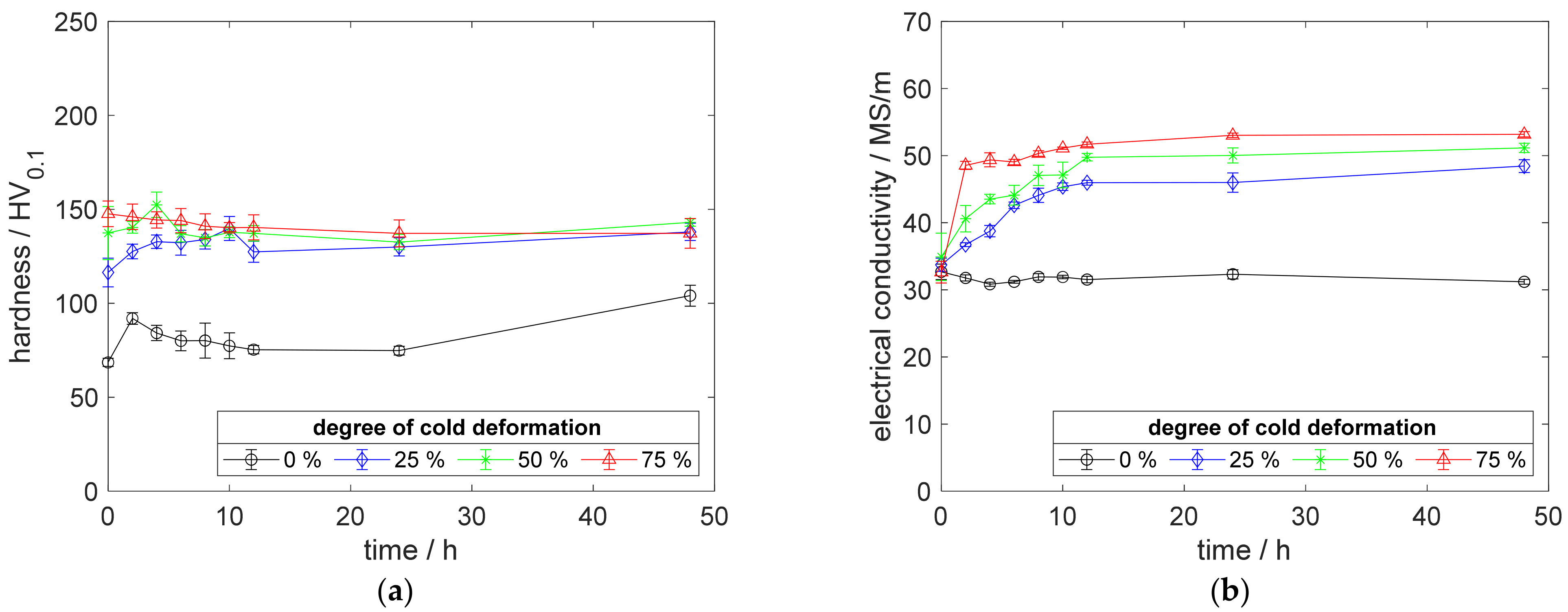
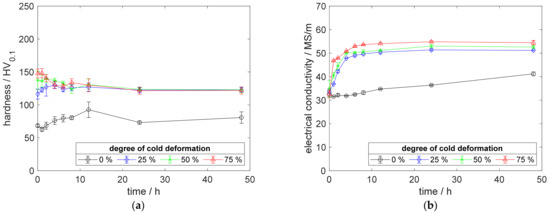
Figure A6.
Property development during aging of CuSc0.15 at 400 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A6.
