Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting
Abstract
:1. Introduction
2. Experimental Procedure
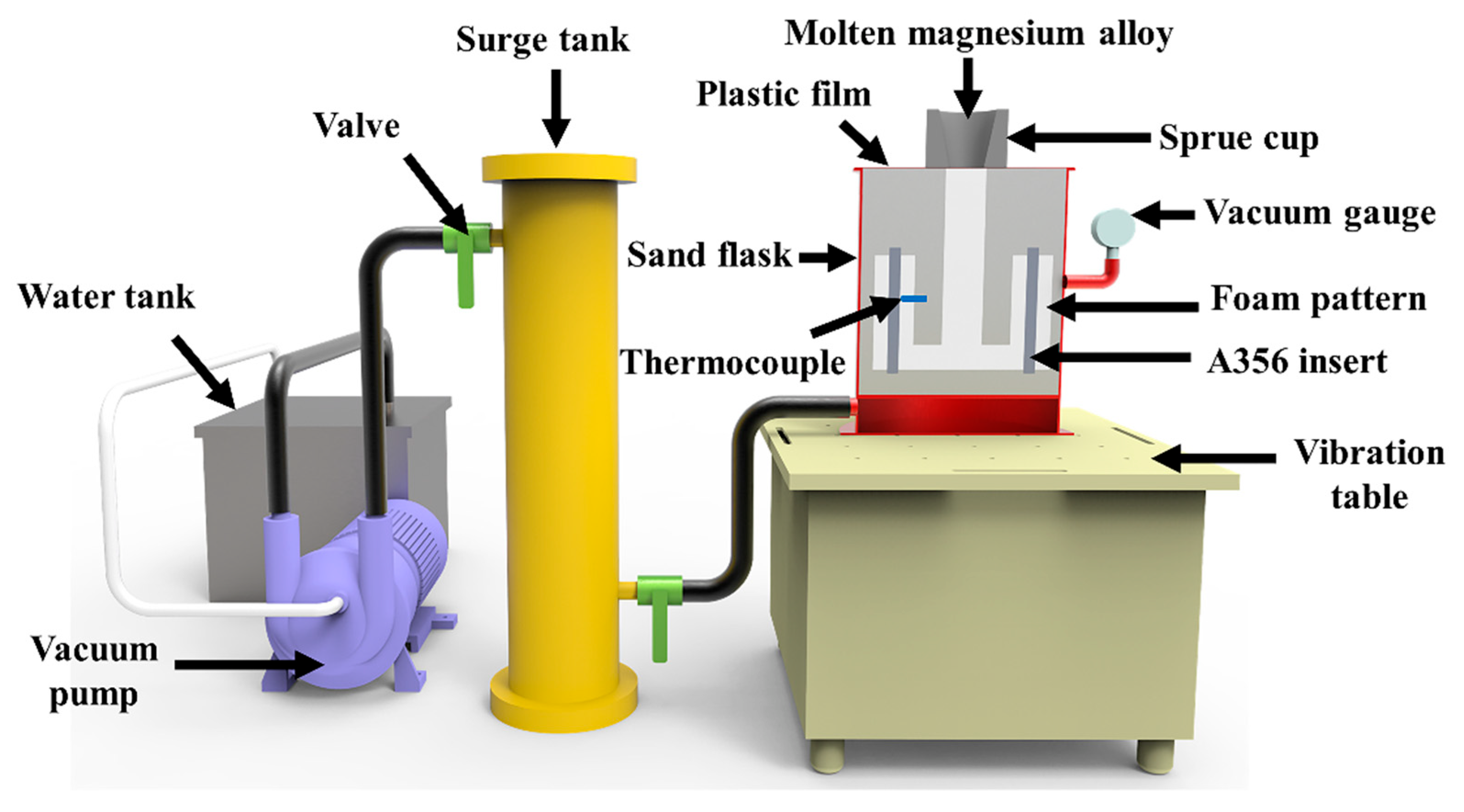
2.1. Materials and Fabrication Process
2.2. Characterization
3. Results
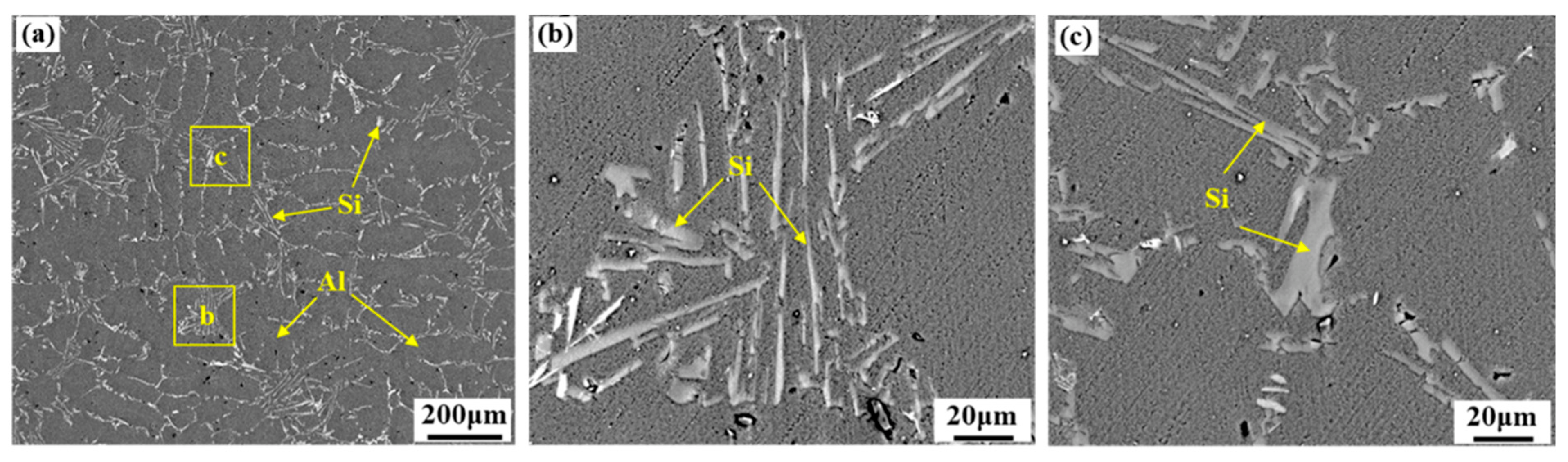
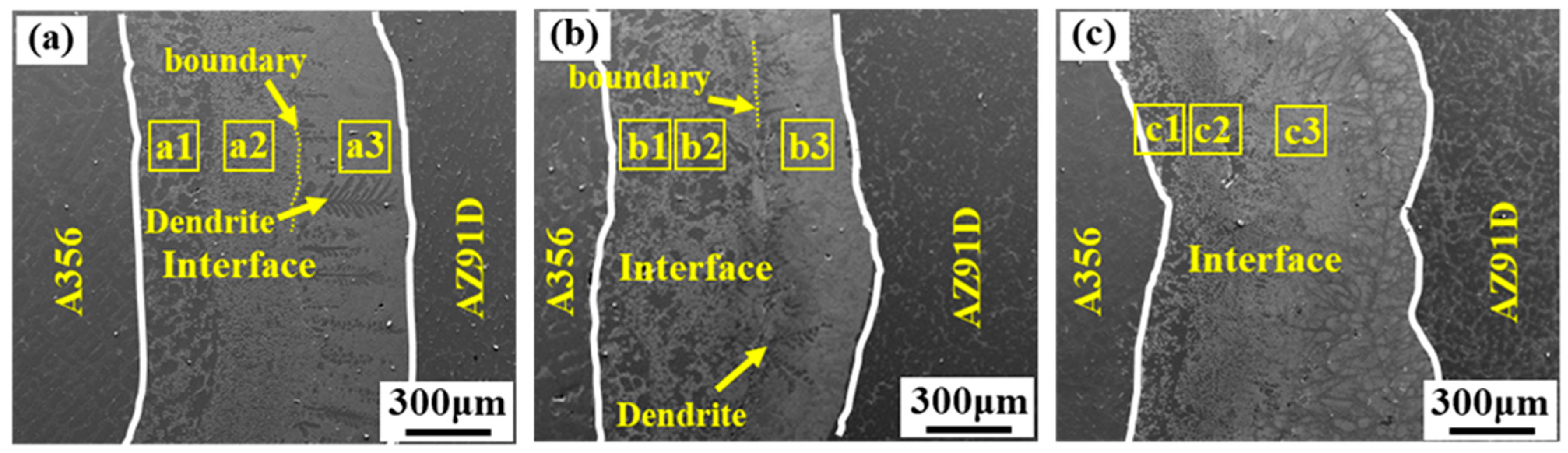
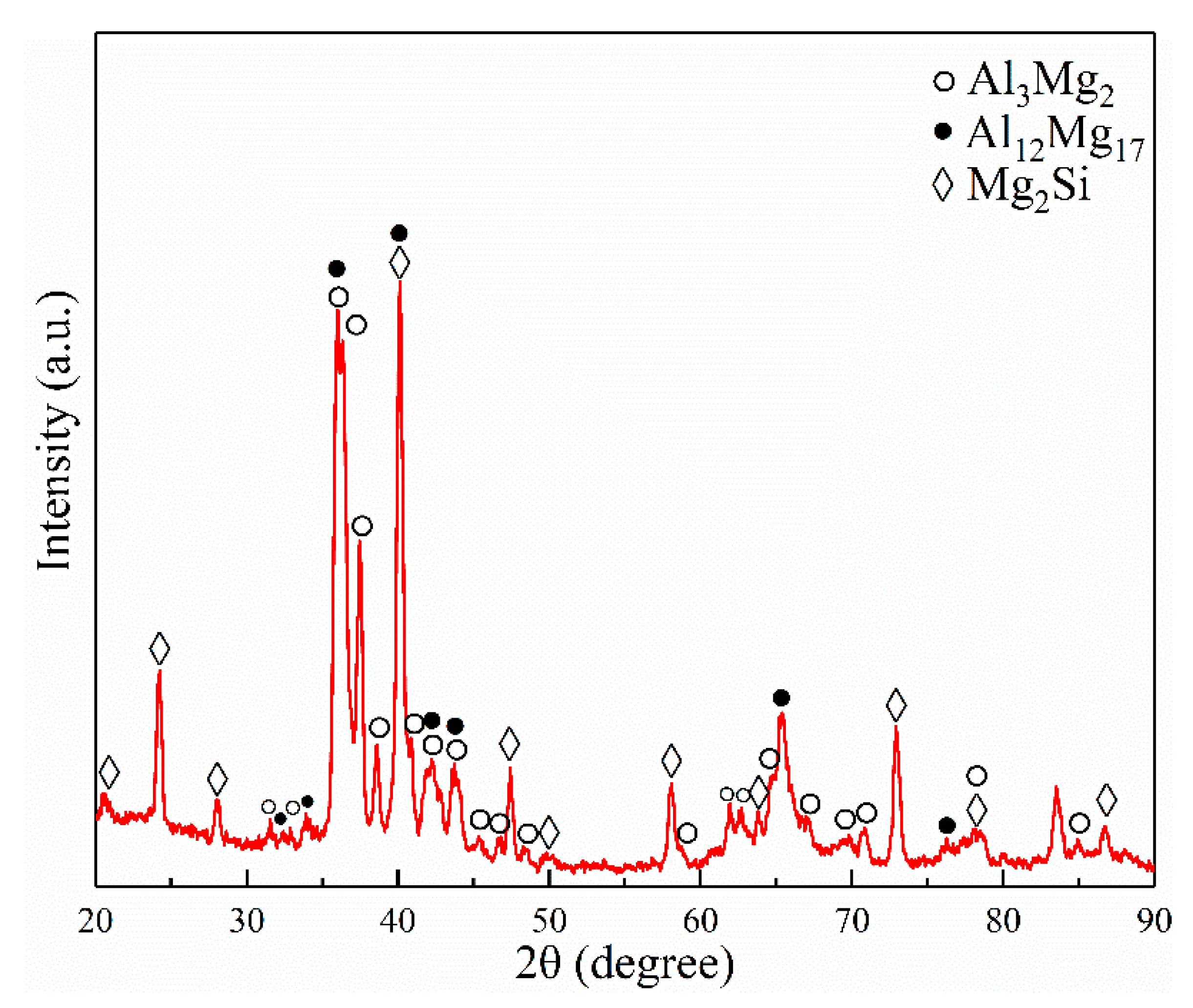
3.1. Effects of the Vibration Acceleration on Microstructure of the Mg–Al Bimetal
