Use of Ion-Exchange Resins to Adsorb Scandium from Titanium Industry’s Chloride Acidic Solution at Ambient Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Procedure
2.2. Mathematical Modelling
2.2.1. Mathematical Description of the Fixed-Bed Columns
2.2.2. Modelling of Experimental Results through Regression Method
3. Results and Discussion
3.1. Breakthrough Curve Data Fitting
3.2. Resins Adsorption Behavior
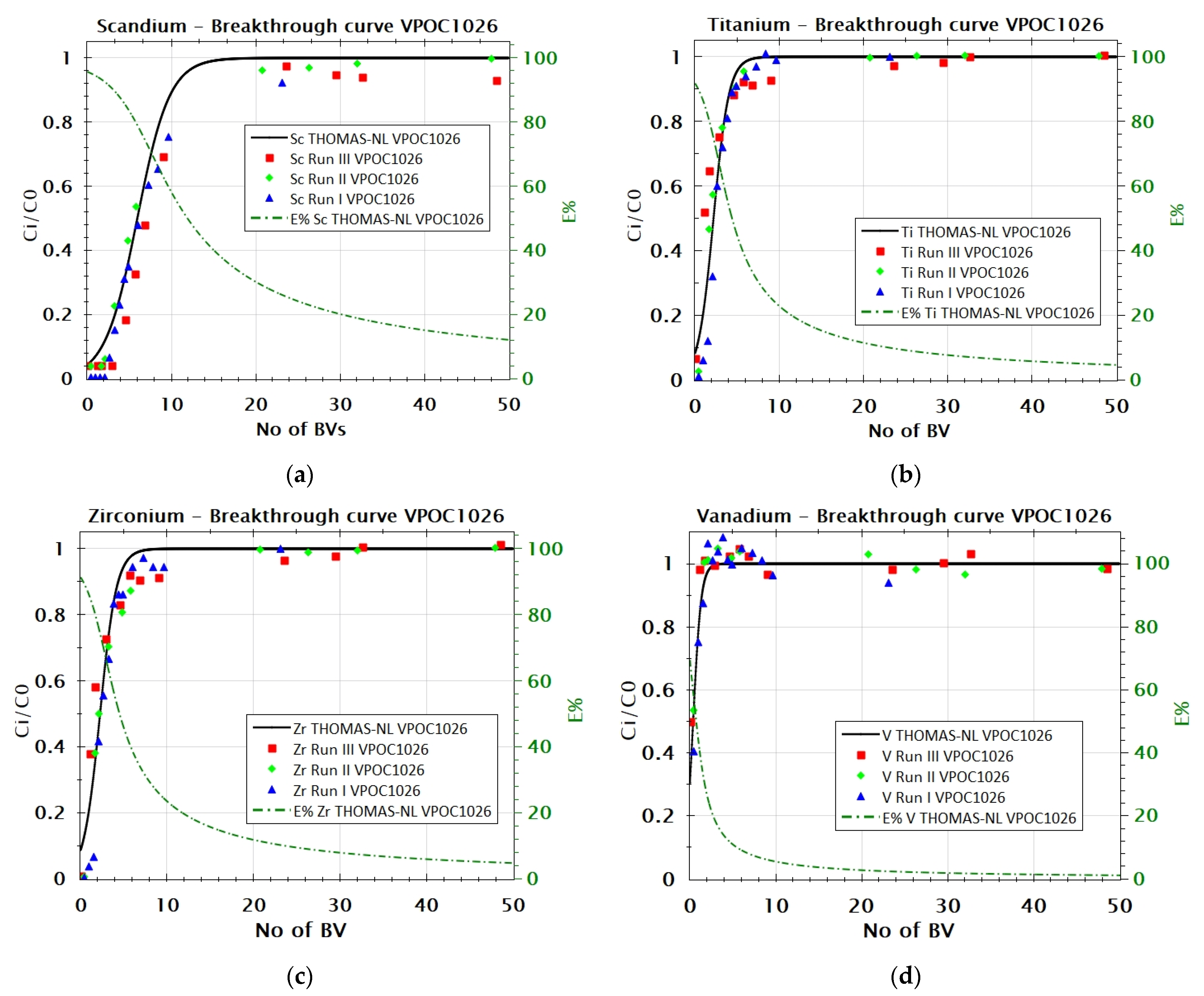
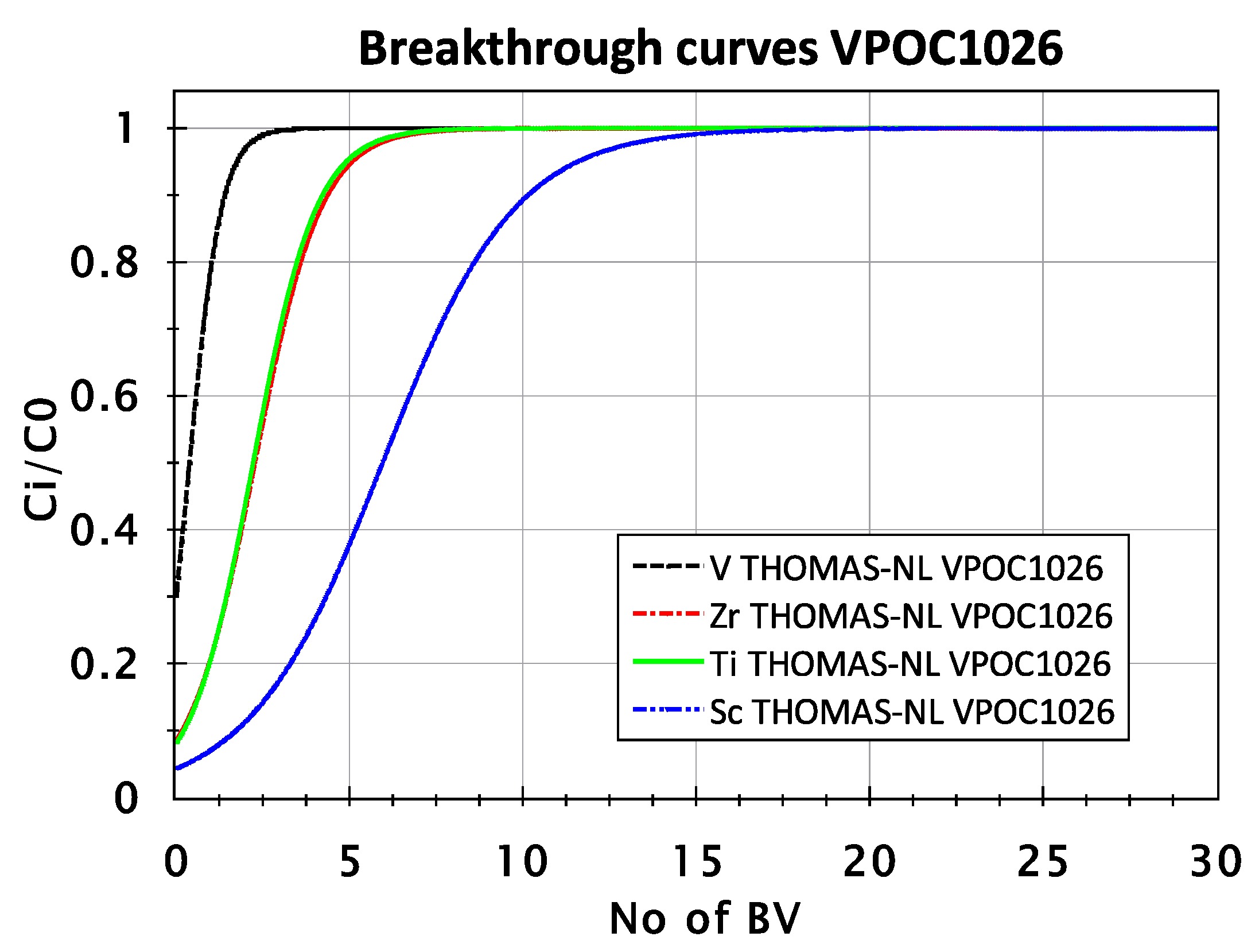
3.2.1. Adsorption Behavior of Sc, Ti, Zr, and V from FeCl2 Solution with VP OC 1026 Resin
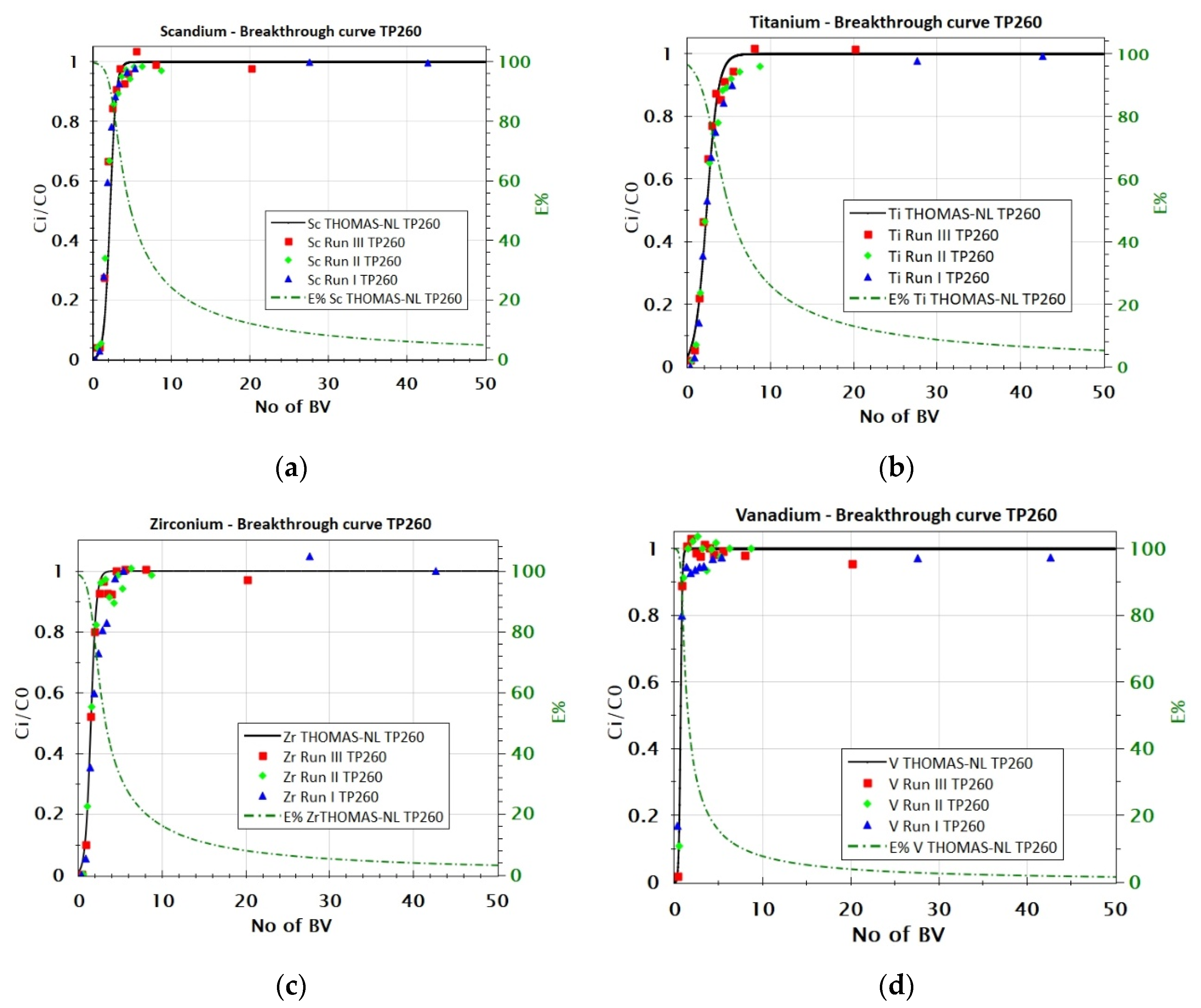
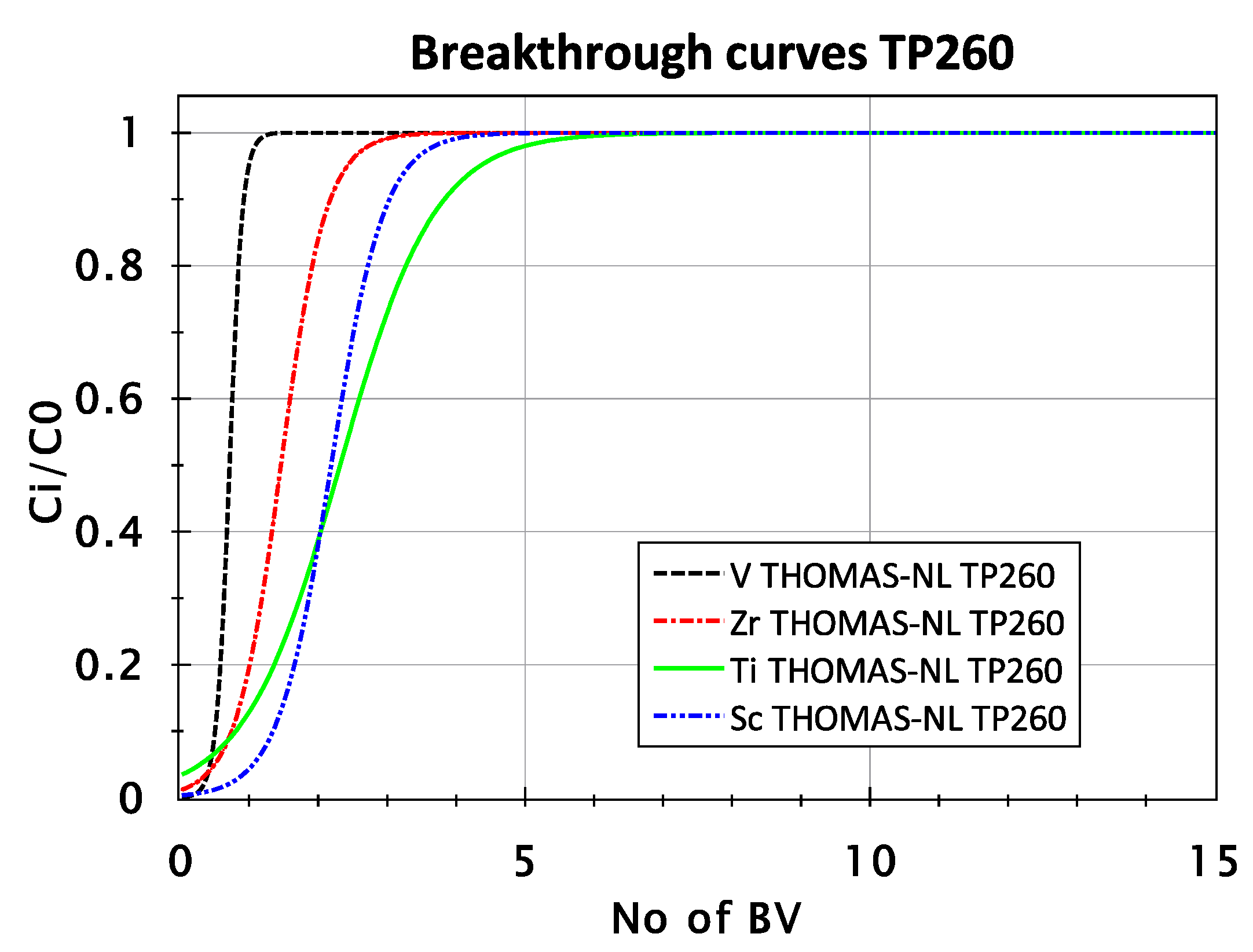
3.2.2. Adsorption Behavior of Sc, Ti, Zr, and V from FeCl2 Solution with TP 260 Resin
3.2.3. Adsorption Behavior of Fe with VP OC 1026 and TP 260 Resins
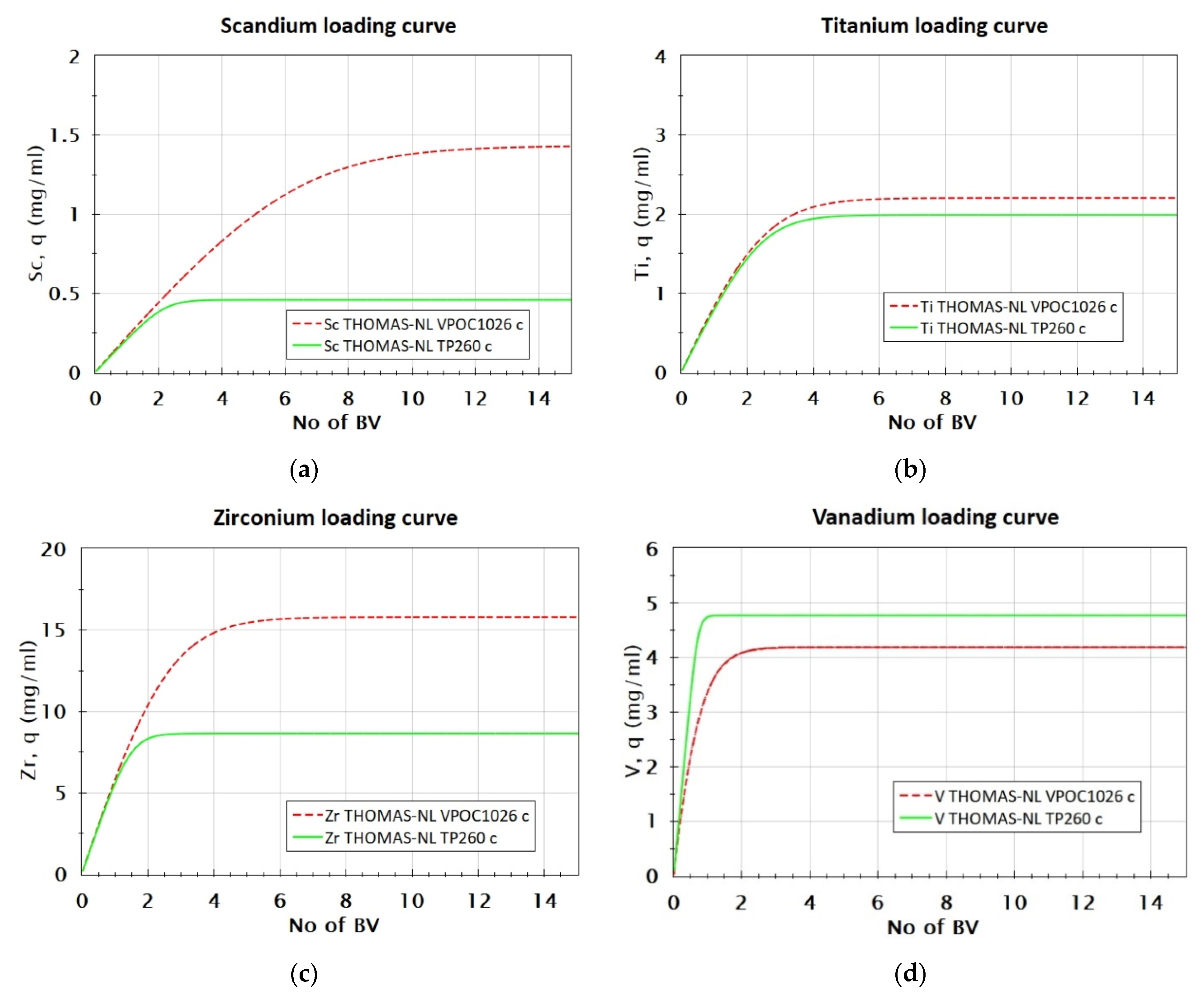
3.2.4. Comparison of VP OC 1026 and TP 260 Resins’ Loading Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibata, J.; Murayama, N. Solvent Extraction of Scandium from the Waste Solution of TiO2 Production Process. Trans. Indian Inst. Met. 2016, 70, 471–477. [Google Scholar] [CrossRef]
- Hartley, C.J.; Hazen, W.W.; Baughman, D.R.; Bemelmans, C.M.A.; Belits, P.F.; Lanyk, T.J.; Porter, B.F.; Liao, L.; McAllister, J.; Yang, M.S.-Y. Methods of Recovering Scandium from Titanium Residue Streams. U.S. Patent No. 9,102,999, 11 August 2015. [Google Scholar]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Vaughan, J.; Tenório, J.A.S. Recovery of scandium from various sources: A critical review of the state of the art and future prospects. Miner. Eng. 2021, 172, 107148. [Google Scholar] [CrossRef]
