Characteristic of Precipitate Evolution during High Temperature Annealing in Grain-Oriented Silicon Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
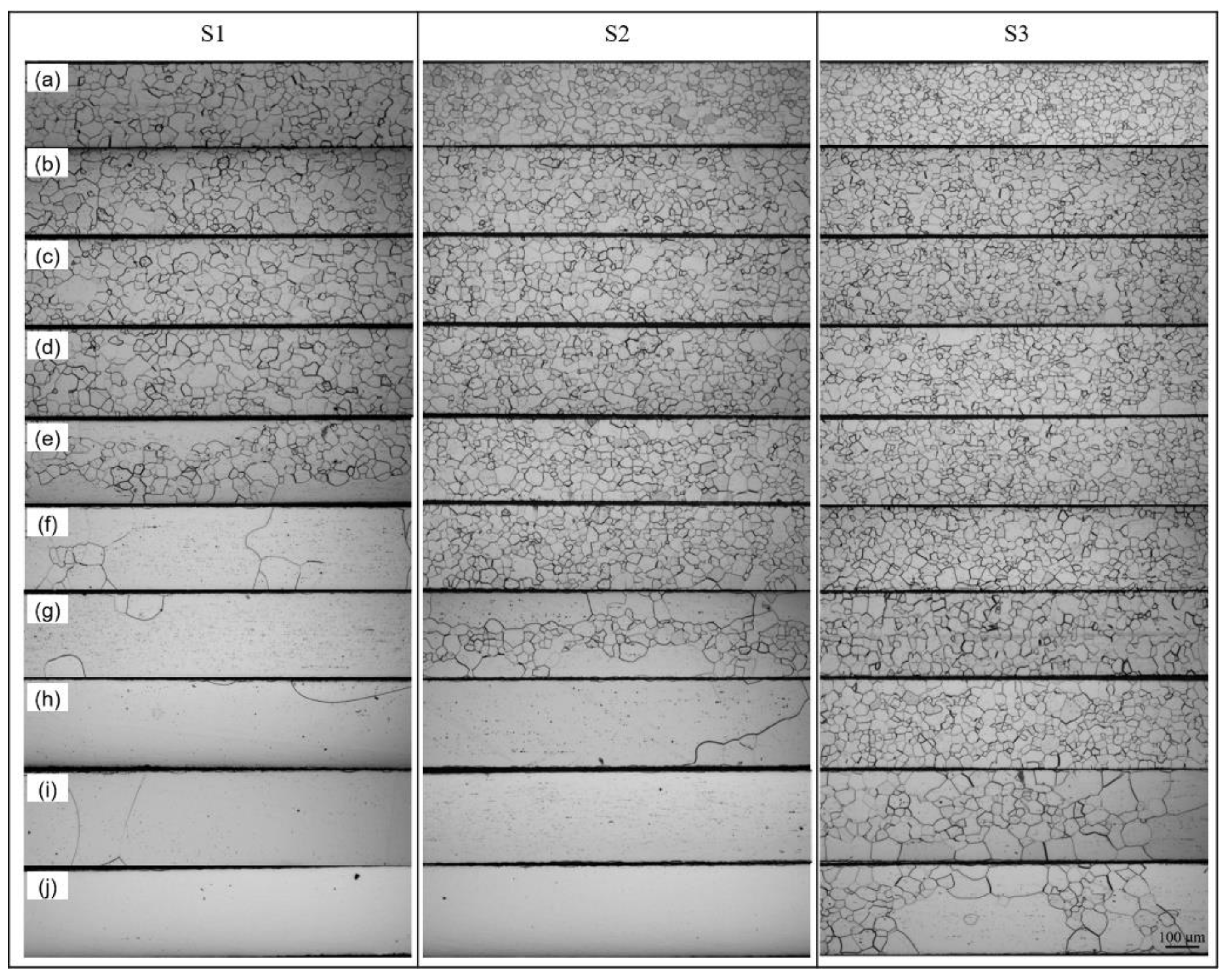
3.1. Metallography
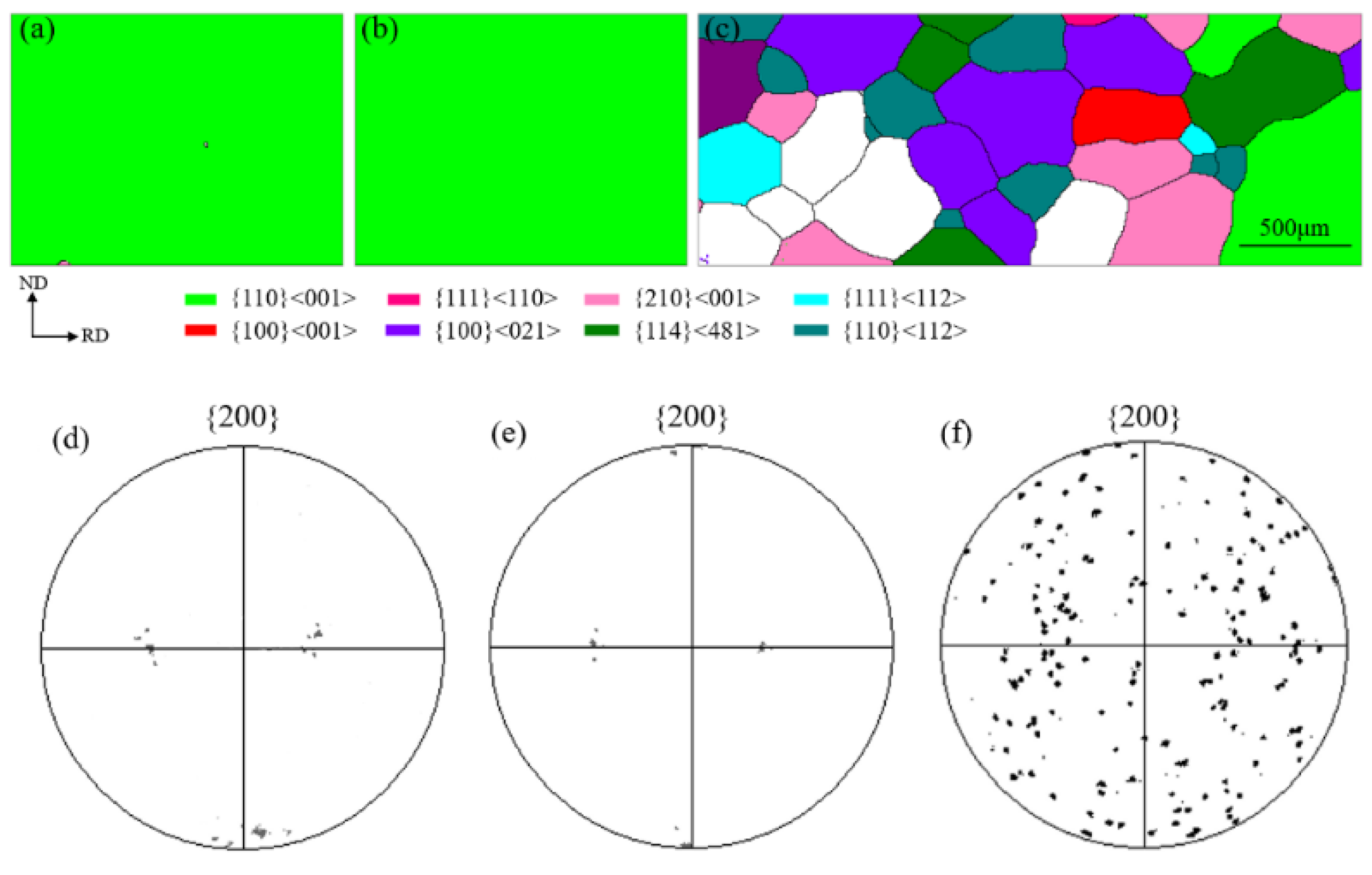
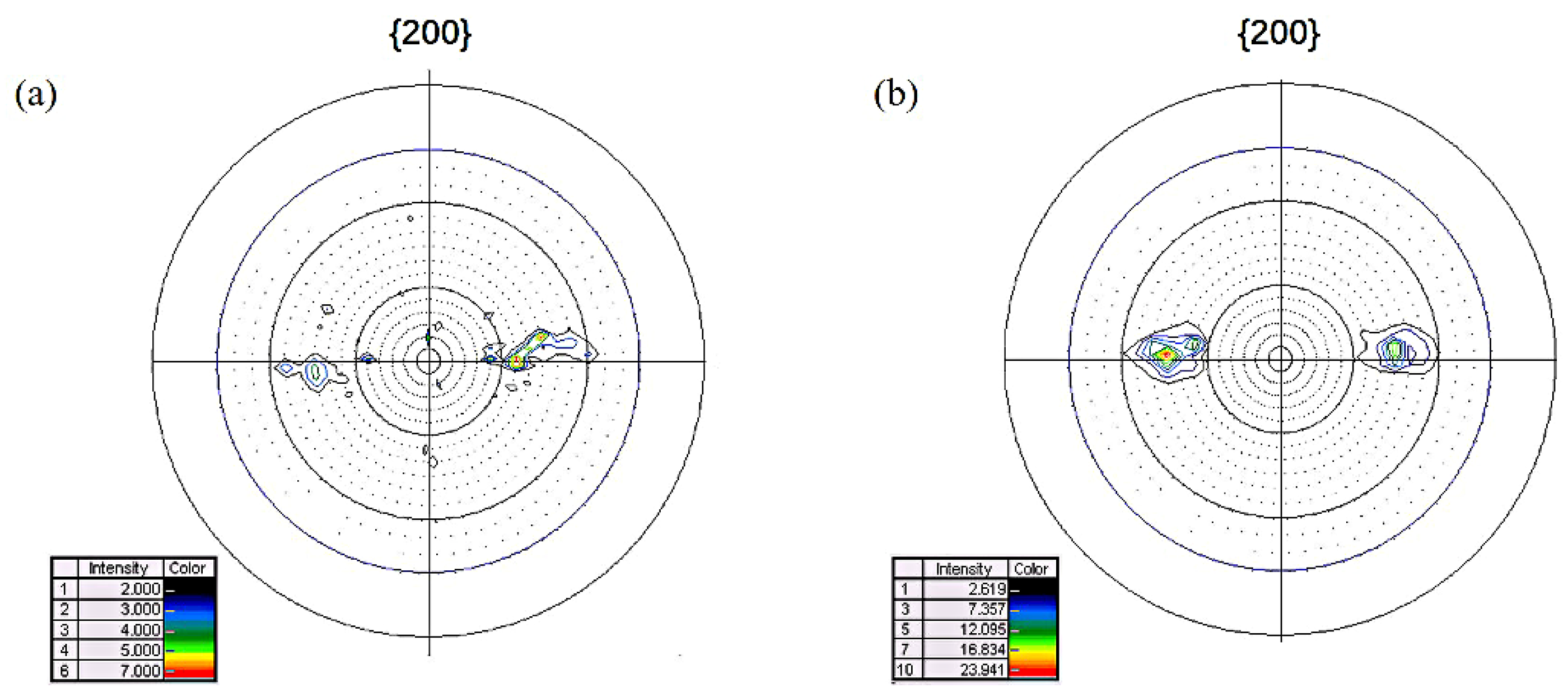
3.2. Magnetic Property and Texture
