Preparation and Application of a New Two-Component Superhydrophobic Coating on Aluminum Alloy
Abstract
:1. Introduction
2. Preparation of the New Two-Component Superhydrophobic Coating
3. Continuous Hitting of Water Droplets on the Two-Component Superhydrophobic Coating
4. Corrosion of the Two-Component Superhydrophobic Coating by Acid and Alkali
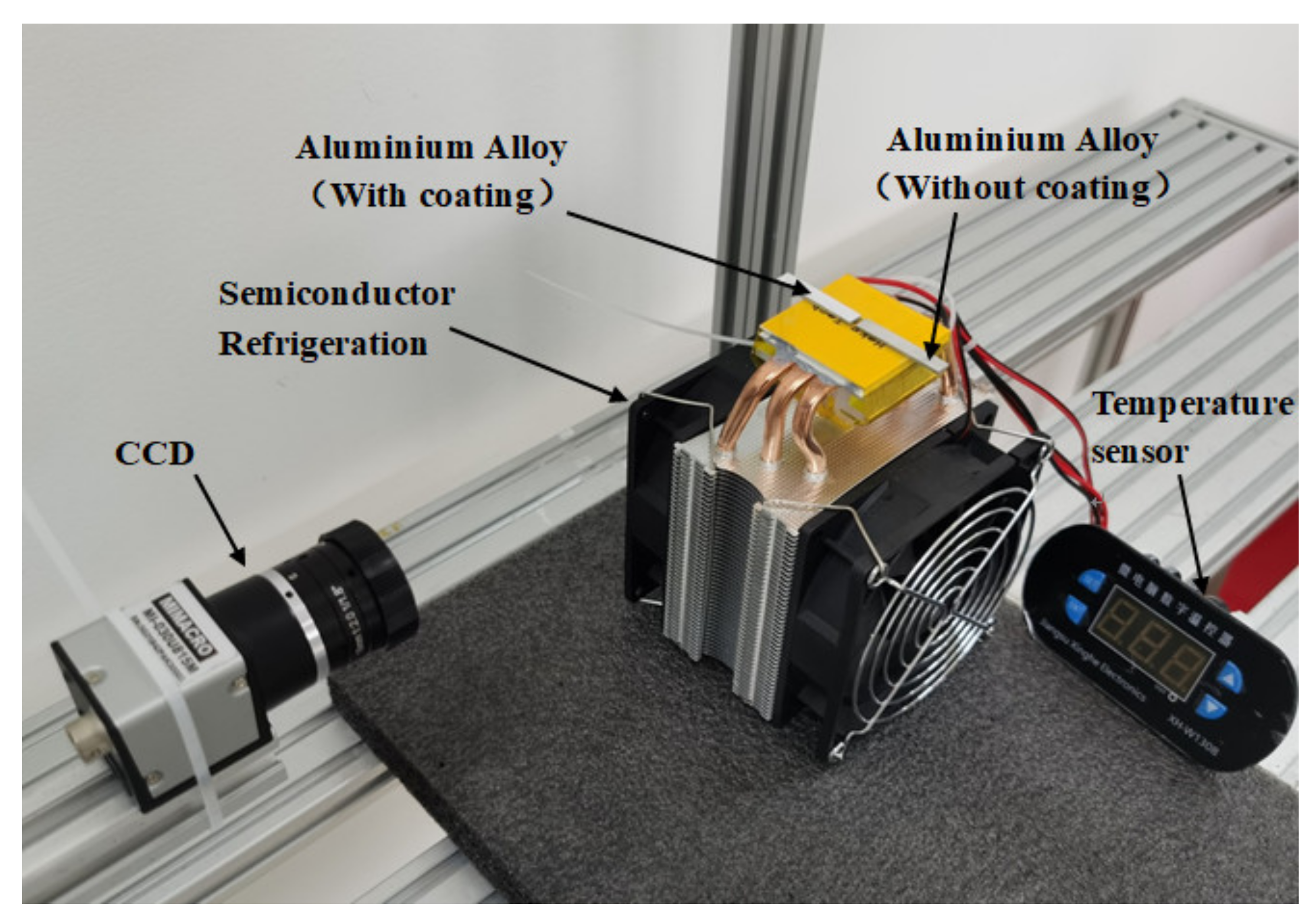
5. Icing and Frosting on the Two-Component Superhydrophobic Coating
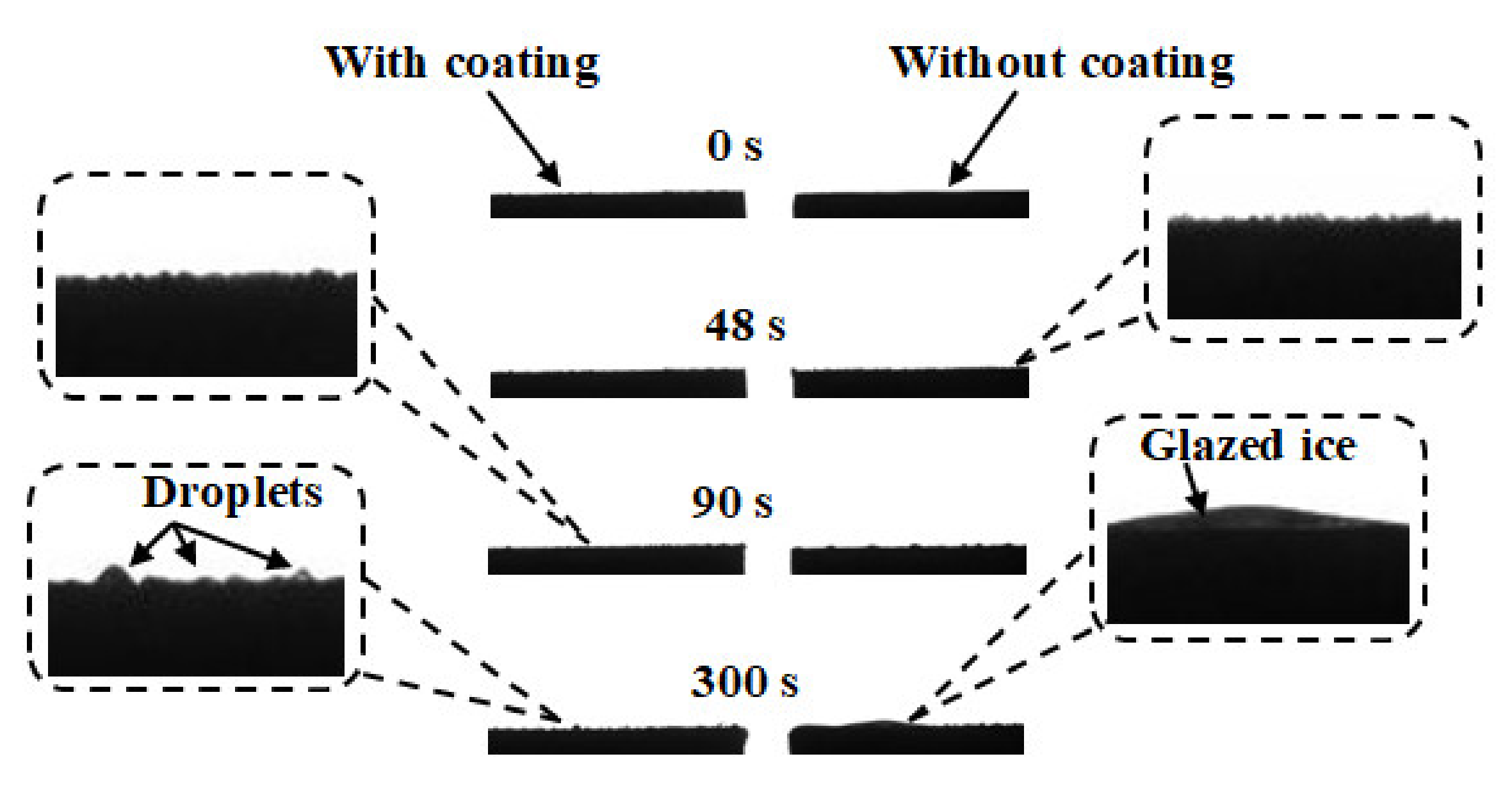
5.1. The Growth of Frost on the Two Aluminum Alloys
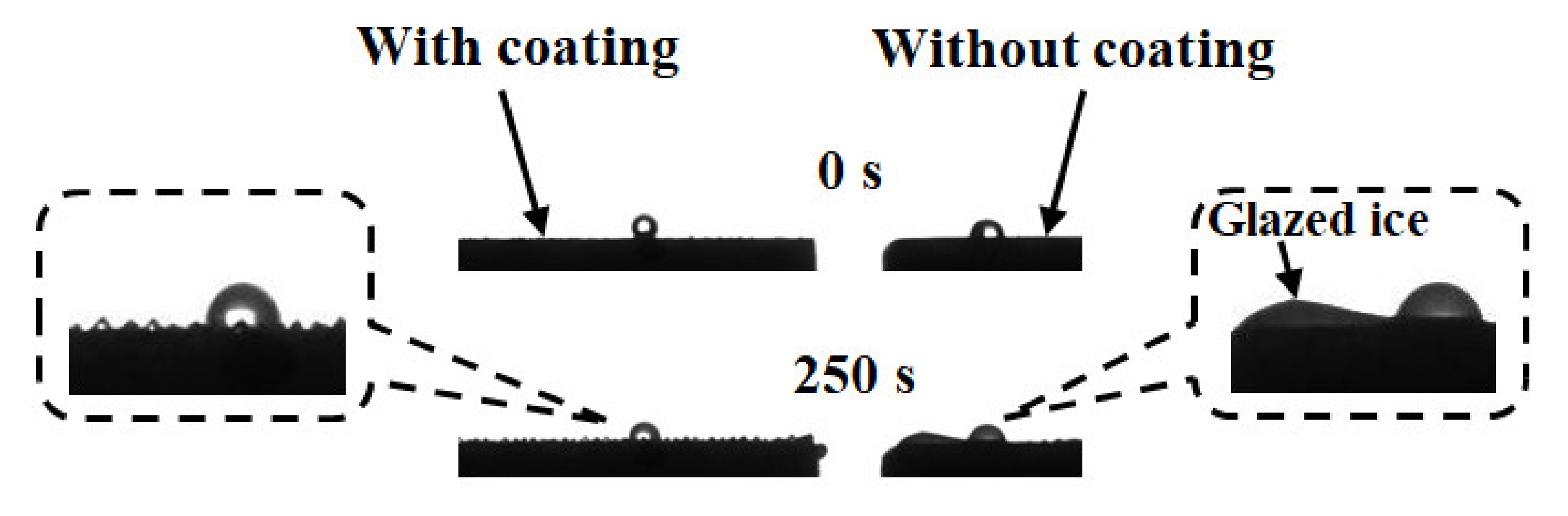
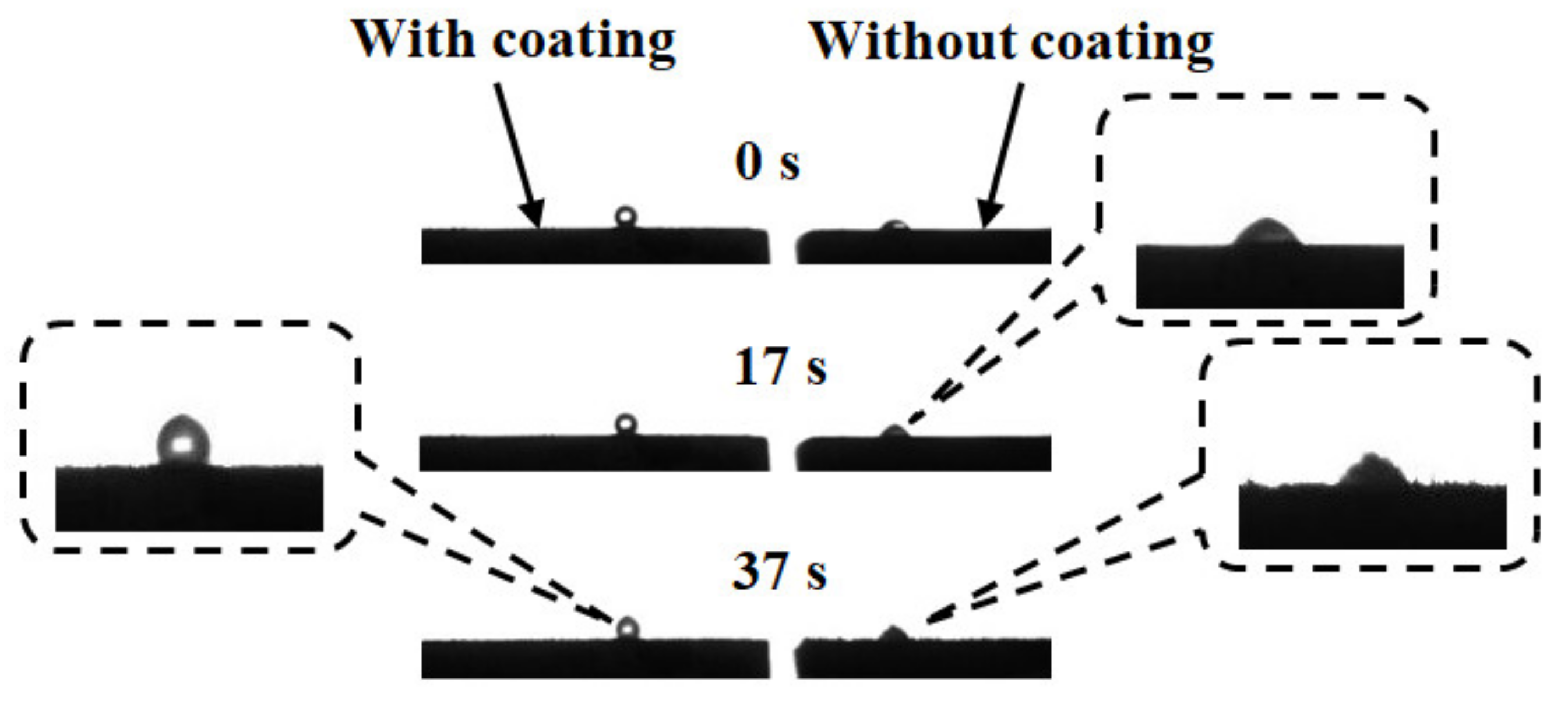
5.2. Freezing of a Droplet on the Two Aluminum Alloys
5.3. The Effect of Icing and Frosting on the Two-Component Superhydrophobic Coating
6. Conclusions
- (1)
- The two-component superhydrophobic coating has a good resistance to water droplets hitting. Continuous falling droplets can still rebound off the two-component superhydrophobic coating after hitting it for 30 min, and will not cause any damage to the two-component superhydrophobic coating.