- European Commision. Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability; European Commision: Brussels, Belgium, 2020. [Google Scholar]
- Petrakova, O.; Kozyrev, A.; Suss, A.; Panov, A.; Gorbachev, S.; Perestoronina, M.; Vishnyakov, S. BR04-Industrial Trials Results of Scandium Oxide Recovery from Red Mud at UC RUSAL Alumina Refineries. Ser. Geol. Tech. Sci. 2020, 4, 156–165. [Google Scholar]
- Zhang, L.; Zhang, T.-A.; Lv, G.; Zhang, W.; Li, T.; Cao, X. Separation and Extraction of Scandium from Titanium Dioxide Waste Acid. JOM 2021, 73, 1301–1309. [Google Scholar] [CrossRef]
- Altinsel, Y.; Topkaya, Y.; Kaya, Ş.; Şentürk, B. Extraction of Scandium from Lateritic Nickel-Cobalt Ore Leach Solution by Ion Exchange: A Special Study and Literature Review on Previous Works; Springer: Cham, Switzerland, 2018; pp. 1545–1553. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, G.; Zeng, L.; Cao, Z.; Guan, W.; Wang, L. Separation and recovery of scandium and titanium from spent sulfuric acid solution from the titanium dioxide production process. Hydrometallurgy 2018, 178, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Ning, S.; Zhong, Y.; Wang, X.; Fujita, T.; Wei, Y. Highly efficient recovery and purification of scandium from the waste sulfuric acid solution from titanium dioxide production by solvent extraction. J. Environ. Chem. Eng. 2021, 9, 106226. [Google Scholar] [CrossRef]