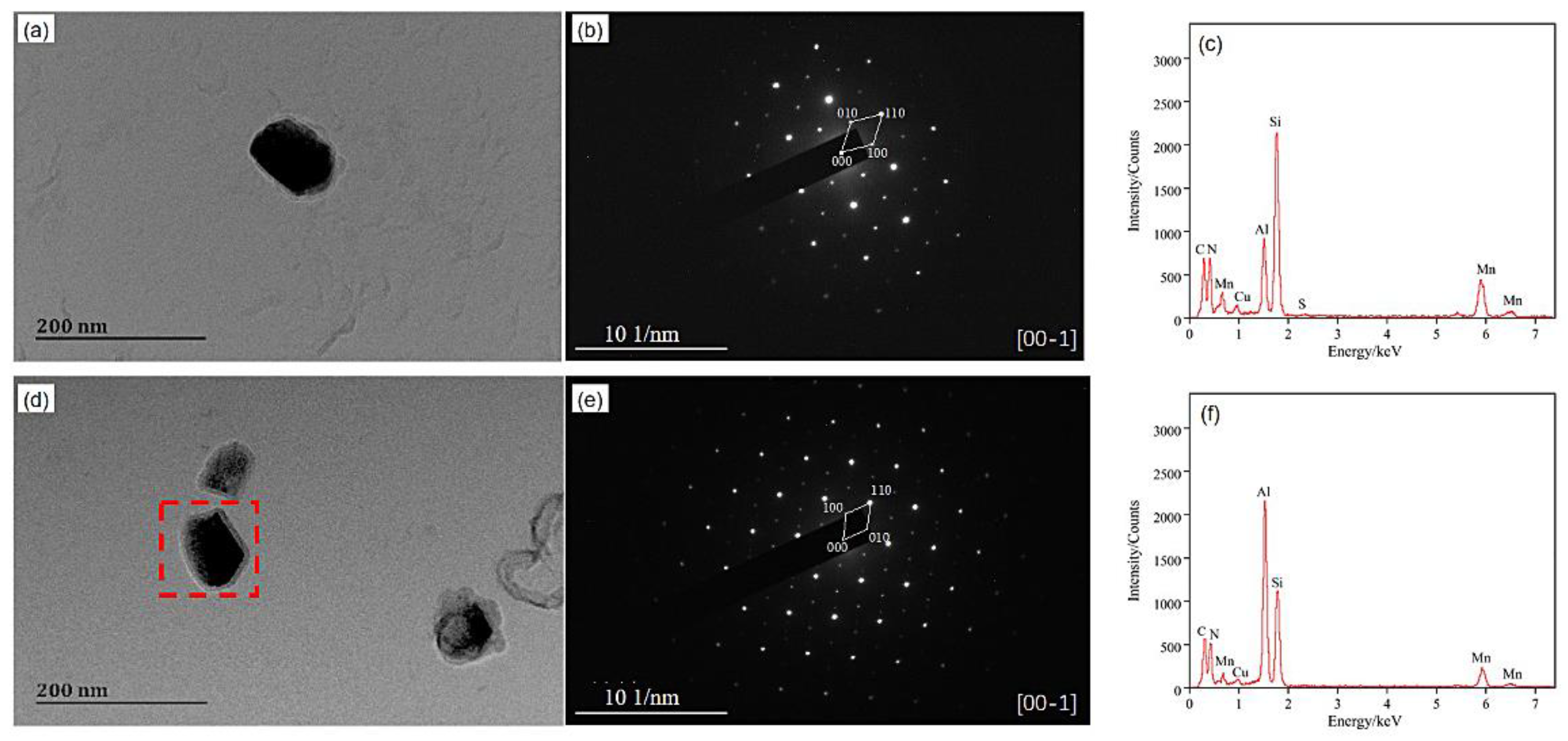
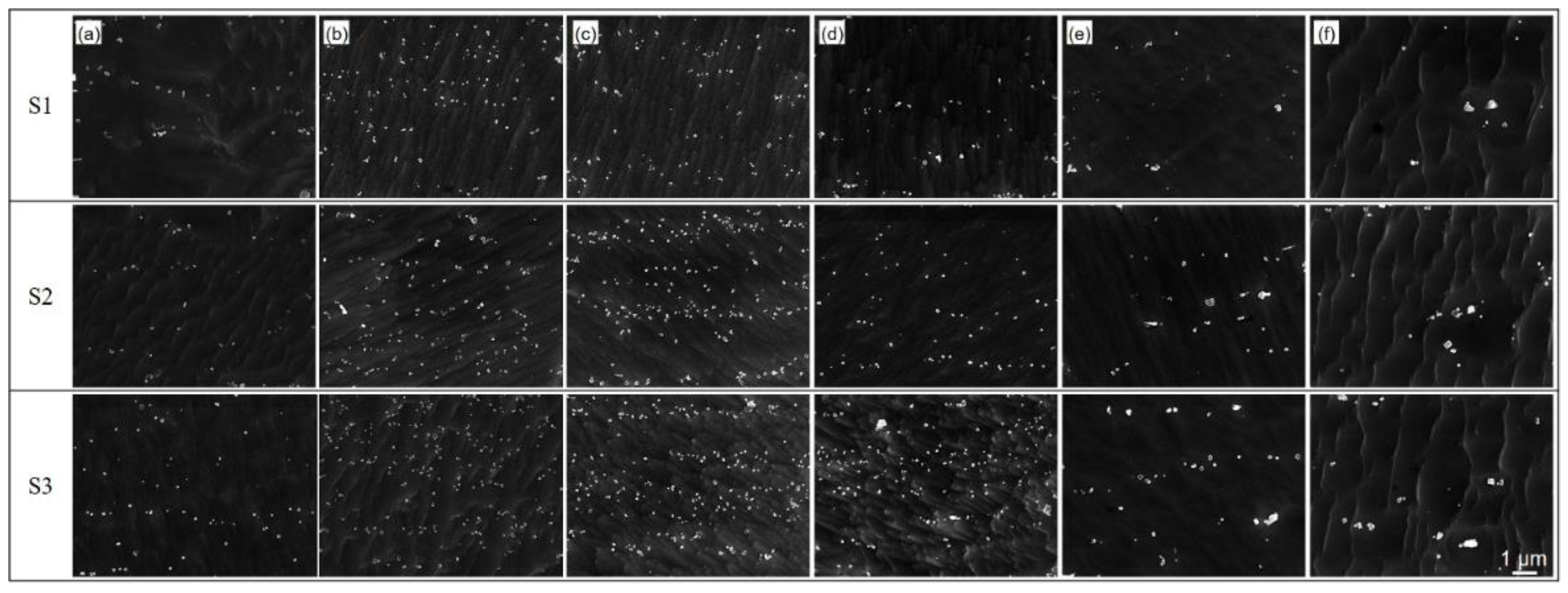
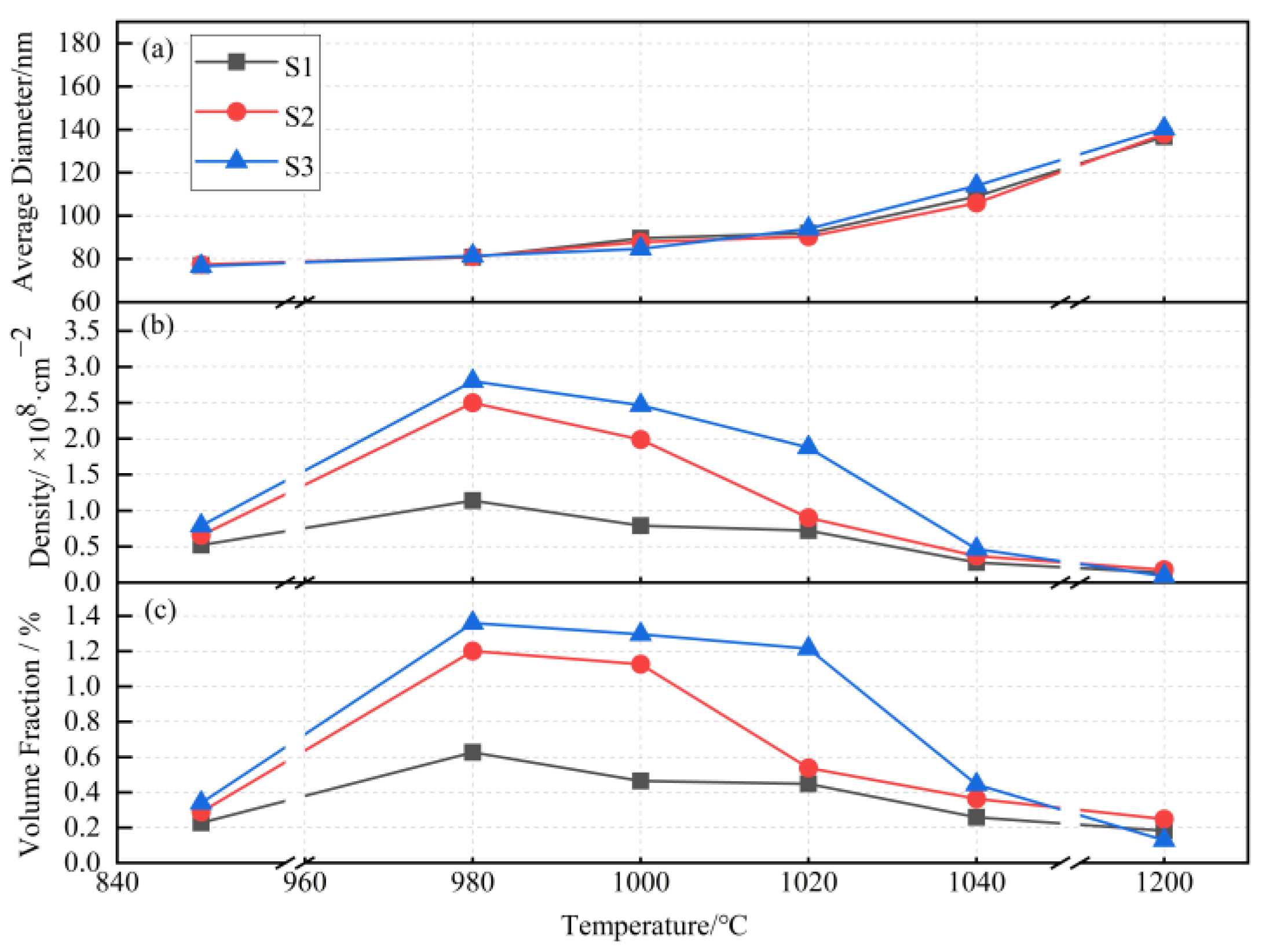
3.3. Precipitate