- (2)
- The two-component superhydrophobic coating has a good resistance to most acids, such as sulfuric acid, hydrochloric acid and nitric acid. Therefore, it can prevent the aluminum alloy from being corroded by acid rain. However, the two-component superhydrophobic coating has a poor resistance to the sodium hydroxide, the rough structure of which is easy to be corroded.
- (3)
- The two-component superhydrophobic coating can not only reduce the freezing point on it more than 5 °C, but also delay the growth of ice and frost on it. On the other hand, the growth of ice or frost will make an extremely minor mechanical damage to the two-component superhydrophobic coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeon, D.K.; Ko, S.; Jeong, S.; Hong, S.P.; Kang, S.M.; Cho, W.K. Oxidation-Mediated, Zwitterionic Polydopamine Coatings for Marine Antifouling Applications. Langmuir 2019, 35, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Cen, R.Q.; Zhang, C. Development of Inner Drag Reduction Coatings with Multi-Functional Anticorrosive Performance for Oil and Gas Transport Pipeline. Coat. Prot. 2020, 329, 12–17. [Google Scholar]
- Peng, W.; Gou, X.; Qin, H.; Zhao, M.; Zhao, X.; Guo, Z. Creation of a multifunctional superhydrophobic coating for composite insulators. Chem. Eng. J. 2018, 352, 774–781. [Google Scholar] [CrossRef]