- Chernoburova, O.; Changes, A. The Future of Scandium Recovery from Wastes. In Proceedings of the International Conference on Raw Materials and Circular Economy, Athens, Greece, 5–9 September 2021. [Google Scholar]
- Zhou, J.; Ning, S.; Meng, J.; Zhang, S.; Zhang, W.; Wang, S.; Chen, Y.; Wang, X.; Wei, Y. Purification of scandium from concentrate generated from titanium pigments production waste. J. Rare Earths 2020, 39, 194–200. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, M.; Xie, Y.; Jianfeng, S.; Huang, T.; Li, X.-M. From trace to pure: Recovery of scandium from the waste acid of titanium pigment production by solvent extraction. Process Saf. Environ. Prot. 2018, 121, 118–124. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Orberger, B.; Dittrich, C.; Croisé, G.; Scharfenberg, R.; Balomenos, E.; Panias, D.; Mikeli, E.; Maier, C.; Schneider, R.; et al. Sustainable Supply of Scandium for the EU Industries from Liquid Iron Chloride Based TiO2 Plants. Mater. Proc. 2021, 5, 86. [Google Scholar]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Zhou, G.; Li, Q.; Sun, P.; Guan, W.; Zhang, G.; Cao, Z.; Zeng, L. Removal of impurities from scandium chloride solution using 732-type resin. J. Rare Earths 2018, 36, 311–316. [Google Scholar] [CrossRef]