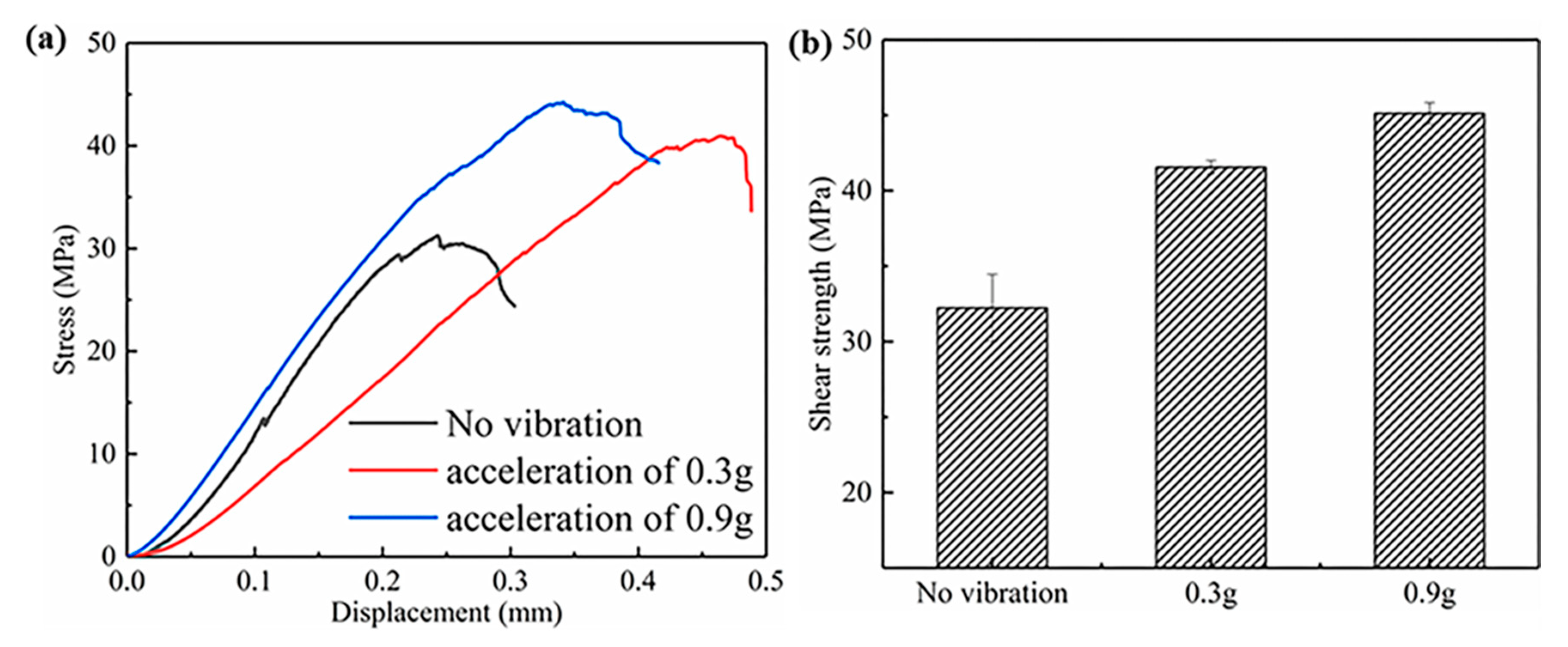
3.2. Effect of the Vibration Acceleration on Bonding Strength of the Mg–Al Bimetal
4. Discussion
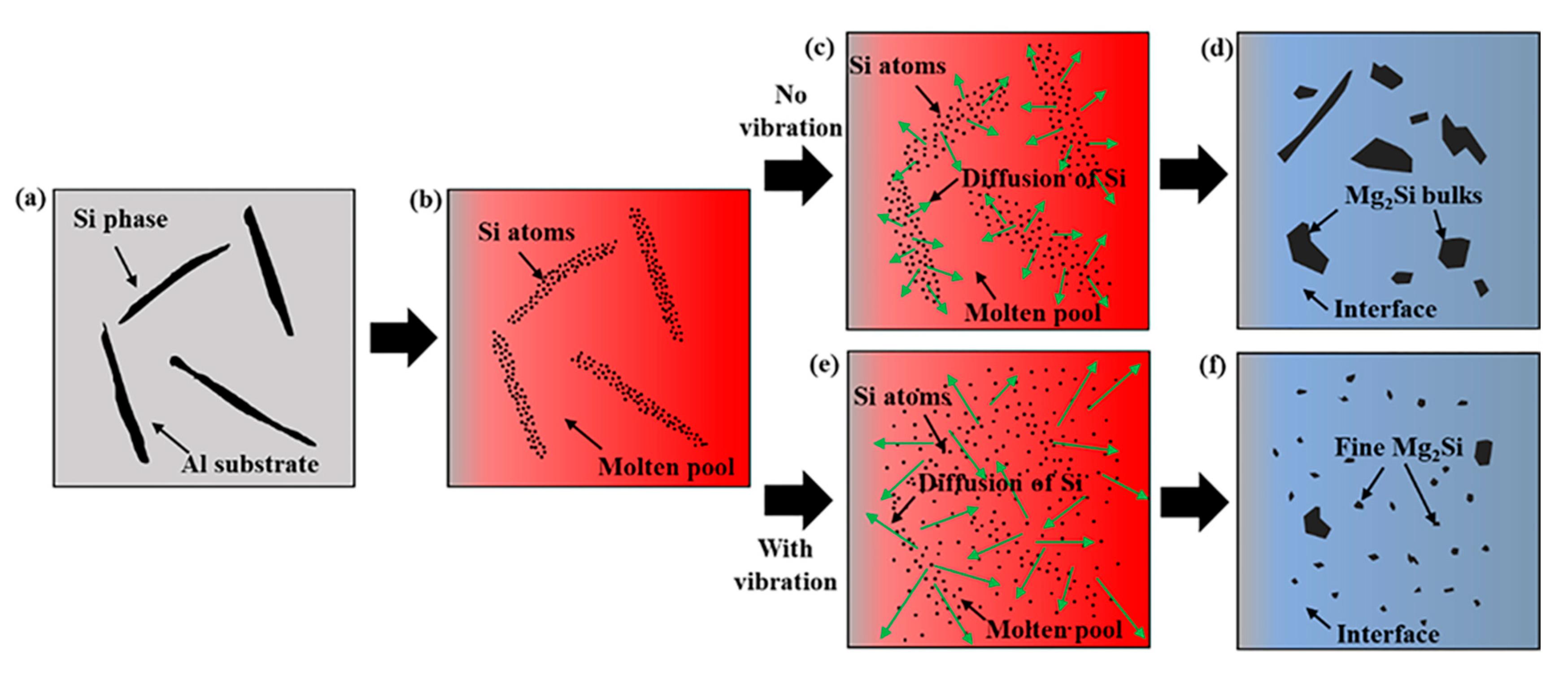
4.1. The Effect of Vibration Acceleration on the Microstructure of Mg–Al Bimetallic Interface
4.2. Strengthening Mechanism of the Mg–Al Bimetal
5. Conclusions
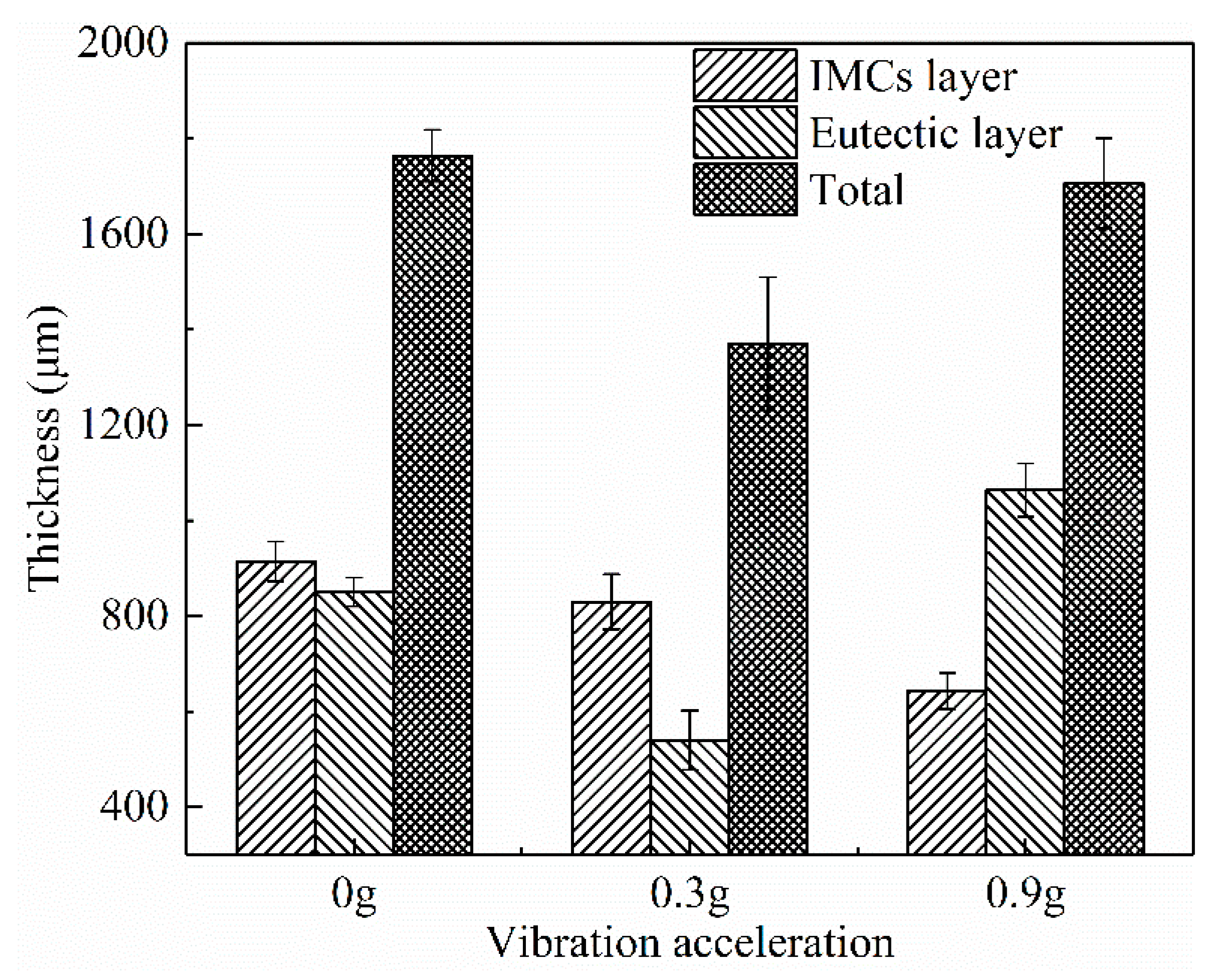