Property development during aging of CuSc0.15 at 400 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
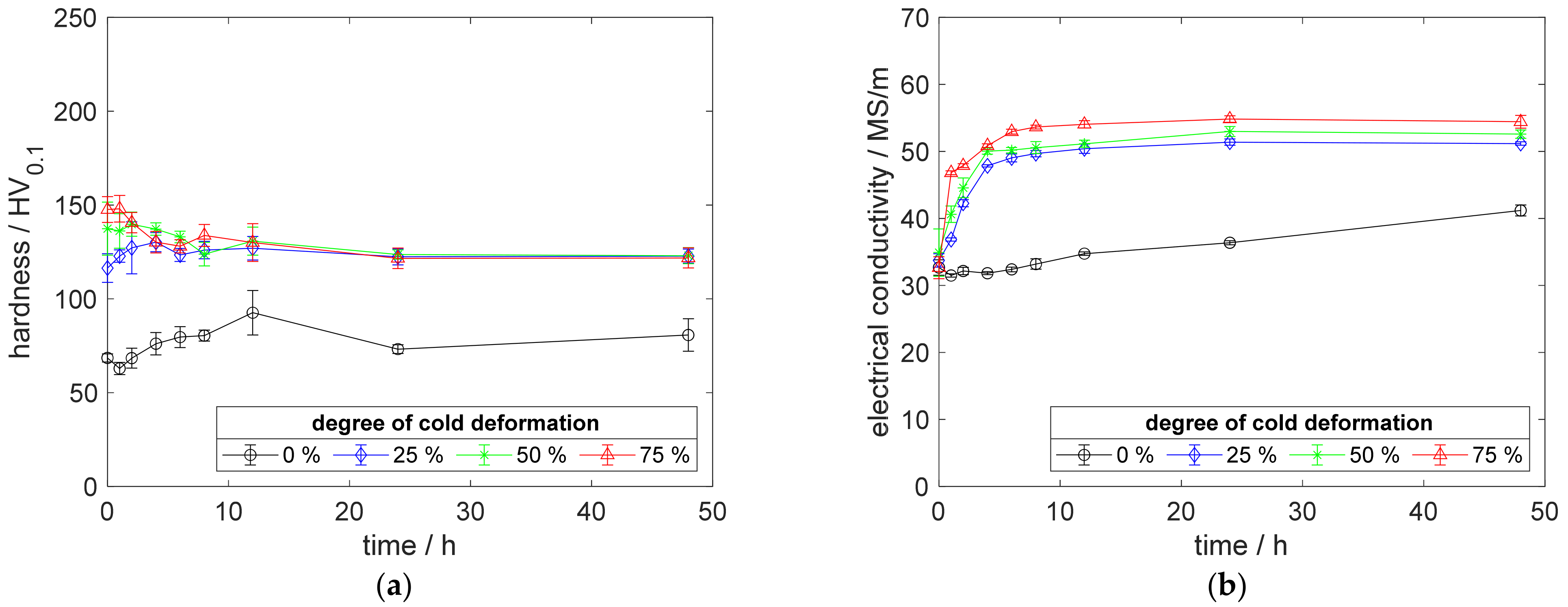
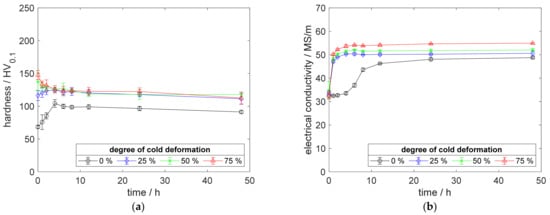
Figure A7.
Property development during aging of CuSc0.15 at 450 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A7.
Property development during aging of CuSc0.15 at 450 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
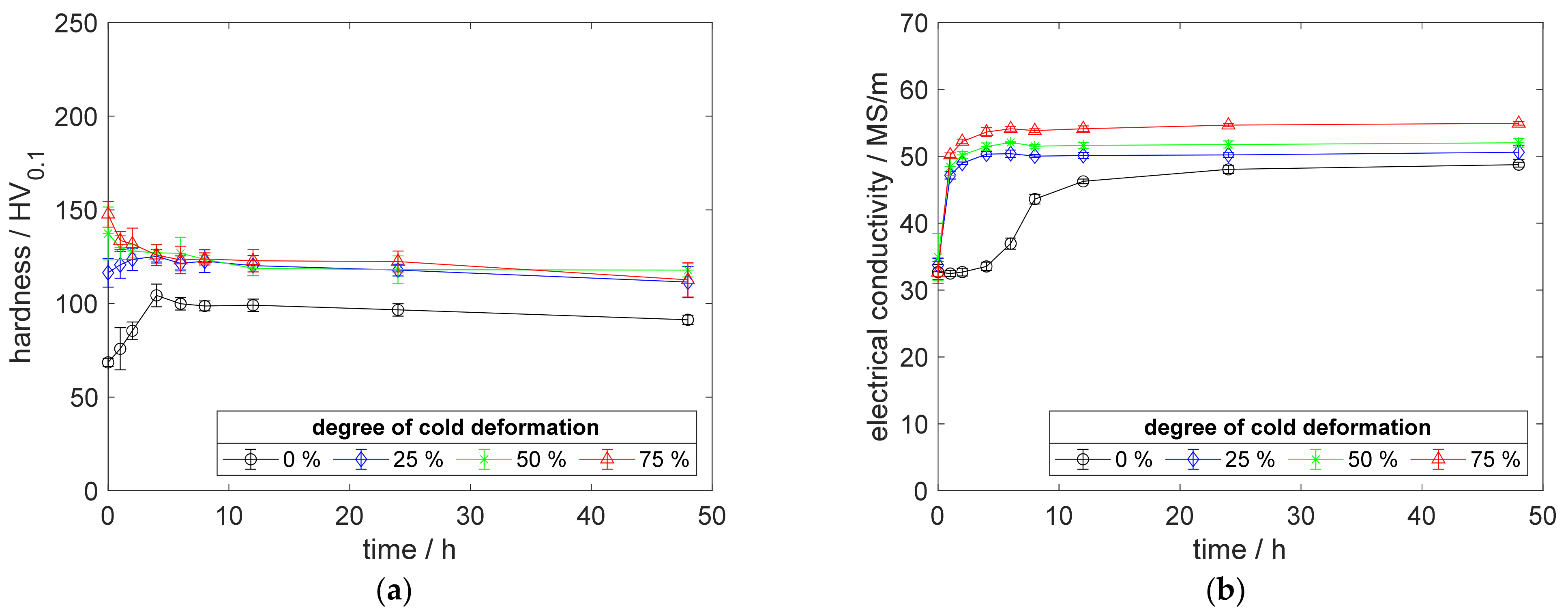
Figure A8.