- Shaoquan, X.; Suqing, L. Review of the extractive metallurgy of scandium in China (1978–1991). Hydrometallurgy 1996, 42, 337–343. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Iizuka, A.; Shibata, E.; Nakamura, T. Study of adsorption behavior of a new synthesized resin containing glycol amic acid group for separation of scandium from aqueous solutions. Hydrometallurgy 2016, 165, 51–56. [Google Scholar] [CrossRef]
- Hérès, X.; Blet, V.; Di Natale, P.; Ouaattou, A.; Mazouz, H.; Dhiba, D.; Cuer, F. Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins. Metals 2018, 8, 682. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.; Titova, S.; Rychkov, V.; Bunkov, G.; Semenishchev, V.; Kirillov, E.; Poponin, N.; Svirsky, I. Study of scandium and thorium sorption from uranium leach liquors. J. Radioanal. Nucl. Chem. 2017, 312, 277–283. [Google Scholar] [CrossRef]
- Ivanov, N.; Abilmagzhanov, A.; Shokobayev, N.; Adelbayev, I.; Nurtazina, A. Scandium Extraction By Phosphorus-Containing Sorbents. NAS RK. Ser. Geol. Tech. Sci. Sci. J. 2020, 4, 156–165. [Google Scholar]
- Rychkov, V.N.; Nalivayko, K.A.; Titova, S.M.; Abakumova, E.V.; Yakovleva, O.V.; Kirillov, E.V.; Skripchenko, S.Y. Kinetics of scandium sorption from sulfuric acid solutions by ampholyte Lewatit TP260. AIP Conf. Proc. 2020, 2313, 050028. [Google Scholar] [CrossRef]
- Bao, S.; Hawker, W.; Vaughan, J. Scandium Loading on Chelating and Solvent Impregnated Resin from Sulfate Solution. Solvent Extr. Ion Exch. 2018, 36, 100–113. [Google Scholar] [CrossRef]