- Prakash, C.; Prasanth, R. Recent trends in fabrication of nepenthes inspired SLIPs: Design strategies for self-healing efficient anti-icing surfaces. Surf. Interfaces 2020, 21, 100678. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, H.; Shen, Y.; Tao, J.; Jin, M.; Wu, Y.; Hou, W. Rational Fabrication of Superhydrophobic Nanocone Surface for Dynamic Water Repellency and Anti-icing Potential. J. Bionic Eng. 2019, 16, 27–37. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, C.H.; Huang, C.L.; Peng, Y.; Li, Y.B. Bioinspired Fabrication of Superhydrophobic and Self-cleaning Surface of Locust Leaf. J. Gannan Norm. Univ. 2020, 241, 49–53. [Google Scholar]
- Sun, X.; Damle, V.G.; Liu, S.; Rykaczewski, K. Bioinspired Stimuli-Responsive and Antifreeze-Secreting Anti-Icing Coatings. Adv. Mater. Interfaces 2015, 2, 1400479. [Google Scholar] [CrossRef]
- Ma, M.L.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys:A review. J. Magnes. Alloy. 2018, 6, 1–12. [Google Scholar]
- Li, L.; Huang, T.; Lei, J.; He, J.; Qu, L.; Huang, P.; Zhou, W.; Li, N.; Pan, F. Robust biomimetic-structural super-hydrophobic surface on aluminum alloy. ACS Appl. Mater. Interfaces 2014, 7, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.D.; Zhou, T.; Wang, R.Y. Preparation of superhydrophobic surface on metal substrate and its mechanical durability. Surf. Technol. 2019, 48, 68–86. [Google Scholar]