Property development during aging of CuSc0.15 at 500 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A8.
Property development during aging of CuSc0.15 at 500 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
A.3. Results for Copper–Scandium Alloy CuSc0.3
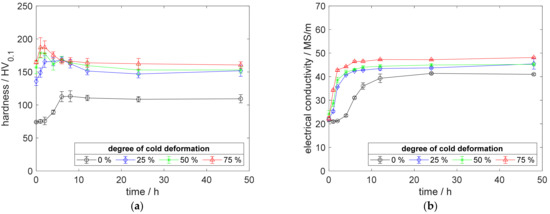
Figure A9.
Property development during aging of CuSc0.3 at 400 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A9.
Property development during aging of CuSc0.3 at 400 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
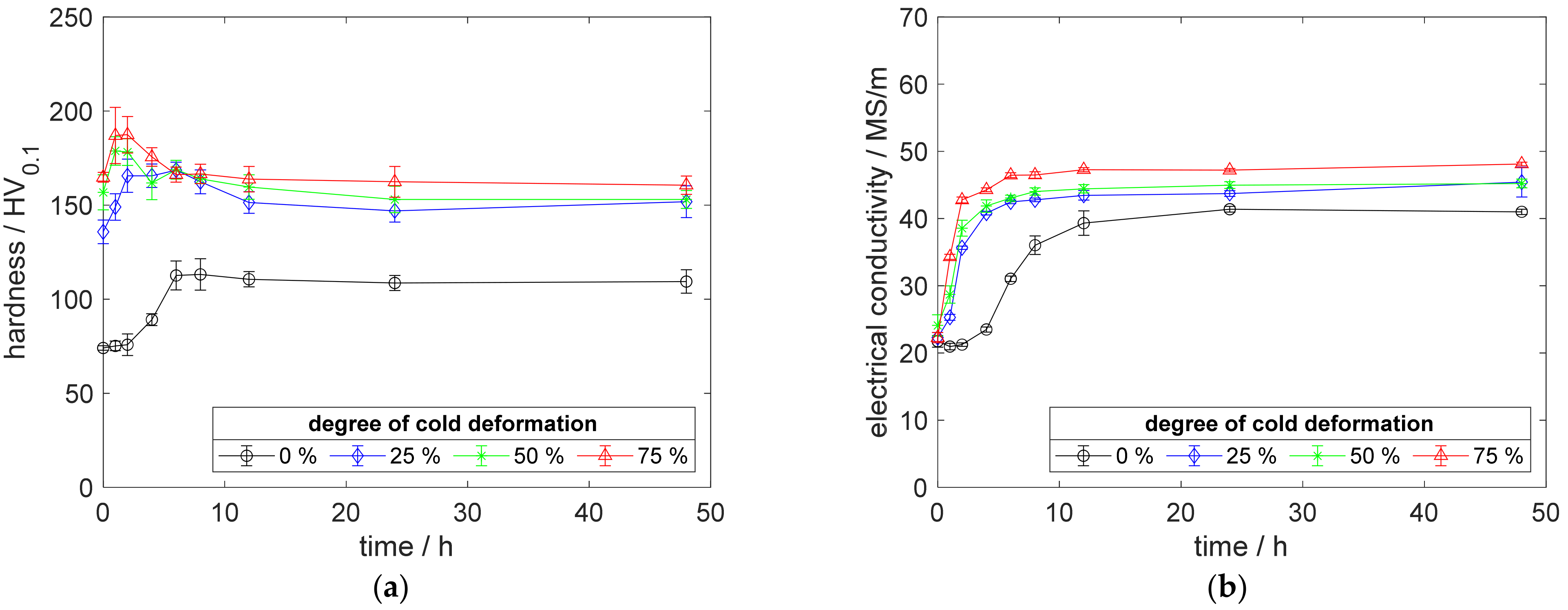
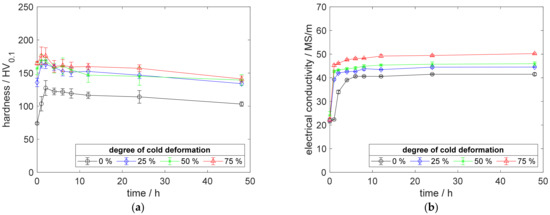
Figure A10.
Property development during aging of CuSc0.3 at 450 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A10.