- Mostajeran, M.; Bondy, J.-M.; Reynier, N.; Cameron, R. Mining value from waste: Scandium and rare earth elements selective recovery from coal fly ash leach solutions. Miner. Eng. 2021, 173, 107091. [Google Scholar] [CrossRef]
- Meshkov, E.Y.; Akimova, I.; Bobyrenko, N.; Solov’ev, A.; Klochkova, N.; Savel’ev, A. Separation of Scandium and Thorium in the Processing of Scandium Rough Concentrate Obtained from Circulating Solutions of In-Situ Leaching of Uranium. Radiochemistry 2020, 62, 652–657. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Chen, J.; Liu, W. Extraction of Scandium(III) Using Ionic Liquids Functionalized Solvent Impregnated Resins. J. Appl. Polym. Sci. 2011, 120, 3284–3290. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, C.Y. Separation and purification of scandium by solvent extraction and related technologies: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Roosen, J.; Van Roosendael, S.; Borra, C.R.; Van Gerven, T.; Mullens, S.; Binnemans, K. Recovery of scandium from leachates of Greek bauxite residue by adsorption on functionalized chitosan–silica hybrid materials. Green Chem. 2016, 18, 2005–2013. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Reynier, N.; Gagné-Turcotte, R.; Coudert, L.; Costis, S.; Cameron, R.; Blais, J.-F. Bioleaching of Uranium Tailings as Secondary Sources for Rare Earth Elements Production. Minerals 2021, 11, 302. [Google Scholar] [CrossRef]