- Zheng, Q.; Ding, H.H.; He, J.H.; Guo, J.; Liu, Q.Y. Research Progress of Ice/Snow-Proof Design for High-speed Train Bogie. Surf. Technol. 2020, 49, 64–74. [Google Scholar]
- Li, S.J.; Lv, J.Y.; Jiang, Z.G. Study on Anti-Icing Coatings for Train Bogiesin Alpine Regions. Paint Coat. Ind. 2020, 50, 37–40. [Google Scholar]
- Li, D.; Wang, X.; Gao, S.W.; Zhan, T.; Zhao, B.X.; Chen, Z.Q. Rebounding and splashing behavior of single water droplet impacting on cold superhydrophobic surface. CIESC J. 2017, 68, 2473–2482. [Google Scholar]
- Yeong, Y.H.; Burton, J.; Loth, E.; Bayer, I.S. Drop Impact and Rebound Dynamics on an Inclined Superhydrophobic Surface. Langmuir 2014, 30, 12027–12038. [Google Scholar] [CrossRef]
- Chen, M.J.; Liu, H.Z.; Chen, D.Q. Investigation of Dynamic Wetting Behavor on Different RoughSurfaces during Droplet Impacting. Nucl. Power Eng. 2019, 40, 50–55. [Google Scholar]
- Chu, F.; Wu, X. Fabrication and condensation characteristics of metallic superhydrophobic surface with hierarchical micro-nano structures. Appl. Surf. Sci. 2016, 371, 322–328. [Google Scholar] [CrossRef]