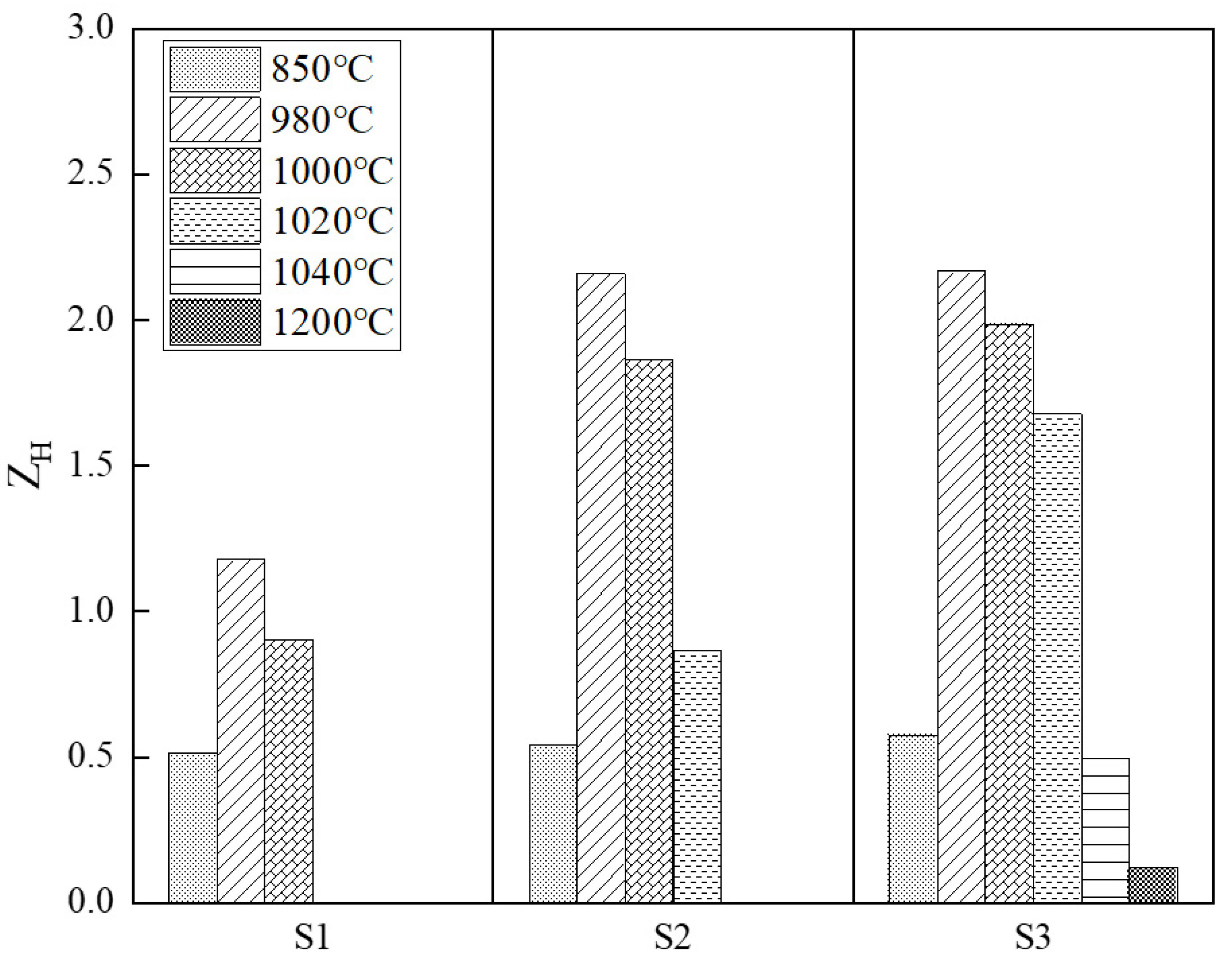
3.4. Effect of Precipitate on Microstructure
4. Conclusions
- The precipitates of slab low temperature reheating grain-oriented silicon steel during high temperature annealing were polygonal, the main composition of which was (Al,Si)N. Both the distribution density and volume fraction of precipitates showed increasing first before 980 °C and then decreasing tendencies, while the precipitate size remained approximately constant before 980 °C and then increased.