Property development during aging of CuSc0.3 at 450 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
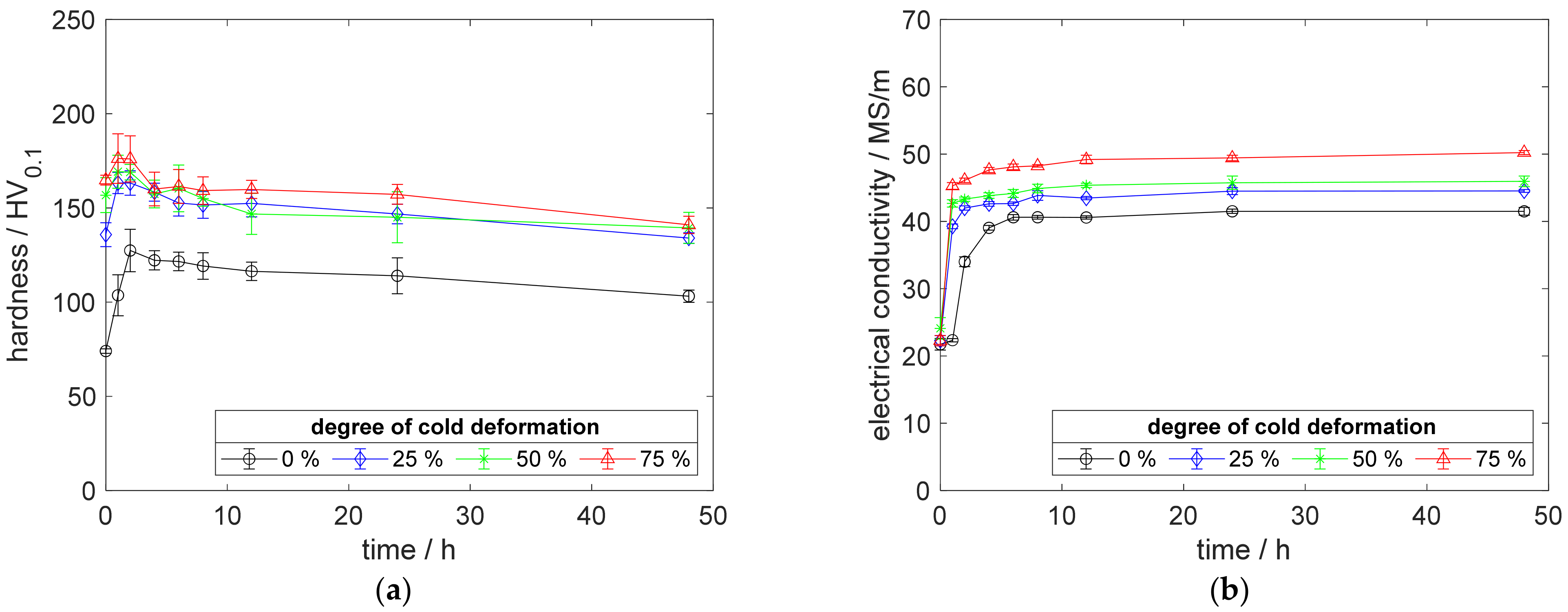
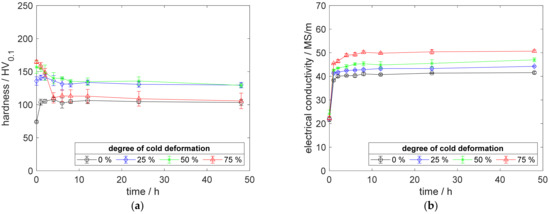
Figure A11.
Property development during aging of CuSc0.3 at 500 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
Figure A11.
Property development during aging of CuSc0.3 at 500 °C (solution annealing, quenching, cold working, aging): (a) hardness; (b) electrical conductivity.