- Larssen, T.; Lydersen, E.; Tang, D.; He, Y.; Gao, J.; Liu, H.; Duan, L.; Seip, H.M.; Vogt, R.D.; Mulder, J.; et al. Acid Rain in China. Environ. Sci. Technol. 2006, 40, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Snoeijer, J.H.; Brunet, P. Pointy ice-drops: How water freezes into a singular shape. Am. J. Phys. 2012, 80, 764–771. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Li, H.; Song, Y. Super-hydrophobic surfaces to condensed micro droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 2011, 7, 3993–4000. [Google Scholar] [CrossRef]
- Fan, Y.J.; Zhang, X.F.; Zhang, L. Tribological properties of PTFE coatings under reciprocating sliding condition. Lubric. Eng. 2016, 4, 101–105. [Google Scholar]
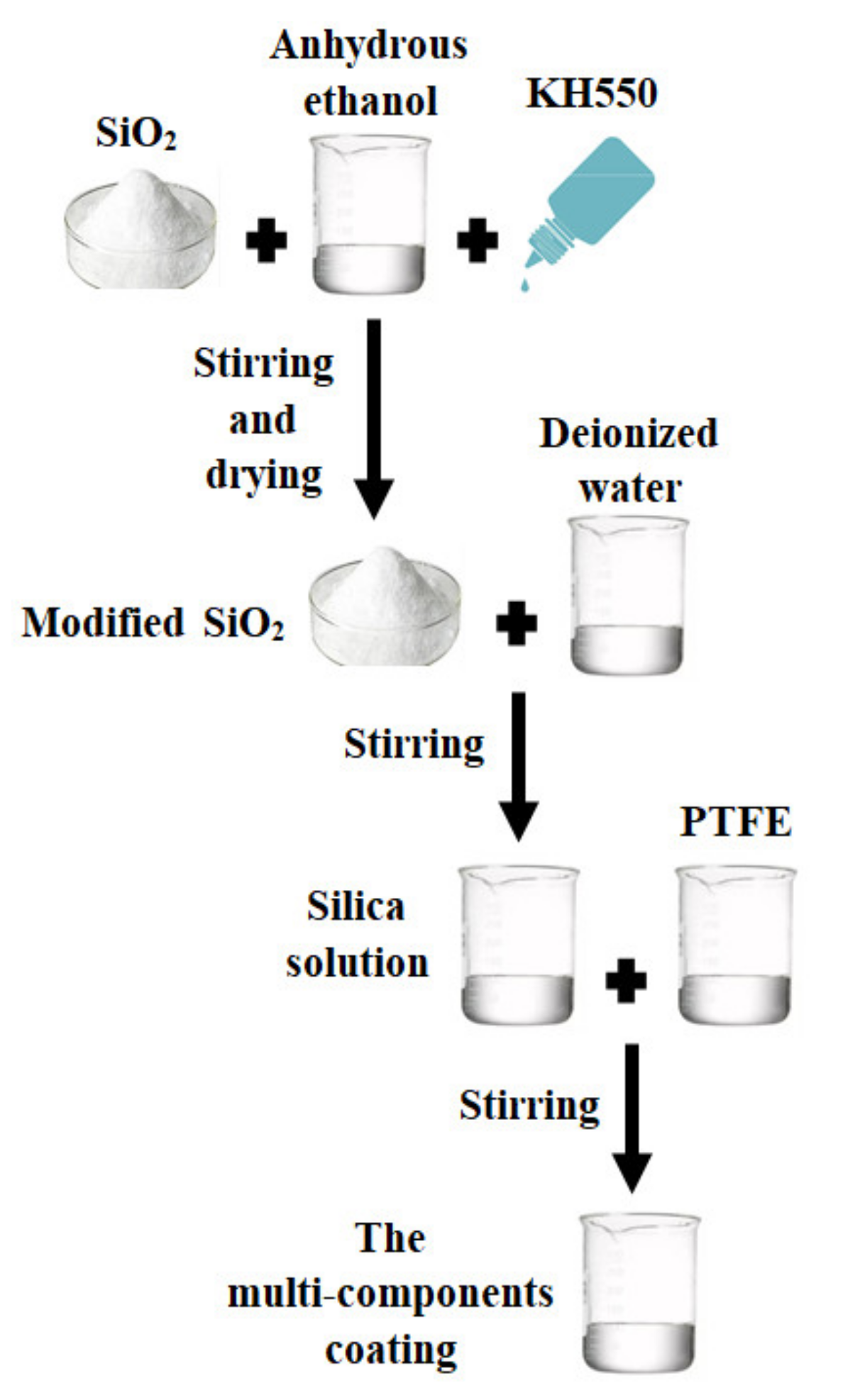
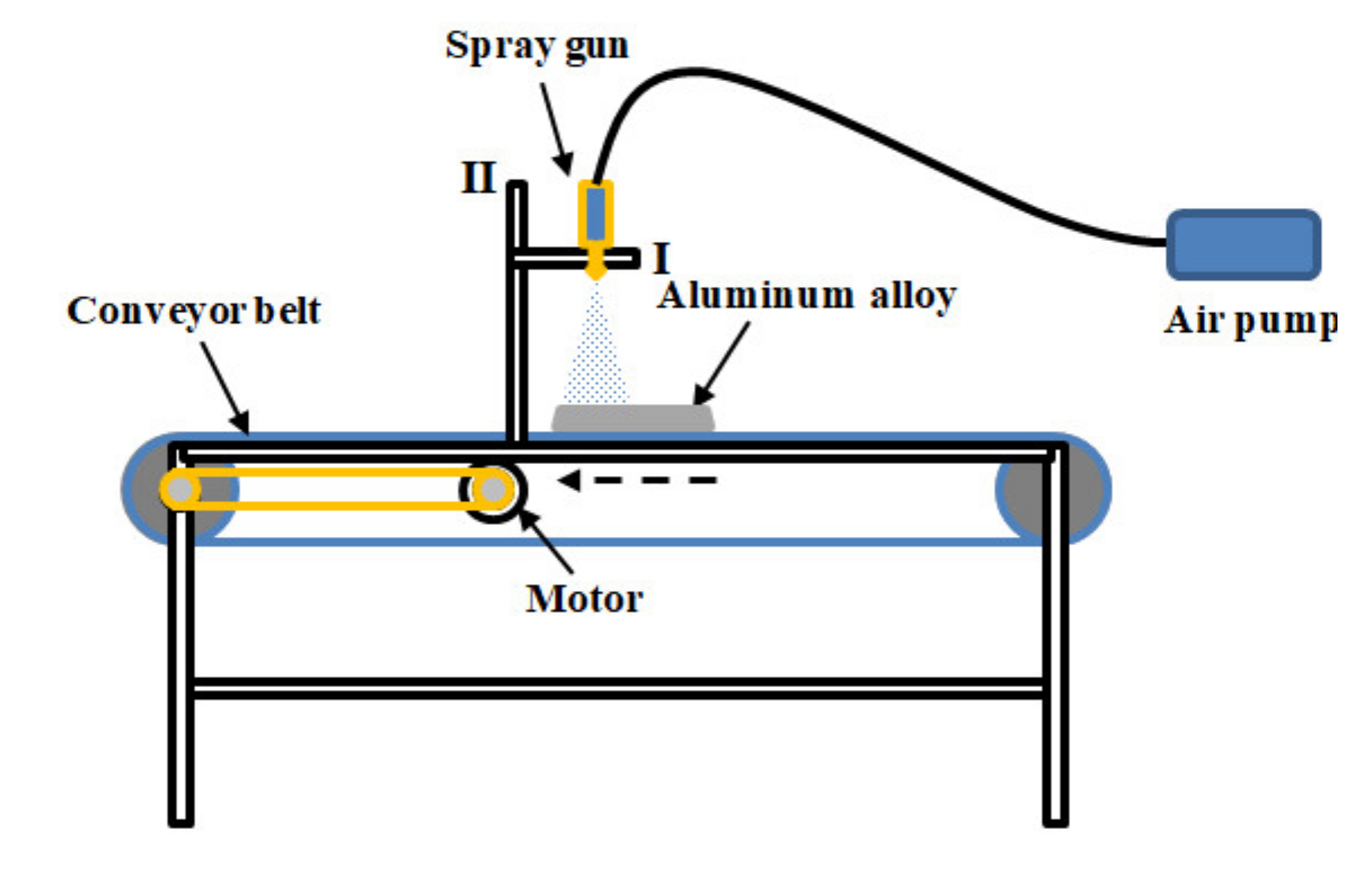

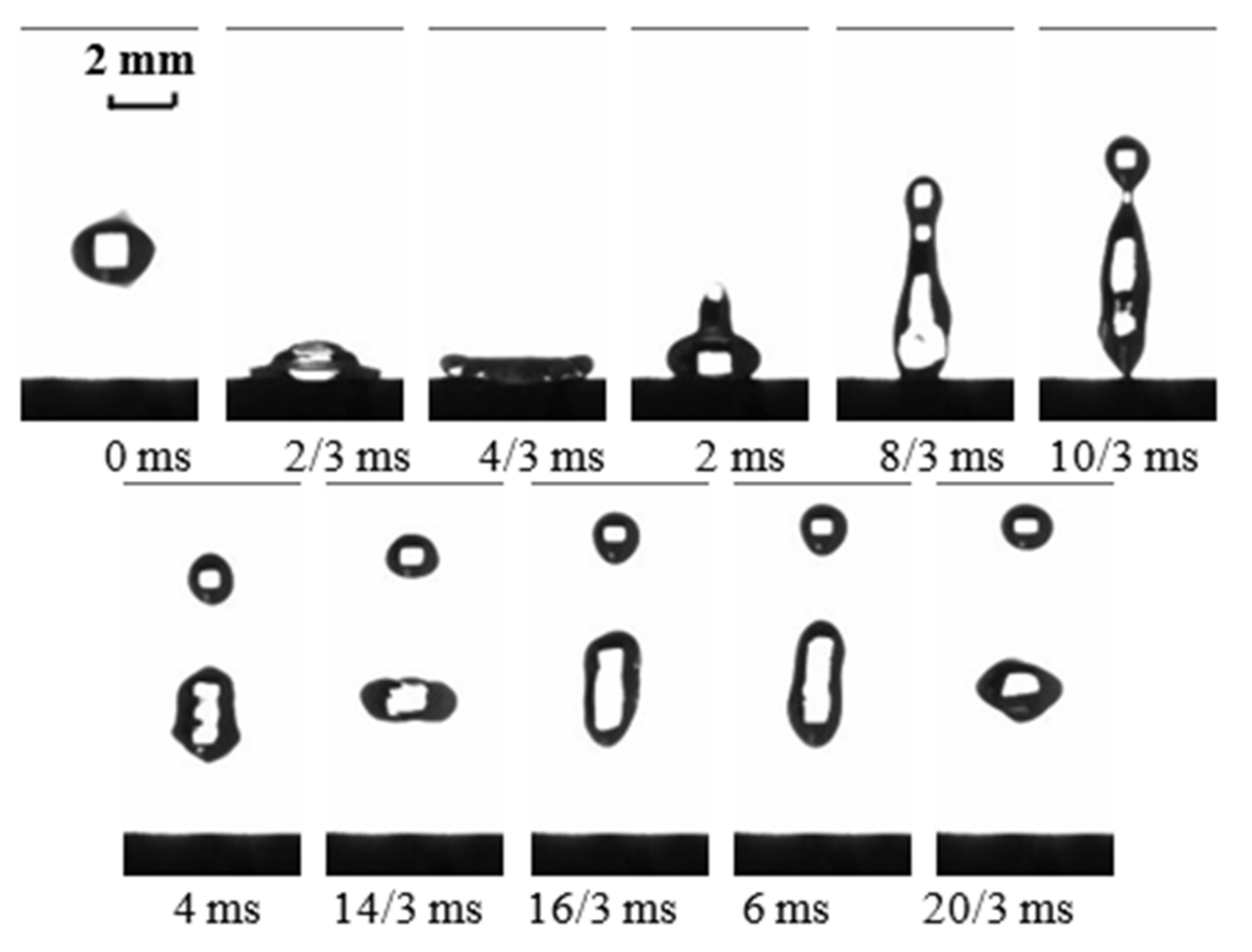
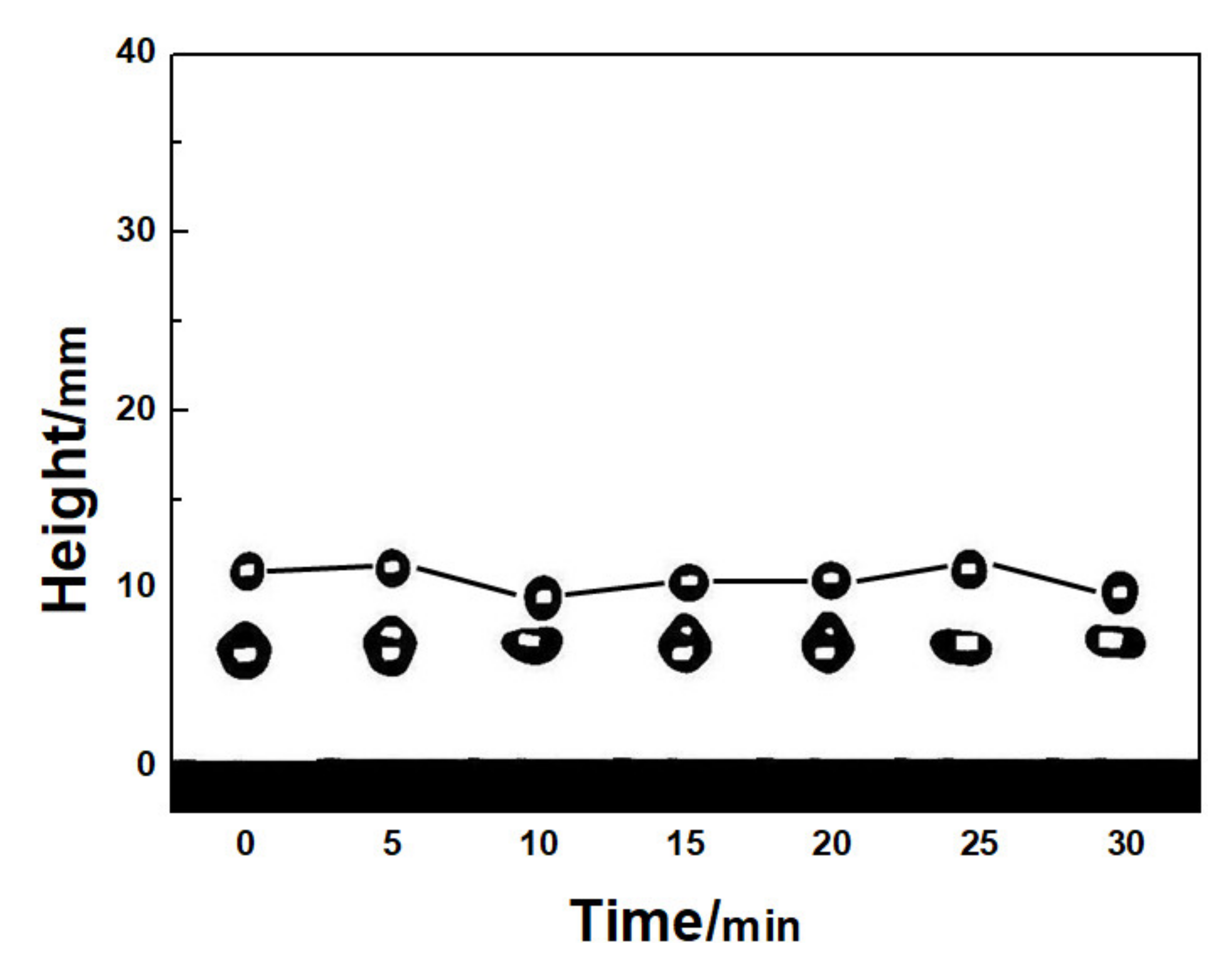




| Components of Coating | Contact Angle | Sliding Angle |
|---|---|---|
| 0.2 g SiO2 + 10 mL H2O+ 5 mL PTFE | 145.2° | — |
| 0.5 g SiO2 + 10 mL H2O+ 5 mL PTFE | 156.5° | 5.4° |
| 0.8 g SiO2 + 10 mL H2O+ 5 mL PTFE | 164.4° | 1° |
| 1.1 g SiO2 + 10 mL H2O+ 5 mL PTFE | 154.4° | 7° |
| Without coating | 74.3° | — |
| Acid or Alkali | Concentration | Time of Corrosion | Contact Angles | |
|---|---|---|---|---|
| Before Corrosion | After Corrosion | |||
| Sulfuric acid | 1 mol/L | 300 min | 163.4° | 159.6° |
| Hydrochloric acid | 1 mol/L | 300 min | 163.2° | 159.0° |