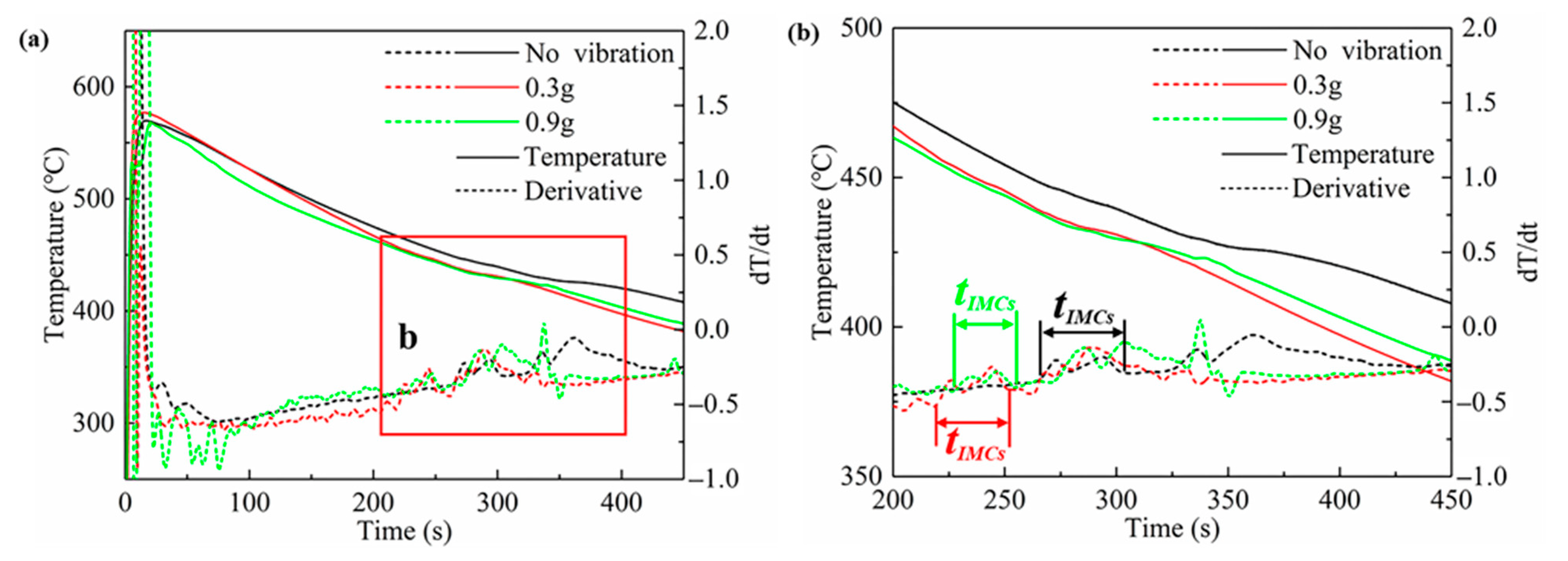
- The interface of the Mg–Al bimetal fabricated by the LFCC process was divided into three areas, named layer I (Al3Mg2 and Mg2Si phases), layer II (Al12Mg17, and Mg2Si phases), and layer III (Al12Mg17 + δ-Mg eutectic structure). With the increase in the vibration acceleration, the cooling rate of the Mg–Al bimetal increased, and the reaction duration of the