A.4. Further Investigations on the Recrystallization Behavior
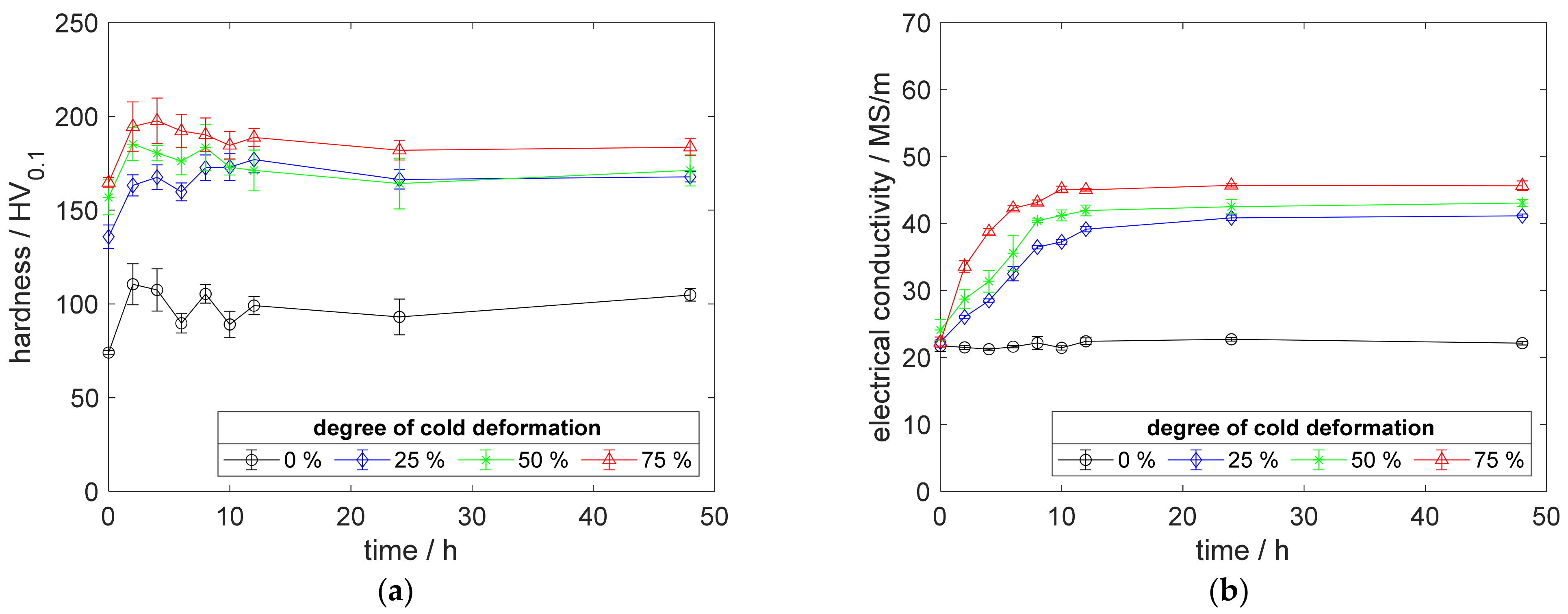
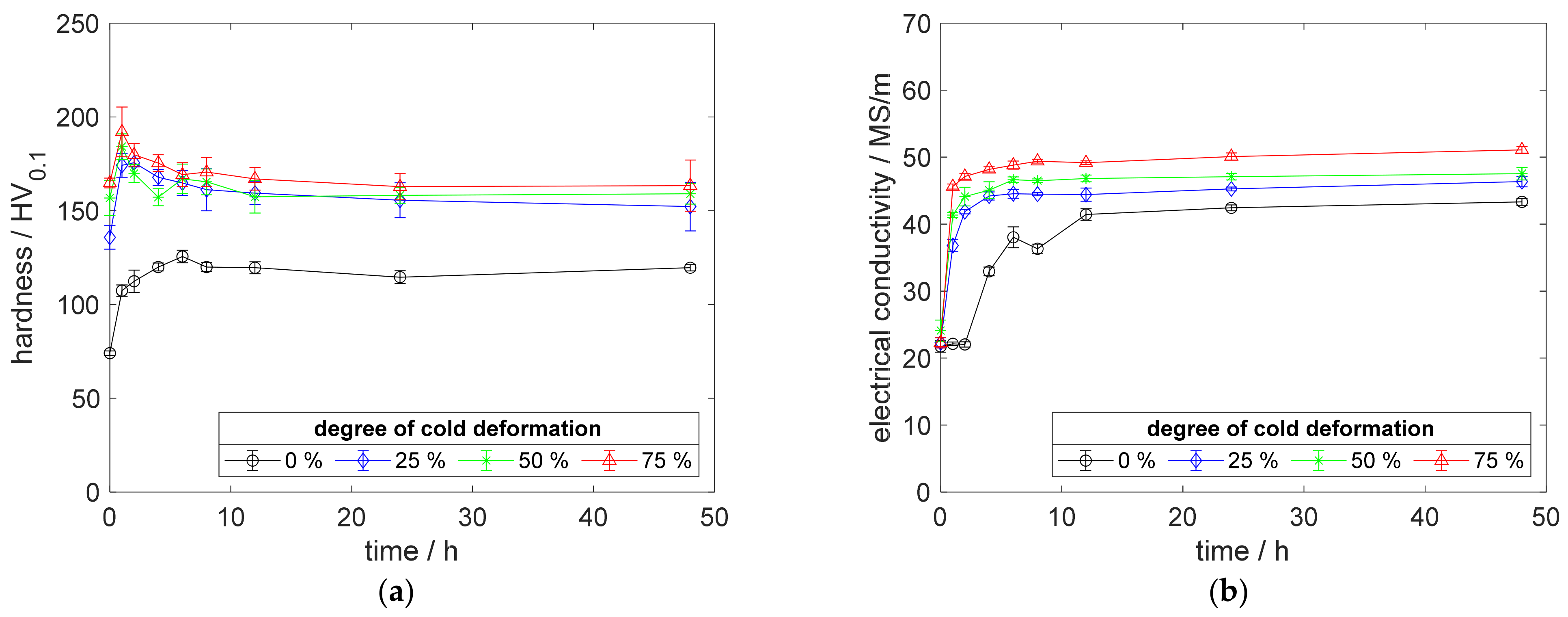
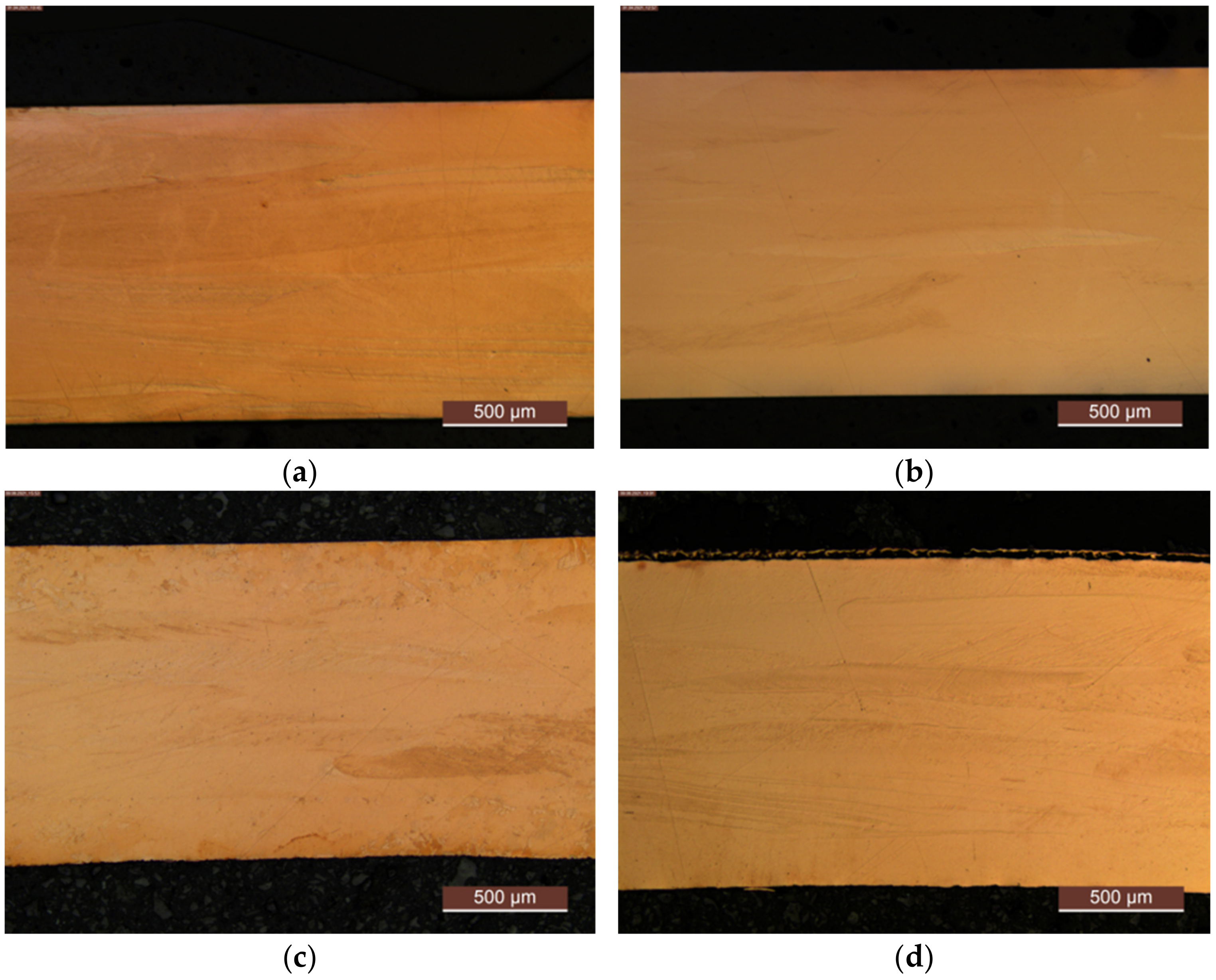
For deeper insight into the described differences in recrystallization behavior, an example, after aging at 500 °C for 48 h, is given in Figure A12.
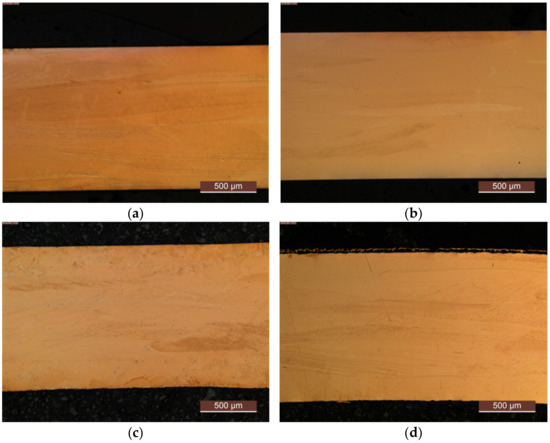
Figure A12.
Impressions on recrystallization behavior: (a) CuZr0.15: annealed, quenched, and 75% cold worked; (b) CuSc0.3: annealed, quenched, and 75% cold worked; (c) CuZr0.15: annealed, quenched, 75% cold worked, and aged at 500 °C for 48 h; (d) CuSc0.3: annealed, quenched, 75% cold worked, and aged at 500 °C for 48 h.
Figure A12.
Impressions on recrystallization behavior: (a) CuZr0.15: annealed, quenched, and 75% cold worked; (b) CuSc0.3: annealed, quenched, and 75% cold worked; (c) CuZr0.15: annealed, quenched, 75% cold worked, and aged at 500 °C for 48 h; (d) CuSc0.3: annealed, quenched, 75% cold worked, and aged at 500 °C for 48 h.