- Sokolova, Y.V. Sorption of Sc(III) on phosphorus-containing cation exchangers. Russ. J. Appl. Chem. 2006, 79, 573–578. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Zhu, Z.; Zhang, X.; Wang, D.; Xie, L. Fixed-Bed Column Adsorption Of Arsenic(V) By Porous Composite Of Magnetite/Hematite/Carbon With Eucalyptus Wood Microstructure. J. Environ. Eng. Landsc. Manag. 2018, 26, 38–56. [Google Scholar] [CrossRef] [Green Version]
- González-López, M.E.; Laureano-Anzaldo, C.M.; Pérez-Fonseca, A.A.; Arellano, M.; Robledo-Ortíz, J.R. A discussion on linear and non-linear forms of Thomas equation for fixed-bed adsorption column modeling. Rev. Mex. De Ing. Química 2021, 20, 875–884. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; Courier Corporation: Toronto, Canada, 1995. [Google Scholar]
- Han, R.; Wang, Y.; Zou, W.; Wang, Y.; Shi, J. Comparison of linear and nonlinear analysis in estimating the Thomas model parameters for methylene blue adsorption onto natural zeolite in fixed-bed column. J. Hazard. Mater. 2007, 145, 331–335. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Kumar Garg, V. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Gallindo, A.D.A.S.; Silva Junior, R.A.D.; Rodrigues, M.G.F.; Ramos, W.B. Modelling and simulation of the ion exchange process for Zn2+(aq) removal using zeolite NaY. Res. Soc. Dev. 2021, 10, e310101220362. [Google Scholar] [CrossRef]
- Lin, L.-C.; Li, J.-K.; Juang, R.-S. Removal of Cu(II) and Ni(II) from aqueous solutions using batch and fixed-bed ion exchange processes. Desalination 2008, 225, 249–259. [Google Scholar] [CrossRef]