- The microstructures after the decarburizing and nitriding processing stages were single ferrite equiaxed grains, with the consistent sizes along the thickness direction of the experimental materials. During the high temperature annealing, the microstructures remained as single ferrite without phase transformation to austenite due to the ultra-low carbon content of below 0.0020 wt.%.
- The primary grain size remained almost unchanged before the onset secondary recrystallization temperature. Abnormal growth started from the surfaces and the growth rate was anisotropic. The appropriate onset temperature for secondary recrystallization ranged from 1000 °C to 1020 °C, when the Goss texture could be well developed and perfect magnetic property could be obtained.
- The aluminum element had a significant effect on the distribution density and volume fraction of precipitates during high temperature annealing, which further affected the onset secondary recrystallization temperature as well as the magnetic property. The more the aluminum content, the higher the onset secondary recrystallization temperature and the better the magnetic property. However, when the aluminum content was as high as 0.035 wt.%, the excessive precipitates made ZH quickly miss the range 0.2 < ZH < 1 which was suitable for secondary recrystallization, leading to both normal and abnormal grain growth, as a result, the secondary recrystallization structure could not be developed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoyka, V.; Kováč, F.; Stupakov, O.; Petryshynets, I. Texture evolution in Fe–3% Si steel treated under unconventional annealing conditions. Mater. Charact. 2010, 61, 1066–1073. [Google Scholar] [CrossRef]
- Kubota, T.; Fujikura, M.; Ushigami, Y. Recent progress and future trend on grain-oriented silicon steel. J. Magn. Magn. Mater. 2000, 215–216, 69–73. [Google Scholar] [CrossRef]
- Liao, C.C.; Hou, C.K. Effect of nitriding time on secondary recrystallization behaviors and magnetic properties of grain-oriented electrical steel. J. Magn. Magn. Mater. 2010, 322, 434–442. [Google Scholar] [CrossRef]
- Kumano, T.; Haratani, T.; Fujll, N. Effect of nitriding on grain oriented silicon steel bearing aluminum. ISIJ Int. 2005, 45, 95–100. [Google Scholar] [CrossRef]
- Xia, Z.; Kang, Y.; Wang, Q. Developments in the production of grain-oriented electrical steel. J. Magn. Magn. Mater. 2008, 320, 3229–3233. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Li, G.; Liu, Y.; Liu, Y. Effect of decarburisation and nitriding on the carbon content, precipitates, microstructure and texture of Nb-bearing grain-oriented silicon steel. Mater. High Temp. 2020, 37, 155–164. [Google Scholar] [CrossRef]
- Morawiec, A. On abnormal growth of Goss grains in grain-oriented silicon steel. Scr. Mater. 2011, 64, 466. [Google Scholar] [CrossRef]
- Horký, P.; Pácl, P. Effective Grain Growth Inhibition in Silicon Steel. J. Magn. Magn. Mater. 1984, 41, 14–16. [Google Scholar] [CrossRef]
- Song, H.Y.; Liu, H.T.; Lu, H.H.; An, L.Z.; Zhang, B.G.; Liu, W.Q.; Cao, G.M.; Li, C.G.; Liu, Z.Y.; Wang, G.D. Fabrication of grain-oriented silicon steel by a novel way: Strip casting process. Mater. Lett. 2014, 137, 475–478. [Google Scholar] [CrossRef]
- Bao, S.; Xu, Y.; Zhao, G.; Huang, X.; Xiao, H.; Ye, C.; Song, N.; Chang, Q. Microstructure, texture and precipitates of grain-oriented silicon steel produced by thin slab casting and rolling process. J. Iron Steel Res. Int. 2017, 24, 91–96. [Google Scholar] [CrossRef]
- Alcântara, F.L.D.; Barbosa, R.A.N.M.; Cunha, M.A.D. Aluminium nitride precipitation in Fe-3%Si steel. ISIJ Int. 2013, 53, 1211–1214. [Google Scholar] [CrossRef]
- Ling, C.; Xiang, L.; Qiu, S.; Gan, Y. Effects of Normalizing annealing on grain-oriented silicon steel. J. Iron Steel Res. Int. 2014, 21, 690–694. [Google Scholar] [CrossRef]
- Li, C.; Yang, H.; Wang, Y.; Yu, Y. Texture of Hot Rolled Strip for Fe-3Si Steel Produced by Thin Slab Casting and Rolling. J. Iron Steel Res. Int. 2010, 17, 46–53. [Google Scholar] [CrossRef]