IMCs layer (including layers I and II) decreased. In addition, the thickness of the IMCs reduced.
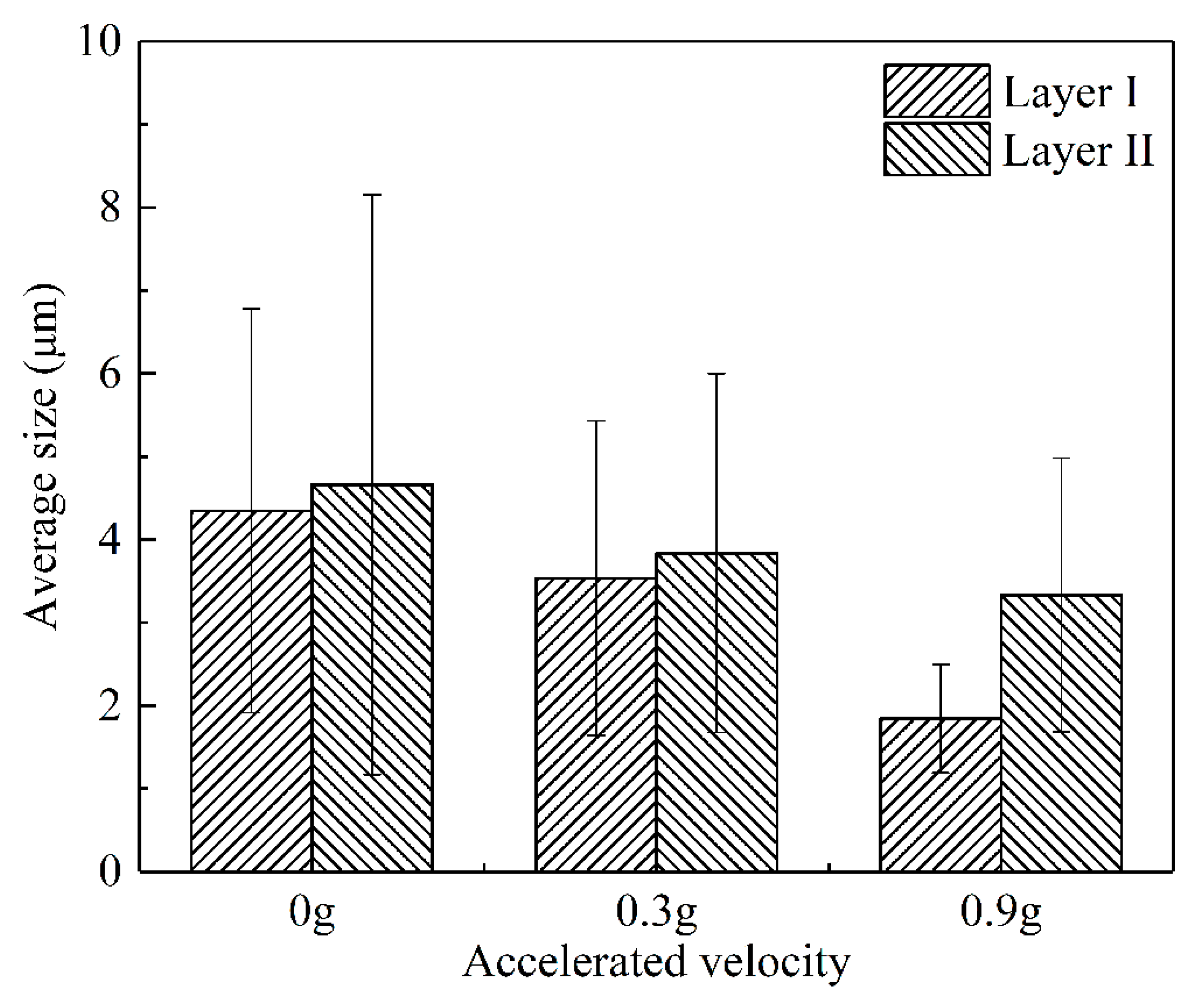
- The vibration promoted the refinement and dispersion of the Mg2Si phase. After applying the vibration, the distribution of the Mg2Si in the IMCs layer became more uniform, and the size of the Mg2Si phase decreased with the increase in the vibration acceleration.