| Nitric acid | 1 mol/L | 300 min | 165.5° | 159.5° |
| Sodium hydroxide | 1 mol/L | 100 min | 164.3° | 143.7° |
| Type | Temperature | Humidity | Duration of Freezing or Frosting | Result | Contact Angles | |
|---|---|---|---|---|---|---|
| Before | After Drying | |||||
| Frosting | −20 °C | 50% | 10 min | Frosted | 160.1° | 155.5° |
| Icing | −20 °C | 50% | 10 min | Frozen | 160.0° | 157.4° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Liang, S.; Li, M.; Cheng, H.; Qin, W. Preparation and Application of a New Two-Component Superhydrophobic Coating on Aluminum Alloy. Metals 2022, 12, 850. https://doi.org/10.3390/met12050850
Qiu C, Liang S, Li M, Cheng H, Qin W. Preparation and Application of a New Two-Component Superhydrophobic Coating on Aluminum Alloy. Metals. 2022; 12(5):850. https://doi.org/10.3390/met12050850
Chicago/Turabian StyleQiu, Chao, Shuai Liang, Meng Li, Han Cheng, and Wenfeng Qin. 2022. "Preparation and Application of a New Two-Component Superhydrophobic Coating on Aluminum Alloy" Metals 12, no. 5: 850. https://doi.org/10.3390/met12050850
APA StyleQiu, C., Liang, S., Li, M., Cheng, H., & Qin, W. (2022). Preparation and Application of a New Two-Component Superhydrophobic Coating on Aluminum Alloy. Metals, 12(5), 850. https://doi.org/10.3390/met12050850