- Xiao, Y.; Azaiez, J.; Hill, J.M. Erroneous Application of Pseudo-Second-Order Adsorption Kinetics Model: Ignored Assumptions and Spurious Correlations. Ind. Eng. Chem. Res. 2018, 57, 2705–2709. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. Continuous fixed-bed column study and adsorption modeling: Removal of cadmium (II) and lead (II) ions in aqueous solution by dead calcareous skeletons. Biochem. Eng. J. 2014, 87, 50–61. [Google Scholar] [CrossRef]
- Fallah, N.; Taghizadeh, M. Continuous fixed-bed adsorption of Mo(VI) from aqueous solutions by Mo(VI)-IIP: Breakthrough curves analysis and mathematical modeling. J. Environ. Chem. Eng. 2020, 8, 104079. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
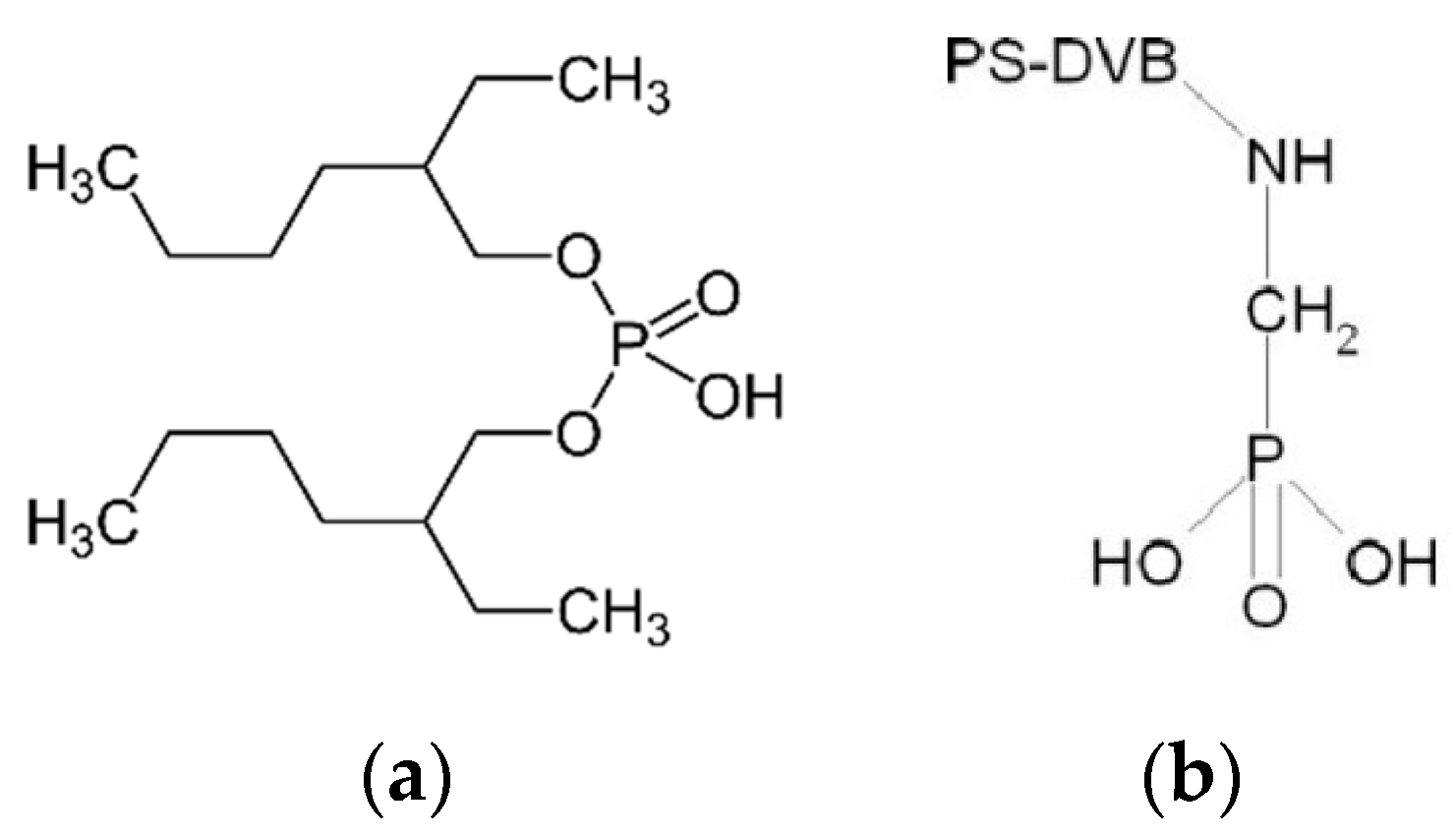

| Resin Name | Lewatit VP OC 1026 | Lewatit TP 260 |
|---|---|---|
| Functional group | bis(2-ethylhexyl)phosphoric acid (D2EHPA) | aminomethyl-phosphonic acid (AMPA) |
| Matrix | Cross-linked polystyrene | Cross-linked polystyrene |
| As received form | H+ | Na+ |
| Bead size range | 0.3–1.6 mm | 0.4–1.25 mm |
| Metals | Fe (II) | Fe (III) | Mn | Al | Mg | Na | V | Zr | Ca | Cr | Ti | Sc |
| Concentration (g/l) | 110.5 | 1.5 | 19.09 | 9.11 | 8.34 | 6.41 | 4.20 | 3.68 | 2.36 | 1.69 | 0.528 | 0.130 |
| Resin | Metal | R2 | KTh (mL/min g) | qTh (mg/mL) |
|---|---|---|---|---|
| VP OC 1026 | Sc | 0.981 | 0.069 | 1.46 |
| Ti | 0.965 | 0.036 | 2.2 | |
| Zr | 0.959 | 0.005 | 15.9 | |
| V | 0.887 | 0.009 | 4.06 | |
| TP 260 | Sc | 0.991 | 0.310 | 0.46 |