A.5. Further Investigations on the Grain Size
For documentation of the described grain size, measurements in Figure A13 summarizes the different materials.
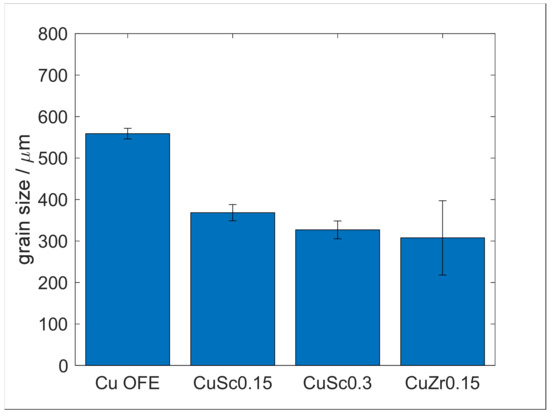
Figure A13.
Grain size in as-cast condition.
Figure A13.
Grain size in as-cast condition.
References
- Davis, J.R. Copper and Copper Alloys, 2nd ed.; ASM International: Novelty, OH, USA, 2008; ISBN 0871707268. [Google Scholar]
- Dies, K. Kupfer und Kupferlegierungen in der Technik; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-48932-7. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. (Eds.) Phase Transformations in Metals and Alloys, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781003011804. [Google Scholar]
- Miyake, J.; Fine, M. Electrical conductivity versus strength in a precipitation hardened alloy. Acta Met. Mater. 1992, 40, 733–741. [Google Scholar] [CrossRef]
- Gottstein, G. Materialwissenschaft und Werkstofftechnik: Physikalische Grundlagen, 4th ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-36602-4. [Google Scholar]
- ASM International. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials, 10th ed.; ASM International: Novelty, OH, USA, 2000; ISBN 0871703785. [Google Scholar]
- Kupferinstitut, D. Niedriglegierte Kupferwerkstoffe: Eigenschaften-Verarbeitung-Verwendung. TechnologieForum Kupfer d. DKI 2012, 8, 1–36. [Google Scholar]
- Molodova, X.; Khorashadizadeh, A.; Gottstein, G.; Winning, M.; Hellmig, R.J. Thermal stability of ECAP processed pure Cu and CuZr. Int. J. Mater. Res. 2007, 98, 269–275. [Google Scholar] [CrossRef]
- Arias, D.; Abriata, J.P. Cu-Zr (Copper-Zirconium). J. Phase Equilibria Diffus. 1990, 11, 452–459. [Google Scholar] [CrossRef]
- Hashmi, S. (Ed.) Copper Alloys: Properties and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Watanabe, H.; Miyamoto, K.; Kunimine, T.; Monzen, R.; Muramatsu, N.; Nomura, K.; Ueno, S. Effect of Thermo-Mechanical Treatment on Electrical Conductivity and Strength of Cu–0.29 mass%Zr Alloy Wires. Mater. Trans. 2021, 62, 1710–1715. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Li, Y.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and its effects on properties of a Cu−0.12 wt% Zr alloy. Mater. Sci. Eng. A 2015, 633, 28–34. [Google Scholar] [CrossRef]
- Nakashima, K.; Miyamoto, K.; Kunimine, T.; Monzen, R.; Muramatsu, N. Precipitation behavior of Cu–Zr compounds in a Cu-0.13 wt%Zr alloy. J. Alloys Compd. 2019, 816, 152650. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Wang, R.-C.; Peng, C.-Q.; Feng, Y.; Wang, X.-F.; Wu, X.; Cai, Z.-Y. Effect of elevated-temperature annealing on microstructureand properties of Cu−0.15Zr alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3772–3784. [Google Scholar] [CrossRef]
- Riva, S.; Yusenko, K.V.; Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R. The scandium effect in multicomponent alloys. Int. Mater. Rev. 2016, 61, 203–228. [Google Scholar] [CrossRef] [Green Version]
- Totten, G.E. (Ed.) Alloy Phase Diagrams, 1st ed.; ASM International: Novelty, OH, USA, 2017; ISBN 978-1-62708-070-5. [Google Scholar]
- Murray, J.L. The Al-Sc (aluminum-scandium) system. J. Phase Equilibria Diffus. 1998, 19, 380–384. [Google Scholar] [CrossRef]
- Ostermann, F. Anwendungstechnologie Aluminium, 3rd ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-662-43806-0. [Google Scholar]
- Xu, C.; Xiao, W.; Hanada, S.; Yamagata, H.; Ma, C. The effect of scandium addition on microstructure and mechanical properties of Al–Si–Mg alloy: A multi-refinement modifier. Mater. Charact. 2015, 110, 160–169. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Yang, J.; Dang, J.; Xu, H.; Du, Z. Effects of Sc content on the microstructure of As-Cast Al-7wt.% Si alloys. Mater. Charact. 2012, 66, 104–110. [Google Scholar] [CrossRef]
- Pramod, S.L.; Rao, A.P.; Murty, B.S.; Bakshi, S.R. Effect of Sc addition and T6 aging treatment on the microstructure modification and mechanical properties of A356 alloy. Mater. Sci. Eng. A 2016, 674, 438–450. [Google Scholar] [CrossRef]