- The shear strength of the Mg–Al bimetal increased with the increase in the vibration acceleration. As the acceleration grew to 0.9 g, the shear strength of the Mg–Al bimetal continued to rise to 45.1 MPa, which was 40% higher than that of the Mg–Al bimetal without vibration. The significant improvement of the shear strength of the Mg–Al bimetal might be attributed to the decrease in the IMC’s thickness, as well as the refinement and uniform distribution of the Mg2Si phase.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, H.M.; Li, W.; Tan, Y.; Tan, Y.B. The Effect of Annealing on the Microstructure and Properties of Ultralow-Temperature Rolled Mg–2Y–0.6Nd–0.6Zr Alloy. Metals 2021, 11, 315. [Google Scholar] [CrossRef]
- Yang, X.K.; Xiong, B.Q.; Li, X.W.; Yan, L.Z.; Li, Z.H.; Zhang, Y.A.; Li, Y.N.; Wen, K.; Liu, H.W. Effect of Li addition on mechanical properties and ageing precipitation behavior of extruded Al−3.0Mg−0.5Si alloy. J. Cent. South Univ. 2021, 28, 2636–2646. [Google Scholar] [CrossRef]
- Li, Z.C.; Deng, Y.L.; Yuan, M.F.; Zhang, J.; Guo, X.B. Effect of isothermal compression and subsequent heat treatment on grain structures evolution of Al-Mg-Si alloy. J. Cent. South Univ. 2021, 28, 2670–2686. [Google Scholar] [CrossRef]
- Zhang, D.D.; Liu, C.M.; Wan, Y.C.; Jiang, S.N.; Zeng, G. Microstructure and anisotropy of mechanical properties in ring rolled AZ80-Ag alloy. J. Cent. South Univ. 2021, 28, 1316–1323. [Google Scholar] [CrossRef]
- Suhuddin, U.F.H.; Fischer, V.; dos Santos, J.F. The Thermal Cycle during the Dissimilar Friction Spot Welding of Aluminum and Magnesium Alloy. Scr. Mater. 2013, 68, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Zhang, Z.; Fan, Z. Microstructure, Mechanical Properties and Corrosion Resistance of A356 Aluminum/AZ91D Magnesium Bimetal Prepared by a Compound Casting Combined with a Novel Ni-Cu Composite Interlayer. J. Mater. Process. Technol. 2021, 288, 116874. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Li, J.; Ma, Z.; Yan, J. Control Al/Mg Intermetallic Compound Formation during Ultrasonic-Assisted Soldering Mg to Al. Ultrason. Sonochem. 2018, 46, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Xin, Y.; Guo, F.; Yu, H.; Wu, Y.; Liu, Q. Compressive Mechanical Behavior of Al/Mg Composite Rods with Different Types of Al Sleeve. Acta Mater. 2016, 120, 379–390. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, R.; Klarner, A.D.; Luo, A.A.; Chen, Y. Predicting and Controlling Interfacial Microstructure of Magnesium/Aluminum Bimetallic Structures for Improved Interfacial Bonding. J. Magnes. Alloys 2020, 8, 578–586. [Google Scholar] [CrossRef]
- He, K.; Zhao, J.; Li, P.; He, J.; Tang, Q. Investigation on Microstructures and Properties of Arc-Sprayed-Al/AZ91D Bimetallic Material by Solid–Liquid Compound Casting. Mater. Des. 2016, 112, 553–564. [Google Scholar] [CrossRef]
- Hajjari, E.; Divandari, M.; Razavi, S.H.; Emami, S.M.; Homma, T.; Kamado, S. Dissimilar Joining of Al/Mg Light Metals by Compound Casting Process. J. Mater. Sci. 2011, 46, 6491–6499. [Google Scholar] [CrossRef]