- Zhou, B.; Zhu, C.; Li, G.; Wan, X.; Schneider, J. Effect of Acid-Soluble Aluminum Content on Precipitates and Microstructure of Annealed Hot-Rolled Grain-Oriented Silicon Steel. Steel Res. Int. 2016, 87, 1702–1714. [Google Scholar] [CrossRef]
- Fan, L.; Xiang, L.; Tang, G.; Qiu, S. Evolution of texture and precipitates in low temperature high magnetic induction grain-oriented silicon steel during high temperature annealing. Funct. Mat. 2013, 44, 3486–3491. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Li, J.; Li, B. Evolution of inhibitor in the annealing process at high temperature of low temperature hot roll and nitriding grain-oriented silicon steel. Trans. Mater. Heat Treat. 2011, 32, 84–87. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, Z.; Wang, Q.; Xu, X.; Pan, L. Precipitating characteristics of inhibitors in grain-oriented electrical steel produced by low slab reheating temperature techniques during secondary recrystallization annealing. Heat Treat. Met. 2012, 37, 42–48. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Mao, J.H.; Wang, R.P.; Qiao, X.L. Effect of precipitated phase particles on secondary recrystallization in grain oriented silicon steel. Heat Treat. Met. 2009, 34, 29–32. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Li, J.; Gong, J.; Li, B. Effect of aluminum on secondary recrystallization texture and magnetic properties of grain-oriented silicon steel. J. Iron Steel Res. Int. 2021, 28, 479–487. [Google Scholar] [CrossRef]
- Ushigami, Y.; Kurosawa, F.; Masui, H.; Suga, Y.; Takahashi, N. Precipitation behaviors of injected nitride inhibitors during secondary recrystallization annealing in grain oriented silicon steel. Mater. Sci. Forum. 1996, 204–206, 593–598. [Google Scholar] [CrossRef]
- Marqusee, J.A.; Ross, J. Theory of Ostwald ripening: Competitive growth and its dependence on volume fraction. J. Chem. Phys. 1984, 80, 536. [Google Scholar] [CrossRef]
- Gladman, T. On the Theory of the Effect of Precipitate Particles on Grain Growth in Metals. Proc. R. Soc. Lond. A 1966, 294, 298–309. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five Decades of the Zener Equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Kumano, T.; Ushigami, Y. Grain boundary characteristics of isolated grains in conventional grain oriented silicon steel. ISIJ Int. 2007, 47, 890–897. [Google Scholar] [CrossRef][Green Version]
- Lin, P.; Palumbo, G.; Harase, J.; Aust, K.T. Coincidence site lattice (CSL) grain boundaries and Goss texture development in Fe-3% Si alloy. Acta Mater. 1996, 44, 4677–4683. [Google Scholar] [CrossRef]
- Shibayanagi, T.; Ichimiya, K.; Umakoshi, Y. Grain boundary migration in Fe-3mass% Si alloy bicrystals with twist boundary. Sci. Technol. Adv. Mater. 2000, 1, 87–95. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, K.S.; Woo, J.S.; Chang, S.K.; Rajmohan, N.; Szpunar, J.A. Influence of primary annealing condition on texture development in grain oriented electrical steels. Acta Mater. 2002, 50, 1825–1834. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Li, J.; Wang, X.; Gong, J.; Li, B. Characteristic of Precipitate Evolution during High Temperature Annealing in Grain-Oriented Silicon Steel. Metals 2022, 12, 824. https://doi.org/10.3390/met12050824
Gao Q, Li J, Wang X, Gong J, Li B. Characteristic of Precipitate Evolution during High Temperature Annealing in Grain-Oriented Silicon Steel. Metals. 2022; 12(5):824. https://doi.org/10.3390/met12050824
Chicago/Turabian StyleGao, Qian, Jun Li, Xianhui Wang, Jian Gong, and Bo Li. 2022. "Characteristic of Precipitate Evolution during High Temperature Annealing in Grain-Oriented Silicon Steel" Metals 12, no. 5: 824. https://doi.org/10.3390/met12050824
APA StyleGao, Q., Li, J., Wang, X., Gong, J., & Li, B. (2022). Characteristic of Precipitate Evolution during High Temperature Annealing in Grain-Oriented Silicon Steel. Metals, 12(5), 824. https://doi.org/10.3390/met12050824