- Shevchenko, S.V.; Neklyudov, I.M.; Lopata, A.T.; Voevodin, V.; Sytin, V.I. Influence of Microadditions of Yttrium on the Structure, Mechanical Properties, and Conductivity of Copper. Mater. Sci. 2000, 36, 901–909. [Google Scholar] [CrossRef]
- Arzhavitin, V.M.; Korotkova, I.M.; Sytin, V.I. Grain-boundary internal friction of yttrium- or scandium-microalloyed copper. Russ. Met. Met. 2016, 2016, 229–234. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Qin, N.; Zhang, Y.; Li, S.; Xiao, Z.; Lei, Q.; Li, Z. Effects of minor rare earths on the microstructure and properties of Cu-Cr-Zr alloy. J. Alloys Compd. 2020, 847, 155762. [Google Scholar] [CrossRef]
- Goncharuk, L.V.; Sidorko, V.R. Thermodynamic properties of scandium-copper compounds. Sov. Powder Met. Met. Ceram. 2006, 45, 72–75. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E.; Chakrabarti, D.J. The Cu-Sc (Copper-Scandium) System. Bull. Alloy. Phase Diagr. 1988, 9, 378–382. [Google Scholar] [CrossRef]
- Predel, B. Cu-Sc (Copper-Scandium). In Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys—Group IV Physical Chemistry, 5th ed.; Madelung, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. The Cr-Cu (Chromium-Copper) system. Bull. Alloy Phase Diagrams 1984, 5, 59–68. [Google Scholar] [CrossRef]
- Fuxiang, H.; Jusheng, M.; Honglong, N.; Zhiting, G.; Chao, L.; Shumei, G.; Xuetao, Y.; Tao, W.; Hong, L.; Huafen, L. Analysis of phases in a Cu–Cr–Zr alloy. Scr. Mater. 2003, 48, 97–102. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Xu, G.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloys Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lv, L. Quantum chemical calculations of thermodynamic and mechanical properties of the intermetallic phases in copper–scandium alloy. J. Theor. Comput. Chem. 2017, 16, 1750056. [Google Scholar] [CrossRef]
- Turchanin, M.A. Phase equilibria and thermodynamics of binary copper systems with 3d-metals. I. The copper-scandium system. Sov. Powder Met. Met. Ceram. 2006, 45, 143–152. [Google Scholar] [CrossRef]
- Franczak, K.; Kwaśniewski, P.; Kiesiewicz, G.; Zasadzińska, M.; Jurkiewicz, B.; Strzępek, P.; Rdzawski, Z. Research of mechanical and electrical properties of Cu–Sc and Cu–Zr alloys. Arch. Civ. Mech. Eng. 2020, 20, 28. [Google Scholar] [CrossRef] [Green Version]
- Shubin, A.B.; Shunyaev, K.Y. Copper-scandium system: Thermodynamic properties of intermetallics and liquid alloys. Russ. Met. Met. 2010, 2010, 672–677. [Google Scholar] [CrossRef]
- Hao, Z.; Xie, G.; Liu, X.; Tan, Q.; Wang, R. The precipitation behaviours and strengthening mechanism of a Cu-0.4 wt% Sc alloy. J. Mater. Sci. Technol. 2021, 98, 1–13. [Google Scholar] [CrossRef]
- Dölling, J.; Zilly, A. Niedriglegierte festigkeitsoptimierte Kupferbasislegierungen mit hohen Leitfähigkeitseigenschaften: Un-tersuchung des Potentials binärer CuSc-Legierungen. Metall 2021, 75, 328–331. [Google Scholar]
- Petzow, G. Metallographisches, Keramographisches, Plastographisches Ätzen, 7th ed.; Gebrüder Borntraeger: Stuttgart, Germany, 2015; ISBN 9783443230197. [Google Scholar]
- Li, H.; Xie, S.; Wu, P.; Mi, X. Study on improvement of conductivity of Cu-Cr-Zr alloys. Rare Met. 2007, 26, 124–130. [Google Scholar] [CrossRef]
- Kozeschnik, E. Modeling Solid-State Precipitation; Momentum Press: New York, USA, 2012; ISBN 9781606500644. [Google Scholar]
- Russell, K.C. Nucleation in solids: The induction and steady state effects. Adv. Colloid Interface Sci. 1980, 13, 205–318. [Google Scholar] [CrossRef]
- Paul, A.; Divinski, S.V. (Eds.) Handbook of Solid State Diffusion, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128042878. [Google Scholar]
- Perez, M.; Dumont, M.; Acevedo-Reyes, D. Implementation of classical nucleation and growth theories for precipitation. Acta Mater. 2008, 56, 2119–2132. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 60, 4575–4585. [Google Scholar] [CrossRef] [Green Version]
- Robson, J. Modelling the evolution of particle size distribution during nucleation, growth and coarsening. Mater. Sci. Technol. 2004, 20, 441–448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).