- Emami, S.M.; Divandari, M.; Hajjari, E.; Arabi, H. Comparison between Conventional and Lost Foam Compound Casting of Al/Mg Light Metals. Int. J. Cast Met. Res. 2013, 26, 43–50. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Yang, L.; Liu, X. Effects of Melt-to-Solid Insert Volume Ratio on the Microstructures and Mechanical Properties of Al/Mg Bimetallic Castings Produced by Lost Foam Casting. Metall. Mater. Trans. A 2016, 47, 6487–6497. [Google Scholar] [CrossRef]
- Jiang, W.; Li, G.; Fan, Z.; Wang, L.; Liu, F. Investigation on the Interface Characteristics of Al/Mg Bimetallic Castings Processed by Lost Foam Casting. Metall. Mater. Trans. A 2016, 47, 2462–2470. [Google Scholar] [CrossRef]
- Fan, S.; Jiang, W.; Li, G.; Mo, J.; Fan, Z. Fabrication and Microstructure Evolution of Al/Mg Bimetal Using a near-Net Forming Process. Mater. Manuf. Processes 2017, 32, 1391–1397. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Jiang, W.; Guan, F.; Jiang, H.; Wu, Y.; Fan, Z. The Role of Vacuum Degree in the Bonding of Al/Mg Bimetal Prepared by a Compound Casting Process. J. Mater. Process. Technol. 2019, 265, 112–121. [Google Scholar] [CrossRef]
- Abdollahzadeh, A.; Shokuhfar, A.; Cabrera, J.M.; Zhilyaev, A.P.; Omidvar, H. The Effect of Changing Chemical Composition on Dissimilar Mg/Al Friction Stir Welded Butt Joints Using Zinc Interlayer. J. Manuf. Processes 2018, 34, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luo, G.; Wang, Y.; Xiao, Y.; Shen, Q.; Zhang, L. Effect of Al Thin Film and Ni Foil Interlayer on Diffusion Bonded Mg–Al Dissimilar Joints. J. Alloys Compd. 2013, 556, 139–142. [Google Scholar] [CrossRef]
- Wang, Y.; Prangnell, P.B. Evaluation of Zn-Rich Coatings for IMC Reaction Control in Aluminum-Magnesium Dissimilar Welds. Mater. Charact. 2018, 139, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, Z.; Zhao, D.; Liu, X.; Yan, J. Effects of Zn on Intermetallic Compounds and Strength of Al/Mg Joints Ultrasonically Soldered in Air. J. Mater. Process. Technol. 2019, 271, 384–393. [Google Scholar] [CrossRef]
- Mola, R.; Bucki, T.; Gwoździk, M. The Effect of a Zinc Interlayer on the Microstructure and Mechanical Properties of a Magnesium Alloy (AZ31)–Aluminum Alloy (6060) Joint Produced by Liquid–Solid Compound Casting. JOM 2019, 71, 2078–2086. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Liu, C.; Liang, C.; Zhang, Y. Influence of Ni Interlayer on Microstructure and Mechanical Properties of Mg/Al Bimetallic Castings. Met. Mater. Trans. A 2018, 49, 3556–3564. [Google Scholar] [CrossRef]
- Xu, G.; Chen, Y.; Liu, L.; Luo, A.; Ma, L. Effect of La on Structures and Properties of the Liquid-Solid Diffusion Bonding Interface of Magnesium/Aluminum. Chin. J. Nonferrous Met. 2014, 24, 2743–2748. [Google Scholar]
- Wu, L.; Kang, H.; Chen, Z.; Liu, N.; Wang, T. Horizontal Continuous Casting Process under Electromagnetic Field for Preparing AA3003/AA4045 Clad Composite Hollow Billets. Trans. Nonferrous Met. Soc. China 2015, 25, 2675–2685. [Google Scholar] [CrossRef]
- Tayal, R.K.; Kumar, S.; Singh, V. Experimental Investigation and Optimization of Process Parameters for Shear Strength of Compound Cast Bimetallic Joints. Trans. Indian Inst. Met. 2018, 71, 2173–2183. [Google Scholar] [CrossRef]
- Tayal, R.K.; Kumar, S.; Singh, V.; Gupta, A.; Ujjawal, D. Experimental Investigation and Evaluation of Joint Strength of A356/Mg Bimetallic Fabricated Using Compound Casting Process. Int. Metalcast. 2019, 13, 686–699. [Google Scholar] [CrossRef]