| Ti | 0.978 | 0.043 | 1.98 | |
| Zr | 0.988 | 0.013 | 8.7 | |
| V | 0.973 | 0.039 | 4.86 |
| Metal, Me | MeVPOC1026/MeTP260 |
|---|---|
| Sc | 3.16 |
| Zr | 1.83 |
| Ti | 1.11 |
| V | 0.84 |
| Ratio, Me/Sc | FeCl2 | VP OC 1026 | TP 260 |
|---|---|---|---|
| Zr/Sc | 27.69 | 10.91 | 18.89 |
| Ti/Sc | 4.06 | 1.51 | 4.30 |
| V/Sc | 32.31 | 2.79 | 10.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikeli, E.; Marinos, D.; Toli, A.; Pilichou, A.; Balomenos, E.; Panias, D. Use of Ion-Exchange Resins to Adsorb Scandium from Titanium Industry’s Chloride Acidic Solution at Ambient Temperature. Metals 2022, 12, 864. https://doi.org/10.3390/met12050864
Mikeli E, Marinos D, Toli A, Pilichou A, Balomenos E, Panias D. Use of Ion-Exchange Resins to Adsorb Scandium from Titanium Industry’s Chloride Acidic Solution at Ambient Temperature. Metals. 2022; 12(5):864. https://doi.org/10.3390/met12050864
Chicago/Turabian StyleMikeli, Eleni, Danai Marinos, Aikaterini Toli, Anastasia Pilichou, Efthymios Balomenos, and Dimitrios Panias. 2022. "Use of Ion-Exchange Resins to Adsorb Scandium from Titanium Industry’s Chloride Acidic Solution at Ambient Temperature" Metals 12, no. 5: 864. https://doi.org/10.3390/met12050864
APA StyleMikeli, E., Marinos, D., Toli, A., Pilichou, A., Balomenos, E., & Panias, D. (2022). Use of Ion-Exchange Resins to Adsorb Scandium from Titanium Industry’s Chloride Acidic Solution at Ambient Temperature. Metals, 12(5), 864. https://doi.org/10.3390/met12050864