- Babaee, M.H.; Maleki, A.; Niroumand, B. A Novel Method to Improve Interfacial Bonding of Compound Squeeze Cast Al/Al−Cu Macrocomposite Bimetals: Simulation and Experimental Studies. Trans. Nonferrous Met. Soc. China 2019, 29, 1184–1199. [Google Scholar] [CrossRef]
- Wang, C.; Cui, J. Application and Research of Vibration Technology in Metal Casting Process. Foundry Technol. 2018, 39, 2240–2243. [Google Scholar]
- Yuanyang, L.I.; Chen, W.; Zheng, K.; Wang, J.; Zheng, Z. Effect of Mechanical Vibration on Bimetallic Compound Casting Behavior. Foundry Technol. 2017, 38, 377–381. [Google Scholar]
- Zhao, J.; Shang, Z.; Wang, L.; Hou, Z.; Wang, Z. Effect of Mechanical Vibration on Heat Transfer of Casting-Mold Interface in Filling and Solidification Process of AZ91 Magnesium Alloy. Rare Met. Mater. Eng. 2015, 44, 3141–3146. [Google Scholar]
- Promakhov, V.; Khmeleva, M.; Zhukov, I.; Platov, V.; Khrustalyov, A.; Vorozhtsov, A. Influence of Vibration Treatment and Modification of A356 Aluminum Alloy on Its Structure and Mechanical Properties. Metals 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Jin, Y.; Ji, S.; Yan, D. Improving Friction Stir Weldability of Al/Mg Alloys via Ultrasonically Diminishing Pin Adhesion. J. Mater. Sci. Technol. 2018, 34, 1817–1822. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Improving mechanical properties of AZ91D magnesium/A356 aluminum bimetal prepared by compound casting via a high velocity oxygen fuel sprayed Ni coating. J. Magnes. Alloys 2021, in press. [Google Scholar] [CrossRef]
- Wen, F.; Zhao, J.; Yuan, M.; Wang, J.; Zheng, D.; Zhang, J.; He, K.; Shangguan, J.; Guo, Y. Influence of Ni interlayer on interfacial microstructure and mechanical properties of Ti-6Al-4V/AZ91D bimetals fabricated by a solid–liquid compound casting process. J. Magnes. Alloys 2021, 9, 1382–1395. [Google Scholar] [CrossRef]
- Feng, B.; Feng, X.; Yan, C.; Xin, Y.; Wang, H.; Wang, J.; Zheng, K. On the rule of mixtures for bimetal composites without bonding. J. Magnes. Alloys 2020, 8, 1253–1261. [Google Scholar] [CrossRef]
- Jia, B.R.; Liu, L.B.; Yi, D.Q.; Jin, Z.P.; Nie, J.F. Thermodynamic Assessment of the Al–Mg–Sm System. J. Alloys Compd. 2008, 459, 267–273. [Google Scholar] [CrossRef]
- Yan, X.-Y.; Chang, Y.A.; Zhang, F. A Thermodynamic Analysis of the Mg-Si System. J. Phase Equilibria 2000, 21, 379. [Google Scholar] [CrossRef]
- Zhao, X.; Bhushan, B. Material Removal Mechanisms of Single-Crystal Silicon on Nanoscale and at Ultralow Loads. Wear 1998, 223, 66–78. [Google Scholar] [CrossRef]
- Rodríguez-González, B.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Bending Contours in Silver Nanoprisms. J. Phys. Chem. B 2006, 110, 11796–11799. [Google Scholar] [CrossRef]
- Imai, M.; Isoda, Y.; Udono, H. Thermal Expansion of Semiconducting Silicides β-FeSi2 and Mg2Si. Intermetallics 2015, 67, 75–80. [Google Scholar] [CrossRef]
- Trebin, H.-R. Quasicrystals: Structure and Physical Properties; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 3-527-40399-X. [Google Scholar]
- Lyubimova, T.P.; Parshakova, Y.N. Effect of Rotational Vibrations on Directional Solidification of High-Temperature Binary SiGe Alloys. Int. J. Heat Mass Transf. 2018, 120, 714–723. [Google Scholar] [CrossRef]
- Ihsan-ul-haq; Shin, J.-S.; Lee, Z.-H. Computer-Aided Cooling Curve Analysis of A356 Aluminum Alloy. Met. Mater. Int. 2004, 10, 89–96. [Google Scholar] [CrossRef]
- Xiao, Y.; Ji, H.; Li, M.; Kim, J. Ultrasound-Induced Equiaxial Flower-like CuZn5/Al Composite Microstructure Formation in Al/Zn–Al/Cu Joint. Mater. Sci. Eng. A 2014, 594, 135–139. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Jiang, H.; Fan, Z. Effect of Insert Materials on Microstructure and Mechanical Properties of Al/Mg Bimetal Produced by a Novel Solid-Liquid Compound Process. J. Manuf. Process. 2019, 47, 62–73. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Zhu, Z.; Xu, G.; Luo, A.A.; Liu, L. Inhibiting Brittle Intermetallic Layer in Magnesium/Aluminum Bimetallic Castings via In Situ Formation of Mg2Si Phase. Met. Mater. Trans. B 2019, 50, 1547–1552. [Google Scholar] [CrossRef]
- Zamani, R.; Mirzadeh, H.; Emamy, M. Mechanical Properties of a Hot Deformed Al-Mg2Si in-Situ Composite. Mater. Sci. Eng. A 2018, 726, 10–17. [Google Scholar] [CrossRef]
- Maleki, M.; Mirzadeh, H.; Emamy, M. Improvement of Mechanical Properties of in Situ Mg-Si Composites via Cu Addition and Hot Working. J. Alloy Compd. 2022, 905, 164176. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, G.; Ren, L.; Liu, B.; Al-Ezzi, S.; Pan, H. Effect of Zn Interlayer Thickness on the Microstructure and Mechanical Properties of Two-Step Diffusion Bonded Joint of ZK60Mg and 5083Al. Vacuum 2019, 161, 353–360. [Google Scholar] [CrossRef]
- Lloyd, D.J. Aspects of Fracture in Particulate Reinforced Metal Matrix Composites. Acta Met. Mater. 1991, 39, 59–71. [Google Scholar] [CrossRef]
- Wang, Z.; Song, M.; Sun, C.; He, Y. Effects of Particle Size and Distribution on the Mechanical Properties of SiC Reinforced Al–Cu Alloy Composites. Mater. Sci. Eng. A 2011, 528, 1131–1137. [Google Scholar] [CrossRef]

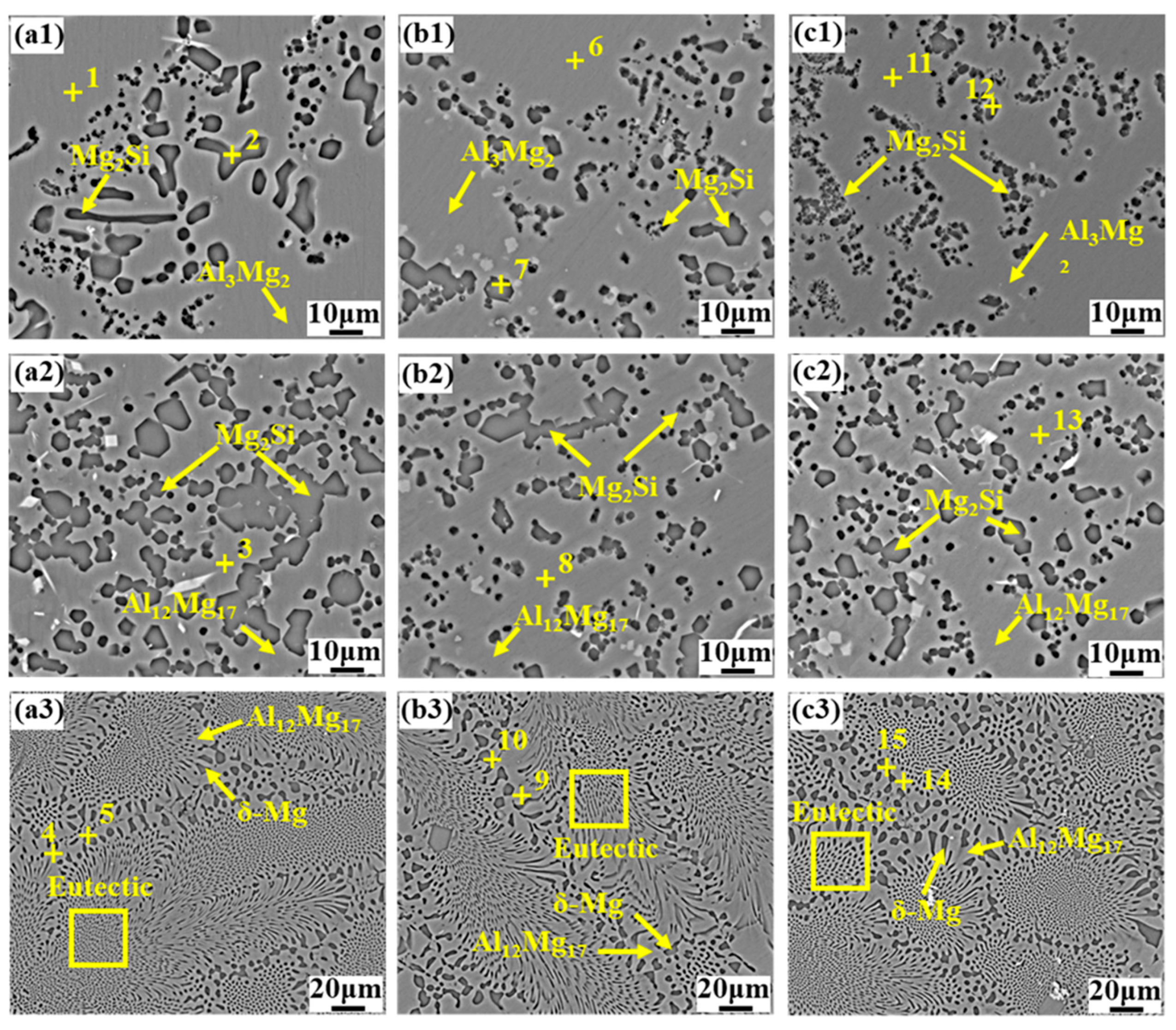

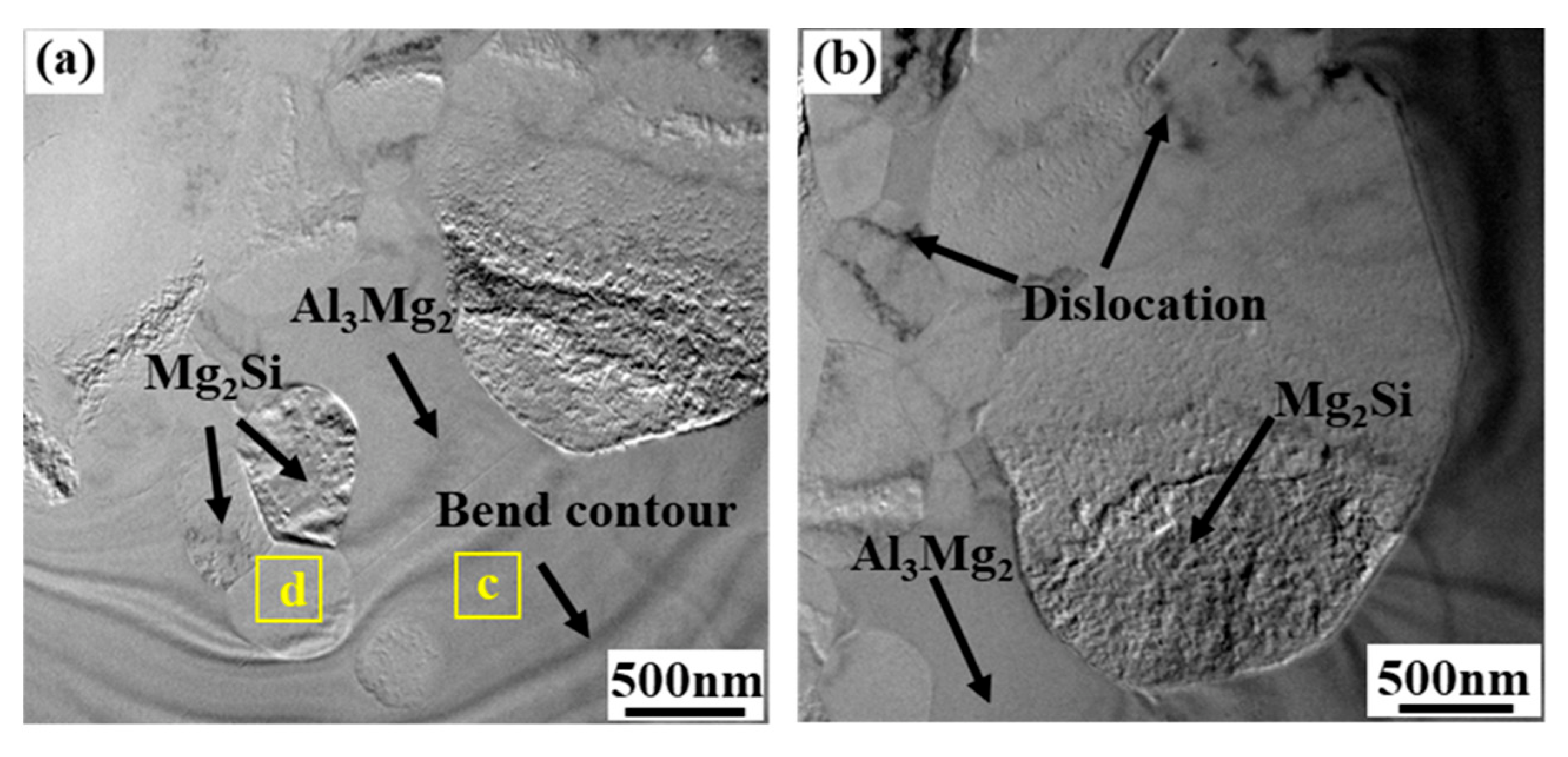
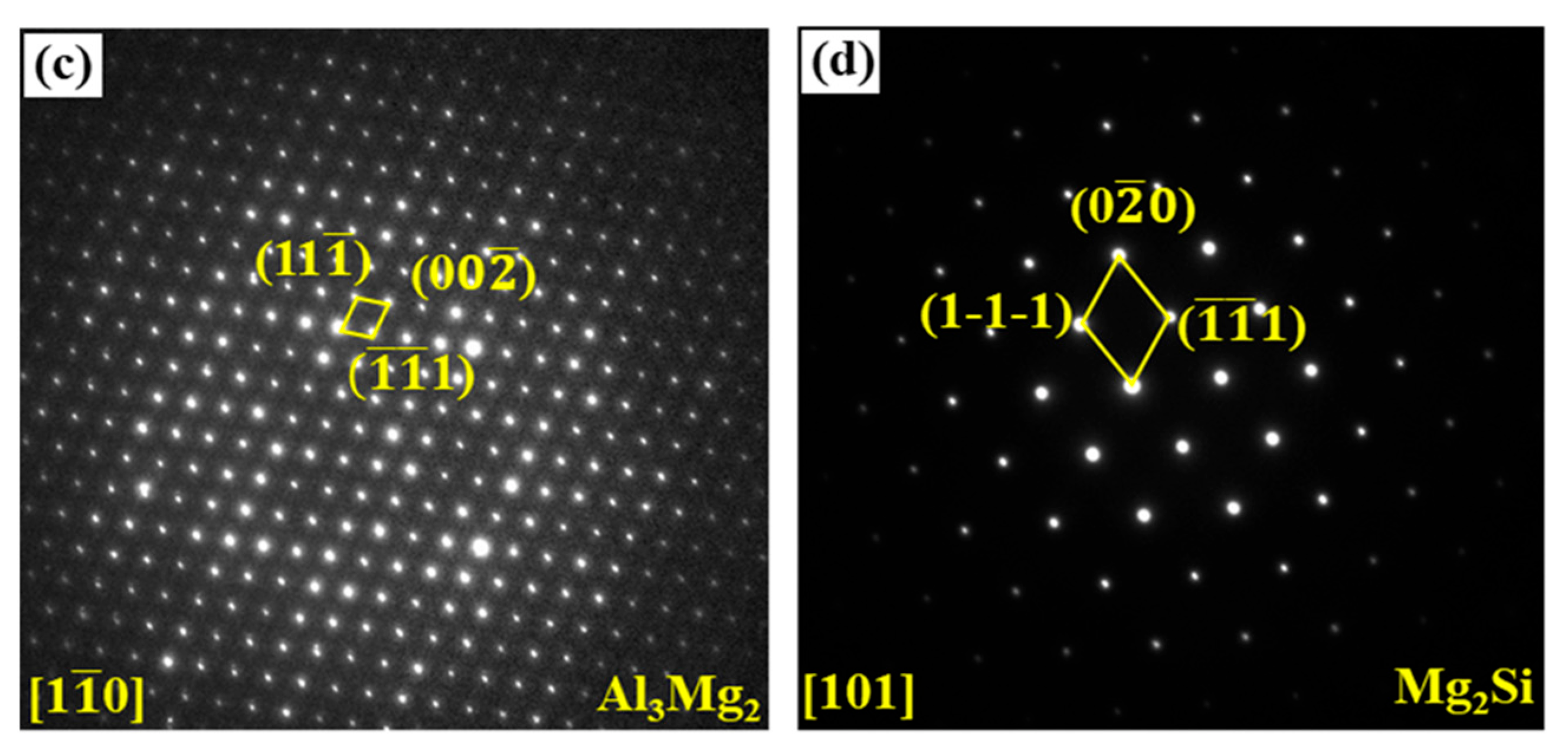

| Area No. | Element Compositions (At.%) | Possible Phase | ||
|---|---|---|---|---|
| Mg | Al | Si | ||
| 1 | 38.73 | 61.27 | - | Al3Mg2 |
| 2 | 58.02 | 12.56 | 29.43 | Mg2Si |
| 3 | 50.82 | 49.18 | - | Al12Mg17 |
| 4 | 63.94 | 36.06 | - | Al12Mg17 |
| 5 | 83.66 | 16.34 | - | δ-Mg |
| 6 | 38.73 | 61.27 | - | Al3Mg2 |
| 7 | 61.23 | 14.84 | 23.93 | Mg2Si |
| 8 | 49.45 | 50.55 | - | Al12Mg17 |
| 9 | 65.65 | 34.35 | - | Al12Mg17 |
| 10 | 77.70 | 22.30 | - | δ-Mg |
| 11 | 38.74 | 61.26 | - | Al3Mg2 |
| 12 | 61.87 | 22.09 | 16.03 | Mg2Si |
| 13 | 49.28 | 50.72 | - | Al12Mg17 |
| 14 | 63.43 | 36.57 | - | Al12Mg17 |
| 15 | 85.78 | 14.22 | - | δ-Mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, F.; Fan, S.; Wang, J.; Li, G.; Zhang, Z.; Jiang, W. Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting. Metals 2022, 12, 766. https://doi.org/10.3390/met12050766
Guan F, Fan S, Wang J, Li G, Zhang Z, Jiang W. Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting. Metals. 2022; 12(5):766. https://doi.org/10.3390/met12050766
Chicago/Turabian StyleGuan, Feng, Suo Fan, Junlong Wang, Guangyu Li, Zheng Zhang, and Wenming Jiang. 2022. "Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting" Metals 12, no. 5: 766. https://doi.org/10.3390/met12050766
APA StyleGuan, F., Fan, S., Wang, J., Li, G., Zhang, Z., & Jiang, W. (2022). Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting. Metals, 12(5), 766. https://doi.org/10.3390/met12050766






